Abstract
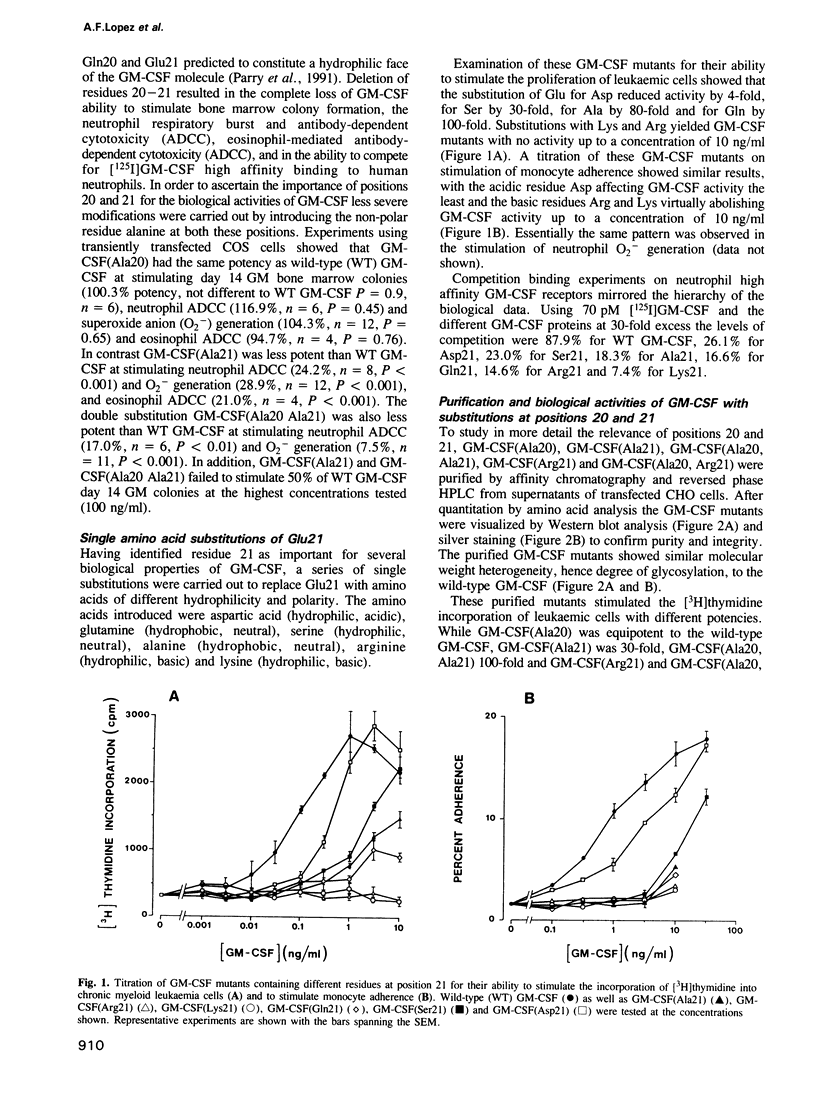
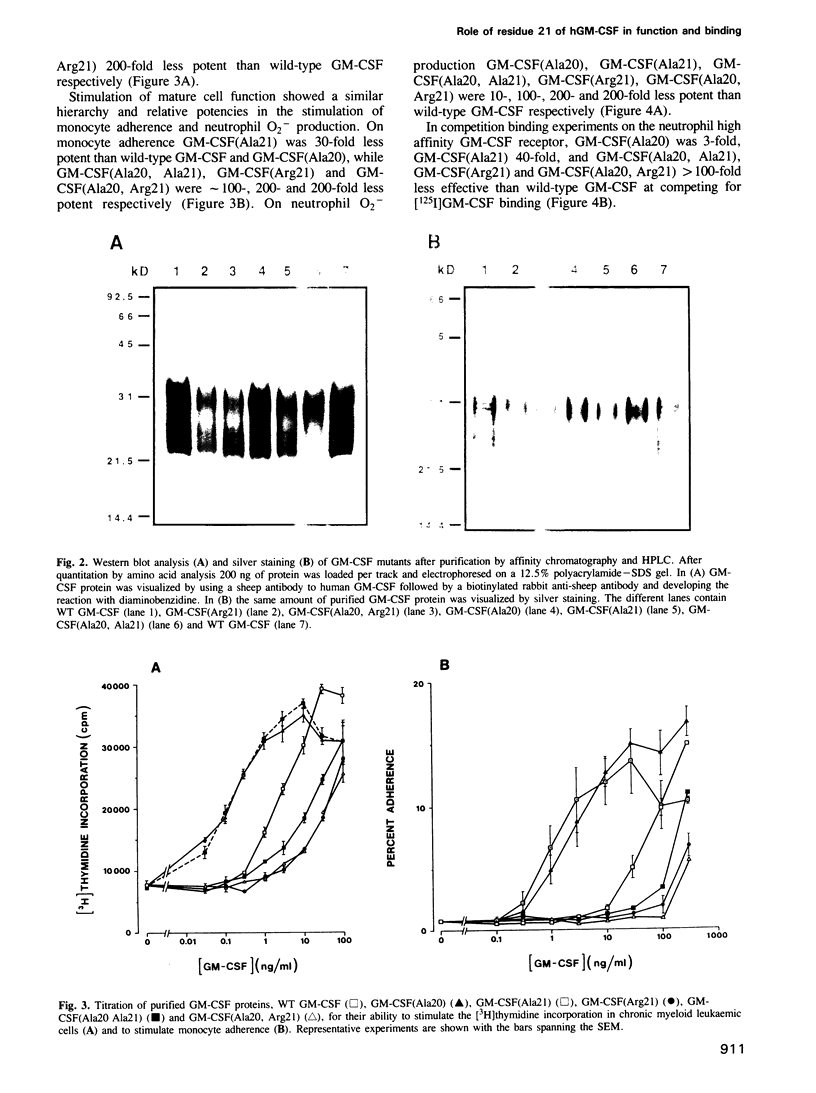
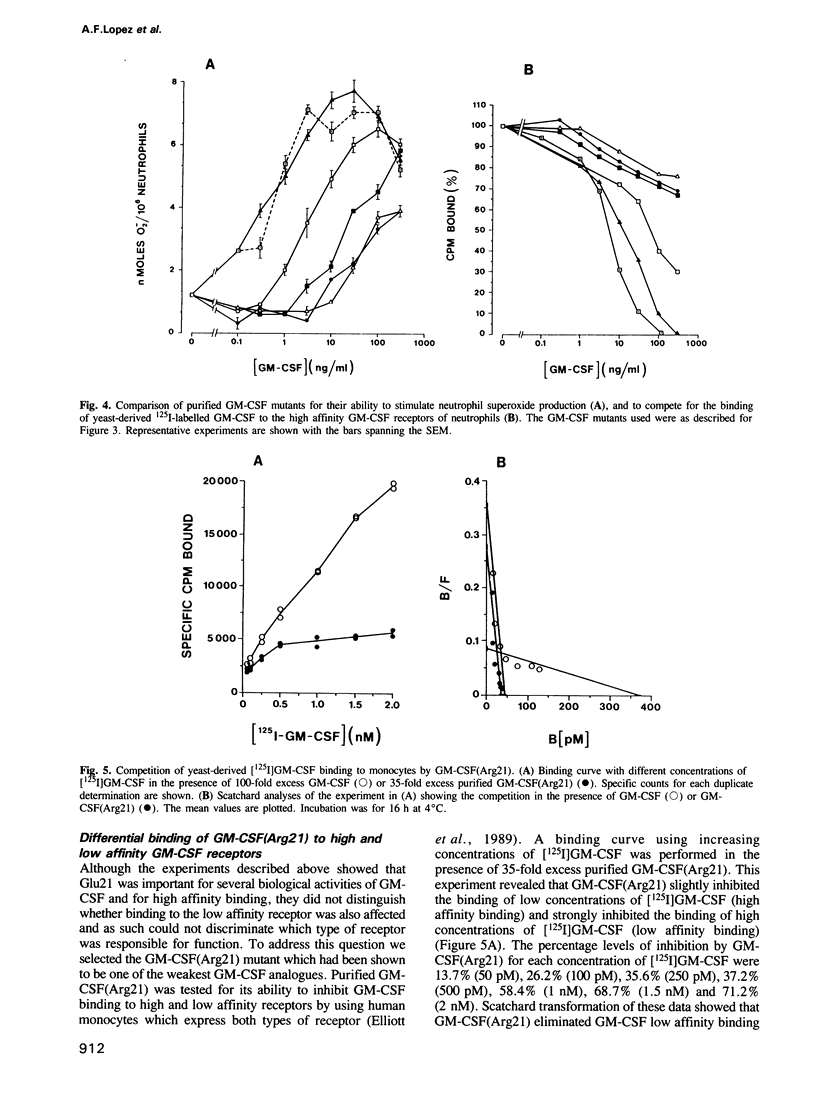
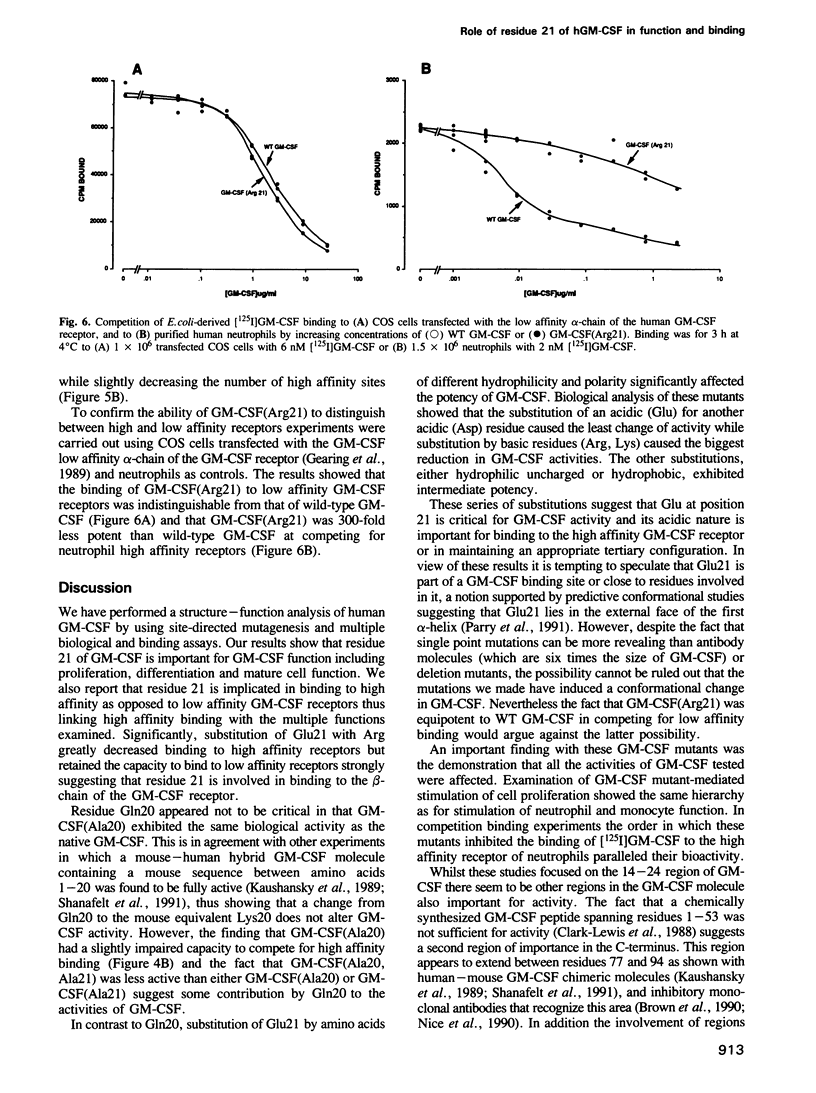
The functional role of the predicted first alpha-helix of human granulocyte-macrophage colony-stimulating factor (GM-CSF) was analysed by site-directed mutagenesis and multiple biological and receptor binding assays. Initial deletion mutagenesis pointed to residues 20 and 21 being critical. Substitution mutagenesis showed that by altering Gln20 to Ala full GM-CSF activity was retained but that by altering Glu21 for Ala GM-CSF activity and high affinity receptor binding were decreased. Substitution of different amino acids for Glu21 showed that there was a hierarchy in the ability to stimulate the various biological activities of GM-CSF with the order of potency being Asp21 greater than Ser21 greater than Ala21 greater than Gln21 greater than Lys21 = Arg21. To distinguish whether position 21 was important for GM-CSF binding to high or low affinity receptors, GM-CSF (Arg21) was used as a competitor for [125I]GM-CSF binding to monocytes that express both types of receptor. GM-CSF (Arg21) exhibited a greatly reduced capacity to compete for binding to high affinity receptors, however, it competed fully for [125I]GM-CSF binding to low affinity receptors. Furthermore, GM-CSF (Arg21) was equipotent with wild-type GM-CSF in binding to the cloned low affinity alpha-chain of the GM-CSF receptor. These results show that (i) this position is critical for high affinity but not for low affinity GM-CSF receptor binding thus defining two functional parts of the GM-CSF molecule; (ii) position 21 of GM-CSF is critical for multiple functions of GM-CSF; and (iii) stimulation of proliferation and mature cell function by GM-CSF are mediated through high affinity receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antman K. S., Griffin J. D., Elias A., Socinski M. A., Ryan L., Cannistra S. A., Oette D., Whitley M., Frei E., 3rd, Schnipper L. E. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on chemotherapy-induced myelosuppression. N Engl J Med. 1988 Sep 8;319(10):593–598. doi: 10.1056/NEJM198809083191001. [DOI] [PubMed] [Google Scholar]
- Baldwin G. C., Gasson J. C., Kaufman S. E., Quan S. G., Williams R. E., Avalos B. R., Gazdar A. F., Golde D. W., DiPersio J. F. Nonhematopoietic tumor cells express functional GM-CSF receptors. Blood. 1989 Mar;73(4):1033–1037. [PubMed] [Google Scholar]
- Baldwin G. C., Gasson J. C., Quan S. G., Fleischmann J., Weisbart R., Oette D., Mitsuyasu R. T., Golde D. W. Granulocyte-macrophage colony-stimulating factor enhances neutrophil function in acquired immunodeficiency syndrome patients. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2763–2766. doi: 10.1073/pnas.85.8.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Haemopoietic receptors and helical cytokines. Immunol Today. 1990 Oct;11(10):350–354. doi: 10.1016/0167-5699(90)90139-z. [DOI] [PubMed] [Google Scholar]
- Berdel W. E., Danhauser-Riedl S., Steinhauser G., Winton E. F. Various human hematopoietic growth factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood. 1989 Jan;73(1):80–83. [PubMed] [Google Scholar]
- Brown C. B., Hart C. E., Curtis D. M., Bailey M. C., Kaushansky K. Two neutralizing monoclonal antibodies against human granulocyte-macrophage colony-stimulating factor recognize the receptor binding domain of the molecule. J Immunol. 1990 Mar 15;144(6):2184–2189. [PubMed] [Google Scholar]
- Cebon J., Dempsey P., Fox R., Kannourakis G., Bonnem E., Burgess A. W., Morstyn G. Pharmacokinetics of human granulocyte-macrophage colony-stimulating factor using a sensitive immunoassay. Blood. 1988 Oct;72(4):1340–1347. [PubMed] [Google Scholar]
- Cebon J., Nicola N., Ward M., Gardner I., Dempsey P., Layton J., Dührsen U., Burgess A. W., Nice E., Morstyn G. Granulocyte-macrophage colony stimulating factor from human lymphocytes. The effect of glycosylation on receptor binding and biological activity. J Biol Chem. 1990 Mar 15;265(8):4483–4491. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I., Lopez A. F., To L. B., Vadas M. A., Schrader J. W., Hood L. E., Kent S. B. Structure-function studies of human granulocyte-macrophage colony-stimulating factor. Identification of residues required for activity. J Immunol. 1988 Aug 1;141(3):881–889. [PubMed] [Google Scholar]
- Contreras M. A., Bale W. F., Spar I. L. Iodine monochloride (IC1) iodination techniques. Methods Enzymol. 1983;92:277–292. [PubMed] [Google Scholar]
- Dedhar S., Gaboury L., Galloway P., Eaves C. Human granulocyte-macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9253–9257. doi: 10.1073/pnas.85.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. J., Vadas M. A., Cleland L. G., Gamble J. R., Lopez A. F. IL-3 and granulocyte-macrophage colony-stimulating factor stimulate two distinct phases of adhesion in human monocytes. J Immunol. 1990 Jul 1;145(1):167–176. [PubMed] [Google Scholar]
- Elliott M. J., Vadas M. A., Eglinton J. M., Park L. S., To L. B., Cleland L. G., Clark S. C., Lopez A. F. Recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor show common biological effects and binding characteristics on human monocytes. Blood. 1989 Nov 15;74(7):2349–2359. [PubMed] [Google Scholar]
- Gamble J. R., Elliott M. J., Jaipargas E., Lopez A. F., Vadas M. A. Regulation of human monocyte adherence by granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7169–7173. doi: 10.1073/pnas.86.18.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. R., Rand T. H., Lopez A. F., Clark-Lewis I., Vadas M. A. Heterogeneity of recombinant granulocyte-macrophage colony-stimulating factor-mediated enhancement of neutrophil adherence to endothelium. Exp Hematol. 1990 Sep;18(8):897–902. [PubMed] [Google Scholar]
- Gasson J. C., Kaufman S. E., Weisbart R. H., Tomonaga M., Golde D. W. High-affinity binding of granulocyte-macrophage colony-stimulating factor to normal and leukemic human myeloid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):669–673. doi: 10.1073/pnas.83.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson J. C., Weisbart R. H., Kaufman S. E., Clark S. C., Hewick R. M., Wong G. G., Golde D. W. Purified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophils. Science. 1984 Dec 14;226(4680):1339–1342. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Metcalf D., Gough J., Grail D., Dunn A. R. Structure and expression of the mRNA for murine granulocyte-macrophage colony stimulating factor. EMBO J. 1985 Mar;4(3):645–653. doi: 10.1002/j.1460-2075.1985.tb03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Groopman J. E., Mitsuyasu R. T., DeLeo M. J., Oette D. H., Golde D. W. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on myelopoiesis in the acquired immunodeficiency syndrome. N Engl J Med. 1987 Sep 3;317(10):593–598. doi: 10.1056/NEJM198709033171003. [DOI] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K., Shoemaker S. G., Alfaro S., Brown C. Hematopoietic activity of granulocyte/macrophage colony-stimulating factor is dependent upon two distinct regions of the molecule: functional analysis based upon the activities of interspecies hybrid growth factors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1213–1217. doi: 10.1073/pnas.86.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Hayashida K., Sakamaki K., Yokota T., Arai K., Miyajima A. Reconstitution of functional receptors for human granulocyte/macrophage colony-stimulating factor (GM-CSF): evidence that the protein encoded by the AIC2B cDNA is a subunit of the murine GM-CSF receptor. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5082–5086. doi: 10.1073/pnas.88.12.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., O'Brien W. A., Zhao J. Q., Golde D. W., Gasson J. C., Chen I. S. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988 Sep 23;241(4873):1673–1675. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Dyson P. G., To L. B., Elliott M. J., Milton S. E., Russell J. A., Juttner C. A., Yang Y. C., Clark S. C., Vadas M. A. Recombinant human interleukin-3 stimulation of hematopoiesis in humans: loss of responsiveness with differentiation in the neutrophilic myeloid series. Blood. 1988 Nov;72(5):1797–1804. [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Lyons A. B., Tapley P. M., To L. B., Park L. S., Clark S. C., Vadas M. A. Human interleukin-3 inhibits the binding of granulocyte-macrophage colony-stimulating factor and interleukin-5 to basophils and strongly enhances their functional activity. J Cell Physiol. 1990 Oct;145(1):69–77. doi: 10.1002/jcp.1041450111. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Vadas M. A., Woodcock J. M., Milton S. E., Lewis A., Elliott M. J., Gillis D., Ireland R., Olwell E., Park L. S. Interleukin-5, interleukin-3, and granulocyte-macrophage colony-stimulating factor cross-compete for binding to cell surface receptors on human eosinophils. J Biol Chem. 1991 Dec 25;266(36):24741–24747. [PubMed] [Google Scholar]
- Lopez A. F., Williamson D. J., Gamble J. R., Begley C. G., Harlan J. M., Klebanoff S. J., Waltersdorph A., Wong G., Clark S. C., Vadas M. A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986 Nov;78(5):1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Vadas M. A., Lopez A. F., Williamson D. J., Wong G. G., Clark S. C., Wang E. A. Biologic properties in vitro of a recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1986 Jan;67(1):37–45. [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A., Gearing D. P., Gough N. M. Low-affinity placenta-derived receptors for human granulocyte-macrophage colony-stimulating factor can deliver a proliferative signal to murine hemopoietic cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4670–4674. doi: 10.1073/pnas.87.12.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nice E., Dempsey P., Layton J., Morstyn G., Cui D. F., Simpson R., Fabri L., Burgess A. Human granulocyte-macrophage colony-stimulating factor (hGM-CSF): identification of a binding site for a neutralizing antibody. Growth Factors. 1990;3(2):159–169. doi: 10.3109/08977199009108278. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Minasian E., Leach S. J. Conformational homologies among cytokines: interleukins and colony stimulating factors. J Mol Recognit. 1988 Jun;1(3):107–110. doi: 10.1002/jmr.300010302. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Minasian E., Leach S. J. Cytokine conformations: predictive studies. J Mol Recognit. 1991 Mar-Jun;4(2-3):63–75. doi: 10.1002/jmr.300040205. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanafelt A. B., Johnson K. E., Kastelein R. A. Identification of critical amino acid residues in human and mouse granulocyte-macrophage colony-stimulating factor and their involvement in species specificity. J Biol Chem. 1991 Jul 25;266(21):13804–13810. [PubMed] [Google Scholar]
- Sieff C. A., Emerson S. G., Donahue R. E., Nathan D. G., Wang E. A., Wong G. G., Clark S. C. Human recombinant granulocyte-macrophage colony-stimulating factor: a multilineage hematopoietin. Science. 1985 Dec 6;230(4730):1171–1173. doi: 10.1126/science.3877981. [DOI] [PubMed] [Google Scholar]
- Tavernier J., Devos R., Cornelis S., Tuypens T., Van der Heyden J., Fiers W., Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991 Sep 20;66(6):1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadas M. A., Nicola N. A., Metcalf D. Activation of antibody-dependent cell-mediated cytotoxicity of human neutrophils and eosinophils by separate colony-stimulating factors. J Immunol. 1983 Feb;130(2):795–799. [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]




