Abstract
The cerebellum is involved in sensorimotor operations, cognitive tasks and affective processes. Here, we revisit the concept of the cerebellar syndrome in the light of recent advances in our understanding of cerebellar operations. The key symptoms and signs of cerebellar dysfunction, often grouped under the generic term of ataxia, are discussed. Vertigo, dizziness, and imbalance are associated with lesions of the vestibulo-cerebellar, vestibulo-spinal, or cerebellar ocular motor systems. The cerebellum plays a major role in the online to long-term control of eye movements (control of calibration, reduction of eye instability, maintenance of ocular alignment). Ocular instability, nystagmus, saccadic intrusions, impaired smooth pursuit, impaired vestibulo-ocular reflex (VOR), and ocular misalignment are at the core of oculomotor cerebellar deficits. As a motor speech disorder, ataxic dysarthria is highly suggestive of cerebellar pathology. Regarding motor control of limbs, hypotonia, a- or dysdiadochokinesia, dysmetria, grasping deficits and various tremor phenomenologies are observed in cerebellar disorders to varying degrees. There is clear evidence that the cerebellum participates in force perception and proprioceptive sense during active movements. Gait is staggering with a wide base, and tandem gait is very often impaired in cerebellar disorders. In terms of cognitive and affective operations, impairments are found in executive functions, visual-spatial processing, linguistic function, and affective regulation (Schmahmann’s syndrome). Nonmotor linguistic deficits including disruption of articulatory and graphomotor planning, language dynamics, verbal fluency, phonological, and semantic word retrieval, expressive and receptive syntax, and various aspects of reading and writing may be impaired after cerebellar damage. The cerebellum is organized into (a) a primary sensorimotor region in the anterior lobe and adjacent part of lobule VI, (b) a second sensorimotor region in lobule VIII, and (c) cognitive and limbic regions located in the posterior lobe (lobule VI, lobule VIIA which includes crus I and crus II, and lobule VIIB). The limbic cerebellum is mainly represented in the posterior vermis. The cortico-ponto-cerebellar and cerebello-thalamocortical loops establish close functional connections between the cerebellum and the supratentorial motor, paralimbic and association cortices, and cerebellar symptoms are associated with a disruption of these loops.
Keywords: Cerebellum, Ataxia, Eye movements, Dysmetria, Tremor, A- or Dysdiadochokinesia, Hypotonia, Speech, Dysarthria, Language, Cognition, Affect, Loops, Functional topography, Cerebellar syndrome
Introduction
The cerebellum is a complex part of the brain that functions as a co-processor of movement, working in concert with the cerebral cortex and basal ganglia [1]. In humans, between 60 and 80 % of brain neurons are found within the cerebellum [2, 3], highlighting the importance of this brain structure.
The terminology of the cerebellar syndrome first included pure motor deficits [4, 5]. The works of Gordon Holmes are often cited as having a foundational influence on our understanding of the clinical symptoms and signs of cerebellar lesions [4, 6, 7]. Holmes combined a careful approach to clinical observation with exposure to excellent patient material to produce a series of descriptions of cerebellar dysfunction, which have become classic [7]. Ataxia initially referred to a lack of motor coordination (lack of order in Greek), but the list of symptoms and signs attributed to the cerebellar circuits has increased over the last three decades [8]. In addition to its function in the coordination of movement, a growing body of evidence indicates that the cerebellum contributes to the regulation of a range of linguistic, cognitive and affective functions; hence, cerebellar symptoms and signs include those in nonmotor domains, although these have not been defined with the same degree of specificity as those within the motor system [1, 3, 8–12]. In other words, the cerebellar syndrome now encompasses a range of symptoms initially considered to be of extracerebellar origin.
Routine bedside neurological examination of cerebellar function is typically based on the clinical detection of oculomotor disorders, speech deficits, limb ataxia, tremor, and postural/gait deficits [13]. The classic cerebellar motor syndrome includes a broad array of signs, with the most commonly noted being dysmetria, asynergia or dyssynergy, a- or dysdiadochokinesia, overshoot/impairment of the check reflex, tremor, oculomotor abnormalities, speech disturbances, abnormalities of posture and gait, and hypotonia [4–16]. A variety of neurological examination maneuvers are used to elicit these signs (Table 1). Clinical rating scales have been developed with the aim of providing an accurate estimation of the clinical deficits, in particular the International Cooperative Ataxia Rating Scale (ICARS) [13], the Scale for the Assessment and Rating of Ataxia (SARA) [17], and the Brief Ataxia Rating Scale (BARS) [18]. Each scale has advantages and disadvantages [19]. The ICARS scale is relatively long and the SARA scale does not include an assessment of eye movements. The BARS scale covers the essential features of the cerebellar motor examination (changes in gait, speech, eye movements, arm and leg movements; Table 2), is fast and accurate for clinical purposes. Because cerebellar patients may also exhibit so-called extracerebellar signs (for instance pyramidal or extrapyramidal signs) that contribute to disability and may even interfere with the evaluation of ataxic symptoms, non- ataxic signs are assessed as well [20]. Neuropsychological tests often complement this clinical assessment.
Table 1.
Most commonly used clinical maneuvers to evaluate the cerebellar motor syndrome
| Clinical test | Signs looked for/evaluated |
| Oculomotor tests | |
| Ocular stability | Nystagmus, saccadic intrusions |
| Ocular pursuit | Saccadic pursuit |
| Saccades | Dysmetric saccades |
| Head-impulse test (HIT) | Impaired vestibulo-ocular response (VOR) |
| Ocular alignment | Skew deviation, esotropia |
| Speech | |
| To repeat a standard sentence or normal conversation | Dysarthric speech |
| Upper limb movements | |
| Finger-to-nose test | Decomposition of movement, dysmetria, kinetic tremor, intention tremor |
| Finger chase | Dysmetria |
| Finger-to-finger test (index-index test) | Kinetic tremor, intention tremor |
| Fast alternating movements | Adiadochokinesia |
| Stewart-Holmes maneuver | Rebound phenomenon |
| Lower limb movements | |
| Heel-to-shin test (knee-tibia test) | Decomposition of movement, kinetic tremor, dysmetria |
| Trunk movements | |
| Quality of sitting | Increased sway of the trunk |
| Muscle tone | |
| Passive stretch of joints | Hypotonia |
| Knee jerk | Pendular knee jerk |
| Stance and gait | |
| Standing in natural position, feet together | Ataxia of stance, titubation, lateropulsion |
| Regular gait/walking capacities | Ataxic gait |
| Tandem gait (heels to toes) | Ataxic gait |
| Handwriting | |
| Standard sentence | Irregular writing, megalographia, kinetic tremor |
| Archimedes’ spiral | Kinetic tremor, dysmetria |
Action tremor (which refers to any tremor produced by voluntary contraction of the muscles) includes postural and kinetic tremor
Table 2.
Brief ataxia rating scale (BARS)
| Gait |
| 0: Normal |
| 1: Almost normal naturally, but unable to walk with feet in tandem position |
| 2: Walking without support, but clearly abnormal and irregular |
| 3: Walking without support but with considerable staggering; difficulties in half turn |
| 4: Walking without support not possible; uses support of the wall for 10-m test. |
| 5: Walking possible only with one cane |
| 6: Walking possible only with two canes or with a stroller |
| 7: Walking possible only with one accompanying person |
| 8: Walking impossible with one accompanying person (2-person assist; wheelchair) |
| Knee-tibia test (decomposition of movement and intention tremor) (Left and Right scored) |
| 0: Normal |
| 1: Lowering of heel in continuous axis, but movement is decomposed in several phases, without real jerks, or abnormally slow |
| 2: Lowering jerkily in the axis |
| 3: Lowering jerkily with lateral movements |
| 4: Lowering jerkily with extremely long lateral movements, or test impossible |
| Finger-to-nose test (decomposition and dysmetria of arm and hand) (left and right scored) |
| 0: Normal |
| 1: Oscillating movement of arm and/or hand without decomposition of the movement |
| 2: Segmented movement in 2 phases and/or moderate dysmetria in reaching nose |
| 3: Segmented movement in more than 2 phases and/or considerable dysmetria in reaching nose |
| 4: Dysmetria preventing the patient from reaching nose |
| Dysarthria |
| 0: Normal |
| 1: Mild impairment of rate/rhythm/clarity |
| 2: Moderate impairment of rate/rhythm/clarity |
| 3: Severely slow and dysarthric speech |
| 4: Speech absent or unintelligible |
| Oculomotor abnormalities |
| 0: Normal |
| 1: Slightly slowed pursuit, saccadic intrusions, hypo/hypermetric saccade, nystagmus |
| 2: Prominently slowed pursuit, saccadic intrusions, hypo/hypermetric saccade, nystagmus |
| TOTAL (out of 30): …………. |
The aim of this Consensus paper is to revisit the cerebellar syndrome from a clinical point of view in light of recent progress in our understanding of the operations that involve the cerebellar circuitry. We also integrate recent advances in lesion-symptom mapping. We focus on the deficits observed in pure cerebellar lesions.
Vertigo and Dizziness in Cerebellar Disorders (Michael Strupp and Caroline Tilikete)
Vertigo, dizziness, and imbalance can be caused by lesions of the vestibulo-cerebellar, vestibulo-spinal, or cerebellar ocular motor systems [21–23]. Clinically, they can manifest in three ways: first, with an acute onset of symptoms lasting days, weeks, or even longer (e.g., cerebellar stroke, bleeding, or encephalitis); second, as recurrent attacks lasting for seconds (e.g., central positioning or positional vertigo) or minutes up to days (e.g., episodic ataxias); third, as a chronic/permanent, often progressive syndrome (e.g., downbeat nystagmus (DBN) syndrome, hereditary ataxias, multiple sclerosis, cerebellar tumors, paraneoplastic syndromes, or inherited metabolic disorders) [21]. Since a classification based on the three types of manifestation is clinically helpful, it is used in this section to describe the different types of cerebellar-related vertigo and dizziness.
Acute Onset of Cerebellar Vertigo and Dizziness: Cerebellar Strokes Associated With Vertigo and Dizziness
This is an important and challenging differential diagnosis in patients with acute, even isolated attacks of vertigo. It can be caused by an infarction in the territory of the posterior inferior cerebellar artery (PICA), anterior inferior cerebellar artery (AICA), and superior cerebellar artery (SCA). PICA infarctions are characterized by variable clinical findings in terms of nystagmus and central ocular motor signs [22–24]. AICA infarctions can manifest as at least eight different types, most often with a combined impairment of vestibular and audiological functions (see [23]). SCA infarctions are associated with vertigo in about 50 % of patients, and in 25 % there is a spontaneous nystagmus to the side of the lesion [25]. Even isolated infarctions of the flocculus, the nodulus or tonsils may lead to acute vertigo [26–28]. The key to the diagnosis in such cases is a careful neuro-ophthalmological and neuro-otological examination to look for cerebellar ocular motor signs, including nystagmus and ocular tilt reaction which combines a head tilt, skew deviation, and cyclotorsion [29].
Recurrent Attacks of Cerebellar Vertigo and Dizziness
Duration of the Symptoms
Seconds
Lesions of the cerebellum affecting the nodulus [30] or the ocular motor vermis [31] can lead to central positioning vertigo, induced by changes of head position relative to gravity, lasting seconds to minutes. Patients may also suffer from short attacks of vertigo induced by head-shaking associated with a cross-coupling of head-shaking nystagmus.
Minutes to Days
If the attacks of dizziness last many minutes or up to days, important differential diagnoses of underlying cerebellar disorders are episodic ataxias (EAs), in particular EA 1 and 2, which are typically associated with cerebellar ocular motor disorders, in particular DBN in EA 2 [32, 33].
Chronic/Permanent Cerebellar Vertigo and Dizziness
Chronic or permanent cerebellar vertigo and dizziness is found in the majority of spinocerebellar ataxias [34]. It is also often associated with cerebellar eye-movement disorders, which may arise from an impairment of the flocculus/paraflocculus, vermis, nodulus, or fastigial nucleus. Posturography with an analysis by a neuronal network has proven diagnostically helpful [35] because patients typically have a 3-Hz sway. This sway may be the only sign indicating cerebellar dysfunction as the cause of dizziness.
Finally, an important syndrome is cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS). It is most likely a neurodegenerative disorder that affects the cerebellar and vestibular systems in particular, leading to a high-frequency deficit of the vestibulo-ocular reflex and a double impairment due to the simultaneous sensory and cerebellar deficit [36–38].
Oculomotor Deficits in Cerebellar Disorders (Caroline Tilikete and Michael Strupp)
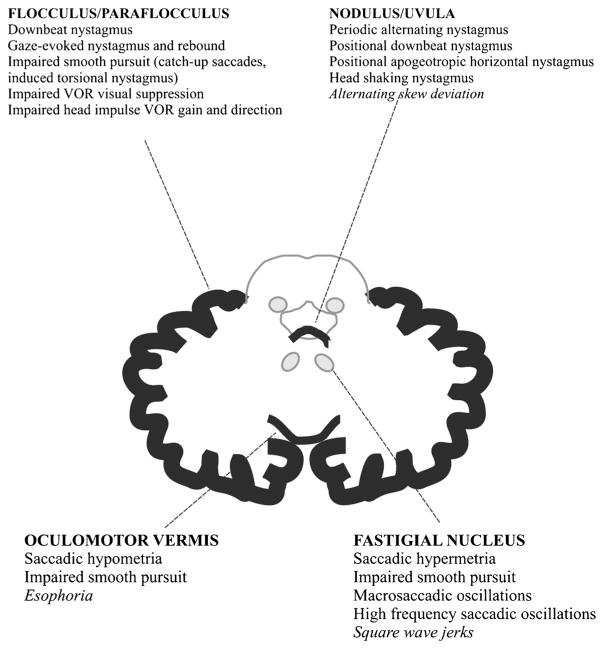
The purpose of eye movements is to optimize vision by promptly bringing images to the fovea, where visual acuity is best, using saccades and vergence, then stabilizing images on the retina/fovea even when the target or body are displaced, using fixation, smooth pursuit (SP) and the vestibulo-ocular reflex (VOR). The cerebellum plays a pivotal role in the online to long-term control of all these eye movements in order to provide best calibration, to reduce eye instability and to maintain ocular alignment [16, 39]. This oculomotor control is ensured by three main cerebellar areas: the flocculus/paraflocculus, the nodulus-ventral uvula (lobules IX and X of the cerebellar vermis), and the dorsal oculomotor vermis (lobules V–VII) which underlies the fastigial oculomotor region.
The following approach is based on the oculomotor deficits that can be observed at bedside. Figure 1 completes this approach by showing the structural-clinical correlation [40]. Another consensus paper provides a more exhaustive list of involvement of the cerebellum in the different types of eye movements and the resulting deficits that can be found [39].
Fig. 1.
Structural-clinical correlation of oculomotor deficits associated with cerebellar disorders. In italics, provisional localization. Adapted from Leigh and Zee [40]
Ocular Instability: Nystagmus and Saccadic Intrusions
Cerebellar oculomotor syndrome can give rise to five main types of central nystagmus and to inappropriate saccades. Except for positional and horizontal nystagmus which are often associated with positional vertigo, ocular instability manifests as oscillopsia and decreased vision.
-
Downbeat nystagmus (DBN)
DBN is one of the most common forms of cerebellar nystagmus [41] in which the eyes drift up (slow phase) and are brought back by a corrective downward saccade (quick phase) [42]. It generally increases or can only be observed when the subject looks to the side and down. DBN can be increased by hyperventilation [43].
-
Gaze-evoked nystagmus and rebound nystagmus (GEN and RN)
GEN is one of the most common forms of central nystagmus (not cerebellar-specific), in which the eyes drift centripetally (slow phase) and are brought back by a corrective centrifugal saccade (quick phase) in sustained eccentric gaze [42]. It therefore changes direction according to changes in gaze position and beats in the direction of eccentric gaze. GEN can be associated with rebound nystagmus (RN) when the eyes return to the central position following sustained eccentric gaze. RN beats in the opposite direction to the prior gaze-evoked nystagmus, i.e., the slow phase is toward the prior eccentric gaze position.
-
Periodic alternating nystagmus (PAN)
PAN presents as a spontaneous, primary-position, horizontal-jerk nystagmus which changes direction approximately every 2 min [44]. The velocity of the nystagmus increases and decreases with a sinusoidal aspect before changing direction. Beats of vertical nystagmus or square-wave jerks can be observed at the transition phase.
-
Central positional or positioning nystagmus (PN)
PN, especially downbeat and apogeotropic horizontal nystagmus [30], occurring in different head-hanging positions, may be due to a lesion of the nodulusuvula or ocular motor vermis region [31]. This phenomenon is often accompanied by slight vertigo and has to be differentiated from benign paroxysmal positional vertigo (BPPV, see section on vertigo and dizziness) in which the nystagmus correlates with the semicircular canal affected. Other types of PN with either horizontal, torsional or vertical directions have been described in posterior fossa lesions, for instance, affecting the vermis [41], but the exact location of the lesions within the posterior fossa is not well understood [45].
-
Spontaneous and head-shaking nystagmus (HSN)
Ipsilesional or perverted head-shaking nystagmus can be observed in cerebellar stroke [46]. A study of patients with stroke in the SCA territory disclosed frequent (30 %) horizontal nystagmus beating toward the side of the lesion [25]. However, this is a rare condition. The nystagmus is dampened by visual fixation and frequently associated with vertigo.
Square-wave jerks, saccadic intrusions and oscillations.
Saccadic Intrusions
Square-wave jerks consist of small, horizontal saccades around 0.5° which take the eye away from the fixation position and return it to the position about 200 ms later. Macrosquare-wave jerks are larger than 5°, more frequent and return earlier. Both of these saccadic intrusions are not specific to cerebellar disorders.
Macrosaccadic oscillations consist of horizontal oscillations of the eyes around a fixation target, building up and then decreasing in amplitude, with a 200-ms interval. They are usually associated with saccadic hypermetria and are triggered by a saccadic shift.
High-Frequency Saccadic Oscillations
Flutter and opsoclonus consist of saccadic oscillations without an intersaccadic interval [47]. Flutter occurs in the horizontal direction only whereas opsoclonus occurs in multiple directions. They appear as bursts of saccades with a frequency of 10 to 25 cycles/s. They are more often intermittent and triggered by normal saccades.
Deficits in Slow Eye Movements
-
Impaired smooth pursuit (SP)
Bedside examination can reveal impaired SP, i.e., tracking of a small object with catch-up saccades instead of a smooth eye movement. Another way to test smooth pursuit is to examine eye-head tracking, i.e., by asking the patient to fixate a target moving simultaneously with the head rotation (visual fixation suppression of the vestibulo-ocular reflex VOR). Deficits in pursuit lead to impaired VOR suppression by fixation, i.e., the eyes are continuously taken off target by VOR and corrective saccades to the target are observed [48]. Torsional nystagmus during vertical smooth pursuit can be observed in lesions of the middle cerebellar peduncle.
Impaired smooth pursuit is not specific to cerebellar lesions, but is of great help in the diagnosis of a subtle cerebellar syndrome.
-
Impaired vestibulo-ocular reflex (VOR)
The VOR can be easily tested clinically using the head-impulse test (HIT) or preferably the video-HIT. The patient is asked to fix the gaze on the examiner’s nose, while the head is actively and briskly rotated 15°. In normal VOR, the gaze is held steady on the nose; in abnormal VOR, a corrective saccade will help to refixate gaze on the nose at the end of the rotation. In cerebellar dysfunction, VOR can show impaired gain (decrease or increase) and direction (i.e., horizontal head thrust induces vertical slow phase) [16, 26, 49, 50]. Impaired VOR can give rise to oscillopsia during head and body motion.
Deficits in Saccades
Saccades are examined by asking the patient to fixate a central target (i.e., the examiner’s nose) then a peripheral target (i.e., the examiner’s finger or the tip of a pen) and back. Lesions of the dorsal oculomotor vermis and fastigial oculomotor region are responsible for abnormal accuracy and trajectory of saccades that can be easily observed at bedside.
-
Saccadic dysmetria
Saccades can be either hypometric or hypermetric, leading to a forward or backward correction respectively, which can be clinically detected [51].
-
Saccadic lateropulsion
Saccadic eye movements can also show horizontal lateropulsion: saccadic hypermetria on one side and hypometria on the other side associated with a horizontal deviation of pure vertical saccades. The syndrome of saccadic lateropulsion is mainly observed with Wallenberg syndrome but can be observed with cerebellar dysfunction as well [52].
Ocular Misalignment
Cerebellar disorders of ocular alignment that can be observed at bedside include skew deviation, alternating skew deviation and esotropia [53, 54]. These deficits are not anatomically specific.
-
Alternating skew deviation
Skew deviation corresponds to a nonparalytic vertical ocular misalignment that is therefore observed in all gaze directions [55]. Skew deviation is typically caused by lesions of the central graviceptive pathways, but has been observed with focal cerebellar lesions as well. Skew deviation in cerebellar lesions may more frequently manifest as alternating skew deviation, i.e., changing direction with changes in horizontal eye position, the abducting eye being the higher.
-
Esotropia
Esophoria and esotropia, i.e., inward non-aralytic latent or manifest strabismus also occurs in cerebellar disorders [54].
In conclusion, cerebellar oculomotor deficits are numerous and can affect ocular stability during fixation, metrics of slow eye movements and saccades and ocular alignment. Recognizing oculomotor deficits is important since some of them are anatomically specific and thus greatly aid a subtle or a topographical diagnosis of cerebellar syndrome.
Cerebellar Tone (Mark Hallett)
Tone is the resistance to passive stretch when a limb is relaxed, and there are three main factors that influence it [56]. One is the mechanical properties of the muscle and joint on which it operates. There is a low, but measurable resistance of normal muscle, and this can be increased in situations such as contractures. The second factor is background contraction. If a person is relaxed, there should be only little if any contraction. The third factor is reflex contraction, which can arise from short latency or long latency reflexes, and such reflexes are normally absent when a limb is at rest. Taken all together, normal individuals have very low, barely perceptible muscle tone when fully relaxed. States of increased tone such as spasticity and rigidity are easily identified.
There is a theme in the literature that patients with cerebellar dysfunction have a reduction in tone, called hypotonia. This idea arose from the work of Holmes, who presented his ideas in his first Croonian Lecture [6]. He ascribed the origin of the idea to Luciani, but then noted that other investigators including Ferrier, Babinski, André-Thomas, and Myers had not found any change in tone. His evidence came from acutely injured soldiers with penetrating wounds to the cerebellum, but he admitted that all such injuries did not lead to hypotonia and that it is not demonstrable in all affections of the cerebellum. Nevertheless, Holmes viewed hypotonia as a fundamental abnormality that underlies many cerebellar motor deficits [57]. This hypotonia tended to be characteristic especially in the upper extremities and to normalize gradually over weeks to months depending on the severity of the injury. A transient hypotonia can also be found in animal models with cerebellar lesions. Gilman [58] showed in monkeys that this change parallels the recovery of muscle spindle sensitivity which was acutely depressed by loss of cerebellar fusimotor facilitation. This deficit would lead to reduction in reflex responsiveness. Hypotonia should be differentiated from the atonia of Luciani, which is a decrease of the supporting reaction of limb extensor muscles seen in experimental animals [58].
In humans, Holmes distinguished the weakness that followed acute massive damage to the cerebellar hemispheres, asthenia, from that associated with corticospinal tract lesions, paresis [57]. Asthenia did not affect specific muscle groups more than others and was not necessarily associated with changes in tendon reflex sensitivity. Profound muscle weakness (or complete lack of contraction), as seen in the floppy infant, can give rise to the sense of hypotonia. Weakness, however, is not a common complaint in cerebellar patients, and asthenia may be more fatigability. Fatigue may affect an individual body part, but may also be sensed more globally. Electrophysiologic studies of fatigue in non-depressed patients with cerebellar ataxia showed decreased post-exercise facilitation of motor potentials evoked from transcranial magnetic stimulation [59].
The hypertonia observed in some patients with chronic cerebellar syndromes might well arise from lesions in other brain systems since degenerations or other lesions do not necessarily only affect the cerebellum [60]. The so-called cerebellar fits are spasms that have been observed in posterior fossa tumors, Chiari malformations and stroke involving the cerebellar cortex but sparing the nuclei [61, 62]. The mechanism of this rare phenomenon is presumably an extensor tone disinhibition. It should be noted that some experts in the panel have observed that the cerebellar type of multiple system atrophy (MSAc) may be characterized by hypotonia rather than hypertonia. This is thought to reflect the involvement of the cerebellum.
Alternate Movements in Cerebellar Disorders (Katharina Marie Steiner and Dagmar Timmann)
It was Joseph Babinski who introduced the assessment of rapid successive movements (succession rapide de mouvements) as part of the neurological examination to reveal cerebellar disease [5]. Rapid succession is most easily assessed during rapid alternating movements (RAMs) at a single joint. In his classic paper, Babinski examined rapid supination and pronation of the forearm at the elbow joint. He coined the term diadochokinesis or diadochokinesia (diadococinésie) from the Greek diadochos = succeeding and kinesis = movement for this kind of test. It quickly became part of the standard neurological examination, and remains so to this day [63–65]. Commonly, patients are asked to imitate screwing in a light bulb. Another common test is to have patients alternately slap the palm and dorsum of the hand on their thigh. The first is one of the test items of the ICARS scale [13], the latter is part of the SARA scale [17]. As pointed out by Holmes there are many other ways to test rapid alternating movements, for example flexion and extension of the fingers or forearm, clapping or tapping the hand on the table, or stamping the foot [6]. Repetition of syllables is a way to test diadochokinesis in speech [66]. Most commonly, patients are asked to repeat the syllables pa-ta-ka, which involves rapid succession of lip, tongue and soft palate movements. Difficulties in performing these tests are called adiadochokinesia (or dysdiadochokinesia) or bradydiadochokinesis.
Babinski pointed out that patients with cerebellar disease have no obvious problems pronating or supinating the forearm as a single movement. Succession of alternating movements, however, is markedly slowed. He hypothesized that cerebellar patients have problems stopping one movement and quickly initiating the next in a rapid succession [5]. Holmes went beyond the clinical examination and measured RAMs using a light-line registration method (Fig. 2) [6]. He also found that slowness is a key feature of adiadochokinesia. Irregularity of movement frequency (rate) and amplitude (range) are additional characteristics. Rapid fatigability is common. Disorders are more prominent when both limbs are moved simultaneously. Holmes also observed that patients were unable to fix movements in adjacent limbs. He explained cerebellar adiadochokinesia by impairments in muscle tone (hypotonia), but also by dissociation in time and force of contractions in muscles which should act synergetically, with delays in initiating the contractions.
Fig. 2.
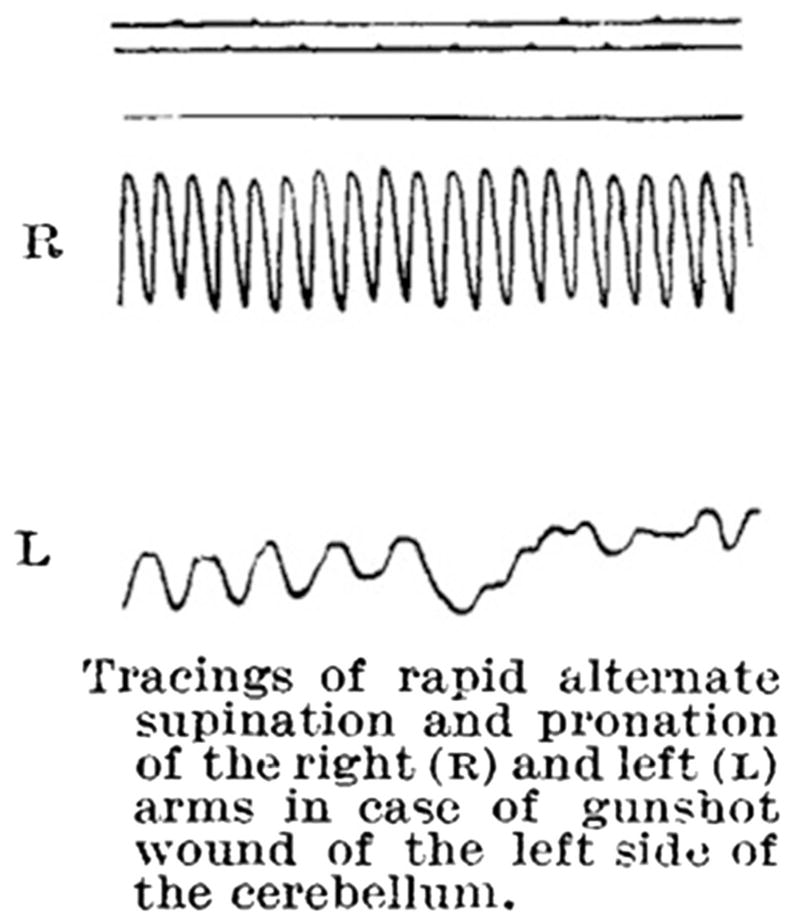
Rapid alternate movements (RAMs) in cerebellar disorders. The figure is taken from Holmes [6]. RAMs are slowed on the left (L) side, ipsilaterally to a gunshot injury of the cerebellum, compared to the right (R) side. Increasing variability in movement frequency and amplitude is also seen, which becomes more prominent during the course of the movements due to fatigue (permission granted)
In subsequent years, surface electromyography (EMG) recordings of RAMs have been performed both in humans and animals. In patients with cerebellar disease, EMG activity in the agonist exceeds the start of the movement of the antagonist at the turning points [67]. Earlier findings of Hallett et al. [68] further support the idea that delayed cessation of the agonist is an important cause of adiadochokinesia. The authors examined rapid elbow flexion in cerebellar patients. A weight was attached to the arm which pulled the arm in the flexion direction. Tonic activation of the triceps muscle was needed to maintain the start position. Triceps activation had to be inhibited prior to onset of the flexion movement. In cerebellar disease the inhibition of the triceps muscle prior to flexion onset was delayed. Likewise, Conrad and Brooks [69] found that cooling of the dentate nucleus in monkeys resulted in delayed termination of the agonist, and consecutively delayed initiation of antagonist movements in RAMs. A primary delay of movement initiation may also contribute [70]. According to Thach, the critical function of the cerebellum is not to control prime movers of a single joint, but to coordinate movement synergies, which includes the braking of the antagonist in RAMs [71]. Following up on Babinski’s view, he postulated that adiadochokinesia is a direct consequence of asynergia. In his detailed studies in monkeys, he found that although discharge of interposed and dentate nuclei was modulated by RAMs, lesions of the nuclei had little effect upon movements restricted to single joints. Disorders are more prominent in RAMs involving multiple joints. Thus, adiadochokinesia can be observed in the imitation of screwing in a light bulb because it includes synergistic fixation of the shoulder and wrist joints. The test of alternatively slapping the dorsum and palm on the thigh is more sensitive because additional elevation and depression of the shoulder is required. Asynergia in cerebellar disease has been explained by an inability to predict the consequences of movements. This is more devastating in multijoint movements because interaction torques need to be controlled [72]. Furthermore, it is of particular importance in fast movements with no time to use afferent feedback.
There is consensus that diadochokinesis (or diadochokinesia) is a vital clinical test to reveal cerebellar dysfunction. Adiadochokinesia can easily be observed in the upper limb, the lower limb and in speech. Adiadochokinesia, however, like any other clinical sign of motor incoordination, is not a specific cerebellar sign, and sensory loss, paresis, and extrapyramidal disorders have to be excluded.
Tremor (Elan D. Louis)
As discussed in previous sections of this article, diseases of the cerebellum are associated with a variety of motor features. This section focuses attention on tremor phenomenology in cerebellar disease. The mechanisms for cerebellar tremor are not clear. A number of mechanisms have been suggested, including correction for defective postural fixations and the presence of a central oscillator with disordered cerebellarthalamic loops. Some have even proposed a contribution of peripheral mechanisms [73, 74].
With the passage of time, there has been an unfortunate tendency for tremor phenomenology to have become summarily packaged in an over-simplified manner. Indeed, in modern times, cerebellar tremor has become equated exclusively with intention tremor. This is a tremor that occurs with goal-directed movement (e.g., finger-to-nose maneuver) and which worsens when approaching a target [75]. However, it is important to recall that Holmes described several other types of tremor in the setting of cerebellar lesions, and these seem to have been largely forgotten [6]. Indeed, Holmes wrote, “But all tremors occurring in cerebellar disease are not of the same nature [6].” First, Holmes described what he termed static tremor [6]; this could be elicited during arm extension. In more modern parlance, this would be a postural tremor. Holmes described this as an irregular tremor, which affected proximal greater than distal muscles, and occurred mostly in the line of gravity [6]. He also described what he referred to as kinetic tremor in the setting of cerebellar disease [6, 57]. In stark contrast to intention tremor or terminal tremor, this movement occurred at the commencement of movement [6].
Our concept of cerebellar disorders has expanded in recent years to include entities such as essential tremor (ET) [76–78], which joins the more classic spinocerebellar ataxias and other disorders. It is therefore worthwhile re-acquainting ourselves with Holmes’ work, as well as devoting additional efforts to the study of tremor phenomenology as it occurs within a broader context of cerebellar disease. In the case of ET, while intention tremor does occur in as many as 50 % of patients [79, 80], the most prominent features are kinetic followed by postural tremors [76], and it is likely that these, too, are cerebellar signs. Of interest is that Holmes noted that with lesions of the superior cerebellar peduncles, kinetic tremor could be quite marked and abrupt [6]. The superior cerebellar peduncle mainly contains efferent fibers from the deep cerebellar nuclei (e.g., dentate nucleus), which continue to the thalamus. Of interest in ET is that it is precisely problems with cerebellar outflow that are posited to be of primary importance [76, 81].
Leaving aside ET, more broadly, there is a need to reappraise the basis for our understanding of cerebellar symptoms and signs. The classic cerebellar syndrome, as defined by Holmes, was grounded on observations of a small and very narrowly defined group of patients who had largely suffered from posttraumatic cerebellar lesions. Subsequent evaluation of patients with cerebellar degenerations has served to confirm many of these findings. However, these patients represent just the tip of the cerebellar disease iceberg; in many ways, they are outliers, with very destructive pathologies. Aside from ET, we know that the cerebellum is not normal in patients with dystonia [82] as well as those with Parkinson’s disease [79], Alzheimer’s disease [83], and a broad range of other disorders. Indeed, in Alzheimer’s disease, loss of Purkinje cells and astrocytosis has been reported, particularly in familial disease [83]. In a small post-mortem study of patients with cervical dystonia, loss of Purkinje cells was noted [82]. In Parkinson’s disease, torpedoes (Purkinje cell axonal swellings) occurred to a greater extent than in controls [79]. The impact of a more gradual, partial lesioning of the cerebellum is likely to produce a very different pattern of symptoms and signs than sudden, violent, traumatic lesioning or rapidly progressive, severely degenerative lesioning. In summary, it is time to move beyond the classics, and to consider the presentation of cerebellar lesions outside of our somewhat myopic field of view.
Grasping Deficits in Cerebellar Disorders (Dennis Nowak)
Reaching and grasping an object is an essential part of the daily motor repertoire. Clumsiness is a common complaint in cerebellar disorders, however, grasping movements are not systematically tested by clinical ataxia rating scales [13, 17, 18]. Grasping for an object in the environment involves at least two different movement components [84]: (1) a transport movement of the hand toward the object (hand transport component) and (2) shaping of the fingers according to the specific object characteristics, such as size and shape (grasp formation component). Once the fingers make contact with the object, sufficient grip forces have to be established to ensure a stable grasp when the object is lifted and moved around in space. Grip forces are scaled according to the object’s weight, shape, surface friction, and other physical constraints, and depend on the inertial loads arising from lifting and transporting movements [85].
Several signs and symptoms of cerebellar disease may impact on reaching and grasping. Dysmetria, decomposition of movements and tremor (both intention and kinetic tremor) cause increased path curvatures, corrective movements, slowed and highly variable velocity profiles of the hand transport component as well as increased grasp apertures and multiple peaks in grasp aperture when cerebellar subjects reach for and grasp an object [86, 87]. In addition, oculomotor deficits, such as problems in fixation, dysmetric saccades and nystagmus, may hamper the accurate processing and performance of both the hand transport and grasp formation components. The ICARS scale scores show significant correlations with deficient kinematic measures of hand transport (prolonged movement times) and grasp formation (prolonged grasp formation times, number of peaks in grasp formation) [13, 86]. The deficits in the kinematics of reaching and grasping also correlate with the amount of atrophy within the lateral and intermediate cerebellum in cerebellar degeneration [86].
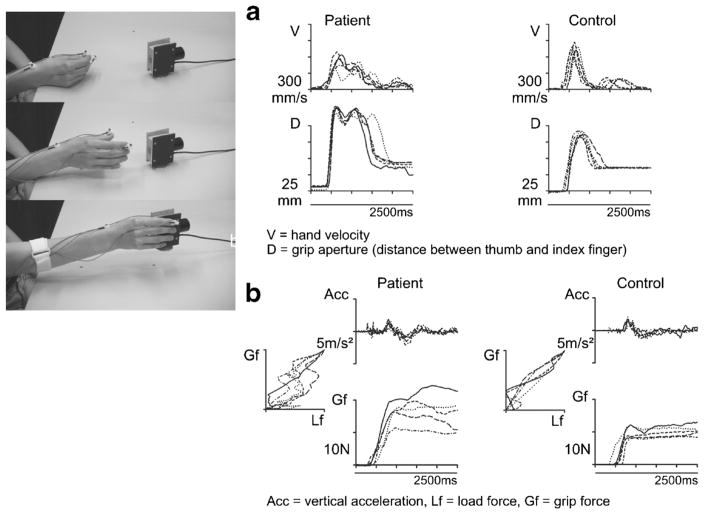
Cerebellar symptoms cause a deterioration of the accurate scaling and coordination of grip and load forces when a grasped object is lifted and moved. In particular, prolonged finger-object contact times before a stable grasp is established, exaggerated and highly variable grip forces in relation to inertial loads and inaccurate coupling between grip and load force profiles have been described [86, 88, 89]. Grip and load force profiles also show additional peaks due to kinetic and intention tremor [88]. Importantly, none of these deficits in the kinetics of grasping correlates with ataxia rating scales [13, 17]. Figure 3 illustrates the pathologic features of reaching for and grasping an object as well as grip force control when lifting an object in cerebellar degeneration.
Fig. 3.
a Profiles of hand transport velocity and grasp formation during repetitive reaching to grasp movements performed by a subject with cerebellar degeneration and a healthy control subject. b Profiles of lifting acceleration and grip force during repetitive grasp and lift movements performed by a subject with cerebellar degeneration and a healthy control subject. Modified according to Brandauer et al. [86]
The control of grip forces when manipulating hand-held objects involves different modes of control, which rely on prediction and sensory feedback to different extents [90]. When, for example, the load of a hand-held object is increased by a self generated action, such as dropping a weight from one hand into a receptacle held by the opposite hand, grip forces increase in parallel with load forces without an obvious time delay [85, 90]. This represents predictive coupling of grip and load forces. When, on the other hand, a weight is unexpectedly dropped by another person into a hand-held receptacle, sensory feedback provides the most useful source to signal a change in load. In this case, grip forces tend to lag behind load forces [90]. The predictive control, but not the reactive control, of grip forces is severely impaired in subjects with cerebellar disorders [88, 89, 91, 92].
Essentially, the motor impairments specified above cannot be sufficiently assessed by routine clinical examination or conventional ataxia rating scales, but only by a detailed analysis of both the kinematics and kinetics of grasping. Technical analysis of the kinematics (hand transport and grasp formation) and kinetics (grip and load force) of grasping widen the spectrum of diagnostic tools in cerebellar disorders. They open a window into the pathologic changes of grasping performance in cerebellar disease, which remain hidden to the naked eye of the clinical examiner.
Cerebellar Dysmetria (Florian Bodranghien and Mario Manto)
Dysmetria is a cardinal sign of cerebellar motor dysfunction. Holmes considered dysmetria as an error in trajectory due to an impaired range, rate and force of movement [4, 6]. Cerebellar patients attempting to perform a goal-directed limb movement either overshoot (hypermetria) or undershoot (hypometria) the target.
Dysmetria is not specific to a cerebellar disorder, although hypermetria is very suggestive. Dysmetria may be observed in lesions of the brainstem, thalamus, and subcortical white matter along the afferent (cortico-ponto-cerebellar) or efferent pathways (cerebello-thalamo-cortical) of the cerebellum [93]. Ataxic hemiparesis is characterized by a combination of pyramidal tract signs with a homolateral ataxic syndrome [94, 95]. Dysmetria is more striking when the pyramidal deficit is mild; lesions involve the pons, corona radiata, thalamus, internal capsule, and rarely the cerebral cortex itself [95]. A crossed cerebellar diaschisis due to deafferentation might explain the cerebellar syndrome in large extracerebellar infarcts [95]. For infarcts of the pons, the topography of the motor representation in the basis pontis explains the cerebellar symptoms [96]. Dysmetria in these cases is caused by lesions of the basilar pons, interrupting the axons from the cerebral cortex to the contralateral cerebellar hemisphere.
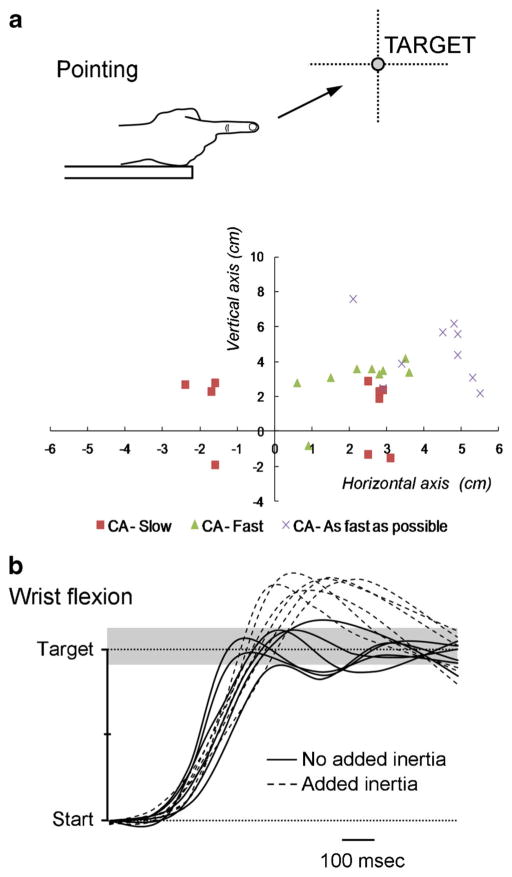
The five main features of cerebellar limbs dysmetria are summarized in Table 3. The overshoot is larger when the movement is performed at a higher speed [97] (Fig. 4a) and when the inertia is artificially increased [98]. The defective adaptation to increased inertia can even be used to unravel a silent cerebellar lesion (Fig. 4b). Spontaneously, cerebellar patients often perform movements at a slower speed, presumably to reduce the overshoot. Dysmetria occurs both for proximal (shoulder, hip) and distal movements (hand, foot), and is often followed by corrective movements in an attempt to reach the target [99]. Hypermetria tends to be more severe in those patients in whom oscillations are not prominent during the second phase of movement, as reported initially in monkeys [100]. The variability from trial to trial is increased, for instance for finger opening during throwing [101, 102]. The variability very often subsides as the patient recovers from an acute injury, whereas it worsens with time in so-called progressive degenerative ataxias. It is usual to observe that recovery of dysmetria remains partial in the case of cerebellar lesions, especially when the lesion involves cerebellar nuclei [103]. Although the focus is often placed on the features of the terminal phase of movement, the preparation and initiation of a voluntary movement are also impaired in ataxic disorders. Initiation is often delayed [67]. Reaction times are increased and dysmetria is correlated with the early velocity profiles [104, 105]. The initiation of combined eye and arm movements is prolonged during tasks of coordinated visuo-motor tracking [106].
Table 3.
Main features of limb dysmetria
| Speed-sensitive |
| Inertia-sensitive |
| Increased inter-trial variability |
| Increased curvature of movement |
| Impaired initiation |
Fig. 4.
a Pointing movements of the right upper limb in a patient presenting with a stroke in the territory of the right superior cerebellar artery (CA). End movements (corresponding to the first zero-crossing value of the velocity curve) are represented. The target is located at a distance of 85 % of the upper limb length, at the height of the shoulder. Series of 10 movements performed at slow speed (CA-Slow red squares), at fast speed (CA-Fast green triangles) and as fast as possible (CA-As fast as possible X). Note that the initial dysmetria (motion at slow speed) is transformed into a genuine hypermetria (overshoot) as the velocity increases. b Fast goal-directed wrist movements in a patient with a cerebellar stroke which is clinically silent. Adding inertia (+300 g) triggers an overshoot of the target (dotted lines) located at 20° from the start position. Gray zone corresponds to the range of control values
EMG studies have shown delayed onset latencies of antagonist activities [68], abnormal rates of rise of agonist/antagonist EMG activities [100] and impaired adaptation to mechanical conditions such as increased inertia or damping [107]. The agonist EMG activity is characterized by smaller magnitudes (smaller torques to generate a launching force) and prolonged duration [68, 100]. Cerebellar patients cannot generate the appropriate muscle torques to control the mechanical consequences of dynamic interaction forces that occur when a movement is executed [108].
Dysmetria is best explained by an impairment of prediction of movement, in agreement with the hypothesis that a critical role of the cerebellum is to maintain accurate internal models of dynamics [105]. Adaptable internal models relate motor commands to the changes of states of limbs. Dysmetric movements would be induced by defects in updates and storages of internal models [105]. Dysmetria appears as the manifestation of a misrepresentation of limb dynamics. It remains to be defined whether different lesion locations cause distinct biases [105]. Because human motion is built on sequences of motor actions, another key question is to elucidate how sequences of elementary commands are built in the cerebellum [109]. The process of abnormal sequencing for motor actions might underlie the pathogenesis of motor dysmetria as well.
Hypermetria is generally observed in lesions of cerebellar lobules I–VIII [3]. It is more severe in the early phases of acute cerebellar lesions such as a cerebellar stroke. The most pronounced hypermetria is observed after lesions of the anterior lobe and cerebellar nuclei (interposed nuclei, dentate nuclei). Upper limb dysmetria correlates with lesions of lobules IV–VI and lower limb ataxia correlates with lesions of lobules III–IV. A plausible explanation is the peculiar representation of the cerebellar homunculus in these lobules, with the upper limbs represented below the lower limbs [39, 103, 110]. In patients with cerebellar cortical atrophy, the clinical deficits correlate with the degree of atrophy of the intermediate and lateral cerebellum.
Sensory Function (Amy Bastian)
There is clear evidence that the cerebellum is important for human somatosensory function. Most studies to date have focused on proprioceptive and force sensing abilities. The cerebellum is perhaps most critical for improving sensory precision during active movement, or when elements of timing are important for the required judgment.
Historically, the idea that the cerebellum contributes to somatosensory function has been controversial. Classic descriptions of cerebellar dysfunction did not include basic deficits in somatosensation [4, 64] and more recent studies have shown that cerebellar patients have normal proprioception when tested passively [111]. Yet, the human cerebellum is active during somatosensory processing [112] and some neurophysiological studies in rats have shown cerebellar cortical activity to be better correlated with tactile inputs compared with movement (e.g., [113]). Here, we review some of the work that demonstrates specific somatosensory deficits in people with cerebellar damage.
An early description of cerebellar deficits in somatosensation comes from a case study of an individual with hemiataxia due to damage in the right cerebellar hemisphere [114]. This individual had difficulty discriminating the weight of objects (i.e., barognosis) when he was asked to actively lift them with his ataxic arm. Angel suggested that the perception of a load during movement might depend on corollary discharge of the motor command, which could be affected by cerebellar damage. Bhanpuri and colleagues [115] studied a group of cerebellar patients performing active force and torque discrimination tasks, as well as a passive elbow position discrimination task. Here, patients had deficits in active force and torque discrimination but no deficits in the passive position discrimination. The authors suggested that the observed pattern of deficits could be due to dysfunction of cerebellum-dependent sensory predictions of self-produced movements, or perhaps due to a deficit in increasing receptor sensitivity via muscle contraction during active movements.
A recent study directly tested whether human cerebellar patients had proprioceptive deficits during active versus passive elbow movements [116]. In this task, subjects had to discriminate the amplitude of two consecutive elbow motions and report which was larger. Cerebellar patients showed clear deficits compared to controls in discrimination during active movements, but not when the arm was passively moved. Further, when control subjects actively performed the task in a force field with unpredictable dynamics, they had active proprioceptive deficits similar to cerebellar patients. Based on these results, the authors suggested that cerebellar patients have an active proprioceptive deficit consistent with disrupted movement prediction rather than an inability to generally enhance peripheral proprioceptive signals during active muscle contraction.
Interestingly, one older study has demonstrated some deficits in passive movements. In 1994, Grill and colleagues [117] found that cerebellar patients had difficulty with specific types of passive proprioceptive discrimination tests: judgments of movement duration and movement velocity were impaired but discrimination of movement amplitude was normal. Maschke and colleagues [111] also showed that cerebellar patients have normal passive discrimination of movement amplitude. One interpretation of this group of studies is that cerebellar damage impairs somatosensory judgments that require some temporal processing regardless of whether the movement was active or passive. Further work should be done to test whether this is, in fact, the case.
In sum, there is clear evidence that cerebellar damage impairs force perception and proprioceptive sense during active movements. Multiple studies show that cerebellar damage does not impair passive proprioceptive sense during tests requiring amplitude discrimination. However, one study showed that passive proprioceptive judgments that involve the sense of time (i.e., duration and velocity) are impaired in cerebellar patients. Further work should more clearly address whether a general deficit in sensory prediction and/or timing can parsimoniously explain these findings, and whether this extends to other somatosensory abilities (e.g., tactile discrimination, stereognosis).
Gait/Posture in Cerebellar Disorders (Carlo Casali and Mariano Serrao)
Gait abnormalities in cerebellar disorders have long been acknowledged and described as clumsy, staggering movements with a wide-based gait. When standing still, the patient’s body may swagger back and forth and from side to side; this is known as titubation. Patients are not able to walk from heel to toe or in a straight line. The gait of cerebellar disease resembles the gait of acute alcohol intoxication. While such traditional description still retains its clinical utility enabling clinicians to identify cerebellar disease, modern motion analysis systems have been used to quantitatively characterize the nature and degree of walking dysfunction. These findings have revealed biomechanical abnormalities in spatiotemporal parameters, center of mass (CoM) and center of pressure (CoP) trajectories, joint kinematics and kinetics, muscles activation pattern and upper body control [118–123]. Patients have difficulties in steady state linear walking, but also in gait initiation, termination, and turning [124–129]. All of these locomotor abnormalities reflect poor limb coordination and impaired balance, which greatly restrict patients in their activities of daily life and predispose them to falls [130].
Various temporal (time-dependent) and spatial (distance-dependent) parameters have been defined: increased step width, decreased step length, low gait speed, reduced cadence, and increased durations in stance and double support phases [118, 120, 123]. In fact, most of these abnormalities seem to represent compensatory mechanisms aimed at reducing dynamic imbalance. For instance, a wider base is a typical compensatory mechanism aimed at increasing the margin of stability by moving the center of gravity far from the foot border; the increase of double support duration and the reduction of the step length may represent an attempt to reduce the sway in the less stable configuration (monopodalic stance).
Reduced motion and torques of hip, knee, and ankle joints have been reported by several authors, although with contrasting results [118–120]. It should be noted that the temporal variability of the joint kinematics and kinetics across the gait cycle (see below) may represent a confounding factor when examining joint kinematics and torques as discrete variables. When these parameters are analyzed as continuous variables, only minimal differences, mainly involving the ankle joints, are revealed between patients and controls [123]. Abnormal intra-limb joint coupling during walking, in terms of both joint movements and interaction torques, has been reported in several studies [122, 123, 131–133]. Particularly, increased temporal variability of intra-limb coordination has been found to be related with dynamic balance and irregular foot trajectories in ataxic gait [132]. More recently, joint coordination has been analyzed as both angular joint displacement and velocity in any given instant of gait cycle by using the continuous relative phase (CRP) method [123]. It has been shown that impaired inter-joint coordination leads to an extremely irregular pattern of alternating proximal/distal joint as compared to the well-shaped pattern of healthy controls [123]. These studies support the notion that, as seen for the upper limb [131, 134], one of the most important features of cerebellar ataxia is the lack of inter-joint coordination in which the temporal aspect is particularly important [123, 132, 135].
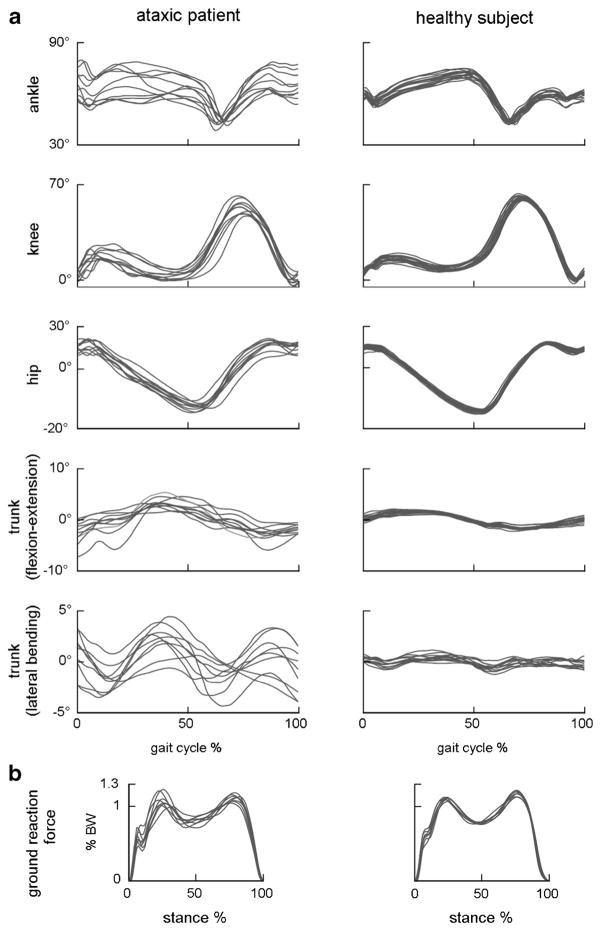
A marked variability of all global and segmental kinematic and kinetic gait parameter values has been observed in almost all studies [118–120, 123, 132] (Fig. 5). Gait variability is somehow related to the stability of the locomotion, meaning the capacity to maintain the dynamic balance through a regular walking pattern [136]. Stride-to-stride variability has been associated with an increased risk of falls in cerebellar ataxia [137]. It is likely that this characteristic is due to the complex interaction between the primary deficit and the adaptive and compensatory mechanisms adopted, resulting in a continuous step-by-step adjustment of the gait strategy.
Fig. 5.
Examples of joint and trunk orientation angles (a) and vertical ground reaction force (b) in one ataxic patient (left) and one age-, sex- and gait speed-matched control (right) during overground walking. Every trace refers to a single gait cycle. Stride-by-stride temporal variability of both kinematic and kinetic patterns is consistently larger in ataxic gait
So far, few studies have provided a detailed analysis of muscle activity patterns during locomotion in cerebellar ataxia. In two recent studies investigating the spatial and temporal profiles of EMG activity in individual muscles [133] as well as in paired antagonist muscles [138], a common pattern has been shown. Specifically, a marked widening of the EMG peaks and a high level of ankle and knee joint muscles co-contraction were observed during all four gait sub-phases. In essence, ataxic patients seem to activate both individual and paired antagonist muscles more intensely and for a longer time, possibly in an attempt to stiffen the limb and to compensate for the instability due to poor muscle coordination.
Although postural changes have been fully described in stance [139], only a few studies have addressed them while walking. Pitch over roll instability has been observed both in tandem gait and normal walking with simultaneous head rotations [140]. Very recently, an abnormal increase of the head and trunk range of motion has been shown in sagittal (flexion-extension), frontal (lateral bending), and yaw planes (rotations) [141]. Such abnormalities correlated with disease severity and duration and gait variability, suggesting that impaired control of the upper body is important and that inter-segment coordination may play a pivotal role in explaining several aspect of gait ataxia. Interestingly, patients displace their trunk forward while walking to reduce instability in the backward direction at the expense of increasing it in the forward direction [141].
The Cerebellar Cognitive Affective Syndrome (Jeremy D. Schmahmann)
The cerebellum is organized into a primary sensorimotor region in the anterior lobe and adjacent part of lobule VI, and a second sensorimotor region in lobule VIII. Current evidence indicates that cognitive and limbic regions are in the posterior lobe (lobule VI, lobule VIIA which includes crus I and crus II, and lobule VIIB); lobule IX may also be part of this network. Cognitively relevant areas are situated more laterally in these lobules whereas the limbic cerebellum is represented in the posterior vermis [142–145]. Lesions of the sensorimotor cerebellum and related nuclei result in the cerebellar motor syndrome. Lesions of the cognitive and limbic cerebellum and related nuclei lead to the cerebellar cognitive affective syndrome (CCAS; [146]), reflecting disruption of reciprocal cerebellar interconnections with cerebral association areas and paralimbic cortices [143, 146, 147]. The CCAS is characterized by (1) impaired executive function, (2) impaired visual-spatial processing, (3) linguistic deficits, and (4) affective dysregulation (see Table 4). CCAS occurs in children [148] as well as adults following many types of injury, can be prominent following acute lesions including stroke, hemorrhage, and infectious or post-infectious cerebellitis, more insidious in the neurodegenerative ataxias [149], and pervasive in the infantile-onset developmental form of the CCAS [150, 151].
Table 4.
Deficits characterizing the cerebellar cognitive affective syndrome (Schmahmann’s syndrome) (derived from [146])
| 1. Executive function Deficient planning, motor or ideational set shifting, abstract reasoning, working memory. Decreased verbal fluency, sometimes to the point of telegraphic speech or mutism. Perseverative ideation in thought and/or action |
| 2. Spatial cognition Visuospatial disintegration with impaired attempts to draw or copy a diagram. Disorganized conceptualization of figures. Impaired visual-spatial memory. Simultanagnosia in some |
| 3. Linguistic difficulties Anomia, agrammatic speech and abnormal syntactic structure, with abnormal prosody |
| 4. Personality change Aberrant modulation of behavior and personality with posterior lobe lesions that involve midline structures. Manifests as flattening or blunting of affect alternating or coexistent with disinhibited behaviors such as overfamiliarity, flamboyant and impulsive actions, and humorous but inappropriate and flippant comments. Regressive, childlike behaviors and obsessive-compulsive traits can be observed (see Table 2) |
The net effect of these disturbances in cognitive functioning is a general lowering of overall intellectual function
Executive function deficits may be the earliest and most notable and pervasive aspects of the syndrome. Patients may demonstrate concrete thinking, poor problem-solving strategies, and impaired ability to multitask, which affects correct trouble planning, sequencing, and organizing their activities [146]. Cognitive testing may also reveal problems with working memory as demonstrated by an impaired performance on (reverse) digit span, disruption of mental flexibility as shown by tasks of set shifting (e.g., Wisconsin Card Sorting Test), and perseverative behavior as shown by bedside assessments of mental control. Executive control significantly impacts a variety of cognitive and behavioral processes, including memory [152, 153].
Short-term memory impairments include difficulty learning and spontaneously recalling new information, reflecting deficient strategies for organizing verbal or visual-spatial material for encoding, and difficulty locating information in memory stores. Successful recall is aided by a structured approach to the task utilizing clues and other prompts. Conditional associative learning is degraded, as shown in studies of classical conditioning in cerebellar patients as well as in animal models [154]. Mental arithmetic is impaired. Ideational apraxia and hemi-inattention have been reported.
Visual-spatial deficits manifest as impaired mental representation of spatial relationships between and among objects. Visuospatial disintegration is apparent when attempting to copy or recall visual images or judge distances. These laboratory measures reflect abstract thinking, conceptualization, and internal models of the complexities of the visual world (present or imagined).
Expressive language can be abnormal, characterized by long response latency, brief responses, reluctance to engage in conversation, and word finding difficulties. Verbal fluency is decreased affecting phonemic (letter) more than semantic (category) naming [146, 155, 156]. Language deficits include abnormal syntax resulting in a grammatism [11, 146]. Discourse, which is the essence of verbal communication, can be disrupted by impaired metalinguistic ability [157]. This manifests as deficits in understanding metaphor, ambiguity, and inferential thinking, and difficulty expressing thoughts verbally. Mutism occurs particularly in children following surgical resection of midline tumors (posterior fossa syndrome [158]), as well as in adults subsequent to cerebellitis, infarction, and hemorrhage. Degraded control of volume, pitch, and tone can produce high-pitched, hypophonic speech.
The affective component of the CCAS occurs when lesions involve the limbic cerebellum in the posterior vermis and fastigial nucleus [146, 148, 159]. Patients exhibit difficulty modulating behavior and personality style, have flattened affect, or disinhibition manifesting as overfamiliarity, and flamboyant or impulsive actions. Behavior may be regressive and childlike, sometimes with obsessive-compulsive traits. Patients can be irritable, with labile affect and poor attentional and behavioral modulation. Acquired panic disorder may follow midline cerebellar lesions, and pathologic laughing and/or crying occur in some ataxias, as in MSAc [160]. Social cognition may be impaired, as can appreciation and demonstration of empathy [161, 162], which is potentially destructive to relationships and families. There appear to be five domains of behavioral dysregulation caused by cerebellar damage: impairments of attentional control, emotional control, autism spectrum disorders, psychosis spectrum disorders, and difficulties with social skill set. Within each of these domains, there are hypometric/diminished behaviors, and hypermetric/exaggerated behaviors (Table 5) [150].
Table 5.
Neuropsychiatric features of the cerebellar cognitive affective syndrome. Five domains, each with hyper/hypometric manifestations (from [150])
| Positive (exaggerated) symptoms | Negative (diminished) symptoms | |
|---|---|---|
| Attentional Control | Inattentiveness | Ruminativeness |
| Distractibility | Perseveration | |
| Hyperactivity | Difficulty shifting focus of attention | |
| Compulsive and ritualistic behaviors | Obsessional thoughts | |
| Emotional control | Impulsiveness, disinhibition | Anergy, anhedonia |
| Lability, unpredictability | Sadness, hopelessness | |
| Incongruous feelings, pathological laughing/crying | Dysphoria | |
| Anxiety, agitation, panic | Depression | |
| Autism spectrum | Stereotypical behaviors | Avoidant behaviors, tactile defensiveness |
| Self stimulation behaviors | Easy sensory overload | |
| Psychosis spectrum | Illogical thought | Lack of empathy |
| Paranoia | Muted affect, emotional blunting | |
| Hallucinations | Apathy | |
| Social skill set | Anger, aggression | Passivity, immaturity, childishness |
| Irritability | Difficulty with social cues and interactions | |
| Overly territorial | Unawareness of social boundaries | |
| Oppositional behavior | Overly gullible and trusting |
Focal damage to the cognitive and limbic cerebellum in the posterior lobe may cause intellectual and emotional impairments in the absence of the motor syndrome if the anterior lobe is spared. The nature of these deficits provides new avenues for conceptualizing mental illnesses including autism, schizophrenia, bipolar disorder, attention deficit disorder, and dyslexia, all of which have been linked to the cerebellum. Together with the underlying dysmetria of thought theory [1, 8, 163] that provides a conceptual framework for these findings, the CCAS underscores the role of the cerebellum in the distributed neural circuits subserving neurological function. It is incumbent on the clinician to know and recognize the cardinal features of the CCAS, and incorporate their management into the overall plan of patient care.
From Ataxic Dysarthria to Cerebellar-Induced Nonmotor Language Disorders (Peter Mariën and Kim van Dun)
As pointed out in previous sections, clinical and experimental research on cerebellar function has been dominated for more than two centuries by an overwhelming scientific interest in the role of the cerebellum in sensorimotor control, including speech [11, 39]. In his classic 1917 paper, Holmes described impaired muscular control of speech production after traumatic cerebellar lesions and added evidence to the view that the cerebellum plays an essential role in motor speech control [4]. Holmes described motor speech symptoms following cerebellar damage as typically slow, monotonous, staccato, scanned, indistinct, remarkable irregular, jerky, explosive, slurred, and labored. These pathological alterations in phonation and articulation were designated as ataxic dysarthria by Darley, Aronson, and Brown [164]. Although Holmes, as well as many investigators after him, maintained that the causative lesion for ataxic dysarthria could be situated in either one or both cerebellar hemispheres [4, 6], it was observed in a large study of 122 patients that dysarthria resulted more frequently from left superior than right cerebellar lesions [165]. Although a laterality effect has not yet been clearly demonstrated [166], clinical and experimental neuroimaging studies addressing the topographic aspects of motor speech processing demonstrate that ataxic dysarthria frequently follows a damage to the right superior anterior vermal and paravermal regions, supplied by the SCA artery [167, 168]. As PICA and AICA infarctions are often associated with brainstem damage, it is not possible to attribute—unambiguously—motor speech deficits to involvement of the cerebellar regions supplied by these arteries [169]. The role of the cerebellum in motor speech production has primarily been defined as the regulation of the temporal, online sequencing, and adaptation of overlearned, basic speech movement patterns (mental syllables) into linguistically larger segments such as words, phrases, and sentences [170].
Involvement of the cerebellum in the sequencing phase of speech computation is in agreement with the recently acknowledged role of the cerebellum in a broad variety of nonmotor cognitive and linguistic functions. Indeed, in addition to its long-established role in motor speech production, clinical and experimental studies have identified a role for the cerebellum in a variety of nonmotor language functions, including aspects of articulatory and graphomotor planning, language dynamics, verbal fluency, phonological, and semantic word retrieval, expressive and receptive syntax, and various other aspects of reading and writing.
Apraxia of speech (AoS) (anarthria) is a motor speech planning and coordination disorder that follows from injury to the motor speech regions of the language dominant hemisphere (anterior insula, inferior premotor and motor cortex, BA 44 of Broca’s area). From a semiological point of view, AoS shares a number of symptoms with ataxic dysarthria, such as inconsistent misarticulations, phonetic alterations of vowel and consonant production, sequential errors, prosodic abnormalities, slow articulation, and scanning of speech. This might suggest that both conditions are related phenomena resulting from disruption of the motor speech planning and coordinating network, subserved by a close functional interplay between the anterior motor speech region of the language dominant hemisphere and the contralateral right cerebellum. Indeed, AoS has been reported in a handful of cases with etiologically heterogeneous cerebellar disorders but the link between both conditions remains to be clarified [171, 172].
Impaired verbal fluency is frequently observed in patients with focal or degenerative cerebellar lesions [11, 155]. It remains to be shown whether disruption of sequence processing as a general mechanism might also account for disruption of language dynamics at other linguistic levels as well ((morpho)syntax, pragmatics). This could result in dynamic or a transcortical-like aphasia, even to the point of mutism due to inhibition of speech production [173].
The cerebellum also contributes to expressive and receptive syntax processing [174]. In their much cited study on cerebellar cognitive affective syndrome, Schmahmann and Sherman included agrammatism in the cluster of linguistic disturbances following focal cerebellar lesions [146, 172]. Several studies have shown that the right cerebellar hemisphere is embedded within a distinct neural network devoted to the processing of grammar, together with the basal ganglia and the language-dominant left prefrontal, temporal and parietal cortex [175].
In contrast to its developmental variant acquired dyslexia (alexia) has only scarcely been studied in the context of cerebellar disorders. Moretti et al. showed that patients with (para)vermian lesions had a lower degree of accuracy in reading and made true aphasic reading errors [176]. In a later study, the authors suggested that alexia may be related either to an imperfect oculomotor control or to disruption of the cerebellar-encephalic projections connecting the cerebellum to the supratentorial areas implicated in language and attentional and alerting processes [177].
Only a few cases exist in which central agraphia was found after cerebellar damage. Following a right SCA infarction, the patient of Mariën et al. presented with surface dysgraphia [178]. The authors hypothesized that the linguistic deficits resulted from functional disruption of the cerebellar-encephalic pathways connecting the cerebellum to the frontal supratentorial areas which subserve attentional and planning processes. Clinical evidence suggests involvement of the cerebellum in the neural network of graphomotor planning. Distortion of the spatiotemporal features of handwriting (apraxic agraphia) has been observed after disruption of the cerebello-cerebral network subserving the planning and execution of skilled motor actions [179].
The co-occurrence of deficits affecting different linguistic levels may give rise to cerebellar-induced aphasia [173]. As supported by the findings of (functional) neuroimaging studies, impairment of linguistic functions after cerebellar lesions may result from a decrease of excitatory impulses through the cerebello-ponto-thalamo-cortical pathways causing functional depression of the supratentorial regions subserving linguistic functions.
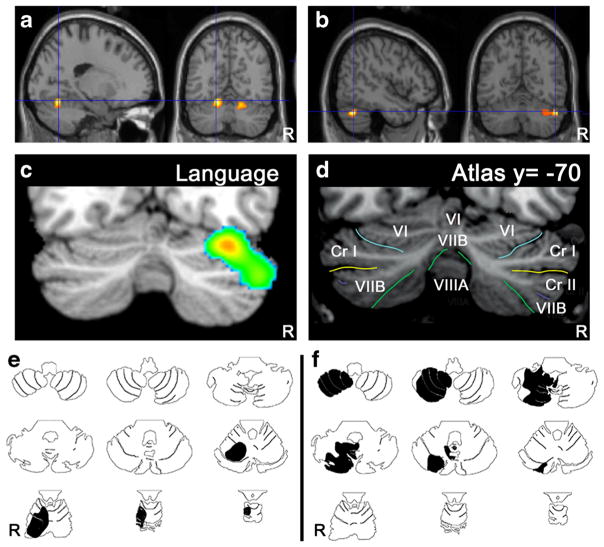
The neuroanatomical substrate of cerebellar involvement in nonmotor language processing is a dense and reciprocal network of crossed cerebro-cerebellar pathways consisting of cortico-ponto-cerebellar and cerebello-thalamo-cortical loops that establish a close connection between the cerebellum and the supratentorial motor, paralimbic, and association cortices. Contemporary lesion-behavior and neuroimaging studies have demonstrated that the human cerebellum is topographically organized for higher-order cognitive and affective functions. A meta-analysis of neuroimaging studies has provided support for a dichotomy between the sensorimotor cerebellum—geographically organized in distinct regions in the anterior lobe—and the neurocognitive and affective cerebellum—represented in distinct parts in the posterior lobe (for a review, see [9, 11]). In addition, the majority of anatomoclinical studies of patients with linguistic impairments following focal cerebellar lesions and the majority of neuroimaging studies typically show a lateralized involvement of lateral, posterior cerebellar regions (including lobules VI and Crus I/II) in nonmotor linguistic processes (Fig. 6).
Fig. 6.
Topographic arrangement in cerebellum of speech versus language representation. Functional MRI localizes articulation (a) to medial parts of lobule VI bilaterally, whereas verb generation (b) activates lateral regions of lobule VI and crus I on the right. In a meta-analysis of functional imaging studies [156] higher-level language tasks engage the right lateral posterior cerebellum, lobules VI and crus I (c) according to the lobule identification in (d; [184]). Case studies of cerebellar stroke patients reveal topography for articulation vs. higher-level language tasks. A patient with stroke in the territory of the right superior cerebellar artery (e, black shading) involving lobules I–VI was dysarthric; whereas a patient with stroke in the territory of the right posterior inferior cerebellar artery (f, black shading) involving lobules VII–IX was not dysarthric but performed poorly on the Boston Naming Test [185] (from Stoodley and Schmahmann in ref. [11])
Despite continued efforts devoted to further refine typology and anatomoclinical configurations of cerebellar-induced nonmotor language dysfunctions, the exact underlying pathophysiological mechanisms remain to be elucidated.
Discussion
This consensus paper focuses on the neurological symptoms and signs observed in cerebellar patients. The current concepts on cerebellar functions indicate that cerebellar circuitry contributes to timing, motor learning and prediction [39]. The terminology of cerebellar ataxia now extends from a pure motor syndrome to a semiological constellation of cognitive and affective deficits as in Schmahmann’s syndrome (CCAS) [180].
Lesion-symptom mapping (also called lesion-behavior mapping) in patients presenting focal cerebellar lesions such as stroke or tumors has been a helpful procedure to localize function within the human cerebellum [181]. Localization in the so-called degenerative cerebellar disorders is often considered less accurate. Ataxia of stance and gait is correlated with atrophy of the medial/intermediate cerebellum, oculomotor disorders with the medial cerebellum, dysarthria with the intermediate cerebellum and limb ataxia with atrophy of the intermediate and lateral cerebellum [182]. In agreement with animal experiments and fMRI studies performed in healthy control subjects, a somatotopy in the superior cerebellar cortex is observed in patients with acute focal lesions. Furthermore, lesion site appears important for motor recovery. In particular, recovery after lesions to the nuclei of the cerebellum is often less complete [182]. New MRI techniques and development of data analysis tools add to the precision of cerebellar maps and will thus help to extract meaningful correlations [183].
The panel of contributors to this Consensus Paper agree that:
The cerebellum is involved not only in motor operations but also in cognitive tasks and tasks related to emotional and affective regulation.
Vertigo, dizziness, and imbalance are associated with lesions of the vestibulo-cerebellar, vestibulo-spinal, or cerebellar ocular motor systems. The cerebellum controls calibration, reduction of eye instability, and maintenance of ocular alignment. The following deficits occur in cerebellar lesions: ocular instability, nystagmus, saccadic intrusions, impaired smooth pursuit, impaired VOR, and ocular misalignment. Recent advances helped to identify reliable structural-clinical correlations.
Ataxic dysarthria is highly suggestive of a cerebellar disorder. Speech is under the control of the superior paravermal region, and the intermediate and lateral cerebellar cortex.
The following signs occur in various degrees when cerebellar patients perform limb movements: a- or dysdiadochokinesia, dysmetria, grasping deficits, asynergia and various types of tremor.
Hypotonia is difficult to assess but may be observed in cerebellar patients, especially in acute conditions with extensive damage as observed in the post-surgery phase.
The cerebellum participates in force perception and proprioceptive sense during active movements.
Cerebellar gait is staggering and wide-based. Tandem gait is very often impaired. Movement analysis discloses a marked variability of global and segmental kinematic and kinetic gait parameters, impaired inter-joint intra-limb coordination as well as increased compensatory antagonist muscle co-contraction.
Cerebellar cognitive deficits include impairments in executive function, visual-spatial processing, linguistic deficits, and affective dysregulation as defined in Schmahmann’s syndrome.
Cerebellar circuitry is organized into (1) a primary sensorimotor region in the anterior lobe and adjacent part of lobule VI, (2) a second sensorimotor region in lobule VIII, (3) cognitive and limbic regions located in the posterior lobe (lobule VI, lobule VIIA which includes crus I and crus II, and lobule VIIB). The limbic cerebellum is located in the posterior vermis.
Cortico-ponto-cerebellar and cerebello-thalamo-cortical loops establish close functional connections between the cerebellum and the supratentorial motor, paralimbic, and association cortices.
The panel identified several points that deserve attention and further investigation:
The definition of prodromal symptoms/signs of progressive cerebellar disorders associated with atrophy of the cerebellum remains unclear. More specific studies are required. Advances in neuroimaging are likely to play an important role in better defining the pre-clinical features of cerebellar disorders.
Well-controlled studies on the role of the cerebellum in sensorimotor operations are needed, taking into account pure cerebellar lesions and the presence of associated factors such as depression.
A modern classification of cerebellar tremors is currently missing. It remains unclear why some patients exhibit tremor and others do not, and why there is a range in type of tremors.
The contribution of the human cerebellum to autonomic control deserves to be better delineated, because there are indications of its possible involvement.
Further studies are needed to develop clinical rating scales for the detection and assessment of severity of Schmahmann’s syndrome, and to explore structure-function correlations of these neurobehavioral and neuropsychiatric manifestations and their relationship to the cerebellar motor syndrome.
Efforts should be devoted to expanding our knowledge of the neuropsychiatric signs in cerebellar patients and demonstrating their temporal profile. The possible patterns of recovery of neuropsychiatric manifestations deserve further studies.
Footnotes
Conflicts Of Interest The authors declare no relevant conflict of interest.
References
- 1.Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13(1):151–77. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herculano-Houzel S. Coordinated scaling of cortical and cerebellar numbers of neurons. Front Neuroanat. 2010;4:12. doi: 10.3389/fnana.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimaldi G, Manto M. Topography of cerebellar deficits in humans. Cerebellum. 2012;11(2):336–51. doi: 10.1007/s12311-011-0247-4. [DOI] [PubMed] [Google Scholar]
- 4.Holmes G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain. 1917;40:461–535. [Google Scholar]
- 5.Babinski J. Sur le role du cervelet dans les actes volitionnels nécessitant une succession rapide de mouvements (1) (diadococinésie) Rev Neurol. 1902;10:1013–5. [Google Scholar]
- 6.Holmes G. The Croonian lectures on the clinical symptoms of cerebellar disease and their interpretation. Lecture III. Lancet. 1922;200:59–65. [Google Scholar]
- 7.Haines DE, Manto MU. Clinical symptoms of cerebellar disease and their interpretation. Cerebellum. 2007;6(4):360–74. doi: 10.1080/14734220701798199. [DOI] [PubMed] [Google Scholar]
- 8.Schmahmann JD. An emerging concept: the cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–87. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- 9.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 10.Timmann D, Daum I. Cerebellar contributions to cognitive functions: a progress report after two decades of research. Cerebellum. 2007;6(3):159–62. doi: 10.1080/14734220701496448. [DOI] [PubMed] [Google Scholar]
- 11.Mariën P, Ackermann H, Adamaszek M, Barwood CH, Beaton A, Desmond J, et al. Consensus paper: language and the cerebellum: an ongoing enigma. Cerebellum. 2014;13(3):386–410. doi: 10.1007/s12311-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis RF, Zee DS. Ocular motor disorders associated with cerebellar lesions: pathophysiology and topical localization. Rev Neurol (Paris) 1993;149(11):665–77. [PubMed] [Google Scholar]
- 13.Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. J Neurol Sci. 1997;145:205–11. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- 14.Wartenberg R. Cerebellar signs. J Am Med Assoc. 1954;156(2):102–5. doi: 10.1001/jama.1954.02950020008003. [DOI] [PubMed] [Google Scholar]
- 15.Haerer AF. DeJong’s the neurological examination. 5. Lippincott: Raven; 1992. [Google Scholar]
- 16.Kheradmand A, Zee DS. Cerebellum and ocular motor control. Front Neurol. 2011;2:53. doi: 10.3389/fneur.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–20. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 18.Schmahmann JD, Gardner R, MacMore J, Vangel MG. Development of a brief ataxia rating scale (BARS) based on a modified form of the ICARS. Mov Disord. 2009;24(12):1820–8. doi: 10.1002/mds.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bürk K. Clinical scales of cerebellar ataxias. In: Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N, editors. Handbook of the cerebellum and cerebellar disorders. Dordrecht: Springer; 2013. pp. 1785–1798. [Google Scholar]
- 20.Jacobi H, Rakowicz M, Rola R, Fancellu R, Mariotti C, Charles P, et al. Inventory of Non-Ataxia Signs (INAS): validation of a new clinical assessment instrument. Cerebellum. 2013;12(3):418–28. doi: 10.1007/s12311-012-0421-3. [DOI] [PubMed] [Google Scholar]
- 21.Brandt T, Dieterich M, Strupp M. Vertigo and dizziness—common complaints. 2. London: Springer; 2013. [Google Scholar]
- 22.Choi KD, Lee H, Kim JS. Vertigo in brainstem and cerebellar strokes. Curr Opin Neurol. 2013;26:90–5. doi: 10.1097/WCO.0b013e32835c5edd. [DOI] [PubMed] [Google Scholar]
- 23.Kim JS, Lee H. Vertigo due to posterior circulation stroke. Semin Neurol. 2013;33(3):179–84. doi: 10.1055/s-0033-1354600. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Sohn SI, Cho YW, Lee SR, Ahn BH, Park BR, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology. 2006;67(7):1178–83. doi: 10.1212/01.wnl.0000238500.02302.b4. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Kim HA. Nystagmus in SCA territory infarction: pattern and a possible mechanism. J Neurol Neurosurg Psychiatry. 2013;84:446–51. doi: 10.1136/jnnp-2012-303177. [DOI] [PubMed] [Google Scholar]
- 26.Park HK, Kim JS, Strupp M, Zee DS. Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol. 2013;260:1576–82. doi: 10.1007/s00415-013-6837-y. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Lee SH, Park JH, Choi JY, Kim JS. Isolated vestibular nuclear infarction: report of two cases and review of the literature. J Neurol. 2014;261(1):121–9. doi: 10.1007/s00415-013-7139-0. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Park SH, Kim JS, Kim HJ, Yunusov F, Zee DS. Isolated unilateral infarction of the cerebellar tonsil: ocular motor findings. Ann Neurol. 2014;75:429–34. doi: 10.1002/ana.24094. [DOI] [PubMed] [Google Scholar]
- 29.Baier B, Dieterich M. Ocular tilt reaction: a clinical sign of cerebellar infarctions? Neurology. 2009;72(6):572–3. doi: 10.1212/01.wnl.0000342123.39308.32. [DOI] [PubMed] [Google Scholar]
- 30.Kim HA, Yi HA, Lee H. Apogeotropic central positional nystagmus as a sole sign of nodular infarction. Neurol Sci. 2012;33(5):1189–91. doi: 10.1007/s10072-011-0884-x. [DOI] [PubMed] [Google Scholar]
- 31.Kremmyda O, Zwergal A, La FC, Brandt T, Jahn K, Strupp M. 4-Aminopyridine suppresses positional nystagmus caused by cerebellar vermis lesion. J Neurol. 2013;260(1):321–3. doi: 10.1007/s00415-012-6737-6. [DOI] [PubMed] [Google Scholar]
- 32.Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics. 2007;4:267–73. doi: 10.1016/j.nurt.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Jen JC. Hereditary episodic ataxias. Ann N Y Acad Sci. 2008;1142:250–3. doi: 10.1196/annals.1444.016. [DOI] [PubMed] [Google Scholar]
- 34.Yu-Wai-Man P, Gorman G, Bateman DE, Leigh RJ, Chinnery PF. Vertigo and vestibular abnormalities in spinocerebellar ataxia type 6. J Neurol. 2009;256(1):78–82. doi: 10.1007/s00415-009-0068-2. [DOI] [PubMed] [Google Scholar]
- 35.Krafczyk S, Tietze S, Swoboda W, Valkovic P, Brandt T. Artificial neural network: a new diagnostic posturographic tool for disorders of stance. Clin Neurophysiol. 2006;117(8):1692–8. doi: 10.1016/j.clinph.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 36.Szmulewicz DJ, Waterston JA, Halmagyi GM, Mossman S, Chancellor AM, Mclean CA, et al. Sensory neuropathy as part of the cerebellar ataxia neuropathy vestibular areflexia syndrome. Neurology. 2011;76(22):1903–10. doi: 10.1212/WNL.0b013e31821d746e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirchner H, Kremmyda O, Hufner K, Stephan T, Zingler V, Brandt T, et al. Clinical, electrophysiological, and MRI findings in patients with cerebellar ataxia and a bilaterally pathological head-impulse test. Ann N Y Acad Sci. 2011;1233(1):127–38. doi: 10.1111/j.1749-6632.2011.06175.x. [DOI] [PubMed] [Google Scholar]
- 38.Szmulewicz DJ, Mclean CA, Rodriguez ML, Chancellor AM, Mossman S, Lamont D, et al. Dorsal root ganglionopathy is responsible for the sensory impairment in CANVAS. Neurology. 2014;82:1410–5. doi: 10.1212/WNL.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SN, Gerwig M, et al. Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11:457–87. doi: 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leigh RJ, Zee DS. The neurology of eye movements. 5. Oxford: University Press; 2015. [Google Scholar]
- 41.Wagner JN, Glaser M, Brandt T, Strupp M. Downbeat nystagmus: aetiology and comorbidity in 117 patients. J Neurol Neurosurg Psychiatry. 2008;79:672–7. doi: 10.1136/jnnp.2007.126284. [DOI] [PubMed] [Google Scholar]
- 42.Zee DS. Mechanisms of nystagmus. Am J Otol. 1985;(Suppl):30–4. [PubMed] [Google Scholar]
- 43.Walker MF, Zee DS. The effect of hyperventilation on downbeat nystagmus in cerebellar disorders. Neurology. 1999;53(7):1576–9. doi: 10.1212/wnl.53.7.1576. [DOI] [PubMed] [Google Scholar]
- 44.Waespe W, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. 1985;228:199–202. doi: 10.1126/science.3871968. [DOI] [PubMed] [Google Scholar]
- 45.Brandt T. Positional and positioning vertigo and nystagmus. J Neurol Sci. 1990;95:3–28. doi: 10.1016/0022-510x(90)90113-2. [DOI] [PubMed] [Google Scholar]
- 46.Huh YE, Kim JS. Patterns of spontaneous and head-shaking nystagmus in cerebellar infarction: imaging correlations. Brain. 2011;134:3662–71. doi: 10.1093/brain/awr269. [DOI] [PubMed] [Google Scholar]
- 47.Ramat S, Leigh RJ, Zee DS, Optican LM. What clinical disorders tell us about the neural control of saccadic eye movements. Brain. 2007;130:10–35. doi: 10.1093/brain/awl309. [DOI] [PubMed] [Google Scholar]
- 48.Sharpe JA. Neurophysiology and neuroanatomy of smooth pursuit: lesion studies. Brain Cogn. 2008;68:241–54. doi: 10.1016/j.bandc.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Kremmyda O, Kirchner H, Glasauer S, Brandt T, Jahn K, Strupp M. False-positive head-impulse test in cerebellar ataxia. Front Neurol. 2012;3:162. doi: 10.3389/fneur.2012.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thurston SE, Leigh RJ, Abel LA, Dell’Osso LF. Hyperactive vestibulo-ocular reflex in cerebellar degeneration: pathogenesis and treatment. Neurology. 1987;37:53–7. doi: 10.1212/wnl.37.1.53. [DOI] [PubMed] [Google Scholar]
- 51.Selhorst JB, Stark L, Ochs AL, Hoyt WF. Disorders in cerebellar ocular motor control. I. Saccadic overshoot dysmetria. An oculographic, control system and clinico-anatomical analysis. Brain. 1976;99:497–508. doi: 10.1093/brain/99.3.497. [DOI] [PubMed] [Google Scholar]
- 52.Helmchen C, Straube A, Buttner U. Saccadic lateropulsion in Wallenberg’s syndrome may be caused by a functional lesion of the fastigial nucleus. J Neurol. 1994;241:421–6. doi: 10.1007/BF00900959. [DOI] [PubMed] [Google Scholar]
- 53.Versino M, Hurko O, Zee DS. Disorders of binocular control of eye movements in patients with cerebellar dysfunction. Brain. 1996;119(Pt 6):1933–50. doi: 10.1093/brain/119.6.1933. [DOI] [PubMed] [Google Scholar]
- 54.Hüfner K, Frenzel C, Kremmyda O, Adrion C, Bardins S, Glasauer S, et al. Esophoria or esotropia in adulthood: a sign of cerebellar dysfunction? J Neurol. 2015;262(3):585–92. doi: 10.1007/s00415-014-7614-2. [DOI] [PubMed] [Google Scholar]
- 55.Brandt T, Dieterich M. Skew deviation with ocular torsion: a vestibular brainstem sign of topographic diagnostic value. Ann Neurol. 1993;33:528–34. doi: 10.1002/ana.410330518. [DOI] [PubMed] [Google Scholar]
- 56.Hallett M. Electrophysiological evaluation of movement disorders. In: Aminoff MJ, editor. Aminoff’s electrodiagnosis in clinical neurology. 6. Amsterdam: Elsevier; 2012. pp. 437–53. [Google Scholar]
- 57.Holmes G. The cerebellum of man. Brain. 1939;62:1–30. [Google Scholar]
- 58.Gilman S, Bloedel JR, Lechtenberg R. Disorders of the cerebellum. Philadelphia: Davis; 1981. [Google Scholar]
- 59.Samii A, Wassermann EM, Hallett M. Decreased postexercise facilitation of motor evoked potentials in patients with cerebellar degeneration. Neurology. 1997;49(2):538–42. doi: 10.1212/wnl.49.2.538. [DOI] [PubMed] [Google Scholar]
- 60.Koeppen AH. The pathogenesis of spinocerebellar ataxia. Cerebellum. 2005;4(1):62–73. doi: 10.1080/14734220510007950. [DOI] [PubMed] [Google Scholar]
- 61.Stewart TG, Holmes G. Symptomatology of cerebellar tumors: a study of forty cases. Brain. 1904;27:522–91. [Google Scholar]
- 62.Manto M. A practical approach to diagnosis and management. Cambridge: Cambridge University Press; 2010. Cerebellar disorders. [Google Scholar]
- 63.Goldstein K, Reichmann F. Beiträge zur Kasuistik und Symptomatologie der Kleinhirnerkrankungen (im besonderen zu den Störungen der Bewegungen, der Gewichts-, Raum- und Zeiteinschätzung) Arch Psychiat Nervenkr. 1916;56:466–521. [Google Scholar]
- 64.Dow RS, Moruzzi G. The physiology and pathology of the cerebellum. Minneapolis: University of Minnesota Press; 1958. [Google Scholar]
- 65.Campbell WW. DeJong’s the neurologic examination. 6. Philadelphia: Lippencott Williams & Wilkens; 2005. [Google Scholar]
- 66.Ziegler W, Wessel K. Speech timing in ataxic disorders: sentence production and rapid repetitive articulation. Neurology. 1996;47:208–14. doi: 10.1212/wnl.47.1.208. [DOI] [PubMed] [Google Scholar]
- 67.Diener HC, Dichgans J. Pathophysiology of cerebellar ataxia. Mov Disord. 1992;7:95–109. doi: 10.1002/mds.870070202. [DOI] [PubMed] [Google Scholar]
- 68.Hallett M, Bhagwan T, Shahani BT, Young RR. EMG analysis of patients with cerebellar deficits. J Neurol Neurosurg Psychiatry. 1975;38:1163–9. doi: 10.1136/jnnp.38.12.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conrad B, Brooks VB. Effects on dentate cooling on rapid alternating arm movements. J Neurophysiol. 1973;37:792–804. doi: 10.1152/jn.1974.37.4.792. [DOI] [PubMed] [Google Scholar]
- 70.Spidalieri G, Busby L, Lamarre Y. Fast ballistic arm movements triggered by visual, auditory, and somesthetic stimuli in the monkey. II. Effects of unilateral dentate lesion on discharge of precentral cortical neurons and reaction time. J Neurophysiol. 1983;50:1359–79. doi: 10.1152/jn.1983.50.6.1359. [DOI] [PubMed] [Google Scholar]
- 71.Thach WT, Perry JG, Kane SA, Goodkin HP. Cerebellar nuclei: rapid alternating movement, motor somatotopy, and a mechanism for the control of muscle synergy. Rev Neurol (Paris) 1993;149:607–28. [PubMed] [Google Scholar]
- 72.Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- 73.Deuschl G, Raethjen J, Lindemann M, Krack P. The pathophysiology of tremor. Muscle Nerve. 2001;24:716–35. doi: 10.1002/mus.1063. [DOI] [PubMed] [Google Scholar]
- 74.Flament D, Hore J. Comparison of cerebellar intention tremor under isotonic and isometric conditions. Brain Res. 1988;439:179–86. doi: 10.1016/0006-8993(88)91474-6. [DOI] [PubMed] [Google Scholar]
- 75.Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: prevalence and association with disease duration. Mov Disord. 2009;24(4):626–7. doi: 10.1002/mds.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Louis ED. The primary type of tremor in essential tremor is kinetic rather than postural: cross-sectional observation of tremor phenomenology in 369 cases. Eur J Neurol. 2013;20(4):725–7. doi: 10.1111/j.1468-1331.2012.03855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Louis ED. From neurons to neuron neighborhoods: the rewiring of the cerebellar cortex in essential tremor. Cerebellum. 2014;13(4):501–12. doi: 10.1007/s12311-013-0545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28(13):1759–61. doi: 10.1002/mds.25645. [DOI] [PubMed] [Google Scholar]
- 79.Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Rajput A, et al. Torpedoes in Parkinson’s disease, Alzheimer’s disease, essential tremor, and control brains. Mov Disord. 2009;24(11):1600–5. doi: 10.1002/mds.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leegwater-Kim J, Louis ED, Pullman SL, Floyd AG, Borden S, Moskowitz CB, et al. Intention tremor of the head in patients with essential tremor. Mov Disord. 2006;21(11):2001–5. doi: 10.1002/mds.21079. [DOI] [PubMed] [Google Scholar]
- 81.Paris-Robidas S, Brochu E, Sintes M, Emond V, Bousquet M, Vandal M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135(Pt 1):105–16. doi: 10.1093/brain/awr301. [DOI] [PubMed] [Google Scholar]
- 82.Prudente CN, Pardo CA, Xiao J, Hanfelt J, Hess EJ, Ledoux MS, et al. Neuropathology of cervical dystonia. Exp Neurol. 2013;241:95–104. doi: 10.1016/j.expneurol.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fukutani Y, Cairns NJ, Rossor MN, Lantos PL. Purkinje cell loss and astrocytosis in the cerebellum in familial and sporadic Alzheimer’s disease. Neurosci Lett. 1996;214(1):33–6. doi: 10.1016/0304-3940(96)12875-5. [DOI] [PubMed] [Google Scholar]
- 84.Castiello U. The neuroscience of grasping. Nat Rev Neurosci. 2005;6:726–36. doi: 10.1038/nrn1744. [DOI] [PubMed] [Google Scholar]
- 85.Flanagan JR, Johansson RS. Hand movements. In: Ramashandran VS, editor. Encyclopedia of the human brain. USA: Elsevier Science; 2002. pp. 399–414. [Google Scholar]
- 86.Brandauer B, Hermsdörfer J, Beck A, Aurich V, Gizewski ER, Marquardt C, et al. Impairments of prehension kinematics and grasping forces in patients with cerebellar degeneration and the relationship to cerebellar atrophy. Clin Neurophysiol. 2008;119:2528–37. doi: 10.1016/j.clinph.2008.07.280. [DOI] [PubMed] [Google Scholar]
- 87.Nowak DA, Timmann D, Hermsdörfer J. Dexterity in cerebellar agenesis. Neuropsychologia. 2007;45:696–703. doi: 10.1016/j.neuropsychologia.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 88.Nowak DA, Hermsdörfer J, Marquardt C, Fuchs HH. Grip and load force coupling during discrete vertical movements in cerebellar atrophy. Exp Brain Res. 2002;145:28–39. doi: 10.1007/s00221-002-1079-8. [DOI] [PubMed] [Google Scholar]
- 89.Rost K, Nowak DA, Timmann D, Hermsdörfer J. Preserved and impaired aspects of predictive grip force control in cerebellar subjects. Clin Neurophysiol. 2005;54:23–7. doi: 10.1016/j.clinph.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 90.Wolpert DM, Flanagan JR. Motor prediction. Curr Biol. 2001;11:R729–32. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]
- 91.Nowak DA, Topka H, Timmann D, Boecker H, Hermsdörfer J. The role of the cerebellum for predictive control of grasping. Cerebellum. 2007;6:7–17. doi: 10.1080/14734220600776379. [DOI] [PubMed] [Google Scholar]
- 92.Nowak DA, Timmann D, Hermsdörfer J. Deficits of grasping in cerebellar disorders. In: Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N, editors. Handbook of the cerebellum and cerebellar disorders. Dordrecht: Springer; 2013. pp. 1657–1667. [Google Scholar]
- 93.Marek M, Paus S, Allert N, Mädler B, Klockgether T, Urbach H, et al. Ataxia and tremor due to lesions involving cerebellar projection pathways: a DTI tractographic study in six patients. J Neurol. 2015;262(1):54–8. doi: 10.1007/s00415-014-7503-8. [DOI] [PubMed] [Google Scholar]
- 94.Fisher CM, Cole M. Homolateral ataxia and crural paresis: a vascular syndrome. J Neurol Neurosurg Psychiatry. 1965;28:48–55. doi: 10.1136/jnnp.28.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hiraga A. Ataxic hemiparesis. In: Manto M, Gruol DL, Schmahmann JD, Koibuchi N, Rossi F, editors. Handbook of the cerebellum and cerebellar disorders. Doordrecht: Springer; 2013. pp. 1669–86. [Google Scholar]
- 96.Schmahmann JD, Ko R, MacMore J. The human basis pontis: motor syndromes and topographic organization. Brain. 2004;127(Pt 6):1269–91. doi: 10.1093/brain/awh138. [DOI] [PubMed] [Google Scholar]
- 97.Grimaldi G. Cerebellar motor disorders. In: Manto M, Gruol DL, Schmahmann JD, Koibuchi N, Rossi F, editors. Handbook of the cerebellum and cerebellar disorders. Dordrecht: Springer; 2013. [Google Scholar]
- 98.Manto M, Godaux E, Jacquy J. Cerebellar hypermetria is larger when the inertial load is artificially increased. Ann Neurol. 1994;35(1):45–52. doi: 10.1002/ana.410350108. [DOI] [PubMed] [Google Scholar]
- 99.Hore J, Wild B, Diener HC. Cerebellar dysmetria at the elbow, wrist and fingers. J Neurophysiol. 1991;65:563–71. doi: 10.1152/jn.1991.65.3.563. [DOI] [PubMed] [Google Scholar]
- 100.Flament D, Hore J. Movement and electromyographic disorders associated with cerebellar dysmetria. J Neurophysiol. 1986;55(6):1221–33. doi: 10.1152/jn.1986.55.6.1221. [DOI] [PubMed] [Google Scholar]
- 101.Timmann D, Watts S, Hore J. Failure of cerebellar patients to time finger opening precisely causes ball high-low inaccuracy in overarm throws. J Neurophysiol. 1999;82(1):103–14. doi: 10.1152/jn.1999.82.1.103. [DOI] [PubMed] [Google Scholar]
- 102.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119(Pt 4):1183–98. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- 103.Schoch B, Dimitrova A, Gizewski ER, Timmann D. Functional localization in the human cerebellum based on voxelwise statistical analysis: a study of 90 patients. Neuroimage. 2006;30(1):36–51. doi: 10.1016/j.neuroimage.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 104.Brunamonti E, Chiricozzi FR, Clausi S, Olivito G, Giusti MA, Molinari M, et al. Cerebellar damage impairs executive control and monitoring of movement generation. PLoS One. 2014;9(1):e85997. doi: 10.1371/journal.pone.0085997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhanpuri NH, Okamura AM, Bastian AJ. Predicting and correcting ataxia using a model of cerebellar function. Brain. 2014;137(Pt 7):1931–44. doi: 10.1093/brain/awu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown SH, Kessler KR, Hefter H, Cooke JD, Freund HJ. Role of the cerebellum in visuomotor coordination. I. Delayed eye and arm initiation in patients with mild cerebellar ataxia. Exp Brain Res. 1993;94(3):478–88. doi: 10.1007/BF00230206. [DOI] [PubMed] [Google Scholar]
- 107.Manto M, Van Den Braber N, Grimaldi G, Lammertse P. A new myohaptic instrument to assess wrist motion dynamically. Sensors. 2010;10:3180–94. doi: 10.3390/s100403180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Topka H, Konczak J, Schneider K, Boose A, Dichgans J. Multijoint arm movements in cerebellar ataxia: abnormal control of movement dynamics. Exp Brain Res. 1998;119(4):493–503. doi: 10.1007/s002210050365. [DOI] [PubMed] [Google Scholar]
- 109.Molinari M, Leggio M. Cerebellar sequencing for cognitive processing. In: Manto M, Gruol DL, Schmahmann JD, Koibuchi N, Rossi F, editors. Handbook of the cerebellum and cerebellar disorders. Doordrecht: Springer; 2013. pp. 1701–15. [Google Scholar]
- 110.Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 2001;13(2):55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003;126:2312–22. doi: 10.1093/brain/awg230. [DOI] [PubMed] [Google Scholar]
- 112.Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272(5261):545–7. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- 113.Hartmann MJ, Bower JM. Tactile responses in the granule cell layer of cerebellar folium crus IIa of freely behaving rats. J Neurosci. 2001;21(10):3549–63. doi: 10.1523/JNEUROSCI.21-10-03549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Angel RW. Barognosis in a patient with hemiataxia. Ann Neurol. 1980;7:73–7. doi: 10.1002/ana.410070113. [DOI] [PubMed] [Google Scholar]
- 115.Bhanpuri NH, Okamura AM, Bastian AJ. Active force perception depends on cerebellar function. J Neurophysiol. 2012;107:1612–20. doi: 10.1152/jn.00983.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhanpuri NH, Okamura AM, Bastian AJ. Predictive modeling by the cerebellum improves proprioception. J Neurosci. 2013;33(36):14301–6. doi: 10.1523/JNEUROSCI.0784-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grill SE, Hallett M, Marcus C, McShane L. Disturbances of kinaesthesia in patients with cerebellar disorders. Brain. 1994;117(Pt 6):1433–47. doi: 10.1093/brain/117.6.1433. [DOI] [PubMed] [Google Scholar]
- 118.Palliyath S, Hallett M, Thomas SL, Lebiedowska MK. Gait in patients with cerebellar ataxia. Mov Disord. 1998;13:958–64. doi: 10.1002/mds.870130616. [DOI] [PubMed] [Google Scholar]
- 119.Mitoma H, Hayashi R, Yanagisawa N, Tsukagoshi H. Characteristics of parkinsonian and ataxic gaits: a study using surface electromyograms, angular displacements and floor reaction forces. J Neurol Sci. 2000;174:22–39. doi: 10.1016/s0022-510x(99)00329-9. [DOI] [PubMed] [Google Scholar]
- 120.Stolze H, Klebe S, Petersen G, Raethjen J, Wenzelburger R, Witt K, et al. Typical features of cerebellar ataxic gait. J Neurol Neurosurg Psychiatry. 2002;73:310–2. doi: 10.1136/jnnp.73.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morton SM, Bastian AJ. Relative contributions of balance and voluntary leg-coordination deficits to cerebellar gait ataxia. J Neurophysiol. 2003;89:1844–56. doi: 10.1152/jn.00787.2002. [DOI] [PubMed] [Google Scholar]
- 122.Earhart GM, Bastian AJ. Selection and coordination of human locomotor forms following cerebellar damage. J Neurophysiol. 2001;85:759–69. doi: 10.1152/jn.2001.85.2.759. [DOI] [PubMed] [Google Scholar]
- 123.Serrao M, Pierelli F, Ranavolo A, Draicchio F, Conte C, Don R, et al. Gait pattern in inherited cerebellar ataxias. Cerebellum. 2012;11:194–211. doi: 10.1007/s12311-011-0296-8. [DOI] [PubMed] [Google Scholar]
- 124.Timmann D, Horak FB. Perturbed step initiation in cerebellar subjects. 1. Modifications of postural responses. Exp Brain Res. 1998;119:73–84. doi: 10.1007/s002210050321. [DOI] [PubMed] [Google Scholar]
- 125.Serrao M, Conte C, Casali C, Ranavolo A, Mari S, Di Fabio R, et al. Reply to comment “Why do patients with cerebellar ataxia not use environmental cues for reducing unpredictability of sudden gait stopping?” on “Sudden stopping in patients with cerebellar ataxia”. Cerebellum. 2013;12:958–9. doi: 10.1007/s12311-013-0501-z. [DOI] [PubMed] [Google Scholar]
- 126.Serrao M, Conte C, Casali C, Ranavolo A, Mari S, Di Fabio R, et al. Sudden stopping in patients with cerebellar ataxia. Cerebellum. 2013;12:607–16. doi: 10.1007/s12311-013-0467-x. [DOI] [PubMed] [Google Scholar]
- 127.Serrao M, Mari S, Conte C, Ranavolo A, Casali C, Draicchio F, et al. Strategies adopted by cerebellar ataxia patients to perform U-turns. Cerebellum. 2013;12:460–8. doi: 10.1007/s12311-012-0441-z. [DOI] [PubMed] [Google Scholar]
- 128.Conte C, Serrao M, Casali C, Ranavolo A, Mari S, Draicchio F, et al. Planned gait termination in cerebellar ataxias. Cerebellum. 2012;11:896–904. doi: 10.1007/s12311-011-0348-0. [DOI] [PubMed] [Google Scholar]
- 129.Mari S, Serrao M, Casali C, Conte C, Ranavolo A, Padua L, et al. Turning strategies in patients with cerebellar ataxia. Exp Brain Res. 2012;222:65–75. doi: 10.1007/s00221-012-3197-2. [DOI] [PubMed] [Google Scholar]
- 130.van de Warrenburg BP, Steijns JA, Munneke M, Kremer BP, Bloem BR. Falls in degenerative cerebellar ataxias. Mov Disord. 2005;20:497–500. doi: 10.1002/mds.20375. [DOI] [PubMed] [Google Scholar]
- 131.Bastian AJ, Zackowski KM, Thach WT. Cerebellar ataxia: torque deficiency or torque mismatch between joints? J Neurophysiol. 2000;83:3019–30. doi: 10.1152/jn.2000.83.5.3019. [DOI] [PubMed] [Google Scholar]
- 132.Ilg W, Golla H, Thier P, Giese MA. Specific influences of cerebellar dysfunctions on gait. Brain. 2007;130:786–8. doi: 10.1093/brain/awl376. [DOI] [PubMed] [Google Scholar]
- 133.Martino G, Ivanenko YP, Serrao M, Ranavolo A, d’Avella A, Draicchio F, et al. Locomotor patterns in cerebellar ataxia. J Neurophysiol. 2014;112:2810–21. doi: 10.1152/jn.00275.2014. [DOI] [PubMed] [Google Scholar]
- 134.Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–42. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- 135.Morton SM, Bastian AJ. Mechanisms of cerebellar gait ataxia. Cerebellum. 2007;6:79–86. doi: 10.1080/14734220601187741. [DOI] [PubMed] [Google Scholar]
- 136.Hausdorff JM. Stride variability: beyond length and frequency. Gait Posture. 2004;20:304. doi: 10.1016/j.gaitpost.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 137.Schniepp R, Wuehr M, Schlick C, Huth S, Pradhan C, Dieterich M, et al. Increased gait variability is associated with the history of falls in patients with cerebellar ataxia. J Neurol. 2014;261:213–23. doi: 10.1007/s00415-013-7189-3. [DOI] [PubMed] [Google Scholar]
- 138.Mari S, Serrao M, Casali C, Conte C, Martino G, Ranavolo A, et al. Lower limb antagonist muscle co-activation and its relationship with gait parameters in cerebellar ataxia. Cerebellum. 2014;13:226–36. doi: 10.1007/s12311-013-0533-4. [DOI] [PubMed] [Google Scholar]
- 139.Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist. 2004;10:247–59. doi: 10.1177/1073858404263517. [DOI] [PubMed] [Google Scholar]
- 140.Van de Warrenburg BP, Bakker M, Kremer BP, Bloem BR, Allum JH. Trunk sway in patients with spinocerebellar ataxia. Mov Disord. 2005;20:1006–13. doi: 10.1002/mds.20486. [DOI] [PubMed] [Google Scholar]
- 141.Conte C, Pierelli F, Casali C, Ranavolo A, Draicchio F, Martino G, et al. Upper body kinematics in patients with cerebellar ataxia. Cerebellum. 2014;13(6):689–97. doi: 10.1007/s12311-014-0586-z. [DOI] [PubMed] [Google Scholar]
- 142.Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–98. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 143.Schmahmann JD, Pandya DN. The cerebrocerebellar system. In: Schmahmann JD, editor. The cerebellum and cognition. Vol. 41. San Diego: Academic Press Int Rev Neurobiol; 1997. pp. 31–60. [DOI] [PubMed] [Google Scholar]
- 144.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59(2):1560–70. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moulton EA, Elman I, Pendse G, Schmahmann JD, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci. 2011;31(10):3795–804. doi: 10.1523/JNEUROSCI.6709-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 147.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 148.Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumor resection in children: cerebellar cognitive affective syndrome in a pediatric population. Brain. 2000;123:1041–50. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- 149.Tedesco AM, Chiricozzi FR, Clausi S, Lupo M, Molinari M, Leggio MG. The cerebellar cognitive profile. Brain. 2011;134(Pt 12):3672–86. doi: 10.1093/brain/awr266. [DOI] [PubMed] [Google Scholar]
- 150.Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum. 2007;6:254–67. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 151.Brossard-Racine M, du Plessis AJ, Limperopoulos C. Developmental cerebellar cognitive affective syndrome in expreterm survivors following cerebellar injury. Cerebellum. 2015;14(2):151–64. doi: 10.1007/s12311-014-0597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gottwald B, Wilde B, Mihajlovic Z, Mehdorn HM. Evidence for distinct cognitive deficits after focal cerebellar lesions. J Neurol Neurosurg Psychiatry. 2004;74:1524–31. doi: 10.1136/jnnp.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baillieux H, De Smet HJ, Dobbeleir A, Paquier PF, De Deyn PP, Mariën P. Cognitive and affective disturbances following focal cerebellar damage in adults: a neuropsychological and SPECT study. Cortex. 2010;46:869–79. doi: 10.1016/j.cortex.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 154.Thompson RF, Bao S, Chen L, Cipriano BD, Grethe JS, Kim JJ. Associative learning. In: Schmahmann JD, editor. The cerebellum and cognition. San Diego: Academic; 1997. pp. 151–89. [DOI] [PubMed] [Google Scholar]
- 155.Leggio MG, Silveri MC, Petrosini L, Molinari M. Phonological grouping is specifically affected in cerebellar patients: a verbal fluency study. J Neurol Neurosurg Psychiatry. 2000;69(1):102–6. doi: 10.1136/jnnp.69.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stoodley CJ, Schmahmann JD. The cerebellum and language: evidence from patients with patients with cerebellar degeneration. Brain Lang. 2009;110:149–53. doi: 10.1016/j.bandl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 157.Guëll X, Hoche F, Schmahmann JD. Metalinguistic deficits in patients with cerebellar dysfunction: empirical support for the dysmetria of thought theory. Cerebellum. 2015;14(1):50–8. doi: 10.1007/s12311-014-0630-z. [DOI] [PubMed] [Google Scholar]
- 158.Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery. 1995;37:885–93. doi: 10.1227/00006123-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 159.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–44. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Parvizi J, Joseph J, Press DZ, Schmahmann JD. Pathological laughter and crying in patients with multiple system atrophycerebellar type. Mov Disord. 2007;22:798–803. doi: 10.1002/mds.21348. [DOI] [PubMed] [Google Scholar]
- 161.Sokolovsky N, Cook A, Hunt H, Giunti P, Cipolotti L. A preliminary characterisation of cognition and social cognition in spinocerebellar ataxia types 2, 1, and 7. Behav Neurol. 2010;23(1–2):17–29. doi: 10.3233/BEN-2010-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hoche F, Harding JA, Vangel M, Schmahmann JD. Soc Neurosci Abstracts. 2014. The cerebellar contribution to social cognition; p. 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20(3):236–60. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- 164.Darley FL, Aronson AE, Brown JR. Motor speech disorders. Philadelphia: Saunders; 1975. [Google Scholar]
- 165.Lechtenberg R, Gilman S. Speech disorders in cerebellar disease. Ann Neurol. 1978;3:285–90. doi: 10.1002/ana.410030402. [DOI] [PubMed] [Google Scholar]
- 166.Ackermann H, Mathiak K, Riecker A. The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum. 2007;6:202–13. doi: 10.1080/14734220701266742. [DOI] [PubMed] [Google Scholar]
- 167.Ackermann H, Vogel M, Petersen D, Poremba M. Speech deficits in ischaemic cerebellar lesions. J Neurol. 1992;239:223–7. doi: 10.1007/BF00839144. [DOI] [PubMed] [Google Scholar]
- 168.Urban PP, Marx J, Hunsche S, Gawehn J, Vucurevic G, Wicht S, et al. Cerebellar speech representation: lesion topography in dysarthria as derived from cerebellar ischemia and functional magnetic resonance imaging. Arch Neurol. 2003;60:965–72. doi: 10.1001/archneur.60.7.965. [DOI] [PubMed] [Google Scholar]
- 169.Urban PP. Speech motor deficits in cerebellar infarctions. Brain Lang. 2013;127:323–6. doi: 10.1016/j.bandl.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 170.Ackermann H. Cerebellar contributions to speech production and speech perception: psycholinguistic and neurobiological perspectives. Trends Neurosci. 2008;31:265–72. doi: 10.1016/j.tins.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 171.De Smet HJ, Baillieux H, Catsman-Berrevoets C, De Deyn PP, Mariën P, Paquier PF. Postoperative motor speech production in children with the syndrome of ‘cerebellar’ mutism and subsequent dysarthria: a critical review of the literature. Eur J Paediatr Neurol. 2007;11:193–207. doi: 10.1016/j.ejpn.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 172.Mariën P, Verhoeven J, Engelborghs S, Rooker S, Pickut BA, De Deyn PP. A role for the cerebellum in motor speech planning: evidence from foreign accent syndrome. Clin Neurol Neurosurg. 2006;108:518–22. doi: 10.1016/j.clineuro.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 173.Mariën P, Saerens J, Nanhoe R, Moens E, Nagels G, Pickut B, et al. Cerebellar induced aphasia: case report of cerebellar induced prefrontal aphasic language phenomena supported by SPECT findings. J Neurol Sci. 1996;144:34–43. doi: 10.1016/s0022-510x(96)00059-7. [DOI] [PubMed] [Google Scholar]
- 174.Adamaszek M, Strecker K, Kessler C. Impact of cerebellar lesion on syntactic processing evidenced by event-related potentials. Neurosci Lett. 2012;12(2):78–82. doi: 10.1016/j.neulet.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 175.Mariën P, Engelborghs S, Fabbro F, De Deyn PP. The lateralized linguistic cerebellum: a review and a new hypothesis. Brain Lang. 2001;79:580–600. doi: 10.1006/brln.2001.2569. [DOI] [PubMed] [Google Scholar]
- 176.Moretti R, Bava A, Torre P, Antonello RM, Cazzato G. Reading errors in patients with cerebellar vermis lesions. J Neurol. 2002;249:461–8. doi: 10.1007/s004150200040. [DOI] [PubMed] [Google Scholar]
- 177.Moretti R, Torre P, Antonello RM, Carraro N, Zambito-Marsala S, Ukmar MJ, et al. Peculiar aspects of reading and writing performances in patients with olivopontocerebellar atrophy. Percept Mot Skills. 2002;94:677–94. doi: 10.2466/pms.2002.94.2.677. [DOI] [PubMed] [Google Scholar]
- 178.Mariën P, Baillieux H, De Smet HJ, Engelborghs S, Wilssens I, Paquier P, et al. Cognitive, linguistic and affective disturbances following a right superior cerebellar artery infarction: a case study. Cortex. 2009;45:527–36. doi: 10.1016/j.cortex.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 179.De Smet HJ, Engelborghs S, Paquier PF, De Deyn PP, Mariën P. Cerebellar-induced apraxic agraphia: a review and three new cases. Brain Cogn. 2011;76(3):424–34. doi: 10.1016/j.bandc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 180.Manto M, Mariën P. Schmahmann’s syndrome—identification of the third cornerstone of clinical ataxiology. Cerebellum Ataxias. 2015;2:2. doi: 10.1186/s40673-015-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Timmann D, Konczak J, Ilg W, Donchin O, Hermsdörfer J, Gizewski ER, et al. Current advances in lesion-symptom mapping of the human cerebellum. Neuroscience. 2009;162:836–51. doi: 10.1016/j.neuroscience.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 182.Timmann D, Brandauer B, Hermsdörfer J, Ilg W, Konczak J, Gerwig M, et al. Lesion-symptom mapping of the human cerebellum. Cerebellum. 2008;7:602–6. doi: 10.1007/s12311-008-0066-4. [DOI] [PubMed] [Google Scholar]
- 183.Timmann D, Küper M, Gizewski ER, Schoch B, Donchin O. Lesion-symptom mapping of the human cerebellum. In: Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N, editors. Handbook of the cerebellum and cerebellar disorders. Dordrecht: Springer; 2013. pp. 1627–1656. [Google Scholar]
- 184.Schmahmann JD, Doyon J, Toga A, Petrides M, Evans A. MRI Atlas of the human cerebellum. San Diego: Academic; 2000. [DOI] [PubMed] [Google Scholar]
- 185.Schmahmann JD, Macmore J, Vangel M. Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience. 2009;162:852–61. doi: 10.1016/j.neuroscience.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]