Abstract
Introduction
People with COPD have a decline in functional status, but little is known about the rate of decline and factors that contribute. Of particular concern is the decline in cognitive and functional performance. Decrease in cognitive and functional performance will finally lead to decreased health status, sedentary life style and premature frailty.
Aim
The aim of this study is to compare functional performance and cognitive status in patients with COPD of different ages and to examine the changes in extrapulmonary effects.
Patients and methods
This study included 62 patients with COPD risk class D who were divided into two groups (<70 years, N=30 and >70 years, N=32). Patients first completed the Montreal Cognitive Assessment (MoCA), which is a 30-point test that assesses different cognitive domains, while isometric knee extension (IKE) was measured using a digital handheld dynamometer, and functional exercise level was assessed using the 6-minute walking distance (6MWD) test.
Results
The patients’ older age (age higher than 70 years) was associated with a significantly lower body mass index (BMI, 27.50 vs 24.24 kg/m2; P=0.020), higher vital capacity parameters, forced vital capacity (FVC, 2.74 vs 2.82 L; P=0.799), FVC (%) (73.00 vs 66.50, P=132), forced expiratory volume in the first second (FEV1, 0.93 vs 1.13 L; P=0.001) and FEV1 (%) (28.50 vs 30.50, P=0.605). In addition, patients at older age presented a significantly reduced physical activity capacity, 6MWD (385.93 vs 320.84 m, P<0.001) and IKE (24.75 vs 22.55 kgf, P=0.005), as well as higher values for inflammatory biomarkers, C-reactive protein (8.77 vs 3.34 mg/L, P=0.022). Moreover, patients at older age presented significantly lower score at the cognitive assessment, MoCA (23.50 vs 20.00, P<0.001).
Conclusion
Elderly COPD patients have reduced exercise capacity and muscle strength, deteriorated cognitive function and increased inflammatory markers. Furthermore, inflammation markers were significantly correlated with muscle strength, walking distance and cognitive impairment.
Keywords: aging, inflammation, strength, pulmonary function
Introduction
The rise in life expectancy worldwide has been accompanied by an increased incidence of age-related diseases, representing a high burden on society and health care services. All vital organs lose function with age, the lungs having a progressive decline in pulmonary function after the age of 25 years. A very common pulmonary disease such a COPD shows age-associated features, such as increased cellular senescence and oxidative stress, alteration in the extracellular matrix, etc.1
Aging has been defined as “the progressive decline of homeostasis that occurs after the productive phase of life is complete resulting in increased risk of disease or death”.2
For a long time, there is growing evidence suggesting that smokers and patients with COPD are prone to early aging, even those who do not smoke are at risk of developing airflow obstruction and reduced elastic recoil that progress with age.3
The Global Initiative for chronic Obstructive Lung Disease (GOLD) report defines COPD as a common preventable and treatable disease that is characterized by persistent airflow limitation which is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases.4
The disease is associated in many patients with several systemic manifestations that can result in impaired functional capacity, reduced health-related quality of life, worsening dyspnea, exacerbations, cognitive impairment and mortality. A common factor such as systemic inflammation has been widely reported to be an important link between COPD and other diseases. Important recognized manifestations include the presence of cardiovascular diseases that in large part are due to atherosclerosis, neurocognitive and psychiatric effects which have hypoxia as the central feature and skeletal muscle dysfunction that appears due to inactivity.
These patients have increased risk of neuronal injury, either due to hypoxia or associated comorbidities such as cardiovascular diseases. Studies suggest that up to 77% of patients with COPD hypoxemic or non-hypoxemic have some form of cognitive impairment.5,6
People with COPD have a decline in functional status but little is known about the rate of decline and factors that contribute. Of particular concern is the decline in cognitive and functional performance. Decrease in cognitive and functional performance will finally lead to decreased health status, sedentary life style and premature frailty.
Given the abovementioned factors, the purpose of this research was to compare functional performance and cognitive status in patients with COPD of different ages and to examine changes in extrapulmonary effects.
Study design
This research was an observational study that included patients with COPD who came for routine investigations. The study was approved by the ethical committee of the Infectious Diseases and Pneumophtisiology “Dr Victor Babes”, Timisoara hospital. All the patients signed an informed consent before entering the study. No invasive interventions were applied, and the medication and treatment were not modified for the purpose of this study. On admission day, blood samples were collected, and all the tests were performed.
Subjects
We included 62 patients with COPD risk class D (former smokers >10 packs-year who met the criteria according to the international guideline)7 who were divided into two groups (<70 years, N=30 and >70 years, N=32). All the patients were male and diagnosed with COPD for at least 10 years. They were classified according to GOLD guidelines as COPD risk class D. Inclusion criteria: COPD risk class D, no exacerbations in the past 3 months and similar educational level. Exclusion criteria: illiteracy, alcoholism, known cognitive impairment, syncope, severe cardiovascular comorbidities (myocardial infarction, congestive heart failure), medication such as antidepressant drugs and lower-extremity joint replacements.
Patients and methods
Patients first completed the Montreal Cognitive Assessment (MoCA), which is a 30-point test that assesses different cognitive domains such as visual-spatial abilities, executive functions, language, short-term memory, attention, concentration and at the end of the test, orientation. The cutoff score for mild cognitive impairment (MCI) is 26 points. This test is an efficient instrument to use for screening, diagnosis and tracking of MCI. It has become a widely used screening instrument in response to the poor sensitivity of the Mini-Mental State Examination (MMSE) test.8,9
Isometric knee extension (IKE) was measured using a digital handheld dynamometer (Chatillon K-FCE-200, USA). Knee extension was tested three times on the dominant foot preferred by the patient with rest period between each test. Only the highest value of the tests was used. Patients were seated upright on the testing chair with the knee bent at 90° with the lower leg hanging down the front of the chair.10
Functional exercise level was assessed using the 6-minute walking distance test (6MWD) according to the guidelines of the American Thoracic Society (ATS). The strongest indication for the 6MWD is for measuring the response to medical interventions in patients with moderate to severe heart or lung disease. It is also used as a measure of functional status of patients as well as a predictor of morbidity and mortality.11
Statistical analysis
Data were presented as mean and standard deviations (SDs) for continuous variables with Gaussian distribution, median and interquartile range (IQR) for continuous variables without Gaussian distribution or percentage (absolute frequency) for categorical variables. Continuous variable distributions were tested for normality using the Shapiro–Wilk test and for equality of variances by using Levene’s test.
To assess the significance of the differences between groups, the Student’s t-test (means, Gaussian populations), Mann–Whitney U test (median, non-Gaussian populations) and Pearson chi-square or Fisher’s exact test (proportions) were used.
The correlation between studied variables was evaluated using Spearman’s rank sum correlation coefficient (non-Gaussian distributed variables), its statistical significance being assessed using the t-distribution score test.
Data were analyzed using the SPSS v.17 software (SPSS Inc., Chicago, IL, USA). A P-value of 0.05 was considered as the threshold for statistical significance, and a confidence level of 0.95 was considered for estimating intervals.
Results
The study sample included 62 male COPD patients with age ranging from 55 to 80 years, mean 67.82±0.75. The detailed characteristics of the studied sample are presented in Table 1.
Table 1.
Patients’ baseline characteristics
| Number of patients | 62 |
| Age (years)a | 67.82±0.75 |
| Education levelb | 12.00 (11.00–12.00) |
| BMI (kg/m2)a | 25.92±0.71 |
| FVC (L)b | 2.79 (2.45–3.07) |
| FVC (%) | 72.00 (54.00–76.00) |
| FEV1 (L)b | 1.01 (0.87–1.14) |
| FEV1 (%) | 29.00 (27.00–33.00) |
| FEV1/FVCb | 35.50 (31.00–44.00) |
| 6MWD (m)b | 360.00 (315.00–400.00) |
| IKE (kgf)b | 22.95 (20.60–24.30) |
| ESR (mm/h)b | 15.00 (10.00–30.00) |
| Fibrinogen (g/L)b | 2.96 (2.44–3.49) |
| C-reactive protein (mg/L)b | 4.16 (2.73–18.72) |
| MoCAb | 21.50 (20.00–24.00) |
Notes:
Continuous variables (with Gaussian distribution) are indicated by their mean and standard deviations.
Continuous variables (with non-Gaussian distribution) are indicated by their median (interquartile range).
Abbreviations: BMI, body mass index; ESR, erythrocyte sedimentation rate; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; IKE, isometric knee extension; MoCA, Montreal Cognitive Assessment; 6MWD, 6-minute walking distance.
The patients’ older age (age higher than 70 years) was associated with a significantly lower body mass index (BMI, 27.50 vs 24.24 kg/m2; P=0.020), higher vital capacity parameters, forced vital capacity (FVC, 2.74 vs 2.82 L; P=0.799), FVC (%) (73.00 vs 66.50, P=132), forced expiratory volume in the first second (FEV1, 0.93 vs 1.13 L; P=0.001), FEV1 (%) (28.50 vs 30.50, P=0.605) and FEV1/FVC (34.00 vs 40.50, P=0.005). In addition, patients at older age presented a significantly reduced physical activity capacity, 6MWD (385.93 vs 320.84 m, P<0.001) and IKE (24.75 vs 22.55 kgf, P=0.005), as well as higher values for inflammatory biomarkers, C-reactive protein (CRP), (8.77 vs 3.34 mg/L, P=0.022). Moreover, patients at older age presented significantly lower score at the cognitive assessment, MoCA (23.50 vs 20.00, P<0.001) (Table 2).
Table 2.
Comparison between groups: patients aged <70 years versus patients aged >70 years
| Parameter | Group 1 (age <70) | Group 2 (age ≥70) | P-valuec |
|---|---|---|---|
| Number of patients | 30 | 32 | |
| BMI (kg/m2) | 27.50±0.96 | 24.24±0.96 | 0.020 |
| FVC (L)a | 2.74 (2.50–3.07) | 2.82 (2.25–3.13) | 0.799 |
| FVC (%)b | 73.00 (59.75–77.25) | 66.50 (59.00–74.75) | 0.132 |
| FEV1 (L)b | 0.93±0.03 | 1.13±0.05 | 0.001 |
| FEV1 (%)a | 28.50 (25.25–31.50) | 30.50 (27.25–33.75) | 0.605 |
| FEV1/FVCb | 34.00 (26.75–37.00) | 40.50 (33.50–46.00) | 0.005 |
| 6MWD (m)a | 385.93±9.67 | 320.84±13.34 | <0.001 |
| IKE (kgf)b | 24.75 (21.87–27.95) | 22.55 (20.20–23.40) | 0.005 |
| ESR (mm/h)b | 15.00 (10.00–26.25) | 17.50 (10.00–40.00) | 0.592 |
| Fibrinogen (g/L)b | 2.93 (2.35–3.30) | 2.95 (2.45–3.50) | 0.799 |
| C-reactive protein (mg/L)b | 8.77 (3.74–28.37) | 3.34 (1.30–5.61) | 0.022 |
| MoCAb | 23.50 (22.00–24.25) | 20.00 (19.00–21.00) | <0.001 |
Notes:
Continuous variables (with Gaussian distribution) are indicated by their mean and standard deviations.
Continuous variables (with non-Gaussian distribution) are indicated by their median (interquartile range).
P-value was computed using t-test for equality of mean values for continuous variables with non-Gaussian distribution and Mann–Whitney U test for continuous variables with non-Gaussian distribution.
Abbreviations: BMI, body mass index; ESR, erythrocyte sedimentation rate; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; IKE, isometric knee extension; MoCA, Montreal Cognitive Assessment; 6MWD, 6-minute walking distance.
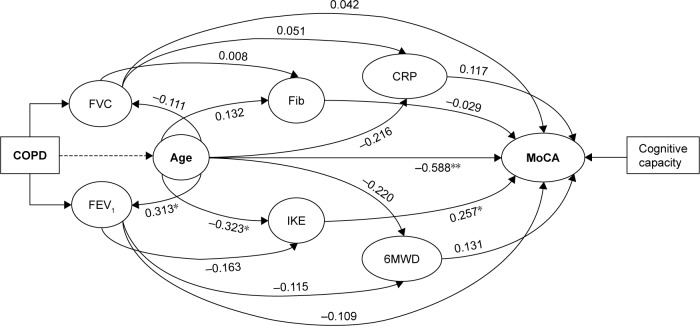
We observed that both 6MWD and IKE scores were negatively correlated with the vital capacity parameters, FVC and FEV1; an observation which points out that the physical activity capacity decreases along with the pulmonary impairment (Table 3). We observed that fibrinogen and CRP very poorly correlated with the vital capacity parameters. Similarly, the cognitive assignment score very poorly correlated with the vital capacity parameters, FVC and FEV1 (Table 3). However, patients’ age significantly reversely correlated with the cognitive capacity (Figure 1).
Table 3.
Correlation analysis of the relationships between the vital capacity and physical activity capacity, inflammatory biomarkers and cognitive function
| Explanatory variables | FVC (L)
|
FEV1 (L)
|
FEV1/FVC
|
|||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| Physical activity capacity | ||||||
| 6MWD (m) | −0.011 | 0.930 | −0.115 | 0.373 | 0.034 | 0.792 |
| IKE | −0.252 | 0.48 | −0.163 | 0.207 | 0.117 | 0.365 |
| Inflammatory biomarkers | ||||||
| Fibrinogen (g/L) | 0.008 | 0.954 | 0.063 | 0.625 | −0.046 | 0.725 |
| C-reactive protein (mg/L) | 0.051 | 0.694 | −0.131 | 0.311 | −0.138 | 0.286 |
| ESR (mm/h) | 0.099 | 0.444 | 0.060 | 0.645 | 0.013 | 0.919 |
| Cognitive capacity | ||||||
| MoCA | 0.042 | 0.748 | −0.109 | 0.401 | −0.171 | 0.183 |
Abbreviations: ESR, erythrocyte sedimentation rate; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; IKE, isometric knee extension; MoCA, Montreal Cognitive Assessment; 6MWD, 6-minute walking distance.
Figure 1.
The correlation between the parameters of the study.
Notes: *Correlation is significant at the 0.05 level (two tailed t-test). **Correlation is significant at the 0.01 level (two tailed t-test).
Abbreviations: CRP, C-reactive protein; FEV1, forced expiratory volume in the first second; Fib, fibrinogen; FVC, forced vital capacity; IKE, isometric knee extension; MoCA, Montreal Cognitive Assessment score; 6MWD, 6-minute walked distance.
We applied a hierarchical multiple regression to determine if the addition of physical activity capacity, MoCA score, age and BMI improved the prediction of FEV1/FVC over and above inflammatory biomarkers alone. Table 4 presents the details of the full regression model.
Table 4.
Summary of the full hierarchical multiple regression analysis
| Variable | B | SEB | β |
|---|---|---|---|
| Intercept | −51.748 | 22.607 | |
| C-reactive protein | −0.108 | 0.043 | −0.311* |
| Fibrinogen | 1.536 | 0.398 | 0.432** |
| ESR | −0.044 | 0.033 | −0.156 |
| MoCA score | 0.953 | 0.518 | 0.237 |
| Age | 0.835 | 0.205 | 0.535** |
| BMI | 0.364 | 0.188 | 0.231 |
Notes:
P<0.05;
P<0.0005. B, unstandardized regression coefficient; SEB, standard error of the coefficient; β, standardized regression coefficient.
Abbreviations: BMI, body mass index; ESR, erythrocyte sedimentation rate; MoCA, Montreal Cognitive Assessment.
Presence of linearity by inspecting the partial regression plots and a plot of studentized residuals against the predicted values was observed. At the same time, the residuals were independent, as assessed by a Durbin-Watson statistic of 1.925. In addition, we observed homoscedasticity, as assessed by visual inspection of a plot of studentized residuals versus unstandardized predicted values. There were three studentized deleted residuals greater than ±2 SD.
The full model including inflammatory biomarkers, age and BMI to predict FEV1/FVC was statistically significant, R2 =0.467, F(6, 49) =7.161, P<0.0005; adjusted R2 =0.402. The addition of IKE and 6MWD parameters to the prediction led to an unsignificant change in R2. The addition of age and BMI to the prediction led to a statistically significant increase in R2 of 0.222, F(4, 51) =3.638, P=0.011. Finally, three of the six variables added significantly to the prediction. Regression coefficients and standard errors are listed in Table 4.
We observed that an increase in CRP and erythrocyte sedimentation rate (ESR) of one SD was significantly associated with a decrease in FEV1/FVC of 0.108 SD and 0.044 SD, respectively. At the same time, an increase in fibrinogen of one SD was associated with an increase in FEV1/FVC of 1.536 SD. In addition, we observed that an increase in MoCA score, age and BMI of one SD was associated with an increase in FEV1/FVC of 0.953 SD, 0.835 SD, and 0.364 SD, respectively (Table 4).
Discussion
Like any other organ, the lung ages with progressive functional impairment and has reduced capacity to environmental stresses and injury. COPD is an age-related condition and accumulating evidence suggests a relationship with a global process of accelerated aging. In this study, we compared two groups of COPD patients with a difference of 10 years between them. We observed significant reductions in functional capacity, lower limb muscle force and cognitive assessment as well as increased inflammatory markers in the older group.
Airflow limitation measured by reduced FEV1 progresses very slowly over several decades, so that most patients with symptomatic COPD are in late middle age or elderly. The prevalence of COPD is age dependent suggesting an intimate relationship between the pathogenesis of COPD and aging.12
Thus, it would seem that older patients with COPD compared to younger would have a reduced FEV1. Interestingly, we observed that the older patients group had improved FEV1 and FVC values. Compared to our results, Vestbo et al13 found in their study that in more than a half of their patients, the rate of decline in FEV1 over a period of 3 years was not greater than that which has been in people without lung disease. We have to mention that we did not study the same patients so that the decrease in FEV1 is dependent on other factors such as time from the first diagnosis, treatment, number of exacerbations, etc.
Even though it is commonly assumed that physical activity declines as the disease progresses, there is little information about the natural course of it over time in COPD. The process of decline and aging can be described as a downward spiral of increasing disease severity, symptoms, decreasing functional capacity and functional performance.14
A study that analyzed the changes in physical activity in healthy individuals and COPD patients revealed a significant decrease in activity in elderly individuals and COPD patients; in the latter ones, the decrease being worse and paralleled to the severity of the disease.15
We observed a significant reduced physical activity capacity evaluated by the 6MWD test in the elderly group. Our findings are consistent with those of Waschki et al who found in two longitudinal studies a decrease in physical capacity in 1.5 years follow-up. The decrease is proportional with the severity of the disease, the lowest physical activity being assessed in patients with severe COPD. The results of our study are consistent with the findings of Corlateanu et al,16 who also compared COPD patients under and above 70 years. We found the same result as they did, that is, elderly COPD patients have a more severe deterioration of functional status. Age is an independent risk factor for reducing functional capacity in COPD patients.16
In healthy subjects, age is known to negatively affect contraction speed and muscle strength. It also induces a shift from muscle type II to type I fibers and atrophy of type II fibers. As a result, the limb muscles of elderly are smaller and contain more connective tissue and fat.17 In patients with COPD, increasing age was found to reduce strength, and this loss of strength was more pronounced than that of age-matched healthy individuals.18
We observed that decreased leg muscle strength was associated with reduced exercise capacity, elderly patients walking less during the 6MWD test. Furthermore, reductions in leg muscle strength (kgf) were associated with decreased exercise capacity. Our findings are consistent with those of Hamilton et al19 who analyzed muscle strength in patients with cardiorespiratory disorders over a period of 5 years. They demonstrated that patients with respiratory disorders have peripheral muscle weakness and that muscle strength is a significant contributor to work capacity.19
Cognitive impairment is an important concern for elderly because it can decrease quality of life and, in advanced stages, it might cause functional disabilities. Crişan et al6 demonstrated in a study which compared severe COPD patients in normal and acute phases (using the same MoCA questionnaire) a significant decrease in cognitive function even in patients with a borderline hypoxemia.
Another small study suggested that cognitive impairment can occur in the absence of hypoxemia or mild disease.20 A study from Finland found that a self-reported diagnosis of COPD in midlife was associated with increased odds of developing minor cognitive impairment in the later life.21
Compared to these studies we found a significant decrease in cognitive function in the elderly COPD group. Our findings confirm those of other authors who found that a longer duration of COPD (>5 years) is associated with higher odds of minor cognitive impairment and that the relationship is strongest in men.22
These results can be explained by the fact that longer duration of COPD could make the brain more vulnerable to hypoxic insults that can result in generation of free radicals, inflammation and neuronal damage.
The age-dependent increase in the prevalence of COPD and the dysregulation of the inflammatory and immune responses have suggested that changes related to aging may contribute to COPD pathogenesis.2
Several studies have shown that low-grade chronic systemic inflammation characterizes aging and that inflammatory markers are significant predictors of mortality in old individuals. Chronic inflammation has been correlated with many diseases and most of them are age related.23
We observed in our study that elderly patients had significantly higher inflammatory markers compared to the other group. These markers also significantly correlated with reduced walking distance and IKE force. The presence of inflammation in the skeletal muscle of patients with COPD is still a controversial issue.24
Our results confirm the findings of Ferrari et al,25 who also found a significant correlation between lower limb muscle strength and inflammation markers.
In another study that divided the COPD patients into subgroups of age, Tudorache et al26 also found significant correlations between inflammatory markers and decreased muscle strength (IKE) and exercise endurance (6MWD), with the patients aged over 70 years having the lowest results.
We have to mention that an important limitation of this study is the fact that the patients were not followed longitudinal. Although other authors have followed patients with COPD over a period of 5 years, our patients were in severe stages of the disease. Few of these patients would remain if we were to follow them over a period of 10 years. As mentioned above many factors influence the evolution of COPD but if we would extend our statistical analysis and suppose that these were the same patients we observed a decrease by 16.86%, 95% CI (13.46; 20.92) in the 6MWD, and 8.89%, 95% CI (2.61; 26.21) in the IKE, in patients older than 70 years.
Another limiting factor of this study is the absence of the control group. It would have been relevant to compare the data of the two COPD groups with at least one control group thus being able to highlight the accelerating aging process in COPD patients.
Conclusion
In summary, our findings demonstrate that elderly COPD patients have reduced exercise capacity and muscle strength, deteriorated cognitive function and increased inflammatory markers. Furthermore, inflammation markers were significantly correlated with muscle strength, walking distance and cognitive impairment. A better knowledge of the normal aging process can be essential to understand the mechanism of age-related lung diseases and thus could develop effective therapeutic strategies for lung diseases in elderly population. Besides pharmacological interventions that can have an effect on aging, exercise is the only non-pharmacological intervention associated with antiaging effect.
Footnotes
Disclosure
The authors report no conflicts of interest or any financial interests related to the study. The authors alone are responsible for the content and writing of the article.
References
- 1.Nicolas M, Kazuhiro I, Peter JB. Accelerated ageing of the lung in COPD: new concepts. Thorax. 2015;70(5):482–489. doi: 10.1136/thoraxjnl-2014-206084. [DOI] [PubMed] [Google Scholar]
- 2.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135(1):173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Strategy for the Diagnosis, Management and Prevention of COPD. 2016. pp. 12–14. [Google Scholar]
- 5.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35(4):913–922. doi: 10.1183/09031936.00125109. [DOI] [PubMed] [Google Scholar]
- 6.Crişan AF, Oancea C, Timar B, Fira-Mladinescu O, Crişan A, Tudorache V. Cognitive impairment in chronic obstructive pulmonary disease. PLoS One. 2014;9(7):e102468. doi: 10.1371/journal.pone.0102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 8.Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry. 2007;52(5):329–332. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- 9.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim WK, Kim DK, Seo KM, Kang SH. Reliability and validity of isometric knee extensor strength test with hand-held dynamometer depending on its fixation: a pilot study. Ann Rehabil Med. 2014;38(1):84–93. doi: 10.5535/arm.2014.38.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 12.Kazuhiro I, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 13.Vestbo J, Lisa E, Paul S, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 14.Larson J, Kapella M, Wirtz S, Covey M, Berry J. Reliability and validity of the Functional Performance Inventory in patients with moderate to severe chronic obstructive pulmonary disease. J Nurs Meas. 1998;6(1):55–73. [PubMed] [Google Scholar]
- 15.Tudorache V, Oancea C, Avram C, Fira-Mlădinescu O. Changes in physical activity in healthy people and COPD patients. Wien Klin Wochenschr. 2014;126(1–2):30–35. doi: 10.1007/s00508-013-0452-x. [DOI] [PubMed] [Google Scholar]
- 16.Corlateanu A, Montanari G, Botnar V. Influence of age in the functional status of COPD patients. Eur Respir J. 2014;44:1456. [Google Scholar]
- 17.Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 18.Spruit MA, Franssen FM, Rutten EP, Wagers SS, Wouters EF. Age-graded reductions in quadriceps muscle strength and peak aerobic capacity in COPD. Rev Bras Fisioter. 2012;16(2):148–156. doi: 10.1590/s1413-35552012005000011. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton AL, Killian KJ, Summers E, et al. Muscle strength, symptoms intensity and exercise capacity in patients with cardiopulmonary disorders. Am J Respir Crit Care Med. 1995;152:2021–2031. doi: 10.1164/ajrccm.152.6.8520771. [DOI] [PubMed] [Google Scholar]
- 20.Liesker JJ, Postma DS, Beukema RJ, et al. Cognitive performance in patients with COPD. Respir Med. 2004;98(4):351–356. doi: 10.1016/j.rmed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Rusanen M, Ngandu T, Laatikainen T, Tuomilehto J, Soininen H, Kivipelto M. Chronic obstructive pulmonary disease and asthma and the risk of mild cognitive impairment and dementia: a population based CAIDE study. Curr Alzheimer Res. 2013;10(5):549–555. doi: 10.2174/1567205011310050011. [DOI] [PubMed] [Google Scholar]
- 22.Balwinder S, Ajay KP, Michelle M, et al. COPD is associated with mild cognitive impairment: the Mayo clinic study of aging. Mayo Clin Proc. 2013;88(11):1222–1230. doi: 10.1016/j.mayocp.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kundu JK, Surh Y-J. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Maltais F, Decramer M, Casaburi R, et al. ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–e62. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari R, Caram LM, Faganello MM, Sanchez FF, Tanni SE, Godoy I. Relation between systemic inflammatory markers, peripheral muscle mass, and strength in limb muscles in stable COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:1553–1558. doi: 10.2147/COPD.S85954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tudorache E, Oancea C, Avram C, et al. Balance impairment and systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1847–1852. doi: 10.2147/COPD.S89814. [DOI] [PMC free article] [PubMed] [Google Scholar]