Abstract
Background
The aim of this research was to study the function of NLRP3 in the pathogenesis and development of diabetic nephropathy (DN).
Material/Methods
We compared the expression of NLRP3-related protein in human glomerular mesangial cells under high glucose conditions at different times and in rats with DN of different ages. We also compared changes in IL-18 and IL-1β expression levels at different stages of DN.
Results
After six hours, 12 hours, and 24 hours of high glucose stimulation, the secretion of IL-1β in human glomerular mesangial cells, compared to unstimulated cells, was 1.85-fold, 3.04-fold, and 4.14-fold; the expression of NLRP3 increased by 2.20-fold, 4.62-fold, and 8.32-fold; and the expression of caspase-1 was increased by 1.60-fold, 2.72-fold, and 3.67-fold. The expression levels of nephrin in eight-week-old and 12-week-old DN rats compared to 4-week rats were 49.60% and 21.20%, respectively. The IL-1β levels compared to four-week DN rats were 2.57-fold and 4.17-fold, respectively; NLRP3 levels were 1.29-fold and 2.17-fold respectively, and caspase-1 levels were 3.37-fold and 4.16-fold, respectively. The serum levels of IL-18 and IL-1β in the DN group were the highest at 218.53±30.69 pg/mL and 62.47±9.36 pg/mL, respectively; followed by the mild DN group at 177.07±32.88 pg/mL and 28.13±5.37 pg/mL, respectively, with the diabetic mellitus (DM) group having the lowest levels at 141.47±9.49 pg/mL and 15.53±3.26 pg/mL, respectively. The healthy control group levels were 99.40±22.72 pg/mL and 12.40±5.08 pg/mL, respectively.
Conclusions
NLRP3 and high glucose activation may participate in the occurrence and development of DN by mediating the inflammatory response.
MeSH Keywords: Diabetic Nephropathies, Mesangial Cells, Systemic Inflammatory Response Syndrome
Background
Diabetic nephropathy (DN) is one of the most important complications of diabetes mellitus (DM). In recent years, with the increase of DM patients and morbidity in China, patients of DN have been also increased annually [1]. At present, the pathogenesis of DN remains unclear. However, the inflammatory theory has been recognized as the core of DN prevalence in the academic world [2]. The inflammatory theory holds that persistent high-glucose environment inside DM patients leads to the increase of inflammatory factors secreted by renal cells, such as interleukin 1β (IL-1β), interleukin-18 (IL-18) and C-reactive protein (CRP), and further results in persistent inflammatory reaction, which is an important pathological basis of DN prevalence [3,4].
NLRP3 inflammasome is a compound composed of NLRP3, apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1. Activated NLRP3 inflammasome can activate multiple inflammatory factors including IL-1β and IL-18 through split caspase-1 [5]. However, the acting mechanisms of NLRP3 inflammasome in the prevalence and the progression of DN remain unclear at present. Wang et al. [6] and Qiu et al. [7] pointed out that urate and lipid accumulated in DM patients can activate NLRP3 inflammasome and cause inflammatory reaction, which results in kidney injury. It was also found that quercetin and allopurinol can reduce urate and lipid accumulated in DN patients, thereby protecting the kidney of DN patients. A new study further proposes that inhibiting the expression and activation of NLRP3 inflammasome in DN patients can improve the renal function of patients [8]. This indicates that NLRP3 inflammasome not only participates in the morbidity of DN, but also may be closely related to further progression of DN. In the present study, the role of NLRP3 inflammasome in the progression of DN was further investigated through cell models, animal models, and blood samples of DN patients in different stages of DN.
Material and Methods
Patients
Forty-five type 2 DM patients hospitalized in Luhe Hospital Affiliated to Capital Medical University between September 2014 and October 2015 were selected for this study. The study was approved by the Ethics Committee of Luhe Hospital. All of the participants signed informed consent before research.
The patients were divided into pure DM group (Alb <20 mg/L), mild DN group (20 mg/L ≤ Alb ≤200 mg/L) and moderate and severe DN group (Alb >200mg/L), with 15 cases in each group. Meanwhile, 10 healthy subjects who received the physical examination in our hospital during the same period were selected as the controls. The difference between each group with respect to general data such as age and sex was not statistically significant (p>0.05) (Table 1).
Table 1.
Clinical data of subjects in each group.
| Groups | N (case) | Age (year) | Male (case) | Alb (mg/L) | IL-18 (pg/ml) | IL-1β (pg/ml) |
|---|---|---|---|---|---|---|
| Healthy control group | 10 | 51.20±6.14 | 6 | 7.00±3.81 | 99.40±22.72 | 12.40±5.08 |
| Pure DM group | 15 | 55.27±7.24 | 9 | 11.27±3.56* | 141.47±9.49* | 15.53±3.26* |
| Mild DN group | 15 | 55.93±7.34 | 8 | 130.33±41.53*# | 177.07±32.88*# | 28.13±5.37*# |
| Moderate and severe DN group | 15 | 55.20±7.05 | 9 | 298.33±51.00*#& | 218.53±30.69*#& | 62.47±9.36*#& |
| F | 1.212 | 9.856 | 161.010 | 35.279 | 137.598 | |
| P | 0.317 | 0.129 | 0.000 | 0.000 | 0.000 |
P<0.05, compared with healthy control group;
P<0.05, compared with pure DM group;
P<0.05, compared with mild DN group.
Cell and animal models
Forty male Wistar rats six to eight weeks old, 175–185 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. Human mesangial cells were purchased from Guangzhou DOCLAB Biotechnology Co., Ltd. (HBZY-1). Animal protocols were approved by the Ethics Committee of Luhe Hospital.
Human IL-18 and human IL-1β detection kits were purchased from Shanghai Xin Yu Biotech Co., Ltd. (xy-E10119, xy-E10110). IL-1β (sc-23460), IL-18 (sc-6177-Y), NLRP3 (sc-66846), caspase-1 (sc-1780) primary antibodies and goat anti-rabbit secondary antibodies (sc-3846) were purchased from Santa Cruz Biotechnology (USA).
Collection and detection of clinical specimens
Blood samples of 10 mL fasting venous blood were collected in the morning from all patients. After centrifugation at room temperature (1,000 rpm, 10 minutes), the separated serum was stored at −80°C or directly used for IL-1β and IL-18 detection (kit detection); 24-hour urine was collected from 8: 00 a.m. to 8: 00 a.m. on the next day. Methylbenzene was added to the collected urine for antisepsis. Then 5 mL of urine was used to detect albumin (Alb) by the colloidal gold method. The Alb/L of urine was calculated.
Preparation and grouping of DN rats
After the rats were acclimated for one week, 10 rats were randomly selected as the control group, and 30 rats were used to establish DN models through intraperitoneal injection of streptozotocin (STZ) (30 mg/kg). The DN model was established successfully if the fasting blood glucose maintained above 16.7 mol/L, the blood glucose was positive, and the urine amount was more than 1.5 times that of the original [9]. At four, eight and 12 weeks after modeling, the kidneys of five rats were removed for the detection of each index.
Mesangial cells stimulated by high glucose
Mesangial cells were thawed and subcultured. Passage was conducted one day before the experiment using cells in good conditions. When the cell grew to 60% density, the medium was changed to serum-free, low-glucose, DMEM medium. Then 30 mol/L glucose was added at the ratio of 1: 1,000. The cells were cultured for 0 hours, six hours, 12 hours, and 24 hours. After the cells were treated by glucose for different time points, they were digested and the total protein (TP) extracted using the extraction kit. The TP concentration was determined using the BCA kit. Then 100 ug of protein was used for SDS-PAGE. Then wet transfer was conducted; then blocking, color development following the addition of primary antibodies and secondary antibodies, and band analysis by ImagJ software were conducted successively. Moreover, the supernatant was collected and the IL-1β content in the supernatant was detected by the kit (xy-E10110, Shanghai Xin Yu Biotech Co., Ltd., China).
Expression of related genes in the renal tissue of DN rats
DN rats were sacrificed at four weeks, eight weeks, and 12 weeks after modeling, and their kidneys were removed. After the kidney was cleaned by normal saline, the TP of the kidney tissues was extracted using the extraction kit. The TP concentration was determined using the BCA kit. 100 ug of protein was used for SDS-PAGE. Then wet transfer was conducted; then blocking, color development following the addition of primary antibodies and secondary antibodies, and band analysis by ImagJ software were conducted successively.
DN rats were sacrificed at four weeks, eight weeks, and 12 weeks after modeling, and their kidneys were removed. After having been cleaned by normal saline, the kidney was fixed by 10% formalin solution for over 24 hours, and then subjected to dehydration, clearing, waxing, embedding, and slicing. The immumohistochemical staining was conducted as per kit instructions. PBS was used as the negative control.
Statistical analysis
Statistical analysis of the data was conducted using SPSS 20.0 software. Measurement data were presented as mean ± standard deviation (χ̄±s). The independent-samples t-test was used for group comparisons, with p<0.05 indicating significant difference. One-way ANOVA was adopted for multi-group comparisons, with p<0.05 indicating significant difference.
Results
Influence of high glucose on the expression of NLRP3 inflammasome-related proteins in mesangial cells
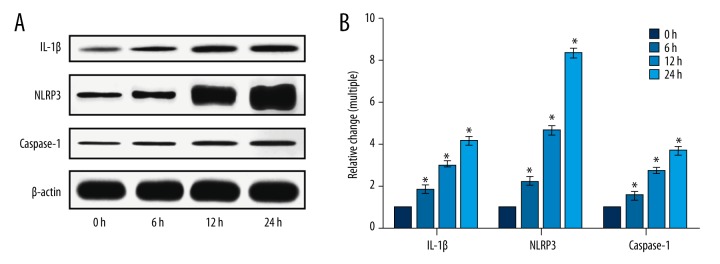
After mesangial cells were stimulated by high glucose for different time points, the expression of IL-1β, NLRP3, and caspase-1 was increased significantly (p<0.05). After high glucose stimulation for six hours, 12 hours, and 24 hours, IL-1β expression was increased by 1.85 times, 3.04 times, and 4.14 times, respectively; NLRP3 expression was increased by 2.20 times, 4.62 times, and 8.32 times, respectively; caspase-1 expression was increased by 1.60 times, 2.72 times, and 3.67 times, respectively, as shown in Figure 1.
Figure 1.
Influence of high glucose on the expression of IL-1β, NLRP3, and caspase-1 in mesangial cells. (A) Proteins IL-1β, NLRP3, and caspase-1 determined by Western blot assay. (B) The relative change analysis of the Western blot result. * p<0.05, compared with 0 hours.
Expression of NLRP3 inflammasome-related proteins in DN rats of different age
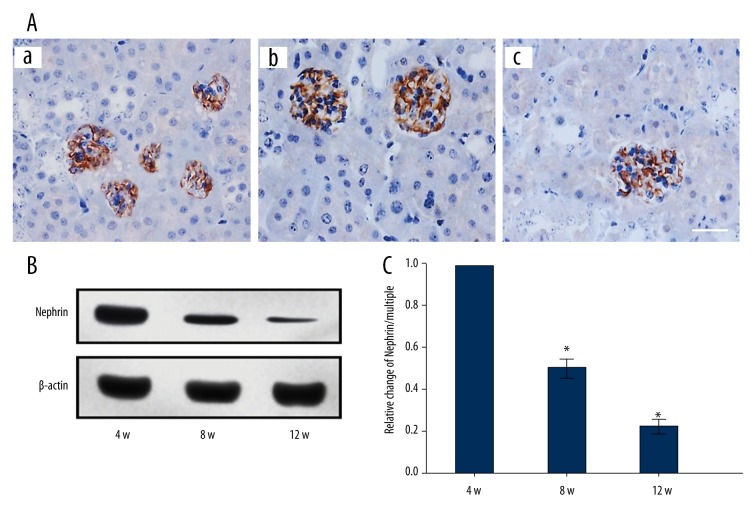
The expression of nephrin in DN rats was decreased significantly with an increase in age (p<0.05). Nephrin expression of the eight-week rats and the 12-week rats was 49.60% and 21.20% respectively, compared to the four-week rats (Figure 2).
Figure 2.
Expression of nephrin in DN rats of different ages. (A) The protein nephrin determined by immunohistochemistry. (a–c) Nephrin expression of DN four-week rats, eight-week rats, and 12-week rats. (B) The expression of nephrin determined by Western blot assay. (C) Analysis of the result B. * p<0.05, compared with 4-week rats. Bar: 50 μm.
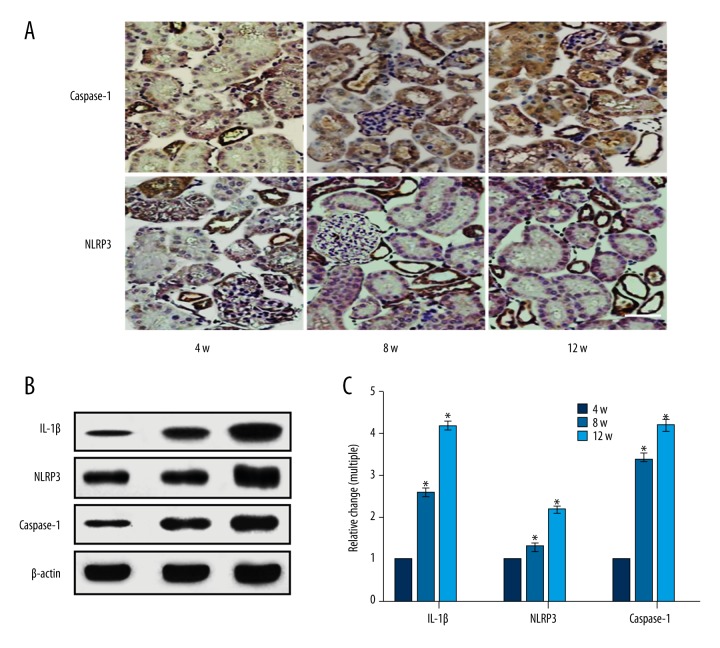
The expression of renal IL-1β, NLRP3, and caspase-1 in DN rats was increased significantly with an increase in age (p<0.05). The IL-1β expression of 8-week rats and 12-week rats was 2.57 times and 4.17 times that of 4-week rats, respectively. The NLRP3 expression of 8-week rats and 12-week rats was 1.29 times and 2.17 times that of 4-week rats, respectively. The caspase-1 expression of 8-week rats and 12-week rats was 3.37 times and 4.16 times that of 4-week rats, respectively (Figure 3).
Figure 3.
Expression of NLRP3 inflammasome-related proteins in DN rats of different ages. (A) The NLRP3 and caspase-1 expression of DN rats determined by immunohistochemistry. (B) IL-1β, NLRP3 and caspase-1 determined by Western blot assay. (C) Analysis of the result of B. * p<0.05, compared with 4-week rats. Bar: 50 μm.
Serum IL-18 and IL-1β of DN patients at different stages
There was significant difference in Alb, IL-18, and IL-1β between groups (p<0.05). Alb, IL-18, and IL-1β of the healthy control group, the pure DM group, the mild DN group, and the moderate and severe DN group were increased gradually (Table 1).
Discussion
The concept of inflammasome was proposed by the Tschopp research group in 2002. They pointed out that inflammasome is a compound composed of multiple proteins, and it is an important part of innate immune system of organism. Pathogen-associated molecular patterns (PAMP) or other risk signals of organism may activate the inflammasome. The activated inflammasome may promote the activation of multiple inflammatory factors through regulating the activation of caspase-1 protein, or perform the immunization effects through inducing the inflammatory necrosis of cells [10]. NLRP3 inflammasome is currently one of the most studied inflammasomes. It is mainly composed of NLRP3 protein which identifies pathogens or risk signals, caspase-1 effector protein which promotes the secretion and activation of inflammatory factors, and ASC protein which connects NLRP3 receptor protein and caspase-1 effector protein [11]. Gross et al. [12] considered that in fungus, NLRP3 inflammasome may be activated by the signal pathway mediated by tyrosine kinase. Meanwhile, in the human body, influenza virus may activate the NLRP3 inflammasome through changing the M2 ion channel inside the cells [13]. The persistent high glucose and high acid environment of DM patients was identified as “risk signals” by the NLRP3 inflammasome, thus the NLRP3 inflammasome was activated [6,7].
In the present study, it was found that after mesangial cells were stimulated by high glucose for different time points, the expression of NLRP3 and caspase-1 was increased significantly, and increased with the rise of stimulating time. These factors indicate that a high glucose environment may serve as a “risk factor” to stimulate the expression and activation of NLRP3 inflammasome in human mesangial cells, and that the expression and the activation of NLRP3 inflammasome depends on high glucose stimulation time.
In the further animal experiment, with the increased age of DN rats, the nephrin expression in the kidney was decreased gradually, and the expression of NLRP3 and caspase-1 was increased gradually. Nephrin is a protein specifically expressed in the glomerular epithelial cells, which is essential for the filtration of glomeruli. A previous study on DN rats indicated that nephrin protein was one important index of kidney injury of DN rats, and was involved in the occurrence and development of proteinuria of DN rats [14]. The present study found that the expression of nephrin protein was decreased with increased age of DN rats, suggesting that the older the DN rat is the more severe the kidney injury will be. DN rats of different ages are similar to DN rats at different stages to a certain extent. It was also found that the expression of NLRP3 and caspase-1 was increased with the increased age of DN rats, which suggests the followings: NLRP3 inflammasome is significantly different in DN rats at different stages; and the expression of NLRP3 inflammasome in the kidney tissue is increased with the increased severity of kidney injury of DN rats.
Our study also found that IL-1β content in the culture medium was increased with the extension of high glucose stimulation, and that IL-1β expression in the kidney tissue was also increased with the increased age of DN rats. IL-1 is a cytokine synthesized and secreted by monocyte-macrophages. There are two forms, i.e., IL-α and IL-β. Initially, the form of IL-1β is a 30 KDa precursor, i.e., Pro-IL-1β. After the NLRP3 inflammasome is stimulated by pathogen-related factors or “risk signals”, Pro-IL-1β is degraded into IL-1β by the effector protein caspase-1 of the NLRP3 inflammasome. IL-1β is an activated 17 KDa cytokine composed of 153 amino acids. When IL-1β is secreted, it performs its effects outside the cell [15]. IL-1β is an important inflammatory factor, and high IL-1β content indicates strong inflammatory reaction [16]. It has been shown that high glucose stimulation can activate the NLRP3 inflammasome inside the cells or the kidney of DN rats. The activated NLRP3 inflammasome may promote the secretion of inflammatory factors such as IL-1β, leading to persistent inflammatory reaction of cells or DN rats. Yuan et al. pointed out that NLRP4 inflammasome may aggravate disease progression by upregulating the IL-1β of DN patients [17]. NLRP3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy [18]. This indicates that NLRP inflammasome and IL-1β play an important role in the progression of diabetic nephropathy.
After we analyzed the content of inflammatory factors such as serum IL-18 and IL-1β in DN patients at different stages, we found that serum IL-18 and IL-1β were increased with increased urine Alb. Alb is a normal protein in the blood. Since it can be blocked by the kidney, there is only a minute quantity of Alb in the urine under physiological conditions. In clinical settings, microalbuminuria is regarded as the symptom of early kidney injury, including DN and hypertensive nephropathy. More Alb in the urine indicates more severe kidney injury [19–22].
Conclusions
For DM patients, a “risk signal” is a persistent high glucose environment as it stimulates the activation of NLRP3 inflammasome. The activated NLRP3 inflammasome may promote the secretion of inflammatory factors including IL-1β. Persistent inflammatory reaction leads to kidney injury in DM patients and the development of DN. NLRP3 inflammasome activated by high glucose is involved in the development of DN through mediating the inflammatory reaction inside DN patients. The limitation of this study was that we could not obtain renal biopsy samples from patients with DN. We only detect the inflammatory factors in renal tissue in the animal model. In order to get more information there is a need for further research.
Footnotes
Source of support: Departmental sources
Declaration of conflict of interest
None.
References
- 1.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 2.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2013;124:139–52. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 3.Vaarala O, Yki-Jarvinen H. Diabetes: Should we treat infection or inflammation to prevent T2DM? Nat Rev Endocrinol. 2012;8(6):323–25. doi: 10.1038/nrendo.2012.31. [DOI] [PubMed] [Google Scholar]
- 4.Chen HY, Huang XR, Wang W, et al. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes. 2011;60:590–601. doi: 10.2337/db10-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youm YH, Adijiang A, Vandanmagsar B, et al. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152:4039–45. doi: 10.1210/en.2011-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Pan Y, Zhang QY, et al. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS One. 2012;7:11. doi: 10.1371/journal.pone.0038285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu YY, Tang LQ. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol Res. 2016;114:251–64. doi: 10.1016/j.phrs.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Sakai N, Furuichi K, Wada T. Inhibition of NLRP3 inflammasome as a therapeutic intervention in crystal-induced nephropathy. Kidney Int. 2016;90:466–68. doi: 10.1016/j.kint.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Xie X, Li Z, Pi M, et al. [Down-regulation of p38 MAPK and collagen by 1, 25-(OH)2-VD3 in rat models of diabetic nephropathy]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32:931–35. [in Chinese] [PubMed] [Google Scholar]
- 10.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 11.Hutton HL, Ooi JD, Holdsworth SR, et al. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology. 2016;21:736–44. doi: 10.1111/nep.12785. [DOI] [PubMed] [Google Scholar]
- 12.Gross O, Poeck H, Bscheider M, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–36. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 13.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010;11:404–10. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhai L, Gu J, Yang D, et al. Metformin ameliorates podocyte damage by restoring renal tissue nephrin expression in type 2 diabetic rats. J Diabetes. 2016 doi: 10.1111/1753-0407.12437. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Traba J, Sack MN. The role of caloric load and mitochondrial homeostasis in the regulation of the NLRP3 inflammasome. Cell Mol Life Sci. 2016;10:10. doi: 10.1007/s00018-016-2431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie M, Hu A, Luo Y, et al. Interleukin-4 and melatonin ameliorate high glucose and interleukin-1beta stimulated inflammatory reaction in human retinal endothelial cells and retinal pigment epithelial cells. Mol Vis. 2014;20:921–28. [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan F, Kolb R, Pandey G, et al. Involvement of the NLRC4-inflammasome in diabetic nephropathy. PLoS One. 2016;11(10):e0164135. doi: 10.1371/journal.pone.0164135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahzad K, Bock F, Dong W, et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015;87:74–84. doi: 10.1038/ki.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belcher JM, Sanyal AJ, Peixoto AJ, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622–32. doi: 10.1002/hep.26980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belcher JM. Acute kidney injury in liver disease: Role of biomarkers. Adv Chronic Kidney Dis. 2015;22:368–75. doi: 10.1053/j.ackd.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol. 2016;65:809–24. doi: 10.1016/j.jhep.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Tan HL, Yap JQ, Qian Q. Acute kidney injury: Tubular markers and risk for chronic kidney disease and end-stage kidney failure. Blood Purif. 2016;41:144–50. doi: 10.1159/000441269. [DOI] [PubMed] [Google Scholar]