Abstract
In many cases, cancer patients do not die of a primary tumor, but rather because of metastasis. Although numerous rodent models are available for studying cancer metastasis in vivo, other efficient, reliable, low-cost models are needed to quickly access the potential effects of (epi)genetic changes or pharmacological compounds. As such, we illustrate and explain the feasibility of xenograft models using human breast cancer cells injected into zebrafish embryos to support this goal. Under the microscope, fluorescent proteins or chemically labeled human breast cancer cells are transplanted into transgenic zebrafish embryos, Tg (fli:EGFP), at the perivitelline space or duct of Cuvier (Doc) 48 h after fertilization. Shortly afterwards, the temporal-spatial process of cancer cell invasion, dissemination, and metastasis in the living fish body is visualized under a fluorescent microscope. The models using different injection sites, i.e., perivitelline space or Doc are complementary to one another, reflecting the early stage (intravasation step) and late stage (extravasation step) of the multistep metastatic cascade of events. Moreover, peritumoral and intratumoral angiogenesis can be observed with the injection into the perivitelline space. The entire experimental period is no more than 8 days. These two models combine cell labeling, micro-transplantation, and fluorescence imaging techniques, enabling the rapid evaluation of cancer metastasis in response to genetic and pharmacological manipulations.
Keywords: Cancer Research, Issue 122, embryonic zebrafish, human breast cancer, metastasis, intravasation, extravasation, perivitelline space, duct of Cuvier
Introduction
Overt cancer metastasis in the clinic comprises a series of complex and multi-step events known as the "metastatic cascade". The cascade has been extensively reviewed and can be dissected into successive steps: local invasion, intravasation, dissemination, arrest, extravasation, and colonization1,2. A better understanding of the pathogenesis of cancer metastasis and the development of potential treatment strategies in vivo require robust host models of cancer cell spread. Rodent models are well established and are widely used to evaluate metastasis3, but these approaches have low efficiency and ethical limitations and are costly as a forefront model to determine whether a particular manipulation could affect the metastatic phenotype. Other efficient, reliable, low-cost models are needed to quickly access the potential effects of (epi)genetic changes or pharmacological compounds. Due to their high genetic homology to humans and the transparency of their embryos zebrafish (Danio rerio) have emerged as an important vertebrate model and are being increasingly applied to the study of developmental processes, microbe-host interactions, human diseases, drug screening, etc.4. The cancer metastasis models established in zebrafish may provide an answer to the shortcomings of rodent models5,6.
Although spontaneous neoplasia is scarcely seen in wild zebrafish7, there are several longstanding techniques to induce the desired cancer in zebrafish. Carcinogen-induced gene mutations or signaling pathway activation can histologically and molecularly model carcinogenesis, mimicking human disease in zebrafish7,8,9. By taking advantage of diverse forward and reverse genetic manipulations of oncogenes or tumor suppressors, (transgenic) zebrafish have also enabled potential studies of cancer formation and maintenance6,10. The induced cancer models in zebrafish cover a broad spectrum, including digestive, reproductive, blood, nervous system, and epithelial6.
The utilization of zebrafish in cancer research has expanded recently due to the establishment of human tumor cell xenograft models in this organism. This was first reported with human metastatic melanoma cells that were successfully engrafted in zebrafish embryos at the blastula stage in 200511. Several independent laboratories have validated the feasibility of this pioneering work by introducing a diverse range of mammalian cancer cells lines into zebrafish at various sites and developmental stages5. For example, injections near the blastodisc and blastocyst of the blastula stage; injections into the yolk sac, perivitelline space, duct of Cuvier (Doc), and posterior cardinal vein of 6-h- to 5-day-old embryos; and injections into the peritoneal cavity of 30-day-old immunosuppressed larvae have been performed5,12. Additionally, allogeneic tumor transplantations were also reported in zebrafish12,13. One of the great advantages of using xenografts is that the engrafted cancer cells can be easily fluorescently labeled and distinguished from normal cells. Hence, investigations into the dynamic behaviors of microtumor formation14, cell invasion and metastasis15,16,17, tumor-induced angiogenesis15,18, and the interactions between cancer cells and host factors17 can be clearly visualized in the live fish body, especially when transgenic zebrafish lines are applied5.
Inspired by the high potential of zebrafish xenograft models to evaluate metastasis, we demonstrated the transvascular extravasation properties of different breast cancer cell lines in the tailfin area of Tg (fli:EGFP) zebrafish embryos through Doc injections16. The role of transforming growth factor-β (TGF-β)16 and bone morphogenetic protein (BMP)19 signaling pathways in pro-/anti-breast cancer cell invasion and metastasis were also investigated in this model. Moreover, we also recapitulated the intravasation ability of various breast cancer cell lines into circulation using xenograft zebrafish models with perivitelline space injections.
This article presents detailed protocols for zebrafish xenograft models based upon the injection of human breast cancer cells into the perivitelline space or Doc. Using high-resolution fluorescence imaging, we show the representative process of intravasation into blood vessels and the invasive behavior of different human breast cancer cells, which move from the blood vessels into the avascular tailfin area.
Protocol
All research using the transgenic fluorescent zebrafish Tg (fli:EGFP) strain, which has enhanced green fluorescent protein (EGFP)-labeled vasculature20, including housing and experiments, was carried out according to the international guidelines and was approved by the local Institutional Committee for Animal Welfare (Dier Ethische Commissie (DEC) of the Leiden University Medical Center.
NOTE: As summarized in Figure 1, the protocol is roughly broken down into four steps: embryo collection (Figure 1A), microinjection (Figure 1B), screening (Figure 1C), and analysis (Figure 1D).
1. Prepare the Injection Needles
Prepare injection needles with borosilicate glass microcapillaries. Put a microcapillary into a micropipette puller device with the following settings: air pressure, 500; heat, 650; pull, 100; velocity, 200; time, 40. Keep the injection needles in a needle holder plate until they are used for injection.
2. Prepare the Fluorescent, Genetically Labeled Breast Cancer Cells for Injection
Culture human breast cancer MDA-MB-231 cells at 37 °C in DMEM-high glucose medium containing L-glutamine, 10% fetal bovine serum, and 1:100 penicillin-streptomycin (pen-strep).
Culture the breast epithelial cell lines, MCF10A (M1) and MCF10A-Ras (M2), at 37 °C in DMEM/F12 media containing L-glutamine with 5% horse serum, 20 ng/mL epidermal growth factor, 10 mg/mL insulin, 100 ng/mL cholera enterotoxin, 0.5 mg/mL hydrocortisone, and 1:100 pen-strep.
Produce mCherry lentivirus by co-transfecting PLV-mCherry, pCMV-VSVG21, pMDLg-RRE (gag/pol)22, and pRSV-REV22 plasmid into HEK293T cells. Harvest cell supernatants 48 h after transfection and store at -80 °C.
Infect MDA-MB-231, M1, and M2 cells at 30% confluence for 24 h with lentiviral supernatants diluted 1:1 with normal culture medium in the presence of 5 ng/mL polybrene.
Select single-cell clones by diluting cells in a 96-well plate, which allows the outgrowth of isolated cell clones, until obtaining the stable mCherry-expressing cell lines.
Culture one T75 flask of cells for injection. Harvest the cells at 80% confluence with a 0.5% trypsin-EDTA treatment. Wash the cells with 1x PBS 2-3 times.
Re-suspend the cells in about 200 µL of PBS. Store at them 4 °C for less than 5 h before injection.
3. Prepare Zebrafish Embryos for Injection
Set up zebrafish breeding pairs and collect embryos, as shown in a previous JoVE article by Rosen et al.23.
Select the embryos that are at 0-4 hpf by removing the unfertilized and abnormal embryos. Keep the embryos in a Petri dish full of egg water (60 µg/mL sea salts; ~60 embryos/dish) and incubate at 28 °C.
Dechorionate the embryos with fine tweezers at 48 hpf.
Anesthetize the embryos by transferring them to 40 µg/mL tricaine (3-aminobenzoic acid) containing egg water approximately 2 min prior to injection, but no longer than 2 h prior to injection. NOTE: Tricaine stock solution (4 mg/mL, 100x) is prepared as 400 mg of tricaine powder in 97.9 mL of double-distilled water and 2.1 mL of 1 M Tris-base (pH 9), with the pH adjusted to 7.4. Store in the -20 °C freezer.
4. Inject Human Breast Cancer Cells into the Perivitelline Space
Load 15 µL of the cell suspension into an injection needle. Mount the needle onto the micromanipulator and break off the needle tip with fine tweezers to obtain a tip opening diameter of 5-10 µm.
Use a pneumatic picopump and a manipulator to perform the microinjection. Adjust the picopump to inject 400 cells each time. Prior to injection, count the cell numbers manually by injecting the cells on the top of a Petri dish containing 1% agarose.
Line up anesthetized embryos (2-3 days post fertilization (dpf)) on a flat, 1% agarose injecting plate, around 10 embryos each time.
Orient the injection plate by hand during the injections to place the embryos in the preferred position for inserting the needle (i.e., diagonally).
Point the needle tip at the injection site and gently insert the needle tip into the perivitelline space between the yolk sac and the periderm of the zebrafish embryo (Figure 2A).
Inject approximately 400 mCherry-labeled tumor cells. Make sure that the yolk sac is not ruptured to avoid implantation into the yolk sac.
5. Inject Human Breast Cancer Cells into the Doc
Prepare the injection needle and zebrafish embryos as described in protocol steps 1, 2, and 3.
Use a 45° needle angle so that the Doc can be approached from the dorsal side of the embryo.
Insert the needle into the starting point of the Doc (Figure 3A), just dorsal to where the duct starts broadening over the yolk sac, and inject approximately 400 cells; the injection is correct if the volume within the duct expands directly after the pulse and the yolk sac. NOTE: Several consecutive injections can be performed without extracting the needle.
Transfer the injected zebrafish embryos to egg water. NOTE: As considerable variation exists among individual zebrafish embryos, and as the death of embryos after injection can occur, a relatively large number of zebrafish embryos (around 100) should be injected with cancer cells.
Maintain the zebrafish embryos at 33 °C to accommodate the optimal temperature requirements for fish and mammalian cells.
6. Screen the Injected Embryos
Screen each fish under a fluorescence stereomicroscope at 2 h post-injection (hpi) for the perivitelline space injection (Figure 2) or at 2-24 hpi for the Doc injection (Figure 2) to ensure that all of the embryos are injected with a similar number of tumor cells. Remove the embryos with injection errors, such as ruptures (Figure 2B) or injections (Figure 3B) of the yolk sac, and pick out embryos with injected cells below (Figures 2C and 3B) or above (Figures 2D and 3B) threshold. Keep only the embryos with approximately 400 cells in culture.
Rule out the possibility that the cells are introduced directly into circulation during the injection process by removing the embryos with cells already in circulation. Also, remove any embryo with a cell mass close to the Doc (Figure 2D).
7. Image and Analyze the Metastatic Process
Collect several anesthetized embryos with a wide-tip Pasteur pipette and transfer them to the glass bottom of a polystyrene dish.
Remove excess water and keep a limited amount of egg water. Manipulate the embryo into position with a hair loop tool and place a cover on top of the glass.
Use an inverted confocal microscope in combination with water-immersion or long-distance dry objectives. Position the embryo such that the region of interest is as close to the objective as possible.
- Perform imaging immediately after anesthesia to reduce the risk of death due to liquid evaporation.
- Capture signals from EGFP-labeled vasculature and mCherry-labeled tumor cells at the same position on the embryos to co-register injected cells with blood vessels by merging the two imaging channels.
- For each zebrafish embryo, collect two different sets of images from the head region and tail region.
- Quantify the number of disseminated cells.
- For perivitelline space injections, count the number of cells in each fish that have disseminated from the cell mass towards the embryonic fish body within the head and tail regions4,15; the regions are beyond the boundaries of the heart cavity frontally, on top of the swim bladder dorsally, and beyond the urogenital opening caudally.
- For the Doc injection, count the number of individual cells that have invaded the collagen fibers of the tailfin from circulation (MDA-MB-231) or the number of clusters formed by cells collectively (M2) in the caudal hematopoietic tissue (CHT) of each zebrafish19.
- Study invasion and metastasis in more detail by using confocal microscopy (highly recommended).
- Use low magnification (4X objective) to image the whole body and to obtain an overview of the tumor cell dissemination pattern. NOTE: Higher magnification (20X and 40X objectives) is suitable for studying intra- and peri-tumoral angiogenesis and the precise localization of disseminated cells in the embryo body.
- Use a 488 nm laser to scan the zebrafish embryo vasculature and a 543 nm laser to scan implanted tumor cells labeled with red fluorescence. Obtain a high-quality image by scanning each embryo in eight to ten steps. Scan and average each step six times.
Carefully place the embryo back into the egg water if it is required for further experiments.
8. Perform Statistical Analysis Using One-way Analysis of Variance (ANOVA) Followed by Post Hoc Analysis
Representative Results
In the embryonic xenograft zebrafish model with a perivitelline space injection, the hematogenous dissemination of labeled cancer cells in the fish body is considered as active migration. This process can be detected and quantified under a fluorescent microscope, as described in the methods above. To illustrate this xenograft model, we followed the dissemination process of different breast cancer cell lines with known (or without) invasion/metastasis potential according to in vitro and in vivo mouse studies, including the benign normal breast epithelial M1 cells, HRAS-transformed premalignant M2 cells, and highly metastatic MDA-MB-231 cells, 1 day post injection (dpi) onward. A high-resolution confocal microscopy image showed that MDA-MB-231 cells (red) exhibit an aggressive phenotype, with irregular borders in the perivitelline space. Pseudopodia-like protrusions and invasive fronts were also frequently present (Figure 4A, left). A few cells disseminated into blood circulation as early as 1 dpi (Figure 4A, right). At 2 dpi, clear dissemination was observed in the distal parts of the fish (Figure 4A, right). The number of disseminated cells increased further at 3 dpi (Figure 4A and 4D). In contrast, when M2 cells were challenged in zebrafish, they exhibited modest spread in the fish body after 2 dpi (Figure 4B). They also showed increased dissemination after time passed (Figure 4F). As shown in Figure 4C and 4G, M1 cells infrequently disseminated into zebrafish circulation, and even active local migration within the perivitelline space was infrequent during the period of observation. The M1 cell mass was virtually detained at the original injection site. If defining positive dissemination or metastasis as >5 cells in the fish body4, MDA-MB-231 and M2 cell metastasis was observed in 92% and 57% of fish, respectively, at 3 dpi (Figure 4G). In contrast, no positive dissemination was observed with M1 cells. Therefore, this zebrafish model of human cancer cell progression accurately reflects the relative level of metastatic potential of the different cells in mice. Neovascularization (green) that sprouted from the subintestinal plexus of the embryonic zebrafish and penetrated the MDA-MB-231 or M2 cell mass was also present after the perivitelline space injection of tumor cells followed by 3 days of incubation (Figure 4A and 4B, left). Consistent with the disability in dissemination, only slight neovascularization was detected upon M1 cell implantation (Figure 4C).
In the embryonic xenograft zebrafish model with mCherry-labeled MDA-MB-231 cells and the Doc injection, the labeled cancer cells in the tailfin of the zebrafish are considered representative of active extravasation. The mCherry-labeled MDA-MB-231 cells were injected at 2 dpf. At 3 dpi, the cells started to migrate out of the vessels to the tailfin, which is enriched with collagen. Single MDA-MB-231 cells migrated one by one, independently from the vessels, to the distant tailfin (Figure 5A). At 6 dpi, the invasion could be quantified by counting the number of cells that migrated into the tailfin tissue. In the mCherry-labeled M2 cell Doc injection model, the injection was also performed at 2 dpf. However, a clustered phenotype was observed during the active extravasation process. At 1 dpi, M2 cells started to migrate out from the vessels into the CHT of the zebrafish. At 2 dpi, the migrated M2 cells started to form a cluster between the vessels in the CHT (Figure 5B). Quantification of the M2 invasive cell cluster number in the CHT region could be conducted at 6 dpi.
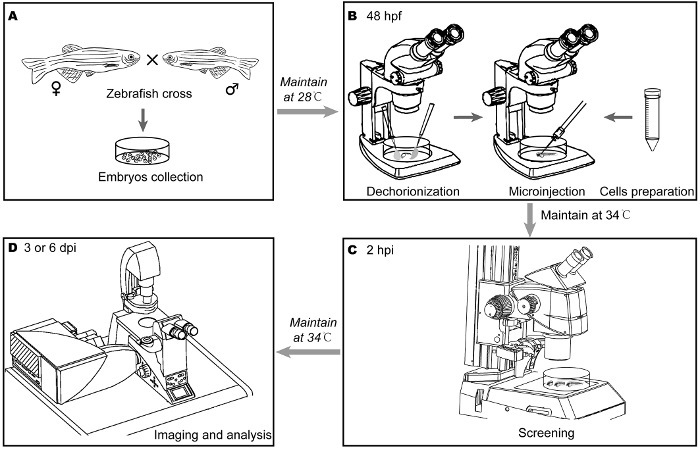 Figure 1:Main steps for investigating the invasive behavior of breast cancer cells in embryonic zebrafish. (A) After crossing parental zebrafish overnight, Tg(fli1:EGFP) zebrafish embryos were collected the following morning and were maintained at 28 °C. (B) The embryos were dechorionated with fine tweezers under a stereomicroscope 48 h post fertilization (hpf). The labeled breast cancer cells were collected and re-suspended in a small amount of PBS. After well-preparation, suspended cells were loaded into one needle. Approximately 400 cells were injected into the duct of Cuvier (Doc) of the perivitelline space under a stereomicroscope. The injected embryos were maintained at 33 °C. (C) 2 h post injection (hpi), the embryos were subjected to careful screening under a fluorescence stereomicroscope. The embryos were maintained at 33 °C for 3 or 6 days. During the interval, the embryos were subjected to the designed treatment. (D) Cancer cell dissemination by perivitelline space injection or invasion by Doc injection was detected, counted, and imaged by confocal microscopy 3 or 6 days post injection (dpi). Please click here to view a larger version of this figure.
Figure 1:Main steps for investigating the invasive behavior of breast cancer cells in embryonic zebrafish. (A) After crossing parental zebrafish overnight, Tg(fli1:EGFP) zebrafish embryos were collected the following morning and were maintained at 28 °C. (B) The embryos were dechorionated with fine tweezers under a stereomicroscope 48 h post fertilization (hpf). The labeled breast cancer cells were collected and re-suspended in a small amount of PBS. After well-preparation, suspended cells were loaded into one needle. Approximately 400 cells were injected into the duct of Cuvier (Doc) of the perivitelline space under a stereomicroscope. The injected embryos were maintained at 33 °C. (C) 2 h post injection (hpi), the embryos were subjected to careful screening under a fluorescence stereomicroscope. The embryos were maintained at 33 °C for 3 or 6 days. During the interval, the embryos were subjected to the designed treatment. (D) Cancer cell dissemination by perivitelline space injection or invasion by Doc injection was detected, counted, and imaged by confocal microscopy 3 or 6 days post injection (dpi). Please click here to view a larger version of this figure.
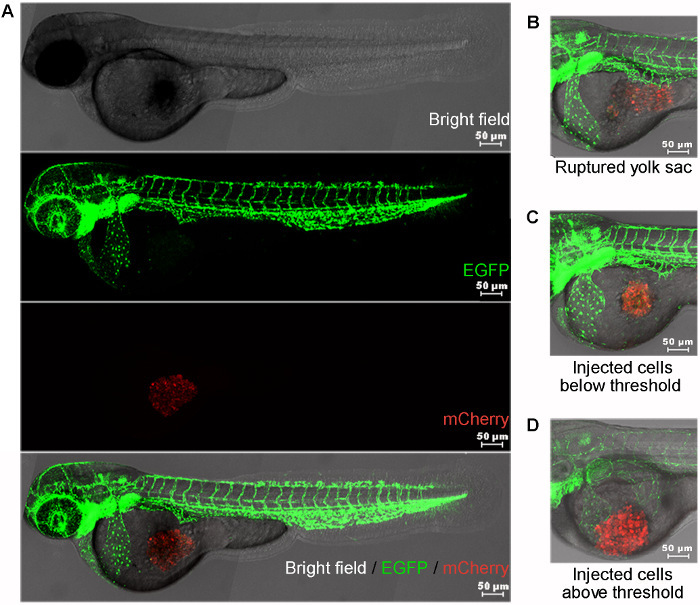 Figure 2:Perivitelline space injection site and common errors. (A) Approximately 400 mCherry-labeled cells (MDA-MB-231) were injected into the perivitelline space. The brightfield (uppermost), green vasculature (middle upper), and red cell mass (middle lower) of injected zebrafish embryos were captured by confocal microscope. The merged image (lowermost) of the three channels shows the stereo location of the cell mass in the embryo. (B) The cells did not target the perivitelline space appropriately. The yolk sac was ruptured. (C) Injected cells below threshold (much less than 400). (D) Injected cells above threshold (much more than 400). The cell mass was too close to the duct of Cuvier, which has a broad blood stream. Scale bar = 50 µm. Please click here to view a larger version of this figure.
Figure 2:Perivitelline space injection site and common errors. (A) Approximately 400 mCherry-labeled cells (MDA-MB-231) were injected into the perivitelline space. The brightfield (uppermost), green vasculature (middle upper), and red cell mass (middle lower) of injected zebrafish embryos were captured by confocal microscope. The merged image (lowermost) of the three channels shows the stereo location of the cell mass in the embryo. (B) The cells did not target the perivitelline space appropriately. The yolk sac was ruptured. (C) Injected cells below threshold (much less than 400). (D) Injected cells above threshold (much more than 400). The cell mass was too close to the duct of Cuvier, which has a broad blood stream. Scale bar = 50 µm. Please click here to view a larger version of this figure.
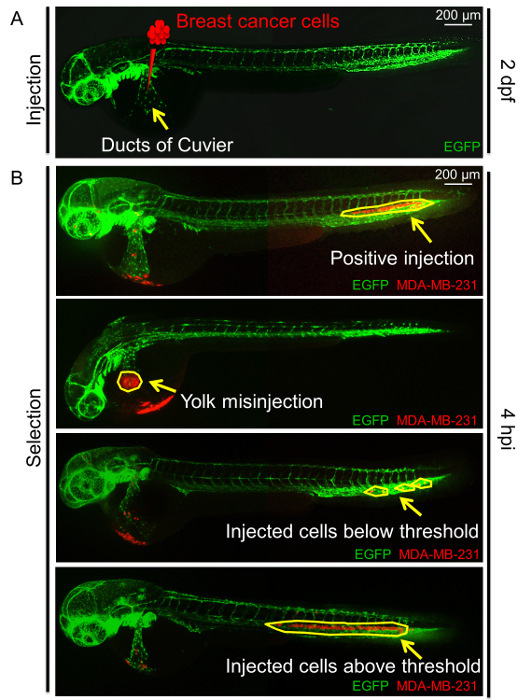 Figure 3:Overview of the duct of Cuvier (Doc) injection. (A) Schematic of the Doc injection at 2 days post-fertilization (dpf) with breast cancer cells in zebrafish embryos. The arrow indicates the Doc. (B) Examples of positive injections, with around 400 breast cancer cells; negative injections, including the yolk misinjection; and an incorrect number of cells injected at 4 hpi. Arrows and circles indicate injected cells. Please click here to view a larger version of this figure.
Figure 3:Overview of the duct of Cuvier (Doc) injection. (A) Schematic of the Doc injection at 2 days post-fertilization (dpf) with breast cancer cells in zebrafish embryos. The arrow indicates the Doc. (B) Examples of positive injections, with around 400 breast cancer cells; negative injections, including the yolk misinjection; and an incorrect number of cells injected at 4 hpi. Arrows and circles indicate injected cells. Please click here to view a larger version of this figure.
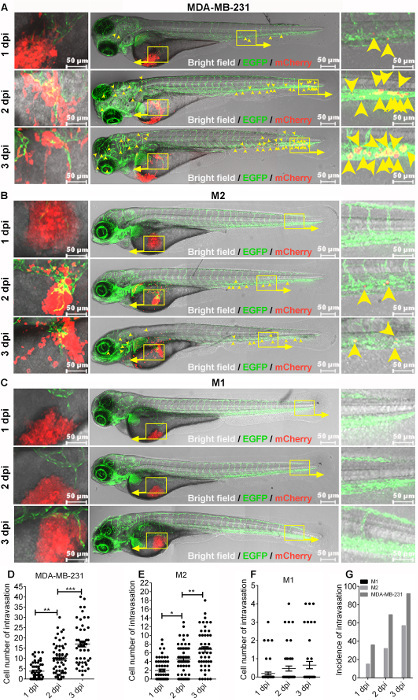 Figure 4:Comparison of dissemination ability among various breast cell lines. Approximately 400 mCherry-labeled MDA-MB-231, MCF10Aras (M2), or MCF10A (M1) cells were injected into the perivitelline space of zebrafish embryos 48 hpf. The injected embryos were followed for 3 days. (A, B, and C) High-resolution micrographs showing the representative migration and dissemination process of MDA-MB-231 (A), M2 (B), and M1 (C) cells in individual embryonic bodies 1, 2, and 3 days post-injection (dpi). Left, cell migration in the perivitelline space (red) and the peritumoral and intratumoral vasculature (green). Yellow signals indicate the overlap of microvessels and cells. Middle, the whole image of the embryo. Right, visualization of disseminated cells in the posterior of the embryo. Yellow arrowheads indicate single disseminated cells. Scale bar = 50 µm. (D, E, and F) Quantification of the number of disseminated cells in each embryonic body at 1, 2, and 3 dpi. Results are expressed as the mean ± SEM. Results from one-way analysis of variance (ANOVA) followed by the post-hoc analysis are shown. P < 0.05 was accepted as statistically significant (*0.01 < P < 0.05; **0.001 < P < 0.01; *** P < .001. (G) Comparison of the incidences of intravasation for MDA-MB-231, M2, and M1 cells in embryonic bodies at 1, 2, and 3 dpi. Please click here to view a larger version of this figure.
Figure 4:Comparison of dissemination ability among various breast cell lines. Approximately 400 mCherry-labeled MDA-MB-231, MCF10Aras (M2), or MCF10A (M1) cells were injected into the perivitelline space of zebrafish embryos 48 hpf. The injected embryos were followed for 3 days. (A, B, and C) High-resolution micrographs showing the representative migration and dissemination process of MDA-MB-231 (A), M2 (B), and M1 (C) cells in individual embryonic bodies 1, 2, and 3 days post-injection (dpi). Left, cell migration in the perivitelline space (red) and the peritumoral and intratumoral vasculature (green). Yellow signals indicate the overlap of microvessels and cells. Middle, the whole image of the embryo. Right, visualization of disseminated cells in the posterior of the embryo. Yellow arrowheads indicate single disseminated cells. Scale bar = 50 µm. (D, E, and F) Quantification of the number of disseminated cells in each embryonic body at 1, 2, and 3 dpi. Results are expressed as the mean ± SEM. Results from one-way analysis of variance (ANOVA) followed by the post-hoc analysis are shown. P < 0.05 was accepted as statistically significant (*0.01 < P < 0.05; **0.001 < P < 0.01; *** P < .001. (G) Comparison of the incidences of intravasation for MDA-MB-231, M2, and M1 cells in embryonic bodies at 1, 2, and 3 dpi. Please click here to view a larger version of this figure.
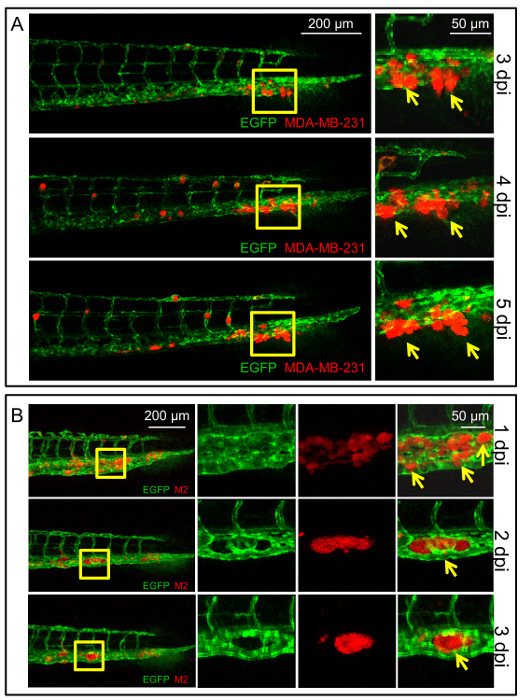 Figure 5:Different behaviors of MDA-MB-231 and M2 cell metastasis in zebrafish with duct of Cuvier injection. (A) Representative confocal images of the zebrafish followed at 3, 4, and 5 dpi to show the single-cell migration behavior of the MDA-MB-231 cells in zebrafish. Arrows indicate invasive MDA-MB-231 cells that migrated out of the vessels into the tailfins. Scale bar = 200 µm in the left column, 50 µm in the right column. (B) Representative confocal images of the zebrafish followed at 1, 2, and 3 dpi to show the cell cluster migration behavior of M2 cells in zebrafish. Arrows indicate invasive M2 cells that migrated out of the vessels to the caudal hematopoietic tissue (CHT) and formed a cluster between the vessels. Please click here to view a larger version of this figure.
Figure 5:Different behaviors of MDA-MB-231 and M2 cell metastasis in zebrafish with duct of Cuvier injection. (A) Representative confocal images of the zebrafish followed at 3, 4, and 5 dpi to show the single-cell migration behavior of the MDA-MB-231 cells in zebrafish. Arrows indicate invasive MDA-MB-231 cells that migrated out of the vessels into the tailfins. Scale bar = 200 µm in the left column, 50 µm in the right column. (B) Representative confocal images of the zebrafish followed at 1, 2, and 3 dpi to show the cell cluster migration behavior of M2 cells in zebrafish. Arrows indicate invasive M2 cells that migrated out of the vessels to the caudal hematopoietic tissue (CHT) and formed a cluster between the vessels. Please click here to view a larger version of this figure.
Discussion
Here, we described two methods to investigate the invasive behavior of breast cancer cells in Tg (fli1:EGFP) zebrafish embryos, with perivitelline space and Doc injections. By injecting cancer cells labeled with chemical dye or fluorescent protein into transgenic zebrafish embryos, the dynamic and spatial characteristics of invasion and metastasis can be clearly tracked in real-time at the single-cell or cluster level under a fluorescence microscope. In most cases, the rapid progression of metastasis in zebrafish ensures that the assay can be performed within 1 week after transplantation. Moreover, powerful statistics can be obtained with large cohorts of fish.
Early and late events of the metastatic cascade could be simulated and recapitulated by injecting cancer cells into the perivitelline space or Doc, respectively. The perivitelline space is the confined space between the periderm of the fish and the yolk sac, which allows one to monitor dissemination of single tumor cells from primary sites in the living body. After implantation, the cancer cells undergo local migration and invasion within the perivitelline space (considered the primary site) and then they intravasate into blood vessels and disseminate along with the circulation. At the head and tailfin (considered distant target sites), cancer cells accumulate in narrow capillary beds and extravasate. Therefore, the number of cells that are found at the distant sites in the fish body is a measurement of metastatic capability. In addition, more extravasated cells can be observed at later time points, which is also true of the Doc injection assay.
The Doc is an enlarged common cardinal vein with an extensive blood stream24. Directly targeting the Doc as an injection site introduces cancer cells into the circulatory system. In practice, breast cancer cells diffuse throughout the embryonic body via the blood stream instantly after Doc injection. The cells then arrest at the caudal vein and dorsal aorta. Extravasation, invasion, and micrometastasis formation can be observed successively within 6 days. As reported previously16, metastatic MDA-MB-231 cells and premalignant mammary M2 cells exhibit different invasive phenotypes. MDA-MB-231 cells undergo single-cell invasion of the collagen matrix-rich tailfin. Thus, the invasion potential of MDA-MB-231 cells can be measured by counting the number of cells that have extravasated and invaded the tailfin tissue. In contrast, M2 cells form clusters of different sizes and undergo collective invasion of the CHT. Quantifying the invasion potential of M2 cells by counting the number of clusters in this protocol is difficult and is preferably performed by making a 3D image using confocal microscopy and determining the volume of clustered tumor cells.
The technical challenge in cancer cell microinjection is successfully targeting the perivitelline space or Doc. The microinjection of large numbers of embryos is a tedious procedure requiring a highly skilled and patient operator. Factors that contribute to variations in the results in individual fish include the developmental stage of the embryo when injecting, differences in the number of cells injected, and the leakage of cells into the yolk sac. Though rare, the manipulation could unintentionally penetrate the vasculature and introduce cells into the circulatory system directly, especially in the perivitelline space injection. To further reduce variation and to ensure the reliability of the analyses, microscopic examination is necessary to exclude unqualified fish at time points throughout the process. In addition, blinded analysis by a professional without knowledge of the setting is strongly suggested to achieve unbiased quantification.
In summary, the two models we introduced here shed light on visualizing the processes of cell invasion and metastasis in vivo without invasive procedures. Although we only studied breast cancer cells in two models regarding metastatic potential, they could be extrapolated to other types of cancer. Moreover, the models could have broader applications in determining the mechanisms and new molecular targets controlling cancer cell metastasis using (epi)genetic manipulation. Due to the higher penetrability of zebrafish embryos by small-molecule compounds as compared to the feeding or injection of rodents25, the two presented models also have advantages in terms of the high-throughput screening of potential new anti-invasion/metastasis drugs.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Studies on TGF-β family members are supported by the Cancer Genomics Centre Netherlands. Sijia Liu and Jiang Ren are supported by the China Scholarship Council for 4 years of study at the University of Leiden. We thank Dr. Fred Miller (Barbara Ann Karmanos Cancer Institute, Detroit, MI, USA) for the MCF10A cell lines.
References
- Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat. Med. 2013;19(11):1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- Obenauf AC, Massagué J. Surviving at a distance: Organ-specific metastasis. Trends Cancer. 2015;1(1):76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena M, Christofori G. Rebuilding cancer metastasis in the mouse. Mol. Oncol. 2013;7(2):283–296. doi: 10.1016/j.molonc.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Xie X, Walker S, et al. Evaluating human cancer cell metastasis in zebrafish. BMC cancer. 2013;13(1):453. doi: 10.1186/1471-2407-13-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konantz M, Balci TB, Hartwig UF, et al. Zebrafish xenografts as a tool for in vivo studies on human cancer. Ann N Y Acad Sci. 2012;1266(1):124–137. doi: 10.1111/j.1749-6632.2012.06575.x. [DOI] [PubMed] [Google Scholar]
- Zhao S, Huang J, Ye J. A fresh look at zebrafish from the perspective of cancer research. J Exp Clin Cancer Res. 2015;34(1):80. doi: 10.1186/s13046-015-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton MF. Diethylnitrosamine-induced hepatic degeneration and neoplasia in the aquarium fish, Brachydanio rerio. J. Natl. Cancer Inst. 1965;34(1):117–130. doi: 10.1093/jnci/34.1.117. [DOI] [PubMed] [Google Scholar]
- Lam SH, Wu YL, Vega VB, et al. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat. Biotechnol. 2006;24(1):73–75. doi: 10.1038/nbt1169. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research-advantages and current limitations. Toxicol. Pathol. 2003;31(Suppl 1):62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoletov K, Klemke R. Catch of the day: zebrafish as a human cancer model. Oncogene. 2008;27(33):4509–4520. doi: 10.1038/onc.2008.95. [DOI] [PubMed] [Google Scholar]
- Lee LM, Seftor EA, Bonde G, et al. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev. Dynam. 2005;233(4):1560–1570. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- Mizgirev I, Revskoy S. Generation of clonal zebrafish lines and transplantable hepatic tumors. Nat. Protoc. 2010;5(3):383–394. doi: 10.1038/nprot.2010.8. [DOI] [PubMed] [Google Scholar]
- Mizgireuv IV, Revskoy SY. Transplantable tumor lines generated in clonal zebrafish. Cancer Res. 2006;66(6):3120–3125. doi: 10.1158/0008-5472.CAN-05-3800. [DOI] [PubMed] [Google Scholar]
- Stoletov K, Montel V, Lester RD, et al. High-resolution imaging of the dynamic tumor cell-vascular interface in transparent zebrafish. Proc. Natl. Acad. Sci. U.S.A. 2007;104(44):17406–17411. doi: 10.1073/pnas.0703446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhi P, Jensen LD, Cao Z, et al. Hypoxia-induced metastasis model in embryonic zebrafish. Nat. Protoc. 2010;5(12):1911–1918. doi: 10.1038/nprot.2010.150. [DOI] [PubMed] [Google Scholar]
- Drabsch Y, He S, Zhang L, et al. Transforming growth factor-β signalling controls human breast cancer metastasis in a zebrafish xenograft model. Breast Cancer Res. 2013;15(6):R106. doi: 10.1186/bcr3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Lamers GE, Beenakker JW, et al. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J. Pathol. 2012;227(4):431–445. doi: 10.1002/path.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoli S, Presta M. The zebrafish/tumor xenograft angiogenesis assay. Nat. Protoc. 2007;2(11):2918–2923. doi: 10.1038/nprot.2007.412. [DOI] [PubMed] [Google Scholar]
- de Boeck M, Cui C, Mulder AA, et al. Smad6 determines BMP-regulated invasive behaviour of breast cancer cells in a zebrafish xenograft model. Sci. Rep. 2016;6:24968. doi: 10.1038/srep24968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002;248(2):307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Stewart SA, Dykxhoorn DM, Palliser D, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9(4):493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, et al. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72(11):8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JN, Sweeney MF, Mably JD. Microinjection of zebrafish embryos to analyze gene function. J. Vis. Exp. 2009. p. e1115. [DOI] [PMC free article] [PubMed]
- Lawson ND, Weinstein BM. Arteries and veins: making a difference with zebrafish. Nat. Rev. Genet. 2002;3(9):674–682. doi: 10.1038/nrg888. [DOI] [PubMed] [Google Scholar]
- Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005;4(1):35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]


