Abstract
Objective
This article reviews recent developments in targeted radionuclide therapy (TRT) approaches directed to malignant liver lesions, bone metastases, neuroendocrine tumors, and castrate-resistant metastatic prostate cancer and discusses challenges and opportunities in this field.
Conclusion
TRT has been employed since the first radioiodine thyroid treatment almost 75 years ago. Progress in the understanding of the complex underlying biology of cancer and advances in radiochemistry science, multimodal imaging techniques including the concept of “see and treat” within the framework of theranostics, and universal traction with the notion of precision medicine have all contributed to a resurgence of TRT.
Keywords: cancer, precision, radionuclide, therapy
Precision medicine depends on identification and use of relevant biologic targets for optimized therapeutic efficacy with the goal of improving patient outcome and diminishing toxicity compared with nontargeted approaches. The rapid pace of advances in the understanding of the complexities of cancer biology has provided unprecedented opportunities for targeted therapies, which are increasingly finding their way into more effective cancer treatment regimens. The targeted approach also allows therapies to be adapted to tackle the temporal evolution of cancer biology and refined in response to various factors including host changes in the tumor microenvironment and stresses from prior and ongoing treatments. Multitargeted approaches may allow even higher therapeutic efficacy through synergism of attacking cancer via multiple pathways and lessening the ability of tumor to adapt.
Targeted radionuclide therapy (TRT) delivers energetic particles (auger, beta, alpha) in close proximity to tumor deposits that are preferentially selected on the basis of specific biologic features [1–3]. The first application of TRT occurred nearly 75 years ago with the use of radioiodine in thyroid disorders including cancer. Since then, many other agents and conditions have employed the TRT concept, including treatments for bone pain palliation; radioimmunotherapy with radiolabeled anti-CD20 monoclonal anti-bodies for relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin lymphoma (e.g., 90Y-inbritumomab tiuxetan, 131I-tositumomab); and radiolabeled metaiodobenzylguanidine in patients with neuroblastoma, paraganglioma, and pheochromocytoma. Numerous excellent articles have been published on these topics [4–10]. More recently, TRT has had a resurgence with growing interest in exploring how various new TRT approaches fit the therapeutic algorithm of cancer. A major contributor has also been the progress with theranostics that couples diagnostic imaging with targeted therapy (the “see and treat” approach). This article briefly reviews recent developments in TRT approaches directed to malignant liver lesions, bone metastases, neuroendocrine tumors (NETs), and metastatic castration-resistant prostate cancer (CRPC) and discusses challenges and opportunities in this exciting and growing field.
Selective Internal Radioembolization of Malignant Liver Lesions
Primary and metastatic liver lesions receive preferential arterial supply from the hepatic arteries, which can be used as conduits for delivery of highly localized radiation treatment. The technique may be combined with concomitant radiosensitizing chemotherapy to shrink lesions that may then become amenable to surgical resection [11]. The toxicity to normal hepatic tissues is limited because these tissues are primarily supplied by the portal venous system. Intraarterial delivery of microsphere therapy has been shown to be safe and effective in a variety of settings [12–28]. Examples include candidates for liver transplant, unresectable hepatocellular carcinoma (HCC) even with concurrent portal vein thrombosis, unresectable or recurrent cholangiocarcinoma, and liver-dominant metastases from colorectal cancer, breast cancer, renal cell carcinoma, pancreatic cancer, melanoma, and NETs even if tumors have been heavily pretreated.
Uliel and colleagues [29] performed an excellent review of the therapy procedure including specifics of commercially available microspheres. SIR-Spheres (Sirtex Medical) are nondegradable pure β-emitter 90Y-loaded resin microspheres ranging from 20 to 60 μm in diameter with specific activity of 50 Bq per microsphere. TheraSpheres (Nordion) are 90Y-assimilated glass microspheres ranging from 20 to 30 μm in diameter with specific activity of 2500 Bq per microsphere. The range of microsphere diameters is similar to that of 99mTc-macroaggregated albumin (MAA), which is used for pretreatment planning.
Selective internal radiation therapy (SIRT) of malignant liver lesions is a multidisciplinary process with coordinated activities of interventional radiology and nuclear medicine services. Pretreatment planning is needed for vascular mapping followed by 99mTc-MAA hepatic perfusion delineation and pulmonary shunt calculation. As described by Uliel et al. [29], the aims of pretreatment angiography are to map the relevant regional arterial supply, identify potential vascular variants, and perform prophylactic embolization of selected vessels as needed to avoid subsequent radioembolization of nontargeted tissues with its associated radiotoxicity (Fig. 1). Procedural complications with vascular mapping are rare [30]. Hepatic perfusion imaging is performed with 99mTc-MAA (5 mCi in sterile normal saline) at the time of pretreatment vascular mapping to allow detection of particles shunted to the lung through unrecognized collateral vessels or intratumoral neovascular arteriovenous connections.
Fig. 1.
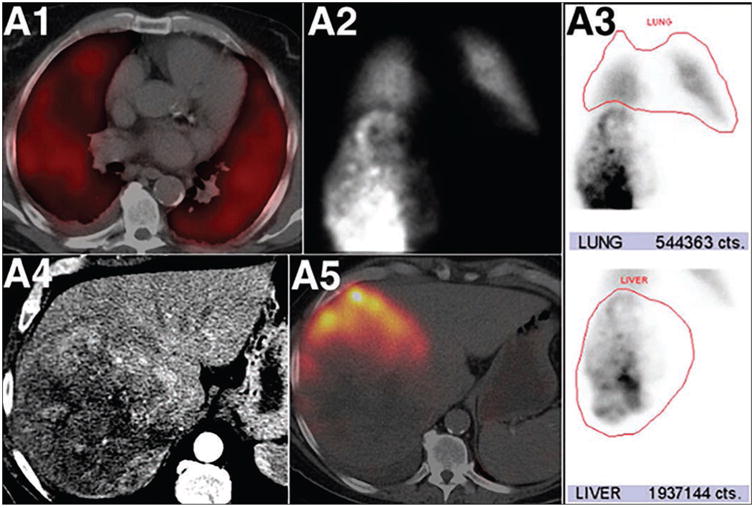
Marked pulmonary 99mTc-macroaggregated albumin (MAA) shunting in patient with hepatocellular carcinoma. Fused SPECT/CT axial image (A1) of chest shows diffuse 99mTc-MAA pulmonary activity in relation to shunted MAA particles. Anterior planar static image (A2) of chest and upper abdomen after 99mTc-MAA administration into right hepatic artery shows heterogeneous 99mTc-MAA distribution activity in liver and diffuse pulmonary activity. ROIs around lung and liver (red outlines, A3) lead to calculation of high lung shunt fraction of 21.9%. Axial contrast-enhanced CT (A4) shows large heterogeneous right liver mass. Fused SPECT/CT image of liver (A5) does not show significant localization of 99mTc-MAA in right liver mass. High lung shunt fraction precluded this patient from radioembolization therapy. This research was originally published in JNM. Uliel L, Royal HD, Darcy MD, et al. From the angio suite to the gamma-camera: vascular mapping and 99mTc-MAA hepatic perfusion imaging before liver radioembolization—a comprehensive pictorial review. J Nucl Med 2012; 53:1736–1747. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.
For SIR-Spheres, the amount of activity is adjusted on the basis of the calculated lung shunt fraction; < 10% shunt, no reduction; 10–15% shunt, amount is reduced by 20%; 15–20% shunt, amount is reduced by 40%; > 20% shunt, no therapy is pursued. For TheraSpheres, the upper limit of allowed activity shunted to the lungs is 16.5 mCi, calculated by multiplying the lung fraction percent by the planned therapeutic activity [29]. For either microsphere, the therapeutic activity dosage is calculated on the basis of the relevant package insert information. A recent report suggested that the prescribed radiation activity might be significantly lower for resin than for glass microspheres, which may in part result from their different specific activities [31, 32]. However, this difference in activity does not appear to lead to a significant survival difference between the two types of treatment [33]. Postprocedural planar, SPECT, or SPECT/CT imaging with the broad-spectrum bremsstrahlung radiation is done to confirm proper localization of the therapeutic activity in the intended hepatic region. However, higher resolution PET may also be performed given the internal pair production that occurs with 90Y decay [34]. Pretherapy 99mTc-MAA and posttherapy 90Y bremsstrahlung mean tumor-to-background ratios may show low correlation, but that should not preclude patients from receiving the radioembolization treatment with low tumor uptake on the 99mTc-MAA scan [35, 36]. A single-session, complete procedure including pretherapy angiography, vascular coil embolization as needed, 99mTc-MAA scintigraphy, and 90Y SIRT appears to be feasible and can facilitate wider therapy adoption, enhance patient comfort, and reduce costs [37]. If the patient is hospitalized after radioembolization, simple methods such as a lead-lined blanket on the patient's abdomen can significantly reduce radiation exposure to hospital staff [38]. Laffont et al. [39] reported that medical staff performing 90Y SIRT procedures are exposed to safe levels of radiation and that the mean finger exposure is less with glass microspheres than with resin microspheres. Riaz et al. [40] and Atassi et al. [41] provided comprehensive reviews of side effects and multimodal imaging, respectively, after radioembolization. Radiation-induced cholecystitis is a rare event occurring in about 0.8% of patients, and radioembolization of HCC activates liver regeneration, produces oxidative stress, and activates the inflammatory cytokines and the coagulation cascade [42, 43]. Procedural guidelines for SIRT have been published [44].
Sangha et al. [45] reported on the ongoing multicenter phase III randomized clinical trial SIRFLOX comparing first-line chemotherapy alone or in combination with 90Y SIRT in patients with liver-dominant metastases from colorectal cancer. Preliminary results have shown that although no significant change in median overall survival and progression-free survival was found between the combined therapy arm and the chemotherapy-only arm, a significant increase in median hepatic progression-free survival favoring the combination arm was seen. Whether improved median hepatic progression-free survival will lead to improved overall survival is still unclear. A potential issue in 90Y SIRT is tumor absorbed-dose inhomogeneity, which may call for more personalized approaches to administered activity in an attempt to achieve optimal clinical response [46, 47].
Assessment of response to SIRT can be challenging and may depend on treatment response criteria employed [48, 49]. Shady et al. [50] showed that the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) have poor sensitivity in this clinical setting and that the Choi criteria or metabolic criteria such as European Organization for Research and Treatment of Cancer or PET Response Evaluation Criteria in Solid Tumors applied to PET with FDG are more relevant. The same group of investigators also showed that metabolic response on FDG PET/CT can predict overall survival [51]. Another group of investigators suggested that dose volume histograms derived from PET/MRI of 90Y distribution in tumor can be helpful in predicting responders and nonresponders [52].
Paprottka and colleagues [53] reported on pretherapy factors that could predict overall survival after 90Y SIRT. Baseline bilirubin and cholinesterase levels, extrahepatic disease, and hepatic tumor content were independent predictors of overall survival. Moreover, a randomized phase II study of patients with HCC found that 90Y SIRT significantly prolongs time to progression compared with chemoembolization (> 26 mo vs 6.8 mo; p = 0.0012) [54]. A recent systematic review and meta-analysis of 21 studies published about 90Y SIRT for intermediate and advanced HCC reported a pooled overall survival of 58% at 1 year and 17% at 3 years after treatment. The observed pooled progression probability was 56% at 1 year and 73% at 2 years after therapy [55]. Another meta-analysis of eight studies involving 1499 patients reported significantly longer overall survival and time to progression with 90Y SIRT than with transarterial chemoembolization in patients with HCC, although the latter may be preferred in patients with low hepatic reserve who are candidates for liver transplant [56]. Similar favorable pooled outcome results have been reported with systematic review and meta-analysis of data on unresectable intrahepatic cholangiocarcinoma and meta-static NETs [57, 58]. Moreover, interestingly, overall survival progressively decreases as lung shunt fraction increases from less than 5% to more than 20% [59, 60].
Hepatic radioembolization therapy is anticipated to experience robust growth as the number of indications increases and additional comparative prognostic data become available.
Bone Metastases Therapy
Bone is the target for metastases for many cancers. The biology of skeletal metastasis is a multifactorial process that involves complex interactions between intrinsic properties of tumor cells and host microenvironment factors [61]. Bone metastases are often associated with skeletal-related events (SREs), which can lead to death or significant morbidity including disability, decreased quality of life, and increased treatment cost [62]. Effective management of bone metastases remains an active area of pharmaceutical research and development [63, 64]. The primary objective of these therapies is aimed at alleviating pain and complications that are associated with bone metastases. Bone-seeking radio-isotopes, such as 153Sm ethylene diamine tetramethylene phosphonate and 89Sr chloride, have been used successfully for palliation of pain from bone metastases [65, 66].
One of the major recent strides has been the development and approval of an alpha-particle bone-seeking radionuclide for treatment of bone metastases. Alpha particles are positively charged helium nuclei (about 8000 times larger than beta particles or electrons) with a short range of about 50–100 μm (vs several mm for beta particles) and high linear transfer energy of about 100 keV/μm (vs 0.2 keV/μm for beta particles) [67]. Alpha particles deposit very high energy in a very small distance, which often leads to irreversible double-strand DNA breaks with per-unit absorbed doses of acute biologic effects that are three to seven times greater than the damage produced by external beam or beta radiation [68, 69]. Cells are not equipped to repair double-strand DNA breaks and typically undergo apoptosis [70]. Given that the targeted cells receive high radiation doses with alpha particles, the conventional medical internal radiation dose dosimetric methods may be inappropriate, and microdosimetric methods have been developed that take into account heterogeneity and protracted exposure to internal radiation [71, 72].
The alpha therapy of choice for treatment of metastatic CRPC is 223Ra dichloride (Xofigo [previously Alpharadin], Bayer HealthCare), which was approved by the U.S. Food and Drug Administration (FDA) on May 15, 2013 [73–76]. Because it is a calcium mimetic, 223Ra dichloride seeks the hydroxyapatite matrix in the bone naturally. Its half-life is 11.43 days, with emitted energy distribution of 93.5% as alpha particle, less than 3.6% as beta particle, and less than 1.1% as gamma radiation [77] (Fig. 2). The Alpharadin in Symptomatic Prostate Cancer (ALSYMPCA) clinical trial was a randomized, double-blind, multinational investigation of 223Ra dichloride versus placebo (saline) in men with CRPC who had symptomatic bone metastases and no visceral metastases (metastatic lymph nodes up to 3 cm in short axis were allowed) and who had adequate bone marrow reserve [78]. The study used 2:1 randomization of the treatment and placebo arms with 614 patients receiving best supportive care plus six IV injections of 223Ra dichloride (each 50 kBq/kg) with each injection 4 weeks apart, and 307 patients in the placebo arm receiving best supportive care plus six IV injections of saline with each injection 4 weeks apart. The primary endpoint was overall survival defined as time from randomization to death from any cause. A number of secondary endpoints were also used, including time to first SRE.
Fig. 2.
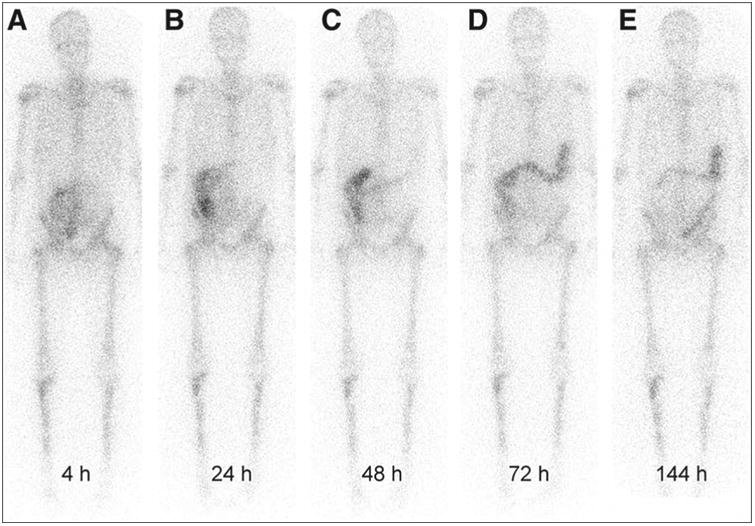
Temporal biodistribution of 223Ra dichloride as imaged by low yield gamma emission of radioisotope. Activity is localized primarily in bone and to some extent in colon. This research was originally published in JNM. Chittenden SJ, Hindorf C, Parker CC, et al. A phase 1, open-label study of the biodistribution, pharmacokinetics, and dosimetry of 223Ra dichloride in patients with hormone-refractory prostate cancer and skeletal metastases. J Nucl Med 2015; 56:1304–1309. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.
The ALSYMPCA clinical trial found a 3.6-month survival benefit with 223Ra dichloride in comparison with placebo (median, 14.9 months vs 11.3 months; hazard ratio, 0.70; 95% CI, 0.58–0.83; p < 0.001). The survival benefit was not affected by prior use of docetaxel chemotherapy (3.1-month survival benefit with prior docetaxel use vs 4.6-month survival benefit without prior docetaxel use). Moreover, the median time to first symptomatic SRE was significantly longer by 5.8 months with 223Ra dichloride in comparison with placebo (median, 15.6 months vs 9.8 months; hazard ratio, 0.66; 95% CI, 0.52– 0.83; p < 0.001). The survival benefit and reduction in time to SRE has also been associated with reduced hospital stay days and improvements in quality of life [79].
Radium-223 dichloride was generally associated with low-grade myelosuppression (primarily thrombocytopenia). Jadvar et al. [80] reviewed the 1-year clinical experience with 223Ra dichloride in 25 men with metastatic CRPC. About 25% of the cohort completed the entire six-dose treatment cycle. Progression of soft-tissue disease was the main reason for the cessation of therapy. Side effects were mild and manageable. A decline in the serum alkaline phosphatase level was more common than a decline in the prostate-specific antigen (PSA) level, with the latter also reflecting concurrent soft-tissue disease. A retrospective investigation of the clinical experience after 532 cycles of 223Ra dichloride administration in 110 patients showed that serum alkaline phosphatase levels significantly reduced with treatment and that progression risk was associated with a decline in the serum PSA doubling time [81]. Skeletal tumor burden as assessed using 18F-NaF PET/CT may be predictive of effectiveness of 223Ra dichloride therapy and overall survival [82].
With regard to radiation exposure and safety, a patient of average weight receiving 3.5 MBq (95 μCi) of 223Ra dichloride will emit radiation at a rate of 0.35 mSv/h (0.035 mrem/h) at 1 m immediately after administration, which entails relatively minimal radiation safety precautions. Fecal excretion is the primary method of clearance. For the sake of simplicity, however, recommendations are similar to those for radioiodine therapy [83].
Given the high efficacy and low toxicity of 223Ra dichloride, the National Comprehensive Cancer Network guideline (v3.2013) incorporates 223Ra dichloride treatment in the management of men with metastatic CRPC. A number of trials are now underway or planned to examine combination or sequencing of 223Ra dichloride with other treatments (e.g., abiraterone acetate and enzalutamide) for metastatic CRPC as well as for other cancers such as bone-dominant metastatic breast cancer and osteosarcoma [84–86].
Peptide Receptor Radionuclide Therapy in Neuroendocrine Tumors
NETs are a heterogeneous group of tumors that stem from the neural crest with main localization in the gastroenteropancreatic tract (e.g., carcinoids, insulinoma, glucagonoma, vasoactive intestinal peptide–secreting tumors, gastrinoma, somatostatinoma). The tumors may be symptomatic through production of various peptides (e.g., diarrhea, flushing, hypoglycemia). Liver metastases are common. Although the bulk of tumor sites may be surgically resected, metastatic disease may be treated with chemotherapy, interferon, and somatostatin analog therapy [87]. FDG PET sensitivity is generally low for well-differentiated tumors, although less-differentiated and dedifferentiated tumors are more metabolically active with higher FDG accumulation [88].
Other relevant PET radiotracers in this clinical space include the 11C- or 18F-labeled amine precursors L–dihydroxyphenylalanine (e.g., 18F-FDOPA) and 5-hydroxy-L-tryptophan (e.g., 11C-5-HTP) and those that bind to the somatostatin receptors (SSTR, particularly subtype 2) using the chelator 1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) including the 68Ga-DOTA-peptides (68Ga-DOTA-d-Phe1-Tyr3-octreotide [DOTATOC[; 68Ga-DOTA-1-Nal3-octreotide [DOTANOC]; and 68Ga-DOTA-DPhe1-Tyr3-octreotate [DOTATATE]) [89]. It is now well recognized that 68Ga-based SSTR PET is superior to 111In-based SSTR planar imaging and SPECT [90]. Procedure guidelines for PET/CT with 68Ga-DOTA–conjugated peptides have been published [91]. In November 2013, the FDA designated 68Ga-DOTATOC an orphan drug, and in June 2016 it approved 68Ga- DOTATATE (Netspot, Advanced Accelerator Applications) for imaging NETs [92].
Along with advances in SSTR imaging, peptide receptor radionuclide therapy (PRRT) has received much attention, particularly as a theranostic companion to the new imaging agents. PRRT has been found to be an effective (with partial and complete response in up to 30% of patients) and well-tolerated treatment of inoperable or metastatic gastroenteropancreatic NETs [93–107] (Fig. 3). The typical therapy radionuclides are 90Y and 177Lu, which are both beta emitters; 90Y (half-life, 64.1 h) has a range in the order of 12 mm and 177Lu (half-life, 6.7 d) has a range of 2 mm. Lutetium-177 also emits gamma rays that allow posttreatment imaging and dosimetry calculations. Typically, one or the other is used, but combination therapy with both isotopes has been studied [108]. The longer path length of the 90Y beta particles may provide better coverage for larger metastases, and the shorter path length of 177 Lu may provide less off-target radiation and radiotoxicity for smaller metastatic lesions. A German multiinstitutional registry study reported on the experience with 450 patients with NET who underwent PRRT with 177Lu, 90Y, or both radiolabels in 54%, 17%, and 29% of cases [109]. Overall survival and progression-free survival were significantly inferior in patients who were treated solely with 90Y-PRRT than in those who received any 177Lu-PRRT. The responses were 5.6% complete remission, 22.4% partial remission, 47.3% stable disease, and 4% progressive disease. With regard to comparative dosimetry of 177Lu-DOTATATE, 177Lu-DOTANOC, and 177Lu-DOTATOC, DOTANOC is associated with higher whole-body dose, whereas DOTA-TOC exhibited the lowest normal organ dose with the best tumor-to-kidney ratio [110].
Fig. 3.
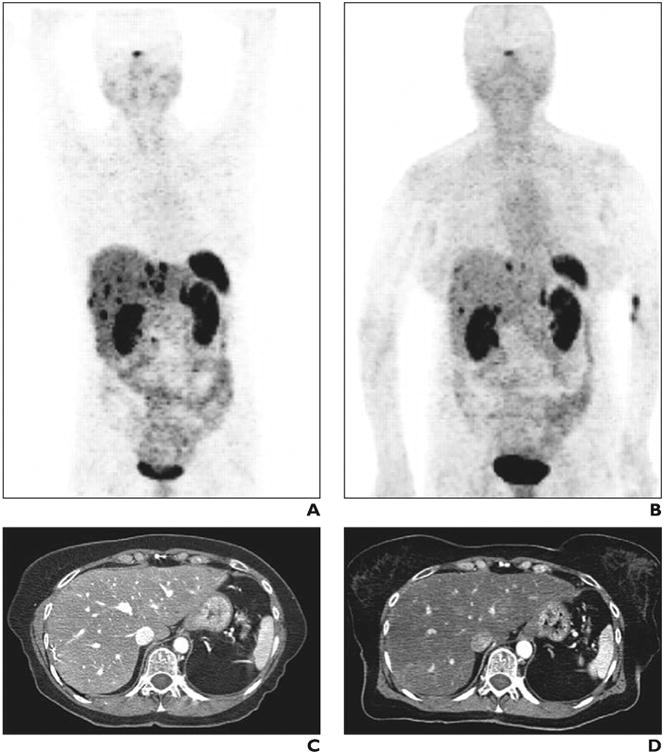
Favorable treatment response to 177Lu-DOTA-dPhe1,Tyr3-octreotate (DOTATATE) therapy in patient with hepatic metastases from resected pancreatic head primary neuroendocrine tumor. This research was originally published in JNM. Gabriel M, Oberauer A, Dobrozemsky G, et al. 68Ga-DOTA-Tyr3- octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med 2009; 50:1427–1434. © by the Society of Nuclear Medicine and Molecular Imaging, Inc. A and B, Gallium-68 DOTATATE PET scans before (A) and after (B) four cycles of 177Lu-DOTATATE show decline in number of intensity of hepatic lesions. C and D, Contrast-enhanced CT scans before (C) and after (D) treatment show no significant interval change in morphology of hepatic lesions.
Strosberg et al. [111] recently reported on the results of NETTER-1, a multicenter, randomized, controlled trial of the efficacy and safety of 177Lu-DOTATATE (Lutathera, Advanced Accelerator Applications) in patients with advanced, progressive, SSTR-positive midgut NETs. This phase III trial randomly assigned 116 patients with well-differentiated, metastatic midgut NETs to receive either 177Lu-DOTATATE at a dose of 7.4 GBq every 8 weeks (four IV infusions plus best supportive care including octreotide long-acting repeatable [LAR] administered intramuscularly at a dose of 30 mg) (177Lu-DOTATATE group) or 113 patients to receive octreotide LAR alone that was administered intramuscularly at a dose of 60 mg every 4 weeks (control group). The primary endpoint was progression-free survival. The rate of progression-free survival at month 20 was 65.2% (95% CI, 50.0–76.8%) in the 177Lu-DOTATATE group and 10.8% (95% CI, 3.5–23.0%) in the control group. Grade 3 or 4 neutropenia, thrombocytopenia, and lymphopenia occurred in 1%, 2%, and 9%, respectively, of patients in the 177Lu-DOTATATE group. None of these conditions occurred in the control group. No evidence of renal toxicity was seen. The authors concluded that treatment with 177Lu-DOTATATE resulted in markedly longer progression-free survival with clinically significant myelo-suppression in less than 10% of patients. A planned final analysis will be performed for confirmation of the preliminary analysis observation of an overall survival benefit. Interestingly, chemotherapy appears to still be considered the mainstay treatment strategy and PRRT utilization is low across NET types. A study by Ezziddin and colleagues [112] showed that PRRT may be useful not only for tumors with Ki-67 tumor proliferation index of < 20% (G1 and G2) but also in patients with poorly differentiated tumors with a Ki-67 proliferation index of > 20% (G3). Given the NETTER-1 results, use of PRRT may increase [113].
The joint practice guideline of the International Atomic Energy Agency, European Association of Nuclear Medicine, and Society of Nuclear Medicine and Molecular Imaging provides an excellent resource for background historical information and practical considerations (e.g., indications, contraindications, pretherapy assessment, therapy procedure, amino acid renal protection) for the administration of PRRT in patients with NET [114].
Targeted alpha therapy of NET has also been studied [115]. Chan et al. [116] reported on the safety and efficacy of 213Bi-DOTATATE in a preclinical study of mice harboring NETs pretreated with L-lysine for renal protection. Lysine reduced the renal uptake of 213Bi-DOTATATE by 50% without effect on the tumor uptake. A significant decline in tumor burden and improved survival were also seen with treatment. Kratochwil and colleagues [117] used intraarterial infusion of 213Bi-DOTATOC in seven patients who harbored progressive NET liver metastases after treatment with 90Y and 177Lu-DOTATOC. Tumor binding could be seen with the 440-keV gamma emission of 213Bi. All seven patients had favorable response to alpha therapy with mild hematotoxicity. With regard to potential nephrotoxicity, infusion of positively charged amino acids seems to be protective and reduce nephrotoxicity, although hyperkalemia may be an issue and needs to be monitored, especially in patients with concurrent heart disease [118]. However, overall nephrotoxicity appears to not be a major concern [119].
Response evaluation to PRRT treatment may be challenging. It has been recognized that the commonly employed structural-based RECIST criteria may be inadequate and other tumor features such as measurements of tumor volume and attenuation in CT may be needed [120]. In one study, contrast-enhanced ultrasound monitoring of perfusion changes after PRRT provided early prediction of response to treatment [121]. Accurate response assessment in this clinical setting is an area of interest and will need additional investigation.
It is readily evident that PRRT will play a major role in the treatment of patients with NETs relatively soon. Future efforts will likely focus on providing benefit to additional populations and with use of alpha particles that may provide improved therapeutic efficacy to tumors resistant to beta particle– based PR RT.
Prostate-Specific Membrane Antigen Radioligand Therapy
Prostate-specific membrane antigen (PSMA) is a type II transmembrane glycoprotein that is overexpressed in metastatic prostate cancer and CRPC. PSMA undergoes constitutive internalization, has no known ligand, is not specific to the prostate gland, and is expressed in other normal (e.g., salivary glands, duodenal mucosa, proximal renal tubular cells, and neuroendocrine cells in the colonic crypts) and neoplastic (e.g., transitional cell carcinoma, renal cell carcinoma, colon carcinoma, and endothelial cells of neovasculature) tissues [122]. Nevertheless, major activity in designing and evaluating various radiolabeled agents for imaging and therapy of metastatic prostate cancer with PSMA as the biologic target has produced encouraging results [123–132] (Fig. 4). These radiolabeled agents (antibodies, antibody fragments, aptamers, and PSMA inhibitors) primarily target the extracellular moiety of the PSMA and are typically radiolabeled with 18F (half-life, 110 min), 68Ga (half-life, 68 min), 64Cu (half-life, 12.7 h), 124I (half-life, 4.2 d), and 89Zr (half-life, 78.4 h) for imaging and with 131I (half-life, 8 d), 90Y (half-life, 64.1 h), and 177Lu (half-life, 6.7 d) for therapy. The common binding domain of these agents to the extracellular moiety of the PSMA involves the glutamate-urea-lysine motif [133].
Fig. 4.
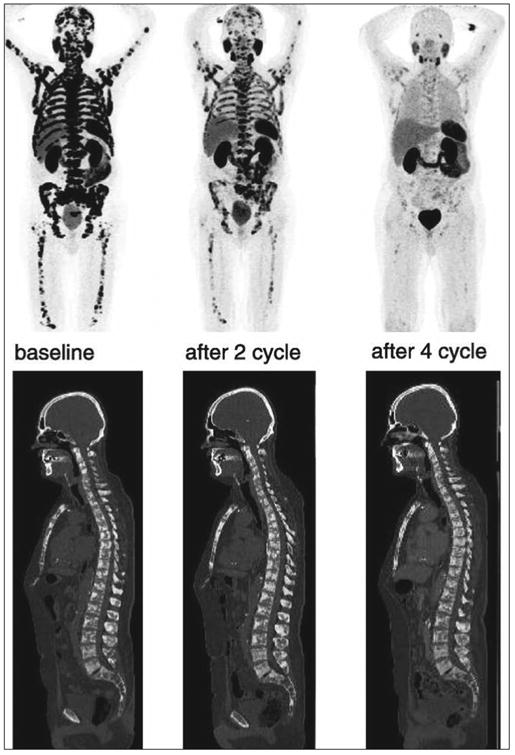
Excellent response to 177Lu prostate-specific membrane antigen (PSMA)-617 therapy in man with extensive metastatic castration-resistant prostate cancer. Gallium-68 PSMA PET/CT was performed at baseline before first, after second, and after fourth cycles of therapy show disappearance of all lesions after fourth therapy cycle. CT did not show major morphologic changes in bone metastases. (Used with permission from [132])
In a theranostic model, the imaging agent is used to localize and assess the extent of disease (“see”), whereas the therapeutic companion is employed to deliver therapy to identified lesions (“treat”). Although false-negatives (small lesions, neuroendocrine differentiation) and false-positives (celiac ganglia, other benign and malignant tumors) can occur with PSMA PET, the tumor-to-background uptake ratios are high, and some studies suggest that other scintigraphic evaluations such as bone scintigraphy may only rarely be needed, if at all [134]. PSMA PET may also be useful as gatekeeper not only for PSMA-based radionuclide therapy but also for other therapies such as 223Ra dichloride treatment [135].
Radiolabeled PSMA-617 has shown favorable kinetics for both imaging (labeled with 68Ga) and radionuclide therapy (labeled with 177Lu). Yadav et al. [136] evaluated the safety and efficacy of 177 Lu-PSMA-617 therapy (mean administered activity, 5059 ± 1845 MBq ranging from one to four cycles) in 31 men with progressive metastatic prostate cancer [136]. Mean serum PSA levels and mean analgesic scores declined from baseline values. Only two patients experienced mild hematotoxicity, and no nephrotoxicity or hepatotoxicity was seen. Rahbar et al. [137] reported on 50 therapies with 177Lu-PSMA-617 in 28 men with metastatic CRPC. Any serum PSA decline and PSA decline of 50% or more occurred in 59% and 32% of patients after one cycle and 75% and 50% of patients after two cycles, respectively. The estimated median survival was 29.4 weeks, which was significantly higher than the historical best supportive care median survival of 19.7 weeks. There were no major toxicities. The same group of authors also published the results of a retrospective study of a cohort of 145 patients with metastatic CRPC who underwent a total of 248 cycles of 177Lu- PSMA-617 radionuclide therapy (one to four treatment cycles with activity range of 2–8 GBq per cycle) and were followed for a median of 16 weeks [138]. The overall biochemical response rate (PSA decline) was 45% after all therapy cycles. Grades 3 and 4 anemia, thrombocytopenia, and leukopenia occurred in 10%, 4%, and 3% of the men, respectively. In another similar study, 30 patients received one to three cycles of 177Lu-PS-MA-617 radionuclide therapy [139]. A decline of more than 50% in serum PSA level was noted in 43% of patients after three cycles, and this level of decline was sustained for over 24 weeks in 27% of all treated patients. Acute hematotoxicity was mild, and xerostomia, nausea, and fatigue occurred in < 10% of patients. The salivary gland received the highest dose at 1.4 Gy/GBq, the kidney at 0.75 Gy/GBq, and the red marrow at 0.03 Gy/GBq irrespective of tumor burden or cycle. With regard to radiation dosimetry, another study reported the highest absorbed dose in the lacrimal glands at 2.82 Gy/GBq, 0.72 Gy/GBq in the salivary glands, and 0.53 Gy/GBq in the kidneys [140]. Others have reported similar results with the parotid glands receiving the highest mean radiation absorbed dose, but this result was associated with only mild reversible xerostomia in some cases [141, 142]. Despite renal clearance of the non–tumor-bound agent, the kidneys do not receive the highest absorbed dose. Nevertheless, PSMA inhibitors such as 2-(phospho-nomethyl)pentanedioic acid have been proposed for nephroprotection to diminish renal PSMA binding [143]. For the imaging counterpart, relatively high physiologic uptake of 68Ga-PSMA-617 in the salivary glands and kidneys has been reported [144].
Other radiolabels being explored for PSMA-617 include auger electrons (125I), alpha particles (e.g., 211At, 213Bi, 225Ac), and cyclotron-produced 44Sc (half-life, 4.04 h) [145–148]. Kratochwil and coworkers [149] reported very early results in two patients with metastatic CRPC who underwent first-in-human treatment with 225Ac-PSMA-617 at a dose of 100 kBq/kg administered bimonthly. Both patients had marked decline in serum PSA levels without hematotoxicity. PSMA imaging and therapy (I&T) agents, which can be radiolabeled with 68Ga for imaging and with 177Lu for therapy, are also being used [150]. From an imaging point of view, the PSMA I&T has been shown to have high detection rates of recurrent prostate cancer comparable with 68Ga-PS-MA [2-hydroxy-5-(carboxyethyl)benzyl] ethylenediamine-N,N′-diacetic acid (equivalent to 68Ga-PSMA-11) [151]. With regard to radiation dosimetry for 177Lu-PSMA I&T agents, the mean absorbed organ doses have been reported as 0.72 ± 0.21 Gy/GBq for the kidneys; 0.12 ± 0.06 Gy/GBq for the liver; and 0.55 ± 0.14 Gy/ GBq for the parotid, 0.64 ± 0.40 Gy/GBq for the submandibular, and 3.8 ± 1.4 Gy/GBq for the lacrimal glands [152]. Therefore, as with 177Lu-PSMA-617, 177Lu-PSMA I&T agents are associated with the highest doses to the lacrimal and salivary glands.
Interestingly, higher PSMA expression has been seen in tumor cells exposed to anti-hormonal treatment [153, 154]. This finding, at least theoretically, provides a possible opportunity to synergize antiandrogen therapy with PSMA-based radionuclide therapy. In summary, the current and steadily growing evidence strongly indicates that PSMA-based theranostics will play a major role in the management of prostate cancer.
Future Prospects
TRT research and clinical use are advancing rapidly. New targets in cancer cells such as gastrin-releasing peptide receptor, integrins, melanocyte-stimulating hormone receptor, melanocortin-1 receptor, glucagonlike peptide-1 receptor, chemokine receptor, folate receptor, and others may provide additional opportunities for more cancer types to move the field forward even further [155–163]. Similarly, incorporation of other high-energy alpha particles (e.g., 213Bi, 211At, 225Ac) is under investigation [164]. Other novel combined treatment methods such as radiovirotherapy (use of oncolytic viruses to deliver radionuclide therapy into infected cancer cells) are also exciting recent developments [165].
Aside from scientific advancements, key issues need to be worked out for TRT to thrive as a legitimate and active component of cancer therapy [166, 167]. Regulatory and reimbursement agencies must coordinate their requirements so that safety, efficacy, and payment follow seamlessly without major delays for the introduction of the therapy into the clinical setting. Reimbursement must be sufficient to ensure that the therapy is not underutilized solely on the basis of financial loss. Access and availability of therapeutic agents must be assured across all geographic areas and clinical practice models. Comparative and cost-effectiveness investigations will need to follow. The medical curricula must also include education on TRT so that the next generation of physicians and ancillary medical personnel are all familiar with this emerging therapy modality.
Conclusion
TRT began with thyroid radioiodine therapy but has expanded to include many other radionuclides and cancers. The current momentum and stimulating results pave the way for TRT to grow its footprint in the care of patients with cancer and likely other diseases in the era of precision medicine.
Acknowledgments
Supported in part by the National Institutes of Health grants R01-CA111613, R21-CA142426, R21-EB017568, and P30-CA014089.
References
- 1.Buchegger F, Perillo-Adamer F, Dupertius YM, et al. Auger radiation targeted into DNA: a therapy perspective. Eur J Nucl Med Mol Imaging. 2006;33:1352–1363. doi: 10.1007/s00259-006-0187-2. [DOI] [PubMed] [Google Scholar]
- 2.Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted alpha particle therapy. J Nucl Med. 2005;46(suppl 1):199S–204S. [PubMed] [Google Scholar]
- 3.Zukotynski K, Jadvar H, Capala J, Fahey F. Targeted radionuclide therapy: practical applications and future prospects. Biomark Cancer. 2016;8(suppl):35–38. doi: 10.4137/BIC.S31804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung JK, Cheon GJ. Radioiodine therapy in differentiated thyroid cancer: the first targeted therapy in oncology. Endocrinol Metab (Seoul) 2014;29:233–239. doi: 10.3803/EnM.2014.29.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pryma DA, Mandel SJ. Radioiodine therapy for thyroid cancer in the era of risk stratification and alternative targeted therapies. J Nucl Med. 2014;55:1485–1491. doi: 10.2967/jnumed.113.131508. [DOI] [PubMed] [Google Scholar]
- 6.Kim JS. Combination radioimmunotherapy approaches and quantification of immune-PET. Nucl Med Mol Imaging. 2016;50:104–111. doi: 10.1007/s13139-015-0392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharkey RM, Goldenberg DM. Cancer radioimmunotherapy. Immunotherapy. 2011;3:349–370. doi: 10.2217/imt.10.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macklis RM. Radioimmunotherapy as a therapeutic option for non-Hodgkin's lymphoma. Semin Radiat Oncol. 2007;17:176–183. doi: 10.1016/j.semradonc.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Gordon LI, Witzig TE, Wiseman GA, et al. Yttrium 90 ibritumomab tiuxetan radioimmunotherapy for relapsed or refractory low-grade non-Hodg-kin's lymphoma. Semin Oncol. 2002;29(suppl 2):87–92. Erratum in Semin Oncol 2003; 30:417. [PubMed] [Google Scholar]
- 10.Troncone L, Rufni V. 131I-MIBG therapy of neural crest tumors. Anticancer Res. 1997;17:1823–1831. [PubMed] [Google Scholar]
- 11.Nicolay NH, Berry DP, Sharma RA. Liver metastases from colorectal cancer: radioembolization with systemic therapy. Nat Rev Clin Oncol. 2009;6:687–697. doi: 10.1038/nrclinonc.2009.165. [DOI] [PubMed] [Google Scholar]
- 12.Bozkurt MF, Salanci BV, Ugur O. Intra-arterial radionuclide therapies for liver tumors. Semin Nucl Med. 2016;46:324–339. doi: 10.1053/j.semnuclmed.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Gulec SA. Y-90 radiomicrosphere therapy for colorectal cancer liver metastases. Semin Nucl Med. 2016;46:126–134. doi: 10.1053/j.semnuclmed.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Jia Z, Paz-Fumagalli R, Frey G, et al. Single-institution experience of radioembolization with yttrium-90 microspheres for unresectable metastatic neuroendocrine liver tumors. J Gastroenterol Hepatol. 2017 Jan 29; doi: 10.1111/jgh.13752. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Fidelman N, Kerlan RK., Jr Transarterial chemo-embolization and 90Y radioembolization for hepatocellular carcinoma: review of current applications beyond intermediate-stage disease. AJR. 2015;205:742–752. doi: 10.2214/AJR.15.14802. [DOI] [PubMed] [Google Scholar]
- 16.Kis B, Shah J, Choi J, et al. Transarterial yttrium-90 radioembolization treatment of patients with liver-dominant metastatic renal cell carcinoma. J Vasc Interv Radiol. 2017;28:254–259. doi: 10.1016/j.jvir.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon AC, Salem R, Lewandowski RJ. Yttrium-90 radioembolization for breast cancer liver metastases. J Vasc Interv Radiol. 2016;27:1316–1319. doi: 10.1016/j.jvir.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Sacco R, Conte C, Tumino E, et al. Transarterial radioembolization for hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:25–29. doi: 10.2147/JHC.S50359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettorre GM, Levi Sandri GB, Laurenzi A, et al. Yttrium-90 radioembolization for hepatocellular carcinoma prior to liver transplantation. World J Surg. 2017;41:241–249. doi: 10.1007/s00268-016-3682-z. [DOI] [PubMed] [Google Scholar]
- 20.Kim AY, Unger K, Wang H, et al. Incorporating yttrium-90 trans-arterial radioembolization (TARE) in the treatment of metastatic pancreatic adenocarcinoma: a single center experience. BMC Cancer. 2016;16:492. doi: 10.1186/s12885-016-2552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosconi C, Gramenzi A, Ascanio S, et al. Yttrium-90 radioembolization for unresectable/ recurrent intrahepatic cholangiocarcinoma: a survival, efficacy and safety study. Br J Cancer. 2016;115:297–302. doi: 10.1038/bjc.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho YY, Lee M, Kim HC, et al. Radioembolization is safe and effective treatment for hepatocellular carcinoma with portal vein thrombosis: a propensity score analysis. PLoS One. 2016;11:e0154986. doi: 10.1371/journal.pone.0154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickey RM, Lewandowski RJ, Salem R. Yttrium-90 radioembolization for hepatocellular carcinoma. Semin Nucl Med. 2016;46:105–108. doi: 10.1053/j.semnuclmed.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Fendler WP, Lechner H, Todica A, et al. Safety, efficacy, and prognostic factors after radioembolization of hepatic metastases from breast cancer: a large single-center experience in 81 patients. J Nucl Med. 2016;57:517–523. doi: 10.2967/jnumed.115.165050. [DOI] [PubMed] [Google Scholar]
- 25.Hickey R, Lewandowski RJ, Prudhomme T, et al. 90Y radioembolization of colorectal hepatic metastases using glass microspheres: safety and survival outcomes from a 531-patient multicenter study. J Nucl Med. 2016;57:665–671. doi: 10.2967/jnumed.115.166082. [DOI] [PubMed] [Google Scholar]
- 26.Braat AJ, Smits ML, Braat MN, et al. 90Y hepatic radioembolization: an update on current practice and recent developments. J Nucl Med. 2015;56:1079–1087. doi: 10.2967/jnumed.115.157446. [DOI] [PubMed] [Google Scholar]
- 27.Kokabi N, Camacho JC, Xing M, et al. Open-label prospective study of the safety and efficacy of glass-based yttrium 90 radioembolization for infiltrative hepatocellular carcinoma with portal vein thrombosis. Cancer. 2015;121:2164–2174. doi: 10.1002/cncr.29275. [DOI] [PubMed] [Google Scholar]
- 28.Memon K, Kuzel TM, Vouche M, et al. Hepatic yttrium-90 radioembolization for metastatic melanoma: a single-center experience. Melanoma Res. 2014;24:244–251. doi: 10.1097/CMR.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 29.Uliel L, Royal HD, Darcy MD, et al. From the angio suite to the gamma-camera: vascular mapping and 99mTc-MAA hepatic perfusion imaging before liver radioembolization—a comprehensive pictorial review. J Nucl Med. 2012;53:1736–1747. doi: 10.2967/jnumed.112.105361. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed O, Patel MV, Masrani A, et al. Assessing intra-arterial complications of planning and treatment angiograms for Y-90 radioembolization. Cardiovasc Intervent Radiol. 2017;40:704–711. doi: 10.1007/s00270-016-1555-3. [DOI] [PubMed] [Google Scholar]
- 31.James T, Hill J, Fahrbach T, et al. Differences in radiation activity between glass and resin 90Y microspheres in treating unresectable hepatic cancer. Health Phys. 2017;112:300–304. doi: 10.1097/HP.0000000000000631. [DOI] [PubMed] [Google Scholar]
- 32.Jha AK, Mithun S, Purandare NC, et al. Impact of the activity calculation method used in trans-arterial radioembolization: a dosimetric comparison between 90Y-SIRSphere and 90Y-TheraSphere therapy. Nucl Med Commun. 2016;37:917–923. doi: 10.1097/MNM.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 33.Van der Gucht A, Jreige M, Denys A, et al. Resin versus glass microspheres for yttrium-90 transarterial radioembolization: comparing survival in unresectable hepatocellular carcinoma using pretreatment partition model dosimetry. J Nucl Med. 2017 Jan 12; doi: 10.2967/jnumed.116.184713. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Gates VL, Esmail AA, Marshall K, Spies S, Salem R. Internal pair production of 90Y permits hepatic localization of microspheres using routine PET: proof of concept. J Nucl Med. 2011;52:72–76. doi: 10.2967/jnumed.110.080986. [DOI] [PubMed] [Google Scholar]
- 35.Ilhan H, Goritschan A, Paprottka P, et al. Predictive value of 99mTc-MAA SPECT for 90Y-labeled resin microsphere distribution in radioembolization of primary and secondary hepatic tumors. J Nucl Med. 2015;56:1654–1660. doi: 10.2967/jnumed.115.162685. [DOI] [PubMed] [Google Scholar]
- 36.Ulrich G, Dudeck O, Furth C, et al. Predictive value of intratumoral 99mTc-macroaggregated albumin uptake in patients with colorectal liver metastases scheduled for radioembolization with 90Y-microspheres. J Nucl Med. 2013;54:516–522. doi: 10.2967/jnumed.112.112508. [DOI] [PubMed] [Google Scholar]
- 37.Gabr A, Kallini JR, Gates VL, et al. Same-day 90Y radioembolization: implementing a new treatment paradigm. Eur J Nucl Med Mol Imaging. 2016;43:2353–2359. doi: 10.1007/s00259-016-3438-x. [DOI] [PubMed] [Google Scholar]
- 38.Law M, Wong KK, Tso WK, et al. Personnel dose reduction in 90Y microspheres liver-directed radio-embolization: from interventional radiology suite to patient ward. Br J Radiol. 2017;90:20160591. doi: 10.1259/bjr.20160591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laffont S, Rolland Y, Ardisson V, et al. Occupational radiation exposure of medical staff performing 90Y-loaded microsphere radioembolization. Eur J Nucl Med Mol Imaging. 2016;43:824–831. doi: 10.1007/s00259-015-3277-1. [DOI] [PubMed] [Google Scholar]
- 40.Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol. 2014;4:198. doi: 10.3389/fonc.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atassi B, Bangash AK, Bahrani A, et al. Multi-modality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. RadioGraphics. 2008;28:81–99. doi: 10.1148/rg.281065721. [DOI] [PubMed] [Google Scholar]
- 42.Sag AA, Savin MA, Lal NR, et al. Yttrium-90 radioembolization of malignant tumors of the liver: gallbladder effects. AJR. 2014;202:1130–1135. doi: 10.2214/AJR.13.10548. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Ros N, Iñarrairaegui M, Paramo JA, et al. Radioembolization of hepatocellular carcinoma activates liver regeneration, induces infammation and endothelial stress and activates coagulation. Liver Int. 2015;35:1590–1596. doi: 10.1111/liv.12592. [DOI] [PubMed] [Google Scholar]
- 44.Giammarile F, Bodei L, Chiesa C, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2011;38:1393–1406. doi: 10.1007/s00259-011-1812-2. [DOI] [PubMed] [Google Scholar]
- 45.Sangha BS, Nimeiri H, Hickey R, et al. Radioembolization as a treatment strategy for metastatic colorectal cancer to the liver: what can we learn from the SIRFLOX trial? Curr Treat Options Oncol. 2016;17:26. doi: 10.1007/s11864-016-0402-8. [DOI] [PubMed] [Google Scholar]
- 46.van den Hoven AF, Rosenbaum CE, Elias SG, et al. Insights into the dose-response relationship of radioembolization with resin 90Y-microspheres: a prospective cohort study in patients with colorectal cancer liver metastases. J Nucl Med. 2016;57:1014–1019. doi: 10.2967/jnumed.115.166942. [DOI] [PubMed] [Google Scholar]
- 47.Pasciak AS, Bourgeois AC, Bradley YC. A microdosimetric analysis of absorbed dose to tumor as a function of number of microspheres per unit volume in 90Y radioembolization. J Nucl Med. 2016;57:1020–1026. doi: 10.2967/jnumed.115.163444. [DOI] [PubMed] [Google Scholar]
- 48.Riaz A, Miller FH, Kulik LM, et al. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. JAMA. 2010;303:1062–1069. doi: 10.1001/jama.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keppke AL, Salem R, Reddy D, et al. Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR. 2007;188:768–775. doi: 10.2214/AJR.06.0706. [DOI] [PubMed] [Google Scholar]
- 50.Shady W, Sotirchos VS, Do RK, et al. Surrogate imaging biomarkers of response of colorectal liver metastases after salvage radioembolization using 90Y-loaded resin microspheres. AJR. 2016;207:661–670. doi: 10.2214/AJR.15.15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shady W, Kishore S, Gavane S, et al. Metabolic tumor volume and total lesion glycolysis on FDG PET/CT can predict overall survival after 90Y radioembolization of colorectal liver metastases: a comparison with SUVmax, SUVpeak, and RECIST 1.0. Eur J Radiol. 2016;85:1224–1231. doi: 10.1016/j.ejrad.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fowler KJ, Maughan NM, Laforest R, et al. PET/MRI of hepatic 90Y microsphere deposition determines individual tumor response. Cardiovasc Intervent Radiol. 2016;39:855–864. doi: 10.1007/s00270-015-1285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paprottka KJ, Schopeppe F, Ingrisch M, et al. Pre-therapeutic factors for predicting survival after radioembolization: a single-center experience in 389 patients. Eur J Nucl Med Mol Imaging. 2017 Feb 14; doi: 10.1007/s00259-017-3646-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Salem R, Gordon AC, Mouli S, et al. Y90 radioem-bolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151:1155–1163.e2. doi: 10.1053/j.gastro.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rognoni C, Ciani O, Sommarvia S, et al. Trans-arterial radioembolization in intermediate-advanced hepatocellular carcinoma: systematic review and meta-analyses. Oncotarget. 2016;7:72343–72355. doi: 10.18632/oncotarget.11644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Li Y, Ji H, et al. Transarterial Y90 radioembolization versus chemoembolization for patients with hepatocellular carcinoma: a meta-analysis. Biosci Trends. 2015;9:289–298. doi: 10.5582/bst.2015.01089. [DOI] [PubMed] [Google Scholar]
- 57.Al-Adra DP, Gill RS, Axford SJ, et al. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and meta-analysis. Eur J Surg Oncol. 2015;41:120–127. doi: 10.1016/j.ejso.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devcic Z, Rosenberg J, Braat AJ, et al. The eff-cacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med. 2014;55:1404–1410. doi: 10.2967/jnumed.113.135855. [DOI] [PubMed] [Google Scholar]
- 59.Narsinh KH, Van Buskirk M, Kennedy AS, et al. Hepatopulmonary shunting: a prognostic indicator of survival in patients with metastatic colorectal adenocarcinoma treated with 90Y radioembolization. Radiology. 2017;282:281–288. doi: 10.1148/radiol.2016152100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xing M, Lahti S, Kokabi N, et al. 90Y radioembolization lung shunt faction in primary and metastatic liver cancer as a biomarker for survival. Clin Nucl Med. 2016;41:21–27. doi: 10.1097/RLU.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 61.Mollabashy A, Scarborough M. The mechanism of metastasis. Orthop Clin North Am. 2000;31:529–535. doi: 10.1016/s0030-5898(05)70172-3. [DOI] [PubMed] [Google Scholar]
- 62.Lange PH, Vessella RL. Mechanisms, hypotheses and questions regarding prostate cancer micrometastases to bone. Cancer Metastasis Rev. 1998–1999;17:331–336. doi: 10.1023/a:1006106209527. [DOI] [PubMed] [Google Scholar]
- 63.Maffioli L, Florimonte L, Costa DC, et al. New radiopharmaceutical agents for the treatment of castration-resistant prostate cancer. Q J Nucl Med Mol Imaging. 2015;59:420–438. [PubMed] [Google Scholar]
- 64.Gartrell BA, Coleman R, Efstathiou E, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol. 2015;68:850–858. doi: 10.1016/j.eururo.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 65.Brady D, Parker CC, O'Sullivan JM. Bone-targeting radiopharmaceuticals including radium-223. Cancer J. 2013;19:71–78. doi: 10.1097/PPO.0b013e318282479b. [DOI] [PubMed] [Google Scholar]
- 66.D'angelo G, Sciuto R, Salvatori M, et al. Targeted “bone-seeking” radiopharmaceuticals for palliative treatment of bone metastases: a systematic review and meta-analysis. Q J Nucl Med Mol Imaging. 2012;56:538–543. [PubMed] [Google Scholar]
- 67.Kim YS, Brechbiel MW. An overview of targeted alpha therapy. Tumour Biol. 2012;33:573–590. doi: 10.1007/s13277-011-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allen B. Systemic targeted alpha radiotherapy for cancer. J Biomed Phys Eng. 2013;3:67–80. [PMC free article] [PubMed] [Google Scholar]
- 69.Sgouros G. Alpha-particles for targeted therapy. Adv Drug Deliv Rev. 2008;60:1402–1406. doi: 10.1016/j.addr.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Kassis AI. Therapeutic radionuclides: biophysical and radiobiologic principles. Semin Nucl Med. 2008;38:358–366. doi: 10.1053/j.semnuclmed.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chouin N, Bardies M. Alpha-particle microdosimetry. Curr Radiopharm. 2011;4:266–280. doi: 10.2174/1874471011104030266. [DOI] [PubMed] [Google Scholar]
- 72.Pouget JP, Lozza C, Deshayes E, et al. Introduction to radiobiology of targeted radionuclide therapy. Front Med (Lausanne) 2015;2:12. doi: 10.3389/fmed.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coleman R. Treatment of metastatic bone disease and the emerging role of radium-223. Semin Nucl Med. 2016;46:99–104. doi: 10.1053/j.semnuclmed.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 74.Jadvar H, Quinn DI. Targeted alpha-particle therapy of bone metastases in prostate cancer. Clin Nucl Med. 2013;38:966–971. doi: 10.1097/RLU.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iagaru AH, Mittra E, Colletti PM, et al. Bone targeted imaging and therapy in prostate cancer. J Nucl Med. 2016;57(suppl 3):19S–24S. doi: 10.2967/jnumed.115.170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turner PG, O'Sullivan JM. 223Ra and other bone-targeting radiopharmaceuticals: the translation of radiobiology into clinical practice. Br J Radiol. 2015;88:20140752. doi: 10.1259/bjr.20140752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chittenden SJ, Hindorf C, Parker CC, et al. A phase 1, open-label study of the biodistribution, pharmacokinetics, and dosimetry of 223Radichloride in patients with hormone-refractory prostate cancer and skeletal metastases. J Nucl Med. 2015;56:1304–1309. doi: 10.2967/jnumed.115.157123. [DOI] [PubMed] [Google Scholar]
- 78.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter Radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 79.Parker C, Zhan L, Cislo P, et al. Effect of radium-223 dichloride (Ra-223) on hospitalization: an analysis from phase 3 randomized Alpharadin in symptomatic prostate cancer patients (ALSYMPCA) trial. Eur J Cancer. 2017;71:1–6. doi: 10.1016/j.ejca.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 80.Jadvar H, Challa S, Quinn DI, et al. One-year postapproval clinical experience with radium-223 dichloride in patients with metastatic castrate-resistant prostate cancer. Cancer Biother Radiopharm. 2015;30:195–199. doi: 10.1089/cbr.2014.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Etchebehere EC, Milton DR, Araujo JC, et al. Factors affecting 223Ra therapy: clinical experience after 532 cycles from a single institution. Eur J Nucl Med Mol Imaging. 2016;43:8–20. doi: 10.1007/s00259-015-3185-4. [DOI] [PubMed] [Google Scholar]
- 82.Etchebehere EC, Araujo JC, Fox PS, et al. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18 F-fluoride PET/CT in predicting overall survival. J Nucl Med. 2015;56:1177–1184. doi: 10.2967/jnumed.115.158626. [DOI] [PubMed] [Google Scholar]
- 83.Dauer LT, Williamson MJ, Humm J, et al. Radiation safety considerations for the use of 223RaCl2 DE in men with castration-resistant prostate cancer. Health Phys. 2014;106:494–504. doi: 10.1097/HP.0b013e3182a82b37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saad F, Carles J, Gillessen S, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17:1306–1316. doi: 10.1016/S1470-2045(16)30173-5. [DOI] [PubMed] [Google Scholar]
- 85.Coleman R, Aksnes AK, Nume B, et al. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone dominant disease. Breast Cancer Res Treat. 2014;145:411–418. doi: 10.1007/s10549-014-2939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson PM, Subbian V, Rohren E. Bone-seeking radiopharmaceuticals as targeted agents of osteosarcoma: samarium-153-EDTMP and radium-223. Adv Exp Med Biol. 2014;804:291–304. doi: 10.1007/978-3-319-04843-7_16. [DOI] [PubMed] [Google Scholar]
- 87.Oberg K, Castellano D. Current knowledge on diagnosis and staging of neuroendocrine tumors. Cancer Metastasis Rev. 2011;30(suppl 1):3–7. doi: 10.1007/s10555-011-9292-1. [DOI] [PubMed] [Google Scholar]
- 88.Jadvar H. Hepatocellular carcinoma and gastro-enteropancreatic neuroendocrine tumors: potential role of other positron emission tomography radiotracers. Semin Nucl Med. 2012;42:247–254. doi: 10.1053/j.semnuclmed.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 89.Breeman WA, de Blois E, Sze Chan H, et al. 68Ga-labeled DOTA peptides and 68Ga-labeled radiopharmaceuticals for positron emission tomography: current status of research, clinical applications, and future perspectives. Semin Nucl Med. 2011;41:314–321. doi: 10.1053/j.semnuclmed.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–1626. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 91.Virgolini I, Ambrosini V, Bomanji JB, et al. Procedure guidelines for PET/CT tumor imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37:2004–2010. doi: 10.1007/s00259-010-1512-3. [DOI] [PubMed] [Google Scholar]
- 92.U.S. Food and Drug Administration website. FDA approves new diagnostic imaging agent to detect rare neuroendocrine tumors. [Accessed March 4, 2017]; www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm504524.htm. Published June 1, 2016.
- 93.Bodei L, Ferone D, Grana CM, et al. Peptide receptor therapies in neuroendocrine tumors. J Endocrinol Invest. 2009;32:360–369. doi: 10.1007/BF03345728. [DOI] [PubMed] [Google Scholar]
- 94.Bodei L, Kwekkeboom DJ, Kidd M, et al. Radio-labeled somatostatin analog therapy of gastroen-teropancreatic cancer. Semin Nucl Med. 2016;46:225–238. doi: 10.1053/j.semnuclmed.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Kwekkeboom DJ, Kenning EP. Peptide receptor radionuclide therapy in the treatment of neuroendocrine therapies. Hematol Oncol Clin North Am. 2016;30:179–191. doi: 10.1016/j.hoc.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 96.Taïeb D, Garrigue P, Bardies M, et al. Application and dosimetric requirements for gallium-68-labeled somatostatin analogues in targeted radionuclide therapy for gastroenteropancreatic neuroendocrine tumors. PET Clin. 2015;10:477–486. doi: 10.1016/j.cpet.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pusceddu S, De Braud F, Festinese F, et al. Evolution in the treatment of gastroenteropancreatic-neuroendocrine neoplasms, focus on systemic therapeutic options: a systematic review. Future Oncol. 2015;11:1947–1959. doi: 10.2217/fon.15.86. [DOI] [PubMed] [Google Scholar]
- 98.Dash A, Chakraborty S, Pillai MR, et al. Peptide receptor radionuclide therapy: an overview. Cancer Biother Radiopharm. 2015;30:47–71. doi: 10.1089/cbr.2014.1741. [DOI] [PubMed] [Google Scholar]
- 99.Graham MM, Menda Y. Radiopeptide imaging and therapy in the United States. J Nucl Med. 2011;52(suppl 2):56S–63S. doi: 10.2967/jnumed.110.085746. [DOI] [PubMed] [Google Scholar]
- 100.Ambrosini V, Fani M, Fanti S, et al. Radiopeptide imaging and therapy in Europe. J Nucl Med. 2011;52(suppl 2):42S–55S. doi: 10.2967/jnumed.110.085753. [DOI] [PubMed] [Google Scholar]
- 101.Druce MR, Lewington V, Grossman AB. Targeted radionuclide therapy for neuroendocrine tumors: principles and application. Neuroendocrinology. 2010;91:1–15. doi: 10.1159/000227808. [DOI] [PubMed] [Google Scholar]
- 102.Lewington VJ. Targeted radionuclide therapy for neuroendocrine tumors. Endocr Relat Cancer. 2003;10:497–501. doi: 10.1677/erc.0.0100497. [DOI] [PubMed] [Google Scholar]
- 103.Cives M, Strosberg J. Radionuclide therapy for neuroendocrine tumors. Curr Oncol Rep. 2017;19:9. doi: 10.1007/s11912-017-0567-8. [DOI] [PubMed] [Google Scholar]
- 104.van Vliet EI, Teunissen JJ, Kam BL, et al. Treatment of gastroenteropancreatic neuroendocrine tumors with peptide receptor radionuclide therapy. Neuroendocrinology. 2013;97:74–85. doi: 10.1159/000335018. [DOI] [PubMed] [Google Scholar]
- 105.Baum RP, Kluge AW, Kulkarni H, et al. [(177)Lu-DOTA](0)-D-Phe(1)-Tyr(3)-Octreotide ((177)Lu-DOTATOC) for peptide receptor radiotherapy in patients with advanced neuroendocrine tumors: a phase-II study. Theranostics. 2016;6:501–510. doi: 10.7150/thno.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hörsch D, Ezziddin S, Haug A, et al. Peptide receptor radionuclide therapy for neuroendocrine tumors in Germany: first results of a multi-institutional cancer registry. Recent Results Cancer Res. 2013;194:457–465. doi: 10.1007/978-3-642-27994-2_25. [DOI] [PubMed] [Google Scholar]
- 107.Gabriel M, Oberauer A, Dobrozemsky G, et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med. 2009;50:1427–1434. doi: 10.2967/jnumed.108.053421. [DOI] [PubMed] [Google Scholar]
- 108.Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuro-endocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19. doi: 10.1007/s00259-014-2893-5. [DOI] [PubMed] [Google Scholar]
- 109.Hörsch D, Ezziddin S, Haug A, et al. Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: a multi-institutional registry study with prospective follow-up. Eur J Cancer. 2016;58:41–51. doi: 10.1016/j.ejca.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 110.Schuchardt C, Kulkarni HR, Prasad V, et al. The Bad Berka dose protocol: comparative results of dosimetry in peptide receptor radionuclide therapy using (177)Lu-DOTATATE, (177)Lu- DOTANOC, and (177)Lu-DOTATOC. Recent Results Cancer Res. 2013;194:519–536. doi: 10.1007/978-3-642-27994-2_30. [DOI] [PubMed] [Google Scholar]
- 111.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumors. N Engl J Med. 2017;12:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ezziddin S, Opitz M, Attassi M, et al. Impact of the Ki-67 proliferation index on response to peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2011;38:459–466. doi: 10.1007/s00259-010-1610-2. [DOI] [PubMed] [Google Scholar]
- 113.Strosberg J, Casciano R, Stern L, et al. United States-based practice patterns and resource utilization in advanced neuroendocrine tumor treatment. World J Gastroenterol. 2013;19:2348–2354. doi: 10.3748/wjg.v19.i15.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bodei L, Mueller-Brand J, Baum RP, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2013;40:800–816. doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chan HS, Konijnenberg MW, de Blois E, et al. Influence of tumor size on the efficacy of targeted alpha therapy with (213)Bi-[DOTA(0), Tyr(3)]-octreotate. EJNMMI Res. 2016;6:6. doi: 10.1186/s13550-016-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chan HS, Konijnenberg MW, Daniels T, et al. Improved safety and efficacy of 213Bi-DOTATATE-targeted alpha therapy of somatostatin receptor-expressing neuroendocrine tumors in mice pre-treated with L-lysine. EJNMMI Res. 2016;6:83. doi: 10.1186/s13550-016-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kratochwil C, Giesel FL, Bruchertseifer F, et al. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumors refractory to beta radiation: a first-I-human experience. Eur J Nucl Med Mol Imaging. 2014;41:2106–2119. doi: 10.1007/s00259-014-2857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Giovacchini G, Nicolas G, Freidank H, et al. Effect of amino acid infusion on potassium serum levels in neuroendocrine tumor patients treated with targeted radionuclide therapy. Eur J Nucl Med Mol Imaging. 2011;38:1675–1682. doi: 10.1007/s00259-011-1826-9. [DOI] [PubMed] [Google Scholar]
- 119.Kulkarni HR, Schuchardt C, Baum RP. Peptide receptor radionuclide therapy with (177)Lu-labeled somatostatin analogs DOTATATE and DOTATOC: contrasting renal dosimetry in the same patient. Recent Results Cancer Res. 2013;194:551–559. doi: 10.1007/978-3-642-27994-2_32. [DOI] [PubMed] [Google Scholar]
- 120.Sowa-Staszczak A, Chrzan R, Pach D, et al. Are RECIST criteria sufficient to assess response to therapy in neuroendocrine tumors? Clin Imaging. 2012;36:360–364. doi: 10.1016/j.clinimag.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 121.Giesel FL, Wulfert S, Zechmann CM, et al. Contrast-enhanced ultrasound monitoring of perfusion changes in hepatic neuroendocrine metastases after systemic versus selective arterial 177Lu/90Y-DOTATOC and 231Bi-DOTATOC radionuclide therapy. Exp Oncol. 2013;35:122–126. [PubMed] [Google Scholar]
- 122.Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 123.Jadvar H. PSMA PET in prostate cancer. J Nucl Med. 2015;56:1131–1132. doi: 10.2967/jnumed.115.157339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pillai MR, Nanabala R, Joy A, et al. Radiolabeled enzyme inhibitors and binding agents targeting PSMA: effective theranostic tools for imaging and therapy of prostate cancer. Nucl Med Biol. 2016;43:692–720. doi: 10.1016/j.nucmedbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 125.Bander NH, Nanus DM, Milowsky MI, et al. Targeted systemic therapy of prostate cancer with a monoclonal antibody to prostate-specific membrane antigen. Semin Oncol. 2003;30:667–676. doi: 10.1016/s0093-7754(03)00358-0. [DOI] [PubMed] [Google Scholar]
- 126.Bouchelouche K, Turkbey B, Choyke PL. PSMA PET and radionuclide therapy in prostate cancer. Semin Nucl Med. 2016;46:522–535. doi: 10.1053/j.semnuclmed.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barrio M, Fendler WP, Czernin J, et al. Prostate specific membrane antigen (PSMA) ligands for diagnosis and therapy of prostate cancer. Expert Rev Mol Diagn. 2016;16:1177–1188. doi: 10.1080/14737159.2016.1243057. [DOI] [PubMed] [Google Scholar]
- 128.Lütje S, Heskamp S, Comelissen AS, et al. PSMA ligands for radionuclide imaging and therapy of prostate cancer: clinical status. Theranostics. 2015;5:1388–1401. doi: 10.7150/thno.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vallabhajosula S, Nikolopoulou A, Jhanwar YS, et al. Radioimmunotherapy of metastatic prostate cancer with 177Lu-DOTAhuJ591 anti prostate specific membrane antigen specific monoclonal antibody. Curr Radiopharm. 2016;9:44–53. doi: 10.2174/1874471008666150313114005. [DOI] [PubMed] [Google Scholar]
- 130.Zechmann CM, Afshar-Oromieh A, Armor T, et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–1292. doi: 10.1007/s00259-014-2713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kulkarni HR, Singh A, Schuchardt C, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka Experience since 2013. J Nucl Med. 2016;57(suppl 3):97S–104S. doi: 10.2967/jnumed.115.170167. [DOI] [PubMed] [Google Scholar]
- 132.Roll W, Bode A, Weckesser M, et al. Excellent response to 177Lu-PSM A-617 radioligand therapy in a patient with advanced metastatic castration resistant prostate cancer evaluated by 68Ga-PSMA PET/CT. Clin Nucl Med. 2017;42:152–153. doi: 10.1097/RLU.0000000000001480. [DOI] [PubMed] [Google Scholar]
- 133.Mease RC, Foss CA, Pomper MG. PET imaging in prostate cancer: focus on prostate-specific membrane antigen. Curr Top Med Chem. 2013;13:951–962. doi: 10.2174/1568026611313080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pyka T, Okamoto S, Dahlbender M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2114–2121. doi: 10.1007/s00259-016-3435-0. [DOI] [PubMed] [Google Scholar]
- 135.Ahmadzadehfar H, Azgomi K, Hauser S, et al. 68Ga-PSMA-11 PET as a gatekeeper for the treatment of metastatic prostate cancer with 223Ra: proof of concept. J Nucl Med. 2017;58:438–444. doi: 10.2967/jnumed.116.178533. [DOI] [PubMed] [Google Scholar]
- 136.Yadav MP, Ballal S, Tripathi M, et al. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44:81–91. doi: 10.1007/s00259-016-3481-7. [DOI] [PubMed] [Google Scholar]
- 137.Rahbar K, Bode A, Weckesser M, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med. 2016;41:522–528. doi: 10.1097/RLU.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 138.Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. doi: 10.2967/jnumed.116.183194. [DOI] [PubMed] [Google Scholar]
- 139.Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of meta-static castration-resistant prostate cancer with 177Lu-labeled PSM A- 617. J Nucl Med. 2016;57:1170–1176. doi: 10.2967/jnumed.115.171397. [DOI] [PubMed] [Google Scholar]
- 140.Hohberg M, Eschner W, Schmidt M, et al. Lacrimal glands may represent organs at risk for radionuclide therapy of prostate cancer with 177Lu-DKF Z-PSM A-617. Mol Imaging Biol. 2016;18:437–445. doi: 10.1007/s11307-016-0942-0. [DOI] [PubMed] [Google Scholar]
- 141.Kabasakal L, AbuQbeitah M, Aygun A, et al. Pre-therapeutic dosimetry of normal organs and tissues of 177Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:1976–1983. doi: 10.1007/s00259-015-3125-3. [DOI] [PubMed] [Google Scholar]
- 142.Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–1013. doi: 10.2967/jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
- 143.Kratochwil C, Giesel FL, Leotta K, et al. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J Nucl Med. 2015;56:293–298. doi: 10.2967/jnumed.114.147181. [DOI] [PubMed] [Google Scholar]
- 144.Afshar-Oromieh A, Hetzheim H, Kratchwil C, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: bio-distribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56:1697–1705. doi: 10.2967/jnumed.115.161299. [DOI] [PubMed] [Google Scholar]
- 145.Sathekge M, Knoesen O, Meckel M, et al. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017 Mar 2; doi: 10.1007/s00259-017-3657-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Umbricht CA, Benesova M, Schmid RM, et al. 44Sc-PSMA-617 for radiotheranostics in tandem with 177Lu-PSM A-617 preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA- 617. EJNMMI Res. 2017;7:9. doi: 10.1186/s13550-017-0257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kiess AP, Minn I, Chen Y, et al. Auger radio-pharmaceutical therapy targeting prostate-specific membrane antigen. J Nucl Med. 2015;56:1401–1407. doi: 10.2967/jnumed.115.155929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kiess AP, Minn I, Vaidyanathan G, et al. (2S)-2-(3-(1-carboxy-5(4-211At-astatobenzamide)pentyl) ureido)-pentanedioic acid for PSMA-targeted alpha-particle radiopharmaceutical therapy. J Nucl Med. 2016;57:1569–1575. doi: 10.2967/jnumed.116.174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kratochwil C, Buchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 150.Weineisen M, Schottelius M, Simecek J, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–1176. doi: 10.2967/jnumed.115.158550. [DOI] [PubMed] [Google Scholar]
- 151.Berliner C, Tienken M, Frenzel T, et al. Detection rate of PET/CT in patients with biochemical relapse of prostate cancer using [68Ga]PSMA I&T and comparison with published data of [68Ga]PSMA HBED-CC. Eur J Nucl Med Mol Imaging. 2017;44:670–677. doi: 10.1007/s00259-016-3572-5. [DOI] [PubMed] [Google Scholar]
- 152.Okamoto S, Thieme A, Allmann J, et al. Radiation dosimetry for 177Lu-PSMA I&T in meta-static castration-resistant prostate cancer: absorbed dose in normal organs and tumor lesions. J Nucl Med. 2017;58:445–450. doi: 10.2967/jnumed.116.178483. [DOI] [PubMed] [Google Scholar]
- 153.Wright GL, Jr, Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 154.Hope TA, Truillet C, Ehman EC, et al. 68Ga-PSMA-11 PET imaging of response to androgen receptor inhibition: first human experience. J Nucl Med. 2017;58:81–84. doi: 10.2967/jnumed.116.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Reynolds TS, Bandari RP, Jiang Z, et al. Lutetium-177 labeled bombesin peptides for radionuclide therapy. Curr Radiopharm. 2016;9:33–43. doi: 10.2174/1874471008666150313112922. [DOI] [PubMed] [Google Scholar]
- 156.Panigone S, Nunn AD. Lutetium-177-labeled gastrin releasing peptide receptor binding analogs: a novel approach to radionuclide therapy. Q J Nucl Med Mol Imaging. 2006;50:310–321. [PubMed] [Google Scholar]
- 157.Dadachova E, Casadevall A. Melanin as a potential target for radionuclide therapy of metastatic melanoma. Future Oncol. 2005;1:541–549. doi: 10.2217/14796694.1.4.541. [DOI] [PubMed] [Google Scholar]
- 158.Dadachova E. Cancer therapy with alpha-emitters labeled peptides. Semin Nucl Med. 2010;40:204–208. doi: 10.1053/j.semnuclmed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 159.Herrmann K, Schottelius M, Lapa C, et al. First-in-human experience of CXCR4-directed endo-radiotherapy with 177Lu- and 90Y-labeled pentixather in advanced-stage multiple myeloma with extensive intra- and extramedullary disease. J Nucl Med. 2016;57:248–251. doi: 10.2967/jnumed.115.167361. [DOI] [PubMed] [Google Scholar]
- 160.Norain A, Dadachova E. Targeted radionuclide therapy of melanoma. Semin Nucl Med. 2016;46:250–259. doi: 10.1053/j.semnuclmed.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 161.Miao Y, Quinn TP. Peptide-targeted radionuclide therapy for melanoma. Crit Rev Oncol Hematol. 2008;67:213–228. doi: 10.1016/j.critrevonc.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nock BA, Kaloudi A, Lymperis E, et al. Theranostic perspectives in prostate cancer with the gastrin-releasing peptide receptor antagonist NeoBOMB1: preclinical and first clinical results. J Nucl Med. 2017;58:75–80. doi: 10.2967/jnumed.116.178889. [DOI] [PubMed] [Google Scholar]
- 163.Müller C, Schilbil R. Prospects in folate receptor-targeted radionuclide therapy. Front Oncol. 2013;3:249. doi: 10.3389/fonc.2013.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guerard F, Barbel J, Chatal JF, et al. Which radionuclide, carrier molecule and clinical indication for alpha-immunotherapy? Q J Nucl Med Mol Imaging. 2015;59:161–167. [PubMed] [Google Scholar]
- 165.Touchefeu Y, Franken P, Harrington KJ. Radiovirotherapy: principles and prospects in oncology. Curr Pharm Des. 2012;18:3313–3320. doi: 10.2174/1381612811209023313. [DOI] [PubMed] [Google Scholar]
- 166.Fahey F, Zukotynski K, Capala J, et al. Targeted radionuclide therapy: proceedings of a joint workshop hosted by the National Cancer Institute and the Society of Nuclear Medicine and Molecular Imaging. J Nucl Med. 2014;55:337–348. doi: 10.2967/jnumed.113.135178. [DOI] [PubMed] [Google Scholar]
- 167.Fahey F, Zukotynski K, Jadvar H, et al. Proceedings of the second NCI-SNMMI workshop on targeted radionuclide therapy. J Nucl Med. 2015;56:1119–1129. doi: 10.2967/jnumed.115.159038. [DOI] [PubMed] [Google Scholar]


