Abstract
Gemcitabine is a widely-used anti-cancer drug with a well-defined mechanism of action in normal and transformed epithelial cells. However, its effect on endothelial cells is largely unknown. Acid sphingomyelinase (ASMase) is highly expressed in endothelial cells, converting plasma membrane sphingomyelin to pro-apoptotic ceramide upon activation by diverse stresses. In the current study, we investigated gemcitabine impact in primary cultures of endothelial cells. We find baseline ASMase increases markedly in bovine aortic endothelial cells (BAEC) as they transit from a proliferative to a confluent growth-arrested state. Further, gemcitabine activates ASMase and induces release of a secretory ASMase form into the media only in proliferating endothelial cells. Additionally, proliferative, but not growth-arrested BAEC, are sensitive to gemcitabine-induced apoptotic death, an effect blocked by inhibiting ASMase with imipramine or by binding ceramide on the cell surface with an anti-ceramide Ab. Confluent growth-arrested BAEC can be re-sensitized to gemcitabine-induced apoptosis by provision of exogenous sphingomyelinase. A highly similar phenotype was observed in primary cultures of human coronary artery endothelial cells. These findings reveal a previously-unrecognized mechanism of gemcitabine cytotoxicity in endothelium that may well contribute to its clinical benefit, and suggest the potential for further improvement of its clinical efficacy via pharmacologic modulation of ASMase/ceramide signaling in proliferative tumor endothelium.
Keywords: Ceramide, Gemcitabine, Vascular endothelium, Apoptosis
1. Introduction
A large body of evidence supports tumor endothelial cells, while non-transformed, as being phenotypically distinguished from normal resting endothelium in healthy tissue [1, 2]. Tumor cells secrete substantial amounts of endothelial mitogens, amongst which VEGF-A is predominant [3], that “educate” their neo-vasculature. These angiogenic factors induce many alterations in endothelial function of which proliferation, as it relates to response to chemotherapy, is the topic of the current studies. While the large majority of endothelial cells in normal mammalian tissues are in G0, endothelial cell turnover is elevated in tumors (estimates range from 20 to 2000x fold of that in normal tissue [4]) and vessels remain immature, in sharp contrast to the pericyte-covered, mature endothelial monolayer of the resting normal microvasculature [5, 6]. Tumor endothelium has been recognized as a target for anti-cancer therapy for over 50 years [7]. Thus far, mainstream approaches aimed at either halting angiogenesis via VEGF blockage [8], or enhancing endothelial death, have had limited clinical success [9].
Acid sphingomyelinase (ASMase), which initiates the Sphingomyelin-Ceramide Signaling Pathway, is highly enriched in endothelial cells [10, 11] and known therein to be responsive to diverse cellular stresses [12]. ASMase is a sphingomyelin-specific phospholipase C that hydrolyzes sphingomyelin to generate the second messenger ceramide [13]. While there is only one asmase gene in mammalian cells, the primary gene product is post-translationally processed to give rise to two distinct forms: a form found in classic lysosomes that is independent of exogenous zinc having acquired it during intracellular transport; and a secreted “zinc-dependent” lysosomal form found in secretory lysosomes [11, 14]. While classic lysosomal ASMase is critical for catabolism of membrane sphingomyelin during physiologic turnover of membranes, secretory ASMase generates ceramide at the outer plasma membrane to initiate transmembrane signal transduction. Ceramide generated via secretory ASMase signals diverse cellular functions including initiation of vesicular transport [15, 16], activation of NADPH-dependent vasodilatory function in the vasculature [17, 18], facilitation of plasma membrane repair [19], and the often reported induction of apoptotic cell death [20, 21], to list a few.
Gemcitabine (2′,2′-difluoro 2′-deoxycytidine; dFdC) is a widely-used chemotherapeutic agent employed as first-line treatment for locally-advanced pancreatic cancer, inoperable lung cancer, relapsed ovarian carcinoma, and in various instances in breast cancer and metastatic sarcoma. The drug is a fluorinated analogue of the nucleoside cytidine, which upon phosphorylation by nucleoside kinases, is incorporated into DNA, causing DNA replication fork stalling and cytotoxicity. This is considered the main mechanism of its cytotoxic activity, thus targeting malignant cells of rapid cellular division. Evidence exists, however, that gemcitabine also acts on endothelial cells, already at low drug concentrations [22–24]. In addition, ceramide has been linked to gemcitabine efficacy, and recently reported as a biomarker for response effectiveness [25]. Here, we investigate impact of gemcitabine on endothelial cells and show that it acts on proliferating, but not growth-arrested, endothelium by a mechanism distinct from DNA damage involving activation of ASMase signaling, providing an additional rationale for its therapeutic index in cancer therapy.
2. Materials and Methods
2.1. Materials
Lyophilized gemcitabine (Sun Pharmaceutical Industries LTD, Gujarat, India) was reconstituted in 0.9% NaCl and stored at room temperature, according to manufacturer’s instructions. Mouse IgM monoclonal anti-ceramide antibody MID 15B4 was from Enzo Life Science (Farmingdale, NY, USA). MTT cell proliferation kit was from Roche (Mannheim, Germany). Bacterial sphingomyelinase (Bacillus cereus) was purchased as a 371 u/ml stock in 50% glycerol from Sigma Aldrich. Unless indicated otherwise, all other reagents and chemicals were from Sigma Aldrich (Milwaukee, WI, USA).
2.2. Cell culture
Primary bovine aortic endothelial cells (BAEC) were established from the intima of bovine aorta as described [26] and maintained in 1.0 g/l glucose DMEM (HyClone™, GE Healthcare, South Logan, UT, USA) supplemented with 10% bovine calf serum (GemCell™, Gemini Bio-Products, West Sacramento, CA, USA), 50 IU/ml penicillin and 50 μg/ml streptomycin. Cells were subcultured by trypsinization up to passage 18 and were maintained at 37°C in a water-saturated atmosphere containing 10% CO2. Human coronary artery endothelial cells (HCAEC) were obtained from Lonza (Walkersville, MD, USA) and maintained in EGM-2 full medium (Lonza) at 5% CO2. HCAEC were sub-cultured up to passage 7. For experiments, EGM-2 medium was replaced with DMEM (1.0 g/l glucose) without supplements.
2.3. ASMase activity in cells and culture medium
For ASMase activity studies, cells were placed on serum-free DMEM for at least 4 hours. For measurement of secreted ASMase, culture medium was collected and the cells were washed 1x with cold PBS. The cells were then lysed in 0.5% Triton X100 and protein content of each lysate was determined by DC protein assay (Biorad, Hercules, CA, USA). The cell lysates were used for the measurement of cellular ASMase activity. For secreted ASMase activity, the PBS wash and the collected medium were pooled, cleared by a single centrifugation step at 3200xg for 5 minutes, and concentrated in a 30,000 molecular weight cutoff centrifugal unit (Merck Millipore, Tullagreen, Ireland). Samples were concentrated at least 40-fold by volume.
ASMase activity was quantified by radioenzymatic assay, measuring conversion of bovine [14C-methylcholine]sphingomyelin (Perkin Elmer, Waltham, MA, USA) according to [27]. Briefly, a film of unlabeled bovine brain sphingomyelin (Avanti Polar Lipids, Alabaster, AL, USA) and radioactive [14C]sphingomyelin (39:1 molar ratio) was hydrated in a 3X acidic assay buffer. 30 μl of this radiolabeled assay buffer was added to 60 μl sample (i.e. concentrated medium, or a 60 μl lysate of 25 μg protein). The final assay concentrations are: 1% triton X-100, 250 mM sodium acetate and 128 μM sphingomyelin, pH 5.0. Zinc chloride was added to 0.1 mM for total ASMase, while 5.0 mM EDTA was added to measure zinc-independent ASMase. After 2 hours incubation at 37°C, the enzymatic reaction was terminated by adding 125 μl of a 2:1 (v/v) chloroform:methanol solution. Samples were well mixed and centrifuged for 5 min at 7000 x g. The aqueous (upper) phase was transferred into scintillation vials and 14C-radioactivity counted in ScintiVerse™ BD Cocktail (Fisher Scientific, Waltham, MA, USA) in a 2200CA Tri-Carb analyzer (Packard Instrument, Downers Grove, IL, USA). For each assay, a buffer only and a sample boiled for 5 min at 95°C were included as negative controls.
2.2. Cell viability
BAEC, cultured in flat-bottom 96-well plates, were incubated with gemcitabine from serial dilutions in 0.9% NaCl. After 16 hours, cells were washed and medium (10% BCS) was refreshed for an additional 6 hours. MTT reagent was added and formazan was allowed to develop over 2–4 hours. The formazan crystals were solubilized by adding 100 μl of 10% SDS, 0.01M HCl to each well. Absorbance was measured at 570 nm with the reference wavelength at 680 nm. As controls, wells were supplemented with vehicle (0.9% NaCl; set to 100%), or 1.0 mM arsenic oxide to kill all cells (0%).
2.3. Apoptosis quantification
Cells were harvested by trypsinization 16 hours after start of treatment. Detached cells and medium were pooled, and cells were collected by centrifugation. After a PBS wash to remove remaining serum and trypsin, cells were fixed in 3% paraformaldehyde and stained in 30 μg/ml bis-benzimide trihydrochloride (Hoechst #33258; Sigma Aldrich, Milwaukee WI, USA). Apoptosis was counted on an Olympus BH2 fluorescence microscope using nuclear compaction and fragmentation as criteria for apoptosis according Lazebnik et al. [28].
3. Results
3.1. ASMase activity increases with cell density
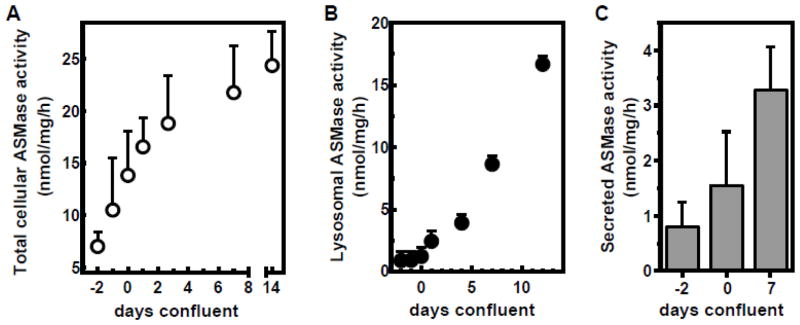
These studies utilized BAEC, a cultured endothelial cell system shown by us to closely mimic endothelial responses observed in vivo in murine allografts and human xenografts [29, 30]. Initial studies examined the impact of cell cycle kinetics on ASMase activity. As in vivo, endothelial cells undergo contact inhibition in cell culture [31]. Fig. S1A shows that BAEC growth slows as confluence is approached, and that the cells fully growth arrest 3 days after reaching confluence under the conditions of our assay. Confluence (day 0) for the purpose of these studies is defined as the day the monolayer occupies the entire surface of the culture plate. After confluence is reached, BAEC density continues to increase from 2.5 (day 0) to 4.0 x 105 cells/cm2 (Fig. S1A), as individual cells continue to pack more tightly. While 50% of sub-confluent BAEC are in the G2/S phase of the cell cycle as detected by BrdU staining, growth-arrested BAEC display ≤5% BrdU staining (Fig. S1B). In the current study, we assessed cellular ASMase activity in relation to these growth states. Figure 1 shows that baseline ASMase activity increases with growth of BAEC. Even after confluence (day 0) and growth arrest (day 4), cellular ASMase activity continues increasing (Fig. 1A), up to 3.5-fold of the initial activity at seeding. Note that the lysosomal form elevates minimally, if at all, in proliferating endothelium and increases linearly over time after cells reach confluence (Fig. 1B). In contrast, secreted ASMase activity increases both before and after endothelial cells reach confluence (Fig. 1C).
Figure 1. ASMase activity elevates over time in cultured endothelial cells.
A: BAEC were harvested at different days after seeding as indicated and ASMase activity was determined in cell lysates using bovine [14C-methylcholine]sphingomyelin. Confluence was judged visually using a phase-contrast microscope (day 0 was defined as the day the monolayer covered 100% of the surface of the well). B: ASMase activity was determined in absence of exogenous zinc, quantifying the lysosomal fraction of cellular ASMase. C: BAEC culture medium (serum-free) was collected after 24 hours and concentrated using 30 kDa molecular weight cut-off filter units. ASMase activity elevates over time in concordance with total cellular levels. Data (mean ± SD) are collated from 9 separate experiments.
3.2. Gemcitabine activates ASMase and induces its secretion into the medium
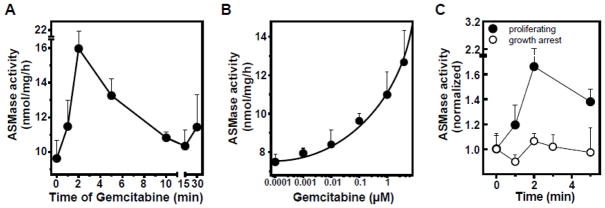
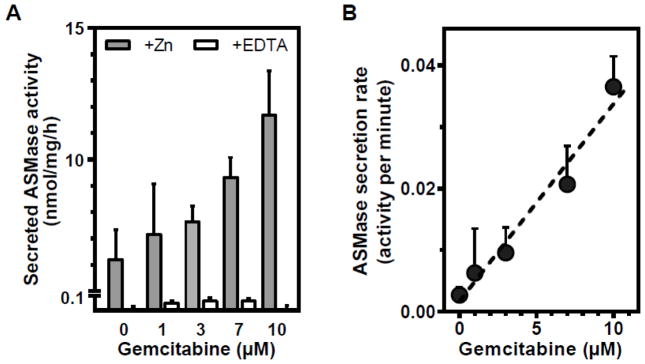
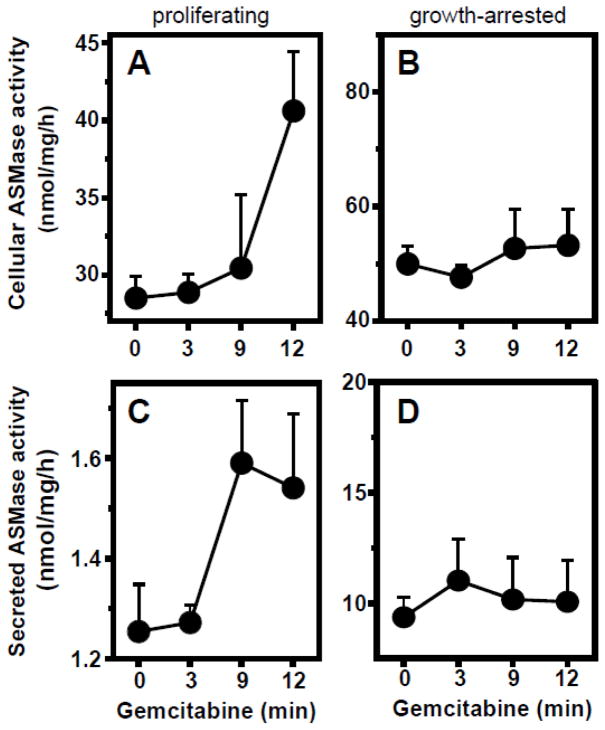
Figure 2 shows that in proliferating BAEC gemcitabine induces rapid time- (Fig. 2A) and dose-dependent (Fig. 2B) ASMase activation. In contrast to proliferating cells, gemcitabine does not activate ASMase in confluent, growth-arrested BAEC (Fig. 2C), despite the higher baseline levels. Furthermore, as displayed in Figure 3, secreted ASMase activity in the medium of proliferating cells increases in a gemcitabine dose-dependent, linear (r2=0.966) manner (Fig. 3B). To confirm that this medium activity indeed represents secretory ASMase, we tested its requirement for exogenous zinc. As displayed by the open bars, enzymatic activity in the medium is absent when zinc in the reaction mixture was replaced by EDTA (Fig. 3A), characteristic for secretory ASMase [14]. When testing another primary culture of endothelial cells, human coronary artery endothelial cells (HCAEC), we observe a similar pattern. As in BAEC, the baseline ASMase level is higher in growth-arrested HCAEC (Fig. 4, compare 0 minute data points). Further, while gemcitabine activates and induces ASMase secretion in proliferating HCAEC (Fig. 4A, C), at three days of confluence growth-arrested HCAEC are unresponsive (Fig. 4B, D).
Figure 2. Gemcitabine activates ASMase exclusively in proliferating BAEC.
A: Time-course of ASMase activity in proliferating BAEC upon addition of 100 nM gemcitabine. B: Dose-response curve of ASMase activation at 5 minutes. C: Gemcitabine does not activate ASMase in growth-arrested BAEC. Data (mean ± SEM) are collated from 3 separate experiments.
Figure 3. Gemcitabine induces ASMase secretion.
A: 15 minutes after adding gemcitabine to proliferating BAEC, medium was harvested and concentrated using a 30 kDa molecular weight cutoff spin column. ASMase activity (sphingomyelin conversion, nmol/mg/h) in concentrated medium samples was determined in the absence (open bars) or presence (grey bars) of exogenous zinc. Activity of the secretory ASMase is dependent on exogenous zinc. B: The rate of ASMase activity secreted per minute is linearly related to gemcitabine concentration. Baseline ASMase secretion rate (0 μM) was subtracted from the gemcitabine-stimulated samples. Data (mean ± SEM) are collated from 5 experiments.
Figure 4. Gemcitabine activates and induces secretion of ASMase exclusively in proliferating HCAEC.
A: Total cellular ASMase activity in proliferating HCAEC upon addition of gemcitabine (10 μM). B: Stable ASMase activity profile upon gemcitabine addition to HCAEC, growth-arrested by 3 days confluence. C: Measurement of ASMase activity secreted into the medium. Gemcitabine induces time-dependent secretion of ASMase in proliferating HCAEC. D: ASMase secretion from growth-arrested HCAEC. Medium was collected and cells were lysed at the indicated time points after gemcitabine (10 μM) addition. Data (mean ± SEM) are collated from 4 separate experiments.
3.3. ASMase activation correlates with gemcitabine-induced endothelial apoptosis
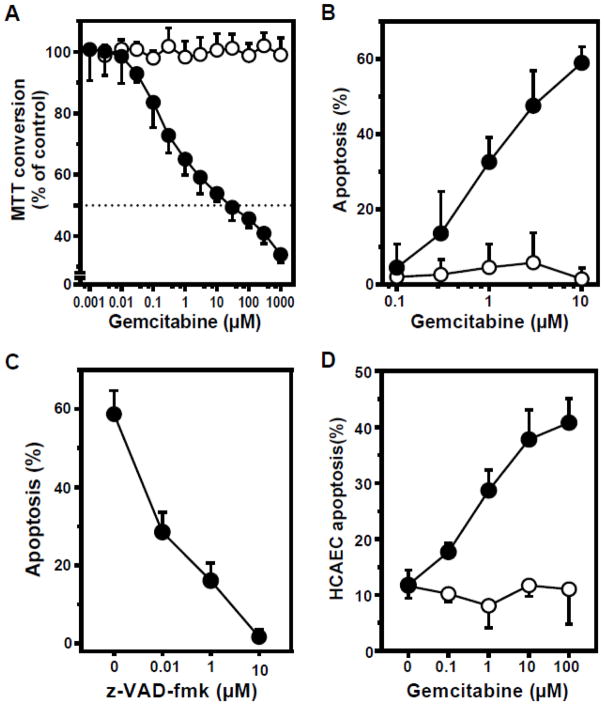
To determine whether ASMase activation correlates with cellular toxicity, cell viability was examined after gemcitabine exposure, using mitochondrial MTT conversion as initial readout. In proliferating BAEC, cell viability is reduced at gemcitabine concentrations ≥100 nM, reaching an IC50 at 25 μM. In sharp contrast, confluent BAEC are completely resistant to gemcitabine, up to concentrations of 1 mM (Fig. 5A). To examine the mechanism of cell death in proliferating endothelial cells we evaluated apoptosis, previously defined in endothelium as the principal mechanism of gemcitabine-induced cell death [32, 33]. Gemcitabine-induced apoptosis, as quantified by bis-benzimide nuclear staining, is time dependent (Fig. S2A) and increases from 0.1 to 10 μM, reaching up to 60% of the total cell population in BAEC (Fig. 5B). However, in growth-arrested BAEC apoptosis was not detected above the baseline level present in untreated cells (Fig. 5B), in agreement with the MTT data. Apoptosis is blocked by the pan-caspase inhibitor z-VAD-fmk, confirming that this represents classic caspase-dependent apoptotic cell death (Fig. 5C). HCAEC likewise showed gemcitabine-induced apoptosis only when proliferating (Fig. 5D). These studies indicate that gemcitabine activates ASMase and induces death by apoptosis exclusively under conditions of active cell division.
Figure 5. Proliferating endothelial cells are gemcitabine sensitive, in contrast to growth-arrested cells.
Endothelial cells were either proliferating (closed circles), or confluent at growth-arrest (open circles). A: BAEC were exposed to gemcitabine and MTT conversion was measured as a readout for cell viability. Cells treated with vehicle as a control were set at 100%. B: After 16 hours gemcitabine exposure, BAEC cells were harvested and apoptosis was quantified by bis-benzimide nuclear staining. C: Pre-treatment (45 min) of BAEC with the pan-caspase inhibitor z-VAD-fmk blocks apoptosis induced by 10 μM gemcitabine. D: HCAEC were exposed to gemcitabine for 16 hours and apoptosis was quantified. Data (mean ± SD) are collated from 3 separate experiments each.
3.4. ASMase is required for apoptosis by gemcitabine
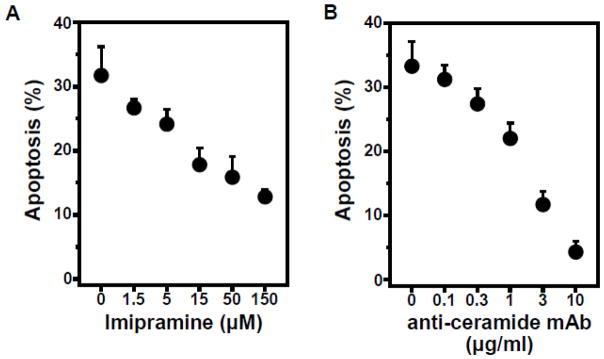
To confirm that activation of the ASMase/ceramide pathway is required for apoptosis by gemcitabine, ASMase was inhibited pharmacologically using imipramine. This cationic drug displaces ASMase from lipid bis(monoacylglycero)phosphate, its natural binding site on the inner lysosomal membrane, resulting in ASMase proteolytic degradation within lysosomes in minutes [34]. We confirmed a 31 ± 4% reduction in cellular ASMase after pre-treatment of BAEC with 50 μM imipramine for 0.5 hours. Under these conditions, imipramine prevents gemcitabine-induced apoptotic death in a dose-dependent manner (Fig. 6A). In a complementary approach, we bound ceramide generated on the surface of BAEC using anti-ceramide antibody MID15B4, a strategy that inhibits monomeric ceramide from coalescing into ceramide-rich platforms, a prerequisite for downstream apoptotic signaling [35, 36]. As displayed in Figure 6B, anti-ceramide antibody fully blocks gemcitabine-induced apoptosis in proliferating BAEC. Note, background levels of apoptosis are 1% or less, either in the absence or presence of imipramine or anti-ceramide antibody alone (Fig. S2B). These data define a requirement for ASMase in proliferating BAEC for gemcitabine induction of endothelial cell apoptosis.
Figure 6. Pharmacologic ASMase inhibition and anti-ceramide antibody prevent gemcitabine-induced apoptosis.
A: Pre-incubation with up to 150 μM imipramine inhibits gemcitabine-induced endothelial apoptosis. Imipramine was added to proliferating BAEC 30 minutes before 1.0 μM gemcitabine. B: Anti-ceramide monoclonal antibody blocks gemcitabine-induced apoptosis in proliferating endothelial cells. BAEC were pre-incubated with anti-ceramide antibody for 15 min prior to 1.0 μM gemcitabine. Cells were harvested after 16 hours and apoptosis was quantified by bis-benzimide staining. Data (mean ± SD) are collated from 3 separate experiments each.
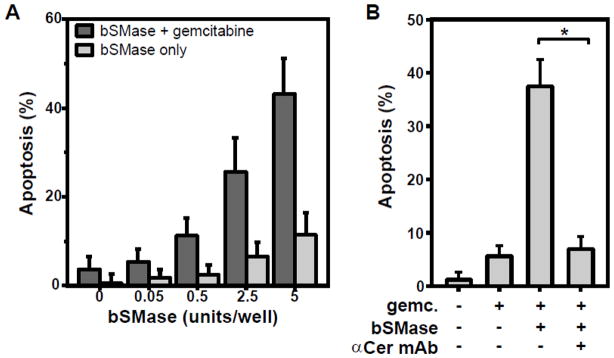
To determine experimentally whether failure of gemcitabine to induce apoptosis in confluent, growth-arrested BAEC relates to failure to activate ASMase, we added exogenous sphingomyelinase isolated from Bacillus cereus simultaneously with gemcitabine. As shown in Figure 7A, addition of sphingomyelinase at a dose that alone fails to induce apoptosis, restores gemcitabine-induced apoptosis in confluent, growth-arrested BAEC. The increase is statistically significant from 2.5 units bSMase on, when comparing cells treated with gemcitabine alone versus cells with bSMase and gemcitabine (p < 0.05). Further, treatment with anti-ceramide antibody fully blocks this bSMase- and gemcitabine-induced apoptosis in confluent BAEC (Fig. 7B). Collectively, these results demonstrate that ceramide generated on the outer plasma membrane leaflet is requisite for gemcitabine-induced endothelial apoptosis.
Figure 7. Exogenous sphingomyelinase restores gemcitabine-induced apoptosis in confluent BAEC.
A: Bacterial sphingomyelinase (Bacillus cereus; bSMase) was applied with or without 1 μM gemcitabine to a confluent monolayer of BAEC in a 2 cm2 well. Apoptosis was quantified after 16 hours. Growth-arrested BAEC otherwise resist gemcitabine (leftmost bar). B: Anti-ceramide antibody blocks the apoptosis, induced by bSMase plus gemcitabine on confluent BAEC. Anti-ceramide antibody (10 μg/ml) was added prior to bSMase (5 units) and gemcitabine (1 μM) treatment. Asterisk denotes a p value < 0.05. The activity of a bSMase unit is defined by the hydrolysis of 1.0 μmol sphingomyelin per minute at 37°C. Data (mean ± CV) are collated from 3 separate experiments each.
4. Discussion
The current study explores the discovery that gemcitabine, a commonly used anti-cancer drug, is preferentially toxic to proliferating endothelial cells, such as might be found in tumors. Gemcitabine-induced cytotoxicity correlates closely with rapid ASMase secretion and activation, an event also only stimulable in proliferating endothelial cells. Further, either pharmacologic inhibition of ASMase using imipramine, which induces ASMase degradation, or binding of ASMase-generated ceramide on the cell surface, preventing the formation of ceramide-rich platforms necessary for transmission of the apoptotic signal across the plasma membrane, confirm these events are not unrelated but rather that gemcitabine-induced endothelial apoptosis has an obligate requirement for ASMase signaling. How the increase in baseline ASMase activity that accompanies transition from proliferating to non-proliferating endothelium relates to the unresponsiveness of ASMase to gemcitabine will require substantive additional investigation. Whether a similar ceramide-mediated mechanism of gemcitabine might be operative in some types of tumor parenchymal cells is also currently unknown.
It should be noted that growth inhibition per se does not prevent gemcitabine cytotoxicity [37]. Hence the cooperative effect of ASMase activation on gemcitabine cytotoxicity likely represents the beginning of the identification of early steps in a pathway required for gemcitabine efficacy. Possible mechanisms by which ASMase/ceramide might confer chemosensitivity include alteration of function of relevant nucleoside transporters CNT1, ENT1 and ENT2 [38], subsequent intracellular vesicular trafficking of the drug, or the enzymatic processing of gemcitabine into active phosphorylated forms (2′,2′-difluoro 2′-deoxycytidine di- and triphosphate) required for incorporation into DNA and nucleoside depletion [39].
The inertness of the resting, confluent endothelium towards gemcitabine is remarkable and could potentially underlie the relatively mild adverse clinical side effects of gemcitabine [40]. This contrasts with many other anti-cancer therapies, such as doxorubicin or radiation, which are known to readily kill resting endothelial monolayers, in some instances by inducing a ceramide stress response [12, 41]. Furthermore, the selective cytotoxicity of gemcitabine towards proliferating endothelium may contribute to the therapeutic window of this anti-cancer drug, facilitating killing of neo-angiogenic tumor vasculature while sparing normal endothelium of healthy tissue.
5. Conclusion
In conclusion, gemcitabine selectively kills proliferating endothelial cells by ASMase activation. This newly-uncovered vascular biology not only provides a potential rationale for its therapeutic index, but suggests the possibility, now being evaluated in our program, that manipulation of ASMase/ceramide signaling may be a viable option for improving efficacy of this commonly used anti-cancer drug.
Supplementary Material
Highlights.
ASMase activity increases in endothelial cells (EC) with increasing cell density
Gemcitabine activates ASMase only in proliferating EC
Gemcitabine induces apoptosis only in proliferating EC
Exogenous ASMase restores gemcitabine-induced apoptosis in growth-arrested EC
Acknowledgments
This work was supported by NIH/NCI grant CA198586 to R.K., Cycle for Survival to W.T. and funded in part through the NIH/NCI Cancer Center Support Core Grant P30 CA008748 to MSKCC.
Abbreviations
- ASMase
acid sphingomyelinase
- BAEC
bovine aortic endothelial cells
- BrdU
5-bromo-2′-deoxyuridine
- bSMase
bacterial sphingomyelinase
- CNT
concentrative nucleoside transporter
- dFdC
2′,2′-difluoro 2′-deoxycytidine
- EDTA
ethylene diamine tetraacetic acid
- ENT
equilibrative nucleoside transporter
- HCAEC
human coronary artery endothelial cells
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- z-VAD-fmk
benzyloxycarbonyl Val-Ala-Asp (OMe) fluoromethylketone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nature Medicine. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aird WC. Molecular heterogeneity of tumor endothelium. Cell and Tissue Research. 2009;335:271–281. doi: 10.1007/s00441-008-0672-y. [DOI] [PubMed] [Google Scholar]
- 3.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature Medicine. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 4.Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. British Journal of Cancer. 1984;49:405–13. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in Pericytes on Blood Vessels and Endothelial Sprouts in Tumors. The American Journal of Pathology. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Algire GH, Chalkley HW, Legallais FY, Park HD. Vasculae Reactions of Normal and Malignant Tissues in Vivo. I. Vascular Reactions of Mice to Wounds and to Normal and Neoplastic Transplants. Journal of the National Cancer Institute. 1945;6:73–85. [Google Scholar]
- 8.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–4. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 9.Jain Rakesh K. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–99. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 11.Marathe S, Schissel SL, Yellin MJ, Beatini N, Mintzer R, Williams KJ, Tabas I. Human Vascular Endothelial Cells Are a Rich and Regulatable Source of Secretory Sphingomyelinase. Journal of Biological Chemistry. 1998;273:4081–4088. doi: 10.1074/jbc.273.7.4081. [DOI] [PubMed] [Google Scholar]
- 12.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–9. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 13.Gorelik A, Illes K, Heinz LX, Superti-Furga G, Nagar B. Crystal structure of mammalian acid sphingomyelinase. Nature Communications. 2016;7 doi: 10.1038/ncomms12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schissel SL, Keesler GA, Schuchman EH, Williams KJ, Tabas I. The Cellular Trafficking and Zinc Dependence of Secretory and Lysosomal Sphingomyelinase, Two Products of the Acid Sphingomyelinase Gene. Journal of Biological Chemistry. 1998;273:18250–18259. doi: 10.1074/jbc.273.29.18250. [DOI] [PubMed] [Google Scholar]
- 15.Zha X, Pierini LM, Leopold PL, Skiba PJ, Tabas I, Maxfield FR. Sphingomyelinase treatment induces ATP-independent endocytosis. The Journal of Cell Biology. 1998;140:39–47. doi: 10.1083/jcb.140.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogasevskaia T, Coorssen JR. Sphingomyelin-enriched microdomains define the efficiency of native Ca(2+)-triggered membrane fusion. Journal of Cell Science. 2006;119:2688–94. doi: 10.1242/jcs.03007. [DOI] [PubMed] [Google Scholar]
- 17.Johns DG, Jin JS, Wilde DW, Webb RC. Ceramide-induced vasorelaxation An inhibitory action on protein kinase C. General Pharmacology: The Vascular System. 1999;33:415–421. doi: 10.1016/s0306-3623(99)00038-5. [DOI] [PubMed] [Google Scholar]
- 18.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide Changes the Mediator of Flow-Induced Vasodilation From Nitric Oxide to Hydrogen Peroxide in the Human Microcirculation. Circulation Research. 2014;115:525–532. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, Schuchman E, Tabas I, Andrews NW. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. The Journal of Cell Biology. 2010;189:1027–38. doi: 10.1083/jcb.201003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Progress in Lipid Research. 2012;51:50–62. doi: 10.1016/j.plipres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Morad SA, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nature Reviews Cancer. 2013;13:51–65. doi: 10.1038/nrc3398. [DOI] [PubMed] [Google Scholar]
- 22.Cham KKY, Baker JHE, Takhar KS, Flexman JA, Wong MQ, Owen DA, Yung A, Kozlowski P, Reinsberg SA, Chu EM, Chang CWA, Buczkowski AK, Chung SW, Scudamore CH, Minchinton AI, Yapp DTT, Ng SSW. Metronomic gemcitabine suppresses tumour growth, improves perfusion, and reduces hypoxia in human pancreatic ductal adenocarcinoma. British Journal of Cancer. 2010;103:52–60. doi: 10.1038/sj.bjc.6605727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bocci G, Danesi R, Marangoni G, Fioravanti A, Boggi U, Esposito I, Fasciani A, Boschi E, Campani D, Bevilacqua G, Mosca F, Del Tacca M. Antiangiogenic versus cytotoxic therapeutic approaches to human pancreas cancer: an experimental study with a vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor and gemcitabine. European Journal of Pharmacology. 2004;498:9–18. doi: 10.1016/j.ejphar.2004.07.062. [DOI] [PubMed] [Google Scholar]
- 24.Veltkamp SA, Beijnen JH, Schellens JH. Prolonged versus standard gemcitabine infusion: translation of molecular pharmacology to new treatment strategy. Oncologist. 2008;13:261–76. doi: 10.1634/theoncologist.2007-0215. [DOI] [PubMed] [Google Scholar]
- 25.Saddoughi SA, Garrett-Mayer E, Chaudhary U, O’Brien PE, Afrin LB, Day TA, Gillespie MB, Sharma AK, Wilhoit CS, Bostick R, Senkal CE, Hannun YA, Bielawski J, Simon GR, Shirai K, Ogretmen B. Results of a Phase II Trial of Gemcitabine Plus Doxorubicin in Patients with Recurrent Head and Neck Cancers: Serum C18-Ceramide as a Novel Biomarker for Monitoring Response. American Association for Cancer Research. 2011;17:6097–6105. doi: 10.1158/1078-0432.CCR-11-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gospodarowicz D, Moran J, Braun D, Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proceedings of the National Academy of Sciences. 1976;73:4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schissel SL, Schuchman EH, Williams KJ, Tabas I. Zn2+-stimulated Sphingomyelinase Is Secreted by Many Cell Types and Is a Product of the Acid Sphingomyelinase Gene. Journal of Biological Chemistry. 1996;271:18431–18436. doi: 10.1074/jbc.271.31.18431. [DOI] [PubMed] [Google Scholar]
- 28.Lazebnik YA, Cole S, Cooke CA, Nelson WG, Earnshaw WC. Nuclear events of apoptosis in vitro in cell-free mitotic extracts: a model system for analysis of the active phase of apoptosis. The Journal of Cell Biology. 1993;123:7–22. doi: 10.1083/jcb.123.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotolo J, Stancevic B, Zhang J, Hua G, Fuller J, Yin X, Haimovitz-Friedman A, Kim K, Qian M, Cardó-Vila M, Fuks Z, Pasqualini R, Arap W, Kolesnick R. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. The Journal of Clinical Investigation. 2012;122:1786–1790. doi: 10.1172/JCI59920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stancevic B, Varda-Bloom N, Cheng J, Fuller JD, Rotolo JA, García-Barros M, Feldman R, Rao S, Weichselbaum RR, Harats D, Haimovitz-Friedman A, Fuks Z, Sadelain M, Kolesnick R. Adenoviral Transduction of Human Acid Sphingomyelinase into Neo-Angiogenic Endothelium Radiosensitizes Tumor Cure. PloS One. 2013;8:e69025. doi: 10.1371/journal.pone.0069025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lampugnani MG, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, β-catenin, and the phosphatase DEP-1/CD148. The Journal of Cell Biology. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laquente B, Lacasa C, Ginestà MM, Casanovas O, Figueras A, Galán M, Ribas IG, Germà JR, Capellà G, Viñals F. Antiangiogenic effect of gemcitabine following metronomic administration in a pancreas cancer model. Molecular Cancer Therapeutics. 2008;7:638–647. doi: 10.1158/1535-7163.MCT-07-2122. [DOI] [PubMed] [Google Scholar]
- 33.Yokoi K, Kim SJ, Thaker P, Yazici S, Nam DH, He J, Sasaki T, Chiao PJ, Sclabas GM, Abbruzzese JL, Hamilton SR, Fidler IJ. Induction of apoptosis in tumor-associated endothelial cells and therapy of orthotopic human pancreatic carcinoma in nude mice. Neoplasia. 2005;7:696–704. doi: 10.1593/neo.05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurwitz R, Ferlinz K, Sandhoff K. The tricyclic antidepressant desipramine causes proteolytic degradation of lysosomal sphingomyelinase in human fibroblasts. Biological Chemistry (Hoppe Seyler) 1994;375:447–50. doi: 10.1515/bchm3.1994.375.7.447. [DOI] [PubMed] [Google Scholar]
- 35.Rotolo J, Stancevic B, Zhang J, Hua G, Fuller J, Yin X, Haimovitz-Friedman A, Kim K, Qian M, Cardó-Vila M, Fuks Z, Pasqualini R, Arap W, Kolesnick R. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. The Journal of Clinical Investigation. 2012;122:1786–1790. doi: 10.1172/JCI59920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Letters. 2010;584:1728–40. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rockwell S, Grindey GB. Effect of 2′,2′-difluorodeoxycytidine on the viability and radiosensitivity of EMT6 cells in vitro. Oncology Research. 1992;4:151–5. [PubMed] [Google Scholar]
- 38.Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Annals of Oncology. 2006;17:v7–v12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 39.Ostruszka LJ, Shewach DS. The role of DNA synthesis inhibition in the cytotoxicity of 2′,2′-difluoro-2′-deoxycytidine. Cancer Chemotherapy and Pharmacology. 2003;52:325–32. doi: 10.1007/s00280-003-0661-5. [DOI] [PubMed] [Google Scholar]
- 40.Zinzani PL, Bendandi M, Stefoni V, Albertini P, Gherlinzoni F, Tani M, Piccaluga PP, Tura S. Value of gemcitabine treatment in heavily pretreated Hodgkin’s disease patients. Haematologica. 2000;85:926–9. [PubMed] [Google Scholar]
- 41.Veldman RJ, Zerp S, van Blitterswijk WJ, Verheij M. N-hexanoyl-sphingomyelin potentiates in vitro doxorubicin cytotoxicity by enhancing its cellular influx. British Journal of Cancer. 2004;90:917–25. doi: 10.1038/sj.bjc.6601581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.