Abstract
A number of animal models have been used to study hypoxic-ischemic injury, traumatic injury, global hypoxia, or permanent ischemia in both the immature and mature brain. Stroke occurs commonly in the perinatal period in humans, and transient ischemia-reperfusion is the most common form of stroke in neonates. The reperfusion phase is a critical component of injury progression, which occurs over a period of days to weeks, and of the endogenous response to injury. This postnatal day 10 (p10) rat model of transient middle cerebral artery occlusion (tMCAO) creates a unilateral, non-hemorrhagic focal ischemia-reperfusion injury that can be utilized to study the mechanisms of focal injury and repair in the full-term-equivalent brain. The injury pattern that is produced by tMCAO is consistent and highly reproducible and can be confirmed with MRI or histological analyses. The severity of injury can be manipulated through changes in occlusion time and other methods that will be discussed.
Keywords: Medicine, Issue 122, Neonatal stroke, MCAO, reperfusion, ischemia, animals models, brain injury
Introduction
Stroke during the neonatal period is a significant cause of death and disability, occurring in as many as 1 in 2,300 live births1. This leads to altered central nervous system development and increased long-term morbidity, including increased incidence of epilepsy, cerebral palsy, mental retardation, and other types of motor or cognitive dysfunction. The lifelong effects of early stroke make translational animal models essential for examining the mechanisms of injury and repair in this population, including strategies to protect the injured brain or to enhance repair.
Different ischemia models have been used to study brain injury in adult animals, and while the Rice-Vannucci (Modified Levine)2 procedure is commonly used to study hypoxic-ischemic injury in the developing brain, focal ischemia-reperfusion is a distinct mechanism of injury causing focal injury, with an injured core and penumbra and uninjured remote tissue. The Koizumi3 and Longa4 models were developed in adult rats to achieve transient middle cerebral artery occlusion via the common carotid artery (CCA) and external carotid artery (ECA), respectively. In both models, permanent ligation and cauterization of artery branches are important to minimize bleeding and to streamline the surgical procedure, which also causes adverse effects on the animal's ability to feed and to gain weight following injury. Furthermore, there are distinct injury mechanisms in the immature brain and specific patterns of injury seen as a result.
More recently, photothrombotic stroke (Rose-Bengal method)5 and permanent MCA ligation6 have been used to study neonatal and adult stroke. Both photothrombotic stroke and MCA ligation create permanent changes in cerebral blood flow that result in a lack of reperfusion. Reperfusion is a critical component of the development and progression of focal injury, with increased excitotoxicity, free radical formation, and nitric oxide production leading to delayed cell death that involves signaling cascades that are distinct from the ischemic phase7. Hypoxia-ischemia involves permanent unilateral carotid ligation followed by global hypoxia, which also differs from the cause of hypoxic-ischemic injury in humans and does not cause a consistent focal injury pattern, making study of the injured core and penumbra more challenging.
We have previously described a non-hemorrhagic ischemia-reperfusion stroke model in the immature rat using transient middle cerebral artery occlusion (MCAO)8,9,10. This is a less invasive method that accesses and occludes the MCA through the internal carotid artery without permanent ligation or cauterization. This provides a model of injury similar to the most common cause of stroke in the perinatal period11,12. This ischemia-reperfusion model of injury results in damage to the ipsilateral striatum and parieto-temporal cortex. This model of tMCAO also allows control over the severity of injury by varying the duration of occlusion. Examination of signaling pathways and histological changes in the injured core and penumbra and in the uninjured ipsilateral and contralateral tissue can further elucidate the mechanisms of injury and repair in the immature brain. This study will demonstrate this important injury model for the developing brain.
Protocol
All animal research was approved by the University of California, San Francisco Committee on Animal Research and was performed in accordance with the Guide for the Care and Use of Laboratory Animals (US Department of Health and Human Services, Publication No. 85-23, 1985). Animals were closely monitored by veterinarians of the UCSF Institutional Animal Care and Use Committee (IACUC), accredited by AAALAC. One female Sprague-Dawley rat with an 8 day-old litter (10 pups per litter) was obtained. The mother and her pups were given food and water ad libitum and housed in a temperature- and light-controlled animal care facility with daily enrichment, per IACUC protocol, until the pups were 10 days old. All surgical instruments used in this procedure were autoclaved to ensure sterility. Sterility of instrument tips is maintained throughout the surgery.
1. Middle Cerebral Artery Occlusion
Weigh the pup and ensure that it is the proper weight (19-21 g). Anesthetize the pup in 3% isoflurane in 100% O2 and ensure that there is no response to a foot pinch. Maintain the body surface temperature between 35.5 °C and 37 °C with the use of a heating pad under the surgical stage.
Secure the animal in a supine position with tape across the shoulder regions. Using sterile cotton swabs, swab the anterior cervical area with povidone-iodine solution followed by a swab of 70% ethanol in double-distilled water, alternating between each solution for four swabs total.
Locally infiltrate 0.25% bupivacaine into the planned incision site. Using a stereoscope, make a midline 5 to 7 mm anterior cervical incision to expose the common carotid artery (CCA). Place 2-4 retractors to keep the cavity open and the artery exposed.
Locate the internal carotid artery (ICA), occipital artery (OA), and external carotid artery (ECA). Groom the arteries to get a clear view. Be careful not to disturb the vagus nerve. NOTE: For sham-operated pups, the inicision is left open and the arteries are exposed, after which the incision is sutured closed. The total anesthesia time is equivalent to that of the occlusion surgery.
Cut 1.5 cm of 6-0 silk braided suture thread. Unbraid the suture, pulling out single strands. Make sure that the single strands are neat and not frayed. NOTE: If necessary, smoothe the frayed ends by dipping the single strand in sterile water and grooming the strand with forcep tips.
While holding the suture strand with 45-degree forceps, move the forceps in a sweeping arc motion to go under the ICA so that the forcep tips emerge between the ICA and OA. NOTE: If the dissection is done well, this step will be relatively easy. If the ICA and OA are touching, be careful not to rupture the arteries when using the forceps to isolate the ICA. If bleeding occurs, apply pressure to the artery with the forceps until the bleeding stops. Absorb the blood with a sterile swab.
Grab the end of the suture strand that is held by the forceps and pull it so that the end is easy to access. Release the strand from the forceps and back the forceps out from under the ICA, reversing the motion in step 1.5 (Figure 1A).
Tie a temporary ligature around the ICA at the base, closest to where it separates from the CCA. NOTE: It is important to tie the knot so that the end of the strand that will be pulled to remove the knot is long enough (greater than 1 mm, less than 3 mm) to easily grasp with forceps, while ensuring that there is an appropriate amount of suture strand on the other side of the knot for retraction.
Carefully retract the ICA laterally and use a clip to secure the strand to excess skin near the axilla region on the side opposite the incision. Ensure that this retracting strand is taut enough to stop blood flow before proceeding to the next step, to minimize the risk of uncontrolled bleeding. Observe that the artery is flat and pale. NOTE: Do not over-retract, as it can cause a partial or full tear of the ICA. The retraction can be adjusted just before making the arteriotomy by pulling on the strand on the side opposite to the clip.
Use 45° forceps to grasp another unbraided suture strand and loop it under and around the ICA, as in step 1.5. Position this strand lateral to the retraction strand (Figure 1B). NOTE: This step can also be done prior to retraction. If unsure about the quality of retraction, this knot can be very loosely tied before the next step.
Cut a 0.2 mm arteriotomy midway between the tied and untied ligatures, erring closer to the tied ligature. NOTE: Blood remaining in the tied-off artery may empty through the arteriotomy but should not exceed 5 µL. If bleeding persists, carefully pull the retraction strand to increase retraction, with care to avoid damaging the artery from excessive tension.
Using a metric ruler, measure the occluding suture and cut the suture with an extra allowance of 2-3 mm for the removal of the occluder during reperfusion. Hold the occluder with 45-degree forceps and use straight forceps to create a bend at the appropriate length to reach the MCA, marking a stopping point for advancement. NOTE: A 10 mm occlusion length from the silicone tip end to the bend is used for P10 Sprague Dawley or Long Evans rat pups in this weight range.
Using 45° forceps, feed the silicone-coated nylon occlusion suture into the arteriotomy and advance the suture to the bend that marks the pre-determined distance to the MCA (Figure 1C). Ensure that advancement feels smooth; immediately stop advancement if resistance is felt. During advancement, aim the suture in a direction that is parallel to the CC/ECA, towards the head. NOTE: If the suture is advanced dorsally, toward the animal's spine, it may run into the pterygopalatine artery (PTA). If resistance is felt after 3-5 mm of advancement, the suture has hit the PTA junction. Back the occluder out of the artery until the silicone head is near the arteriotomy before adjusting the advancement direction. It is not necessary to completely remove the occluder from the arteriotomy.
Secure the occluder by tying a temporary ligature, using the strand from step 1.10 (Figure 1D).
Remove the retractor clip. Trim the strands of both of the temporary ligatures so that the strand of the knot that is pulled to remove the knot is easy to grasp with straight forceps and is longer than the strand that is pulled to tighten the knot. NOTE: The strands must be short enough so that they do not tangle in the cavity after closure.
Remove the retractors and close the cavity using 6-0 braided silk to create three to four interrupted sutures.
Remove the pup from anesthesia and place it on a heating pad in room air. Monitor the pup until it has regained sufficient consciousness to maintain sternal recumbency and ensure that it has fully recovered before returning it to the dam. Ensure that the pup maintains a body surface temperature between 35.5 °C and 37 °C.
During occlusion, diffusion-weighted magnetic resonance imaging (DW-MRI) under isoflurane anesthesia can be used to verify the appropriate induction of injury. Figure 2 demonstrates ongoing ischemic injury detected by DW-MRI during tMCAO9. NOTE: Diffusion-weighted spin echo planar imaging is performed 10-15 min prior to reperfusion. The entire brain is imaged with serial 2-mm-thick coronal sections using the following pulse sequence settings: TR/TE=5,000/60 ms, 4 averages, field of view = 35 mm, data matrix = 128x128, diffusion gradient duration = 20 ms, separation = 29.7 ms, amplitude = 70mT/m, and b-factor = 1,045 s/mm2. Animals exhibiting a lack of cortical involvement or atypical ischemic injury, such as in the brainstem, are excluded.
2. Reperfusion
NOTE: Occlusion is performed for 3 h to cause a moderate-to-severe amount of injury involving the striatum and cortex.
At approximately 2 h and 50 min following occlusion of the MCA, anesthetize the pup in 3% isoflurane in 100% O2. Maintain a body surface temperature between 35.5 °C and 37 °C with the use of a heating pad under the surgical stage.
Remove the interrupted sutures from step 1.16 and locate the junction, which is marked by the two ligatures and the tail end of the occluding suture. NOTE: Sham-operated animals are induced and remain under anesthesia for an equivalent time period to the occluded animals. The incision is once again opened and sutured closed. For sham-operated animals, proceed to step 2.11.
Carefully untie the most lateral knot previously tied in step 1.14 by pulling the longer strand. NOTE: If there is resistance in untying the knot, stop to ensure that the correct strand of the knot is being pulled. If resistance continues, increase magnification for a better view. Be cautious when untying, as it is possible to damage the artery during this step.
Remove the suture strand from the cavity.
At exactly 3 h following MCA occlusion, slowly back the occluding suture out of the artery. There will be no resistance. NOTE: In most cases, there will be no bleeding. If a small amount of bleeding occurs, apply pressure to the artery at the arteriotomy site.
Use forceps to apply pressure to the arteriotomy, as the next step restores ICA blood flow for reperfusion.
Carefully untie the medial knot using the same method as in step 2.3.
Remove the suture strand from the body and apply a hemostatic agent to the arteriotomy to stop bleeding.
Ensure that the bleeding has stopped. NOTE: Return of the artery's original shape and red color confirms that ICA blood flow has been restored.
Remove the retractors and close the cavity with three to four interrupted sutures of 6-0 braided silk.
Remove the pup from anesthesia and place it on a heating pad in room air. Monitor it until it has regained sufficient consciousness to maintain sternal recumbency and ensure full recovery before returning it to the dam. Ensure that the pup maintains a body surface temperature between 35.5 °C and 37 °C.
Inspect the pups daily for 5 days. Recprd their weights and inspect the incision sites closely for appropriate healing. Remove the sutures after 7-14 days. Post-tMCAO injury can be imaged with MRI, if desired9. NOTE: Animals may lose up to 1 g of weight on the first day but will typically regain the weight without intervention and be within a similar weight range to controls by days 4-5. Rarely, a pup may lose excessive weight or have difficulty feeding, requiring oral gavage feeds for 2-3 days.
At P21, injury can be reliably assessed with sensorimotor behavioral tests, such as rotarod or cylinder rearing. Cognitive testing can be performed as early as 4-6 weeks of age using assessments such as novel object recognition or the Morris water maze13.
Euthanize the rats by intraperitoneal injection of Euthasol (50 mg/kg) for brain harvest11,12.
Histological analysis to assess injury volume or response to interventions can be performed with cresyl violet or H&E staining (Figure 3)10.
Representative Results
The severity of injury caused by tMCAO is highly dependent upon both the occlusion time and the experience of the surgeon. A 90-min occlusion often produces a mild to moderate injury pattern, while 3 h produces a moderate-to-severe injury. Severity of injury may be assessed through a variety of methods, including MRI, histology, or short- or long-term behavioral analyses. Figure 2 demonstrates an example of the DW-MRI performed 75 min into a 90-min occlusion, confirming ischemic injury involving the ipsilateral hemisphere. Diffusion weighted imaging demonstrates increased diffusion in the ipsilateral striatum and the majority of the ipsilateral cortex, without contralateral changes, during the acute ischemic phase. This correlates to a moderate level of long-term injury involving both the cortex and the deep gray matter.
Occlusion of the MCA results in cell death that begins in the striatum for less severe injury and develops worsening cortical and hippocampal injury for longer occlusion times and more severe injury. During optimization of the surgical technique, such as determining the suture insertion length, MRI is highly recommended, as it allows for the confirmation of proper suture placement and visualization of edema and injury progression during occlusion14,15,16. If MRI is not available, H&E or cresyl violet staining are simple and reliable histological methods to determine injury morphology and can be used at both early and late time points after the tMCAO. Figure 3 demonstrates a moderate-to-severe injury pattern on histopathological examination following a 3 h occlusion, demonstrating cyst formation and volume in the ipsilateral striatum and cortex.
Unbiased stereological quantification of cresyl-violet-stained sections to calculate the ratio of ipsilateral to contralateral hemispheric volume confirms ipsilateral ischemic-reperfusion injury. Specifically, after anesthetizing the animal with Euthasol, the brain was harvested by transcardiac perfusion in 0.1 M PBS with 4% paraformaldehyde. Following overnight postfixation and equilibration in 30% sucrose, the entire brain was sectioned at 50 µm intervals, and every twelfth section was selected, mounted, and stained with cresyl violet10.
Even with mild injury, locomotor changes, such as circling and hemiparesis, are noted during the occlusion period. With more severe injury, these changes will persist after reperfusion. Additional behavioral testing can be used to assess the severity of injury, including rotarod or cylinder rearing testing for sensorimotor function and the Morris water maze for cognitive function13.
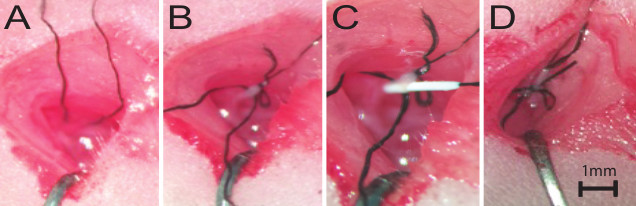 Figure 1:Live Surgical Images of the tMCAO Procedure. (A) The first suture strand is looped around the ICA, as detailed in step 1.6. (B) The first temporary ligature is tied and the ICA is retracted. The second suture strand is looped around the ICA, lateral to the first suture strand, as detailed in step 1.9. (C) The silicone coated occlusion suture is fed into the arteriotomy site, as detailed in step 1.12. (D) The second temporary ligature is tied to secure the occluder in place, as detailed in step 1.14. Scale bar = 1 mm.
Figure 1:Live Surgical Images of the tMCAO Procedure. (A) The first suture strand is looped around the ICA, as detailed in step 1.6. (B) The first temporary ligature is tied and the ICA is retracted. The second suture strand is looped around the ICA, lateral to the first suture strand, as detailed in step 1.9. (C) The silicone coated occlusion suture is fed into the arteriotomy site, as detailed in step 1.12. (D) The second temporary ligature is tied to secure the occluder in place, as detailed in step 1.14. Scale bar = 1 mm.
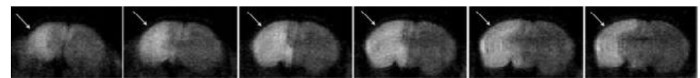 Figure 2:MRI During Occlusion Demonstrates the Appropriate Unilateral Injury. Anterior to posterior, coronal image slices of the DW-MRI, performed during a 90 min occlusion, demonstrate increased diffusion involving the ipsilateral hemisphere (arrows), which is consistent with ongoing ischemic injury in the acute phase. Reprinted with permission from Stroke11. Please click here to view a larger version of this figure.
Figure 2:MRI During Occlusion Demonstrates the Appropriate Unilateral Injury. Anterior to posterior, coronal image slices of the DW-MRI, performed during a 90 min occlusion, demonstrate increased diffusion involving the ipsilateral hemisphere (arrows), which is consistent with ongoing ischemic injury in the acute phase. Reprinted with permission from Stroke11. Please click here to view a larger version of this figure.
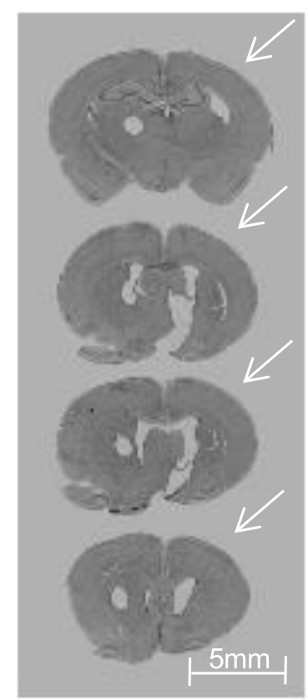 Figure 3:Unilateral Injury Involving the Striatum and Cortex at 4 weeks following tMCAO. Posterior to anterior, cresyl-violet-stained coronal brain sections (each 50 µm) harvested from P38 animals demonstrate fairly severe injury (the arrows show ipsilateral cyst formation and reduced cortical and striatal volume) following a 3 h tMCAO at P10. The round hole on the left side represents a contralateral hemisphere identifier. Scale bar = 5 mm. Reprinted with permission from Neurobiology of Disease12. Please click here to view a larger version of this figure.
Figure 3:Unilateral Injury Involving the Striatum and Cortex at 4 weeks following tMCAO. Posterior to anterior, cresyl-violet-stained coronal brain sections (each 50 µm) harvested from P38 animals demonstrate fairly severe injury (the arrows show ipsilateral cyst formation and reduced cortical and striatal volume) following a 3 h tMCAO at P10. The round hole on the left side represents a contralateral hemisphere identifier. Scale bar = 5 mm. Reprinted with permission from Neurobiology of Disease12. Please click here to view a larger version of this figure.
Discussion
Critical steps within the protocol
First, it is important to maintain normothermia from the initiation of anesthesia until full recovery, as there are known effects of both hypothermia17 and hyperthermia18 on the progression of brain injury in both immature and mature animals. Second, while securing the animal and retracting the incision, optimal positioning to monitor breathing and to ensure that the trachea is free of compression is essential. Third, avoid squeezing or stretching the vagus nerve, as this may cause changes in heart rate with vagal stimulation. Fourth, because retraction of the ICA is necessary to control bleeding during the arteriotomy, attention must be paid to the degree of tension during retraction to avoid damaging the artery. If the artery does tear from retraction, or if there is a poor arteriotomy incision, the animal should be excluded from analysis due to the risk of hemorrhage and poor reperfusion.
Modifications and troubleshooting
Using MRI as a guide, the suture length may be optimized to ensure that the silicone tip properly occludes the MCA to create the focal ischemia. If MRI is not available, pups may be euthanized before reperfusion for dissection to visualize the placement of the suture. Adjust the suture length as needed. The pup weight highly correlates with the occluding suture length requirements. The occlusion time can be modified to adjust the degree of injury severity.
In addition, suture shape and length are critical. For P10 Sprague-Dawley and Long Evans rats weighing 19-21 g, 10 mm is the optimal length of insertion in our experience. Further insertion of the occluding suture may result in perforation of the MCA. Furthermore, the consistency in the shape of the occluding filament in each surgery will result in an increased consistency of injury pattern19,20. For this reason, we recommended using professionally-manufactured sutures for this specific purpose. It is also important to note that the injury pattern may differ between practitioners due to seemingly minute differences in technique.
Limitations of the technique
Performing this technique in a small, developing rodent requires significant experience. If performed correctly, the surgeon is able to cause a very consistent injury pattern across animals of different sizes and attain a survival rate greater than 95%. Furthermore, proper surgical tools are essential. Surgical instruments must be well maintained to ensure that all instrument tips approximate properly.
Significance of this technique with respect to existing or alternative methods
While hypoxia-ischemia, or the Rice-Vannucci model2, is most commonly used to study hypoxic-ischemic injury in the developing brain, it is important to note that this model of tMCAO is distinct from HI in that there is transient focal ischemia without global hypoxia, followed by a reperfusion phase when the obstruction is removed and blood flow is restored. This causes a more consistent and reproducible injury and is more clinically translational by causing an injury pattern similar to that seen in full-term neonatal stroke. This enables the study of focal injury patterns and compensatory responses in uninjured tissue.
Future applications after mastering this technique
This model is similar to the most common cause of stroke in human neonates, a transient occlusive thrombus that occurs during the perinatal period11,21. The etiology is not entirely clear and is most likely multifactorial, but it is presumed in most cases to result from emboli passing from the placenta11. In addition, many newborns with presumed perinatal stroke often present with later seizure activity or subtle focal neurological exam abnormalities22. This makes the use of a consistent, translational injury model to identify mechanisms of injury progression and possible therapeutic strategies crucial.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Funding was provided by the NIH K08 NS064094 and UCSF REAC grants. The authors would like to acknowledge Nikita Derguin, Zinalda Vexler, and Joel Faustino for their assistance in the development of this technique.
References
- Grunt S, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics. 2015;135(5):1220–1228. doi: 10.1542/peds.2014-1520. [DOI] [PubMed] [Google Scholar]
- Rice JE, Vannucci 3rd RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. I: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8(8) [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Labat-gest V, Tomasi S. Photothrombotic ischemia: a minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J Vis Exp. 2013. [DOI] [PMC free article] [PubMed]
- Renolleau S, Aggoun-Zouaoui D, Ben-Ari Y, Charriaut-Marlangue C. A model of transient unilateral focal ischemia with reperfusion in the P7 neonatal rat: morphological changes indicative of apoptosis. Stroke. 1998;29(7):1454–1460. doi: 10.1161/01.str.29.7.1454. [DOI] [PubMed] [Google Scholar]
- Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28(9):1353–1365. doi: 10.1016/j.clinthera.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Derugin N, Ferriero DM, Vexler ZS. Neonatal reversible focal cerebral ischemia: a new model. Neurosci Res. 1998;32(4):349–353. doi: 10.1016/s0168-0102(98)00096-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez FF, et al. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke. 2013;44(3):753–758. doi: 10.1161/STROKEAHA.111.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larpthaveesarp A, Georgevits M, Ferriero DM, Gonzalez FF. Delayed erythropoietin therapy improves histological and behavioral outcomes after transient neonatal stroke. Neurobiol Dis. 2016. [DOI] [PMC free article] [PubMed]
- Rutherford MA, Ramenghi LA, Cowan FM. Neonatal stroke. Arch Dis Child Fetal Neonatal Ed. 2012;97(5):377–384. doi: 10.1136/fetalneonatal-2010-196451. [DOI] [PubMed] [Google Scholar]
- van der Aa NE, Benders MJ, Groenendaal F, de Vries LS. Neonatal stroke: a review of the current evidence on epidemiology, pathogenesis, diagnostics and therapeutic options. Acta Paediatr. 2014;103(4):356–364. doi: 10.1111/apa.12555. [DOI] [PubMed] [Google Scholar]
- Gonzalez FF, et al. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci. 2009;31(5):403–411. doi: 10.1159/000232558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudink J, et al. Evolution of unilateral perinatal arterial ischemic stroke on conventional and diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2009;30(5):998–1004. doi: 10.3174/ajnr.A1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derugin N, et al. Magnetic resonance imaging as a surrogate measure for histological sub-chronic endpoint in a neonatal rat stroke model. Brain Res. 2005;1066(1-2):46–56. doi: 10.1016/j.brainres.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Dzietko M, Wendland M, Derugin N, Ferriero DM, Vexler ZS. Magnetic resonance imaging (MRI) as a translational tool for the study of neonatal stroke. J Child Neurol. 2011;26(9):1145–1153. doi: 10.1177/0883073811408308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso A, Derugin N, Hara K, Sharp FR, Weinstein PR. Mild hypothermia decreases the incidence of transient ADC reduction detected with diffusion MRI and expression of c-fos and hsp70 mRNA during acute focal ischemia in rats. Brain Res. 2000;887(1):34–45. doi: 10.1016/s0006-8993(00)02963-2. [DOI] [PubMed] [Google Scholar]
- Kasdorf E, Hyperthermia Perlman JM. Inflammation, and Perinatal Brain Injury. Pediatric Neurology. 2013;49(1):8–14. doi: 10.1016/j.pediatrneurol.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Bouley J, Fisher M, Henninger N. Comparison between coated vs. uncoated suture middle cerebral artery occlusion in the rat as assessed by perfusion/diffusion weighted imaging. Neurosci Lett. 2007;412(3):185–190. doi: 10.1016/j.neulet.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Shimamura N, Matchett G, Tsubokawa T, Ohkuma H, Zhang J. Comparison of silicon-coated nylon suture to plain nylon suture in the rat middle cerebral artery occlusion model. J Neurosci Methods. 2006;156(1-2):161–165. doi: 10.1016/j.jneumeth.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Kirton A, deVeber G. Paediatric stroke: pressing issues and promising directions. Lancet Neurol. 2015;14(1):92–102. doi: 10.1016/S1474-4422(14)70227-3. [DOI] [PubMed] [Google Scholar]
- Nelson KB. Perinatal ischemic stroke. Stroke. 2007;38:742–745. doi: 10.1161/01.STR.0000247921.97794.5e. [DOI] [PubMed] [Google Scholar]


