Abstract
The high amount of pp60c-src in platelets has led to speculation that this kinase is responsible for tyrosine-specific phosphorylation of cellular proteins during platelet activation by different agonists, and is, therefore, implicated in signal transduction of these cells. Unlike pp60v-src, the association of which with the cytoskeleton appears to be a prerequisite for transformation, pp60c-src is detergent-soluble in fibroblasts overexpressing the c-src gene, and its role in normal cellular function remains elusive. To gain a better understanding of the function of pp60c-src we have investigated the subcellular distribution of pp60c-src and its relationship to the cytoskeleton during platelet activation. Quantitative immunoblotting and immunoprecipitation have revealed that pp60c-src is detergent-soluble in resting platelets, while 40% of total platelet pp60c-src becomes associated with the cytoskeletal fraction upon platelet activation. We have also shown that a small pool of pp60c-src is associated with the membrane skeletal fraction which remains unchanged during the activation process. The interaction of pp60c-src with cytoskeletal proteins strongly correlates with aggregation and is mediated by GPIIb/IIIa receptor-fibrinogen binding. We suggest that the translocation of pp60c-src to the cytoskeleton and its association with cytoskeletal proteins may regulate tyrosine phosphorylation in platelets.
Full text
PDF
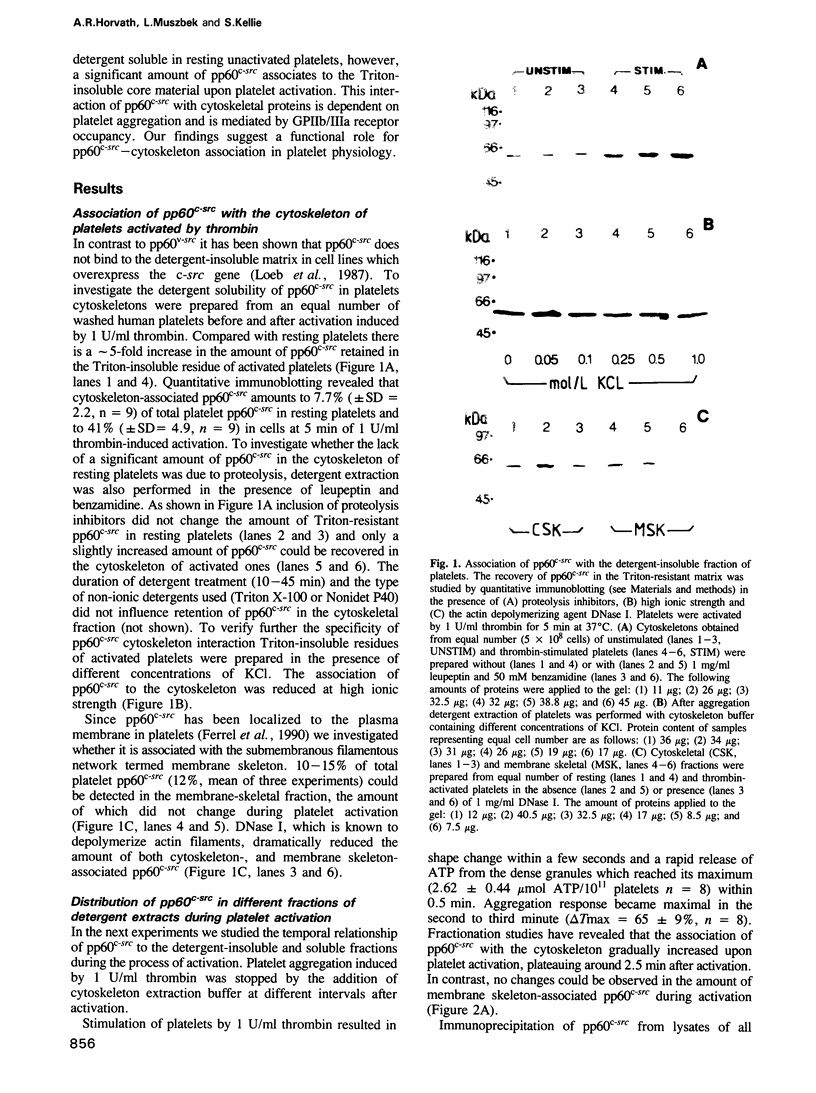
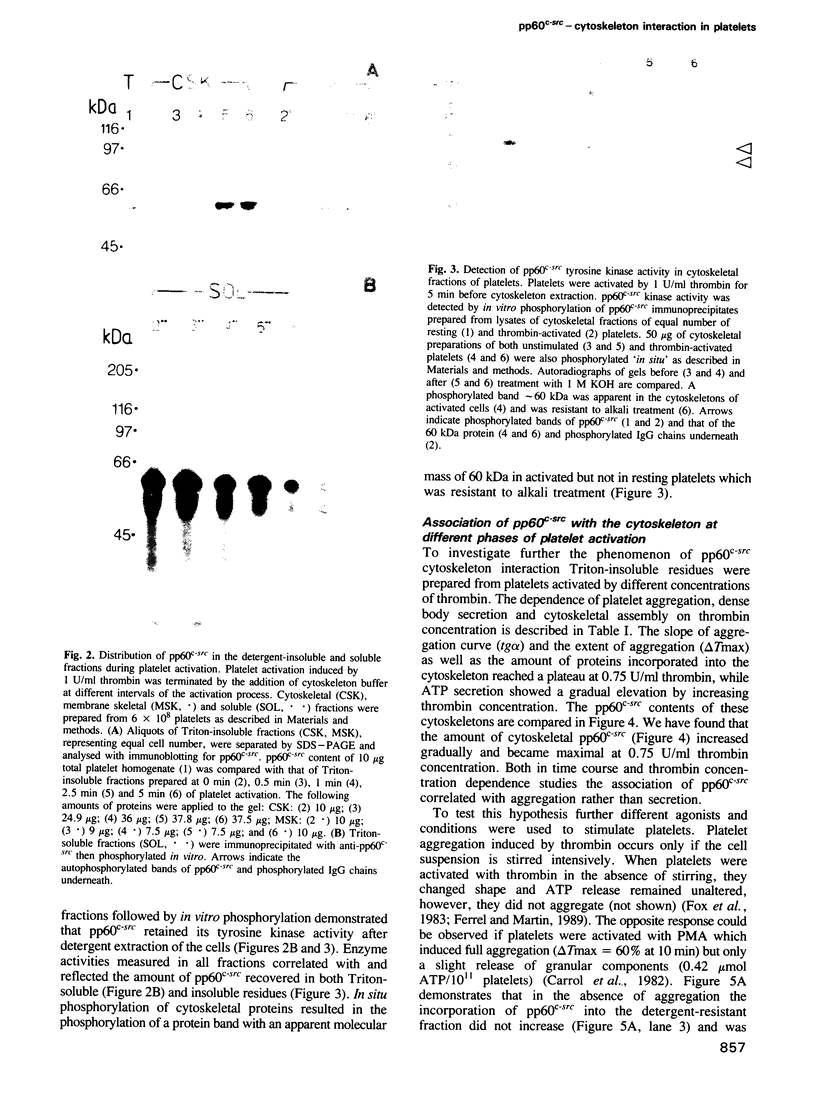
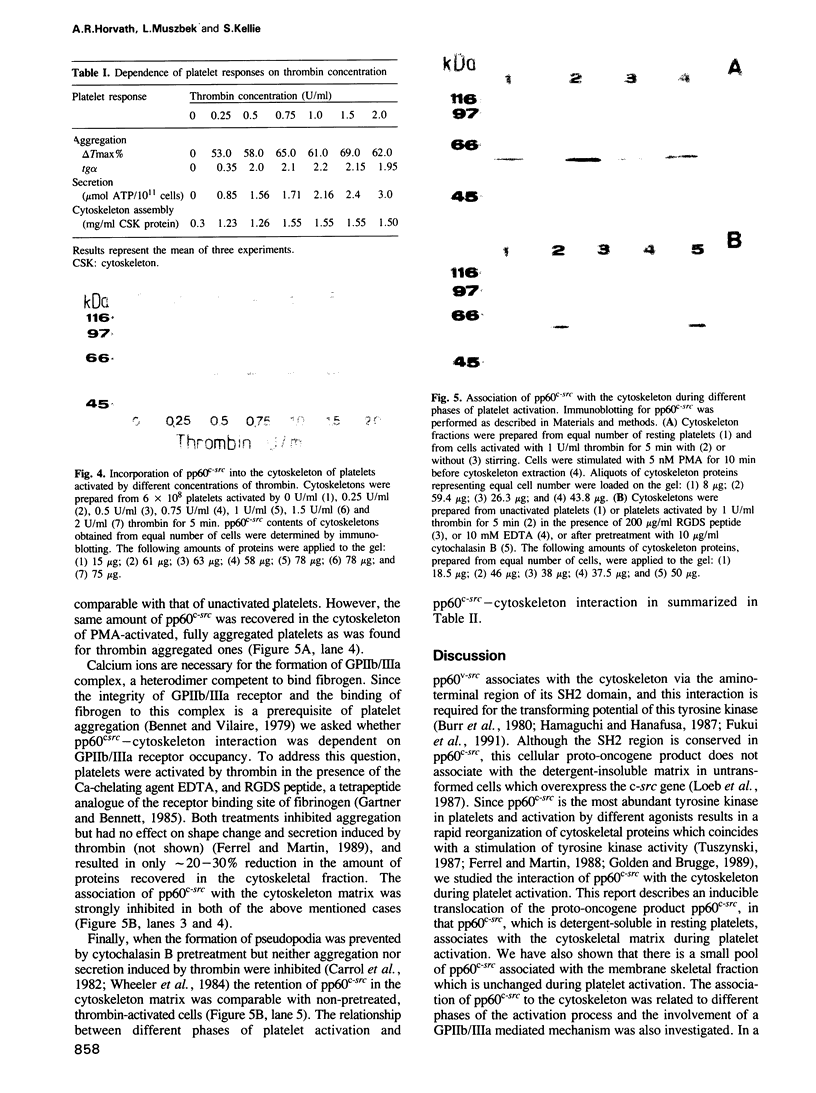



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagrodia S., Chackalaparampil I., Kmiecik T. E., Shalloway D. Altered tyrosine 527 phosphorylation and mitotic activation of p60c-src. Nature. 1991 Jan 10;349(6305):172–175. doi: 10.1038/349172a0. [DOI] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Burr J. G., Dreyfuss G., Penman S., Buchanan J. M. Association of the src gene product of Rous sarcoma virus with cytoskeletal structures of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3484–3488. doi: 10.1073/pnas.77.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. C., Butler R. G., Morris P. A., Gerrard J. M. Separable assembly of platelet pseudopodal and contractile cytoskeletons. Cell. 1982 Sep;30(2):385–393. doi: 10.1016/0092-8674(82)90236-7. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens P. M., Cooper J. A., Hunter T., Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60c-src relative to pp60v-src. Mol Cell Biol. 1985 Oct;5(10):2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore M. A., Anand R., Horvath A. R., Kellie S. Tyrosine-specific phosphorylation of gpIIIa in platelet membranes. FEBS Lett. 1990 Sep 3;269(2):283–287. doi: 10.1016/0014-5793(90)81177-p. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Platelet tyrosine-specific protein phosphorylation is regulated by thrombin. Mol Cell Biol. 1988 Sep;8(9):3603–3610. doi: 10.1128/mcb.8.9.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Tyrosine-specific protein phosphorylation is regulated by glycoprotein IIb-IIIa in platelets. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2234–2238. doi: 10.1073/pnas.86.7.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Noble J. A., Martin G. S., Jacques Y. V., Bainton D. F. Intracellular localization of pp60c-src in human platelets. Oncogene. 1990 Jul;5(7):1033–1036. [PubMed] [Google Scholar]
- Findik D., Reuter C., Presek P. Platelet membrane glycoproteins IIb and IIIa are substrates of purified pp60c-src protein tyrosine kinase. FEBS Lett. 1990 Mar 12;262(1):1–4. doi: 10.1016/0014-5793(90)80138-9. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Boyles J. K., Berndt M. C., Steffen P. K., Anderson L. K. Identification of a membrane skeleton in platelets. J Cell Biol. 1988 May;106(5):1525–1538. doi: 10.1083/jcb.106.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Reynolds C. C., Phillips D. R. Calcium-dependent proteolysis occurs during platelet aggregation. J Biol Chem. 1983 Aug 25;258(16):9973–9981. [PubMed] [Google Scholar]
- Fukui Y., O'Brien M. C., Hanafusa H. Deletions in the SH2 domain of p60v-src prevent association with the detergent-insoluble cellular matrix. Mol Cell Biol. 1991 Mar;11(3):1207–1213. doi: 10.1128/mcb.11.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner T. K., Bennett J. S. The tetrapeptide analogue of the cell attachment site of fibronectin inhibits platelet aggregation and fibrinogen binding to activated platelets. J Biol Chem. 1985 Oct 5;260(22):11891–11894. [PubMed] [Google Scholar]
- Golden A., Brugge J. S. Thrombin treatment induces rapid changes in tyrosine phosphorylation in platelets. Proc Natl Acad Sci U S A. 1989 Feb;86(3):901–905. doi: 10.1073/pnas.86.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin P., Plantavid M., Sultan C., Breton M., Mauco G., Chap H. Interaction of pp60c-src, phospholipase C, inositol-lipid, and diacyglycerol kinases with the cytoskeletons of thrombin-stimulated platelets. J Biol Chem. 1991 Aug 25;266(24):15705–15709. [PubMed] [Google Scholar]
- Hamaguchi M., Hanafusa H. Association of p60src with Triton X-100-resistant cellular structure correlates with morphological transformation. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2312–2316. doi: 10.1073/pnas.84.8.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., McClenaghan M. D. Cytochalasin B, the blood platelet release reaction and cyclic GMP. Nature. 1975 Feb 6;253(5491):455–457. doi: 10.1038/253455a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb D. M., Woolford J., Beemon K. pp60c-src has less affinity for the detergent-insoluble cellular matrix than do pp60v-src and other viral protein-tyrosine kinases. J Virol. 1987 Aug;61(8):2420–2427. doi: 10.1128/jvi.61.8.2420-2427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M. F., Koch C. A., Anderson D., Ellis C., England L., Martin G. S., Pawson T. Src homology region 2 domains direct protein-protein interactions in signal transduction. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8622–8626. doi: 10.1073/pnas.87.21.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszbek L., Laposata M. Glycoprotein Ib and glycoprotein IX in human platelets are acylated with palmitic acid through thioester linkages. J Biol Chem. 1989 Jun 15;264(17):9716–9719. [PubMed] [Google Scholar]
- Nada S., Okada M., MacAuley A., Cooper J. A., Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991 May 2;351(6321):69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990 Jan 12;60(1):167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Parsons S. J., Creutz C. E. p60c-src activity detected in the chromaffin granule membrane. Biochem Biophys Res Commun. 1986 Jan 29;134(2):736–742. doi: 10.1016/s0006-291x(86)80482-x. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Edwards H. H. Identification of membrane proteins mediating the interaction of human platelets. J Cell Biol. 1980 Jul;86(1):77–86. doi: 10.1083/jcb.86.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pho D. B., Vasseur C., Desbruyeres E., Olomucki A. Evidence for the presence of tropomyosin in the cytoskeletons of ADP- and thrombin-stimulated blood platelets. FEBS Lett. 1984 Jul 23;173(1):164–168. doi: 10.1016/0014-5793(84)81039-x. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H., Williams N. G., Cheng S. H., Roberts T. M. Regulation of pp60c-src and its interaction with polyomavirus middle T antigen in insect cells. J Virol. 1990 Jan;64(1):61–68. doi: 10.1128/jvi.64.1.61-68.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presek P., Reuter C., Findik D., Bette P. High-yield purification of a pp60c-src related protein-tyrosine kinase from human platelets. Biochim Biophys Acta. 1988 May 13;969(3):271–280. doi: 10.1016/0167-4889(88)90062-6. [DOI] [PubMed] [Google Scholar]
- Rendu F., Lebret M., Danielian S., Fagard R., Levy-Toledano S., Fischer S. High pp60c-src level in human platelet dense bodies. Blood. 1989 May 1;73(6):1545–1551. [PubMed] [Google Scholar]
- Shalloway D., Coussens P. M., Yaciuk P. Overexpression of the c-src protein does not induce transformation of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7071–7075. doi: 10.1073/pnas.81.22.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S. J., Hoxie J. A., Cunningham M., Brass L. F. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985 Sep 15;260(20):11107–11114. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Talmage D. A., Freund R., Young A. T., Dahl J., Dawe C. J., Benjamin T. L. Phosphorylation of middle T by pp60c-src: a switch for binding of phosphatidylinositol 3-kinase and optimal tumorigenesis. Cell. 1989 Oct 6;59(1):55–65. doi: 10.1016/0092-8674(89)90869-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuy F. P., Henry J., Rosenfeld C., Kahn A. High tyrosine kinase activity in normal nonproliferating cells. 1983 Sep 29-Oct 5Nature. 305(5933):435–438. doi: 10.1038/305435a0. [DOI] [PubMed] [Google Scholar]
- Welham M. J., Wyke J. A. A single point mutation has pleiotropic effects on pp60v-src function. J Virol. 1988 Jun;62(6):1898–1906. doi: 10.1128/jvi.62.6.1898-1906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M. E., Cox A. C., Carroll R. C. Retention of the glycoprotein IIb-IIIa complex in the isolated platelet cytoskeleton. Effects of separable assembly of platelet pseudopodal and contractile cytoskeletons. J Clin Invest. 1984 Sep;74(3):1080–1089. doi: 10.1172/JCI111475. [DOI] [PMC free article] [PubMed] [Google Scholar]








