Abstract
Importance
Intracameral injection of cefuroxime sodium (1 mg/0.1 mL) has been reported to reduce the risk of endophthalmitis following cataract surgery. In the United States it must be compounded, which is subject to dilution error. We describe a series of 13 eyes that received intracameral injection of cefuroxime sodium, 9 mg/0.1 mL, intraoperatively.
Observations
On postoperative day 1, 6 of 13 eyes (46%; 95% CI, 19%-75%) had visual acuity of 20/70 or worse and macular edema. Spectral-domain optical coherence tomography of 2 eyes revealed central subfield thicknesses of 909 and 873 μm. On postoperative day 4, the mean (SD) central subfield thickness was 309 (78) μm in the 6 eyes with diagnosed macular edema, 279 (23) μm in the fellow eyes, and 271 (38) μm in the 7 exposed eyes without macular edema. The mean (SD) time to resolution of macular edema was 5.2 (1.3) days; the final central subfield thickness ranged from 193 to 293 μm. All eyes, except 2 with preexisting ocular comorbidity, had a best-corrected final visual acuity at 1 month of 20/30 or better. Significant corneal edema was not observed.
Conclusions And Relevance
Intracameral injection of cefuroxime sodium at a dose of 9 mg/0.1 mL was associated with transient macular edema and diminished visual acuity in 6 of 13 exposed eyes (46%), resolving largely within 1 week.
Cefuroxime sodium (1 mg/0.1 mL) appears to be effective in preventing endophthalmitis.1,2 Kaiser Permanente surgeons began injecting intracameral cefuroxime in 2007, with resulting declining rates of infection.3,4 Cefuroxime is available in Europe as Aprokam, a manufactured product for intracameral injection. In the United States, no intracameral antibiotic preparation is approved to prevent endophthalmitis, thus creating a need for compounding for each surgical case. Reports have shown a long-term deleterious effect on the retina and cornea of injecting cefuroxime sodium at concentrations greater than 50 mg,5,6 while no adverse effect has been observed at 3 mg.7 We describe transient macular edema from injection of 9 mg of cefuroxime sodium.
Methods
Following Kaiser Permanente Institutional Review Board approval, we reviewed medical records of 11 patients (13 eyes) who underwent cataract surgery by a single surgeon (D.C.W.) on a single day. In this article, exposed eye refers to an eye that underwent surgery and was injected with cefuroxime sodium at a dose of 9 mg/0.1mL on that day. Reduced vision refers to Snellen visual acuity of 20/70 or worse on postoperative day 1 following uncomplicated clear-cornea cataract surgery. Data were collected and analyzed between June 2014 and January 2015. Informed consent was not required owing to the retrospective nature of the study.
All patients were prescribed topical prednisolone acetate, 1%, diclofenac, 0.1%, and ofloxacin, 0.3%, 4 times daily in the eye undergoing surgery beginning 3 days prior to surgery. Two of the 11 patients underwent bilateral same-day surgery. Ordinarily, 750 mg of cefuroxime sodium injectable powder is reconstituted with 7.5 mL of preservative-free normal saline (sodium chloride, 0.9%). Three milliliters (300 mg) of the resulting solution is then injected into an empty 30-mL sterile interior vial. To this, 27 mL of preservative-free normal saline is added, resulting in a final cefuroxime sodium concentration of 1 mg/0.1 mL.8 At each procedure's conclusion, 0.1 mL of compounded cefuroxime sodium solution was injected through the paracentesis. However, on this day, the final concentration was 9 mg/0.1 mL.
Results
Postoperative Day 1
On the first postoperative day, 6 of 13 eyes (46%; 95% CI, 19%-75%) had reduced vision with uncorrected distance visual acuity of 20/70 (0.54 logMAR) or worse (Table). On slitlamp bio-microscopy, 2 of 13 exposed eyes (15%) had mild central corneal edema. No eyes had more than mild cell and flare in the anterior chamber.
Table. Results of 13 Eyes of 11 Patients Who Received Cefuroxime Sodium at a Dose of 9 mg/0.1 mL at the Close of Phacoemulsificationa.
| Patient No. | Exposed Eye at 1-4 d | Fellow Eye, CST at 1-4 d, μm | Exposed Eye, CST at 6-7 d, μm | Exposed Eye, 1-3 mo | CST Resolution, d | Ocular Comorbidity | |||
|---|---|---|---|---|---|---|---|---|---|
| UDVA at 1 d, LogMAR (Snellen) | CST at 1 d, μm | CST at 4 d, μm | CST at 1-3 mo, μm | BCVA at 1 mo, LogMAR (Snellen) | |||||
| Eyes without macularedema | |||||||||
| 1 | 0.18 (20/30) | … | 290 | 298 | … | 293 | 0.00 (20/20) | NA | DM, no NPDR |
| 2 | 0.30 (20/40) | … | 195 | 155 | … | 193 | 0.30 (20/40) | NA | Birdshot chorio-retinopathy |
| 3 | 0.30 (20/40) | … | 261 | 259 | … | … | 0.30 (20/40) | NA | ERM, DM, NPDR |
| 4 | 0.40 (20/50) | … | 277 | 284 | … | 282 | 0.00 (20/20) | NA | DM, no NPDR |
| 5 | 0.40 (20/50) | … | 319 | 297 | … | … | 0.18 (20/30) | NA | VMT, DM, PRP |
| 6 | |||||||||
| Right eye | 0.40 (20/50) | … | 272 | NAb | … | 269 | 0.00 (20/20) | NA | DM, no NPDR |
| Left eye | 0.40 (20/50) | … | 285 | NAb | … | 262 | 0.00 (20/20) | NA | DM, no NPDR |
| Mean (SD) | 0.34 (0.08) [20/40]c | NA | 271 (38) | 259 (60) | NA | 260 (39) | 0.11 (0.14) [20/25]c | NA | NA |
| Eyes with macular edema | |||||||||
| 7 | 0.54 (20/70) | … | 434 | 246 | 239 | 240 | 0.10 (20/25) | 6 | None |
| 8 | |||||||||
| Left eye | 0.54 (20/70) | Clin Dxd | 235 | NAb | … | 237 | 0.00 (20/20) | 4 | None |
| Right eye | 0.60 (20/80) | Clin Dxd | 269 | NAb | … | 240 | 0.00 (20/20) | 4 | None |
| 9 | 0.88 (20/150) | Clin Dxd | 375 | 281 | 247 | … | 0.10 (20/25) | 7 | None |
| 10 | 0.88 (20/150) | 909 | 270 | 291 | 257 | 292 | 0.18 (20/30) | 6 | None |
| 11 | 1.00 (20/200) | 873 | 271 | 296 | … | … | 0.10 (20/25) | 4 | None |
| Mean (SD) | 0.74 (0.20) [20/100]c | 891 (25) | 309 (78) | 279 (23) | 248 (9) | 252 (27) | 0.08 (0.07) [20/25]c | 5.2 (1.3) | NA |
Abbreviations: BCVA, best-corrected visual acuity; Clin Dx, clinical diagnosis; CST, central subfield thickness; DM, diabetes mellitus; ERM, epiretinal membrane; NA, not applicable; NPDR, nonproliferative diabetic retinopathy; PRP, history of panretinal photocoagulation; UDVA, uncorrected distance visual acuity; VMT, vitreomacular traction; ellipses, optical coherence tomography not performed at this time.
Normative CST range for Spectralis spectral-domain optical coherence tomography (Heidelberg Engineering GmbH) is 270 ± 22.5 μm.
Both eyes of the same patient were exposed to cefuroxime sodium at a dose of 9 mg/0.1 mL.
Expressed as logMAR mean (SD) [Snellen equivalent].
Macular edema was clinically diagnosed on slitlamp biomicroscopy.
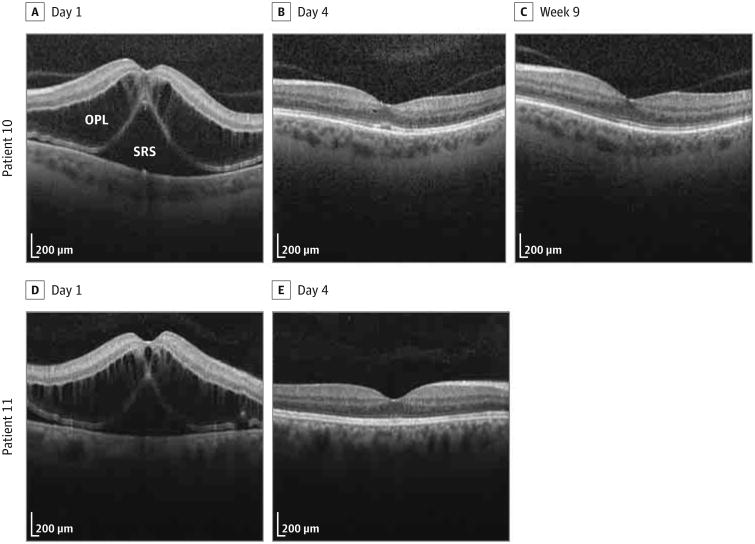
Spectral-domain optical coherence tomography (OCT) (Spectralis; Heidelberg Engineering GmbH) was performed on 2 patients with uncorrected distance visual acuity of 20/150 and 20/200. The central subfield thicknesses (CSTs) of the 2 exposed eyes were 909 and 873 μm, respectively (Figure).
Figure. Postoperative Spectral-Domain Optical Coherence Tomographic Images From Patients 10 and 11.
A-C, Patient 10 had severe thickening of the outer plexiform layer (OPL) and severe and broad thickening of the subretinal space (SRS) on day 1 (A), a small hyporeflective pocket in the OPL and trace shallow thickening of the SRS on day 4 (B), and no abnormalities at week 9 (C). D and E, Patient 11 had severe thickening of the OPL and severe and broad thickening of the SRS on day 1 (D), and no abnormalities on day 4 (E).
No fluorescein angiography was performed on these patients during the period under study. Therefore, vascular abnormalities could not be evaluated as a cause of vision loss.
That same day, the surgeon investigated the cause of the cluster. On pharmacy review, dilution error in the preparation of cefuroxime was uncovered as described later. The operating surgeon promptly notified all patients of the findings.
Postoperative Day 4
All exposed eyes were tested with spectral-domain OCT on postoperative day 4. Among the 6 exposed eyes with reduced vision on the first postoperative day, the mean (SD) CST of the 6 eyes was 309 (78) μm (range, 235-434 μm). The mean (SD) CST of the 7 exposed eyes without reduced vision on the first postoperative day was 271 (38) μm (range, 195-319 μm), including 1 eye with mild vitreomacular traction that was present 2 months prior to surgery (CST, 327 μm). The mean CSTs of the 2 groups were not statistically different (t test, P = .28). The mean(SD) CST of the fellow eyes was 279(23) μm.
Postoperative Days 6 and 7
A retina specialist (M.D.W.) examined 5 of 6 eyes diagnosed as having macular edema within 7 days following surgery. Patient 11 did not follow up as planned. The vitreous was documented as clear in all eyes. Additional spectral-domain OCT imaging acquired in 2 eyes that had demonstrated elevated CST on day 4 and in 1 of the 2 patients with elevated CST on day 1 showed resolution of retinal thickening and a mean (SD) CST of 248 (9) μm (range, 239-257 μm). Patient 10 had a CST of 257 μm with trace hyporeflective spaces in the outer plexiform layer and subretinal space, which resolved by week 9.
All exposed eyes with macular edema had resolution of thickening with a CST of 271 μm or less (range, 235-271 μm) within 1 week of surgery (mean [SD], 5.2 [1.3] days). The mean (SD) final best-corrected visual acuity at 1 month for exposed eyes with diagnosed macular edema was 0.08 (0.07) logMAR (Snellen equivalent, 20/25) and did not differ significantly from exposed eyes without diagnosed edema (0.11 [0.14] logMAR; Snellen equivalent, 20/25) (P = .64). Two eyes had visual acuity worse than 20/30 (20/40 in each) at 1 month due to preexisting retinal problems. The morphology of all retinal layers in the final OCT images was normal except in patients with preexisting conditions. The final CSTs were all within 95% normal limits except in the patient with preexisting birdshot chorioretinopathy. On OCT, the final CST ranged from 193 to 293 μm.
Cefuroxime Compounding
Root cause analysis identified protocol variances for in-house cefuroxime compounding. The usual product of powdered cefuroxime sodium, 750 mg, was unavailable from the manufacturer prior to compounding on the morning of surgery. Instead, a substituted product contained 1.5 g of cefuroxime sodium. The technician compounding the mixture mis-interpreted the updated dilution formula, which was prepared by another pharmacy staff member, and the second dilution step was omitted. The final check of product by pharmacy personnel was inadvertently skipped. Corrective action was instituted immediately. Subsequently, cefuroxime compounding has been moved to a US Food and Drug Administration–registered outsourcing facility.
Discussion
While uneventful cataract surgery has been shown to cause a subclinical increase in foveal thickness by OCT without visual impairment on postoperative day 1,9 intracameral cefuroxime sodium at a concentration of 1 mg/0.1 mL has been shown to be efficacious2 and safe10 without a significant effect on macular thickness by OCT11 or corneal endothelial cell density.12 Inadvertent injection of cefuroxime sodium, 3 mg, caused by substitution of a 750-mg vial for the presumed 250-mg product caused no harm to the cornea or retina on clinical examination in 6 patients, who had a final best-corrected visual acuity of 20/20 at 1 week postoperatively.7 Doses ranging from 50 to 60 mg have produced transient or permanent damage to the cornea and retina.13
While doses as low as 10 mg injected into the vitreous in rabbits have induced structural changes to the retina,14 no reports as yet show deleterious clinical effects in humans at this dose. This is the first report, to our knowledge, of the effects on the human eye of intracameral injection of cefuroxime sodium at a dose of 9 mg. In this series of eyes, the hyperosmolarity of the solution may have contributed to the development of macular edema; however, there were no signs of toxic anterior segment syndrome, which would be expected if osmolarity were causative.15
Conclusions
These data show that intracameral injection of cefuroxime sodium at a dose of 9 mg/0.1 mL can result in transient macular edema and diminished visual acuity likely in 19% to 75% of exposed eyes, resolving largely within 1 week. The safe therapeutic concentration window currently appears to be between 1 and 3 mg.
At a Glance.
Dilution errors in preparation of cefuroxime sodium for intracameral injection may occur.
Injection of 9 mg/0.1 mL may cause temporary macular edema and diminished visual acuity.
Resolution of this macular edema occurs largely within 1 week.
Acknowledgments
Funding/Support: This work was supported by grant R21EY022989 from the National Eye Institute. Drs Shorstein and Herrinton were also supported by the Community Benefit Grant from Kaiser Permanente.
Role of the Funder/Sponsor: The funding sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Wong and Waxman had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Wong, Waxman, Shorstein.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Wong, Waxman, Shorstein.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Wong, Herrinton, Shorstein.
Obtained funding: Herrinton.
Study supervision: Wong, Shorstein.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
References
- 1.Montan PG, Wejde G, Koranyi G, Rylander M. Prophylactic intracameral cefuroxime: efficacy in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2002;28(6):977–981. doi: 10.1016/s0886-3350(01)01269-x. [DOI] [PubMed] [Google Scholar]
- 2.Endophthalmitis Study Group, European Society of Cataract and Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978–988. doi: 10.1016/j.jcrs.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39(1):8–14. doi: 10.1016/j.jcrs.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Carnahan MC, Chang WJ, Shorstein NH, Herrinton LJ. New benchmark in preventing phacoemulsification-related endophthalmitis. J Cataract Refract Surg. 2014;40(9):1568. doi: 10.1016/j.jcrs.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi F, Clark D. Macular infarction after inadvertent intracameral cefuroxime. J Cataract Refract Surg. 2011;37(6):1168–1169. doi: 10.1016/j.jcrs.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Çiftçi S, Çiftçi L, Dağ U. Hemorrhagic retinal infarction due to inadvertent overdose of cefuroxime in cases of complicated cataract surgery: retrospective case series. Am J Ophthalmol. 2014;157(2):421–425. e2. doi: 10.1016/j.ajo.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Sakarya Y, Sakarya R. Cefuroxime dilution error. Eur J Ophthalmol. 2010;20(2):460–461. doi: 10.1177/112067211002000232. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen ET, Shorstein NH. Preparation of intracameral antibiotics for injection. J Cataract Refract Surg. 2013;39(11):1778–1779. doi: 10.1016/j.jcrs.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Jagow B, Ohrloff C, Kohnen T. Macular thickness after uneventful cataract surgery determined by optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2007;245(12):1765–1771. doi: 10.1007/s00417-007-0605-6. [DOI] [PubMed] [Google Scholar]
- 10.Montan PG, Wejde G, Setterquist H, RylanderM, Zetterström C. Prophylactic intracameral cefuroxime: evaluation of safety and kinetics in cataract surgery. J Cataract Refract Surg. 2002;28(6):982–987. doi: 10.1016/s0886-3350(01)01270-6. [DOI] [PubMed] [Google Scholar]
- 11.Gupta MS, McKee HD, Saldaña M, Stewart OG. Macular thickness after cataract surgery with intracameral cefuroxime. J Cataract Refract Surg. 2005;31(6):1163–1166. doi: 10.1016/j.jcrs.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 12.Lam PT, Young AL, Cheng LL, Tam PM, Lee VY. Randomized controlled trial on the safety of intracameral cephalosporins in cataract surgery. Clin Ophthalmol. 2010;4:1499–1504. doi: 10.2147/OPTH.S15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delyfer MN, Rougier MB, Leoni S, et al. Ocular toxicity after intracameral injection of very high doses of cefuroxime during cataract surgery. J Cataract Refract Surg. 2011;37(2):271–278. doi: 10.1016/j.jcrs.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 14.Shahar J, Zemel E, Perlman I, Loewenstein A. Physiological and toxicological effects of cefuroxime on the albino rabbit retina. Invest Ophthalmol Vis Sci. 2012;53(2):906–914. doi: 10.1167/iovs.11-8053. [DOI] [PubMed] [Google Scholar]
- 15.Brightbill FS, McDonnell PJ, McGhee CNJ, Farjo AA, Serdarevic O. Corneal Surgery: Theory, Technique and Tissue. Philadelphia, PA: Elsevier Health Sciences; 2008. [Google Scholar]