Abstract
Background
Locomotor training using treadmills or robotic devices is commonly utilized to improve gait in cerebral palsy (CP); however, effects are inconsistent and fail to exceed those of equally intense alternatives. Possible limitations of existing devices include fixed non-variable rhythm and too much limb or body weight assistance.
Objective
Quantify and compare effectiveness of a motor-assisted cycle and a novel alternative, an elliptical, in CP to improve interlimb reciprocal coordination through intensive speed-focused leg training.
Methods
27 children with bilateral CP, 5–17 years, were randomized to 12 weeks of 20 minutes, 5 days per week home-based training (elliptical =14; cycle =13) at a minimum 40 RPMs with resistance added when speed target achieved. Primary outcomes were self-selected and fastest voluntary cadence on the devices and gait speed. Secondary outcomes included knee muscle strength, selective control and functional mobility measures.
Results
Cadence on trained but not non-trained devices increased, demonstrating task specificity of training and increased exercise capability. Mean gait speed did not increase in either group nor did parent-reported functional mobility. Knee extensor strength increased in both. An interaction between group and time was seen in selective control with scores slightly increasing for the elliptical and decreasing for the cycle, possibly related to tighter limb coupling with cycling.
Conclusions
Task-specific effects were similarly positive across groups, but no transfer was seen to gait or function. Training dose was low (≤20 hours) compared to intensive upper limb training recommendations and may be insufficient to produce appreciable clinical change. NCT01086670: Physical, functional and neural effects of two lower extremity exercise programs in children with cerebral palsy
Keywords: locomotor, gait, elliptical, cycling, pediatric
Introduction
Cerebral palsy (CP) is a group of disorders comprising the most prevalent childhood onset physical disability. The defining characteristic is a motor impairment that limits daily activities such as self-care and mobility. Intensive task-specific upper limb training has been shown to be efficacious for improving hand function in children primarily with unilateral CP1,2. However, intensive task-specific approaches in the lower extremity utilizing motorized treadmills or robotic devices have largely failed to produce similarly consistent positive outcomes or those superior to equally intense non-device alternatives in CP3,4 or stroke5. This has prompted some researchers to recommend reconsideration of these approaches in neurorehabilitation6.
Despite the lack of scientific consensus, the advent of intensive motor training paradigms has transformed neurorehabilitation practices and stimulated consideration of training characteristics that may predict effectiveness,7,8. Nudo9 emphasized that both quantity and quality of motor experience can influence brain plasticity and functional recovery after brain injury. With respect to quantity, the amount of repetition or practice is a key ingredient in fostering motor learning; however, he noted that the amount of repetition in clinical trials is often far less than in animal studies, suggesting that rehabilitation may be largely under-dosed. Constraint-Induced Movement Therapy (CIMT) is one of the highest dosed therapies in neurorehabilitation. Dose-response in CIMT has been evaluated in CP and minimum dosing thresholds for producing clinically important changes in functioning have been proposed10,11. Insights from trials in CP suggest that 30 hours is insufficient, and while 60 hours can produce clinically significant effects, 90 hours may produce greater and more lasting benefits10. Interestingly, results from CIMT are similar to other intensive paradigms such as bilateral training when doses are equivalent11,12, supporting the importance of sufficient repetition.
Several qualitative aspects to enhance treatment effectiveness have been proposed including greater salience or meaningfulness to the learner8, more physical and mental engagement during training8, and a more flexible and variable underlying rhythm that may reduce spinal habituation which can occur during rote, repetitive tasks13. Therefore, treadmill training at a constant belt speed or using a motorized gait orthosis that can passively move the limbs at a fixed pace may understandably not prove superior to alternatives such as over ground walking or activity-based therapies that require more effort. An electroencephalography (EEG) study during treadmill walking in adults demonstrated greater activity in brain regions involved in motor planning and performance when subjects actively controlled treadmill speed rather than responding passively to speed changes14. Recent implementation of more adaptive and engaging control strategies or feedback in robotic gait training are demonstrating positive outcomes15,16 yet comparisons of these with equivalent dose alternatives or previous control systems have not yet been reported.
In addition to robotic or treadmill-based gait training, functional electrical stimulation (FES) assisted or standard stationary cycles have been utilized to improve lower extremity strength, endurance and/or function in CP. Three studies17,18,19 have reported mostly positive mean results after approximately 15 hours of cycling in each study, yet results failed to be significantly greater than those from a randomized non-intervention group in one study17. While not as directly task-specific to walking as treadmill training20, cycling shares the same neural circuitry as walking21,22,23. Therefore, it is reasonable to propose that repetitive practice on a cycle could improve reciprocal interlimb coordination24 and thereby enhance stepping performance. Elliptical training is even more closely task-related to walking20, but no studies have yet been published utilizing this device for locomotor training in CP. Additionally, unlike treadmill protocols, pedal resistance can be added to both devices to increase the strengthening load and/or the aerobic level,18. In contrast, harnessed or other weight-support systems such as robotic exoskeletons may reduce limb loading and the degree of lower limb strengthening. Harnessed walking also restricts trunk movements. In those with CP who can already perform stepping movements, step training alone may not be very beneficial, but the combination of this with postural training, versus support, and leg strengthening could potentially be more effective. Adequate postural control is a rate limiting factor in the development of independent walking25 and is directly related to functional mobility in CP26. Use of a stationary cycle, particularly when performed in a supported or recumbent sitting position, also does not require postural control and coordination with leg movements; whereas an elliptical challenges balance control and requires coordination of the trunk and limbs with no weight support provided. The device most similar to an elliptical investigated in CP was an electromechanical Gait Trainer that also supports and moves with the feet; however, in contrast, the speed is motor-driven. A randomized trial comparing 10 30-minute sessions on the device focused primarily on increasing movement speed plus 10 minutes of joint mobilization and stretching to 10 sessions of 40 minutes of conventional therapy found improvement in the 10-m and 6-min Walk Tests for the experimental group after only 5 total hours on the device27.
The main objective here was to evaluate and compare two alternatives to treadmill or robotic-assisted gait training, a motor-assisted stationary cycle designed specifically for rehabilitation and a pediatric elliptical device, for improving gait function in children with bilateral CP. The primary outcome of interest was improved lower limb reciprocal coordination, with the ultimate aim to improve stepping cadence and thereby increase gait speed. Training focused primarily on having the children move their legs as quickly as possible in the devices and progressively increase speed first, then resistance.
We hypothesized that both device groups would demonstrate improved reciprocal coordination, as measured by increased voluntary cadence on each device as well as increased gait speed at both a self-selected and “as fast as possible” pace. Within-subject changes during training would also be greater than during a 3 month no-exercise period. We further hypothesized that all would show improvements in secondary outcomes at the International Classification of Functioning, Disability and Health (ICF) levels of Body Structures and Functions, i.e. knee extensor strength and Selective Control Assessment of the Lower Extremity (SCALE) 29, and Activity and Participation, i.e. the computer-adapted version of the Pediatric Evaluation of Disability Inventory (PEDI-CAT) 30 and the Pediatric Outcomes Data Collection Instrument (POCCI) 31. Finally, we hypothesized that outcomes for the elliptical would be significantly greater than for cycling due to its greater task-specificity to walking and active use of all four limbs and the trunk.
Methods
Participants
Twenty seven children with bilateral spastic CP, mean age of 10.3 years, ranging from 5.0 to 17.6 years, 8 male, 19 female, GMFCS Levels I-III, participated in this study from 2010 to 2015 with no drop-outs or withdrawals. A power analysis based on pilot (unpublished) motor-assisted cycling data in children with CP, GMFCS III and IV, indicated that 24 subjects, 12 per group, were needed to detect a 20 RPM gain with 80% power. Table 1 presents participant characteristics separated by device group. While mean weight, height and age were higher in the elliptical group (p= 0.09, 0.09, and 0.13, respectively) the differences were not significant. Children were recruited through our laboratory database and through IRB- approved flyers sent to local physicians and therapists who care for children with CP.
Table 1.
Participant characteristics and baseline values for all outcome measures by device group with associated p-values and significance indicated in bold (p<0.05). Device cadence is based on Revolutions per Minute (RPM). Torque is in Newton-meters (Nm).
| Cycle (n=13) | Elliptical (n=14) | p | |
|---|---|---|---|
| Age | 9.2 (2.9) | 11.4 (4.0) | 0.13 |
| Height (cm) | 127.4 (16.3) | 139.7 (20.7) | 0.09 |
| Mass (kg) | 30.8 (12.7) | 41.3 (18.1) | 0.09 |
| GMFCS I | 1 | 1 | |
| II | 4 | 11 | |
| III | 8 | 2 | |
| Elliptical cadence - free | 34.6 (30.7)* | 53.9 (22.6) | 0.18 |
| Elliptical cadence – fast | 73.0 (24.5)* | 77.9 (34.8) | 0.06 |
| Cycle cadence – free | 87.5 (34.4) | 116.7(15.1) | 0.04 |
| Cycle cadence - fast | 113.1 (50.1) | 175.3 (32.9) | 0.19 |
| Gait speed m/sec– free | 0.81 (0.29) | 0.92 (0.29) | 0.29 |
| Gait speed m/sec– fast | 1.18 (0.15) | 1.37 (0.33) | 0.11 |
| PEDI Self-care | 49.0 (6.22) | 56.6 (8.35) | 0.01 |
| PEDI Mobility | 44.2 (7.51) | 50.2 (8.31) | 0.06 |
| PODCI Global | 62.5 (12.4) | 75.8 (12.8) | 0.01 |
| PODCI Transfers | 67.9 (20.3) | 85.1 (17.8) | 0.03 |
| PODCI Sports | 37.9 (19.2) | 55.9 (11.9) | 0.01 |
| PODCI UE | 57.0 (24.0) | 76.5 (17.7) | 0.03 |
| PODCI Pain | 87.2 (11.6) | 85.6 (15.9) | 0.78 |
| KE Torque 30°/s (Nm) | 18.0 (12.8) | 39.8 (22.6) | 0.01 |
| KE Torque 90°/s (Nm) | 11.1 (4.5) | 22.5 (11.1) | 0.01 |
| Device Resistance Level | 4.2 (3.9) | 6.4 (3.8) | 0.18 |
| SCALE | 7.0 (2.6) | 10.4 (2.3) | 0.001 |
indicates incomplete dataset with n=7 for each
Inclusion criteria were: 1) ages 5–17 years inclusive; 2) diagnosis of bilateral CP; 3) GMFCS levels I-III; 4) preterm birth (34 weeks of gestation or less); 5) periventricular leukomalacia (PVL) verified by MRI; 6) no surgery within a year and no lower limb muscle injections within 4 months; 7) ability to follow simple directions and perform and complete the exercise program as judged by a parent and/or referring therapist or physician; 8) not currently participating in locomotor device training. The inclusion criteria of preterm birth and PVL were to increase the homogeneity of the study population with all likely to have white manner injury. The protocol (#10-CC-0073) was approved by the Institutional Review Board at the National Institutes of Health and written informed consent was obtained from a parent and verbal assent was obtained from each child.
Rationale for Choice of Training Devices
The primary training goal was to encourage active self-paced reciprocal leg movement at increasingly faster velocities with minimal external assistance. The cycling device selected here (MOTOMed gracile, RECK, Germany) has a unique servomotor that can initiate the motion and maintain it at a low cadence for those who cannot start or maintain a continuous cycling rhythm; however, to encourage children to control their own pace as much as possible, the target for all children was to exceed the cadence on the machine to take over control of the cadence themselves. Once in control, the rhythm becomes more flexible, variable and requires greater voluntary effort, as opposed to a rote, invariable speed that encourages greater passivity or automaticity. We anticipated that all children with bilateral CP GMFCS Levels I, II and III would be capable of using the cycle with minimal practice. Since no study had yet been performed in CP using elliptical devices, we were uncertain whether all would be able to use this well enough to participate in training. Elliptical cadence is totally driven by the user and therefore more challenging. However, the elliptical has the attractive advantage that upper limbs can assist lower limb movement as needed until the child can more fully use their lower limbs. The ability to increase pedal resistance in both devices may not only improve leg strength in addition to coordination18, but also increase the amount of muscle (electrical) activation or sensory input ascending from spinal pathways to the cortex, thus enhancing the neuroplastic effects of training28. The elliptical further requires reciprocation of all four limbs, which could further enhance sensorimotor stimulation. The elliptical device selected for this study (Cardiokids, Kidsfit, Huger, South Carolina) was designed specifically for children and had resistance settings from 1–10.
Procedures
The study design consisted of randomization of participants after enrollment using a randomly generated, by a statistician, order of assignment to one of two device groups, elliptical or cycle. This list was maintained by the PI and not revealed to the members of the study team who enrolled all participants until after enrollment. However, at the initial assessment, six participants were found to be either too short to comfortably reach the elliptical handles, even though it was designed for those 5 years of age and older, or were unable to move the elliptical pedals in a continuous manner for 30 seconds with no resistance. Three of those had been assigned to the elliptical group (1 was too small and 2 could not pedal continuously; all were GMFCS level III). Randomization had to be broken in those cases and the statistician recommended they be assigned instead to the next cycle condition on the list. That condition regardless of location on the list was then replaced with the unfilled elliptical condition for future assignments. The other three had been assigned to the cycle group, but were all also GMFCS level III with one who was 5 years old having difficulty reaching and holding onto the handles. However, two other 5 year old children, both GMFCS level II, one of whom was the smallest child in the study, were able to train with the elliptical. No child was too small for or unable to use the cycle.
Each participant was tested three times at three month intervals, and training occurred either in the first or second three month interval depending on device availability at enrollment. The number of devices for this study was limited because of their expense (@ $3000 each). If a device was available at enrollment, the child started training immediately, and was followed for another three months after the training ended. If a device was not yet available, the child was followed for a three month baseline period, then received a second assessment prior to training with their last visit being the post-training assessment.
Exercise Intervention
In this study, the cycle servo motor was set to only assist cadences below 20 rpm. Families were given written instructions on how to use the computerized cycle that were reviewed prior to having the device delivered to their home. The elliptical device was simpler to operate and this was also reviewed with the family prior to delivery. The resistance settings were 1–20 on the cycle, however to be more consistent with the elliptical we used only even numbers as increments for progression. Each family was contacted every 2 weeks throughout training by the therapist to evaluate exercise safety and compliance, and to ascertain training parameters such as current speed and duration, as well as level of resistance if any. If a child had met the target cadence and had maintained it for a week, the resistance was increased by one increment, unless the child went below the target speed and the level of exertion was so high that the child could not talk easily while training. If either occurred, the child was to continue working on increasing speed and to try to add resistance the following week.
For all participants, the assigned device was delivered to their home and they were instructed to exercise above 40 rpm for 20 minutes, 5 days a week for 12 weeks. Children were instructed to wear shoes when exercising to protect their feet, but not ankle foot orthoses. The goal was to complete at least 90% or 54 of 60 total sessions allowing them to miss some sessions due to illness or vacation. Through parent report (24/27 participants with completed log sheets (with 2 in the cycle group and 1 in the elliptical group failing to complete them) the mean number of completed sessions was 57.3 with only two participants (both in the elliptical group) reporting less than 54 sessions completed.
Data Collection
All children participated in three assessments approximately three months apart at a national research hospital in Bethesda, MD. Assessments were to be performed within one week prior to the start and two weeks after completion of the intervention. Primary outcomes, voluntary device cadence and gait speed, were based on 3-D gait analysis and the same standardized data collection procedures were followed for all three assessments. A 10 camera motion capture system (Vicon Motion Systems, Denver, CO) was used to quantify self-selected and fast paces for barefoot walking and voluntary cycling and elliptical cadences. All children were tested on both devices, unless they were too small for or unstable on the elliptical. Those who could stand on the elliptical safely but had difficulty pedaling were still assessed each time. Five cycles of each condition were collected and only the temporal-spatial data are reported here. However, revolutions during device trials were determined by tracking foot motion over time, averaged within each trial. The secondary strength or selective control measures were not blinded and the same therapist who performed these, with assistance as needed from other members of the research team, also trained children on each device and followed up with the family. To reduce bias, results of prior assessments were not available during subsequent assessments. One therapist-administered assessment was the SCALE which compares voluntary ability to move five lower extremity joints per limb independently of others within passive limits 29. Scores per joint range from 0–2, with 10 points the maximum (best) score per limb and 20 for the total score.
Knee flexor and extensor muscle group isokinetic peak torques (Newton-meters) at 30° and 90°/sec were recorded at each assessment. All subjects were positioned on the Biodex System 3 (Shirley, NY) isokinetic dynamometer in a supported semi-reclined sitting position with the knee joint aligned with the dynamometer axis. A resistance test on the trained device was also performed at each assessment to evaluate the ability to withstand and maintain pedal resistance at a relatively fast pace initially and after training. Subjects were asked to maintain pedal speed at 20 RPMs as resistance was added by one level every 30 seconds until the rate dropped below 20 RPMs for more than 5 seconds. The highest resistance level achieved prior to that was recorded.
Two computer-based functional parent (proxy) reported outcome measures, the PODCI (version 1.36) and the Computer Adapted Test version of the PEDI-CAT (version 2.5), both in English, were used to determine the child’s functional capabilities. The same parent completed these at all assessments. Both measure functional mobility as well as self-care or upper extremity function. The PEDI-CAT yields a single score scaled from 0–100 for both Mobility and Self-care with higher scores indicating greater function. The PODCI has a Global Function Score that includes four subscales which also can be evaluated separately: Transfers and Basic Mobility, Sports and Physical Function, Upper Extremity and Comfort/Pain. Scores ranged from 0–100 on each with higher scores indicating less disability.
Statistics
A general linear mixed-model design was used to assess the effects of time (pre versus post training) and group (elliptical or cycle) on the primary and secondary outcomes. Change scores for the primary outcomes were also compared during the non-exercise (three month baseline or follow up) and exercise period across device groups using a general linear mixed model with p<0.05 (SPSS Version 22; IBM Corporation, Armonk, NY). Change scores for device cadences over the training period were compared across groups and were also correlated with changes in knee extensor torque and the increase in the amount of pedal resistance during training using Pearson’s r (p<0.05) to assess the extent to which strength changes or greater training effort were related to outcomes.
Results
Primary outcome measures
Voluntary coordination on the trained device, assessed by device cadence at self-selected and fast speeds for all participants, improved markedly and significantly as a result of training (See Table 2). With the exception of the self-selected cycle condition, results differed across device groups and all device comparisons showed a significant interaction indicating that the groups behaved differently over time. Specifically, this indicated that the elliptical group had greater gains on the elliptical and the cycle group on the cycle, which reflected the task specificity of the training. No significant transfer to increased cadence on the non-trained devices was seen; however, a trend towards increased elliptical cadence was found in the cycle group (p=0.063 free and 0.054 fast).
Table 2.
Mean values of primary outcomes before and after training in each device group with p-values for main effects for group and time and interaction of group by time (G X T). Device cadence is based on Revolutions per Minute (RPM). All device outcomes increased significantly over time. Group differences were seen for 2 of 4 device conditions, each showing greater change in the group that trained on the tested device. This same task specific effect was shown by the significant interaction in 3 of 4 device conditions. Gait outcomes showed no differences
| Outcome | Device Group | Pre Mean (SD) | Post Mean (SD) | p-value (Time) | p-value (Group) | p-value (G X T) |
|---|---|---|---|---|---|---|
| Cycle Cadence FREE | Cycle | 87.5 (34.4) | 114.8 (28.4) | 0.002 | 0.20 | 0.03 |
| Elliptical | 116.7 (15.9) | 129.0 (32.0) | ||||
| Cycle Cadence FAST | Cycle | 113.1 (50.1) | 155.8 (41.1) | <0.001 | 0.006 | 0.009 |
| Elliptical | 175.3 (33.0) | 186.4 (43.0) | ||||
| Elliptical Cadence FREE | Cycle | 23.2 (28.5) | 35.0 (29.9) | <0.001 | 0.01 | <0.001 |
| Elliptical | 53.9 (22.6) | 84.5 (22.9) | ||||
| Elliptical Cadence FAST | Cycle | 29.9 (38.7) | 42.5 (40.9) | <0.001 | 0.002 | <0.001 |
| Elliptical | 77.9 (34.8) | 119.2 (35.0 | ||||
| Gait Speed m/s FREE | Cycle | 0.81 (0.29) | 0.81 (0.31) | 0.21 | 0.12 | 0.20 |
| Elliptical | 0.92 (0.24) | 1.01 (0.09) | ||||
| Gait Speed m/s FAST | Cycle | 1.18 (0.19) | 1.18 (0.44) | 0.84 | 0.82 | 0.13 |
| Elliptical | 1.37 (0.33) | 1.40 (0.31) |
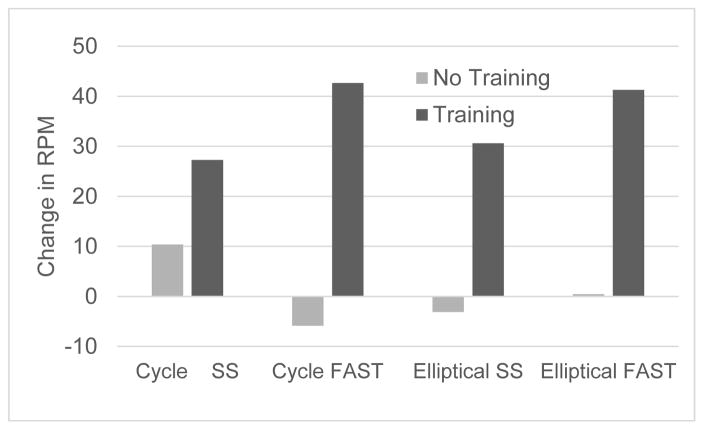
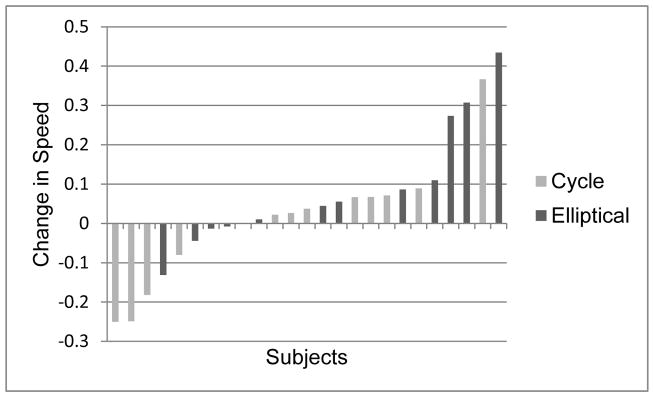
Since some children had their three month no-exercise period before and some after the training, changes in outcomes for each period were first compared using paired t-tests and not found to be statistically different. We then combined the change scores for these periods and compared these to the changes as a result of training. Again with the exception of the self-selected cycle condition (mean difference = +17.5 steps/min during training; p = 0.25), gains during training were significantly higher than during the no-training period (Figure 1). Notably, only two subjects had a decrease in device cadence after training and, in both the trained device was the cycle and the decrease was only in the self-selected pace while a large increase was seen for each in the fast pace. No significant changes were seen in gait speed for time or group with the means for the two periods virtually identical in the cycle group; however, a strong trend towards increased self-selected gait speed (data missing for one participant who only had fast speed data) was seen for the elliptical group (p=0.054) (Table 2). Figure 2 shows individual results for that condition by group.
Figure 1.
This shows the mean change in Revolutions per Minute (RPM) within device groups for both self-selected (SS) and FAST speeds with the darker bars representing the change after the three month training and the lighter bars showing the amount of change in the three month no-training period.
Figure 2.
This shows the individual results for change in freely selected gait speed for subjects by device group, with those in the cycle group represented by the lighter bars and those in the elliptical group by the darker bars.
Secondary outcome measures
Complete datasets were available for all secondary outcomes with results summarized in Table 3. Isometric and isokinetic knee extensor and flexor strength at 30°/s did not increase significantly but there was a significant increase at 90°/s in the knee extensors only over time. No group interaction was found suggesting that the groups behaved similarly; however, the device groups did differ in absolute strength with the elliptical group having consistently higher values. No significant correlations between changes in device cadence with knee extensor torques at 30° or 90° were found (p=0.62 and 0.63 for free cadence; p=0.87 and 0.97 for fast cadence).
Table 3.
Results for secondary outcomes showing that strength and device resistance increased over time in the entire sample. Group differences were related mainly to baseline differences in function across groups with the exception of the SCALE which demonstrated the groups behaved differently in response to training (interaction effect)
| Outcomes | Main Effect (Time) | Main Effect (Group) | Interaction (Group X Time) |
|---|---|---|---|
| PEDI-CAT Self-care | 0.21 | 0.65 | 0.65 |
| PEDI-CAT Mobility | 0.68 | 0.05 | 0.78 |
| PODCI Global | 0.56 | 0.02 | 0.73 |
| PODCI Transfers | 0.54 | 0.034 | 0.30 |
| PODCI Sports | 0.43 | 0.017 | 0.43 |
| PODCI UE | 0.20 | 0.045 | 0.61 |
| PODCI Pain | 0.71 | 0.97 | 0.39 |
| KE Torque 30°/s (Nm) | 0.45 | 0.006 | 0.65 |
| KE Torque 90°/s (Nm) | 0.02 | 0.01 | 0.19 |
| Device Resistance Level | 0.005 | 0.34 | 0.30 |
| SCALE | 0.97 | <0.001 | <0.001 |
The resistance test results were similar to the faster speed isokinetic test in that the resistance increased over time, with no differences between groups and no interaction. A greater increase in resistance over the training period measured in the laboratory was moderately correlated with greater gains in fast device cadence (r=0.60; p<0.05). We also tracked progression in resistance during training and found that increased device cadence was related to progressive increases in training effort as measured by increased levels of resistance with r=0.53 (p<0.01) for free and r=0.54 (p<0.01) for fast cadences. There were no differences across groups in the amount of progression in training resistance (p= 0.30)
SCALE results showed no main effect for time (p=0.97); however, a significant difference for group (p<0.001) and a group by time interaction (p<0.001) were seen with the elliptical group showing a small increase and the cycle group a small decrease in scores after training.
No significant main effects for training compared to no training periods were seen in the PEDI-CAT Self-care and Mobility domains or in the PODCI Global Function Score or subscales. A significant main effect for device group was seen for all scales and subscales except for PEDI-CAT Self-care and PODCI Comfort/Pain which likely reflected the higher baseline function of the elliptical group.
Only the cycle group showed negative mean differences after training in some outcomes, none of which was significantly worse (Table 4).
Table 4.
Pre to post training results by device group including mean difference, 95% Confidence Interval (CI) and effect size. Device cadence is based on Revolutions per Minute (RPM)
| Outcomes | Elliptical Mean Diff | Elliptical 95% CI | Elliptical Effect Size | Cycle Mean Diff | Cycle 95% CI | Cycle Effect Size |
|---|---|---|---|---|---|---|
| Cycle Cadence FREE | 12.3 | 5.43–29/9 | 0.15 | 27.3 | 10.2–44.4 | 0.50 |
| Cycle Cadence FAST | 11.0 | 5.50–27.5 | 0.14 | 42.6 | 27.4–57.0 | 0.76 |
| Elliptical Cadence FREE | 30.6 | 22.5–38.7 | 0.84 | 11.8 | 0.74–24.5 | 0.26 |
| Elliptical Cadence FAST | 41.3 | 29.4–53.1 | 0.81 | 12.6 | 0.28–25.4 | 0.28 |
| Gait Speed m/s FREE | 0.09 | 0.01–0.18 | 0.24 | −0.001 | −0.11–0.11 | 0.00 |
| Gait Speed m/s FAST | 0.02 | −0.07–0.11 | 0.02 | −0.001 | −0.21–0.20 | 0.00 |
| PEDI-CAT Self-care | 0.81 | −0.83–2.46 | 0.08 | 0.39 | −0.63–1.40 | 0.05 |
| PEDI-CAT Mobility | 0.47 | −0.10–1.95 | 0.01 | 0.08 | −1.40–1.63 | 0.00 |
| PODCI Global | 1.15 | −1.73–4.04 | 0.06 | 0.25 | −4.70–5.20 | 0.00 |
| PODCI Transfers | 2.15 | 0.83–5.10 | 0.17 | −0.58 | −5.30–4.20 | 0.01 |
| PODCI Sports | 0.00 | −2.78–2.78 | 0.00 | 2.30 | 3.50–8.20 | 0.05 |
| PODCI UE | 1.23 | −4.80–7.30 | 0.02 | 3.00 | 1.16–7.16 | 0.00 |
| PODCI Pain | 1.39 | −5.72–8.49 | 0.02 | −3.50 | −13.8–6.7 | 0.05 |
| KE Torque 30°/s | 0.60 | −9.1–10.3 | 0.00 | 2.80 | 0.55–6.16 | 0.24 |
| KE Torque 90°/s | 3.82 | 0.53–8.17 | 0.28 | 4.38 | −1.50–10.3 | 0.20 |
| Device Resistance Level | 2.00 | −0.01–3.99 | 0.29 | 4.09 | 0.02–8.20 | 0.33 |
| SCALE | 0.71 | 0.23–1.67 | 0.17 | −0.69 | −1.45–0.06 | 0.25 |
Discussion
The first hypothesis stated that device cadence would increase as a task-specific effect of training. This was supported in that for both device groups, the major change after training was a marked increase in device cadence with the high maximum cadences achieved particularly remarkable. A potential advantage of each of these devices over treadmills that may have facilitated this outcome is the apparently greater ease in producing a faster cadence that is closer to normal walking cadence as well as a more variable one since the pace was self-initiated rather than externally imposed.
The underlying neural premise here was that children with bilateral CP often seem to experience difficulty when required to perform asynchronous leg tasks that may be due in part to an underlying deficit in reciprocal coordination. Abnormal development of spinal interneurons after early brain injury have been identified and have been found to affect motor prognosis and the later development of the corticospinal tract and the motor map32. Speed-focused training may help children to more rapidly turn their muscles on and off as needed to perform alternating leg tasks. The ability to increase speed or cadence with training was clearly and dramatically shown here, but was specific to the device, at least at the dose delivered here and was related to progressively greater effort (resistance) during training. Interestingly, peak torque production at the fastest isokinetic speed also increased significantly, although was not related to speed outcomes. This increase is notable because a deficit in the rate of producing force is more pronounced and functionally relevant than weakness in CP 33 and has been shown to respond differentially to high velocity training 34. However, increased training resistance accounted for only a small portion of the variance (R2 = 0.36) in increased device cadence, suggesting that additional factors such as improved reciprocal coordination may have been responsible for the change. It is also possible that the increased resistance produced greater electrical (EMG) input to the brain which may have helped to improve motor coordination as well; however, these hypotheses will require further investigation. The maintenance of device cadence three months after training in those with a post-intervention follow-up further suggests that the change was more likely due to improved motor coordination rather than strength changes which would have been more transient.
Reciprocal or interlimb coordination for walking primarily involves the proximal hip musculature to either advance the limb forward or be stretched passively as the opposite limb advances. Intralimb coordination is fundamentally different, i.e. whether one joint can move independently from others in the same limb. We utilized the SCALE here to assess selective control before and after training; however, this test primarily evaluates intralimb control which may help explain why both device groups could have had similar increases in cadence due to improvements in interlimb coordination while their results on the SCALE, while not statistically or clinically significant35 in the group comparison, were in opposite directions which led to a significant interaction. Ankle movements on the elliptical are not tightly coupled to the hip or knee as they are on the cycle, which may have caused the small divergence in changes in intralimb control for the elliptical and cycle. Similarly, individual results for gait velocity were more positively skewed for those in the elliptical group and more negatively skewed for those in the cycle groups, with 4 of 5 participants showing a clinically significant 0.1 m/sec improvement being in the elliptical group and 3 of 4 showing a 0.01 m/sec decrease being in the cycle group. While these individual trends were not supported by significant group differences or a group by time interaction, potential differences in training regimens warrant further evaluation. However, it is important to note that more than two-thirds of all participants in each group improved in gait speed after training.
One major limitation of this study was that the comparison of outcomes across device groups was undermined by the inability to randomize some children to the elliptical group due to smaller physical size and/or greater motor impairments. The two groups, while not significantly different in age, weight or height in this relatively small sample, did show a disproportionate distribution in GMFCS levels II and III with many more in the cycle group classified at lower functional levels; a difference which was reinforced by significantly lower PODCI scores, PEDI Self-care scores and non-normalized strength values in that group as well as lower cycle cadence at free speed. The elliptical required greater baseline coordination and interestingly one child in the cycle group who could not continuously pedal the elliptical at baseline was able to do this quite proficiently after the cycle training, suggesting that children who are less functional could be progressed to this more challenging device. The elliptical cadence for those in the cycle group was the only outcome with incomplete data having no-pre-post data in 6 participants with 1 of the 6 who could not do it at baseline able to do this after training. Other study limitations included non-blinding of therapists who conducted the isokinetic torque and SCALE assessments, and that fact that the 3 month non-training period differed across subjects with some having a double baseline before training and others have a follow-up after training. This was done to expedite enrollment as devices became available.
This randomized trial, by demonstrating that children with bilateral CP could significantly increase both speed and resistance on the trained device, suggests that these devices could provide new options for achieving fitness goals in CP. Cycling places far less compressive stress on the joints than treadmill walking, a factor that will be increasingly important as the length of time these devices are utilized increases. Elliptical trainers involve a motion that is similar to cycling, but involves stepping plus limb loading in upright that more closely simulates over ground or treadmill walking. These also have smaller joint impact forces compared to treadmill walking36. Many individuals with CP experience earlier and greater joint deterioration due to their abnormal loading patterns and the high impact forces they may experience during walking, which may lead to a cessation of walking in adulthood37. Therefore, it is critically important that any long-term exercise or therapy does not significantly exacerbate joint stress and deterioration.
The second half of the first hypothesis that training would lead to improvements in gait speed as a result of training was not supported here. A recent systematic review of interventions for children with CP aimed to improve gait speed demonstrated that gait training was the most effective in increasing gait speed with resistance training not shown to be effective, with the exception of high velocity (power) training38. Given the task specificity of gait training, this finding is not entirely surprising. Frequency of the interventions, but not total doses, were reported in the review. We believe that our lack of significant changes in gait speed here for each group as a whole may have been due to the fact that the maximum total training dose for any participant was only 20 hours. This is far less than the minimal dose hours associated with positive outcomes from upper limb training, so it is possible that with extended training, the elliptical device in particular could ultimately have positive effects on gait and other aspects of gross motor function, assuming the trends were sustained over time Many, if not most, studies on locomotor training and cycling in CP have used even lower total doses; therefore, dosing studies on locomotor training paradigms should be performed in CP before concluding that these are not effective. Similar to strength training in the lower extremities, it may take far more training before functional benefits become evident 39.
The parent (proxy) -report measures of their child’s physical function also did not change after training. The sensitivity of the selected secondary functional measures for measuring changes after physical therapy is also a consideration with the PODCI showing greater responsiveness to change for surgical interventions40 and with the PEDI-CAT not as well studied in older children and those with chronic conditions41,42. It is possible that a condition specific evaluative measure such as the GMFM-66 or computer-adapted test such as the recently published CP LE CAT43 may have been more responsive.
While devices have featured prominently in locomotor training, Bleyenheuft and colleagues 44 challenged this practice by presenting significant positive results on the 6-minute walk test and the ABILICO functional mobility measure from intensive bilateral upper and lower limb training of approximately 80 hours compared to the same dose of conventional therapy, based on the Bobath method which has been shown not to be effective 2. The much higher training dose in that study is notable and warrants comparison to other activity-based or device augmented therapies of equal doses.
The strengths of this study were the implementation of two novel devices in CP, the high compliance rate, a fairly homogeneous study group in that all were born preterm and had diffuse white matter injury with resultant spastic CP, and absence of significant adverse events. Limitations were failed randomization in some subjects that compromised the functional equivalence of study groups. This could be addressed in the future by adding size criteria and giving subjects more opportunity for practice on the elliptical prior to training.
Concluding Remarks
Intense leg training with a motor-assisted cycle and elliptical device produced large task-specific changes that could enable children with CP to exercise at higher speeds and aerobic levels than before training. The primary aim to improve gait and gross motor function was not achieved, although positive individual and mean trends were seen mainly in the elliptical group that may become more evident with longer training. Highly effective therapies for improving gait and gross motor function still remain elusive in CP with inadequate dosing likely one factor.
Acknowledgments
This work was funded by the Intramural Research Program of the National Institutes of Health Clinical Center (Protocol # 10-CC-0073).
Footnotes
COI Statement: All authors declare that there is no conflict of interest.
References
- 1.Huang HH, Fetters L, Hale J, McBride A. Bound for success: a systematic review of constraint-induced movement therapy in children with cerebral palsy supports improved arm and hand use. Phys Ther. 2009;89(11):1126–41. doi: 10.2522/ptj.20080111. [DOI] [PubMed] [Google Scholar]
- 2.Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, Stumbles E, Wilson SA, Goldsmith S. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol. 2013;55:885–910. doi: 10.1111/dmcn.12246. [DOI] [PubMed] [Google Scholar]
- 3.Damiano DL, DeJong SL. A systematic review of the effectiveness of treadmill training and body weight support in pediatric rehabilitation. J Neurol Phys Ther. 2009;33(1):27–44. doi: 10.1097/NPT.0b013e31819800e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willoughby KL, Dodd KJ, Shields N, Foley S. Efficacy of partial body weight-supported treadmill training compared with overground walking practice for children with cerebral palsy: a randomized controlled trial. Arch Phys Med Rehabil. 2010;91(3):333–9. doi: 10.1016/j.apmr.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, Dobkin BH, Rose DK, Tilson JK, Cen S, Hayden SK LEAPS Investigative Team. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364(21):2026–36. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobkin BH, Duncan PW. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil Neural Repair. 2012;26(4):308–17. doi: 10.1177/1545968312439687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidler J, Lum PS. The road ahead for rehabilitation robotics. J Rehabil Res Dev. 2011;48(4):vii–x. doi: 10.1682/jrrd.2011.02.0014. [DOI] [PubMed] [Google Scholar]
- 8.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–39. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 9.Nudo RJ. Recovery after brain injury: mechanisms and principles. Front Hum Neurosci. 2013;7:887. doi: 10.3389/fnhum.2013.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakzewski L, Gordon A, Eliasson AC. The state of the evidence for limb therapy approaches for children with unilateral cerebral palsy. J Child Neurol. 2014;29:1077–90. doi: 10.1177/0883073814533150. [DOI] [PubMed] [Google Scholar]
- 11.Gordon AM. To constrain or not to constrain, and other stories of intensive upper extremity training for children with unilateral cerebral palsy. Dev Med Child Neurol. 2011;53(Suppl 4):56–61. doi: 10.1111/j.1469-8749.2011.04066.x. [DOI] [PubMed] [Google Scholar]
- 12.Dong VA, Tung IH, Siu HW, Fong KN. Studies comparing the efficacy of constraint-induced movement therapy and bimanual training in children with unilateral cerebral palsy: a systematic review. Dev Neurorehabil. 2013;16(2):133–43. doi: 10.3109/17518423.2012.702136. [DOI] [PubMed] [Google Scholar]
- 13.Dimitrijevic MR, Danner SM, Mayr W. Neurocontrol of Movement in Humans With Spinal Cord Injury. Artif Organs. 2015;39:823–33. doi: 10.1111/aor.12614. [DOI] [PubMed] [Google Scholar]
- 14.Bulea TC, Kim J, Damiano DL, Stanley CJ, Park HS. User-driven control increases cortical activity during treadmill walking: an EEG study. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:2111–4. doi: 10.1109/EMBC.2014.6944033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer-Heim A, van Hedel HJ. Robot-assisted and computer-enhanced therapies for children with cerebral palsy: current state and clinical implementation. Semin Pediatr Neurol. 2013 Jun;20:139–45. doi: 10.1016/j.spen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Riener R, Dislaki E, Keller U, Koenig A, Van Hedel H, Nagle A. Virtual reality aided training of combined arm and leg movements of children with CP. Stud Health Technol Inform. 2013;184:349–55. [PubMed] [Google Scholar]
- 17.Fowler EG, Knutson LM, Demuth SK, Siebert KL, Simms VD, Sugi MH, Souza RB, Karim R, Azen SP Physical Therapy Clinical Research Network (PTClinResNet) Pediatric endurance and limb strengthening (PEDALS) for children with cerebral palsy using stationary cycling: a randomized controlled trial. Phys Ther. 2010;90:367–81. doi: 10.2522/ptj.20080364. [DOI] [PubMed] [Google Scholar]
- 18.Chen CL, Hong WH, Cheng HY, Liaw MY, Chung CY, Chen CY. Muscle strength enhancement following home-based virtual cycling training in ambulatory children with cerebral palsy. Res Dev Disabil. 2012;33(4):1087–94. doi: 10.1016/j.ridd.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Williams H, Pountney T. Effects of a static bicycling programme on the functional ability of young people with cerebral palsy who are non-ambulant. Dev Med Child Neurol. 2007;49:522–527. doi: 10.1111/j.1469-8749.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- 20.Damiano DL, Norman T, Stanley CJ, Park HS. Comparison of elliptical training, stationary cycling, treadmill walking and overground walking. Gait Posture. 2011;34:260–4. doi: 10.1016/j.gaitpost.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ting LH, Raasch CC, Brown DA, Kautz SA, Zajac FE. Sensorimotor state of the contralateral leg afferents ipsilateral muscle coordination of pedaling. J Neurophys. 1998b;80:1341–1351. doi: 10.1152/jn.1998.80.3.1341. [DOI] [PubMed] [Google Scholar]
- 22.Ting LH, Kautz SA, Brown DA, Van der Loos HF, Zajac FE. Bilateral integration of sensorimotor signals during pedaling. Ann N Y Acad Sci. 1998;860:513–516. doi: 10.1111/j.1749-6632.1998.tb09091.x. [DOI] [PubMed] [Google Scholar]
- 23.Brooke JD, Mellroy WE, Staines WR, Angerilli PA, Peritore GF. Cutaneous reflexes of the human leg during passive movement. J Physiol. 1999;518:619–628. doi: 10.1111/j.1469-7793.1999.0619p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez MA, Field-Fote EC, Floeter MK. Patterned sensory stimulation induces plasticity in reciprocal Ia inhibition in humans. J Neurosci. 2003;23:2014–2018. doi: 10.1523/JNEUROSCI.23-06-02014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamm K, Thelen E, Jensen JL. A dynamical systems approach to motor development. Phys Ther. 1990;70:763–75. doi: 10.1093/ptj/70.12.763. [DOI] [PubMed] [Google Scholar]
- 26.Curtis DJ, Butler P, Saavedra S, Bencke J, Kallemose T, Sonne-Holm S, Woollacott M. The central role of trunk control in the gross motor function of children with cerebral palsy: a retrospective cross-sectional study. Dev Med Child Neurol. 2015;57:351–7. doi: 10.1111/dmcn.12641. [DOI] [PubMed] [Google Scholar]
- 27.Smania N, Bonetti P, Gandolfi M, Cosentino A, Waldner A, Hesse S, Werner C, Bisoffi G, Geroin C, Munari D. Improved gait after repetitive locomotor training in children with cerebral palsy. Am J Phys Med Rehabil. 2011;90:137–49. doi: 10.1097/PHM.0b013e318201741e. [DOI] [PubMed] [Google Scholar]
- 28.Field-Fote EC. Electrical stimulation modifies spinal and cortical neural circuitry. Exerc Sport Sci Rev. 2004;32:155–60. doi: 10.1097/00003677-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Fowler EG, Staudt LA, Greenberg MB, Oppenheim WL. Selective Control Assessment of the Lower Extremity (SCALE): development, validation, and interrater reliability of a clinical tool for patients with cerebral palsy. Dev Med Child Neurol. 2009;51:607–14. doi: 10.1111/j.1469-8749.2008.03186.x. [DOI] [PubMed] [Google Scholar]
- 30.Haley SM, Raczek AE, Coster WJ, et al. Assessing mobility in children using a computer adaptive testing version of the Pediatric Evaluation of Disability Inventory (PEDI) Arch Phys Med Rehabil. 2005a;86:932–939. doi: 10.1016/j.apmr.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Daltroy LH, Liang MH, Fossel AH, Goldberg M. The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. Pediatric Outcomes Instrument Development Group. Pediatric Orthopaedic Society of North America. J Pediatr Orthop. 1998;18:561–571. doi: 10.1097/00004694-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Friel KM, Williams PT, Serradj N, Chakrabarty S, Martin JH. Activity-Based Therapies for Repair of the Corticospinal System Injured during Development. Front Neurol. 2014;24(5):229. doi: 10.3389/fneur.2014.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreau NG, Falvo MJ, Damiano DL. Rapid force generation is impaired in cerebral palsy and is related to decreased muscle size and functional mobility. Gait Posture. 2012;35:154–8. doi: 10.1016/j.gaitpost.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau NG, Holthaus K, Marlow N. Differential adaptations of muscle architecture to high-velocity versus traditional strength training in cerebral palsy. Neurorehabil Neural Repair. 2013;27:325–34. doi: 10.1177/1545968312469834. [DOI] [PubMed] [Google Scholar]
- 35.Balzer J, Marsico P, Mitteregger E, van der Linden ML, Mercer TH, van Hedel HJ. Construct validity and reliability of the Selective Control Assessment of the Lower Extremity in children with cerebral palsy. Dev Med Child Neurol. 2016;58:167–72. doi: 10.1111/dmcn.12805. [DOI] [PubMed] [Google Scholar]
- 36.Lu TW, Chien HL, Chen HL. Joint loading in the lower extremities during elliptical exercise. Med Sci Sports Exerc. 2007;39:1651–1658. doi: 10.1249/mss.0b013e3180dc9970. [DOI] [PubMed] [Google Scholar]
- 37.Bottos M, Gericke C. Ambulatory capacity for cerebral palsy: prognostic criteria and consequences for intervention. Dev Med Child Neurol. 2004;5:786–90. doi: 10.1017/s0012162203001452. [DOI] [PubMed] [Google Scholar]
- 38.Moreau NG, Bodkin AW, Bjornson K, Hobbs A, Soileau M, Lahasky K. Effectiveness of Rehabilitation Interventions to Improve Gait Speed in Children With Cerebral Palsy: Systematic Review and Meta-analysis. Phys Ther. 2016;96:1938–1954. doi: 10.2522/ptj.20150401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, McCartney N. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord. 2003;41:34–43. doi: 10.1038/sj.sc.3101389. [DOI] [PubMed] [Google Scholar]
- 40.Allen DD, Gorton GE, Oeffinger DJ, Tylkowski C, Tucker CA, Haley SM. Analysis of the pediatric outcomes data collection instrument in ambulatory children with cerebral palsy using confirmatory factor analysis and item response theory methods. J Pediatr Orthop. 2008;28:192–8. doi: 10.1097/BPO.0b013e3181652185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dumas HM, Fragala-Pinkham MA, Rosen EL, Lombard KA, Farrell C. Pediatric Evaluation of Disability Inventory Computer Adaptive Test (PEDI-CAT) and Alberta Infant Motor Scale (AIMS): Validity and Responsiveness. Phys Ther. 2015;95:1559–68. doi: 10.2522/ptj.20140339. [DOI] [PubMed] [Google Scholar]
- 42.Fragala-Pinkham MA, Dumas HM, Lombard KA, O’Brien JE. Responsiveness of the Pediatric Evaluation of Disability Inventory-Computer Adaptive Test in measuring functional outcomes for inpatient pediatric rehabilitation. J Pediatr Rehabil Med. 2016;9:215–22. doi: 10.3233/PRM-160382. [DOI] [PubMed] [Google Scholar]
- 43.Haley SM, Chafetz RS, Tian F, Montpetit K, Watson K, Gorton G, Mulcahey MJ. Validity and reliability of physical functioning computer-adaptive tests for children with cerebral palsy. J Pediatr Orthop. 2010;30:71–5. doi: 10.1097/BPO.0b013e3181c85453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bleyenheuft Y, Arnould C, Brandao MB, Bleyenheuft C, Gordon AM. Hand and arm bimanual intensive therapy including lower extremity (HABIT-ILE) in children with unilateral spastic cerebral palsy: A randomized trial. Neurorehabil Neural Repair. 2015;29:645–57. doi: 10.1177/1545968314562109. [DOI] [PubMed] [Google Scholar]