Abstract
Background
Cancer-associated fibroblasts (CAFs) are key factors in malignant tumor initiation, progression, and metastasis. However, the effect of CAFs autophagy on triple-negative breast cancer (TNBC) cells is not clear. In this study, the growth effect of TNBC cells regulated by CAFs autophagy was evaluated.
Material/Methods
CAFs were obtained from invasive TNBC tumors and identified by Western blot and immunofluorescence staining assay. CAFs were co-cultured with TNBC cells, and migration and invasion were evaluated by Matrigel-coated Transwell and Transwell inserts. TNBC cells growth was detected by MTT assay, and epithelial-mesenchymal transition (EMT) regulated by CAFs was evaluated by Western blot assay.
Results
CAFs were identified by the high expression of α-smooth muscle actin (α-SMA) protein. Autophagy-relevant Beclin 1 and LC3-II/I protein conversion levels in CAFs were higher than those in NFs (P<0.05). TNBC cells migration, invasion, and proliferation levels were significantly improved in the CAFs-conditioned medium (CAFs-CM) group, compared with the other 3 groups (P<0.05). TNBC cells vimentin and N-cadherin protein levels were upregulated and E-cadherin protein level was downregulated in the CAFs-CM group compared with the control group (P<0.05). Further study indicated β-catenin and P-GSK-3β protein levels, which are the key proteins in the Wnt/β-catenin pathway, were upregulated in the CAFs-CM group compared with the control group (P<0.05).
Conclusions
Our data demonstrated CAFs autophagy can enhance TNBC cell migration, invasion, and proliferation, and CAFs autophagy can induce TNBC cells to engage in the EMT process through the Wnt/β-catenin pathway.
MeSH Keywords: Autophagy, Epithelial-Mesenchymal Transition, Triple Negative Breast Neoplasms
Background
Breast cancer is one of the most common malignant tumors in women throughout the world [1]. According to immunohistochemical typing for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2), breast cancer can be divided into 4 types: Luminal A, Luminal B, HER2 overexpression, and triple-negative breast cancer (TNBC). TNBC is a special type of breast cancer in which ER, PR, and HER2 expressions are all negative [2]. Due to this characteristic, efficient therapy for breast cancer, such as endocrine therapy and Herceptin targeted therapy, cannot be used for TNBC in clinical practice [3]. The most common treatment for primary TNBC patients is surgical therapy, followed by chemotherapy. With treatment, patients can quickly achieve complete remission; however, tumor metastasis can easily occur in lungs, liver, bones, and brain [4,5], and metastasis is the most common cause of treatment failure and mortality [6].
To solve this clinical problem, numerous studies have been performed [7–9]. One theory considers that tumor cells do not exist separately, but rather grow in a “tumor microenvironment (TME)” in vivo, and various cells and cytokines cooperate with each other in this microenvironment, activating many aspects of tumorigenesis [10,11]. One such cells type is fibroblasts, which are the most abundant cellular components in TME; they can transform into cancer-associated fibroblasts (CAFs) once activated by tumor cells [11–13]. The characteristics of CAFs are expression of α-smooth muscle actin (α-SMA) and fibroblast activation protein-α (FAP-α), while expressions of these proteins is nearly negative in normal fibroblasts (NFs) [14]. Many researchers reported that CAFs play an important role in malignant tumor initiation, progression, chemoresistance, and metastasis by producing a plethora of chemokines, growth factors, and extracellular matrix (ECM) proteins [15–17].
CAFs can affect the metastatic behavior of breast cancer cells in different ways, such as promoting breast cancer cells to engage in the epithelial–mesenchymal transition (EMT) process [18]. A previous study by our team found that the EMT process can be induced in TNBC cells through the Wnt/β-catenin pathway by overexpression of Beclin 1 gene (an autophagic regulator gene [19]), thus enhancing the autophagic level of cells [20]. As autophagy is a conservative lysosomal degradation pathway that mediates the clearance of cytoplasmic components in most kinds of cells, autophagy can also happen in CAFs [21,22]. However, because CAFs affect breast cancer cell progression in different ways, the precise effect of CAFs autophagy on TNBC cells is still unknown. In the present study, we investigated whether CAFs autophagy can enhance the metastatic potential of TNBC cells through the Wnt/β-catenin pathway, which might be useful for the clinical treatment of TNBC in the future.
Material and Methods
Specimens
Fresh specimens were collected under sterile conditions from 5 patients who had primary invasive TNBC and who accepted radical mastectomy at Zhujiang Hospital of Southern Medical University. Written consent was obtained from the patients and the study was approved by the Ethics Committee of Zhujiang Hospital.
CAFs and NFs culture
Fresh specimens and adjacent normal breast tissue samples (>3–5 cm away from the tumor) were collected from 5 TNBC patients in Zhujiang Hospital. The specimens were sectioned into 1-mm3 pieces and digested with 1 ml 0.12% collagenase A in a 37°C humidified atmosphere containing 95% air and 5% CO2 for 8 h, after which the digestion was stopped by supplementation with Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) plus 10% fetal bovine serum (FBS, Gibco, USA). Tissue debris was removed and cells were collected and cultured in a 37°C humidified atmosphere containing 95% air and 5% CO2. Once cells reached 80% confluence, they were harvested and reseeded.
CAFs conditioned medium (CAFs-CM) and NFs conditioned medium (NFs-CM) were prepared as follows: CAFs and NBFs in logarithmic growth phase were harvested, cell density was adjusted to 1×106/mL, and a total of 20 mL cells were inoculated in a 75-cm2 cell culture flask. When cells reached 80–90% confluency, the supernatants were collected and centrifuged at 1200 rpm for 15 min to remove cell debris, then the suspension was stored at −20°C until use.
TNBC cell lines culture
BT-549, MDA-MB-231, and MDA-MB-468 cell lines were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were routinely maintained in RPMI-1640 medium (Gibco, USA) supplemented with 10% FBS and cultured within a 37°C humidified atmosphere containing 95% air and 5% CO2.
Immunofluorescence staining
CAFs and NFs were seeded on the coverslips and cultured routinely. Once cells reached 50–70% confluence, they were fixed in 4% paraformaldehyde (Beyotime, China) for 15 min. After washing with PBS for 3 times, cells were incubated with 0.1 mL Triton-100 (0.5%) for 20 min and blocked with 10% bovine serum albumin (BSA, Jiawei, China) for 20 min. Then, cells were incubated with primary antibodies (anti-α-SMA 1: 300, anti-E-Cadherin, and anti-vimentin 1: 200 100 μl) at 4°C for 8–10 h, washed with PBS, and incubated with fluorescein-conjugated goat anti-rabbit secondary antibodies (GeneCopoeia, USA) for 1–2 h. Nuclei were stained by 4,6-diamidino-2-phenylindole (DAPI, GeneCopoeia, USA). Cells were observed using a fluorescence inverted microscope (IX71 Olympus, Japan), cytoplasmic staining was excitated with a 495-nm wavelength laser, and nucleus staining was excitated with a 358-nm wavelength laser.
CAFs autophagy inhibition evaluation
CAFs were cultured in DMEM plus 10% FBS, as well as 5 mM 3-Methyladenine (3-MA, an autophagy inhibitors) for 24 h, then culture medium was removed and DMEM plus 10% FBS was added again. Beclin 1 and LC3-II/I conversion were evaluated by Western blot at 24, 48, 72, and 96 h.
TNBC cell migration and invasion analysis
For migration and invasion assay, 8-uM pore size Transwell inserts (BD Biosciences, USA) were used. Invasion ability of TNBC cells was evaluated by Matrigel-coated Transwell and migration ability was evaluated by Transwell inserts. We added 2.5×104 TNBC cells in 200 μl to the upper chamber. For the CAFs group, 5×104 CAFs in 600 μl was added into the lower chamber. For the 3-MA-CAFs group, 5×104 CAFs in 600 μl was added by 5 mM concentration of 3-MA for 24 h, then the medium was changed to DMEM plus 10% FBS. For the NFs group, 5×104 NFs in 600 μl was added. For the control group, 600 μl DMEM plus 10% FBS was added. Cells were routinely co-cultured for 48 h. Migrating or invading cells were fixed in 4% paraformaldehyde for 30 min, followed by crystal violet indicator (Sigma, USA) staining. Cell numbers were counted under a microscope at ×400 magnification.
TNBC cell growth assay
TNBC cell growth was assessed by 3-(4,5-Dimet-hylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT, Amresco, USA) assay. We cultured 2.5×103 TNBC cells in 96-well plates. For the CAFs-CM group, 200 ul CAFs-CM was added. For the 3-MA-CAFs-CM group, CAFs was added by 5-mM concentration of 3-MA for 24 h, then the medium was changed into DMEM plus 10% FBS and a total of 200 ul medium was added. For the NFs-CM group, 200 ul NFs-CM was added. For the control group 200 ul DMEM plus 10% FBS was added. At 24, 48, 72, and 96 h, 20 μl MTT (5 mg/ml in PBS) was added to each well and incubated for 4 h, then 200 μl DMSO (Sigma, USA) was added to dissolve the formazan crystals. Absorption (optical density [OD] value) was measured at 570 nm using a microplate spectrophotometer (Molecular Devices, USA).
Western blot assay
Harvested cells were lysed with newly-prepared protein lysis buffer for 20–25 min on ice, and homogenates were cleared by centrifugation at 12 000 rpm for 25 min at 4. Supernatants were collected and protein content was qualified by use of a bicinchoninic acid (BCA) protein assay kit (Beyotime, China) according to the protocol: Copper was chelated with protein and reacted with BCA, and the BCA/copper complex exhibited a strong linear absorbance at 562 nm with increasing protein concentrations. Each sample of proteins (20 μg) was separated and run on a 10% SDS-PAGE gel electrophoresis, followed by transfer to a polyvinylidene difluoride Polyscreen (PVDF) membrane (Millipore, Germany). Then, the membrane was blocked by 5% nonfat milk dissolved in phosphate buffer solution with Tween-20 (PBST) at room temperature for 2 h and was incubated with primary antibodies anti-α-SMA, anti-Beclin 1, anti-E-cadherin, anti-vimentin, anti-N-cadherin, and anti-P-GSK-3β (Santa Cruz, USA) or 1: 1000 anti-β-Tubulin (Cell Signal Technology, USA) overnight at 4° on a rotator. FITC-labeled secondary anti-rabbit antibody (Santa Cruz, USA) was added, and the membrane was incubated and protected from light for 2 h on a rotator. Protein signals were visualized sing the Odyssey Scan system (LI-COR, USA) in 700–800 nm channel, band intensities were quantified by the software of image j1.44, and fold changes in the intensity of protein signals were the mean value of the results.
Statistics
The data are presented as mean ± standard error for at least 3 separate determinations. One-way ANOVA was used to determine the differences between groups for the results of Western blot, cell migration, and cell invasion assay, and repeated-measures ANOVA was used to determine the difference between groups for the results of MTT. Data were processed with SPSS 13.0 software. P<0.05 was considered as significant.
Results
CAFs isolated from breast cancer tissues exhibited characteristics of CAFs
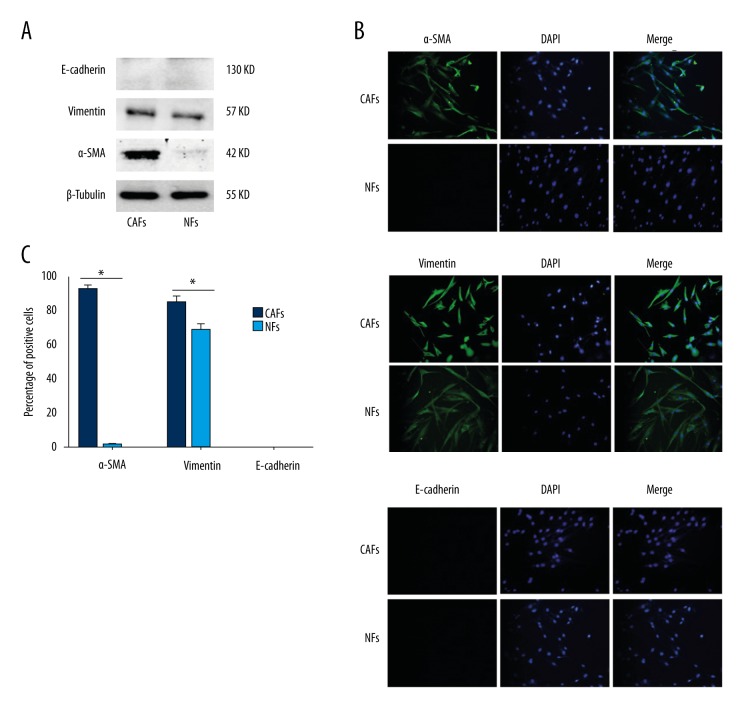
Western blot showed that the mesenchymal marker vimentin could be detected but epithelial marker E-cadherin could not be detected, both in CAFs and NFs. Particularly, myofibroblast marker α-SMA was significantly higher in CAFs than in NFs (P<0.05) (Figure 1A).
Figure 1.
CAFs identification by Western blot and immunofluorescence staining. (A) E-cadherin, vimentin, and α-SMA protein levels of CAFs and NFs were analyzed by Western blot. (B) E-cadherin, vimentin and α-SMA expressions of CAFs and NFs were analyzed by immunofluorescence staining (×200). (C) Percentage of cells was calculated in 3 different fields of vision according to immunofluorescence staining. * P<0.05.
Immunofluorescence staining also showed α-SMA and vimentin expressions were significantly higher in CAFs cytoplasm than in NFs cytoplasm (P<0.05) (Figure 1B, 1C). However, E-cadherin expression was negative in CAFs and NFs (Figure 1B, 1C).
CAFs autophagic level could be detected by relevant proteins
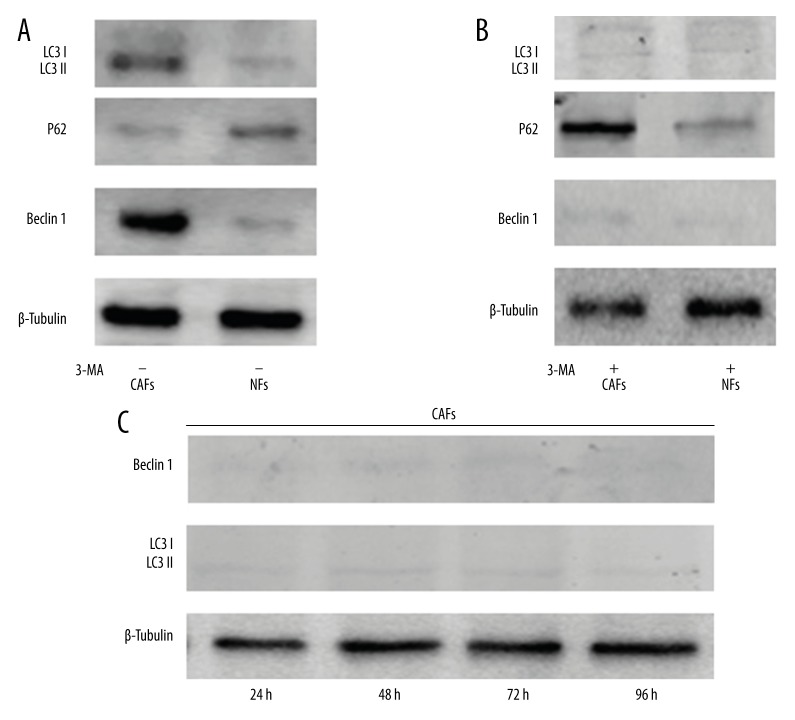
Beclin 1 and LC3-II/I protein conversion, which represented the level of cell autophagy, was detected in CAFs by Western blot and was higher than the level of NFs (P<0.05). P62, which is a downstream protein regulated by LC 3, was significantly lower in CAFs than in NFs (P<0.05) (Figure 2A). However, the expression of Beclin 1, LC3-II/I conversion, and P62 was reversed by 3-MA, indicating that the autophagic level of CAFs can be inhibited by 3-MA (Figure 2B). To detect the duration of the 3-MA inhibiting effect on CAFs autophagy, CAFs were first added to 3-MA for 24 h, then the medium was changed into DMEM plus 10% FBS, Beclin 1, and LC3-II/I conversion levels at 24, 48, 72, and 96 h were significantly lower than without 3-MA (P<0.05) (Figure 2C).
Figure 2.
CAFs and NFs autophagic levels were detected by Western blot. (A) Beclin 1, P62, LC3 I, and LC3 II proteins were detected. (B) Beclin 1, P62, LC3 I, and LC3 II proteins were detected when CAFs and NFs were cultured with 3-MA. (C) Beclin 1, LC3 I, and LC3 II proteins were detected at 24, 48, 72, and 96 h when CAFs were previously cultured with 3-MA for 24 h, then the media was changed into DMEM plus 10% FBS.
CAFs autophagy enhanced migration and invasion of TNBC cells
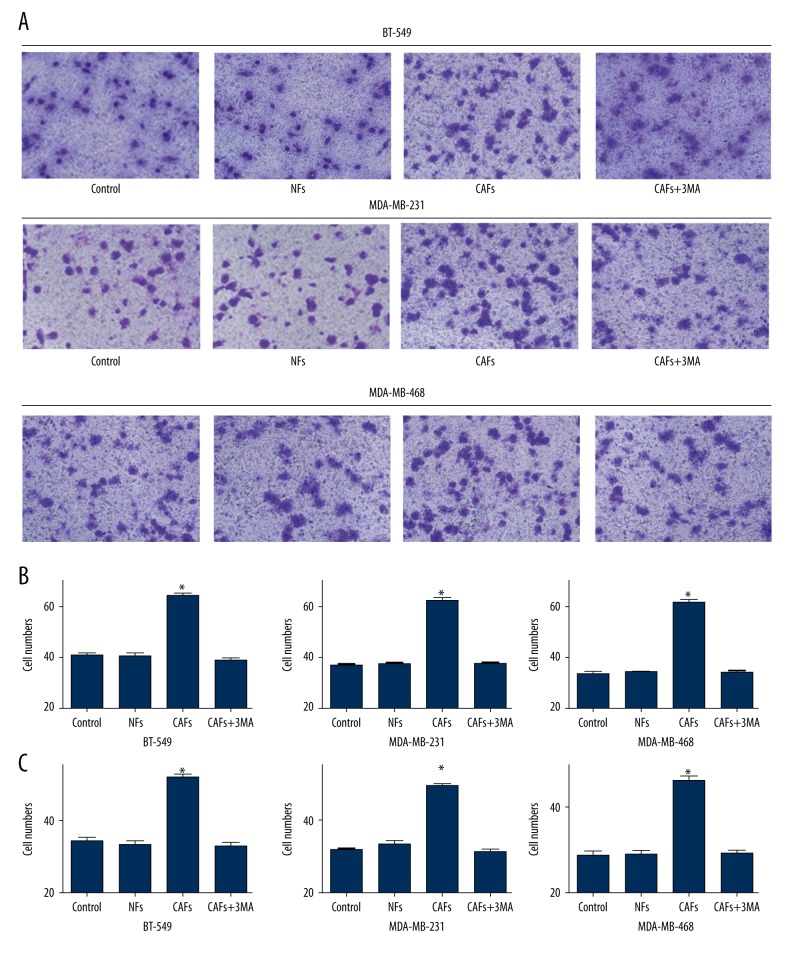
Cell migration assay indicated the numbers of cells in BT-549 were 40.8±2.14 in the control group, 40.2±2.32 in the NFs group, 64.0±2.12 in the CAFs group, and 38.6±1.85 in the 3-MA-CAFs group. The number of cells in the CAFs group was significantly higher than in the other 3 groups (P<0.05) (Figure 3A, 3B, Table 1). For MDA-MB-231 and MDA-MB-468, there were significantly more cells in the CAFs group than in the other 3 groups (P<0.05) (Figure 3B, Table 1).
Figure 3.
TNBC cell lines migration and invasion analysis. (A) TNBC cells migration was detected by Matrigel-coated Transwell. (B) The comparison of migration for different groups of TNBC cells. (C) The comparison of invasion for different groups of TNBC cells. * P<0.05.
Table 1.
Comparison of migration of TNBC cells.
| Groups | Control | NFs | CAFs | 3-MA-CAFs | F | P |
|---|---|---|---|---|---|---|
| BT-549 | 40.8±2.14 | 40.2±2.32 | 64.0±2.12 | 38.6±1.85 | 138.18 | <0.05 |
| MDA-MB-231 | 36.6±1.82 | 37.0±1.87 | 62.0±2.55 | 37.2±1.92 | 184.88 | <0.05 |
| MDA-MB-468 | 33.4±2.07 | 34.0±1.58 | 61.2±2.86 | 33.8±1.92 | 201.78 | <0.05 |
Cell invasion assay indicated there were 34.2±1.72 cells in the BT-549 control group, 33.4±1.50 in the NFs group, 51.8±1.92 in the CAFs group, and 32.8±1.67 in the 3-MA-CAFs group. There were significantly more cells in the CAFs group than in the other 3 groups (P<0.05) (Figure 3C, Table 2). For MDA-MB-231 and MDA-MB-468, there were significantly more cells in the CAFs group than in the other 3 groups (P<0.05) (Figure 3C, Table 2).
Table 2.
Comparison of invasion of TNBC cells.
| Groups | Control | NFs | CAFs | 3-MA-CAFs | F | P |
|---|---|---|---|---|---|---|
| BT-549 | 34.2±1.72 | 33.4±1.50 | 51.8±1.92 | 32.8±1.67 | 122.14 | <0.05 |
| MDA-MB-231 | 31.4±1.52 | 33.0±2.21 | 49.0±1.58 | 31.0±1.58 | 126.62 | <0.05 |
| MDA-MB-468 | 28.8±1.92 | 29.0±1.87 | 46.4±2.07 | 29.2±1.30 | 114.72 | <0.05 |
CAFs autophagy enhanced TNBC cells proliferation
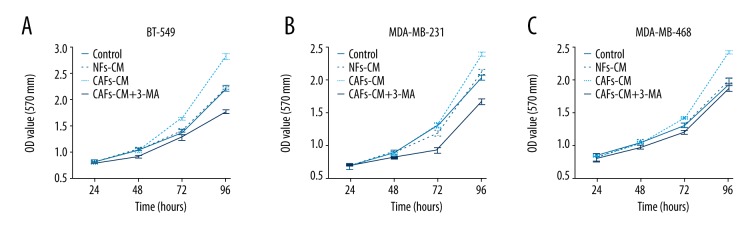
MTT assay indicated that the proliferation rates in the 4 groups of cells in BT-549 were significantly different; among them, the CAFs group rate was the highest and the 3-MA-CAFs group was the lowest (P<0.05) (Figure 4A). The same phenomenon was observed in MDA-MB-231 (P<0.05) (Figure 4B). For MDA-MB-468, the CAFs group rate was significantly higher than in the other 3 groups (P<0.05) (Figure 4C), while the 3-MA-CAFs group was not significantly lower than in the NFs or control groups.
Figure 4.
The comparison of proliferation for different groups in BT-549 (A), MDA-MB-231 (B), and MDA-MB-468 cells (C).
CAFs autophagy promoted the EMT process in TNBC cells
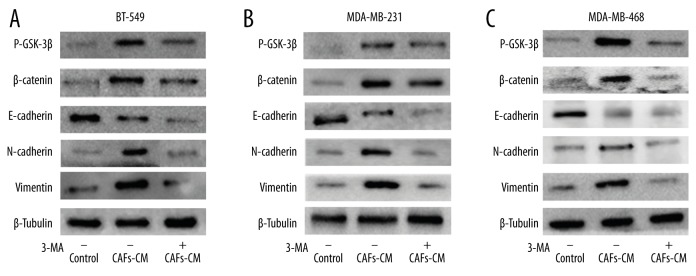
Western blot assay indicated vimentin and N-cadherin protein levels were upregulated and E-cadherin protein level was downregulated in BT-549 when the cells were co-cultured with CAFs-CM, compared with the control group (P<0.05). This effect was reversed when CAFs were previously cultured with 3-MA for 24 h (Figure 5A). Further study showed β-catenin and P-GSK-3β, which are key proteins in the Wnt/β-catenin pathway, were upregulated in the CAFs-CM group compared with the control group (P<0.05). This effect was reversed by 3-MA (Figure 5A). For MDA-MB-231 and MDA-MB-468, the same phenomenon was also observed (Figure 5B, 5C). The results demonstrate that CAFs autophagy can promote TNBC cells to engage in the EMT process through the Wnt/β-catenin pathway.
Figure 5.
EMT relative proteins were detected for different groups in BT-549 (A), MDA-MB-231 (B), and MDA-MB-468 cells (C).
Discussion
Autophagy is an evolutionarily conserved lysosomal process whereby cytoplasmic organelles and macromolecules are enveloped in autophagosomes and degraded by fusion with lysosomes for energy recycling [19,23]. Recent studies show the contradictory roles of autophagy for tumor cells, which means autophagy not only facilitates cell survival and delays apoptotic death under stress, but also promotes a specific form of cell death called autophagic cell death [24,25]. The same effects of autophagy on breast cancer cells can also be found in the literature, so whether autophagy promotes or protects breast cancer cells from death depends on various conditions [24–27]. In a previous study by our team, we demonstrated that elevated autophagic level play a double role in TNBC BT-549 and MDA-MB-231 cell growth in vitro [28]. However, as TNBC cells are not isolated in vivo, but are living in TME, TNBC cells have numerous connections with other cytokines and cells. Among these cells, fibroblasts are thought to be important as they are the most abundant cellular components in TME and they can transform into CAFs when activated by tumor cells [13]. Because autophagy is conservative for most cells and CAFs is a key regulator of paracrine signaling required for cancer progression, autophagic characteristics of CAFs and effects on TNBC cells growth were evaluated in this study.
As α-SMA is a marker of CAFs and its expression is higher than NFs derived from normal breast tissues [14], CAFs obtained from TNBC tumors were identified by α-SMA expression in our study. CAFs autophagic level was detected, showing that Beclin 1 and LC3-II/I protein conversion levels were upregulated and P62 level was downregulated, indicating that the CAFs autophagic level was higher than that of NFs for TNBC tumors. However, as CAFs were harvested from 5 patients in our study, whether CAFs autophagic level is higher than NFs for all TNBC patients needs to be further evaluated. We also found CAFs autophagy improves migration, invasion, and proliferation of TNBC cells, demonstrating the promoting effect of CAFs autophagy on TNBC cells metastasis. Because autophagy is a “double-edged sword” for cell growth, CAFs autophagy might have a negative effect on TNBC cells growth, which depends on the tumor microenvironment.
The activation of CAFs is a key event in tumor metastasis [29], but the exact mechanisms of metastasis caused by CAFs in breast cancer vary [30,31]. For example, one study demonstrated that CAFs enhanced metastatic potential of breast cancer cells through EMT process induced by paracrine TGF-b signaling [16]. Another study indicated that CAFs synthesized ECM, which is a mediator of invasion and migration of cancer cells with EMT phenotype, promoting cancer cells metastasis [12]. Yet another study showed that CAFs can promote aggressive behavior of TNBC cells by inducing EMT in a CXCL12/SDF-1-dependent manner [32]. From these results, we know that the EMT process is an important mechanism for tumor cell metastasis caused by CAFs. Because we already proved that a higher autophagic level improves the EMT process in TNBC cells through the Wnt/β-catenin pathway [19], we also hoped to find whether there is a relationship between CAFs autophagy and TNBC cells EMT process. The present study shows that CAFs autophagy enhances the EMT process for TNBC cells by leading to upregulation of vimentin and N-cadherin protein levels, and by downregulation of E-cadherin. Further analysis showed that β-catenin and P-GSK-3β protein levels in TNBC cells were also upregulated, indicating the Wnt/β-catenin pathway might be induced by CAFs autophagy. In fact, the activation of the Wnt/β-catenin pathway is just one type of mechanisms for tumor progression, and there are various mechanisms induced by CAFs autophagy in breast cancer. For example, TGF-β1 induced an upregulation of α-SMA in CAFs and protected breast cancer cells from nutrient deprivation [33]; BRCA1 gene knocked-down CAFs demonstrated an increase in markers of autophagy and increased ketone body production, promoting MDA-MB-231 cells progression [34]. From these results, we know that when tumor cells are in starvation or in other stress conditions, CAFs autophagy can provide nutrition for tumor cells by paracrine signal pathways, by which tumor cells can survive in such conditions [35–37]. However, most of these results came from in vitro experiments; in vivo research on TME would be more complicated and uncontrollable, so more signal pathways might be found in TNBC cells induced by CAFs [34].
Conclusions
We obtained CAFs from TNBC tumors and found that CAFs autophagic levels were higher than in NFs. We found that CAFs autophagy enhanced the migration, invasion, proliferation, and EMT process of TNBC cell lines, and activation of the Wnt/β-catenin pathway may be a mechanism induced by CAFs autophagy for EMT process of TNBC cells.
Footnotes
Competing interests
There are no conflicts of interest.
Source of support: This study was supported by a grant from the Youth Cultivation Foundation of Southern Medical University (No. PY2014N062)
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 3.O’Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–14. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 4.Wen J, Yeo S, Wang C, et al. Autophagy inhibition re-sensitizes pulse stimulation-selected paclitaxel-resistant triple negative breast cancer cells to chemotherapy-induced apoptosis. Breast Cancer Res Treat. 2015;149:619–29. doi: 10.1007/s10549-015-3283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou L, Chen M, Yang H, et al. MiR-940 inhibited cell growth and migration in triple-negative breast cancer. Med Sci Monit. 2016;22:3666–72. doi: 10.12659/MSM.897731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: Therapeutic options. Lancet Oncol. 2007;8:235–44. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 7.Shu S, Lin CY, He HH, et al. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature. 2016;529:413–17. doi: 10.1038/nature16508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Iliopoulos D, Zhang Q, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508:103–7. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang T, Kwiatkowski N, et al. CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell. 2015;163:174–86. doi: 10.1016/j.cell.2015.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brauer HA, Makowski L, Hoadley KA, et al. Impact of tumor microenvironment and epithelial phenotypes on metabolism in breast cancer. Clin Cancer Res. 2013;19:571–85. doi: 10.1158/1078-0432.CCR-12-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu FL, Mo EP, Yang L, et al. Autophagy is involved in TGF-beta1-induced protective mechanisms and formation of cancer-associated fibroblasts phenotype in tumor microenvironment. Oncotarget. 2016;7:4122–41. doi: 10.18632/oncotarget.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Z, Song P, Li D, et al. Cancer-associated fibroblasts from invasive breast cancer have an attenuated capacity to secrete collagens. Int J Oncol. 2014;45:1479–88. doi: 10.3892/ijo.2014.2562. [DOI] [PubMed] [Google Scholar]
- 13.Gunaydin G, Kesikli SA, Guc D. Cancer associated fibroblasts have phenotypic and functional characteristics similar to the fibrocytes that represent a novel MDSC subset. Oncoimmunology. 2015;4:e1034918. doi: 10.1080/2162402X.2015.1034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ao Z, Shah SH, Machlin LM, et al. Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Cancer Res. 2015;75:4681–87. doi: 10.1158/0008-5472.CAN-15-1633. [DOI] [PubMed] [Google Scholar]
- 15.Amornsupak K, Insawang T, Thuwajit P, et al. Cancer-associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells. BMC Cancer. 2014;14:955. doi: 10.1186/1471-2407-14-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Xiao CH, Tan LD, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. Br J Cancer. 2014;110:724–32. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao Y, Keller ET, Garfield DH, et al. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32:303–15. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soon PS, Kim E, Pon CK, et al. Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells. Endocr Relat Cancer. 2013;20:1–12. doi: 10.1530/ERC-12-0227. [DOI] [PubMed] [Google Scholar]
- 19.Liang XH, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by Beclin 1. Nature. 1999;402:672–76. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 20.Wang MC, Wu AG, Huang YZ, et al. Autophagic regulation of cell growth by altered expression of Beclin 1 in triple-negative breast cancer. Int J Clin Exp Med. 2015;8:7049–58. [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Xue L, Zhang X, et al. Autophagy protects ovarian cancer-associated fibroblasts against oxidative stress. Cell Cycle. 2016;15:1376–85. doi: 10.1080/15384101.2016.1170269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu FL, Mo EP, Yang L, et al. Autophagy is involved in TGF-beta1-induced protective mechanisms and formation of cancer-associated fibroblasts phenotype in tumor microenvironment. Oncotarget. 2016;7:4122–41. doi: 10.18632/oncotarget.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang DH, Choi DS, Ensor JE, et al. The autophagy inhibitor chloroquine targets cancer stem cells in triple negative breast cancer by inducing mitochondrial damage and impairing DNA break repair. Cancer Lett. 2016;376:249–58. doi: 10.1016/j.canlet.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu X, Li S, Wei F, et al. Apogossypolone induces autophagy and apoptosis in breast cancer MCF-7 cells in vitro and in vivo. Breast Cancer. 2014;21:223–30. doi: 10.1007/s12282-012-0372-z. [DOI] [PubMed] [Google Scholar]
- 25.Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–29. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang LH, Yang AJ, Wang M, et al. Enhanced autophagy reveals vulnerability of P-gp mediated epirubicin resistance in triple negative breast cancer cells. Apoptosis. 2016;21:473–88. doi: 10.1007/s10495-016-1214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Shamaladevi N, Jayaprakasha GK, et al. Polyphenol-rich extract of Pimenta dioica berries (Allspice) kills breast cancer cells by autophagy and delays growth of triple negative breast cancer in athymic mice. Oncotarget. 2015;6:16379–95. doi: 10.18632/oncotarget.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang MC, Wu AG, Huang YZ, et al. Autophagic regulation of cell growth by altered expression of Beclin 1 in triple-negative breast cancer. Int J Clin Exp Med. 2015;8:7049–58. [PMC free article] [PubMed] [Google Scholar]
- 29.Tang X, Hou Y, Yang G, et al. Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell Death Differ. 2016;23(1):132–45. doi: 10.1038/cdd.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang S, Hou Y, Zhang H, et al. Oxidized ATM promotes abnormal proliferation of breast CAFs through maintaining intracellular redox homeostasis and activating the PI3K-AKT, MEK-ERK, and Wnt-beta-catenin signaling pathways. Cell Cycle. 2015;14:1908–24. doi: 10.1080/15384101.2015.1041685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goruppi S, Dotto GP. Mesenchymal stroma: Primary determinant and therapeutic target for epithelial cancer. Trends Cell Biol. 2013;23:593–602. doi: 10.1016/j.tcb.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Ansari MM, Hendrayani SF, Shehata AI, Aboussekhra A. p16(INK4A) represses the paracrine tumor-promoting effects of breast stromal fibroblasts. Oncogene. 2013;32:2356–64. doi: 10.1038/onc.2012.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guido C, Whitaker-Menezes D, Capparelli C, et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-beta drives tumor growth: connecting TGF-beta signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle. 2012;11:3019–35. doi: 10.4161/cc.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salem AF, Howell A, Sartini M, et al. Downregulation of stromal BRCA1 drives breast cancer tumor growth via upregulation of HIF-1alpha, autophagy and ketone body production. Cell Cycle. 2012;11:4167–73. doi: 10.4161/cc.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Outschoorn UE, Goldberg A, Lin Z, et al. Anti-estrogen resistance in breast cancer is induced by the tumor microenvironment and can be overcome by inhibiting mitochondrial function in epithelial cancer cells. Cancer Biol Ther. 2011;12:924–38. doi: 10.4161/cbt.12.10.17780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren J, Guo H, Wu H, et al. GPER in CAFs regulates hypoxia-driven breast cancer invasion in a CTGF-dependent manner. Oncol Rep. 2015;33:1929–37. doi: 10.3892/or.2015.3779. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]