Abstract
This protocol describes a centrally catheterized mouse model of prolonged critical illness. We combine the cecal ligation and puncture method to induce sepsis with the use of a central venous line for fluids, drugs and nutrient administration to mimic the human clinical setting. Critically ill patients require intensive medical support in order to survive. While the majority of patients will recover within a few days, about a quarter of the patients need prolonged intensive care and are at high risk of dying from non-resolving multiple organ failure. Furthermore, the prolonged phase of critical illness is hallmarked by profound muscle weakness, and endocrine and metabolic changes, of which the pathogenesis is currently incompletely understood. The most widely used animal model in critical care research is the cecal ligation and puncture model to induce sepsis. This is a very reproducible model, with acute inflammatory and hemodynamic changes similar to human sepsis, which is designed to study the acute phase of critical illness. However, this model is hallmarked by a high lethality, which is different from the clinical human situation, and is not developed to study the prolonged phase of critical illness. Therefore, we adapted the technique by placing a central venous catheter in the jugular vein allowing us to administer clinically relevant supportive care, to better mimic the human clinical situation of critical illness. This mouse model requires an extensive surgical procedure and daily intensive care of the animals, but it results in a relevant model of the acute and prolonged phase of critical illness.
Keywords: Medicine, Issue 123, critical illness, intensive care, cecal ligation and puncture, sepsis, mice, catheter
Introduction
Critical illness is a disease state in which the function of one or more organ systems is hampered to the extent that the patient will die, unless intensive medical support is administered. Whereas the initial cause for admission to the intensive care unit (ICU) can vary, ranging from trauma, complicated surgery, burns, disease exacerbations to sepsis, all critically ill patients suffer from cellular damage, caused by hypoperfusion, hypoxia and excessive inflammation among others, which leads to organ failure. Most patients survive their acute insult, but an important fraction of the patients do not immediately recover and need prolonged intensive care. They are at high risk of death due to non-resolving multiple organ failure. Furthermore, the prolonged phase of critical illness is hallmarked by profound muscle weakness and endocrine and metabolic change, of which the pathogenesis is currently incompletely understood.
Several rodent models are being used in the critical care research setting. The two mostly used models are the exogenous administration of lipopolysaccharide (LPS) and cecal ligation and puncture (CLP). Both models are developed to mimic the acute phase of sepsis, defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection, and one of the primary reasons for admission to the ICU worldwide1,2. The LPS model has several disadvantages as it only transiently affects the release of cytokines and the hemodynamic status of the animal3. Unlike humans, rodents are also particularly resistant to endotoxin and the use of 'high doses' of endotoxin is necessary to produce hypotension and mortality, hereby further raising concerns of the validity of this method4,5. The other model, cecal ligation and puncture model (CLP) features a ligation of a portion of the cecum followed by a needle puncture through-and-through. This procedure causes a polymicrobial abdominal infection with tissue damage, followed by a translocation of the bacteria into the blood compartment. This will trigger a systemic inflammatory response and the development of sepsis. The CLP model has been extensively recognized as an animal model of acute critical illness that reproduces the main features of sepsis: hyperinflammation, vasodilation, hypotension and increased cardiac output6,7. However, this model does not allow study of non-resolving multiple organ failure, muscle wasting, and endocrine and metabolic changes, which are typical for the prolonged phase of critical illness. Furthermore, recently the validity of mouse models for critical illness have been questioned, since findings from mouse models cannot always be translated to the human setting8,9,10. A possible explanation might be that the supportive care that is provided to critically ill human patients differs substantially from the care that is provided to critically ill mice.
Therefore, to resemble the human setting more closely and to allow investigation of the prolonged phase of critical illness, we developed a mouse model that mimics the acute intensive care as given to humans, such as extensive intravenous fluid administration and antibiotic treatment, and which allows to administer supportive care in order to survive the prolonged phase of critical illness, such as nutritional support. For this purpose, we adapted the mouse model of CLP-induced sepsis, being the golden standard for sepsis, and placed a central venous line which enables the administration of fluids, nutrition and drugs.
Protocol
The protocol was approved by the University of Leuven Ethical Review Board for Animal Research.
1. Preparation of the Venous Line
Prepare the tip of the venous catheter by quickly immersing the middle part of 60 cm microrenathane (MRE) tubing in hot (> 220 °C) sesame oil. Subsequently stretch the middle part of the tube to produce a narrow diameter (outer diameter (OD) < 0.5 mm) by gently moving the ends of the tubing away from each other.
Use a scalpel blade to cut the tubing into two parts of 30 cm each (see Figure 1, Table 1).
Connect the MRE tubing to polyethylene tubing PE10 using polyethylene PE50 connector and connect this PE10 tubing to the bottom part of the swivel. Connect the upper part of the swivel to PE10 tubing, and connect this to a Luer stub needle with PE50 connector tubing as per Figure 1.
Apply strong fast-acting adhesive glue to all connections and test the catheter for leakage by flushing with air. Gas sterilize the catheter prior to use.
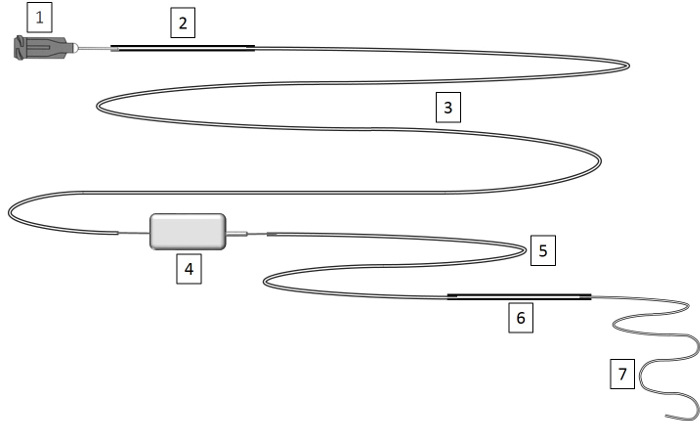 Figure 1: Construction of Venous Line. Venous lines are prepared by stretching the MRE tubing to a small diameter and connecting it via polyethylene tubing to a rodent swivel device. See Table 1 for instructions on lengths of the different parts. Please click here to view a larger version of this figure.
Figure 1: Construction of Venous Line. Venous lines are prepared by stretching the MRE tubing to a small diameter and connecting it via polyethylene tubing to a rodent swivel device. See Table 1 for instructions on lengths of the different parts. Please click here to view a larger version of this figure.
| Legend | Length | Volume |
| 1) Luer stub needle 22 G | ||
| 2) PE50 – polyethylene .023" x .038" | 5 cm | 13 µL |
| 3) PE10 – polyethylene .011" x .024” | 50 cm | 30 µL |
| 4) Rodent Swivel 20 G | ||
| 5) PE-10 | 15 cm | 9 µL |
| 6) PE-50 (connector) | 5 cm | 13 µL |
| 7) MRE025 Tip stretched microrenathane .025" x .012" | 30 cm | 27 µL |
| Total sum | 105 cm | 92 µL |
Table 1: Construction of Venous Line. This table provides a legend for Figure 1.
2. Anesthesia and Pre-surgery Handling
Use 24-week old male C57BL/6J mice (27 - 32 g). NOTE: We use 24-weeks-old mice (mature adult) as this age corresponds better with the mean age of intensive care patients. We use only male mice to avoid the cyclic influence of estrogens. The technique can also be used on younger animals (tested in 16-week old mice) and females, if preferred.
Sterilize all surgical instruments before use. Flush the sterile home-made venous catheter with saline to remove any air bubbles and to test for patency and potential leaks.
Cut a metal wire (0.8 mm diameter) of 80 cm length, double it by folding and make a loop. Lead 3 pearls over the doubled wire to the loop. Attach the catheter to the metal wire by guiding the tip of the catheter through the 3 pearls. NOTE: The metal attachment wire is necessary for rotation of the swivel to which the catheter is connected. As such, the mouse can move freely, without blocking the venous line.
Anesthetize the mouse by an intraperitoneal (IP) injection of a mixture of 0.03 mL ketamine (100 mg/kg) and 0.02 mL xylazine (13 mg/kg). Ensure that anesthesia is adequate by checking the absence of reflexes. Gently pull the mouse tongue outside with forceps to avoid suffocation by swallowing of the tongue. NOTE: If after 10 min, the mouse still attempts to withdraw its limbs give additional ketamine as a 0.01 mL bolus.
Shave the surgical area including abdomen, ventral side of the neck (triangle between chin, sternum and clavicle), and between shoulder blades at the base of the head).
Place the mouse in prone position on a pre-warmed heating pad, disinfect the skin with 70% ethanol and apply a small amount of ophthalmic lubricant to protect eyes from drying out. Infiltrate the surgical sites (abdomen, neck-front and back) with 0.2 mL ropivacaine (0.67 mg/kg).
While the mouse is still in prone position, make a small incision at the base of the dorsal side of the head with scalpel or scissors. Expose the posterior cervical muscles, and tie the muscle to the loop of the attachment wire with 3.0 nylon by leading a 3.0 nylon thread under the muscle.
Place the mouse on its right side. Make a small vertical incision in the skin of the ventral neck with a scalpel or scissors. Under visual guidance, tunnel an 18-gauge needle subcutaneously through this ventral incision towards the incision on the back made previously and thread the catheter through the needle to exteriorize it at the ventral side.
Place the mouse on its back. Pass a 3.0 nylon thread behind the top incisor teeth of the mouse and tape it down to the heating pad. Secure the head in a face mask for delivering oxygen (2 L/min). Fix the mouse in a stretched position by taping down the tail and the two forelimbs. NOTE: If the ketamine-xylazine anesthesia alone is not sufficient, inhaled isoflurane (0.5 - 1.5%) can be delivered during surgery.
3. Placement of a Chronic Indwelling Catheter
Place the mouse under the dissecting microscope. In the incision made previously in the ventral neck, gently tease fat tissue and glands away with forceps until the jugular vein can be visualized.
Bluntly dissect to free the jugular vein from the connective and subcutaneous tissue above and around the vessel by placing the tip of the forceps between the vein and connective tissue and opening the forceps repeatedly in parallel with the vein. As such, damage to the vein will be avoided.
Isolate the jugular vein by placing blunt forceps under the vein. Feed three pieces of 3.0 silk thread under the vein, position one piece proximal to the bifurcation of the jugular vein (cranial ligature) and one piece close to the sternocleidomastoid muscle (caudal ligature). Tighten the cranial ligature and the caudal ligature to stretch the vein and prevent excessive bleeding while placing the catheter. Tie a loose ligature with the middle thread to ensure easy securing of the catheter later on.
Using micro scissors, make an incision along the vein between the cranial and caudal ligatures and large enough to pass the catheter. Grab the catheter with forceps, and insert the catheter 11 mm into the vein. Loosely secure the catheter by tying off the middle ligature and confirm correct placement by gently flushing with sterile saline.
Secure the catheter by firmly tying off the middle and caudal ligature around the vessel and catheter. Tie ends of caudal and middle ligature together to firmly secure the catheter. Ensure that the knots do not occlude the vein, by flushing the catheter after every knot made. Finally, tie the caudal and cranial ligatures. Close the incision with 5.0 silk sutures.
4. Cecal Ligation and Puncture
Make a 1-cm midline incision through skin of the lower half of the abdomen with scalpel or scissors; be careful not to penetrate into the peritoneal cavity.
Identify the linea alba of the abdominal musculature and make an intermuscular incision to gain entry into the peritoneal cavity. Locate the cecum, and use blunt anatomical forceps to isolate the cecum and exteriorize it.
Ligate the cecum at 50% of its length with 3.0 silk sutures. Make sure not to ligate the ileocecal valve so that intestinal continuity is maintained.
Perforate the cecum with an 18-gauge needle by a single through-and-through puncture midway between the ligation and the tip of the cecum. After removing the needle, extrude a small amount of feces from the hole to ensure patency.
Reposition the bowel in the abdominal cavity, and close the peritoneum and skin with 5.0 silk sutures. NOTE: On average, a new trainee requires 10 to 15 animals to be able to smoothly place the venous catheter and perform CLP within 45 min. After the training period, an anesthesia/surgery related mortality of 10% can be expected.
5. Post-surgical Treatment and Fluid Resuscitation
Place the mouse in supine position and secure the catheter to the attachment wire with tape. Move the mouse to an individual cage. Use a stand with adjustable clamp to hold the swivel device 25 cm above the mouse and firmly tape the free part of the metal attachment wire to the rotating point of the swivel.
Attach a syringe containing the mixture of balanced colloids and crystalloids (1:4) to the venous line to start fluid resuscitation.
Place the cage in a temperature controlled (27 °C) animal cabinet with 12 h light and dark cycles and start intravenous fluid resuscitation (10 mL/kg/h) via an accurate syringe-driven infusion pump. Provide cage enrichment such as nesting material and a wooden block.
6. Intensive Care
At 6 h post-operation, subcutaneously inject pain medication and antibiotics (0.3 mL buprenorphine (0.15 mg/kg) and 0.2 mL imipenem (16.67mg/kg)). NOTE: Following standard laboratory animal medicine practice, the first dose of pain meds is given prior to the initial surgical incision and then as directed by the institution’s veterinary policies. Subcutaneous injections should be carefully injected into the subcutaneous space.
After 20 - 24 h of fluid resuscitation, replace the crystalloids/colloids by total parenteral nutrition (6.67 mL/kg/h). NOTE: Total parenteral nutrition administered (5.8 kcal/24 h) covers around 40% of the daily caloric needs of mice, similar to the early caloric intake of patients in the intensive care unit.
Subcutaneously administer pain medication and antibiotics (0.6 mL buprenorphine (0.3 mg/kg) and 0.2 mL imipenem (16.67 mg/kg) every 12 h during the whole period of critical illness.
Check the animals at least every 3 h during the day. Assess suffering by giving a pain score based on the validated mouse grimace scale11. Intensify monitoring for animals with a high pain score.
7. End of the Experiment
NOTE: Approval of and recommendations on the level of severity of the model and guidelines and policies for human endpoints should be sought from the local Institutional Ethical Review Board for Animal Research.
NOTE: In case of a nonfunctional venous line such as blocked catheter, delocalization of the catheter, problems with the syringe pump, the animal is excluded from the study and euthanized.
At the end of the experimental period, deeply anesthetize the mouse using a mixture of 0.03 mL ketamine (100 mg/kg) and 0.02 mL (13 mg/kg) xylazine. Euthanize the mouse by withdrawing blood by cardiac puncture. Store snap frozen tissue samples of interest at -80 °C.
Representative Results
C57BL/6 mice were made critically ill as described above. We performed two experiments to assess post-CLP survival until two time points: survival until day 5 (n = 15) and survival until day 7 (n = 22) post-CLP. Survival curves of the two experiments were not significantly different (compared until day 5), indicating the reproducibility of the experimental setup. Non–surviving animals were found death or euthanized due to reaching human endpoints. Ligation of 50% of the cecum in combination with antibiotics, fluid resuscitation and total parenteral nutrition via a venous catheter in the vena jugularis, as described, resulted in a 13% mortality after 1 day of critical illness, 24% mortality after 3 days of critical illness, 27 - 31% mortality after 5 days of critical illness and 36 % after 7 days of critical illness. The healthy pair-fed mice, which were caloric restricted to the nutrient intake of the critically ill mice, did not show any mortality.
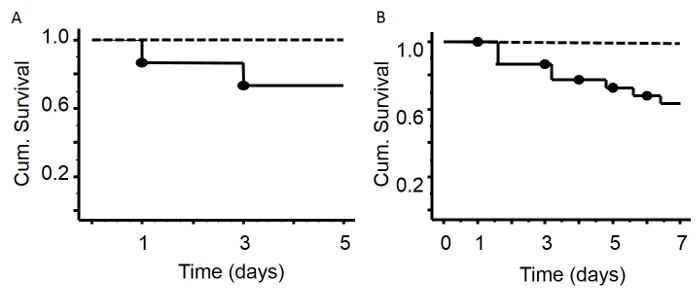 Figure 2: Survival Curves after 5 or 7 Days of Critical Illness. There was no mortality in the group of healthy animals (A, B, dashed line - healthy animals without surgery). Five days after surgery (A), mortality rate was 27% (solid line).Seven days after induction of sepsis (B), the mortality rate was 36%. Mice with leaking or dislodged catheters were excluded from the experiment (15%). Please click here to view a larger version of this figure.
Figure 2: Survival Curves after 5 or 7 Days of Critical Illness. There was no mortality in the group of healthy animals (A, B, dashed line - healthy animals without surgery). Five days after surgery (A), mortality rate was 27% (solid line).Seven days after induction of sepsis (B), the mortality rate was 36%. Mice with leaking or dislodged catheters were excluded from the experiment (15%). Please click here to view a larger version of this figure.
Discussion
We developed a more clinically relevant mouse model of critical illness, by combining the cecal ligation and puncture method to induce sepsis with the use of a central venous line for fluids, drugs and nutrient administration. This experimental setup is reproducible, allows to study the prolonged phase of critical illness and results in a stable mortality rate, hereby mimicking the human clinical situation1.
Cecal ligation and puncture has been extensively recognized as an animal model of acute critical illness that reproduces the main features of sepsis6,7. However, this model of acute critical illness does not allow the study of non-resolving multiple organ failure, muscle wasting, and endocrine and metabolic changes, which are typical for the prolonged phase of critical illness. Furthermore, preclinical findings with this model often fail to translate to the clinical human setting8,9,10. Therefore, we developed a model where placing a central venous line allows the investigator to administer extensive fluid resuscitation, drugs and total parenteral nutrition. These supportive measures are of vital importance for critically ill patients in order to survive the acute phase of critical illness. Despite the placement of this central venous line, the animals are still able to freely move with relatively minor discomfort. Daily pain medication helps alleviate suffering, as assessed by the mouse grimace scale of pain11. This model also incorporates other essential aspects of supportive care for critically ill patients, such as antibiotic treatment and pain medication.
This model has several limitations. First, the extensive surgical procedure is demanding and requires sufficient training. Nevertheless, with sufficient training, on average 10% of the operated animals die during or immediately after the surgery. Furthermore, catheters that are not well-placed, can lead to leakage of resuscitation fluids or parenteral nutrition into the thorax of the animal. On average, 15% of surviving animals have to be excluded during the study due to these catheter related problems. Thus, when one calculates the required number of animals for an experiment, one has to take into account a 25% surplus due to surgery and catheter related losses. Second, with only one venous access point, antibiotics and pain medication still have to be administered subcutaneously twice daily. Indeed, compatibility of parenteral nutrition with antibiotics and pain medication drugs cannot be guaranteed, and therefore co-infusion should be avoided. Flushing of the line followed by a bolus injection is also technically not possible due to limited blood volume of the mice. Third, the anesthesia and surgery needed to place the catheter will induce a severe stress response by itself. We use healthy animals without surgery as controls, and consider the catheter related surgery as a part of the critical illness, comparable to what human surgical ICU patients have to endure. Fourth, whereas post-operative pain medication and antibiotics and the use of a central venous line to allow administration of parenteral nutrition increases the clinical relevance of the murine CLP model, our model still does not completely mimic the clinical human situation. Indeed, due to the small animal size it is challenging to introduce several advanced supportive techniques, such as renal replacement therapy. However, the use of this model allows genetic interference, vital in unraveling the pathogenesis of critical illness.
It has been shown that mortality and severity of the CLP procedure can be manipulated, if deemed necessary, by the portion of the cecum that is ligated, by the size and number of punctures, subcutaneous fluid resuscitation and daily antibiotic administration6,12. We choose a protocol in which we provided extensive fluid resuscitation at 10 mL/kg/h for the first 20 h, as it has been demonstrated previously that this improves mortality13. Our adaptations should be incorporated into the protocol if a model of prolonged critical illness is desired instead of a model of lethality. This mouse model uses a mid-grade CLP, intravenous fluid resuscitation and antibiotic treatment in order to create a more clinically relevant model of critical illness, as is shown by its prolonged survival curves which mimics the survival rate of human sepsis1. The ICU patient population, as the general population, is aging14. In order to mimic the human ICU setting even more closely, one can use mature mice (6 months), as used in this study, in order to enhance clinical relevance of the experimental model.
In conclusion, this mouse model requires an extensive surgical procedure and daily intensive care of the animals, but it results in a model of critical illness, which allows the investigator to study the aspects of the prolonged phase of critical illness.
Disclosures
The authors have nothing to disclose.
Acknowledgments
GVdB, via the University of Leuven (KU Leuven), receives long-term structural research support from the Methusalem Program funded by the Flemish Government (METH08/07) and holds a European Research Council Advanced Grant AdvG-2012-321670 from the Ideas Program of the European Union seventh framework program. SET received a Research Foundation-Flanders (FWO) Research Assistant Fellowship.
References
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13(2):110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- Copeland S, et al. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12(1):60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghari PS, Pinto BB, Dyson A, Singer M. The metabolic phenotype of rodent sepsis: cause for concern. Intensive Care Med Exp. 2013;1(1):25. doi: 10.1186/2197-425X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermacher P, Haouzi P. A mouse is not a rat is not a man: species-specific metabolic responses to sepsis - a nail in the coffin of murine models for critical care research. Intensive Care Med Exp. 2013;1(1):26. doi: 10.1186/2197-425X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting. Crit Care Med. 2009;37(1 Suppl):S30–S37. doi: 10.1097/CCM.0b013e3181922bd3. [DOI] [PubMed] [Google Scholar]
- Osuchowski MF, et al. Abandon the mouse research ship? Not just yet. Shock. 2014;41(6):463–475. doi: 10.1097/SHK.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford DJ1 BA, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7 doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, et al. Characterization of a hyperdynamic murine model of resuscitated sepsis using echocardiography. Am J Respir Crit Care Med. 2001;164(5):891–895. doi: 10.1164/ajrccm.164.5.2010073. [DOI] [PubMed] [Google Scholar]
- Heuer JG, et al. Cecal ligation and puncture with total parenteral nutrition: a clinically relevant model of the metabolic, hormonal, and inflammatory dysfunction associated with critical illness. J Surg Res. 2004;121(2):178–186. doi: 10.1016/j.jss.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Conti M, Merlani P, Ricou B. Prognosis and quality of life of elderly patients after intensive care. Swiss Med Wkly. 2012;142 doi: 10.4414/smw.2012.13671. [DOI] [PubMed] [Google Scholar]


