Abstract
Evoked potentials (EP) characterize signal conduction in selected tracts of the central nervous system in a quantifiable way. Since alteration of signal conduction is the main mechanism of symptoms and signs in multiple sclerosis (MS), multimodal EP may serve as a representative measure of the functional impairment in MS. Moreover, EP have been shown to be predictive for disease course, and thus might help to select patient groups at high risk of progression for clinical trials. EP can detect deterioration, as well as improvement of impulse propagation, independently from the mechanism causing the change. Therefore, they are candidates for biomarkers with application in clinical phase-II trials. Applicability of EP in multicenter trials has been limited by different standards of registration and assessment.
Keywords: Evoked potentials, visual evoked potentials, somatosensory evoked potentials, motor evoked potentials, multimodal evoked potentials, electrophysiology, biomarker, remyelination, phase-II trials
Introduction
Four years ago, the role of evoked potentials (EP) for diagnosis and monitoring of multiple sclerosis (MS) was discussed in this journal.1,2 The bottom line of the commentary by Hutchinson3 was that despite some strong arguments for the use of EP in predicting and monitoring the disease course, emerging magnetic resonance imaging (MRI) techniques would finally become the methods of choice for these purposes. While advances in imaging and the understanding of its biological substrate have made considerable progress and provide a unique avenue for the characterization of tissue damage and repair,4,5 many of the proposed techniques remain to be validated and are available at specialized centers only. Information gained by EP is widely available at low cost, and it is complementary to structural data, as well as to biochemical and metabolic information. Most importantly, direct functional assessment of myelin, axon and synapses in multisynaptic eloquent sensorimotor pathways is only granted by electrophysiological techniques. In this topical review, we will discuss the current and possible future role of EP in MS with a focus on their suitability as biomarkers, especially in phase-II trials.
EP characterize impulse propagation in the central nervous system
Most clinical symptoms typical of MS are closely related to altered impulse generation and conduction in the central nervous system. Abnormal signal propagation can be due to different mechanisms including demyelination, localized conduction block, frequency-dependent block, and axonal damage, which may be due to different causes such as inflammation, axonal transection, or mitochondrial dysfunction6–10 (see also Figures 1 and 2). As an example, slowing and dispersion of conduction speed has been shown recently to interfere with motion perception.11 A demyelinating lesion in the optic nerve of 10-mm length causes a conduction delay of approximately 25 ms.12 Conversely, the exact mechanism for a delayed or diminished EP, for example, slowed conduction, prolongation, or even replacement of spatial by temporal summation at the synapse due to conduction block or axonal loss, cannot be determined with certainty.7
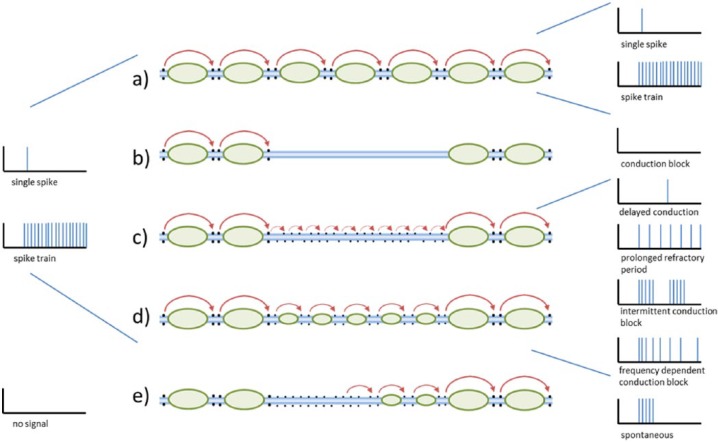
Figure 1.
Signal conduction at the level of single axons; left and right panels: input and output spike and spike trains; middle panel: (a) normal saltatory impulse conduction; (b) conduction block due to demyelinisation; (c) redistribution of sodium channels on demyelinated axon and non-saltatory conduction; (d) partly remyelinated axon with slowed saltatory conduction; and (e) ephaptic/mechanic impulse generation at the demyelinated axon (adapted from Smith8; blue: axon; black dots: sodium channels at the nodes of Ranvier; green: myelin sheath; red arrows: impulse propagation).
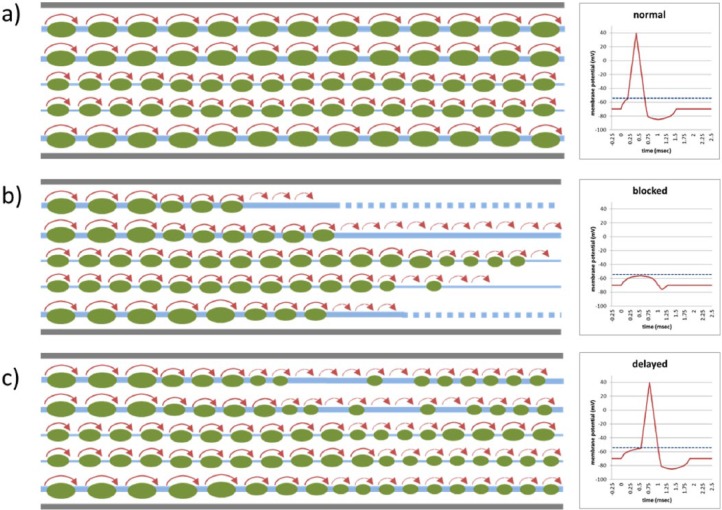
Figure 2.
Signal conduction at the level of tracts (left panels; blue: axons; green: myelin sheath; red: impulse propagation) and membrane potential (red) at the synapse (right panels; blue dotted line: depolarization threshold); (a) normal conduction in axons of different size; (b) blocked conduction as depolarization threshold at the synapse is not reached due to insufficient spatial (too few axons) and temporal (dispersed arrival of volleys) summation; and (c) delayed conduction due to slow impulse propagation but still reaching depolarization threshold.
EP are measures of central signal conduction in vivo and cross at least one central synapse. Sensory EP include brainstem auditory EP (BAEP), visual EP (VEP), and somatosensory EP (SEP). They are elicited by standardized stimuli and recorded over the cortex by averaging the response over a number of repetitions to cancel out background activity. Motor EP (MEP) are recorded over the target muscle in the upper and lower limbs (UL and LL). They are elicited by a short magnetic pulse which induces a depolarizing current in the motor cortex. In SEP and MEP, the duration of peripheral conduction is subtracted from the total latency to deduce the central conduction time (CCT) and central motor conduction time (CMCT), respectively.
Before the advent of MRI, EP were used to document clinically manifest and silent lesions in MS and were part of routine diagnosis (median SEP,13 VEP,14 and MEP15). The sensitivity of an EP study to detect an abnormality depends on the length of the tracts measured and on the probability of the examined functional system suffering from a demyelinating lesion. Therefore, multimodal assessment using a combination of different EP modalities has been proposed.16,17 This approach parallels partly the clinical evaluation and is more appropriate for covering the heterogeneity of MS than single modalities. Several studies using multimodal EP (mmEP) have demonstrated a strong correlation between mmEP score and the Expanded Disability Status Scale (EDSS) cross-sectionally (median rho = 0.64, range: 0.16–0.79 over 13 cohorts; see Table 1).
Table 1.
Overview of studies in MS using EP scores to summarize results from different EP modalities.
| Author (year) | Study type | Disease course (n) | Length of follow up | EP modalities | Score (name; min–max) | EDSS at baseline (mean (SD) or median [range]) | Correlationa with EDSS (c: cross sectional, l: longitudinal) | Prognostic correlationa to EDSS at follow up (p1) or EDSS change (p2) |
|---|---|---|---|---|---|---|---|---|
| O’Connor et al. (1998)18 | Prospective | RR/SP (50) | 2 years | VEP, BAEP, SEP-UL, SEP-LL | Ordinal (EPAS; 0–8) | 2.0 [IQR: 1.0–2.5] | c: 0.68–0.72; l: 0.36 | p2: 0.23 |
| Fuhr et al. (2001)19 | Prospective | RR/SP (25, 5) | 2 years | VEP, MEP-UL, MEP-LL | Quantitative (qEPS) | 4.65 [2–6.5] | c: 0.62–0.72; l: 0.43 | p2: 0.43 |
| Leocani et al. (2006)20 | Retrospective | RR/SP/PP (43, 28, 13) | 2.5 years | VEP, BAEP, SEP-UL, SEP-LL, MEP-UL, MEP-LL | Ordinal (gEPS; 0–36) | RR: 3.0 [1.0–6.0]; SP: 5.0 [2.5–8.0]; PP: 5.5 [2.0–6.5] | c: 0.60; l: 0.18 | p1: 0.46 |
| Kallmann et al. (2006)21 | Retrospective | RR/SP (44, 50) | 10 years | VEP, SEP-LL, MEP-LL | Ordinal (0–12) | RR: 2.0 [0–4.0]; SP: 3.5 [0–7] | RR, c: 0.25; l: NA SP, c: 0.28; l: NA | RR, p1: 0.55, p2: 0.88 SP, p1: 0.56, p2: n.s. |
| Jung et al. (2008)22 | Prospective | RR (37) | 2 years | VEP, SEP-UL, SEP-LL, MEP-UL, MEP-LL | Ordinal (mEPS; 0–70) | 1.5 [0–3.0] | c: 0.28; l: 0.69 | p1: 0.65 |
| Pelayo et al. (2010)23 | Prospective | CIS (245) | 6.4 years | VEP, BAEP, SEP-UL, SEP-LL | Ordinal (0–3) | NA | c: NA; l: NA | Hazard ratio for EDSS ⩾ 3: 7 (95% CI 1.4–34.9) |
| Invernizzi et al. (2011)24 | Retrospective | RR/SP/PP (62, 4, 14) | 8 years | VEP, SEP-LL, MEP-UL, MEP-LL | Ordinal (gEPS; 0–30) | 3.0 [0–6.5] | c: 0.55; l: NA | p1: 0.78 |
| Schlaeger et al. (2012)25 | Prospective | RR/CIS (44, 3) | 3 years | VEP, SEP-UL, SEP-LL, MEP-UL, MEP-LL | Quantitative (qEPS) | RR: 2.0 (0.64); CIS: 2.0 (0.94) | c: 0.64–0.79; l: 0.51 | p1: 0.70 (R2: 0.54), p2: 0.35 |
| Margaritella et al. (2012)26,b | Retrospective | RRb (52) | 2 years | VEP15, VEP30, BAEP, SEP-UL, SEP-LL | Ordinal (mEPS; 0–50) | 2.1 (1.5) | c: 0.34–0.50; l: n.s. | p1: 0.38–53b |
| Ramanathan (2013)27 | Retrospective | RR/SP (53, 10) | 1.6 years | VEP, BAEP, SEP-UL, SEP-LL | Ordinal (CEPS; 0–8) | RR: 1 [1–2]; SP: 6 [3.5–6.5] | c: 0.65; l: NA | p1: 0.57 |
| Schlaeger et al. (2014)28 | Prospective | PP (22) | 3 years | VEP, SEP-UL, SEP-LL, MEP-UL, MEP-LL | Quantitative (qEPS) | 4.0 [2.5–6.5] | c: 0.53–0.68; l: 0.46 | p1: 0.77 (R2: 0.82), p2: 0.42 |
| Giffroy et al. (2016, 2017)29,30 | Retrospective | RR/SP/PP (90, 9, 1) | 6.3 years | VEP, SEP-LL, MEP-UL, MEP-LL | Ordinal (gEPS; 0–30)/quantitative (qEPS) | RR: 2.8 (1.0); SP+PP: 5.0 (0.83) | oEPS, c: 0.67; l: 0.18; qEPS, c: 0.67; l: 0.15 | oEPS p1(R2): 0.72; p2(R2): 0.21 |
| London et al. (2017)31 | Retrospective | RR/CIS (47, 61) | 10 years | VEP, SEP-UL, SEP-LL, MEP-UL, MEP-LL | Ordinal (gEPS; 0–30) | [IQR: 1.0–2.0] | c: 0.16; l: NA | p1: 0.59 |
VEP, BAEP, SEP, MEP: visual, brainstem auditory, somatosensory, motor evoked potentials; UL: upper limbs; LL: lower limbs; EPAS: evoked potential abnormality score; qEPS: quantitative evoked potentials score; mEPS: multimodal evoked potentials score; gEPS: global evoked potentials score; CEPS: combined evoked potentials score.
Correlation coefficients; significant results are given in bold; R2 indicates explained variability in regression models.
Only RR subgroup with complete follow up (bs, y1, y2) reported in the table; p1 for correlation of EP score 1 year prior to EDSS.
In demyelinating disorders, conduction depends not only on the number of intact nerve fibers but can also be altered by temperature and medication interfering with ion channels. The effect of body core temperature on symptoms in MS has been known for a long time.32 Action potentials become shorter when temperature increases as sodium-channel inactivation occurs earlier. The brevity of the action potential decreases the time for accumulation of current to reach the firing threshold of the axonal membrane. In demyelinated axons at the verge of conducting, the time may become too short to reach the threshold resulting in a temperature-dependent conduction block.8 This observation is probably a partial explanation for the fact that an increase of only 0.2C°–0.4C° in body core temperature is sufficient in susceptible subjects to worsen symptoms.33 Therapeutically, the potassium-channel blocking agent 4-aminopyridine (4AP) has been used to improve signal conduction34 which has been shown experimentally to improve signal propagation along demyelinated axons.35,36 Besides clinical effects, short-term effects of 4AP on the elicitability of MEP and VEP latency and amplitude have been demonstrated.37,38 These mechanisms are at the base of the observation that only patients with a prolonged CMCT profited from fampridine medication for improvement of gait.39 Here, MEP were a predictive biomarker for therapeutic response.
Clinical research in MS requires novel biomarkers
To achieve the goal of successful interventional trials, especially for phase-II studies in progressive MS, novel biomarkers are desirable. Requirements include reliability, validity, quantifiability, tolerability, and efficiency. Intraclass correlation of VEP over 1 year in healthy subjects is 0.94 for latency and 0.73 for amplitudes.40 Reliability of any EP can be improved further by standardization of recording procedures and central reading by appropriate tools (e.g. EPMark).41 Construct validity is given by the close relationship between EP, pathological, and clinical alterations in MS. Criterion validity is documented by many observational studies showing significant correlations between mmEP and current state, as well as disease course and prognosis (see Table 1). Quantifiability of mmEP is obtained either by ordinally or numerically scaled scores. Tolerability of mmEP may limit compliance, but has proven not to be a problem in the majority of patients. Efficiency of EP in clinical trials is likely as their sensitivity to change is higher than that of EDSS and as correlations with clinical course are significant even with small numbers of patients (see Table 1).
According to Amur et al.,42 there are four different types of biomarkers: Diagnostic biomarkers distinguish between patients with and without a particular disease, prognostic biomarkers provide information on the likely course of disease in an untreated individual and identify patients who are probabilistically at a faster rate of decline in their health status, predictive biomarkers provide a forecast of the potential for a patient to respond to a given treatment, and response biomarkers show that a biological response has occurred in a patient after having received a therapeutic intervention. Furthermore, the context of use is important: if a biomarker has been validated in progressive patients, it is probably suited for trials in progressive disease, while a biomarker validated in early MS or even in a clinically isolated syndrome may not necessarily be valid for these conditions, as pathomechanisms are not identical.
EP as diagnostic biomarker
Due to its high sensitivity to subclinical lesions and relatively high specificity, MRI has largely replaced EP to demonstrate dissemination in time and space in patients presenting with typical symptoms suspicious of a demyelinating event and to exclude alternative diagnoses.43 However, the capability of EP to detect even subclinical lesions in pathways which are not well explored in routine MRI assessments, such as optic nerve and spinal cord, has been shown in many studies. Summarizing the results from several studies performed in the seventies and eighties (using Schumaker or Poser diagnostic criteria) including about a thousand (BAEP and SEP) or even nearly 2000 (VEP) patients,44 the proportion of abnormal sensory EP was high in clinically possible, probable, and definite MS (SEP: 49%, 67%, 77%; VEP: 37%, 58%, 85%; BAEP: 30%, 47%, 67%, respectively), as well as in patients without a history of prior symptoms in the respective functional system (SEP: 51%; VEP: 51%; BAEP: 38%). The same applies to motor EP15 with a strong correlation between CMCT to lower extremities and EDSS (rho = 0.5345). In patients with primary progressive multiple sclerosis (PPMS), spinal syndromes often predominate, and VEP are frequently abnormal (in about 90%20) even without corresponding clinical signs and, therefore, add diagnostic information.
The added value of mmEP to confirm a clinical diagnosis of MS has been shown in a sample of 189 patients, in which the reclassification sensitivity of a paraclinical test over clinical assessment alone was higher in MEP, SEP, and VEP (91%–96%) compared with conventional MRI (86%); mmEP allowed reclassification in 32% of patients in whom MRI did not change the diagnostic category.46 However, as MRI standards are changing over time, reclassification sensitivity of MRI is probably higher nowadays. Nonetheless, diagnosis of MS may become more difficult in patients not presenting with a classical clinically isolated syndrome (CIS). In these cases, overreliance on imaging results may lead to misdiagnosis:47 the final diagnoses of patients referred to a tertiary center for evaluation of MS were migraine (22%), fibromyalgia (15%), nonspecific symptoms with abnormal MRI (12%), psychogenic disorders (11%), and neuromyelitis optica spectrum disorders (6%). In these cases, normal cerebrospinal fluid and EP studies might have been helpful to the clinician.
EP as prognostic biomarker
Prognosis of disease course is important for individualized counseling and therapeutic decisions. Moreover, mmEP may be useful as a prognostic biomarker to select patients at high risk of progression for clinical trials. Enriching study samples lowers the risk of negative results due to a less-than-expected event rate.
Several studies with a total of more than 1000 patients in 13 cohorts have shown mostly a strong relationship between a baseline mmEP score and future disability measured by the EDSS (median rho = 0.57, range: 0.38–0.82; see Table 1). This general finding applies to all phases of the disease, but prognostic power is more pronounced in the early relapsing remitting phase and in primary progressive patients as compared to CIS or SP.21,22,25,28,31 The relationship increases with the length of the observation period, and mmEP at baseline have been shown to correlate with the EDSS even after 20 years.48
To determine the added value of EP assessment over the EDSS alone, some studies have looked at the relationship between baseline mmEP and change of EDSS over time, and have still shown mainly significant correlations (median rho = 0.39, range: 0.21–0.88, Table 1). Using regression models, EDSS and EP scores at baseline were independent predictors of clinical outcome,26,28,29 and the amount of explained variability to predict EDSS after 3 years increased when EP data were included (EDSS alone: R2 = 0.67, EDSS + EP score + age: R2 = 0.82).28 Interestingly, change of EDSS over 3 years was predicted by change of mmEP over the first 6 months but not by change of EDSS in the same period.28 Therefore, not only a baseline score but also change in EP score over a certain time may be used as a predictor of future disease progression. Furthermore, mmEP added independent information to MRI at disease onset23 as well as in a mixed sample of relapsing-remitting (RR) and secondary progressive multiple sclerosis (SPMS) patients.27
The odds ratio for progression in mixed-patient samples was 4 over 2.5 years (RRMS, SPMS, and PPMS20) and 11 over 10 years (CIS and early RRMS31) in patients with EP score values greater than the median. Receiver-operating characteristic curves have shown sensitivities between 57% and 85% and specificities between 83% and 88% to detect EDSS progression in different cohorts.24,25,30,48 In a small sample of PPMS patients, the positive predictive value for EDSS progression after 3 years was actually 1, and the negative predictive value was 0.62.28 The fact that different centers with different combinations of EP modalities and different scoring systems reached similar conclusions underlines the validity of this approach. However, a generally applicable cut-off value in EP scores remains to be determined, as does the selection of the modalities to be included into mmEP. Since upper limb EP (SEP-UL, MEP-UL) may only be affected in later stages or progressive disease and since lower limb EP (SEP-LL, MEP-LL) may be absent in these patients, the combination for EP depends on the patient sample in question. However, BAEP have shown the weakest association to future disability and have a low overall frequency of abnormal conduction in MS.20,22,23
EP as response biomarker
In MS, the relationship between structural measures from conventional MRI (brain atrophy, development of hypointense T1-lesions) and disease progression is moderate.49 Non-conventional MRI techniques need to be validated in particular for their multicenter applicability.4,5 Measures from optical coherence tomography (OCT) reflect axonal degeneration and seem less sensitive than VEP to early damage from primarily demyelinating disorders in the optic nerve.50 Given the fact that VEP and OCT assess the two main pathological processes in MS in a complementary way, the combination seems to be well suited for proof-of-concept studies in optic neuritis.51 Body fluid markers as neurofilaments among others may reflect global axonal damage or other specific aspects of the disease process but need to be validated.52
EP are more closely related to clinical disability than structural data. Improved signal conduction can be a “symptomatic” effect, for example, due to 4AP or cooling as discussed above. However, signal conduction may deteriorate with fever (Uhthoff’s phenomenon) or with agents acting on ion channels such as antiepileptic drugs. However, when excluding or balancing such confounding factors in clinical studies, improvement of signal conduction most probably reflects a true effect of remyelination or neuroprotection.
To detect treatment effects, the outcome measure must be able to reflect disease progression in the placebo group, and the effect size of the intervention has to be large enough for the chosen sample size. Studies with serial EP assessments have shown mostly significant correlations between change in EP score and change in EDSS, particularly when employing qEPS (median rho = 0.43, range: 0.18–0.69, see Table 1). Studies using EP latencies or EP scores to evaluate treatment effects in relapsing or progressive MS are summarized in Table 2 and studies in the visual system are summarized in Table 3. Possible treatment effects could be identified with EP latencies and mmEP scores in small cohorts of RRMS patients using natalizumab,53 fingolimod,54 and after re-infusion of the patient’s own bone-marrow cells.55 A large study testing azathioprine in progressive MS has shown increasing latencies in sensory EP in the placebo, as well as in the treated group in parallel to clinical deterioration in both groups.17 These studies indicate that EP change with disease course and provide rational rather than ordinal scores as the EDSS does. Therefore, EP may help to differentiate early between possibly effective and futile interventions in phase-II trials and thus may serve as response biomarkers.
Table 2.
Studies using EP to measure treatment effects in relapsing and progressive MS.
| Author (year) | Study design | Disease course (n) | Study duration | Outcome measure | Intervention | Proposed MOA | Result (effect on outcome) |
|---|---|---|---|---|---|---|---|
| Nuwer et al. (1987)17 | RCT | Progressive MS (101) | 3 years | VEP, BAEP, SEP-UL | Azathioprine | Immunosuppression | No |
| Feuillet et al. (2007)56 | Prospective observational | RRMS (15) | 6 months | MEP-UL (RMT, AR, CMCT) | IFN-β-1a | Immunomodulation | Yes |
| Rice et al. (2010)55 | Prospective open-label phase I | Relapsing-progressing MS (6) | 1 year | gEPS (VEP, BAEP, SEP, MEP) | IV re-infusion of own bone-marrow cells | Immunomodulation, possibly remyelination | Yes |
| Meuth et al. (2011)53 | Semi-prospective observational | RRMS (44) | 2 years | Lat of VEP, BAEP, SEP, MEP-LL | Natalizumab in year 2 | Immunomodulation | Yes |
| Iodice et al. (2016)54 | Semi-prospective observational | RRMS (20) | 2 years | gEPS (VEP, SEP, MEP-LL) | Fingolimod in year 2 | Neuroprotection | Yes |
RCT: randomized-controlled-trial; VEP/BAEP/SEP/MEP: visual, brainstem auditory, somatosensory, motor evoked potentials; UL: upper limbs; LL: lower limbs; RMT: resting motor threshold; AR: amplitude ratio; CMCT: central motor conduction time; Lat: latency; IV: intravenous; MOA: mechanism of action
Table 3.
Studies using EP to measure treatment effects of neuroprotective and remyelinating agents in patients with ON.
| Author (year) | Study design | Disease course (n) | Study duration | Outcome measure | Intervention | Proposed MOA | Result (effect on outcome) |
|---|---|---|---|---|---|---|---|
| Tsakiri et al. (2012)57 | RCT | AON in MS (60) or CIS (4) | 6 months | VEP Lat and Amp | Simvastatin | Immunomodulation | VEP: yes |
| Cadavid et al. (2015)58 | RCT | First ever AON (82) | 32 weeks | VEP Lat; RNFL | Opicinumab | Remyelination | VEP: yes; RNFL: trend |
| McKee et al. (2016)59 | RCT | AON in MS or non-MS (46) | 6 months | RNFL; VEP Lat; | Amiloride | Neuroprotection | VEP: no (deterioration); RNFL: no |
| Raftopoulos (2016)60 | RCT | AON in MS (28) or CIS (58) | 6 months | RNFL; VEP Lat | Phenytoin for 3 mths | Neuroprotection | VEP: no; RNFL: yes |
| Green et al. (2016)61 | RCT cross over | MS with ON (50) | 150 days | VEP Lat; RNFL | Clemastine | Remyelination | VEP: yes; RNFL: no |
RCT: randomized controlled trial; AON: acute (unilateral) optic neuritis; VEP: visual evoked potentials; Lat: latency; Amp: amplitude; RNFL: (thickness of the) retinal nerve fiber layer; MOA: mechanism of action.
Studies focusing on the visual system and ON as a model for testing remyelinating or neuroprotective agents are summarized in Table 3.51,62 In these studies, either VEP latency or measures from OCT were used as the primary outcome. Since baseline values in the affected eye are not reliable due to the effects of acute inflammation, for example, conduction block and optic nerve swelling, usually the values of the unaffected eye have been taken as the reference. Comparing the difference in latency change between treated and placebo groups showed probable treatment effects for simvastatin57 and opicinumab,58 whereas phenytoin60 had no effect and amiloride59 actually prolonged VEP latencies; OCT measures showed a probable effect under therapy with phenytoin,60 a trend towards improvement under opicinumab,58 and no effect of amiloride.59 In a cross-over design in patients with MS and chronic demyelinating optic neuropathy, VEP latency but not OCT measures improved during treatment with clemastine while no changes were observed during the off-drug period.61
These studies show that measuring effects of agents with proposed neuroprotective or remyelinating properties is still a challenge even in the well-defined visual system and in quite homogenous patients samples recruited for acute ON. This may mainly be due to the small effects of the tested interventions. However, agents interfering with ion channels may have a direct “symptomatic” effect on the VEP limiting its use, but VEP seem to have a higher sensitivity than OCT to detect effects of putatively remyelinating agents like opicinumab and clemastine.
Whatever the cause of improved signal conduction, EP offer a chance to detect or exclude a significant effect of the intervention. However, ceiling effects in patients with PMS may prevent using EP, especially SEP from and MEP to lower limbs. For this reason, mmEP including the upper extremities is recommended to monitor progressive disease.
Choice of EP scoring systems depends on context of use
Summarizing results from the different EP modalities into a one-number score yields an estimate of overall dysfunction from the different functional systems. Ordinal scores range from a qualitative assessment of the number of abnormal modalities23 and the number of abnormal tests18,27 to graded scores with four20 or six22 steps per test. Accordingly, the dynamic range can be very small (0–323) or quite high (0–7022) as given in Table 1. The global EP score (gEPS, dynamic range: 0–3620) may be an attractive compromise between number of steps and complexity of definitions. The gEPS has shown robust prognostic correlations in four different cohorts.20,24,30,31
However, ordinal scores may be less well suited to detect change over time, as an increase or decrease in latency still within or still above upper limits of normal would not change an ordinal score. Furthermore, latencies are the most reliable or “solid” measures of early EP components;63 consequently, they have been proposed to be used as the principal measure.17 Latencies can be more easily quantified than any other parameter such as amplitudes or configurations. Z-transformation of latencies allows the normalization of all EP modalities summary into one rationally scaled number.19 When comparing with ordinal EP scores, this quantitative EP score (qEPS) has shown similar cross-sectional and predictive correlations with the actual and future EDSS, but more frequently significant longitudinal correlations with EDSS change in three different cohorts (RRMS and SPMS;19,48 early RRMS;25 PPMS28). Figure 3 shows an example of the qEPS over time in a patient with PPMS. Direct comparison of scoring systems has shown equal performance in cross-sectional correlations with the EDSS.64,65 However, the qEPS has a higher sensitivity to change as compared to the gEPS as illustrated by the lower number of patients needed to detect EP deterioration with 90% certainty over 6 months (n = 50 vs n = 22265). The higher sensitivity to change renders quantitative scores promising candidates for response biomarkers.
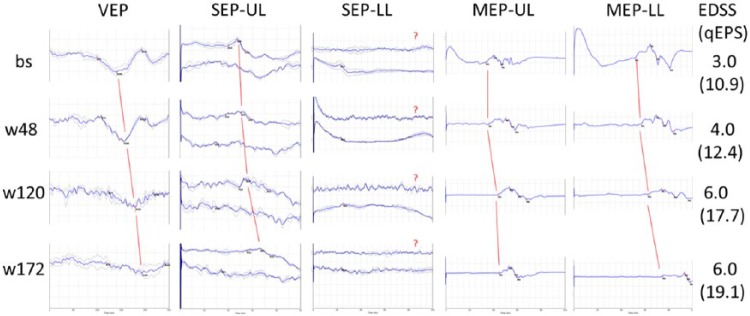
Figure 3.
Multimodal evoked potentials (visual, somatosensory, motor EP; UL/LL: upper/lower limb) over time (baseline, weeks 48, 120, 172) in a sample case (39-year-old male, PPMS, disease duration: 10 month, one side per modality is shown). Red lines signify progressively longer latencies of the main EP components (VEP: P100, SEP-UL: N20, SEP-LL: P40, MEP: shortest cortico-muscular latency), bold blue lines in VEP and SEP are the mean of two replications (gray lines). In the case of SEP-LL, no P40 could be determined as indicated by the question marks; for quantitative analysis, the longest measured latency of the study sample is taken as an approximation.
EDSS: expanded disability status scale; qEPS: quantitative EP score.
Conclusion
What is needed most currently is detection of a safe and highly effective therapy for progressive MS. Efficient response biomarkers could be multidimensional, including mmEP to cover functional aspects of wanted treatment effect. MmEP scores are bi-directional, covering both improvement and deterioration. Furthermore, mmEP can be analyzed by central reading in a multicenter setting and their quantifiability is well suited for statistical analysis. EP recording is time-consuming and may not be tolerated by every patient. However, as sample size can probably be considerably smaller than when using other outcome measures, novel effective treatments may get discovered earlier, at lower cost and with less inconvenience to the whole community of patients suffering from MS. Limitations include insensitivity of EP to cerebellar, frontal, and cognitive dysfunctions and ceiling effects in advanced disease; moreover, they are not validated yet for evaluation of individual patients.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: M.H.’s research is or was supported by the Swiss Multiple Sclerosis Society and the Swiss National Science Foundation SPUM 33CM30_124115 and 33CM30_140338; L.L.’s research is or was supported by Gossweiler Foundation, Telethon, Multiple Sclerosis Italian Foundation, and by unconditional research grants from industry (Almirall, Biogen, Novartis, Merck KGaA); P.F.’s research is or was supported by the Swiss National Science Foundation SPUM 33CM30_124115 and 33CM30_140338 (PI), Swiss Multiple Sclerosis Society, Synapsis Foundation, Parkinson Schweiz, Novartis Research Foundation, Gossweiler Foundation, Freiwillige Akademische Gesellschaft Basel, Mach-Gaensslen-Stiftung, Botnar Foundation, Bangerter Foundation, and by unconditional research grants from industry (Roche, AbbVie, Biogen, General Electrics).
Contributor Information
Martin Hardmeier, Section of Clinical Neurophysiology, Department of Neurology, University Hospital of Basel, Basel, Switzerland.
Letizia Leocani, Neurological Department and Institute of Experimental Neurology (INSPE) Scientific Institute, University Hospital San Raffaele, Milan, Italy.
Peter Fuhr, Section of Clinical Neurophysiology, Department of Neurology, University Hospital of Basel, Basel, Switzerland.
References
- 1. Fernández O, Fernández V. Evoked potentials are of little use in the diagnosis or monitoring of MS: No. Mult Scler 2013; 19: 1822–1823. [DOI] [PubMed] [Google Scholar]
- 2. McGuigan C. Evoked potentials are of little use in the diagnosis or monitoring of MS: Yes. Mult Scler 2013; 19: 1820–1821. [DOI] [PubMed] [Google Scholar]
- 3. Hutchinson M. Evoked potentials are of little use in the diagnosis or monitoring of MS: Commentary. Mult Scler 2013; 19: 1824–1825. [DOI] [PubMed] [Google Scholar]
- 4. Absinta M, Sati P, Reich DS. Advanced MRI and staging of multiple sclerosis lesions. Nat Rev Neurol 2016; 12: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harlow DE, Honce JM, Miravalle AA. Remyelination therapy in multiple sclerosis. Front Neurol 2015; 6: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDonald WI, Sears TA. Effect of demyelination on conduction in the central nervous system. Nature 1969; 221(5176): 182–183. [DOI] [PubMed] [Google Scholar]
- 7. Waxman SG. Clinical course and electrophysiology of multiple sclerosis. In: Waxman SG. (ed.) Advances in neurology, volume 47: functional recovery in neurological disease. New York: Raven Press, 1988, pp. 157–184. [PubMed] [Google Scholar]
- 8. Smith KJ. Conduction properties of central demyelinated and remyelinated axons, and their relation to symptom production in demyelinating disorders. Eye 1994; 8: 224–237. [DOI] [PubMed] [Google Scholar]
- 9. Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338: 278–285. [DOI] [PubMed] [Google Scholar]
- 10. Sadeghian M, Mastrolia V, Rezaei Haddad A, et al. Mitochondrial dysfunction is an important cause of neurological deficits in an inflammatory model of multiple sclerosis. Sci Rep 2016; 6: 33249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raz N, Dotan S, Chokron S, et al. Demyelination affects temporal aspects of perception: An optic neuritis study. Ann Neurol 2012; 71: 531–538. [DOI] [PubMed] [Google Scholar]
- 12. McDonald WI. Pathophysiology of conduction in central nerve fibres. In: Desmedt JE. (ed.) Visual evoked potentials in man: New developments. Oxford: Clarendon Press, 1977, pp. 427–437. [Google Scholar]
- 13. Baker JB, Larson SJ, Sances A, et al. Evoked potentials as an aid to the diagnosis of multiple sclerosis. Neurology 1968; 18: 286. [PubMed] [Google Scholar]
- 14. Halliday AM, McDonald WI, Mushin J. Delayed visual evoked response in optic neuritis. Lancet 1972; 1(7758): 982–985. [DOI] [PubMed] [Google Scholar]
- 15. Hess CW, Mills KR, Murray NM. Measurement of central motor conduction in multiple sclerosis by magnetic brain stimulation. Lancet 1986; 2(8503): 355–358. [DOI] [PubMed] [Google Scholar]
- 16. Khoshbin S, Hallett M. Multimodality evoked potentials and blink reflex in multiple sclerosis. Neurology 1981; 31: 138–144. [DOI] [PubMed] [Google Scholar]
- 17. Nuwer MR, Packwood JW, Myers LW, et al. Evoked potentials predict the clinical changes in a multiple sclerosis drug study. Neurology 1987; 37: 1754–1761. [DOI] [PubMed] [Google Scholar]
- 18. O’Connor P, Marchetti P, Lee L, et al. Evoked potential abnormality scores are a useful measure of disease burden in relapsing-remitting multiple sclerosis. Ann Neurol 1998; 44: 404–407. [DOI] [PubMed] [Google Scholar]
- 19. Fuhr P, Borggrefe-Chappuis A, Schindler C, et al. Visual and motor evoked potentials in the course of multiple sclerosis. Brain 2001; 124: 2162–2168. [DOI] [PubMed] [Google Scholar]
- 20. Leocani L, Rovaris M, Boneschi FM, et al. Multimodal evoked potentials to assess the evolution of multiple sclerosis: A longitudinal study. J Neurol Neurosurg Psychiatry 2006; 77: 1030–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kallmann BA, Fackelmann S, Toyka KV, et al. Early abnormalities of evoked potentials and future disability in patients with multiple sclerosis. Mult Scler 2006; 12: 58–65. [DOI] [PubMed] [Google Scholar]
- 22. Jung P, Beyerle A, Ziemann U. Multimodal evoked potentials measure and predict disability progression in early relapsing-remitting multiple sclerosis. Mult Scler 2008; 14: 553–556. [DOI] [PubMed] [Google Scholar]
- 23. Pelayo R, Montalban X, Minoves T, et al. Do multimodal evoked potentials add information to MRI in clinically isolated syndromes? Mult Scler 2010; 16: 55–61. [DOI] [PubMed] [Google Scholar]
- 24. Invernizzi P, Bertolasi L, Bianchi MR, et al. Prognostic value of multimodal evoked potentials in multiple sclerosis: The EP score. J Neurol 2011; 258: 1933–1939. [DOI] [PubMed] [Google Scholar]
- 25. Schlaeger R, D’Souza M, Schindler C, et al. Combined evoked potentials as markers and predictors of disability in early multiple sclerosis. Clin Neurophysiol 2012; 123: 406–410. [DOI] [PubMed] [Google Scholar]
- 26. Margaritella N, Mendozzi L, Garegnani M, et al. Sensory evoked potentials to predict short-term progression of disability in multiple sclerosis. Neurol Sci 2012; 33: 887–892. [DOI] [PubMed] [Google Scholar]
- 27. Ramanathan S, Lenton K, Burke T, et al. The utility of multimodal evoked potentials in multiple sclerosis prognostication. J Clin Neurosci 2013; 20: 1576–1581. [DOI] [PubMed] [Google Scholar]
- 28. Schlaeger R, D’Souza M, Schindler C, et al. Electrophysiological markers and predictors of the disease course in primary progressive multiple sclerosis. Mult Scler 2014; 20: 51–56. [DOI] [PubMed] [Google Scholar]
- 29. Giffroy X, Maes N, Albert A, et al. Do evoked potentials contribute to the functional follow-up and clinical prognosis of multiple sclerosis? Acta Neurol Belg 2017; 117: 53–59. [DOI] [PubMed] [Google Scholar]
- 30. Giffroy X, Maes N, Albert A, et al. Multimodal evoked potentials for functional quantification and prognosis in multiple sclerosis. BMC Neurol 2016; 16: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. London F, Sankari SE, van Pesch V. Early disturbances in multimodal evoked potentials as a prognostic factor for long-term disability in relapsing-remitting multiple sclerosis patients. Clin Neurophysiol 2017; 128: 561–569. [DOI] [PubMed] [Google Scholar]
- 32. Uhthoff W. Untersuchungen über die bei der multiplen Herdsklerose vorkommenden Augenstörungen [German]. Arch Psychiatr Nervenkr 1890; 21: 303–420. [Google Scholar]
- 33. Frohman TC, Davis SL, Beh S, et al. Uhthoff’s phenomena in MS–clinical features and pathophysiology. Nat Rev Neurol 2013; 9: 535–540. [DOI] [PubMed] [Google Scholar]
- 34. Jones RE, Heron JR, Foster DH, et al. Effects of 4-aminopyridine in patients with multiple sclerosis. J Neurol Sci 1983; 60: 353–362. [DOI] [PubMed] [Google Scholar]
- 35. Sherratt RM, Bostock H, Sears TA. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature 1980; 283: 570–572. [DOI] [PubMed] [Google Scholar]
- 36. Smith KJ, Felts PA, John GR. Effects of 4-aminopyridine on demyelinated axons, synapses and muscle tension. Brain 2000; 123: 171–184. [DOI] [PubMed] [Google Scholar]
- 37. Fujihara K, Miyoshi T. The effects of 4-aminopyridine on motor evoked potentials in multiple sclerosis. J Neurol Sci 1998; 159: 102–106. [DOI] [PubMed] [Google Scholar]
- 38. van Diemen HA, Polman CH, van Dongen MM, et al. 4-Aminopyridine induces functional improvement in multiple sclerosis patients: A neurophysiological study. J Neurol Sci 1993; 116: 220–226. [DOI] [PubMed] [Google Scholar]
- 39. Zeller D, Reiners K, Bräuninger S, et al. Central motor conduction time may predict response to fampridine in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2014; 85: 707–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hardmeier M, Hatz F, Naegelin Y, et al. Improved characterization of visual evoked potentials in multiple sclerosis by topographic analysis. Brain Topogr 2014; 27: 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bousleiman H, Hardmeier M, Fuhr P. EPMark. Presented at meetings of the Progressive MS Alliance, Bethesda, MD, 4 December 2015 and Washington, DC, 16 February 2017. [Google Scholar]
- 42. Amur S, LaVange L, Zineh I, et al. Biomarker qualification: Toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther 2015; 98: 34–46. [DOI] [PubMed] [Google Scholar]
- 43. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chiappa KH. Evoked potentials in clinical medicine (Chapters 8 (6.1, 6.2, 9.1.6) and 9 (1.1.3, 3.2)). 3rd ed. Philadelphia, PA: Lippincott-Raven, 1997. [Google Scholar]
- 45. Kalkers NF, Strijers RL, Jasperse MM, et al. Motor evoked potential: A reliable and objective measure to document the functional consequences of multiple sclerosis? Relation to disability and MRI. Clin Neurophysiol 2007; 118: 1332–1340. [DOI] [PubMed] [Google Scholar]
- 46. Beer S, Rosler KM, Hess CW. Diagnostic value of paraclinical tests in multiple sclerosis: Relative sensitivities and specificities for reclassification according to the Poser committee criteria. J Neurol Neurosurg Psychiatry 1995; 59: 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology 2016; 87: 1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schlaeger R, Schindler C, Grize L, et al. Combined evoked potentials predict MS disability after 20 years. Mult Scler 2014; 20: 1348–1354. [DOI] [PubMed] [Google Scholar]
- 49. Barkhof F, Calabresi PA, Miller DH, et al. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 2009; 5: 256–266. [DOI] [PubMed] [Google Scholar]
- 50. Di Maggio G, Santangelo R, Guerrieri S, et al. Optical coherence tomography and visual evoked potentials: Which is more sensitive in multiple sclerosis? Mult Scler 2014; 20: 1342–1347. [DOI] [PubMed] [Google Scholar]
- 51. Martínez-Lapiscina EH, Sanchez-Dalmau B, Fraga-Pumar E, et al. The visual pathway as a model to understand brain damage in multiple sclerosis. Mult Scler 2014; 20: 1678–1685. [DOI] [PubMed] [Google Scholar]
- 52. Housley WJ, Pitt D, Hafler DA. Biomarkers in multiple sclerosis. Clin Immunol 2015; 161: 51–58. [DOI] [PubMed] [Google Scholar]
- 53. Meuth SG, Bittner S, Seiler C, et al. Natalizumab restores evoked potential abnormalities in patients with relapsing-remitting multiple sclerosis. Mult Scler 2011; 17: 198–203. [DOI] [PubMed] [Google Scholar]
- 54. Iodice R, Carotenuto A, Dubbioso R, et al. Multimodal evoked potentials follow up in multiple sclerosis patients under fingolimod therapy. J Neurol Sci 2016; 365: 143–146. [DOI] [PubMed] [Google Scholar]
- 55. Rice CM, Mallam EA, Whone AL, et al. Safety and feasibility of autologous bone marrow cellular therapy in relapsing-progressive multiple sclerosis. Clin Pharmacol Ther 2010; 87: 679–685. [DOI] [PubMed] [Google Scholar]
- 56. Feuillet L, Pelletier J, Suchet L, et al. Prospective clinical and electrophysiological follow-up on a multiple sclerosis population treated with interferon beta-1 a: A pilot study. Mult Scler 2007; 13: 348–356. [DOI] [PubMed] [Google Scholar]
- 57. Tsakiri A, Kallenbach K, Fuglø D, et al. Simvastatin improves final visual outcome in acute optic neuritis: A randomized study. Mult Scler 2012; 18: 72–81. [DOI] [PubMed] [Google Scholar]
- 58. Cadavid D, Balcer LJ, Galetta SL, et al. Efficacy analysis of the Anti-Lingo-1 monoclonal antibody BIIB033 in Acute Optic Neuritis: The RENEW trial (P7.202). In: 67th meeting of the American academy of neurology, Washington, DC, April 18–25, 2015. [Google Scholar]
- 59. McKee JB, Elston J, Evangelou N, et al. Amiloride does not protect retinalnerve fibre layer thickkness following acute optic neuritis; result from a phase II, double blind randomised controlled trial. In: Presented at 32nd ECTRIMS, London, September 2016. [Google Scholar]
- 60. Raftopoulos R, Hickman SJ, Toosy A, et al. Phenytoin for neuroprotection in patients with acute optic neuritis: A randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15: 259–269. [DOI] [PubMed] [Google Scholar]
- 61. Green A, Gelfand J, Cree B, et al. Positive phase II double-blind randomized placebo-controlled crossover trial of clemastine. In: 68th meeting of the American academy of neurology, Vancouver, BC, Canada, April 2016. [Google Scholar]
- 62. Galetta SL, Villoslada P, Levin N, et al. Acute optic neuritis: Unmet clinical needs and model for new therapies. Neurol Neuroimmunol Neuroinflamm 2015; 2: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Comi G, Leocani L, Medaglini S, et al. Measuring evoked responses in multiple sclerosis. Mult Scler 1999; 5: 263–267. [DOI] [PubMed] [Google Scholar]
- 64. Canham LJ, Kane N, Oware A, et al. Multimodal neurophysiological evaluation of primary progressive multiple sclerosis—An increasingly valid biomarker, with limits. Mult Scler Relat Disord 2015; 4: 607–613. [DOI] [PubMed] [Google Scholar]
- 65. Schlaeger R, Hardmeier M, D’Souza M, et al. Monitoring multiple sclerosis by multimodal evoked potentials: Numerically versus ordinally scaled scoring systems. Clin Neurophysiol 2016; 127: 1864–1871. [DOI] [PubMed] [Google Scholar]