Abstract
Background
Benign paroxysmal positional vertigo (BPPV) is one of the most common and most successfully treated vestibular disorders. However, there is a lack of predictive factors for BPPV in clinical practice. We aimed to explore several possible predictive factors for BPPV in the Chinese population.
Material/Methods
We enrolled 240 patients with BPPV from Beijing Chaoyang Hospital between July 2013 and July 2016. Biochemical and hematological markers were obtained along with the history of cardiovascular and cerebrovascular diseases.
Results
Serum uric acid (SUA) [279.0±84.7 vs. 331.0±82.7], hemoglobin A1C (HbA1c) [5.75±1.17 vs. 6.61±1.00], albumin [38.1±3.71 vs. 40.9±4.1], and creatinine [68.4±19.3 vs. 81.5±24.1] were significantly lower in patients with BPPV compared with controls (P<0.05). Multiple logistic regression analysis showed that lower levels of HbA1c and albumin were independently associated with BPPV (P<0.05), with odds ratio (OR) 0.680 (95% CI 0.551–0.839) and 0.338 (95% CI 0.190–0.603), respectively. However, the level of SUA was not independently related with BPPV [OR=0.999 (95% CI 0.991–1.006), P=0.713]. There were no significant differences between the parameters of systolic blood pressure, diastolic blood pressure, blood routine examination, lipid profiles, homocysteine, pre-albumin, and blood urea nitrogen in patients with BPPV vs. controls (P>0.05).
Conclusions
Lower levels of HbA1c and albumin were independently associated with BPPV. Although the level of SUA was lower in BPPV patients, SUA was not an independent risk factor for BPPV.
MeSH Keywords: Albumins; Hemoglobin A, Glycosylated; Uric Acid; Vertigo
Background
Vertigo is an important public health care issue, especially in the elderly. Benign paroxysmal positional vertigo (BPPV) is one of the most common peripheral vestibular diseases, characterized by brief episodes of vertigo provoked by head movement [1]. BPPV severely decreases quality of life, with reduced ability to perform activities of daily living, increased morbidity, psychosocial impact, and medical costs. Due to the high incidence and complications of BPPV in the general population, it is of utmost importance to be aware of the predictive factors for BPPV at an earlier stage, which may improve prognosis. Although most BPPV is diagnosed according to specific maneuvers, some atypical BPPV is misdiagnosed in clinical practice. As a result, the clinical applications of blood biomarkers may be helpful in diagnosing BBPV. Low level of vitamin D is an independent predictive factor of BPPV [2], but there are no known independent factors for prediction of BPPV in clinical practice.
Serum uric acid (SUA) is one of the products of purine metabolism. Hyperuricemia (HUA) has been widely investigated for its close relationship with hypertension, intracranial atherosclerosis, cardiovascular events, and stroke [3–5], with increased morbidity and mortality [6]. Few observational cross-sectional studies have been performed on the association between levels of SUA and BPPV. In a case-control study of African men, Adam observed a positive association between BPPV and hyperuricemia in 2001 and 2005 [7,8]. Recently, a multiple logistic regression analysis of 50 BPPV patients and 40 controls from Turkey demonstrated that decreased albumin and increased SUA were independently associated with BPPV [9]. However, some inconsistent results were also found. In contrast, a study from the United Kingdom failed to find any difference in SUA levels between 20 patients with BPPV and controls [10]. In the Asian population, a study from Korea found that SUA levels were decreased in 168 patients with BPPV compared with 194 controls; however, it did not demonstrate that hypouricemia is an independent risk factor for BPPV [11].
Due to the size limitations, sex differences, racial background, and variable lifestyles and diet, the exact relationship between SUA and BPPV in the Chinese population remains unclear. The present study investigated the relationships between SUA and BPPV in a larger cohort in China, and explored the underlying risk factors for BPPV.
Material and Methods
Our study involved 240 consecutive BPPV patients (134 females and 106 males) at the Department of Neurology and 72 age- and sex- matched controls (33 females and 39 males) at the Physical Examination Department. Patients with autoimmune diseases, gout, malignant tumors, chronic renal failure, hypohepatia, hyperthyroidism, hypothyroidism, pregnancy morbid obesity, use of allopurinol or diuretics, any history of vestibular and neurologic diseases, history of recent trauma, endolymphatic hydrops, and recent fasting were excluded from our study [9]. All BPPV patients had a typical history of brief attacks of positional vertigo and were diagnosed by using the vestibular test, in which eye movements are recorded by videonystagmography. PSC-BPPV was diagnosed when vertical and torsional nystagmus were induced by the Dix-Hallpike test, whereas HSC-BPPV was performed as horizontal nystagmus with the Roll test, and vertical and torsional nystagmus confirmed ASC-BPPV. The direction of nystagmus reversed when patients returned to sitting position from horizontal position in all 3 circumstances. The duration of nystagmus is usually less than 1 min in canalithiasis and more than 1 min in cupulolithiasis. All the BPPV patients underwent another positional test 1 week after treatment. Vascular risk factors (blood pressure, blood sugar, and blood lipids) were assessed, as well as history of smoking and drinking. Hematological and biochemical analyses were performed in our hospital. The protocol of our study was approved by the Ethics Committee of Beijing Chaoyang Hospital. All patients and subjects signed the informed consent form.
Statistical analysis
The data were analyzed by SPSS 16.0 software. Measurement data with normal distribution are shown as (χ̄±s). We used the independent-samples t test to compare the continuous variables between BPPV and controls. To determine factors predictive of BPPV, multiple binary logistic regression analyses were performed and the odds ratio (OR) was calculated with 95% confidence intervals (CIs). Categorical variables were performed as percentage and the chi-square test was used for its analysis. We used on-parametric receiver operating characteristic (ROC) analyses and area under the curve (AUC) measures were calculated with 95% CIs. Cutoff values were calculated for each factor. Values of P<0.05 were considered statistically significant.
Results
The clinical features and laboratory results are summarized in Table 1. No significant difference was observed between the 2 groups with respect to age, sex, BMI, systolic and diastolic blood pressures, lipid profiles, homocysteine levels, pre-albumin levels, blood panels, and blood urea nitrogen levels (Table 1, P>0.05).
Table 1.
The clinical presentations and laboratory data of patients with BBPV and controls.
| Variables | BPPV (n=240) | Control (n=72) | t/χ2 | P |
|---|---|---|---|---|
| Age (year) | 62.4±12.5 | 63.5±11.9 | −.641 | 0.522 |
| BMI (kg/m2) | 24.9±2.9 | 25.6±2.8 | −1.589 | 0.113 |
| Sex (F/M) | 134/106 | 33/39 | 2.226 | 0.136 |
| SBP (mmHg) | 140.2±17.5 | 137.3±14.9 | 1.235 | 0.218 |
| DBP (mmHg) | 79.8±11.4 | 81.8±6.4 | −1.405 | 0.161 |
| CHOL (mmol/L) | 4.54±0.95 | 4.46±0. 83 | 0.627 | 0.531 |
| HDL (mmol/L) | 1.30±0.32 | 1.38±0.36 | −1.832 | 0.068 |
| LDL (mmol/L) | 2.51±0.81 | 2.51±0.77 | 0.038 | 0.970 |
| SUA (μmol/L) | 279.0±84.7 | 331.0±82.7 | −4.413 | 0.001* |
| HCY (μmol/L) | 15.5±4.3 | 15.8±4.3 | −0.430 | 0.668 |
| HbA1c (%) | 5.75±1.17 | 6.61±1.00 | −4.937 | 0.001* |
| ALB(g/L) | 38.1±3.71 | 40.9±4.1 | −5.347 | 0.001* |
| PreALB (g/L) | 0.25±0.06 | 0.26±0.05 | −1.348 | 0.179 |
| BUN (mmol/L) | 5.1±1.5 | 5.3±1.9 | −1.236 | 0.218 |
| CR (μmol/L) | 68.4±19.3 | 81.5±24.1 | −4.523 | 0.001* |
| WBC (*109/L) | 6.56±1.96 | 6.38±1.72 | 0.524 | 0.601 |
| HGB (g/L) | 132.8±15.1 | 134.8±14.0 | −0.705 | 0.482 |
| PLT (*109/L) | 221.3±56.1 | 203.5±46.7 | 1.793 | 0.074 |
Values are expressed as n (%), mean SD or median. BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; CHOL – total cholesterol; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; SUA – serum uric acid; HCY – homocysteine; HbA1c – hemoglobin A1C; ALB – albumin; PreALB – pre-albumin; BUN – blood urea nitrogen; CR – creatinine; WBC – white blood cells; HGB – hemoglobin; PLT – platelets.
P<0.05.
SUA [279.0±84.7 vs. 331.0±82.7], HbA1c [5.75±1.17 vs. 6.61±1.00], albumin [38.1±3.71 vs. 40.9±4.1], and creatinine [68.4±19.3 vs. 81.5±24.1] were significantly lower in patients with BPPV compared to the controls (P<0.05). According to multiple logistic regression analysis, lower levels of albumin and HbA1c were independently associated with BPPV (P<0.05), with an OR of 0.680 (95% CI 0.551–0.839) and 0.338 (95% CI 0.190–0.603), respectively. However, we did not find that the level of SUA was independently related with the occurrence of BPPV [OR 0.999 (95% CI 0.991–1.006), P=0.163] (Table 2).
Table 2.
A multiple logistic regression analysis to identify independent factors of BPPV.
| Variables | OR (95% CI) | P |
|---|---|---|
| UA | 0.997 (0.992–1.001) | 0.163 |
| HbA1c (%) | 0.562 (0.417–0.758) | 0.001 |
| ALB (g/L) | 0.880 (0.799–0.969) | 0.009 |
| CR | 0.984 (0.966–1.002) | 0.075 |
SUA – serum uric acid; HbA1c – hemoglobin A1C; ALB – albumin; CR – creatinine.
ROC analyses were performed to assess the levels of HbA1c and albumin. The values of AUC for HbA1c and albumin were 0.757 (0.694–0.819) and 0.682 (0.596–0.768), respectively. The cutoff value of HbA1c was 5.89 with a sensitivity of 61.3% and a specificity of 85.5%. The cutoff value of albumin was 40.7 with a sensitivity of 77.3% and a specificity of 56.4% (Figure 1).
Figure 1.
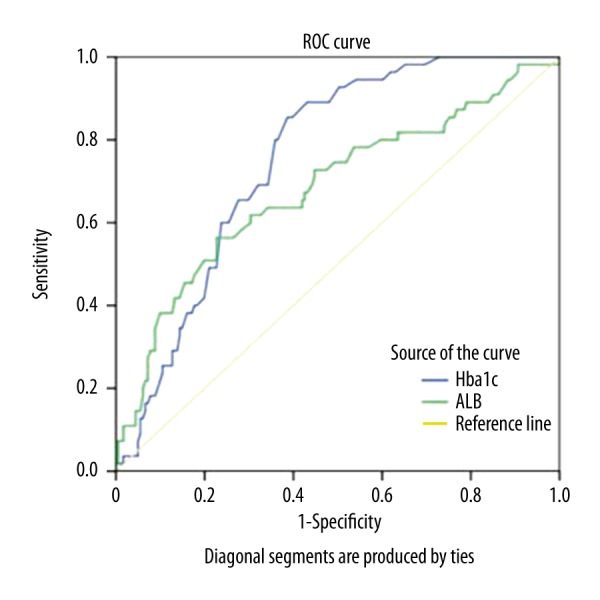
Comparison of ROC curves of glycosylated hemoglobin and albumin to identify BPPV.
Discussion
In present study, the values of SUA, HbA1c, albumin, and creatinine were much lower in patients with BPPV. Although the values of SUA were lower in patients with BPPV, we did not find SUA as an independent risk factor for the occurrence of BPPV in multi-factor logistic regression. We also found that the lower levels of HbA1c and albumin were independently associated with BPPV.
Although BPPV is well defined as a clinical entity, its pathophysiologic mechanisms remain unclear. It is widely accepted that BPPV is also caused when calcium carbonate material originating from the macula of the utricle falls into one of the semicircular canals [12]. BPPV is also thought to be related with some conditions leading to otoconia away from the utricle, such as head trauma, vestibular neuritis, or other inner-ear diseases. It is also hypothesized that the formation of the particle occurs within the semicircular canals when a calcium carbonate crystal becomes dislodged. Despite the above hypothesis, we do not have access to the inner ear in real time, particularly during attacks of BPPV, and we also have no approaches or techniques to access the condition of semicircular canals and vestibula at present. As a consequence, we are limited to clinical observation of symptomatic BPPV patients. Although most BPPV can be treated with specific maneuvers, atypical BPPV may be misdiagnosed. Thus, information helpful in early diagnosis of BBPV from blood tests would be of great value.
Accumulating data have shown a close relationship between the levels of SUA and a wide variety of cardiovascular and cerebrovascular diseases and metabolic syndrome [4,6,13]. In contrast, SUA may also be neuro-protective as an antioxidant in multiple sclerosis [14] and Parkinson’s disease [15]. To the best of our knowledge, however, there are only limited data about the association of SUA with BPPV. On the one hand, a study from Africa showed that levels of SUA were significantly higher in 90 patients with BPPV [7,8]. A recent study from Turkey also indicated an independent positive association between SUA and BPPV in 50 patients with BPPV [9]. On the other hand, however, some inconsistent results were also found. A United Kingdom study involving 20 patients with BPPV found no association between SUA and BPPV [10]. A Korean study involving 168 consecutive patients with BPPV and 194 controls found that SUA levels were decreased in patients with BPPV compared with controls, but it did not find that lower SUA was an independent risk factor for BPPV [11]. Our results are in accordance with the Korean study. The differences and inconsistency of previous studies from Asia and Europe may be partly attributed to the age, sex, racial background, metabolism, or variable lifestyles in different countries [9, 10]. To date, it is still not clear why SUA appears to be associated with BPPV. Therefore, we hypothesize that the results from Asians with decreased SUA were different from results from Turks with increased SUA, and SUA has different effects in different ethnic groups. As a result, the mechanism of otoconia and the exact relationship between SUA and BPPV remain unclear and needs further investigation.
We found that serum albumin was decreased in patients with BPPV and it was independently related with BPPV in our study. Our results are in line with the study from Turkey, showing that the reduced albumin was independently associated with BPPV [9]. We speculate that recurrent attacks of dizziness and constant vomiting cause malnutrition, leading to hypoproteinemia. However, the exact mechanisms underlying the association of albumin with BPPV requires further investigation.
Regarding HbA1c, according to the study from Turkey, there was no difference in fasting blood glucose between BPPV patients and controls, but the indicators of DM severity (e.g., HbA1c levels) were not consistently available in that study [9]. To the best of our knowledge, our study is the first to find that patients with BPPV had lower HbA1c, and HbA1c was independently associated with BPPV. This may be a novelty in the present study. Our findings may be influenced by the nutritional habits in China and the poor nutrition caused by vertigo attacks. However, the exact of relationship between HbA1c and BPPV remains unclear.
Our study has several strengths. First, although we aimed to test the hypothesis that elevated levels of SUA contribute to precipitating crystals, resulting in BPPV in the Chinese population, we did not find that SUA is an independent risk factor of BPPV. To the best of our knowledge, our study has the largest sample size of BPPV patients from Asia. Furthermore, our study further supports that albumin is reduced in patients with BPPV, and the lower albumin is independently associated with BPPV both in Asian and European populations [9]. Furthermore, because the level of HbA1c was not consistently available by previous studies to explore its relationship with BPPV, our study is the first to demonstrate that patients with BPPV have lower HbA1c, and HbA1c is independently associated with BPPV.
However, there are some limitations to be considered in our study. Firstly, some inconsistent results from prior studies have been reported, perhaps due to differences in age, sex, race, or other undetermined environmental and demographic factors, such as different diet or physical exercise habits, which may have a remarkable impact on the metabolism of SUA. Secondly, we did not analyze the levels of SUA according to the 3 different variants of BPPV (PSC, HSC, and ASC) or the position of BPPV (left or right side). Thirdly, we did not perform follow-up to detect the values of SUA at different stages of disease (e.g., acute stage or remission stage). Lastly, due to some technical limitations, we did not test the biomarkers of osteoporosis, such as otolin-1, aminoterminal propeptide of protocollagen type I, aminoterminal telopeptides of collagen, and vitamin D, which are urgently needed in our future studies [16].
Conclusions
In summary, we found that lower levels of HbA1c and albumin were independently associated with BPPV. Although the values of SUA were lower in patients with BPPV, we did not find SUA as an independent risk factor for BPPV. The mechanisms underlying these disorders are unclear. Future studies need to address the underlying mechanisms and biomarkers of BPPV [16], and large clinical trials are needed to investigate other factors associated with BPPV.
Footnotes
Source of support: This study was funded by the National Natural Science Foundation of China (81271309, 81301016) and the Beijing Municipal Administration of Hospitals’ Youth Program (QML20150303)
Conflicts of interest
None.
References
- 1.Kim JS, Zee DS. Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med. 2014;370:1138–47. doi: 10.1056/NEJMcp1309481. [DOI] [PubMed] [Google Scholar]
- 2.Jeong SH, Kim JS, Shin JW, et al. Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. J Neurol. 2013;260:832–38. doi: 10.1007/s00415-012-6712-2. [DOI] [PubMed] [Google Scholar]
- 3.Viazzi F, Leoncini G, Ratto E, Pontremoli R. Hyperuricemia and renal risk. High Blood Press Cardiovasc Prev. 2014;21:189–94. doi: 10.1007/s40292-014-0042-7. [DOI] [PubMed] [Google Scholar]
- 4.Grassi D, Desideri G, Di Giacomantonio AV, et al. Hyperuricemia and cardiovascular risk. High Blood Press Cardiovasc Prev. 2014;21:235–42. doi: 10.1007/s40292-014-0046-3. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Yang C, Zhao Y, et al. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease? A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122. doi: 10.1186/1471-2369-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Hou W, Zhang X, et al. Hyperuricemia and risk of stroke: A systematic review and meta-analysis of prospective studies. Atherosclerosis. 2014;232:265–70. doi: 10.1016/j.atherosclerosis.2013.11.051. [DOI] [PubMed] [Google Scholar]
- 7.Adam AM. Benign positional vertigo as a clinical manifestation of hyperuricemia – a recent discovery. J Neurol Sci. 2001;187:222. [Google Scholar]
- 8.Adam AM. Benign positional vertigo and hyperuricaemia. East Afr Med J. 2005;82:376–78. [PubMed] [Google Scholar]
- 9.Celikbilek A, Gencer ZK, Saydam L, et al. Serum uric acid levels correlate with benign paroxysmal positional vertigo. Eur J Neurol. 2014;21:79–85. doi: 10.1111/ene.12248. [DOI] [PubMed] [Google Scholar]
- 10.Ziavra NV, Bronstein AM. Is uric acid implicated in benign paroxysmal positional vertigo? J Neurol. 2004;251:115. doi: 10.1007/s00415-004-0277-7. [DOI] [PubMed] [Google Scholar]
- 11.Jeong SH, Kim JS. The effect of serum uric acid in generating idiopathic benign paroxysmal positional vertigo. Res Vesti Sci. 2010;9:27–31. [Google Scholar]
- 12.Fife TD. Benign paroxysmal positional vertigo. Semin Neurol. 2009;29(5):500–8. doi: 10.1055/s-0029-1241041. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol. 2013;25(2):210–16. doi: 10.1097/BOR.0b013e32835d951e. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Shen Y, Xiao K, et al. Serum uric acid levels in patients with multiple sclerosis: A meta-analysis. Neurol Res. 2012;34:163–71. doi: 10.1179/1743132811Y.0000000074. [DOI] [PubMed] [Google Scholar]
- 15.Shen C, Guo Y, Luo W, et al. Serum urate and the risk of Parkinson’s disease: Results from a meta-analysis. Can J Neurol Sci. 2013;40:73–79. doi: 10.1017/s0317167100012981. [DOI] [PubMed] [Google Scholar]
- 16.Sacks D, Parham K. Preliminary report on the investigation of the association between BPPV and osteoporosis using biomarkers. Otol Neurotol. 2015;36:1532–36. doi: 10.1097/MAO.0000000000000853. [DOI] [PubMed] [Google Scholar]


