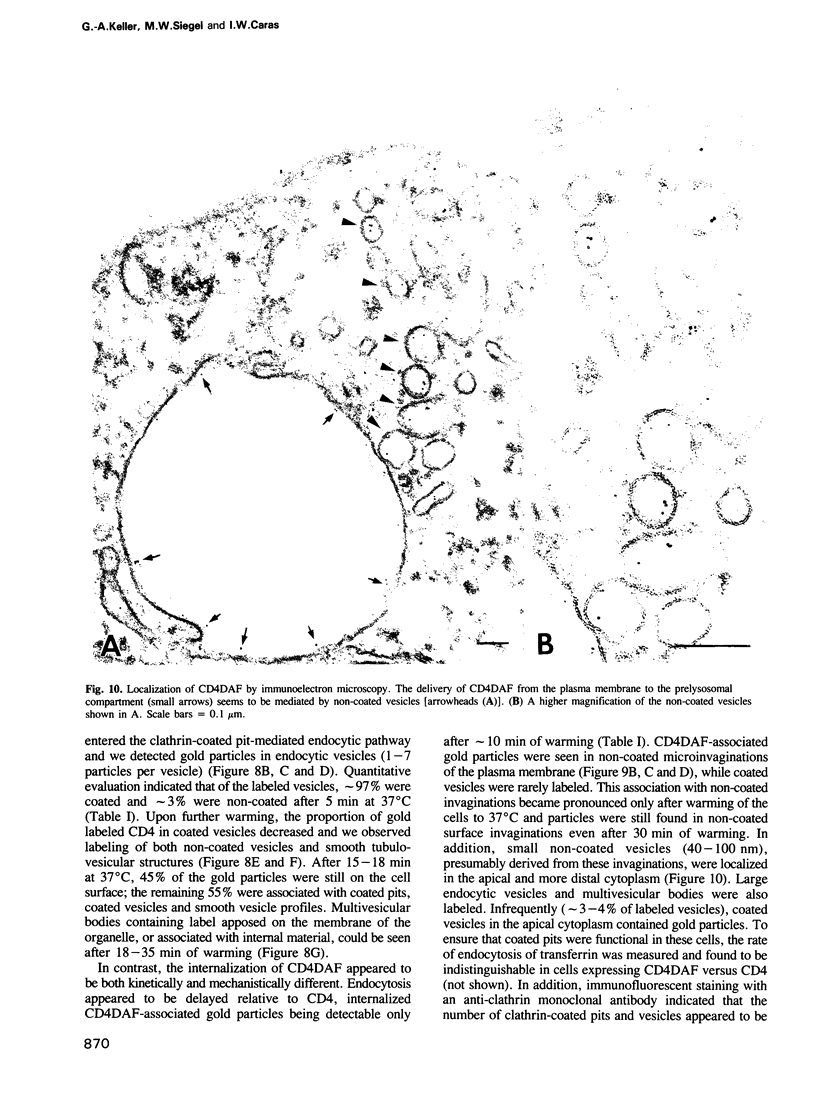
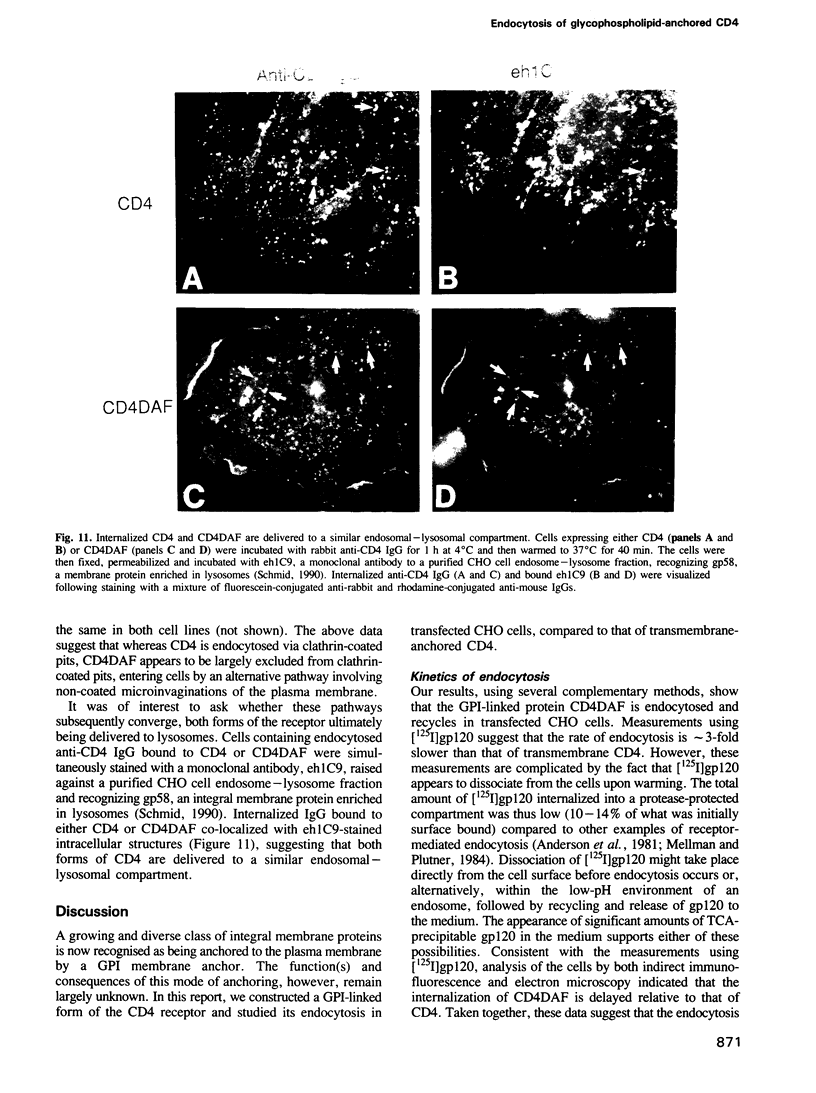
Abstract
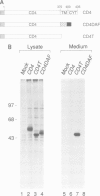
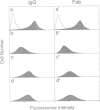
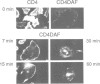
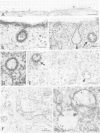
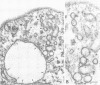
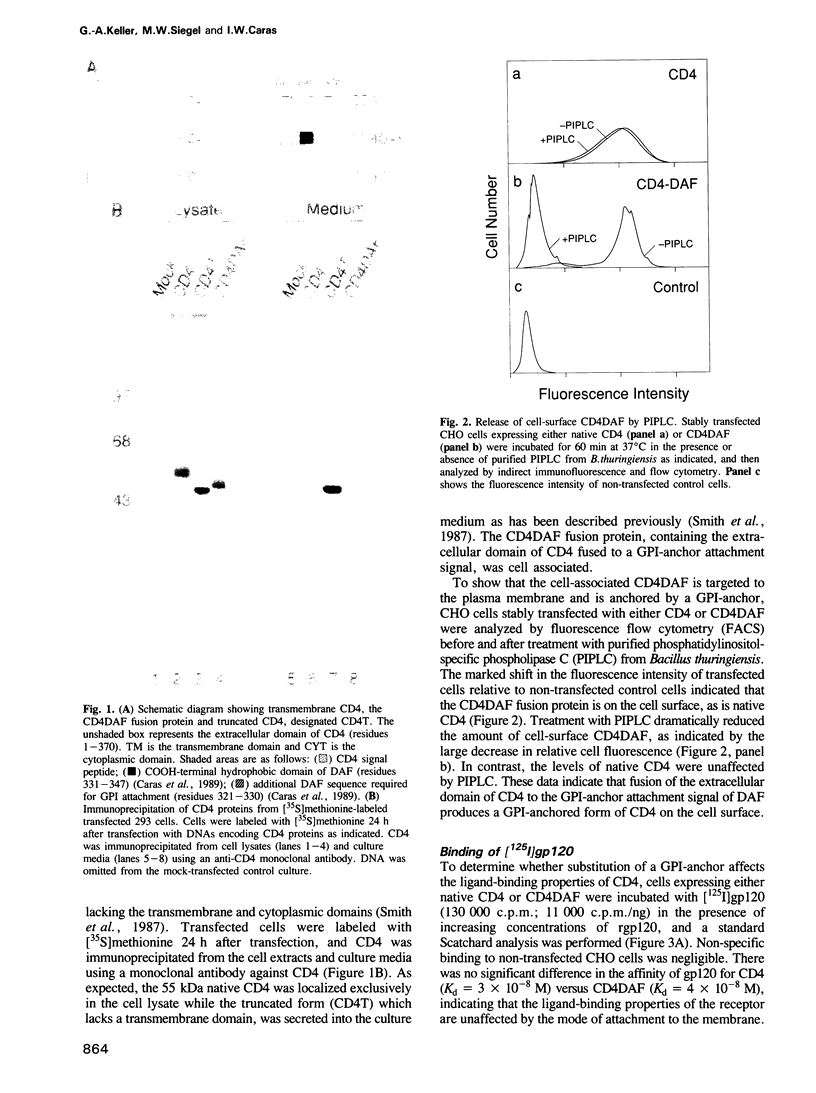
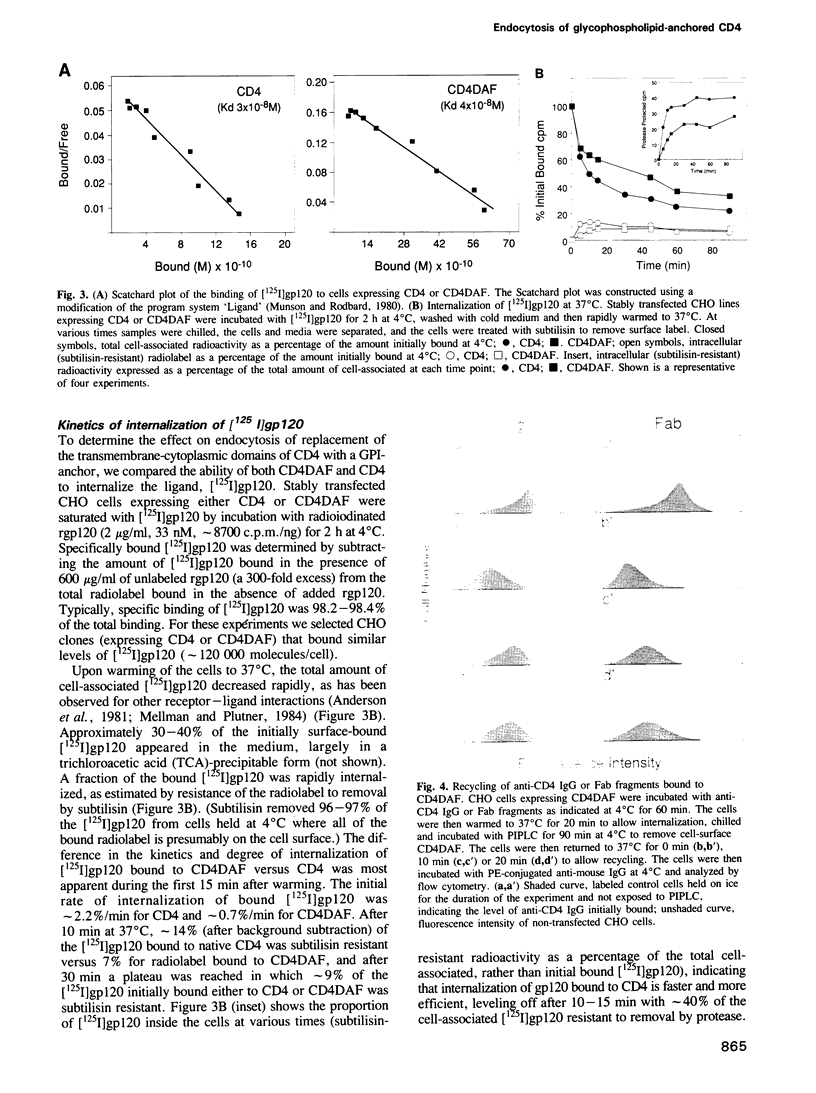
A glycophosphatidylinositol (GPI)-anchored form of the CD4 receptor was constructed by fusing the extracellular domain of CD4 to the COOH-terminus of decay accelerating factor (DAF), containing a signal for GPI-anchor attachment. The internalization of GPI-linked CD4 (CD4DAF) was compared to that of transmembrane CD4 using both [125I]gp120 and anti-CD4 antibodies. We show that transmembrane CD4 is rapidly endocytosed in transfected CHO cells, while CD4DAF is internalized at a rate approximately 3-fold slower. Immunoelectron microscopy suggests that whereas transmembrane CD4 is endocytosed via clathrin-coated vesicles, CD4DAF enters cells by an alternative pathway involving non-coated microinvaginations of the plasma membrane. Following internalization CD4DAF recycles through a primaquine-insensitive compartment, whereas the recycling of transmembrane CD4 is inhibited by primaquine, suggesting that the two receptors may recycle from distinct populations of early endosomes. Colocalization of both CD4DAF and CD4 with an antibody against a lysosomal membrane protein suggests that the two endocytic pathways may converge.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Brown M. S., Goldstein J. L. Inefficient internalization of receptor-bound low density lipoprotein in human carcinoma A-431 cells. J Cell Biol. 1981 Feb;88(2):441–452. doi: 10.1083/jcb.88.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs J. D., Andrews N. W., Hart G. W., Englund P. T. Posttranslational modification and intracellular transport of a trypanosome variant surface glycoprotein. J Cell Biol. 1986 Jul;103(1):255–263. doi: 10.1083/jcb.103.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs J. D., Hereld D., Krakow J. L., Hart G. W., Englund P. T. Rapid processing of the carboxyl terminus of a trypanosome variant surface glycoprotein. Proc Natl Acad Sci U S A. 1985 May;82(10):3207–3211. doi: 10.1073/pnas.82.10.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Thomson J. N., Pearse B. M. Coated pits act as molecular filters. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4156–4159. doi: 10.1073/pnas.77.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras I. W., Weddell G. N., Davitz M. A., Nussenzweig V., Martin D. W., Jr Signal for attachment of a phospholipid membrane anchor in decay accelerating factor. Science. 1987 Nov 27;238(4831):1280–1283. doi: 10.1126/science.2446389. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Weddell G. N., Williams S. R. Analysis of the signal for attachment of a glycophospholipid membrane anchor. J Cell Biol. 1989 Apr;108(4):1387–1396. doi: 10.1083/jcb.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Davis C. G., van Driel I. R., Russell D. W., Brown M. S., Goldstein J. L. The low density lipoprotein receptor. Identification of amino acids in cytoplasmic domain required for rapid endocytosis. J Biol Chem. 1987 Mar 25;262(9):4075–4082. [PubMed] [Google Scholar]
- Ferguson M. A., Duszenko M., Lamont G. S., Overath P., Cross G. A. Biosynthesis of Trypanosoma brucei variant surface glycoproteins. N-glycosylation and addition of a phosphatidylinositol membrane anchor. J Biol Chem. 1986 Jan 5;261(1):356–362. [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Iacopetta B. J., Rothenberger S., Kühn L. C. A role for the cytoplasmic domain in transferrin receptor sorting and coated pit formation during endocytosis. Cell. 1988 Aug 12;54(4):485–489. doi: 10.1016/0092-8674(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Lazarovits J., Roth M. A single amino acid change in the cytoplasmic domain allows the influenza virus hemagglutinin to be endocytosed through coated pits. Cell. 1988 Jun 3;53(5):743–752. doi: 10.1016/0092-8674(88)90092-x. [DOI] [PubMed] [Google Scholar]
- Lemansky P., Fatemi S. H., Gorican B., Meyale S., Rossero R., Tartakoff A. M. Dynamics and longevity of the glycolipid-anchored membrane protein, Thy-1. J Cell Biol. 1990 May;110(5):1525–1531. doi: 10.1083/jcb.110.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. Glycosyl-phosphatidylinositol: a versatile anchor for cell surface proteins. FASEB J. 1989 Mar;3(5):1600–1608. doi: 10.1096/fasebj.3.5.2522071. [DOI] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Mellman I., Plutner H. Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J Cell Biol. 1984 Apr;98(4):1170–1177. doi: 10.1083/jcb.98.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Roth J., Robert A., Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982 Apr 15;296(5858):651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Ockleford C. D., Critchley D. R. Tetanus toxin binding to mouse spinal cord cells: an evaluation of the role of gangliosides in toxin internalization. Brain Res. 1988 Dec 13;475(1):118–127. doi: 10.1016/0006-8993(88)90204-1. [DOI] [PubMed] [Google Scholar]
- Pelchen-Matthews A., Armes J. E., Griffiths G., Marsh M. Differential endocytosis of CD4 in lymphocytic and nonlymphocytic cells. J Exp Med. 1991 Mar 1;173(3):575–587. doi: 10.1084/jem.173.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A., Armes J. E., Marsh M. Internalization and recycling of CD4 transfected into HeLa and NIH3T3 cells. EMBO J. 1989 Dec 1;8(12):3641–3649. doi: 10.1002/j.1460-2075.1989.tb08538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P. A., Watts C. Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature. 1990 Aug 16;346(6285):655–657. doi: 10.1038/346655a0. [DOI] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kolhouse J. F., Kamen B. A., Anderson R. G. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990 Mar;110(3):637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
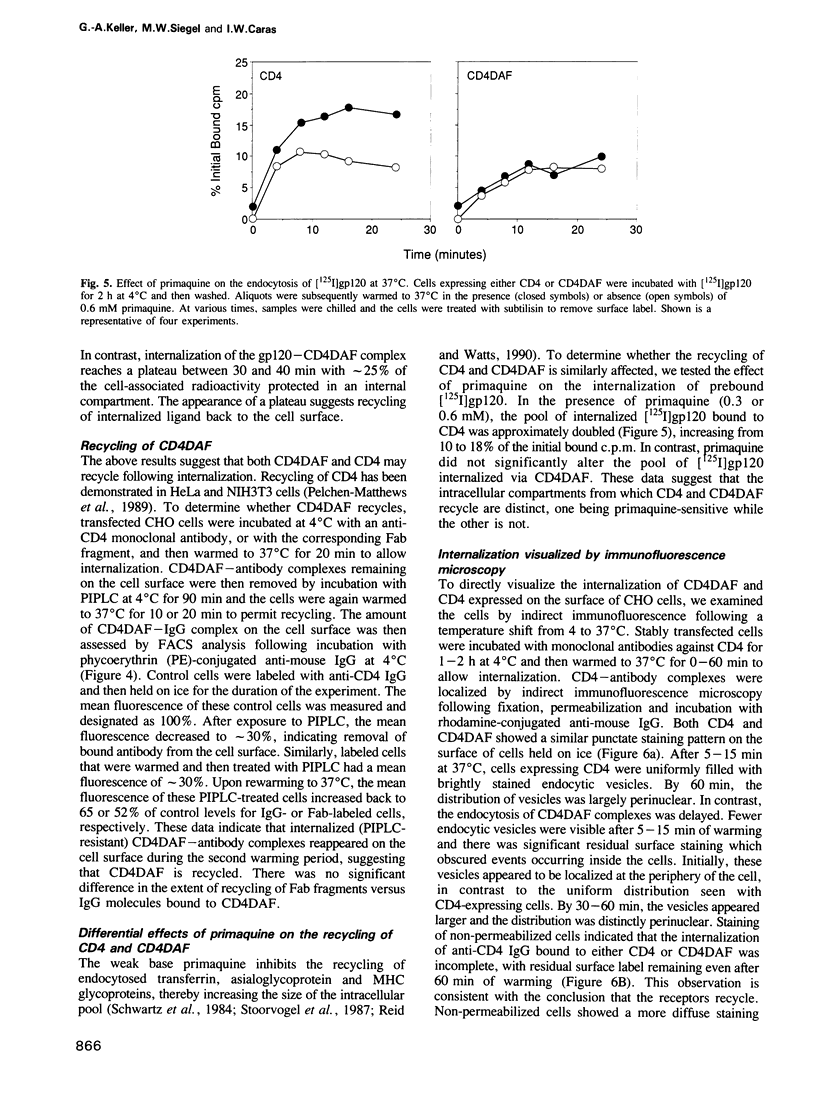
- Schwartz A. L., Bolognesi A., Fridovich S. E. Recycling of the asialoglycoprotein receptor and the effect of lysosomotropic amines in hepatoma cells. J Cell Biol. 1984 Feb;98(2):732–738. doi: 10.1083/jcb.98.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
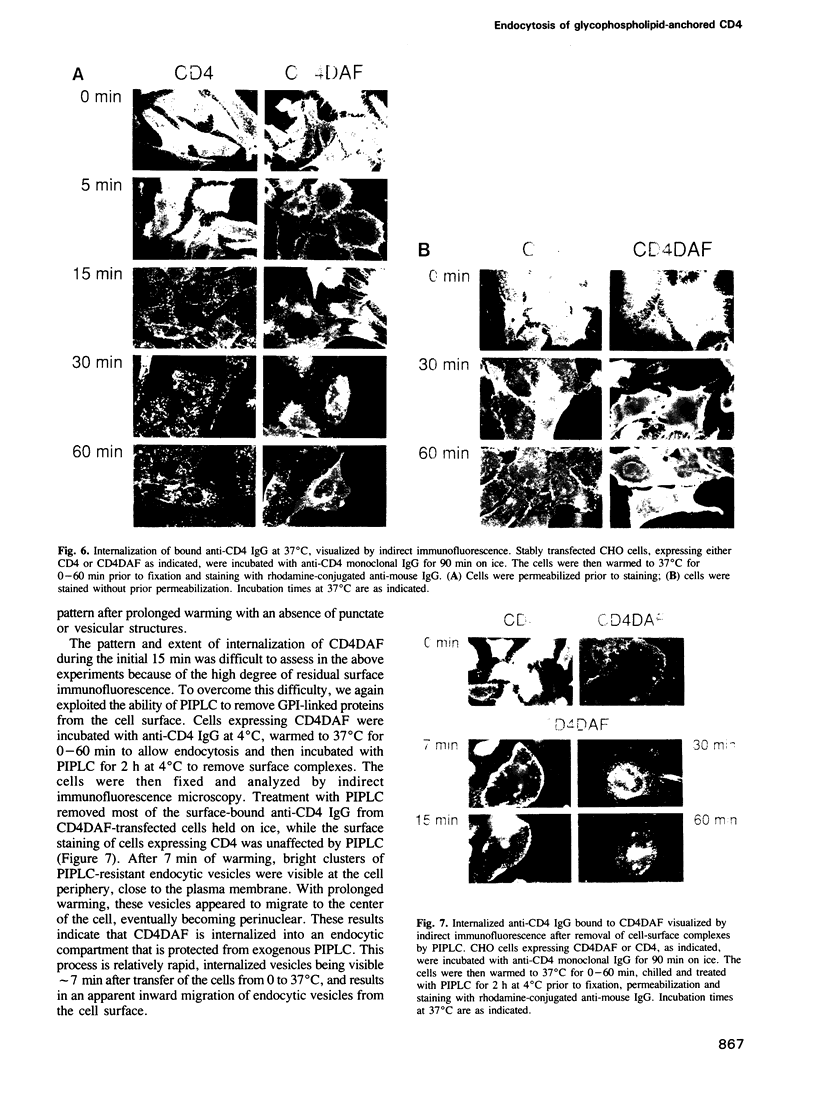
- Smith D. H., Byrn R. A., Marsters S. A., Gregory T., Groopman J. E., Capon D. J. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science. 1987 Dec 18;238(4834):1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W., Geuze H. J., Strous G. J. Sorting of endocytosed transferrin and asialoglycoprotein occurs immediately after internalization in HepG2 cells. J Cell Biol. 1987 May;104(5):1261–1268. doi: 10.1083/jcb.104.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tausk F., Fey M., Gigli I. Endocytosis and shedding of the decay accelerating factor on human polymorphonuclear cells. J Immunol. 1989 Nov 15;143(10):3295–3302. [PubMed] [Google Scholar]
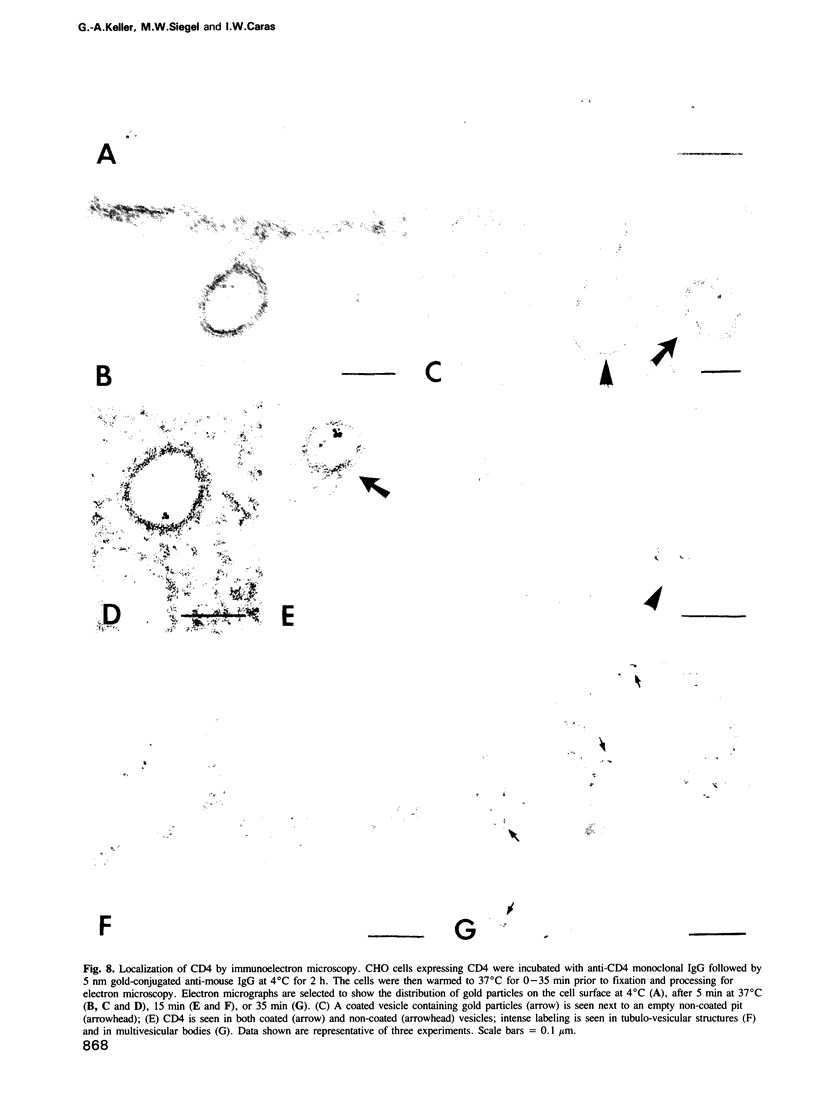
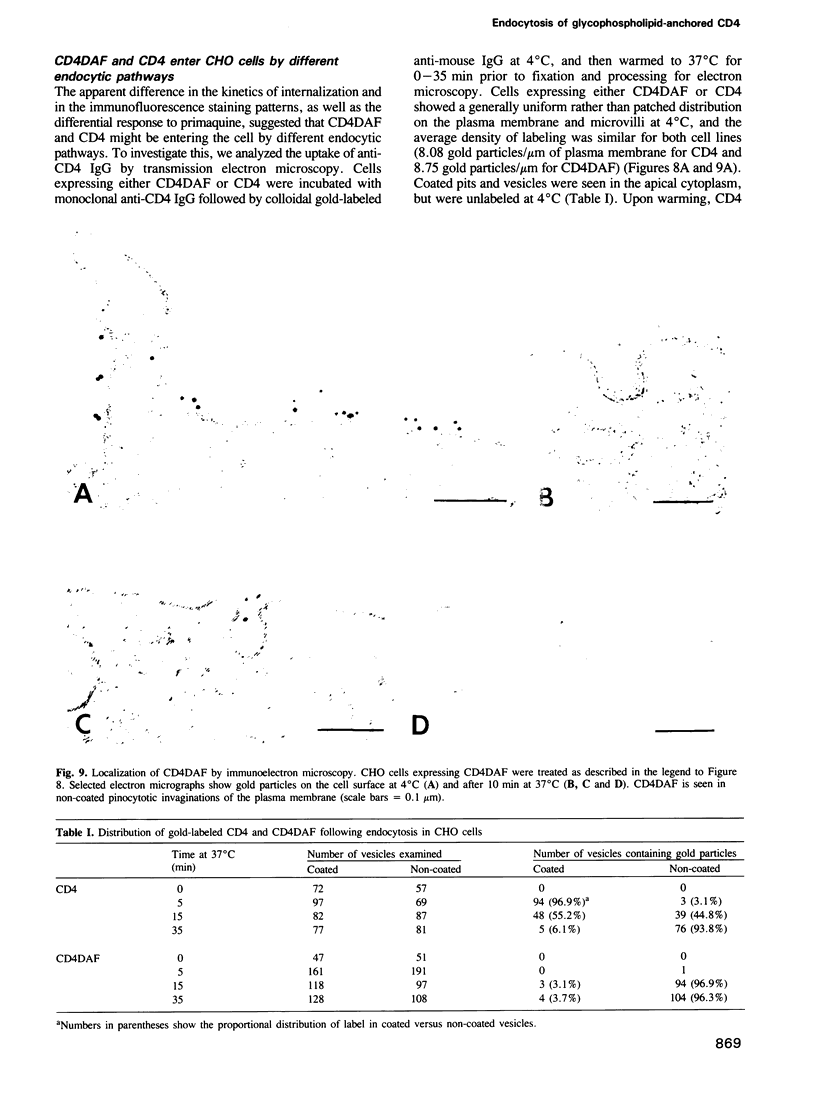
- Tran D., Carpentier J. L., Sawano F., Gorden P., Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B., Petersen O. W., Olsnes S., Sandvig K. The ways of endocytosis. Int Rev Cytol. 1989;117:131–177. doi: 10.1016/s0074-7696(08)61336-4. [DOI] [PubMed] [Google Scholar]
- van den Bosch R. A., du Maine A. P., Geuze H. J., van der Ende A., Strous G. J. Recycling of 5'-nucleotidase in a rat hepatoma cell line. EMBO J. 1988 Nov;7(11):3345–3351. doi: 10.1002/j.1460-2075.1988.tb03206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]