Abstract
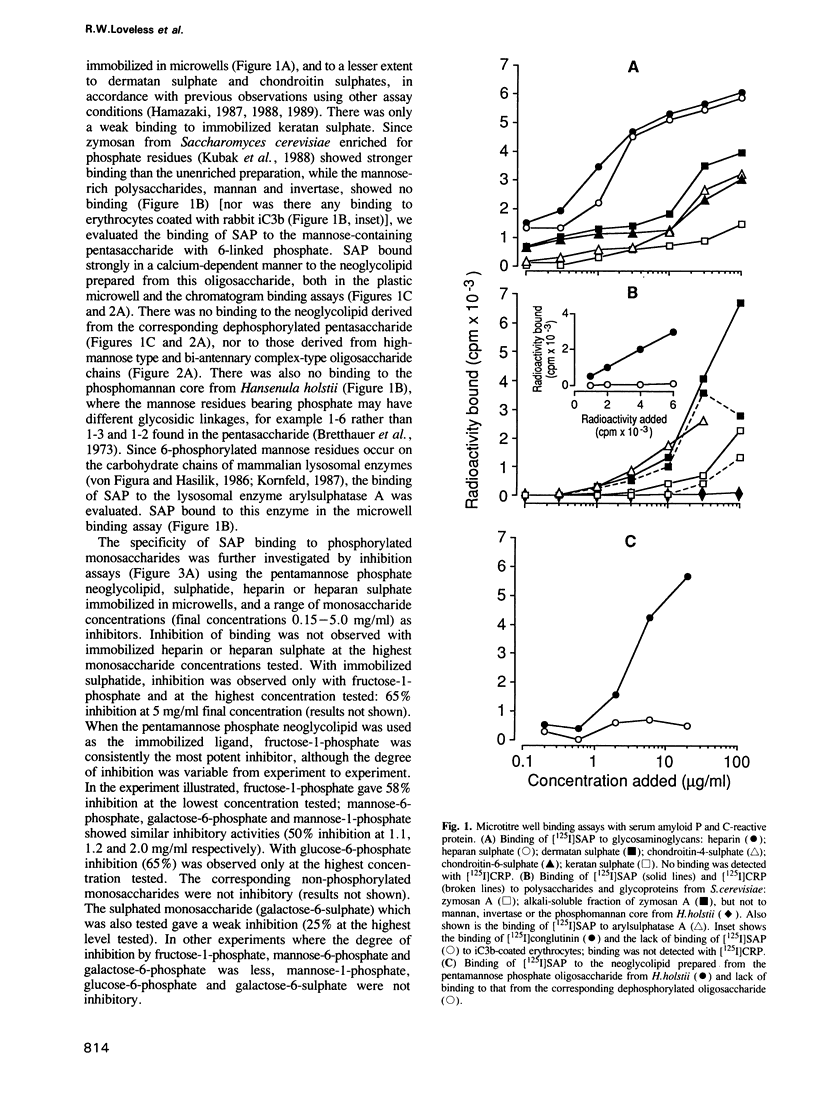
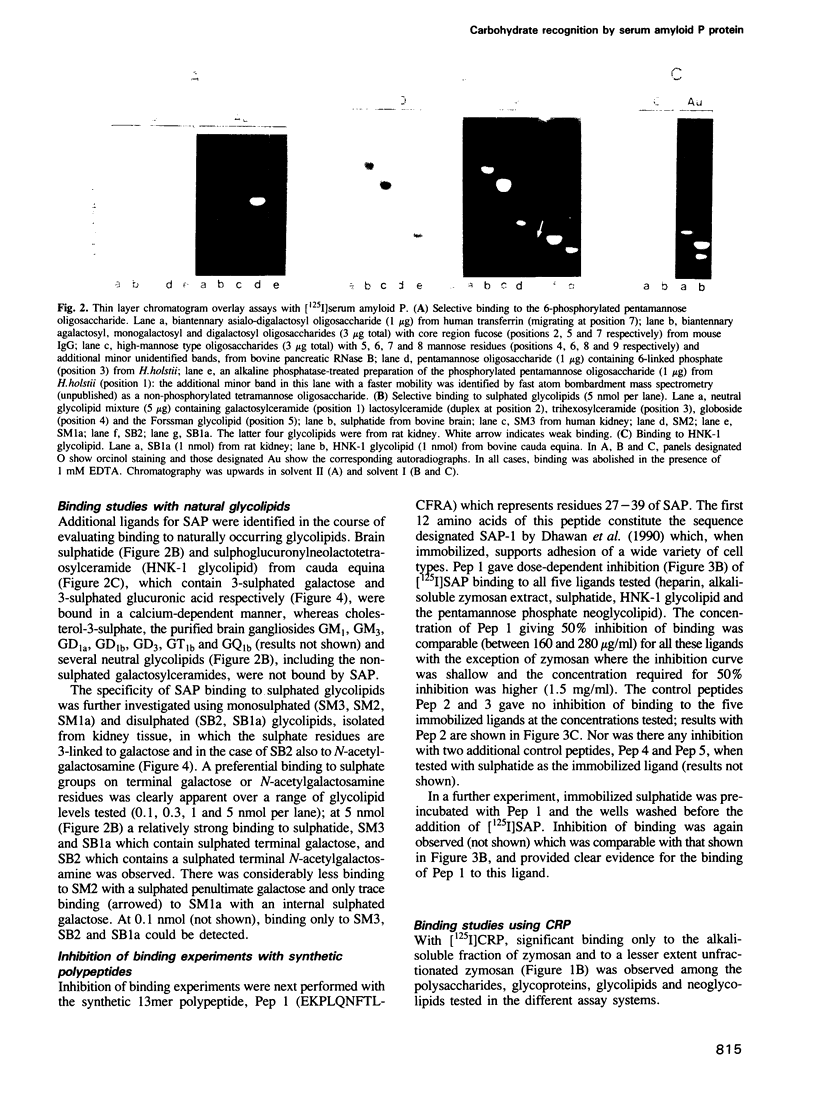
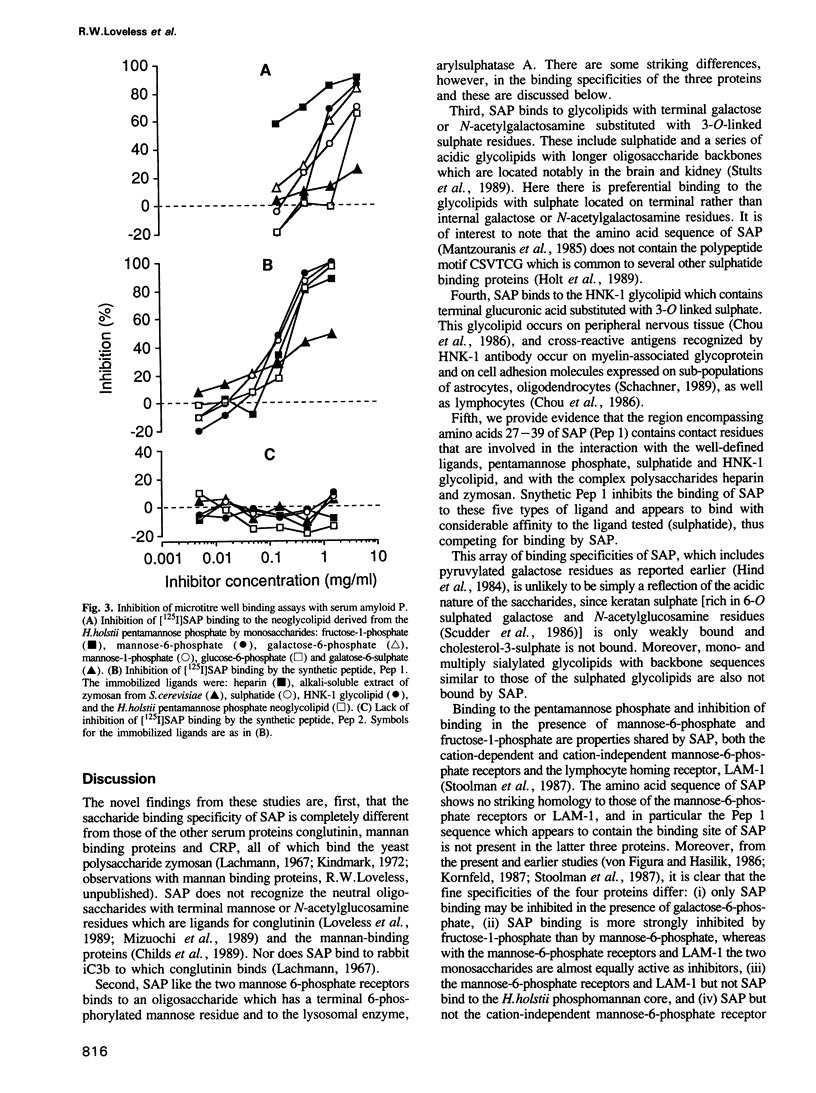
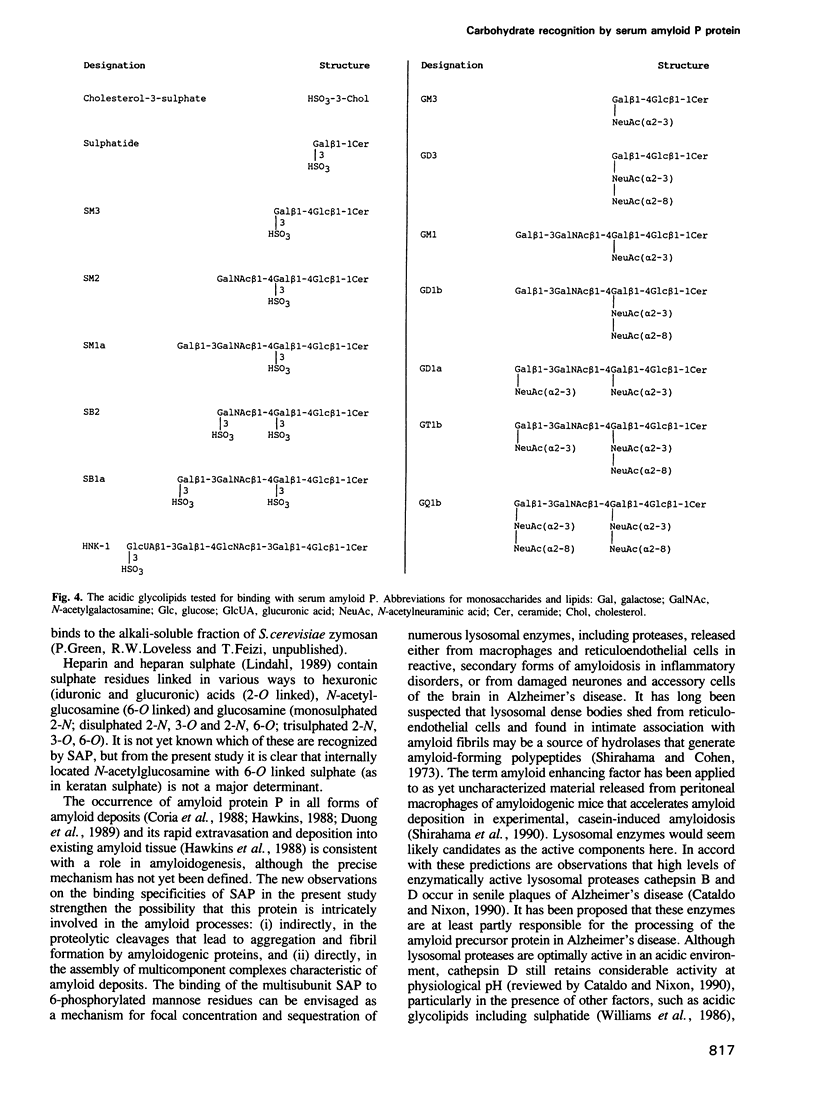
Carbohydrate recognition by amyloid P component from human serum has been investigated by binding experiments using several glycosaminoglycans, polysaccharides and a series of structurally defined neoglycolipids and natural glycolipids. Two novel classes of carbohydrate ligands have been identified. The first is 6-phosphorylated mannose as found on lysosomal hydrolases, and the second is the 3-sulphated saccharides galactose, N-acetyl-galactosamine and glucuronic acid as found on sulphatide and other acidic glycolipids that occur in neural or kidney tissues or on subpopulations of lymphocytes. Binding to mannose-6-phosphate containing molecules and inhibition of binding by free mannose-6-phosphate and fructose-1-phosphate are features shared with mannose-6-phosphate receptors involved in trafficking of lysosomal enzymes. However, only amyloid P binding is inhibited by galactose-6-phosphate, mannose-1-phosphate and glucose-6-phosphate. These findings strengthen the possibility that amyloid P protein has a central role in amyloidogenic processes: first in formation of focal concentrations of lysosomal enzymes including proteases that generate fibril-forming peptides from amyloidogenic proteins, and second in formation of multicomponent complexes that include sulphoglycolipids as well as glycosaminoglycans. The evidence that binding to all of the acidic ligands involves the same polypeptide domain on amyloid P protein, and inhibition data using diffusible, phosphorylated monosaccharides, is potentially important leads to novel drug designs aimed at preventing or even reversing amyloid deposition processes without interference with essential lysosomal trafficking pathways.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breathnach S. M., Melrose S. M., Bhogal B., de Beer F. C., Dyck R. F., Tennent G., Black M. M., Pepys M. B. Amyloid P component is located on elastic fibre microfibrils in normal human tissue. Nature. 1981 Oct 22;293(5834):652–654. doi: 10.1038/293652a0. [DOI] [PubMed] [Google Scholar]
- Bretthauer R. K., Kaczorowski G. J., Weise M. J. Characterization of a phosphorylated pentasaccharide isolated from Hansenula holstii NRRL Y-2448 phosphomannan. Biochemistry. 1973 Mar 27;12(7):1251–1256. doi: 10.1021/bi00731a002. [DOI] [PubMed] [Google Scholar]
- Cataldo A. M., Nixon R. A. Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc Natl Acad Sci U S A. 1990 May;87(10):3861–3865. doi: 10.1073/pnas.87.10.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs R. A., Drickamer K., Kawasaki T., Thiel S., Mizuochi T., Feizi T. Neoglycolipids as probes of oligosaccharide recognition by recombinant and natural mannose-binding proteins of the rat and man. Biochem J. 1989 Aug 15;262(1):131–138. doi: 10.1042/bj2620131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou D. K., Ilyas A. A., Evans J. E., Costello C., Quarles R. H., Jungalwala F. B. Structure of sulfated glucuronyl glycolipids in the nervous system reacting with HNK-1 antibody and some IgM paraproteins in neuropathy. J Biol Chem. 1986 Sep 5;261(25):11717–11725. [PubMed] [Google Scholar]
- Coria F., Castaño E., Prelli F., Larrondo-Lillo M., van Duinen S., Shelanski M. L., Frangione B. Isolation and characterization of amyloid P component from Alzheimer's disease and other types of cerebral amyloidosis. Lab Invest. 1988 Apr;58(4):454–458. [PubMed] [Google Scholar]
- De Beer F. C., Pepys M. B. Isolation of human C-reactive protein and serum amyloid P component. J Immunol Methods. 1982;50(1):17–31. doi: 10.1016/0022-1759(82)90300-3. [DOI] [PubMed] [Google Scholar]
- Dhawan S., Fields R. L., Robey F. A. A novel peptide from amyloid P component supports cell attachment. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1284–1290. doi: 10.1016/0006-291x(90)90825-8. [DOI] [PubMed] [Google Scholar]
- Drickamer K., Dordal M. S., Reynolds L. Mannose-binding proteins isolated from rat liver contain carbohydrate-recognition domains linked to collagenous tails. Complete primary structures and homology with pulmonary surfactant apoprotein. J Biol Chem. 1986 May 25;261(15):6878–6887. [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Duong T., Pommier E. C., Scheibel A. B. Immunodetection of the amyloid P component in Alzheimer's disease. Acta Neuropathol. 1989;78(4):429–437. doi: 10.1007/BF00688180. [DOI] [PubMed] [Google Scholar]
- Dyck R. F., Lockwood C. M., Kershaw M., McHugh N., Duance V. C., Baltz M. L., Pepys M. B. Amyloid P-component is a constituent of normal human glomerular basement membrane. J Exp Med. 1980 Nov 1;152(5):1162–1174. doi: 10.1084/jem.152.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Day L. E., Herman G. A. A human mannose-binding protein is an acute-phase reactant that shares sequence homology with other vertebrate lectins. J Exp Med. 1988 Mar 1;167(3):1034–1046. doi: 10.1084/jem.167.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooi H. C., Picard J. K., Hounsell E. F., Gregoriou M., Rees A. R., Feizi T. Monoclonal antibody (EGR/G49) reactive with the epidermal growth factor receptor of A431 cells recognizes the blood group ALeb and ALey structures. Mol Immunol. 1985 Jun;22(6):689–693. doi: 10.1016/0161-5890(85)90099-9. [DOI] [PubMed] [Google Scholar]
- Hamazaki H. Ca2+-mediated association of human serum amyloid P component with heparan sulfate and dermatan sulfate. J Biol Chem. 1987 Feb 5;262(4):1456–1460. [PubMed] [Google Scholar]
- Hamazaki H. Calcium-dependent polymerization of human serum amyloid P component is inhibited by heparin and dextran sulfate. Biochim Biophys Acta. 1989 Oct 19;998(3):231–235. doi: 10.1016/0167-4838(89)90279-3. [DOI] [PubMed] [Google Scholar]
- Hamazaki H. Calcium-mediated hemagglutination by serum amyloid P component and the inhibition by specific glycosaminoglycans. Biochem Biophys Res Commun. 1988 Jan 15;150(1):212–218. doi: 10.1016/0006-291x(88)90507-4. [DOI] [PubMed] [Google Scholar]
- Hawkins P. N. Amyloidosis. Blood Rev. 1988 Dec;2(4):270–280. doi: 10.1016/0268-960x(88)90016-1. [DOI] [PubMed] [Google Scholar]
- Hawkins P. N., Myers M. J., Epenetos A. A., Caspi D., Pepys M. B. Specific localization and imaging of amyloid deposits in vivo using 123I-labeled serum amyloid P component. J Exp Med. 1988 Mar 1;167(3):903–913. doi: 10.1084/jem.167.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind C. R., Collins P. M., Renn D., Cook R. B., Caspi D., Baltz M. L., Pepys M. B. Binding specificity of serum amyloid P component for the pyruvate acetal of galactose. J Exp Med. 1984 Apr 1;159(4):1058–1069. doi: 10.1084/jem.159.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt G. D., Krivan H. C., Gasic G. J., Ginsburg V. Antistasin, an inhibitor of coagulation and metastasis, binds to sulfatide (Gal(3-SO4) beta 1-1Cer) and has a sequence homology with other proteins that bind sulfated glycoconjugates. J Biol Chem. 1989 Jul 25;264(21):12138–12140. [PubMed] [Google Scholar]
- Hutchcraft C. L., Gewurz H., Hansen B., Dyck R. F., Pepys M. B. Agglutination of complement-coated erythrocytes by serum amyloid P-component. J Immunol. 1981 Mar;126(3):1217–1219. [PubMed] [Google Scholar]
- Ishizuka I., Tadano K. The sulfoglycolipid, highly acidic amphiphiles of mammalian renal tubules. Adv Exp Med Biol. 1982;152:195–214. [PubMed] [Google Scholar]
- Kawasaki N., Kawasaki T., Yamashina I. Isolation and characterization of a mannan-binding protein from human serum. J Biochem. 1983 Sep;94(3):937–947. doi: 10.1093/oxfordjournals.jbchem.a134437. [DOI] [PubMed] [Google Scholar]
- Kawasaki N., Kawasaki T., Yamashina I. Mannan-binding protein and conglutinin in bovine serum. J Biochem. 1985 Nov;98(5):1309–1320. doi: 10.1093/oxfordjournals.jbchem.a135398. [DOI] [PubMed] [Google Scholar]
- Kindmark C. O. In vitro binding of human C-reactive protein by some pathogenic bacteria and zymosan. Clin Exp Immunol. 1972 Jun;11(2):283–289. [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987 Dec;1(6):462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- Kubak B. M., Potempa L. A., Anderson B., Mahklouf S., Venegas M., Gewurz H., Gewurz A. T. Evidence that serum amyloid P component binds to mannose-terminated sequences of polysaccharides and glycoproteins. Mol Immunol. 1988 Sep;25(9):851–858. doi: 10.1016/0161-5890(88)90121-6. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J. Conglutinin and immunoconglutinins. Adv Immunol. 1967;6:479–527. doi: 10.1016/s0065-2776(08)60527-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loveless R. W., Feizi T., Childs R. A., Mizuochi T., Stoll M. S., Oldroyd R. G., Lachmann P. J. Bovine serum conglutinin is a lectin which binds non-reducing terminal N-acetylglucosamine, mannose and fucose residues. Biochem J. 1989 Feb 15;258(1):109–113. doi: 10.1042/bj2580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majocha R. E., Jungalwala F. B., Rodenrys A., Marotta C. A. Monoclonal antibody to embryonic CNS antigen A2B5 provides evidence for the involvement of membrane components at sites of Alzheimer degeneration and detects sulfatides as well as gangliosides. J Neurochem. 1989 Sep;53(3):953–961. doi: 10.1111/j.1471-4159.1989.tb11798.x. [DOI] [PubMed] [Google Scholar]
- Mantzouranis E. C., Dowton S. B., Whitehead A. S., Edge M. D., Bruns G. A., Colten H. R. Human serum amyloid P component. cDNA isolation, complete sequence of pre-serum amyloid P component, and localization of the gene to chromosome 1. J Biol Chem. 1985 Jun 25;260(12):7752–7756. [PubMed] [Google Scholar]
- Mizuochi T., Loveless R. W., Lawson A. M., Chai W., Lachmann P. J., Childs R. A., Thiel S., Feizi T. A library of oligosaccharide probes (neoglycolipids) from N-glycosylated proteins reveals that conglutinin binds to certain complex-type as well as high mannose-type oligosaccharide chains. J Biol Chem. 1989 Aug 15;264(23):13834–13839. [PubMed] [Google Scholar]
- Mårtensson E. Sulfatides of human kidney. Isolation, identification, and fatty acid composition. Biochim Biophys Acta. 1966 Jun 1;116(3):521–531. doi: 10.1016/0005-2760(66)90122-6. [DOI] [PubMed] [Google Scholar]
- Ohkubo I., Sahashi W., Namikawa C., Tsukada K., Takeuchi T., Sasaki M. A procedure for large scale purification of human plasma amyloid P component. Clin Chim Acta. 1986 May 30;157(1):95–101. doi: 10.1016/0009-8981(86)90322-0. [DOI] [PubMed] [Google Scholar]
- Oka S., Ikeda K., Kawasaki T., Yamashina I. Isolation and characterization of two distinct mannan-binding proteins from rat serum. Arch Biochem Biophys. 1988 Jan;260(1):257–266. doi: 10.1016/0003-9861(88)90448-1. [DOI] [PubMed] [Google Scholar]
- Osmand A. P., Friedenson B., Gewurz H., Painter R. H., Hofmann T., Shelton E. Characterization of C-reactive protein and the complement subcomponent C1t as homologous proteins displaying cyclic pentameric symmetry (pentraxins). Proc Natl Acad Sci U S A. 1977 Feb;74(2):739–743. doi: 10.1073/pnas.74.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Dyck R. F., de Beer F. C., Skinner M., Cohen A. S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979 Nov;38(2):284–293. [PMC free article] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Schachner M. Families of neural adhesion molecules. Ciba Found Symp. 1989;145:156-69, discussion 169-72. doi: 10.1002/9780470513828.ch10. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Saitoh T., Cole G. Characterization of an amyloid beta precursor protein that binds heparin and contains tyrosine sulfate. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2066–2069. doi: 10.1073/pnas.86.6.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder P., Tang P. W., Hounsell E. F., Lawson A. M., Mehmet H., Feizi T. Isolation and characterization of sulphated oligosaccharides released from bovine corneal keratan sulphate by the action of endo-beta-galactosidase. Eur J Biochem. 1986 Jun 2;157(2):365–373. doi: 10.1111/j.1432-1033.1986.tb09678.x. [DOI] [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. An analysis of the close relationship of lysosomes to early deposits of amyloid. Ultrastructural evidence in experimental mouse amyloidosis. Am J Pathol. 1973 Oct;73(1):97–114. [PMC free article] [PubMed] [Google Scholar]
- Shirahama T., Miura K., Ju S. T., Kisilevsky R., Gruys E., Cohen A. S. Amyloid enhancing factor-loaded macrophages in amyloid fibril formation. Lab Invest. 1990 Jan;62(1):61–68. [PubMed] [Google Scholar]
- Skinner M., Cohen A. S. Amyloid P component. Methods Enzymol. 1988;163:523–536. doi: 10.1016/0076-6879(88)63048-5. [DOI] [PubMed] [Google Scholar]
- Slodki M. E., Ward R. M., Boundy J. A. Concanavalin A as a probe of phosphomannan molecular structure. Biochim Biophys Acta. 1973 Apr 28;304(2):449–456. doi: 10.1016/0304-4165(73)90264-x. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Willmer J., Kisilevsky R. Sulfated glycosaminoglycans: a common constituent of all amyloids? Lab Invest. 1987 Jan;56(1):120–123. [PubMed] [Google Scholar]
- Stoll M. S., Mizuochi T., Childs R. A., Feizi T. Improved procedure for the construction of neoglycolipids having antigenic and lectin-binding activities, from reducing oligosaccharides. Biochem J. 1988 Dec 1;256(2):661–664. doi: 10.1042/bj2560661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolman L. M., Yednock T. A., Rosen S. D. Homing receptors on human and rodent lymphocytes--evidence for a conserved carbohydrate-binding specificity. Blood. 1987 Dec;70(6):1842–1850. [PubMed] [Google Scholar]
- Stults C. L., Sweeley C. C., Macher B. A. Glycosphingolipids: structure, biological source, and properties. Methods Enzymol. 1989;179:167–214. doi: 10.1016/0076-6879(89)79122-9. [DOI] [PubMed] [Google Scholar]
- Tadano K., Ishizuka I. Bis-sulfoglycosphingolipid containing a unique 3-O-sulfated N-acetylgalactosamine from rat kidney. J Biol Chem. 1982 Aug 25;257(16):9294–9299. [PubMed] [Google Scholar]
- Tadano K., Ishizuka I. Isolation and characterization of the sulfated gangliotriaosylceramide from rat kidney. J Biol Chem. 1982 Feb 10;257(3):1482–1490. [PubMed] [Google Scholar]
- Tadano K., Ishizuka I., Matsuo M., Matsumoto S. Bis-sulfated gangliotetraosylceramide from rat kidney. J Biol Chem. 1982 Nov 25;257(22):13413–13420. [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Williams K. R., Williams N. D., Konigsberg W., Yu R. K. Acidic lipids enhance cathepsin D cleavage of the myelin basic protein. J Neurosci Res. 1986;15(2):137–145. doi: 10.1002/jnr.490150203. [DOI] [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]




