Abstract
Objective
Perspective-taking ability is an essential spatial faculty that is of much interest in both health and neuropsychiatric disorders. There is limited data on the neural correlates of perspective taking in the context of a realistic three-dimensional environment. We report the results of a pilot study exploring the same in eight healthy volunteers.
Methods
Subjects underwent two runs of an experiment in a 3 Tesla magnetic resonance imaging (MRI) involving alternate blocks of a first-person perspective based allocentric object location memory task (OLMT), a third-person perspective based egocentric visual perspective taking task (VPRT), and a table task (TT) that served as a control. Difference in blood oxygen level dependant response during task performance was analyzed using Statistical Parametric Mapping software, version 12. Activations were considered significant if they survived family-wise error correction at the cluster level using a height threshold of p<0.001, uncorrected at the voxel level.
Results
A significant difference in accuracy and reaction time based on task type was found. Subjects had significantly lower accuracy in VPRT compared to TT. Accuracy in the two active tasks was not significantly different. Subjects took significantly longer in the VPRT in comparison to TT. Reaction time in the two active tasks was not significantly different. Functional MRI revealed significantly higher activation in the bilateral visual cortex and left temporoparietal junction (TPJ) in VPRT compared to OLMT.
Conclusion
The results underscore the importance of TPJ in egocentric manipulation in healthy controls in the context of reality-based spatial tasks.
Keywords: Egocentric, Allocentric, Functional magnetic resonance imaging, Temporoparietal junction
INTRODUCTION
Virtual reality environments are increasingly being used to understand the neural process mediating spatial faculties due to their exciting ability to mimic real life scenarios while providing a controlled reproducible experimental setup.1) Few studies have employed this strategy to understand the neural correlates of the overlapping but distinct effects of frame of reference (ego vs. allocentric) and perspective (first- vs. third-person).2–4) The task developed by Amorim1) is particularly interesting as it utilizes a variation in the perspective to produce reliable examples of allocentric and egocentric spatial processing. The object location memory task (OLMT) is an example of a first-person perspective task that encourages an allocentric frame of reference while the visual perspective-taking task (VPRT) is a third-person perspective based task that requires the adoption of an egocentric frame of reference.4) Thus, the tasks differ in the visuospatial strategy that needs to be employed—allocentric processing strategy is used for OLMT while an egocentric processing strategy has to be used for the VPRT. The allocentric-egocentric dichotomy is of special relevance in schizophrenia; the allocentric simulation hypothesis postulates that the pathological referencing in schizophrenia is due to a problem in adopting a “world-centered”—inter-subjective—reference frame.5) Interestingly, in a recent study by our group, patients with schizophrenia were found to have a selective defect in allocentric spatial transformations in line with this hypothesis. Hence, understanding this deficit and its neural correlates further would help further our understanding of cognitive deficits in schizophrenia.6)
While a study has examined the neural and eye movement correlates of the OLMT,3) a comparison of the functional activation patterns between the two tasks has not been performed. In this pilot study, we examined the activation differences between these two tasks in a small group of healthy controls.
METHODS
Subjects
Eight healthy right-handed volunteers (five males, three females; mean age 26.7±3.2 years) participated in the study after giving a written, informed consent. The study was approved by the institute’s ethics committee at National Institute of Mental Health & Neurosciences (NIMHANS), Bangalore (NIMHANS/74IEC/2011).
Stimuli
We used the same set of stimuli that were reported in the original study.1) The stimuli were pictures of a three-dimensional (3D) virtual environment taken from viewpoints distributed at 45° intervals. Of the many conditions described in the paper, stimuli relevant to the OLMT (without avatar), the VPRT, and the table task (TT) were chosen for the experiment (Supplementary Fig. 1). The TT served as the control task.1)
Experiment
Subjects underwent two runs of the task. Before each run began, they were shown a short movie that provided a 360° tour of the virtual environment, once in the clockwise direction and once anticlockwise to help orient themselves to the environment.
The task included 16 alternating blocks of control and active conditions. In the control condition (TT), the subjects had to indicate whether a table was present in the field of view or not. Each control block was 20 s long and involved the presentation of five stimuli for 3.5 s each. A crosshair was displayed for 0.5 s between consecutive stimuli. The active task alternated between OLMT and VPRT. In the first run, subjects performed the OLMT first, while in the second run VPRT was presented first. Each active block was 40 s long and involved the presentation of four trials of OLMT or VPRT. In the OLMT, the subject memorized the position of the lamp from an initial viewpoint displayed for 4 s (priming stimulus). This was followed by a new viewpoint of the environment (probe stimulus). Subjects had to indicate, by means of button presses, if the position of the lamp had changed in the new viewpoint. In the VPRT, an avatar is shown from an initial viewpoint for 4 s (priming stimulus) and the subject is asked to imagine and memorize what the avatar is seeing. Then, a new viewpoint of the environment is displayed (probe stimulus) and the task is to judge if this new viewpoint is identical to the one adopted by the avatar in the first viewpoint. Subjects were given 5.5 s to make a response in both the tasks. The angular difference between the initial and the new viewpoint varied from 0° to 180°. A crosshair was displayed for 0.5 s between consecutive trials. Before each block began, an instruction slide was displayed for 2 s informing the subject about the nature of the task.
Functional Magnetic Resonance Imaging (fMRI)
MRI was performed with a 3-Tesla scanner (Skyra; Seimens, Erlangen, Germany) using a 32 channel coil. T1-weighted 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequence was performed. The scan parameters were as follows: repetition time (TR)=1,900 ms; echo time (TE)=2.43 ms; flip angle=9°; slice thickness=1 mm; slice number=192; voxel size=1 mm isotropic. This was followed by fMRI during the performance of the task. A total of 256 functional scans were acquired. Three scans were rejected before the task began. The functional scan parameters were as follows: TR=2,000 ms; TE=30 ms; flip angle=78°; slice thick-ness=3 mm; slice order: descending; slice number=37; gap=25%; voxel=3 mm isotropic).
Data Analysis
Response accuracy and reaction time (RT) were averaged across the two runs. Given the small sample size, Friedman test was conducted separately on response accuracy and RT to investigate the effect of task type. Post-hoc tests were performed using the Wilcoxon signed-rank test with Bonferroni correction for multiple comparisons. The threshold for statistical significance was set at p<0.05 (two-tailed).
The imaging data were assessed using Statistical Parametric Mapping software, version 12. Standard pre-processing steps—realignment, slice-time correction, indirect normalization (using each subject’s high-resolution T1 MRI scan) and smoothing using 8-mm FWHM kernel were done in that order. Brain activation differences between the tasks were assessed for all subjects in a fixed-effects design using paired t tests. Activations were considered significant if they survived family-wise error correction at the cluster level (p<0.05, no extent threshold) using a height threshold of p<0.001, uncorrected at the voxel level. The Wake Forest University pick atlas (version 3.0.5)7,8) was used to identify regions of significant activation differences.
RESULTS
Behavioral Performance
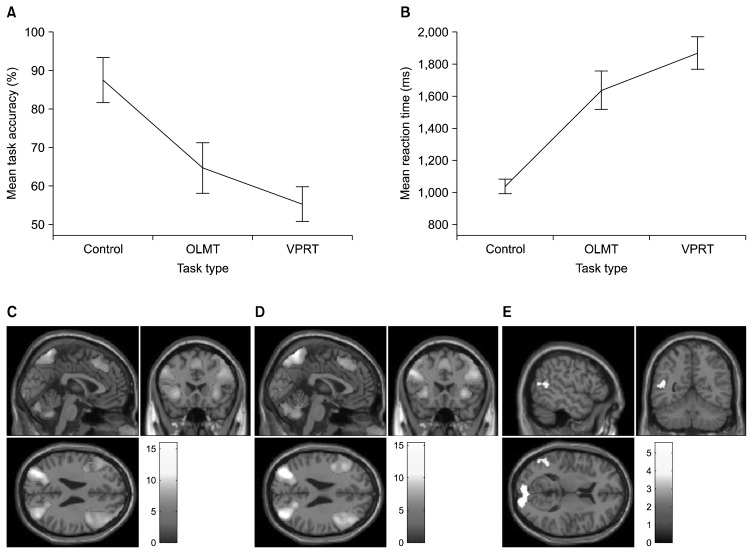
There was a statistically significant difference in response accuracy (χ2(2)=10.75, p=0.005) and RT (χ2(2)= 10.75, p=0.005) depending on the task type. Post hoc tests with Bonferroni correction revealed that subjects had significantly lower accuracy in VPRT (p=0.036) compared to TT (Fig. 1A). Accuracy in the two active tasks was not significantly different. RT in the VPRT (p=0.012) was significantly higher than in TT. RT in the two active tasks was not significantly different (Fig. 1B).
Fig. 1.
Figure showing accuracy (A) and reaction time (B) differences among the three tasks. Brain activation differences (C) between object location memory task (OLMT) and table task (TT), (D) between visual perspective taking task (VPRT) and TT, and (E) between VPRT and OLMT. Error bars, ±1 standard error.
Imaging Analysis
When compared to TT, both OLMT and VPRT showed higher activation of an almost identical network of brain regions consisting of parietal, occipito-temporal and frontal regions (Fig. 1C, 1D). Significantly higher activation was noted in bilateral primary and association visual cortex and the left temporoparietal junction (TPJ) when VPRT was compared against OLMT (Fig. 1E and Table 1). The reverse contrast did not reveal any areas of significant activation.
Table 1.
Brain regions showing significant difference in activation between visual perspective taking task and object location memory task
| Brain region | Broadmann area | MNI coordinates of peak voxel in the cluster (mm) (x, y, z) | T value of the peak voxel | Cluster size (voxel) | Mean F/t value at each region | Number of significant voxels in each region |
|---|---|---|---|---|---|---|
| Bilateral primary and association visual cortices | 17 | 16,−98, 6 | 5.63 | 1,190 | 4.1 | 203 |
| 18 | 16,−98, 6 | 5.63 | 1,190 | 3.6 | 125 | |
| 19 | 16,−98, 6 | 5.63 | 1,190 | 3.2 | 3 | |
| Left temporoparietal junction | 39 | −44,−70, 20 | 4.3 | 238 | 3.4 | 29 |
| 22 | −44,−70, 20 | 4.3 | 238 | 3.3 | 3 | |
| 37 | −44,−, 20 | 4.3 | 238 | 3.2 | 3 |
Reported MNI coordinates refer only to the peak voxel in each cluster and not to the individual brain regions.
DISCUSSION
Our pilot study showed that similar to previous studies, a distributed network including parietal, occipito-temporal and frontal regions was activated during task performance.2,3,9) Interestingly, when VPRT was compared against OLMT, higher activation was found in the visual cortex, and the left TPJ. TPJ has been shown to play an important role in several related cognitive processes like perspective taking, empathy, and theory of mind.10) Furthermore, empathy has been shown to correlate with visuospatial abilities, especially in an egocentric context.11,12) Performing an egocentric task from a third-person perspective would require a flexible self-other transition central to the concept of empathy. Indeed a recent study has found that TPJ is critical to egocentric spatial transformations.13) Thus, our results underscore the importance of TPJ in egocentric manipulation of spatial constructs in the healthy controls assessed in this study.
We have presented findings with obvious caveats of a small sample size and fixed-effects analysis that limits the generalizability of these observations. Owing to these limitations, we were unable to perform a correlation between task performance measures and BOLD signal changes as well. Future studies with a larger sample of subjects will perhaps be able to address these limitations and confirm the novel but preliminary results of this study.
The tasks used in this study explored spatial cognition of perspective change which has associations with multiple interrelated aspects of interest in neuroscience and psychiatry like theory of mind and empathy,14) schizotypy,14,15) and schizophrenia psychopathology.6,16) This pilot study is the first to evaluate the difference between OLMT and VPRT in an fMRI context, and adds to the findings of previous studies,3) wherein OLMT was compared with TT, but a comparison between OLMT and VPRT was not made. In a recent metanalysis of fMRI studies examining perspective taking left TPJ was found to involved in the processing of alternative perspectives, that is important in both visual perspective taking, as well was false belief reasoning.17) It also features consistently whenever other and self-perspectives are compared.18) This task can hence be employed in future systematic studies to further understand and elaborate the interactions between visuospatial perspective taking and cognitive functioning in health and disease, and exploring specifically the role of left TPJ in these states.
Supplementary Information
Acknowledgments
This work is supported by the Wellcome Trust / DBT India Alliance Senior Fellowship (500236/Z/11/Z) and DST Swarnajayanti Fellowship (DST/SJF/LSA-02/2014-15) grants to Ganesan Venkatasubramanian. Sri Mahavir Agarwal, Sunil V Kalmady, Vijay Danivas, Anekal C Amaresha are supported by the Wellcome Trust / DBT India Alliance. Anushree Bose is supported by the DST SJF Grant. Venkataram Shivakumar is supported by the Indian Council of Medical Research (DHR/HRD/Young Scientist/Type-VI-(2)/2015).
REFERENCES
- 1.Amorim MA. “What is my avatar seeing?”: The coordination of “out-of-body” and “embodied” perspectives for scene recognition across views”. Visual Cognition. 2003;10:157–199. doi: 10.1080/713756678. [DOI] [Google Scholar]
- 2.Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness. J Cogn Neurosci. 2004;16:817–827. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt D, Krause BJ, Weiss PH, Fink GR, Shah NJ, Amorim MA, et al. Visuospatial working memory and changes of the point of view in 3D space. Neuroimage. 2007;36:955–968. doi: 10.1016/j.neuroimage.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Lambrey S, Amorim MA, Samson S, Noulhiane M, Hasboun D, Dupont S, et al. Distinct visual perspective-taking strategies involve the left and right medial temporal lobe structures differently. Brain. 2008;131:523–534. doi: 10.1093/brain/awm317. [DOI] [PubMed] [Google Scholar]
- 5.Langdon R, Coltheart M, Ward PB, Catts SV. Visual and cognitive perspective-taking impairments in schizophrenia: A failure of allocentric simulation? Cognitive Neuropsychiatry. 2001;6:241–269. doi: 10.1080/13546800143000005. [DOI] [Google Scholar]
- 6.Agarwal SM, Danivas V, Amaresha AC, Shivakumar V, Kalmady SV, Bose A, et al. Cognitive mapping deficits in schizophrenia: Evidence from clinical correlates of visuospatial transformations. Psychiatry Res. 2015;228:304–311. doi: 10.1016/j.psychres.2015.05.096. [DOI] [PubMed] [Google Scholar]
- 7.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser S, Walther S, Nennig E, Kronmüller K, Mundt C, Weisbrod M, et al. Gender-specific strategy use and neural correlates in a spatial perspective taking task. Neuropsychologia. 2008;46:2524–2531. doi: 10.1016/j.neuropsychologia.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson PH, Rinehart NJ, Enticott PG. Noninvasive stimulation of the temporoparietal junction: A systematic review. Neurosci Biobehav Rev. 2015;55:547–572. doi: 10.1016/j.neubiorev.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Gardner MR, Brazier M, Edmonds CJ, Gronholm PC. Strategy modulates spatial perspective-taking: evidence for dissociable disembodied and embodied routes. Front Hum Neurosci. 2013;7:457. doi: 10.3389/fnhum.2013.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gronholm PC, Flynn M, Edmonds CJ, Gardner MR. Empathic and non-empathic routes to visuospatial perspective-taking. Conscious Cogn. 2012;21:494–500. doi: 10.1016/j.concog.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Ganesh S, van Schie HT, Cross ES, de Lange FP, Wigboldus DH. Disentangling neural processes of egocentric and allocentric mental spatial transformations using whole-body photos of self and other. Neuroimage. 2015;116:30–39. doi: 10.1016/j.neuroimage.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Thakkar KN, Park S. Empathy, schizotypy, and visuospatial transformations. Cogn Neuropsychiatry. 2010;15:477–500. doi: 10.1080/13546801003711350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langdon R, Coltheart M. Visual perspective-taking and schi-zotypy: evidence for a simulation-based account of mentalizing in normal adults. Cognition. 2001;82:1–26. doi: 10.1016/S0010-0277(01)00139-1. [DOI] [PubMed] [Google Scholar]
- 16.Moseley P, Alderson-Day B, Ellison A, Jardri R, Fernyhough C. Non-invasive brain stimulation and auditory verbal hallucinations: New techniques and future directions. Front Neurosci. 2016;9:515. doi: 10.3389/fnins.2015.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schurz M, Aichhorn M, Martin A, Perner J. Common brain areas engaged in false belief reasoning and visual perspective taking: a meta-analysis of functional brain imaging studies. Front Hum Neurosci. 2013;7:712. doi: 10.3389/fnhum.2013.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schurz M, Kronbichler M, Weissengruber S, Surtees A, Samson D, Perner J. Clarifying the role of theory of mind areas during visual perspective taking: Issues of spontaneity and domain-specificity. Neuroimage. 2015;117:386–396. doi: 10.1016/j.neuroimage.2015.04.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.