Abstract
Resurgence is defined as an increase in the frequency of a previously reinforced target response when an alternative source of reinforcement is suspended. Despite an extensive body of research examining factors that affect resurgence, the effects of alternative-reinforcer magnitude have not been examined. Thus, the present experiments aimed to fill this gap in the literature. In Experiment 1, rats pressed levers for single-pellet reinforcers during Phase 1. In Phase 2, target-lever pressing was extinguished, and alternative-lever pressing produced either five-pellet, one-pellet, or no alternative reinforcement. In Phase 3, alternative reinforcement was suspended to test for resurgence. Five-pellet alternative reinforcement produced faster elimination and greater resurgence of target-lever pressing than one-pellet alternative reinforcement. In Experiment 2, effects of decreasing alternative-reinforcer magnitude on resurgence were examined. Rats pressed levers and pulled chains for six-pellet reinforcers during Phases 1 and 2, respectively. In Phase 3, alternative reinforcement was decreased to three pellets for one group, one pellet for a second group, and suspended altogether for a third group. Shifting from six-pellet to one-pellet alternative reinforcement produced as much resurgence as suspending alternative reinforcement altogether, while shifting from six pellets to three pellets did not produce resurgence. These results suggest that alternative-reinforcer magnitude has effects on elimination and resurgence of target behavior that are similar to those of alternative-reinforcer rate. Thus, both suppression of target behavior during alternative reinforcement and resurgence when conditions of alternative reinforcement are altered may be related to variables that affect the value of the alternative-reinforcement source.
Keywords: resurgence, relapse, reinforcer magnitude, extinction, operant behavior
Differential reinforcement of alternative behavior (DRA; see Petscher, Rey, & Bailey, 2009) often is used to eliminate problematic behavior in clinical settings. This intervention strategy entails suspending reinforcement for a target problem behavior while concurrently providing reinforcement for an alternative, socially desirable behavior. Interventions that incorporate DRA typically produce suppression of target behavior so long as treatment contingencies remain in place. When these contingencies are altered in some way(e.g., a lapse in treatment fidelity occurs or treatment is suspended altogether), however, problem behavior is susceptible to relapse (see, e.g., Volkert, Lerman, Call, & Trosclair-Lasserre, 2009; Wacker et al., 2013). Recurrence of previously extinguished behavior following suspension of an alternative source of reinforcement that was made available during extinction is termed resurgence (Epstein, 1983; 1985). Given that resurgence presents a clinically significant challenge to maintenance of positive treatment outcomes following DRA-based interventions, an understanding of variables that affect resurgence could inform applications of these interventions.
In the laboratory, resurgence often is examined using a three-phase procedure (see Leitenberg, Rawson, & Bath, 1970; Leitenberg, Rawson, & Mulick, 1975). In Phase 1, a target response is trained, analogous to reinforcement of problem behavior prior to introduction of DRA-based interventions. Next, during Phase 2, reinforcement is suspended for the target response, and an alternative source of reinforcement is made available contingently on an alternative response. Finally, to test for resurgence in Phase 3, contingencies of alternative reinforcement are changed, usually by suspending alternative reinforcement. Resurgence is said to occur if target behavior increases in frequency relative to terminal Phase-2 response rates. An ‘inactive’ response (i.e., a response alternative never associated with reinforcement) often is available in all three phases of the resurgence preparation. The purpose of this response is to help determine whether an increase in target responding during Phase 3 represents a focal increase in previously reinforced behavior (i.e., resurgence) or a general increase in activity produced by extinction of the alternative behavior.
Using the procedure detailed above, a considerable body of basic and translational research has been dedicated to determining factors that produce resurgence and that affect the magnitude of resurgence when it occurs (for reviews, see Bloom & Lambert, 2015; Lattal & St. Peter Pipkin, 2009; Podle-snik & Kelley, 2015). One of the most extensively examined of these parameters is the rate at which alternative reinforcement is delivered. Craig and Shahan (2016), for example, trained groups of rats to press a target lever for food reinforcement on either variable-interval (VI) 15-s (a relatively high rate) or VI 60-s (a relatively low-rate) schedules of reinforcement during Phase 1. Next, during Phase 2, lever pressing was extinguished and an alternative nose-poke response was made available. Groups of rats received VI 15-s, VI 60-s, or no (an extinction control) alternative reinforcement for nose poking. Finally, alternative reinforcement was suspended for all groups in Phase 3 to test for resurgence. Lever pressing was eliminated more quickly and to a greater extent in groups of rats that received high-rate relative to groups that received low-rate alternative reinforcement during Phase 2. Further, when alternative reinforcement was suspended in Phase 3, resurgence of lever pressing occurred in the high-rate alternative-reinforcement groups, while no resurgence of target-lever pressing was observed in groups that received low-rate alternative reinforcement (for similar findings, see Bouton & Trask, 2015; Craig, Nall, Madden, & Shahan, 2016; Leitenberg, Rawson, & Mulick, 1975; Sweeney & Shahan, 2013; but see also Can-çado & Lattal, 2013; Fujimaki, Lattal, & Saka-gami, 2015; for possible exceptions). Thus, high-rate alternative reinforcement appears to more successfully eliminate target behavior than low-rate alternative reinforcement, but resurgence is more likely following elimination of high-rate alternative reinforcement.
Furthermore, in the absence of complete removal of alternative reinforcement, reductions from higher rate to lower, nonzero rates of alternative reinforcement appear to be associated with resurgence. For example, reductions from initially high-rate to lower-rate alternative reinforcement encountered as a part of alternative reinforcement-rate “thinning” generate increases in target responding in rats and in humans with developmental disabilities (Bouton & Schepers, 2015; Volkert, Lerman, Call, & Trosclair-Lasserre, 2009; see Sweeney & Shahan, 2011, for review). Finally there is some evidence that resurgence could be related to the degree to which alternative reinforcement rate is reduced. For example, Lieving and Lattal (2003) found that a shift from high-rate alternative reinforcement delivered on a VI 30-s schedule to a VI 360-s schedule produced some resurgence of pigeons' key pecking, but the increase in responding was less than in a subsequent condition where alternative reinforcement was completely removed.
Despite the considerable emphasis placed on examining alternative-reinforcer rate effects on response suppression and resurgence in the basic laboratory, dimensions of alternative reinforcement other than rate often are sometimes manipulated to achieve therapeutic outcomes in application. Alternative-reinforcer magnitude is one such dimension. Increasing alternative-reinforcer magnitude has been shown to promote alternative behavior that occurs at a higher rate and that is more persistent in the face of challenges in children with developmental disabilities (e.g., Lerman, Kelley, Vorndran, Kuhn, & LaRue, 2002; Trosclair-Lasserre, Lerman, Call, Addison, & Kodak, 2008). Some limited evidence suggests that, in these populations, large-magnitude alternative reinforcement also promotes faster elimination of problem behavior than small-magnitude alternative reinforcement (Lerman, Kelley, Van Camp, & Roane, 1999). Further, in contingency-management interventions for substance-use disorders (for review, see Higgins & Petry, 1999; Stitzer & Petry, 2015), the duration of abstinence from drug taking achieved during treatment has been shown to be positively related to the magnitude of alternative reinforcement arranged for such abstinence (Higgins et al., 2007; Petry, Barry, Alessi, Rounsaville, & Carroll, 2012; Silverman, Chutuape, Bigelow, & Stitzer, 1999). That is, incentives with larger monetary value tend to produce more sustained abstinence from drug taking than incentives with smaller monetary value.
Although alternative-reinforcer magnitude is a commonly manipulated component of differential-reinforcement based treatments, nothing is known about the effects of alternative-reinforcer magnitude on resurgence. The present experiments aimed to fill this gap in the literature. Experiment 1 examined the effects of different magnitudes of alternative reinforcement during Phase 2 on resurgence in Phase 3 when alternative reinforcement was removed. Experiment 2 examined whether reductions in the magnitude of alternative reinforcement from Phase 2 to Phase 3 would generate resurgence.
Experiment 1
Experiment 1 was designed to determine if variations in alternative-reinforcer magnitude affect resurgence. Three groups of rats pressed target levers for single food-pellet reinforcers during Phase 1. Next, during Phase 2, target-lever pressing was placed on extinction, an alternative-response lever became available, and pressing this lever produced either small-magnitude (one-pellet), large-magnitude (five-pellet), or no (an extinction control) alternative reinforcement. Finally, alternative reinforcement was suspended during Phase 3 to test for resurgence. Because Craig and Shahan (2016) showed that VI 60-s single-pellet alternative reinforcement produced no resurgence, this schedule was used here to determine whether increasing alternative-reinforcer magnitude would generate resurgence under these conditions.
Method
Subjects
Thirty male Long-Evans rats (Harlan, Indianapolis, IN), approximately 90 days of age at the beginning of the experiment, served. Rats were individually housed in a temperature-controlled colony room with a 12:12 light/dark cycle. Rats were maintained at 80% their free-feeding body weights and had free access to water in the home cages. Animal housing and care, and all procedures reported below were conducted in accordance with Utah State University's Intuitional Animal Care and Use Committee.
Apparatus
Four modular Med-Associates (St. Albans, VT) operant chambers (30 cm × 24 cm × 21 cm) housed in sound-attenuating cubicles were used. Two retractable levers on the front wall, with stimulus lights above them, were positioned on either side of a food receptacle. A house light situated above this receptacle was used for general chamber illumination. A third lever and stimulus light were positioned on the center of the back wall. All reinforcer deliveries consisted of a10-s blackout of the chamber and delivery of 45-mg food pellets (BioServ, Flemington, NJ) into the illuminated food receptacle. When multiple food pellets were delivered, as detailed below, one pellet was delivered every 0.5 s. All experimental events and data collection were controlled by Med-PC (Med-Associates) software run on a PC computer in an adjoining control room.
Procedure
Magazine training
Rats first were trained to consume food pellets from the lit food receptacle. Single food pellets were delivered response independently according to a variable-time (VT) 60-s schedule for three 30-min sessions (session durations for the remainder of the experiment were also 30 min), excluding time for reinforcement. All VT and VI schedules were constructed of 10 intervals derived from Fleshler and Hoffman's (1962) constant-probability distribution. Levers were retracted and all stimulus lights remained off throughout magazine training.
Phase 1
During Phase 1, sessions began with insertion of the two front levers and illumination of the house light. The stimulus light situated above the target lever (left–right counterbalanced) was illuminated and presses to the target lever produced a single food pellet according to a VI 60-s schedule. Responses to the other, inactive, lever were recorded but had no programmed consequences. During the first session of Phase 1, the first response to the target lever produced a reinforcer to facilitate acquisition of target-lever pressing, after which the VI schedule commenced. Phase 1 lasted 20 sessions, after which rats were divided into three groups such that mean response rates across the last 5 sessions of Phase 1 were equivalent between groups.
Phase 2
During Phase 2, sessions began with insertion of both front levers and the lever located on the rear wall of the chamber (hereafter the alternative lever). Further, the house light and the stimulus lights positioned above the target and alternative levers were illuminated. Reinforcement for target responses was suspended, and responses on the alternative lever produced reinforcers according to a VI 60-s schedule. Rats in the 5-Pellet group (n = 10) received five pellets (one pellet delivered every 0.5 s), and rats in the 1-Pellet group (n = 10) received one pellet, per reinforcer delivery. Alternative responses were recorded but had no consequence for rats in the No-Alt. group (n = 9, an additional rat was excluded from this group due to an equipment malfunction). For rats that received alternative reinforcement, the first lever press to the alternative lever during the first session of Phase 2 produced a reinforcer to facilitate acquisition, after which the VI schedule commenced. This phase lasted for 15 sessions.
Phase 3
Sessions of Phase 3 began in an identical manner to sessions of Phase 2. Alternative reinforcement for the 5- and 1-Pellet groups was suspended while contingencies for the No-Alt. group were unchanged. This phase lasted five sessions.
Data analyses
All statistical analyses reported below were deemed significant at an a level of .05. When the assumption of sphericity in analyses of variance (ANOVA) was violated, Greenhouse-Geisser corrections to degrees of freedom were used.
Results
Phase 1
Target-lever pressing increased across sessions of Phase 1 to comparable levels for all groups during the final five sessions of the phase. Further, reinforcer rates were similar between groups in these final sessions. Inactive responding occurred at near-zero levels for all groups. See Table 1 for a summary of response and reinforcer rates for each phase of the experiment.
Table 1. Summary of mean response and reinforcer rates from each phase of Experiment 1.
| Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 5 Pellet | 1 Pellet | No Alt. | |||||||
|
|
|
|
|||||||
| Phase 1a | Phase 2b | Phase 3c | Phase 1a | Phase 2b | Phase 3c | Phase 1a | Phase 2b | Phase 3c | |
| Target/Min | 19.97 | 0.92 | 2.02 | 19.62 | 1.55 | 1.46 | 20.23 | 0.50 | 0.51 |
| (SEM) | (3.07) | (0.52) | (0.89) | (2.70) | (0.47) | (0.34) | (4.43) | (0.09) | (0.12) |
| Alt./Min | - | 14.74 | 18.16 | - | 13.09 | 8.88 | - | 0.14 | 0.18 |
| (SEM) | - | (4.66) | (5.37) | - | (3.03) | (1.59) | - | (0.07) | (0.13) |
| Inactive/Min | 0.44 | 0.49 | 0.68 | 1.46 | 0.59 | 0.7 | 0.70 | 0.27 | 0.13 |
| (SEM) | (0.14) | (0.22) | (0.20) | (0.88) | (0.27) | (0.29) | (0.29) | (0.07) | (0.03) |
| Sr /Min | 0.92 | 0.84 | - | 0.91 | 0.86 | - | 0.91 | - | - |
| (SEM) | (0.01) | (0.02) | - | (0.01) | (0.02) | - | (0.01) | - | - |
| Pellets/Min | 0.92 | 4.20 | - | 0.91 | 0.86 | - | 0.91 | - | - |
| (SEM) | (0.01) | (0.11) | - | (0.01) | (0.02) | - | (0.01) | - | - |
Data from the last five sessions of Phase 1 are shown.
Data from the last session of Phase 2 are shown.
Data from the first session of Phase 3 are shown.
Note: “Sr/Min” refers to the number of reinforcement periods per min earned by each group, while “Pellets/Min” is this value multiplied by the magnitude of reinforcement each group earned.
Phase 2
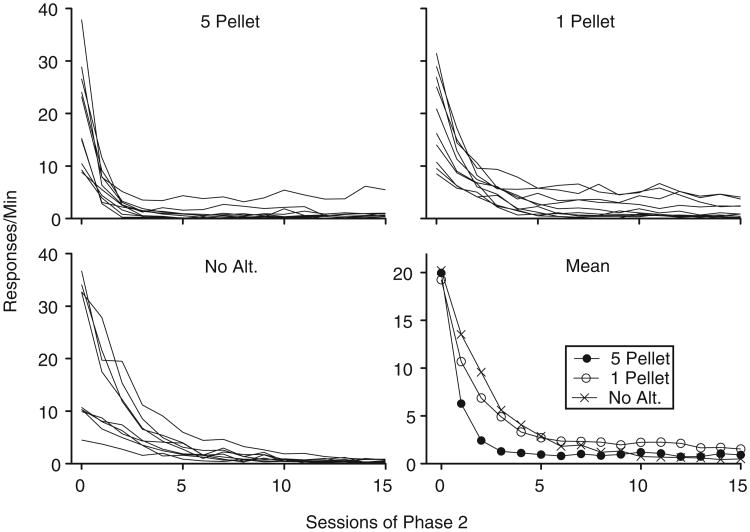
Figure 1 shows mean rates of target-lever pressing during the final five sessions of Phase 1 (labeled Session “0”) and all sessions of Phase 2 for individual subjects and at the group level. Target responding decreased across sessions of Phase 2 for all groups. Across sessions of Phase 2, considerably more variability in target-response rates was present in the 1-Pellet group than in the other groups. Early in Phase 2, responding appeared to decrease faster for the 5-Pellet group than for the 1-Pellet and No-Alt. groups. In addition, responding for roughly half the rats in the 1-Pellet group appeared more persistent than for rats in the No-Alt. group. Statistical assessment of the group-level performance using a 3 × 15 (Group × Session) mixed-model ANOVA showed significant main effects of Group, F(2, 26) = 3.71, MSE = 148.52, and Session, F(1.48, 38.55) = 75.96, MSE = 1752.79, and a Group × Session interaction, F(2.96, 38.55) = 6.95, MSE= 160.26. To determine the source of the interaction, follow-up 2 × 15 (Group × Session) ANOVA were conducted for each pairwise comparison of Group. The interaction term remained significant when responding from the 5-Pellet group was compared to the 1-Pellet, F(2.19, 39.43) =8.51, MSE =52.66, and No-Alt, F (1.33, 22.67) = 10.83, MSE= 352.06, groups. The interaction did not reach statistical significance when responding from the 1-Pellet group was compared to responding from the No-Alt. group, F(1.33, 22.67) = 10.83, p = .09, MSE = 352.06. Finally, response rates across groups were similar in the last session of Phase 2, a conclusion supported by a nonsignificant one-way ANOVA F(2, 26) = 1.53, MSE = 2.65.
Fig. 1.
Target-lever response rates across sessions of Phase 2 in Experiment 1. Data from individual subjects within each group and mean response rates from these sessions aggregated across subjects are shown.
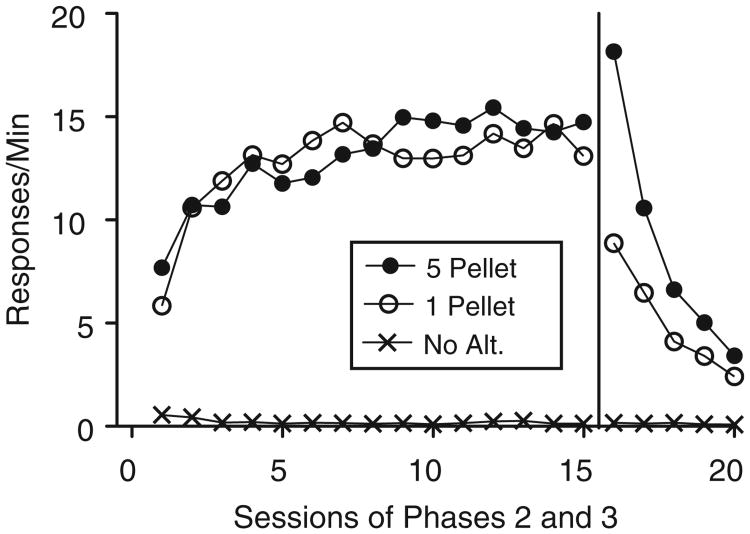
Alternative responding increased at comparable rates across sessions of Phase 2 for the 5-Pellet and 1-Pellet groups and remained low for the No-Alt. group (see Fig. 2). There was no difference in alternative responding on the final session of Phase 2 for the groups that received alternative reinforcement. Alternative responding for the No-Pellet group was at near-zero levels during this session. This observation was supported by a significant one-way ANOVA conducted on alternative response rates during the final session of Phase 2, F (2, 28) = 5.57, MSE = 595.51. Least Significant Difference post hoc analyses revealed that response rates for both the 5-Pellet (p < .01) and 1-Pellet (p < .05) groups were significantly higher than for the No-Alt. group. There was no difference, however, in alternative responding between the 5-Pellet and 1-Pellet groups (p = .72). During this final session of Phase 2, alternative-reinforcer rates were comparable between groups that received alternative reinforcement and inactive responding remained at low levels for each group (see Table 1). The total number of pellets earned by the 5- and 1-Pellet groups, however, differed by about a factor of five in this session.
Fig. 2.
Mean alternative-lever presses per min across sessions of Phases 2 and 3 for each group in Experiment 1.
Phase 3
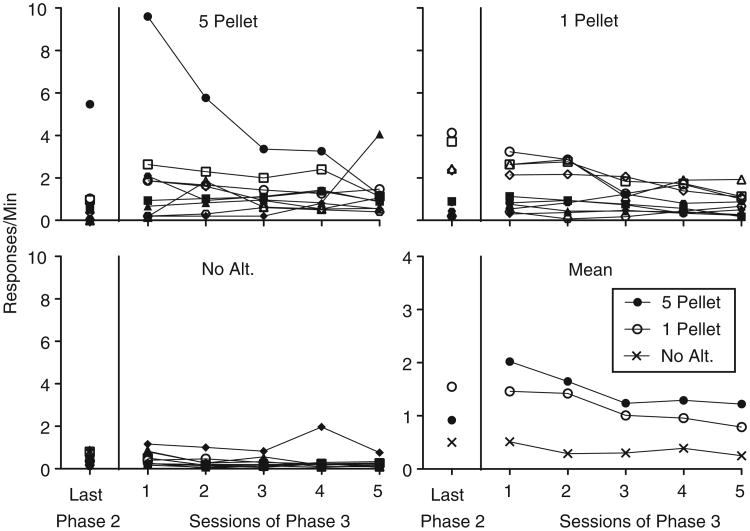
Figure 3 shows rates of target-lever pressing during the final session of Phase 2 and all five sessions of Phase 3 for individual subjects and at the group level. Target-lever pressing increased between the last session of Phase 2 and the first session of Phase 3 (i.e., resurgence of lever pressing occurred) for seven rats in the 5-Pellet group, for two rats in the 1-Pellet group, and for no rats in the No-Alt. group. A 3 × 2 (Group × Phase) mixed-model ANOVA conducted on target response rates during the last session of Phase 2 and the first session of Phase 3 found a significant main effect of Phase, F(1, 26) = 5.44, MSE = 1.71, and a Group × Phase interaction, F (2, 26) = 6.89, MSE = 2.17. The main effect of Group was not significant, F(2, 26) = 1.3, MSE = 5.98. To determine the source of the significant interaction, follow-up 2 × 2 (Group × Phase) ANOVA were conducted for each pairwise comparison of Group. The interaction term remained significant when responding from the 5-Pellet group was compared to both the 1-Pellet, F(1, 18) = 7.99, MSE = 3.56, and No-Alt, F(1, 17) =6.90, MSE =2.84, groups. The interaction term was not significant, however, when target responding from the 1-Pellet group was compared to the No-Alt. group, F(1, 17) = .29, MSE = 0.02. Further, inactive responding did not change between the last session of Phase 2 and the first session of Phase 3. Thus, resurgence was specific to the target response (see Table 1 for inactive responding during these sessions). This observation was supported by a nonsignificant main effect of Phase from a 3 × 2 (Group × Phase) mixed-model ANOVA conducted on inactive responding during the last session of Phase 2 and the first session of Phase 3, F (1, 26) = 1.47, MSE = 0.041.
Fig. 3.
Target-lever response rates during the final sessions of Phase 2 and all sessions of Phase 3 in Experiment 1. Data from individual subjects within each group and mean response rates for these sessions aggregated across subjects are shown.
With respect to target response rates across all five sessions of Phase 3, a 3 × 5 (Group × Session) mixed-model ANOVA revealed a nonsignificant main effect of Session, F(1.48, 38.55) = 2.85, MSE = 4.34, a nonsignificant Group × Session interaction, F(2.97, 38.55) = 0.46, MSE = 0.7, but a significant main effect of Group, F(2, 26) = 4.25, MSE = 15.84. Least Significant Difference post hoc analyses revealed that response rates in the 5-Pellet group were significantly higher than the No-Alt. group (p < .01). There was no difference in response rates, however, between the 5-Pellet and 1-Pellet groups (p=.36), or between the 1-Pellet and No-Alt. groups (p = .06; see Fig. 3).
Alternative responding decreased across sessions of Phase 3 for the groups that received alternative reinforcement and remained low for the No-Alt. group (see Fig. 2). This conclusion was confirmed by a 3 × 5 (Group × Session) mixed-model ANOVA conducted on alternative responding across all five sessions of Phase 3. All effects were significant (for the main effects of Session and Group, and the Group × Session interaction, respectively: F (1.09, 28.28) =14.23, MSE =856.31; F (2, 26) =9.67, MSE = 882.60; and F(2.18, 28.28) = 5.06, MSE = 304.28). To determine the source of the significant Group × Session interaction, follow-up 2 × 5 (Group × Session) mixed-model ANOVA were conducted for each pairwise comparison of Group. The interaction remained significant when responding from the 5-Pellet group, F(1.05, 17.79) = 6.78, MSE = 622.10, and the 1-Pellet group, F(1.77, 30.03) =30.53, MSE =71.43, were compared to the No-Alt. group. The interaction term was not significant, however, when responding from the 5-Pellet group was compared to the 1-Pellet group, F(1.09, 19.58) =2.35, MSE = 203.86.
Discussion
The purpose of Experiment 1 was to determine if differences in alternative-reinforcer magnitude during Phase-2 of a standard resurgence preparation affect resurgence. The results showed that during Phase 2, larger-magnitude (five-pellet) alternative reinforcement produced faster suppression of target behavior than did smaller-magnitude reinforcement. In addition, suspension of large-magnitude, but not small-magnitude, alternative reinforcement produced resurgence of target-lever pressing. These findings are similar to those reported by Craig and Shahan (2016) and others (e.g., Bouton & Trask, 2015; Craig et al., 2016; Leitenberg et al., 1975; Sweeney & Shahan, 2013) with respect to manipulations of alternative-reinforcer rate. Thus, like higher-rate alternative reinforcement, larger-magnitude alternative reinforcement appears to produce greater suppression of target behavior but more resurgence when it is removed.
Experiment 2
As noted above, Lieving and Lattal (2003) found that a shift from high-rate alternative reinforcement to a lower rate generated some resurgence, but less than when alternative reinforcement was removed completely. The purpose of this experiment was to examine the effects of shifts from higher-magnitude to lower-magnitude alternative reinforcement on resurgence. Three groups of rats pressed a target-response lever during Phase 1 for six-pellet reinforcers. Next, during Phase 2, lever pressing was extinguished, a response chain was made available, and pulling the chain produced six-pellet reinforcers at the same rate as during Phase1. Finally, during Phase 3, alternative reinforcement was suspended for one group as in a typical resurgence test, decreased by half (i.e., a shift to three pellets per delivery) for a second group, and decreased by five-sixths (i.e., a shift to one pel-let per delivery) for a third group.
Method
Subjects
Twenty-one male Long-Evans rats (Charles River, Portage, MI), approximately 90 days of age at the beginning of the experiment, served. Animal care and housing were as in Experiment 1.
Apparatus
Four modular Coulbourn (Holliston, MA) operant chambers (29 cm × 24 cm × 29 cm) housed in sound-attenuating cubicles were used. Two bite-proof, nonretractable levers on the front wall, with tri-tone (red, yellow, and green) LED stimulus lights above them, were positioned on either side of a food receptacle. A house light situated above this receptacle was used for general chamber illumination. A small hole was cut in the ceiling of the chamber, through which a ball chain could be inserted that, when inserted, extended to the floor of the chamber. All reinforcer deliveries consisted of a 10-s blackout of the chamber and delivery of 45-mg food pellets (BioServ, Flemington, NJ), as in Experiment 1. All experimental events and data collection were controlled by Med-PC (Med-Associates) software run on a PC computer in an adjoining control room.
Procedure
Magazine training
Magazine-training procedures were identical to those used in Experiment 1. Sessions for all phases of the experiment, however, were limited to 10 min, excluding time for reinforcement, to reduce the likelihood of within-session satiation.
Phase 1
During Phase 1, sessions began with illumination of the house light and the stimulus-light array situated above the target lever (left-right counterbalanced). Presses to the target lever produced six-pellet reinforcers (one pellet delivered every 0.5 s) according to a VI 15-s schedule. Responses to the other, inactive, lever were recorded but had no programmed consequences. During the first session of Phase 1, the first response to the target lever was reinforced to facilitate acquisition, after which the VI schedule commenced. Phase 1 lasted 30 sessions, after which rats were divided into three groups such that the average response rates across the last 5 sessions of Phase 1 were equivalent between groups.
Phase 2
During Phase 2, sessions began as in Phase 1, with the addition that a response-chain was suspended from the ceiling of each chamber. Reinforcement for target responses was removed, and chain pulling produced six-pellet reinforcers according to a VI 15-s schedule for all groups. During the first session of Phase 2, the first chain pull was reinforced to facilitate acquisition, after which the VI schedule commenced. This phase lasted for 10 sessions.
Phase 3
Sessions of Phase 3 began in anidentical manner to sessions of Phase 2. Forthe 6–1 group (n=7), alternative-reinforcer magnitude was decreased to one pellet perdelivery, and for the 6–3 group (n = 7)alternative-reinforcer magnitude was decreased to three pellets per delivery. Alternative reinforcement was suspended altogether for a third, 6–0 group (n = 7). Phase 3 lasted for four sessions.
Data analyses
Data analyses were as in Experiment.
Results
Phase 1
Target-lever pressing increased across sessions of Phase 1 to comparable levels between groups during the final five sessions, and reinforcer rates were comparable between groups during these sessions. Inactive responding occurred at near-zero levels for all groups. See Table 2 for a summary of response and reinforcer rates for each phase of the experiment.
Table 2. Summary of mean response and reinforcer rates from each phase of Experiment 2.
| Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 6-1 | 6-3 | 6-0 | |||||||
|
|
|
|
|||||||
| Phase 1a | Phase 2b | Phase 3c | Phase 1a | Phase 2a | Phase 3c | Phase 1a | Phase 2b | Phase 3c | |
| Target/Min | 31.25 | 0.97 | 10.46 | 30.59 | 2.34 | 4.89 | 30.19 | 1.27 | 9.86 |
| (SEM) | (6.02) | (0.23) | (2.55) | (5.95) | (0.68) | (1.43) | (3.55) | (0.31) | (1.27) |
| Alt./Min | - | 15.49 | 28.10 | - | 12.99 | 25.75 | - | 17.03 | 15.46 |
| (SEM) | - | (2.25) | (3.05) | - | (2.11) | (3.19) | - | (2.14) | (1.31) |
| Inactive/Min | 1.34 | 0.14 | 1.97 | 0.79 | 0.07 | 1.04 | 0.41 | 0.04 | 2.16 |
| (SEM) | (0.73) | (0.07) | (0.94) | (0.27) | (0.05) | (0.47) | (0.13) | (0.03) | (0.78) |
| Sr /Min | 2.99 | 2.84 | 3.3 | 2.95 | 2.7 | 3.31 | 3.01 | 2.86 | - |
| (SEM) | (0.14) | (0.12) 17.06 | (0.16) | (0.06) | (0.12) | (0.12) | (0.07) | (0.11) | - |
| Pellets/Min | 17.91 | 17.06 | 3.3 | 17.67 | 16.2 | 9.94 | 18.03 | 17.14 | - |
| (SEM) | (0.81) | (0.70) | (0.16) | (0.38) | (0.72) | (0.37) | (0.43) | (0.68) | - |
Data from the last five sessions of Phase 1 are shown.
Data from the last session of Phase 2 are shown.
Data from the first session of Phase 3 are shown.
Note: “Sr/Min” refers to the number of reinforcement periods per min earned by each group, while “Pellets/Min” is this value multiplied by the magnitude of reinforcement each group earned.
Phase 2
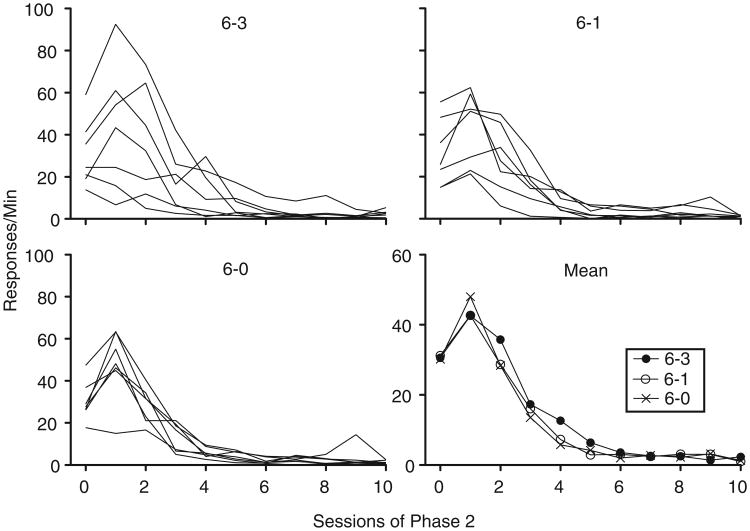
Figure 4 shows mean rates of target-lever pressing during the final five sessions of Phase 1 (labeled Session “0”) and all sessions of Phase 2 for individual subjects and means across groups. Responding was more variable for rats in the 6–1 and 6–3 groups than for the 6–0 group. Despite this differential variability, no meaningful differences were present in the overall pattern of Phase-2 responding between groups: Target-lever response rates initially increased during the first session of Phase 2, after which responding decreased across sessions at comparable rates between groups. This observation was confirmed by a 3 × 10 (Group × Session) mixed-model ANOVA conducted on response rates across sessions of Phase 2. Only the main effect of Session was significant, F(1.63, 29.36) = 60.11, MSE = 103.33 (main effect of Group: F[3.26, 29.36] =0.42, MSE =173.67; Group × Session interaction: F[2, 18] =0.18, MSE = 63.24).
Fig. 4.
Target-lever response rates across sessions of Phase 2 in Experiment 2. Session “0” represents mean rates of responding from the final five sessions of Phase 1. Data from individual subjects within each group and mean response rates from these sessions aggregated across subjects are shown.
Alternative response rates increased at comparable rates across sessions of Phase 2 (see Fig. 5), and alternative-response rates during the final session of Phase 2 did not differ significantly between groups, evidenced by a nonsignificant result from a one-way ANOVA conducted on these data, F(2, 18) = 0.89, MSE = 29.14. During this final session of Phase 2, alternative-reinforcer rates were comparable between groups, and inactive responding remained at low levels for each group (see Table 2).
Fig. 5.
Mean alternative-chain pulls per min across sessions of Phases 2 and 3 for each group in Experiment 2.
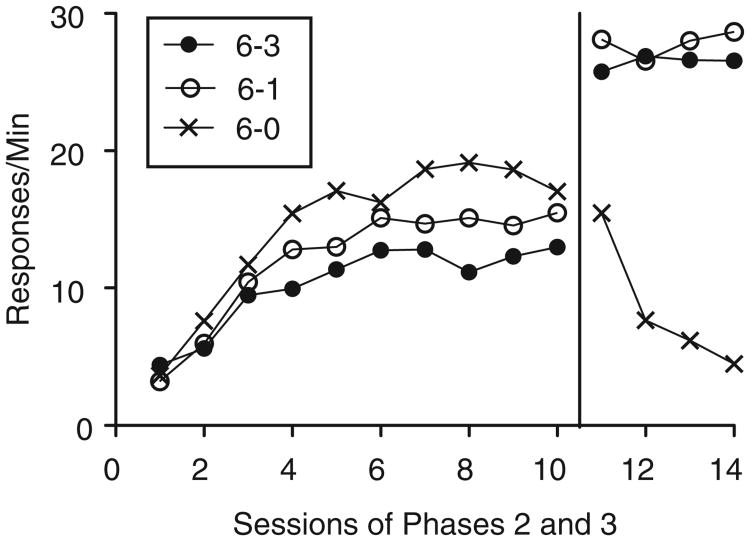
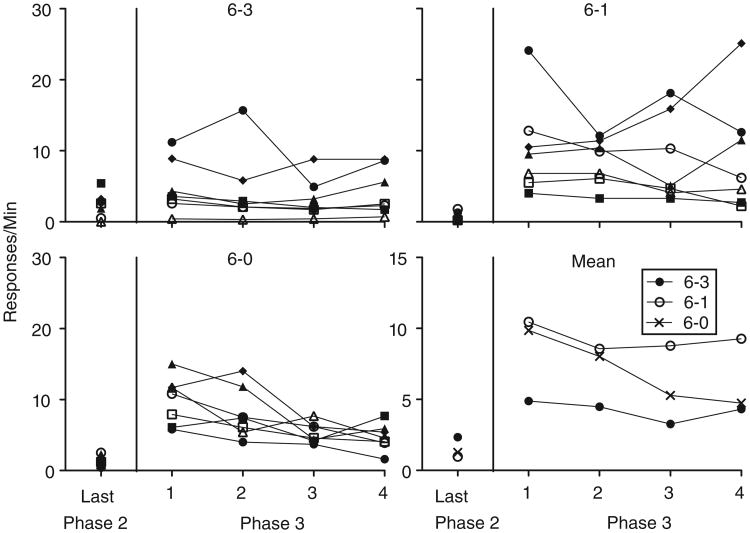
Phase 3
Figure 6 shows individual and group mean rates of target-lever pressing during the final session of Phase 2 and all four sessions of Phase 3. Between the final session of Phase 2 and the first session of Phase 3, target-lever pressing increased for all rats in the 6–1 and 6–0 groups and for four of seven rats in the 6–3 group. On average, the magnitude of resurgence was comparable in the 6–1 and 6–0 groups and smaller in the 6–3 group. A 3 × 2 -(Group × Phase) mixed-model ANOVA found a significant main effect of Phase, F (1, 18) =48.22, MSE = 495.77, and Group × Phase interaction, F(2, 18) =4.85, MSE = 49.89. Themain effect of Group was not significant, F(2,18) = 1.29, MSE = 19.22. To determine the source of the significant interaction, follow-up 2 × 2 (Group × Phase) mixed-model ANOVA were conducted for each pairwise comparison of Group. The interaction term remained significant when responding from the 6–3 group was compared to the 6–1, F (1, 12) =6.43, MSE = 84.36, and the 6–0, F (1, 12) = 12.07, MSE = 63.9, groups. The interaction was not significant, however, when responding from the 6–1 group was compared to the 6–0 group, F(1, 12) = 0.11, MSE = 1.42. Target-lever responding decreased in a uniform manner between groups across sessions of Phase 3. This observation was supported by a 3 × 4 (Group × Session) mixed-model ANOVA showing a significant main effect of Session, F (2.14, 38.51) =3.62, MSE =40.48, but nonsignificant effects of Group, F(2, 18) = 3.01, MSE = 177.72, or Group × Session interaction, F(4.28, 38.51) = 1.24, MSE = 13.8.
Fig. 6.
Target-lever response rates during the final sessions of Phase 2 and all sessions of Phase 3 in Experiment 2. Data from individual subjects within each group and mean response rates for these sessions aggregated across subjects are shown.
Inactive responding also increased significantly and to comparable levels for each group between the final session ofPhase 2 and the first session of Phase 3 (see Table 2 for inactive response rates from these sessions), evidenced by a 3 × 2 (Group × Phase) mixed-model ANOVA conducted on these data. Only the main effect of Phase was significant, F(1, 18) = 15.4, MSE = 28.18 (for the main effect of Group and the Group × Phase interaction: F[2, 18] = 0.59, MSE = 1.28, and F [2, 18] = 0.68, MSE= 1.24, respectively). Thus, a 3 × 2 × 2 (Group × Response Type × Phase) mixed-model ANOVA was conducted to determine if target-lever pressing increased at a significantly faster rate than inactive-lever pressing between the final sessions of Phase 2 and the first session of Phase 3. The Response Type × Phase interaction was significant, F(2, 18) =39.03, MSE = 143.79, suggesting that target-lever responding did, indeed, increase at a faster rate than inactive-lever responding. The Group × Response Type × Phase interaction, however, was also significant, F(2, 18) =4.95, MSE = 18.22. To determine the source of the significant three-way interaction, three follow-up 2 × 2 (Response Type × Phase) mixed-model ANOVA were conducted, one for each group. The interaction term was significant for tests conducted on data form the 6–1 and 6–0 groups (respectively, F[1, 6] =20.97, MSE = 102.61; and F[1, 6] = 26.27, MSE = 73.29), but this term was not significant for the test conducted on data from the 6–3 group, F (1, 6) =1.28, MSE = 4.32. Thus, target-lever responding increased at a faster rate than inactive-lever responding between the final session of Phase 2 and the first session of Phase 3 in the 6–1 and 6–0 groups, but these two response types increased at similar rates in the 6–3 group.
Rates of alternative-chain pulling increased between the final session of Phase 2 and the first session of Phase 3 for the 6–1 and 6–3 groups and decreased between these sessions for the 6–0 group (see Fig. 5). Thereafter, responding remained relatively constant for the 6–1 and 6–3 groups and continued to decrease for the 6–0 group. These observations were supported first by a 3 × 2 (Group × Phase) mixed-model ANOVA conducted on alternative-response rates from the final session of Phase 2 and the first session of Phase 3. The main effects of Phase and the Group × Phase interaction were significant (respectively, F[1, 18] = 55.35, MSE = 661.64; and F[2, 18] = 19.86, MSE = 237.4). The main effect of Group was not significant, F(2, 18) = 1.54, MSE = 108.39. To determine the source of the significant Group × Phase interaction, follow-up 2 × 2 (Group × Phase) mixed-model ANOVA were conducted on each pairwise comparison of Group. The interaction term remained significant when responding from the 6–1, F(1, 12) =30.32, MSE =352.16, and 6–3, F(1, 12) = 38.6, MSE = 360.01, groups was compared to responding from the 6–0 group. The interaction was not significant, however, when responding from the 6–1 group was compared to responding from the 6–3 group, F(1, 12) < 0.01, MSE = 0.04. Alternative responding across sessions of Phase 3 was analyzed using a 3 × 4 (Group × Session) mixed-model ANOVA. All effects were significant (for the main effects of Group and Session, and the Group × Session interaction, respectively: F[2, 18] = 24.95, MSE = 3,275.56; F [3, 54] = 6.24, MSE = 45.76; and F[6, 54] = 8.61, MSE = 63.15). To determine the source of the significant Group × Session interaction, follow-up 2 × 4 (Group × Session) mixed-model ANOVA were conducted for each pair-wise comparison of Group. The interaction remained significant when responding from the 6–1, F(3, 36) = 9.85, MSE = 86.94, and 6–3, F(3, 36) = 17.9, MSE = 97.33, groups was compared to responding from the 6–0 group. The interaction was not significant, however, when responding from the 6–1 group was compared to responding from the 6–3 group, F (3, 36) = 0.67, MSE = 5.18.
Discussion
Based on the findings of the present Experiment 1 and Lieving and Lattal (2003), the present experiment aimed to determine the effects of reductions in alternative-reinforcer magnitude on resurgence. The results showed that shifts from a large magnitude alternative reinforcer to both no alternative reinforcement (i.e., 6–0) and to a small magnitude of alterative reinforcement (i.e., 6–1) generated robust and similar amounts of resurgence of target responding. A 50% reduction in alternative-reinforcement magnitude from six to three pellets, however, produced a smaller increase in target responding that did not exceed an associated increase in inactive-lever pressing.
These data are consistent with those reported by Lieving and Lattal, who showed that decreasing alternative-reinforcer rate produced less resurgence than suspending alternative reinforcement altogether. Thus, as with reductions in alternative-reinforcer rate, it appears that resurgence is related to the degree to which alternative-reinforcer magnitude is reduced.
Lieving and Lattal (2003) showed only modest increases in pigeons' target responding following a 12-fold decrease in alternative-reinforcer rate, while the present experiment demonstrated robust resurgence of rats' lever pressing following a smaller, six-fold decrease in alternative-reinforcer magnitude. At face value, these results suggest that behavior is more sensitive to changes in alternative-reinforcer magnitude than to changes in alternative-reinforcer rate. It could be the case, however, that this apparent differential sensitivity to rate and magnitude represent a species difference between rats and pigeons or any of a number of differences between the Lieving and Lattal's procedures and those reported here. More extensive comparisons of the effects of decreasing alternative-reinforcer rate and magnitude on resurgence are warranted.
One potential limitation to Experiment 2 is worth noting. Rats were assigned to groups based on Phase-1 response rates. Despite this strategy, target responding was considerably more variable in the 6–3 group than in the other groups during Phase 2, and alternative-response acquisition differed visually (but not statistically) between groups. These variations in responding could have impacted the amount of resurgence between groups. We note, however, that target behavior was uniformly low between groups at the end of Phase 2 and that, to our knowledge, no relation between alternative-response rate and resurgence has been demonstrated empirically. Given the robust relation between Phase-1 response rates and resurgence (see, e.g., Sweeney & Shahan, 2013), distributing subjects to groups based on these rates was empirically most appropriate.
General Discussion
In Experiment 1, a larger-magnitude alternative reinforcer produced faster suppression of target behavior during Phase 2 than a smaller magnitude. Further, only removal of the larger-magnitude alternative reinforcer generated resurgence. Thus, delivering larger magnitudes of alternative reinforcement during Phase 2 appears to have effects on response suppression and resurgence that are similar to those of higher rates of alternative reinforcement (see Bouton & Trask, 2015; Craig et al., 2016; Craig & Shahan, 2016; Leitenberg et al., 1975; Sweeney & Shahan, 2013).
In Experiment 2, reducing alternative-reinforcer magnitude from six to three pellets (i.e., 6–3) produced a moderate increase in target behavior that did not exceed associated increases in inactive responding. Further, this increase in target-response rate was significantly smaller than the increase in the group that experienced no alternative reinforcement in Phase 3 (i.e., 6–0). These findings are consistent with those reported by Lieving and Lat-tal (2003), suggesting that downshifts in alternative reinforcer magnitude, like downshifts in rate, can reduce resurgence relative to suspension of alternative reinforcement. A six-fold decrease in alternative-reinforcer magnitude in the present experiment, however, produced resurgence that was comparable to suspension of alternative reinforcement. This finding was unexpected given that Lieving and Lattal showed that a 12-fold decrease in alternative-reinforcer rate produced only modest resurgence of pigeons' key pecking. It is noteworthy, however, that smaller shifts of alternative-reinforcer rate with rats during Phase 2 (e.g., VI 10 s to VI 75 s) have sometimes generated increases in target behavior comparable to complete removal of alternative reinforcement (Schepers & Bouton, 2015). Thus, the precise relation between decreases in alternative-reinforcer rate and increases in target responding is presently unclear, and additional parametric research examining both shifts in rate and magnitude of alternative reinforcement would seem warranted. Regardless, the present experiment demonstrates that robust resurgence is possible following a shift from a larger- to smaller-magnitude alternative reinforcer if the change in reinforcer magnitude is sufficiently large.
In summary, both higher rates and larger magnitudes of alternative reinforcement produce greater suppression of target behavior during extinction and generate greater resurgence when they are removed. Further, reductions in either the rate or magnitude of alternative reinforcement can generate resurgence, and maintaining alternative-reinforcer availability with a decreased rate or magnitude can reduce resurgence. Thus, the data from the present experiments suggest that magnitude and rate of alternative reinforcement similarly affect suppression and resurgence of extinguished target behavior.
The above conclusion is perhaps not surprising, given that recent theoretical accounts of resurgence suggest that alternative-reinforcer magnitude and rate affect target behavior through similar mechanisms. The behavioral-momentum based theory of resurgence (for review, see Craig, Nevin, & Odum, 2014; Nevin & Shahan, 2011; Shahan & Sweeney, 2011), for example, suggests that alternative reinforcement disrupts target behavior while it is available but also strengthens target behavior, thereby increasing resurgence. Both the disruptive and response-strengthening aspects of alternative reinforcement are directly related to alternative-reinforcer rate and magnitude (see Nevin, 1992), so these reinforcement dimensions have proportional effects on response suppression and resurgence. In Experiment 1, momentum theory suggests that delivering larger magnitude alternative reinforcement produced both greater disruption of target-lever pressing and a stronger stimulus–reinforcer relation than delivering smaller magnitude reinforcement. Thus, target behavior was suppressed to a greater degree, and was ultimately more susceptible to resurgence, in the 5-Pellet group than in the 1-Pellet group. In Experiment 2, decreasing alternative-reinforcer magnitude presumably decreased the disruptive impact of alternative reinforcement on target behavior, producing resurgence that was related to the size of the magnitude decrease.
Bouton and colleagues' (Bouton, Winterbauer, & Todd, 2012; Winterbauer & Bouton, 2010; 2011) context theory suggests that resurgence occurs because of changes in the discriminative properties of alternative reinforcement (i.e., reinforcer rate, magnitude, source, quality, etc.; see Bouton & Trask, 2015). More specifically, during Phase 2 of a standard resurgence preparation, organisms learn to inhibit target behavior in the “context” created by the discriminative properties of alternative reinforcement. When those properties change during Phase 3, the inhibitory learning that occurred during Phase 2 might fail to generalize, thus resulting in resurgence of target behavior. Findings from both of the present experiments are reasonably consistent with the assertions of context theory. For example, delivery of large-magnitude alternative reinforcement in Experiment 1 could have produced a Phase-2 conditioning situation that was more discriminable from Phases 1 and 3 than delivery of small-magnitude alternative reinforcement. Thus, target behavior in the 5-Pellet group was eliminated more quickly in Phase 2 and resurged to a greater degree in Phase 3 than behavior in the 1-Pellet group. Further, decreases in alternative-reinforcer magnitude in Experiment 2 could have produced graded contextual changes that depended on the size of the magnitude downshift.
Both of these theories, however, are limited. For example, behavioral momentum theory has failed with respect to many of its predictions about the precise effects of reinforcement rate on target-response persistence and resurgence. Craig and Shahan (2016) provided a detailed discussion of these and other empirical and conceptual challenges for the momentum model. For the sake of brevity, their criticism will not be restated here. Further, context theory is qualitative in nature, limiting its utility as a predictive framework for understanding resurgence (for similar criticism, see Craig et al., 2016; Craig & Shahan, 2016; McConnell & Miller, 2014; Podlesnik & Kelley, 2015). That is, because context is a broadly defined construct, it often is difficult to determine a priori what will and what will not constitute a context change or what will affect the size of any such change. As a result, what defines a context often is determined in a post hoc manner (e.g., Winterbauer & Bouton, 2012; see Shahan & Craig, 2016, for discussion).
Considering the limitations of the theories above, it might be worthwhile to consider a potential alternative view. Shahan and Craig (2016) argued that resurgence is governed by the same basic processes that are thought to govern choice. Full development of Shahan and Craig's “resurgence as choice” (RaC) model is beyond the scope of this paper. In brief, RaC states that target behavior during Phases 2 and 3 of a standard resurgence preparation represent allocation of behavior between the target and alternative options with respect to changes in the relative value of those options over time. The foundation of the model is a restatement of the concatenated matching law (Baum & Rachlin, 1969):
| (1) |
where pT is the conditional probability of a target response. Values of the target (VT) and alternative (VAlt) options in Equation 1 are functions of previously experienced parameters of reinforcement (e.g., reinforcer rates and magnitudes) associated with those options and are determined by a temporal-weighting rule (Devenport & Devenport, 1994; Devenport, Hill, Wilson, & Ogden, 1997) that assigns more weight to recent experiences. Weights decay hyperbolically as these experiences move into the past. Thus, resurgence occurs because some residual VT lingers in Phase 3 and VAlt decreases precipitously when alternative reinforcement is suspended. We note that this approach to understanding resurgence appears to be consistent with the view of Lattal and Wacker (2015) that resurgence might be generated by a “worsening of conditions” of alternative reinforcement.
It is noteworthy that, in Experiment 1, single-pellet alternative reinforcement was delivered at a rate (i.e., VI 60 s) that previously has been shown to produce little suppression of target-lever pressing during Phase 2 (relative to higher rates of alternative reinforcement) and no resurgence when it was removed in Phase 3 (Craig & Shahan, 2016). These outcomes were replicated with VI 60-s single-pellet alternative reinforcement here, but Experiment 1 also demonstrated that increasing the magnitude of alternative reinforcement both increased the suppressive effects of VI 60-s reinforcement in Phase 2 and resulted in resurgence when it was removed in Phase 3. Such symmetric effects of alternative-reinforcer rate and magnitude are consistent with the notion that both the suppressive effects of alternative reinforcement andresurgence with their subsequent removal depend on the value of the alternative sourceof reinforcement as detailed by RaC. According to the RaC model, higher rates or larger magnitudes of alternative reinforcement in Phase 2 result in relatively more allocation of behavior to the alternative-reinforcement source (and, thus, less to the target-reinforcement source). Further, higher rates and larger magnitude of alternative reinforcement produce greater resurgence in part because, in Phase 3, the value of the alternative option decreases in larger absolute terms, producing greater increases in the relative value of the target option according to Equation 1. Similarly, in Experiment 2, the amount of resurgence induced by reductions in the magnitude of the alternative reinforcer could have been related to such reductions in the value of the alternative reinforcer. From the perspective of RaC, such a decrease in the value of the alternative option should increase the conditional probability of target responding (i.e., produce resurgence), and the degree of this increase would depend on the size of the decrease in the value of the alternative option.
Regardless of any potential theoretical implications, the findings of the present experiments could have practical implications. As noted in the Introduction, differential-reinforcement based interventions are among the most commonly used behaviorally based treatment strategies for decreasing problematic behavior in humans. Further, alternative-reinforcer magnitude often is manipulated to achieve suppression of target behavior within these treatments. The results of the present series of experiments offer several insights into the practical issues associated with manipulating alternative-reinforcer magnitude in such application.
First, though using relatively large-magnitude alternative reinforcement may produce faster elimination of problem behavior than using small-magnitude reinforcement (see Lerman et al., 1999; Petry et al., 2012; Silverman et al., 1999), it may also produce behavior that is subsequently more susceptible to relapse. Pre-existing evidence from the contingency-management literature suggests this effect might be apparent in clinical situations. For example, Higgins and colleagues (2007) examined the effects of large- and small-magnitude alternative reinforcement on abstinence from drug taking in cocaine-dependent participants. Larger magnitude alternative reinforcers produced greater abstinence from drug taking during treatment than did smaller magnitude reinforcers. Importantly, however, for participants that achieved similar levels of abstinence during treatment, those that received large-magnitude alternative reinforcement were more likely to relapse to drug taking at 9 to 24 month follow-ups than those that received small-magnitude reinforcers. Thus, additional precautions may need to be taken to decrease the risk of relapse following use of large-magnitude alternative reinforcement.
In addition, thinning of alternative-reinforcer rates across sessions of treatment is a common component of DRA-based interventions for problem behavior (see Hagopian, Boelter, & Jarmolowicz, 2011; Tiger, Hanley, & Bruzek, 2008). Such thinning has been shown to decrease resurgence in animals (e.g., Sweeney & Shahan, 2013; Winterbauer & Bouton, 2012). Though Experiment 2 was not designed to determine the effects of thinning alternative-reinforcer magnitude on resurgence, per se, the findings from this experiment demonstrate the feasibility of this potential approach to preventing resurgence following large-magnitude alternative reinforcement. A two-fold decrease in alternative reinforcement (i.e., 6–3 pellets) generated less resurgence than a six-fold decrease (i.e., 6–1 pellets). Thus, relatively small reductions in alternative-reinforcer magnitude appear to prevent resurgence of extinguished target behavior. Whether further systematic decreases in reinforcer magnitude (i.e., magnitude thinning), followed ultimately by suspension of alternative reinforcement, would prevent resurgence, however, is an empirical question.
Acknowledgments
This research was supported by AQ8 grant 1R21DA037725-01 (Experiment 1) and by an Undergraduate Research and Creative Opportunities grant awarded by Utah State University's Office of Research and Graduate Studies to CMM (Experiment 2).
References
- Baum WM, Rachlin HC. Choice as time allocation. Journal of the Experimental Analysis of Behavior. 1969;12:861–874. doi: 10.1901/jeab.1969.12-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom SE, Lambert JM. Implications for practice: Resurgence and differential reinforcement of alternative responding. Journal of Applied Behavior Analysis. 2015;48:781–784. doi: 10.1002/jaba.266. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Trask S. Role of the discriminative properties of the reinforcer in resurgence. Learning & Behavior. 2015 doi: 10.3758/s13420-015-0197-7. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Winterbauer NE, Todd TP. Relapse processes after the extinction of instrumental learning: Renewal, resurgence, and reacquisition. Behavioral Processes. 2012;90:130–141. doi: 10.1016/j.beproc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cançado CRX, Lattal KA. Response elimination, reinforcement rate, and resurgence of operant behavior. Behavioural Processes. 2013;100:91–102. doi: 10.1016/j.beproc.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Craig AR, Nall RW, Madden GJ, Shahan TA. Higher rate alternative non-drug reinforcement produces greater suppression of cocaine seeking but more resurgence when removed. Behavioral Brain Research. 2016;306:48–51. doi: 10.1016/j.bbr.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AR, Nevin JA, Odum AL. Behavioral momentum and resistance to change. In: McSweeney FK, Murphy ES, editors. The Wiley Blackwell handbook of operant and classical conditioning. Oxford, UK: Wiley-Blackwell; 2014. pp. 249–274. [Google Scholar]
- Craig AR, Shahan TA. Behavioral momentum theory fails to account for the effects of reinforcement rate on resurgence. Journal of the Experimental Analysis of Behavior. 2016;105:375–392. doi: 10.1002/jeab.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport LD, Devenport JA. Time-dependent averaging of foraging information in least chipmunks and golden-mantled ground squirrels. Animal Behaviour. 1994;47:787–802. [Google Scholar]
- Devenport LD, Hill T, Wilson M, Ogden E. Tracking and averaging in variable environments: A transition rule. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:450–460. [Google Scholar]
- Epstein R. Resurgence of previously reinforced behavior during extinction. Behavior Analysis Letters. 1983;3:391–397. [Google Scholar]
- Epstein R. Extinction induced resurgence: Preliminary investigations and possible applications. The Psychological Record. 1985;35:143–153. [Google Scholar]
- Fleshler M, Hoffman HS. A progression for generating variable-interval schedules. Journal of the Experimental Analysis of Behavior. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki S, Lattal KA, Sakagami T. A further look at reinforcement rate and resurgence. Mexican Journal of Behavior Analysis. 2015;41:116–136. [Google Scholar]
- Hagopian LP, Boelter EW, Jarmolowicz DP. Reinforcement schedule thinning following functional communication training: Review and recommendations. Behavior Analysis in Practice. 2011;4:4–16. doi: 10.1007/BF03391770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102:271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Petry NM. Contingency management: Incentives for sobriety. Alcohol Research & Health. 1999;23:122–127. [PMC free article] [PubMed] [Google Scholar]
- Lattal KA, St. Peter Pipkin C. Resurgence of previously reinforced responding: Research and application. The Behavior Analyst Today. 2009;10:254–266. [Google Scholar]
- Leitenberg H, Rawson RA, Bath K. Reinforcement of competing behavior during extinction. Science. 1970;169:301–303. doi: 10.1126/science.169.3942.301. [DOI] [PubMed] [Google Scholar]
- Leitenberg H, Rawson RA, Mulick JA. Extinction and reinforcement of alternative behavior. Journal of Comparative and Physiological Psychology. 1975;88:640–652. [Google Scholar]
- Lerman DC, Kelley ME, Van Camp CM, Roane HW. Effects of reinforcement magnitude on spontaneous recovery. Journal of Applied Behavior Analysis. 1999;32:197–200. doi: 10.1901/jaba.1999.32-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman DC, Kelley ME, Vorndran CM, Kuhn SAC, LaRue RH. Reinforcement magnitude and responding during treatment with differential reinforcement. Journal of Applied Behavior Analysis. 2002;35:29–48. doi: 10.1901/jaba.2002.35-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieving GA, Lattal KA. Recency, repeatability, and reinforcer retrenchment: An experimental analysis of resurgence. Journal of the Experimental Analysis of Behavior. 2003;80:217–233. doi: 10.1901/jeab.2003.80-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BL, Miller RR. Associative accounts of recover-from-extinction effects. Learning and Motivation. 2014;46:1–15. doi: 10.1016/j.lmot.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA. An integrative model for the study of behavioral momentum. Journal of the Experimental Analysis of Behavior. 1992;57:301–316. doi: 10.1901/jeab.1992.57-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, Grace RC. Behavioral momentum and the law of effect. Behavioral and Brain Sciences. 2000;23:73–130. doi: 10.1017/s0140525x00002405. [DOI] [PubMed] [Google Scholar]
- Nevin JA, Shahan TA. Behavioral momentum theory: Equations and applications. Journal of Applied Behavior Analysis. 2011;44:877–895. doi: 10.1901/jaba.2011.44-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. Journal of Consulting and Clinical Psychology. 2012;80:276–285. doi: 10.1037/a0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petscher ES, Rey C, Bailey JS. A review of empirical support for differential reinforcement of alternative behavior. Research in Developmental Disabilities. 2009;30:409–425. doi: 10.1016/j.ridd.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Podlesnik CA, Kelley ME. Translational research on the relapse of operant behavior. Mexican Journal of Behavior Analysis. 2015;41:226–251. [Google Scholar]
- Schepers ST, Bouton ME. Effects of reinforcer distribution during response elimination on resurgence of an instrumental behavior. Journal of Experimental Psychology: Animal Learning and Cognition. 2015;41:179–192. doi: 10.1037/xan0000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahan TA, Craig AR. Resurgence as choice. Behavioural Processes. 2016 doi: 10.1016/j.beproc.2016.10.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psycho-pharmacology. 1999;146:128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Stitzer M, Petry N. Contingency management. In: Galanter M, Kleber HD, Brady KT, editors. The American Psychiatric Publishing textbook of substance abuse treatment. 5th. Arlington, VA: American Psychiatric Publishing, Inc; 2015. pp. 423–439. [Google Scholar]
- Sweeney MM, Shahan TA. Effects of high, low, and thinning rates of alternative reinforcement on response elimination and resurgence. Journal of the Experimental Analysis of Behavior. 2013;100:102–116. doi: 10.1002/jeab.26. [DOI] [PubMed] [Google Scholar]
- Tiger JH, Hanley GP, Bruzek J. Functional communication training: A review and practical guide. Behavior Analysis in Practice. 2008;1:16–23. doi: 10.1007/BF03391716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Schepers ST, Bouton ME. Context change explains resurgence after the extinction of operant behavior. Mexican Journal of Behavior Analysis. 2015;41:187–210. [PMC free article] [PubMed] [Google Scholar]
- Trosclair-Lasserre NM, Lerman DC, Call NA, Addison LR, Kodak T. Reinforcement magnitude: An evaluation of preference and reinforcer efficacy. Journal of Applied Behavior Analysis. 2008;41:203–220. doi: 10.1901/jaba.2008.41-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert VM, Lerman DC, Call NA, Trosclair-Lasserre N. An evaluationof resurgence during treatment with functional communication training. Journal of Applied Behavior Analysis. 2009;42:145–160. doi: 10.1901/jaba.2009.42-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker DP, Harding JW, Morgan TA, Berg WK, Schieltz KM, Lee JF, Padilla YC. An evaluation of resurgence during functional communication training. The Psychological Record. 2013;63:3–20. doi: 10.11133/j.tpr.2013.63.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbauer NE, Bouton ME. Mechanisms of resurgence of an extinguished instrumental behavior. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:343–353. doi: 10.1037/a0017365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbauer NE, Bouton ME. Mechanisms of resurgence II: Response-contingent reinforcers can reinstate a second extinguished behavior. Learning and Motivation. 2011;42:154–164. doi: 10.1016/j.lmot.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbauer NE, Bouton ME. Effects of thinning the rate at which the alternative behavior is reinforced on resurgence of an extinguished instrumental response. Journal of Experimental Psychology: Animal Behavior Processes. 2012;3:279–291. doi: 10.1037/a0028853. [DOI] [PMC free article] [PubMed] [Google Scholar]