Abstract
Background and purpose
Impaired dexterity (fine hand movements) is often present in Parkinson’s disease (PD), even at early to moderate disease stages. It has a detrimental impact on activities of daily living (ADL) such as buttoning, contributing to reduced quality of life. Limb-kinetic apraxia, a loss of the ability to make precise, independent but coordinated finger and hand movements, may contribute to impaired dexterity even more than bradykinesia per se. However, the impact of limb-kinetic apraxia on ADL remains controversial. Our aim was to identify the strongest predictor of buttoning and unbuttoning in PD. It was hypothesized that coin rotation (a surrogate of limb-kinetic apraxia) represents the most important determinant.
Methods
Sixty-four right-handed, early to moderate PD patients were recruited from three movement disorder centers (Hoehn and Yahr stages 1–3). Buttoning, unbuttoning and coin rotation (right and left hand) represented the target tasks. Motor impairment was assessed according to the Unified Parkinson’s Disease Rating Scale.
Results
Multiple linear regression analysis showed that coin rotation with the right hand was the only significant predictor of buttoning (P < 0.001) and unbuttoning (P = 0.002). Notably, measures of bradykinesia or overall motor impairment did not represent significant predictors.
Conclusions
Constituting the novel key finding, limb-kinetic apraxia seems to be particularly relevant for ADL requiring dexterity skills in PD, even at early to moderate disease stages. Our results prompt research into the pathophysiological background and therapeutic options to treat limb-kinetic apraxia. The simple coin rotation test provides valuable information about ADL-related dexterity skills.
Keywords: activities of daily living, buttoning, dexterity, limb-kinetic apraxia, Parkinson’s disease
Introduction
Throughout different Parkinson’s disease (PD) stages, impaired dexterity (fine hand movements) is amongst the most frequently reported disturbing symptoms [1]. It represents a major contributor to the burden of the disease [2]. Deficits of dexterity impair typical activities of daily living (ADL). The existence of impaired dexterity even in mild to moderate PD stages has been shown [3].
Traditionally, limb bradykinesia was regarded as the main source of impaired dexterity. Previous research, however, illustrated that limb-kinetic apraxia (LKA) represents a second source, which seems largely unaffected by dopaminergic therapy [4,5]. LKA is regarded as a loss of the ability to make precise, independent but coordinated finger and hand movements [6]. It has to be delineated from the classical forms of apraxia, namely ideomotor and ideational/conceptual apraxia [7].
Expressing LKA-related dexterity deficits, the coin rotation (CR) task has been utilized by previous studies investigating LKA in PD [4,5,8–10]. Adequate CR performance needs precise, independent but coordinated movements. This is in contrast to the repetitive activation of agonists/antagonists as typical for bradykinesia tests like finger tapping. Indeed, the comparison of CR and finger tapping has been used to underline the existence of LKA in PD [4,5]. CR is also characterized by its simple feasibility [11,12]. Moreover, previous research [13] showed that CR is the only dexterity task lacking a significant response to dopaminergic therapy, which contrasts well with bradykinesia. Ultimately, recent investigations assessed CR thresholds to separate normal from impaired CR performance [11,12]. It is therefore a reasonable approach to use CR in order to examine the impact of LKA on ADL in PD.
The critical question is whether LKA has substantial impact on typical everyday dexterity tasks, thus contributing to the disease burden. Concomitantly, it is necessary to examine the impact of the ‘traditional’ source of impaired dexterity, namely limb bradykinesia, on the same ADL tasks.
Using buttoning/unbuttoning as representative ADL tasks, the first data on the impact of LKA on ADL are provided here. The primary hypothesis is that CR deficits represent the best predictor of dexterity-related ADL deficits. The alternative hypothesis states that bradykinesia represents the best predictor. Our aim was therefore to identify the strongest predictor of buttoning/unbuttoning in PD patients. The selection of buttoning is supported by reports identifying dressing difficulties as a major predictor of reduced quality of life [14] and troublesome buttoning as a sensitive ADL marker of PD [15,16]. Moreover, impaired buttoning was recognized as a typical feature early on [17]. It was therefore included in the ADL part of the Unified Parkinson’s Disease Rating Scale (UPDRS).
Methods/patients
All experiments were conducted in accordance with the Declaration of Helsinki. All subjects gave written informed consent. The study was approved by the institutional review board of all institutions (Vienna/Bern/Bethesda).
Inclusion/exclusion criteria and clinical/behavioral data
Applying a three-site multi-center approach, our aim was to include at least 60 PD patients [18], with every center contributing approximately 20 patients. The centers included (i) the Department of Neurology, Medical University of Vienna, Austria, (ii) the Departments of Neurology and Clinical Research, University Hospital, Inselspital Bern, Switzerland, and (iii) the NIH Parkinson Clinic, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland, USA.
To exclude executive, attentional and visuo-spatial dysfunction, a Montreal Cognitive Assessment score below 26 was an exclusion criterion. Only right-handed participants were recruited. Since ideomotor apraxia may contribute to defective manual skills, it was assessed applying the apraxia screen of the test of upper limb apraxia [19]. Only participants scoring above 8/12 points (normal range) were included. Patients with a history/clinical evidence of another neurological illness or with musculoskeletal disease interfering with task performance were excluded. Intact proprioception [20] represented another inclusion criterion (Data S1). Since buttoning/unbuttoning were performed in an upright position, Hoehn and Yahr stage I–III ON medication was required.
All tests were performed on medication, resembling an everyday life situation. Moreover, bradykinesia and LKA are best separated in the ON condition, since their surrogate tasks differ regarding their dopaminergic response [4,5,13]. Every participant was rated according to the UPDRS III. Patients with UPDRS III item 21 score of ≥2 (moderate amplitude action tremor or worse) were excluded. Since dyskinesias may confound motor performance [21], patients with dyskinesias interfering with any of the tasks were excluded.
Since the overall aim was to examine the predictive value of CR with regard to buttoning/unbuttoning, a subjective impairment of dexterity was not an obligatory inclusion criterion.
Representing an ADL-related measure of quality of life, items 11–16 (ADL sub-scale) of the Parkinson’s Disease Questionnaire 39 (PDQ-39) were assessed. The composite score 11–16 represented the ADL sub-scale score. It was included in the exploration of predictors of buttoning/unbuttoning.
Target tasks
Coin rotation
Participants rotated a US nickel ‘as quickly as possible’ in a clockwise direction with their thumb, index and middle finger. The task was performed in a sitting position, with the coin resting in the patient’s first three fingers as the initial position. Upon the command ‘start’, patients started flipping the coin above a flat table. Every 180° flip was counted as one rotation. Following 20 rotations, patients received the command ‘stop’. The time for completion of 20 rotations (full seconds) represented the outcome measure. Patients picked up the coin in the case of drops. The coin flip associated with a drop was not counted, and the time measurement was interrupted and resumed when the coin was back in the starting position. CR was performed with the right (CR right) and left hand (CR left).
Buttoning
Comparable to a previous study [22] and resembling everyday life, two cardigans, one male and one female, were used (buttons on right/left sides, respectively). The cardigans were custom-tailored. The distances were 65 cm in the vertical axis and 125 cm bottom width for the female version, and 67/153 cm for the male version (Fig. 1). Each cardigan had five vertically aligned buttons of 2.5 cm diameter, with a 10 cm inter-button distance. The time (seconds) taken to do up all five buttons from the lowest to the highest was the outcome variable, measured from the command ‘start’ to the completion of the last button (maximum speed). Buttoning was performed in a standing position, starting with both arms outstretched beside the trunk and all buttons open.
Figure 1.
Cardigans used for buttoning/unbuttoning. The male version is left, the female right.
Unbuttoning
In contrast to buttoning, the time taken to open all buttons from the highest to the lowest represented the outcome variable.
Participants underwent a practice trial (trial 1) with the following order: CR right/CR left/buttoning/unbuttoning. Following the practice trial, participants performed the actual experiment. The individual experiment consisted of two trials, which were composed of the four target tasks in a randomized order. A possible order was ‘CR left/unbuttoning/CR right/buttoning’ as trial 2, and ‘buttoning/CR left/CR right/unbuttoning’ as trial 3. For each target task, the trial with the better performance (shorter duration) entered the statistical work-up. Previous research investigating CR applied a similar approach regarding the characterization of CR performance and the temporal relation between practice trial and experiment [13].
Statistical explorations
Target task data (two-step analysis)
The first step included all 64 patients. Since buttoning/unbuttoning may be influenced by both the overall motor impairment and upper limb bradykinesia, two measures entered further exploration: the UPDRS III score represented overall motor impairment including rigidity; the sum of items 23–25 of the UPDRS represented upper limb bradykinesia. The latter was assessed separately for right (Upper limb bradykinesia right) and left (Upper limb bradykinesia left) [13].
Buttoning was correlated with CR right, CR left, UPDRS III, Upper limb bradykinesia right, Upper limb bradykinesia left, Hoehn and Yahr score, disease duration, age and the ADL sub-scale score of the PDQ-39 (Pearson’s correlation analysis). A significant correlation (P < 0.05) with buttoning qualified a variable as a predictor integrated in the subsequent multiple linear regression analysis. Concerning unbuttoning, an analogous analysis was applied. This approach provided two multiple linear regression analyses with the dependent variables buttoning and unbuttoning, respectively. The quality of the particular model was characterized by its R2. Measures of multicollinearity were assessed to rule out unacceptable collinearities. The above mentioned analysis was applied to a priori rule out unrelated variables (compare [9]). To avoid thresholds of significance that were too rigid, no Bonferroni correction was used for this screening correlation analysis.
In a second step, patients were dichotomized into two groups (control group with intact dexterity and target group with impaired dexterity). Since two independent studies [11,12] suggested a CR right performance >15 s as indicative of impaired dexterity, this threshold was applied. Further analyses were performed for both groups separately. By analogy to the above mentioned approach, significantly correlating variables qualified as predictors for subsequent multiple linear regression analyses. Since no uniform suggestion of a CR threshold for the non-dominant hand exists [11,12], dichotomization according to CR left was not performed.
Relationship of coin rotation/bradykinesia with handwriting
Handwriting represents another important everyday dexterity task. An additional analysis therefore aimed to assess the relationship of CR and bradykinesia with handwriting (represented by PDQ-39 item 14, reporting the frequency of ‘problems writing clearly’). Pearson’s correlation analysis was applied to explore possible correlations of handwriting with CR right and Upper limb bradykinesia right.
Possible learning effects and influence of action tremor on CR
Possible learning effects of buttoning/unbuttoning/CR were assessed. Moreover, a possible influence of action tremor on CR was assessed. The corresponding methods, results and discussion are outlined in Data S1.
Results
Table 1 provides the demographic characteristics and outcome of variables used for inclusion/exclusion. Table 2 shows the outcome of target task and individual clinical data.
Table 1.
Demographic data and outcome of variables used for inclusion/exclusion (64 patients)
| Age | 62.8 ± 9.6 (range 40–78) years |
| Disease duration | 7.6 ± 4.7 (range 1–18) years |
| Dominant side of parkinsonism | All participants: 35 right, 29 left |
| Vienna: 12 right, 10 left | |
| Bern: 13 right, 9 left | |
| Bethesda: 10 right, 10 left | |
| Sex | All participants: 34 male, 30 female |
| Vienna: 12 male, 10 female | |
| Bern: 9 male, 13 female | |
| Bethesda: 13 male, 7 female | |
| Hoehn and Yahr stage | 2.1 ± 0.6 (min 1, max 3); stages normally distributed; Hoehn and Yahr 1: 14.1% |
| Hoehn and Yahr 1.5: 1.6% | |
| Hoehn and Yahr 2: 54.7% | |
| Hoehn and Yahr 2.5: 10.9% | |
| Hoehn and Yahr 3: 18.8% | |
| MoCA | 28.2 ± 1.3 (min 26, max 30) points |
| AST right | 11.5 ± 0.8 (min 9, max 12) points |
| AST left | 11.5 ± 0.9 (min 9, max 12) points |
| Proprioception right | 5.9 ± 0.2 (min 5, max 6) points |
| Proprioception left | 5.9 ± 0.3 (min 5, max 6) points |
AST, apraxia screen of the test of upper limb apraxia; MoCA, Montreal Cognitive Assessment. Values are mean ± SD.
Table 2.
Target task and clinical data (64 patients)
| Upper limb bradykinesia right ON medication | 3.0 ± 2.2 (range 0–9) points |
| Upper limb bradykinesia left ON medication | 3.1 ± 2.3 (range 0–9) points |
| UPDRS III ON medication | 17.9 ± 8.8 (range 3–46) points |
| CR right | 19.7 ± 8.0 (range 10–65) s |
| CR left | 21.3 ± 6.2 (range 13–43) s |
| Buttoning | 14.8 ± 5.8 (range 7–38) s |
| Unbuttoning | 8.7 ± 3.5 (range 4–22) s |
| Items 11–16 of the PDQ-39 | 5.7 ± 4.6 (range 0–16) points |
| Item 14 of the PDQ-39 (handwriting) | 2.0 ± 1.4 (range 0–4) points |
CR, coin rotation; PDQ-39, Parkinson’s Disease Questionnaire 39; UPDRS, Unified Parkinson’s Disease Rating Scale. Values are mean ± SD.
Statistical explorations
Target task data
Step 1
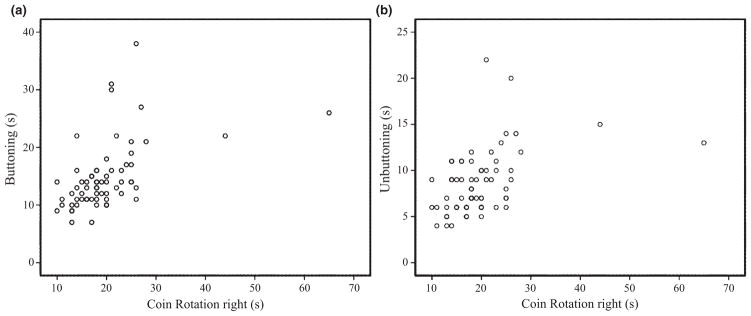
Pearson’s correlation analysis showed a significant positive correlation of buttoning/unbuttoning with CR right (r = 0.51/0.45), CR left (r = 0.41/0.32), UPDRS III (r = 0.48/0.46), Upper limb bradykinesia right (r = 0.49/0.38), Upper limb bradykinesia left (r = 0.40/0.36), Hoehn and Yahr score (r = 0.28/0.40) and the ADL sub-scale score (r = 0.45/0.45). In addition, significant correlations were found for buttoning with disease duration (r = 0.25) and unbuttoning with age (r = 0.29). The subsequent multiple linear regression analysis showed that CR right was the only significant predictor of buttoning (P < 0.001). All other variables did not represent significant predictors (P > 0.05). The created model was very acceptable (R2 = 0.50), with acceptable measures of multicollinearity (tolerance >0.29 and variance inflation factor <3.44 for all predictors). With regard to the analogous analysis of unbuttoning, again CR right represented the only significant predictor (P = 0.002). As before, the assumed model was very acceptable (R2 = 0.42), and measures of collinearity were within the same range (tolerance >0.28, variance inflation factor <3.50). Figure 2 provides a complementary scatter-plot illustrating CR right relative to buttoning/unbuttoning.
Figure 2.
Scatter-plot illustrating buttoning/unbuttoning versus coin rotation right. Coin rotation right versus buttoning ((a), Pearson’s r = 0.51; P < 0.001) and unbuttoning ((b), Pearson’s r = 0.45; P < 0.001); n = 64.
Step 2
In all, 47/64 patients scored above 15 s for CR right, representing the group with impaired dexterity. Compared to the remaining 17 patients, they were not significantly different regarding age, disease duration, UPDRS III, Upper limb bradykinesia right, Upper limb bradykinesia left, Hoehn and Yahr score and the ADL sub-scale score (seven unpaired t tests, all P > 0.05, Bonferroni corrected).
The further exploration of the 47 patients with impaired dexterity showed that both buttoning and unbuttoning correlated significantly (Pearson’s correlation analysis, P < 0.05) with CR right (r = 0.45/0.37), UPDRS III (r = 0.42/0.42), Upper limb bradykinesia right (r = 0.41/0.30), Upper limb brady kinesia left (r = 0.44/0.39), the ADL sub-scale score (r = 0.36/0.36) and Hoehn and Yahr score (only unbuttoning, r = 0.38). The subsequent multiple linear regression analyses identified CR right again as the only significant predictor of buttoning (P = 0.005) and unbuttoning (P = 0.031). The quality of the created models was acceptable (buttoning, R2 = 0.39, tolerance >0.33, variance inflation factor <2.97; unbuttoning, R2 = 0.31, tolerance >0.27, variance inflation factor <3.62).
Regarding the group of 17/64 patients (control group), buttoning/unbuttoning correlated significantly with CR left (r = 0.84/0.70), UPDRS III (r = 0.87/0.71), Upper limb bradykinesia right (r = 0.79/0.61), Upper limb bradykinesia left (r = 0.72/0.62), the ADL sub-scale score (r = 0.62/0.56), the Hoehn and Yahr score (r = 0.73/0.58) and age (only unbuttoning, r = 0.56). Importantly, CR right did not correlate with buttoning/unbuttoning (P > 0.05). Subsequent multiple linear regression analyses did not reveal significant predictors without unacceptable collinearities.
Relationship of coin rotation/bradykinesia with handwriting
Coin rotation did not correlate significantly with the ADL measure of handwriting (P > 0.05), whereas a significant correlation of handwriting with Upper limb bradykinesia right was found (r = 0.34, P = 0.006).
Discussion
Our study aimed to explore the significant determinants of typical everyday dexterity tasks in PD. The buttoning and unbuttoning in a cohort of non-demented PD patients was investigated. CR performance of the dominant hand represented the only significant predictor of buttoning and unbuttoning. Constituting the key finding, CR represents an indicator of buttoning difficulties in PD. Since CR expresses LKA-related deficits, LKA seems to be particularly relevant for this typical ADL task.
Notably, neither overall motor impairment nor upper limb bradykinesia predicted buttoning performance significantly.
At group level, buttoning/unbuttoning showed a positive correlation with bradykinesia, disease stage, duration (buttoning) and age (unbuttoning). This observation underlines that the tasks are more impaired in later disease stages, when disease burden including postural instability increases. Nevertheless, the critical determinant of individual buttoning performance is CR.
Although buttoning/unbuttoning represent bimanual tasks, only right-handed CR performance represented their significant predictor. This finding reflects the particular role of the dominant hand during the bimanual task. The preferred use and increased practice of the right hand represents a straightforward explanation. Accordingly, the average CR performance of our cohort was slightly better right versus left, comparable to previous studies [5]. The finding also corresponds to the notion that the left hemisphere can program independent but precise movements better than the right [6].
Since valid ADL dexterity scales do not exist in PD, the PDQ-39 ADL sub-scale score was generated to investigate the predictive value of a measure of health-related quality of life. Clearly, buttoning/unbuttoning were not predicted by this score. Considering the actual activities evaluated (washing, dressing in general, doing shoe laces, writing, cutting, drinking without spilling), it becomes obvious that they do not depend exclusively on dexterity skills. This consideration may explain why the ADL sub-scale was no significant predictor.
Patients with earlier stage PD often complain of difficulties with handwriting, which represents another everyday dexterity task. The relationship of handwriting (represented by item 14 of PDQ-39) with CR and bradykinesia was therefore analyzed. A significant correlation with bradykinesia was identified, but not with CR. This finding suggests that the main movement components of handwriting (size, speed, acceleration and stroke duration) are predominantly determined by bradykinesia. It is worth mentioning that the handwriting score refers to the self-assessment of patients. The score is not performance-based. It is therefore not possible to draw final conclusions about the relationship of CR with handwriting. To further follow up on this issue, it is necessary to assess the different performance parameters of handwriting as mentioned above and compare them to CR performance.
As our overall aim was to analyze the impact of LKA on a typical ADL task in PD, the current study did not include healthy controls. Corresponding follow-up studies could explore the relationship between buttoning and CR in controls, also exploring a possible ceiling effect. According to previous evidence of reduced CR variability in controls relative to PD patients (with significantly better overall CR performance in controls), a ceiling effect can be expected [4,10].
Since two reports [11,12] showed that 15 s for 20 rotations with the dominant hand represents a threshold with sufficient sensitivity and specificity, this threshold was applied to examine patients with impaired/intact dexterity as defined by supra-/sub-threshold CR performance, respectively. Whereas buttoning/unbuttoning did not even correlate with CR right in the control group with intact dexterity, CR right remained the only significant predictor of buttoning/unbuttoning in the group with reduced CR capabilities, emphasizing the particular impact of LKA on a typical ADL task in PD patients with impaired dexterity. It is another interesting observation that age, disease duration, disease stage and magnitude of the cardinal motor symptoms did not differ between the groups. This finding confirms that LKA represents a largely independent symptom that may be present at all PD stages. Correspondingly, our cohort even includes patients with Hoehn and Yahr stage 1 scoring more than 15 s for CR right, and four patients within 2 years of PD diagnosis scoring more than 15 s.
In conclusion, this is the first study illustrating the dominant impact of LKA on a typical ADL-related dexterity task in PD. The study highlights that LKA represents an important source of dextrous deficits in PD and prompts further research into its origins. Facing a lack of therapeutic options with long-lasting benefit [5], our findings enhance trials investigating targeted training of dexterity skills and different brain stimulation techniques. It is a reasonable suggestion to include the simple CR test in routine PD assessments, providing information on ADL-related dexterity skills.
Supplementary Material
Assessment of intact proprioception, investigation/interpretation of possible learning effects and exploration of possible influence of action tremor on coin rotation (CR) performance.
Acknowledgments
We would like to thank Mrs Pauline Hafner for tailoring the cardigans used in the current study.
Footnotes
Disclosure of conflict of interest
The authors declare no financial or other conflicts of interest.
References
- 1.Nijkrake MJ, Keus SHJ, Quist-Anholts GWL, et al. Evaluation of a patient-specific Index as an outcome measure for physiotherapy in Parkinson’s disease. Eur J Phys Rehabil Med. 2009;45:507–512. [PubMed] [Google Scholar]
- 2.Pohar SL, Allyson Jones C. The burden of Parkinson disease (PD) and concomitant comorbidities. Arch Gerontol Geriatr. 2009;49:317–321. doi: 10.1016/j.archger.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Proud EL, Morris ME. Skilled hand dexterity in Parkinson’s disease: effects of adding a concurrent task. Arch Phys Med Rehabil. 2010;91:794–799. doi: 10.1016/j.apmr.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Quencer K, Okun MS, Crucian G, Fernandez HH, Skidmore F, Heilman KM. Limb-kinetic apraxia in Parkinson disease. Neurology. 2007;68:150–151. doi: 10.1212/01.wnl.0000250331.35912.a5. [DOI] [PubMed] [Google Scholar]
- 5.Gebhardt A, Vanbellingen T, Baronti F, Kersten B, Bohlhalter S. Poor dopaminergic response of impaired dexterity in Parkinson’s disease: bradykinesia or limb kinetic apraxia? Mov Disord. 2008;23:1701–1706. doi: 10.1002/mds.22199. [DOI] [PubMed] [Google Scholar]
- 6.Hanna-Pladdy B, Mendoza JE, Apostolos GT, Heilman KM. Lateralised motor control: hemispheric damage and the loss of deftness. J Neurol Neurosurg Psychiatry. 2002;73:574–577. doi: 10.1136/jnnp.73.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zadikoff C, Lang AE. Apraxia in movement disorders. Brain. 2005;128:1480–1497. doi: 10.1093/brain/awh560. [DOI] [PubMed] [Google Scholar]
- 8.Lee MS, Lyoo CH, Lee MJ, Sim J, Cho H, Choi YH. Impaired finger dexterity in patients with Parkinson’s disease correlates with discriminative cutaneous sensory dysfunction. Mov Disord. 2010;25:2531–2535. doi: 10.1002/mds.23304. [DOI] [PubMed] [Google Scholar]
- 9.Vanbellingen T, Kersten B, Bellion M, et al. Impaired finger dexterity in Parkinson’s disease is associated with praxis function. Brain Cogn. 2011;77:48–52. doi: 10.1016/j.bandc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Foki T, Pirker W, Geissler A, et al. Finger dexterity deficits in Parkinson’s disease and somatosensory cortical dysfunction. Parkinsonism Relat Disord. 2015;21:259–265. doi: 10.1016/j.parkreldis.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Mendoza JE, Apostolos GT, Humphreys JD, Hanna-Pladdy B, O’Bryant SE. Coin rotation task (CRT): a new test of motor dexterity. Arch Clin Neuropsychol. 2009;24:287–292. doi: 10.1093/arclin/acp030. [DOI] [PubMed] [Google Scholar]
- 12.Hill BD, Barkemeyer CA, Jones GN, Santa Maria MP, Minor KS, Browndyke JN. Validation of the coin rotation test: a simple, inexpensive, and convenient screening tool for impaired psychomotor processing speed. Neurologist. 2010;16:249–253. doi: 10.1097/NRL.0b013e3181b1d5b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart KC, Fernandez HH, Okun MS, et al. Effects of dopaminergic medication on objective tasks of deftness, bradykinesia and force control. J Neurol. 2009;256:2030–2035. doi: 10.1007/s00415-009-5235-y. [DOI] [PubMed] [Google Scholar]
- 14.Rahman S, Griffn HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord. 2008;23:1428–1434. doi: 10.1002/mds.21667. [DOI] [PubMed] [Google Scholar]
- 15.Rocca WA, Maraganore DM, McDonnell SK, Schaid DJ. Validation of a telephone questionnaire for Parkinson’s disease. J Clin Epidemiol. 1998;51:517–523. doi: 10.1016/s0895-4356(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 16.Fereshtehnejad S-M, Shafieesabet M, Rahmani A, et al. A novel 6-item screening questionnaire for parkinsonism: validation and comparison between different instruments. Neuroepidemiology. 2014;43:178–193. doi: 10.1159/000368167. [DOI] [PubMed] [Google Scholar]
- 17.Brumlik J, Boshes B. The mechanism of bradykinesia in parkinsonism. Neurology. 1966;16:337–344. doi: 10.1212/wnl.16.4.337. [DOI] [PubMed] [Google Scholar]
- 18.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology. 2001;57:1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 19.Vanbellingen T, Lungu C, Lopez G, et al. Short and valid assessment of apraxia in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:348–350. doi: 10.1016/j.parkreldis.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilman S. Joint position sense and vibration sense: anatomical organisation and assessment. J Neurol Neurosurg Psychiatry. 2002;73:473–477. doi: 10.1136/jnnp.73.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans AH, Farrell MJ, Gibson SJ, Helme RD, Lim SY. Dyskinetic patients show rebound worsening of affect after an acute L-dopa challenge. Parkinsonism Relat Disord. 2012;18:514–519. doi: 10.1016/j.parkreldis.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Soliveri P, Brown RG, Jahanshahi M, Marsden CD. Effect of practice on performance of a skilled motor task in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1992;55:454–460. doi: 10.1136/jnnp.55.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of intact proprioception, investigation/interpretation of possible learning effects and exploration of possible influence of action tremor on coin rotation (CR) performance.