Abstract
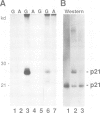
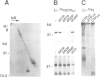
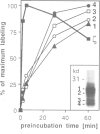
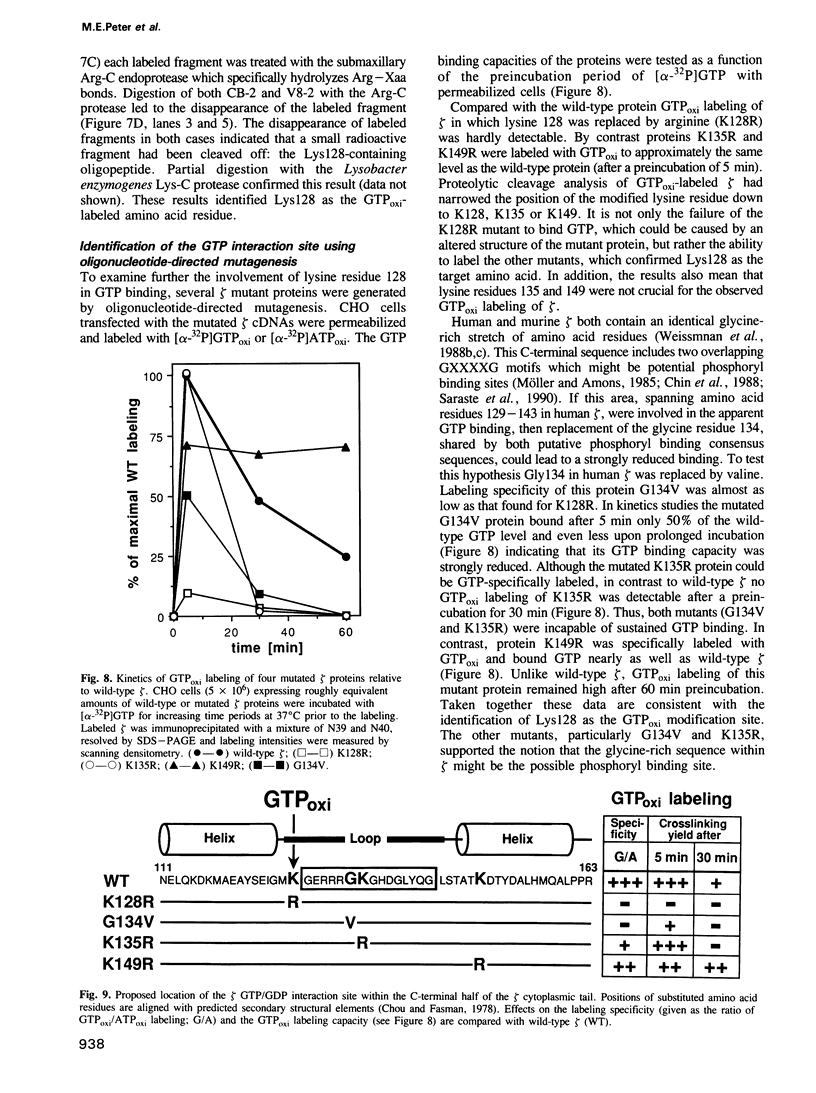
In a search for nucleotide binding proteins associated with the T-cell receptor (TCR)-CD3 complex, a novel labeling technique involving introduction of [alpha-32P]GTP or [alpha-32P]ATP into permeabilized cells followed by in situ periodate oxidation was developed. To test the method we first demonstrated that p21ras and other classical GTP binding proteins could be labeled in a GTP-specific manner. In human T lymphocytes the TCR zeta chain was found to be specifically labeled by GTPoxi but not by ATPoxi or CTPoxi. Labeling kinetics and competition experiments demonstrated that zeta had a capacity to bind GTP and GDP but not GMP or ATP. Proteolytic cleavage experiments identified lysine 128 as the GTP crosslinking site. This result was confirmed by studies using oligonucleotide-directed mutagenesis. Lysine residues 128, 135 and 149 were each replaced by arginine and glycine 134 by valine and mutated proteins were expressed in CHO cells. Labeling of mutants K128R and G134V was abrogated whereas mutant proteins K135R and K148R could still be specifically crosslinked to GTP. We conclude that Lys128 and Gly134 are part of a GTP/GDP binding site suggesting that zeta is a unique GTP/GDP binding structure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcon B., Berkhout B., Breitmeyer J., Terhorst C. Assembly of the human T cell receptor-CD3 complex takes place in the endoplasmic reticulum and involves intermediary complexes between the CD3-gamma.delta.epsilon core and single T cell receptor alpha or beta chains. J Biol Chem. 1988 Feb 25;263(6):2953–2961. [PubMed] [Google Scholar]
- Baniyash M., Garcia-Morales P., Bonifacino J. S., Samelson L. E., Klausner R. D. Disulfide linkage of the zeta and eta chains of the T cell receptor. Possible identification of two structural classes of receptors. J Biol Chem. 1988 Jul 15;263(20):9874–9878. [PubMed] [Google Scholar]
- Baniyash M., Garcia-Morales P., Luong E., Samelson L. E., Klausner R. D. The T cell antigen receptor zeta chain is tyrosine phosphorylated upon activation. J Biol Chem. 1988 Dec 5;263(34):18225–18230. [PubMed] [Google Scholar]
- Büttcher V., Rühlmann A., Cramer F. Improved single-stranded DNA producing expression vectors for protein manipulation in Escherichia coli. Nucleic Acids Res. 1990 Feb 25;18(4):1075–1075. doi: 10.1093/nar/18.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. T., Goff S. A., Webster T., Smith T., Goldberg A. L. Sequence of the lon gene in Escherichia coli. A heat-shock gene which encodes the ATP-dependent protease La. J Biol Chem. 1988 Aug 25;263(24):11718–11728. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Clevers H., Dunlap S., Terhorst C. The transmembrane orientation of the epsilon chain of the TcR/CD3 complex. Eur J Immunol. 1988 May;18(5):705–710. doi: 10.1002/eji.1830180508. [DOI] [PubMed] [Google Scholar]
- DYER J. R. Use of periodate oxidations in biochemical analysis. Methods Biochem Anal. 1956;3:111–152. doi: 10.1002/9780470110195.ch5. [DOI] [PubMed] [Google Scholar]
- Der C. J., Finkel T., Cooper G. M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986 Jan 17;44(1):167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Frank S. J., Niklinska B. B., Orloff D. G., Merćep M., Ashwell J. D., Klausner R. D. Structural mutations of the T cell receptor zeta chain and its role in T cell activation. Science. 1990 Jul 13;249(4965):174–177. doi: 10.1126/science.2371564. [DOI] [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Hall C., Berkhout B., Alarcon B., Sancho J., Wileman T., Terhorst C. Requirements for cell surface expression of the human TCR/CD3 complex in non-T cells. Int Immunol. 1991 Apr;3(4):359–368. doi: 10.1093/intimm/3.4.359. [DOI] [PubMed] [Google Scholar]
- Hol W. G. The role of the alpha-helix dipole in protein function and structure. Prog Biophys Mol Biol. 1985;45(3):149–195. doi: 10.1016/0079-6107(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Jin Y. J., Clayton L. K., Howard F. D., Koyasu S., Sieh M., Steinbrich R., Tarr G. E., Reinherz E. L. Molecular cloning of the CD3 eta subunit identifies a CD3 zeta-related product in thymus-derived cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3319–3323. doi: 10.1073/pnas.87.9.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Klaus G. G. Role of G-proteins in receptor signalling in T and B cells. Semin Immunol. 1990 Mar;2(2):151–157. [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N. H., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. J., Nathans D. Proliferin secreted by cultured cells binds to mannose 6-phosphate receptors. J Biol Chem. 1988 Mar 5;263(7):3521–3527. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Möller W., Amons R. Phosphate-binding sequences in nucleotide-binding proteins. FEBS Lett. 1985 Jul 1;186(1):1–7. doi: 10.1016/0014-5793(85)81326-0. [DOI] [PubMed] [Google Scholar]
- Orloff D. G., Frank S. J., Robey F. A., Weissman A. M., Klausner R. D. Biochemical characterization of the eta chain of the T-cell receptor. A unique subunit related to zeta. J Biol Chem. 1989 Sep 5;264(25):14812–14817. [PubMed] [Google Scholar]
- Pai E. F., Kabsch W., Krengel U., Holmes K. C., John J., Wittinghofer A. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature. 1989 Sep 21;341(6239):209–214. doi: 10.1038/341209a0. [DOI] [PubMed] [Google Scholar]
- Peter M. E., Schirmer N. K., Reiser C. O., Sprinzl M. Mapping the effector region in Thermus thermophilus elongation factor Tu. Biochemistry. 1990 Mar 20;29(11):2876–2884. doi: 10.1021/bi00463a033. [DOI] [PubMed] [Google Scholar]
- Peter M. E., Wittmann-Liebold B., Sprinzl M. Affinity labeling of the GDP/GTP binding site in Thermus thermophilus elongation factor Tu. Biochemistry. 1988 Dec 27;27(26):9132–9139. doi: 10.1021/bi00426a010. [DOI] [PubMed] [Google Scholar]
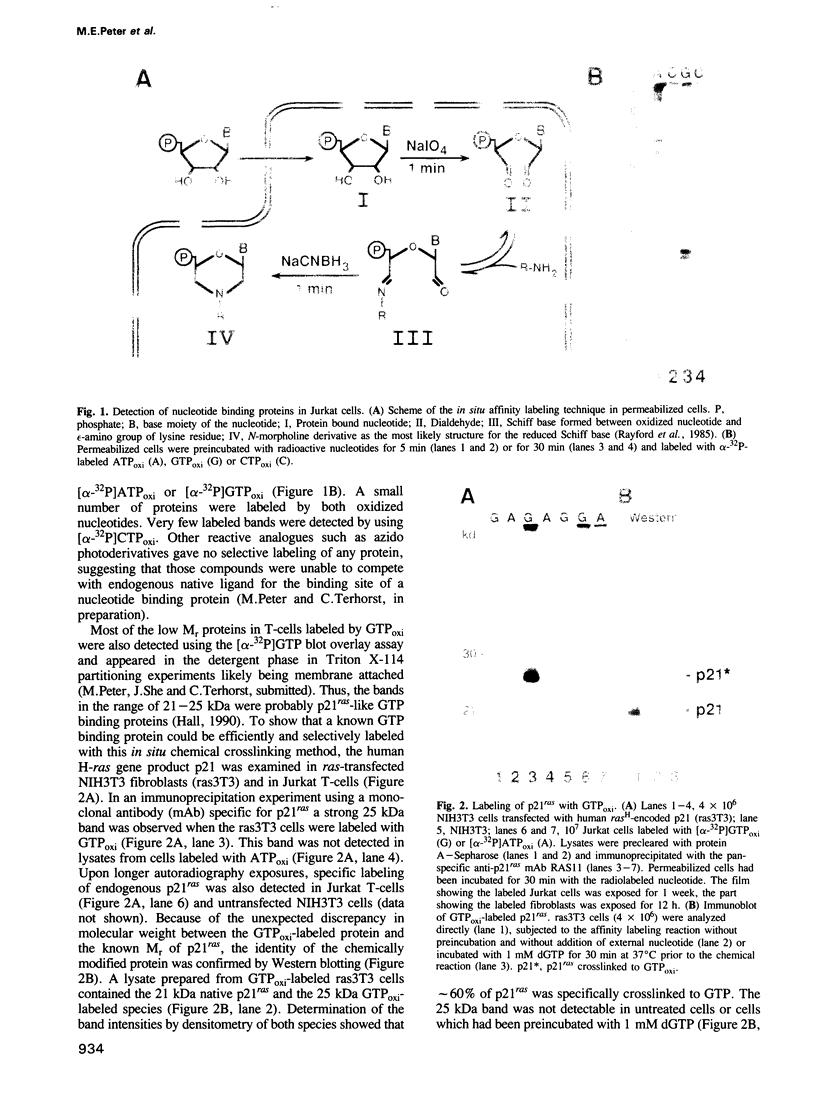
- Rayford R., Anthony D. D., Jr, O'Neill R. E., Jr, Merrick W. C. Reductive alkylation with oxidized nucleotides. Use in affinity labeling or affinity chromatography. J Biol Chem. 1985 Dec 15;260(29):15708–15713. [PubMed] [Google Scholar]
- Romeo C., Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991 Mar 8;64(5):1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Moras D., Olsen K. W. Chemical and biological evolution of nucleotide-binding protein. Nature. 1974 Jul 19;250(463):194–199. doi: 10.1038/250194a0. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Patel M. D., Weissman A. M., Harford J. B., Klausner R. D. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986 Sep 26;46(7):1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- Sancho J., Chatila T., Wong R. C., Hall C., Blumberg R., Alarcon B., Geha R. S., Terhorst C. T-cell antigen receptor (TCR)-alpha/beta heterodimer formation is a prerequisite for association of CD3-zeta 2 into functionally competent TCR.CD3 complexes. J Biol Chem. 1989 Dec 5;264(34):20760–20769. [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
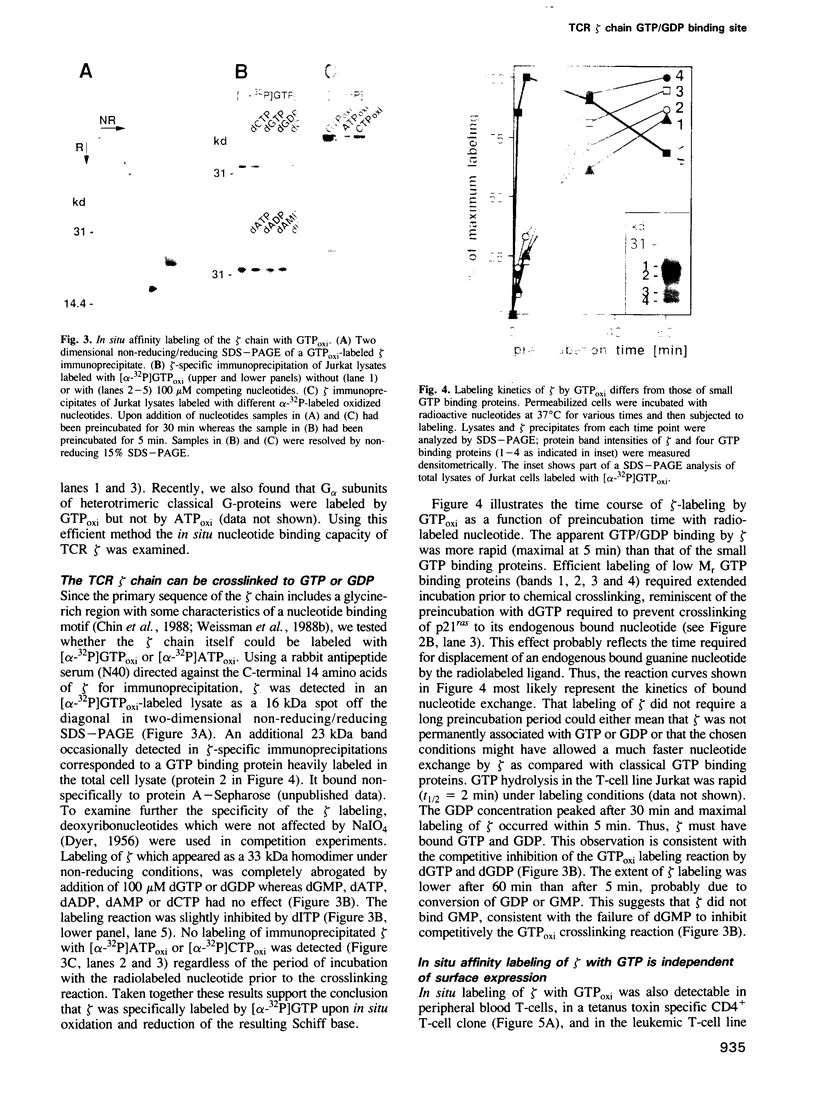
- Schulz G. E., Schiltz E., Tomasselli A. G., Frank R., Brune M., Wittinghofer A., Schirmer R. H. Structural relationships in the adenylate kinase family. Eur J Biochem. 1986 Nov 17;161(1):127–132. doi: 10.1111/j.1432-1033.1986.tb10132.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Baniyash M., Hou D., Samelson L. E., Burgess W. H., Klausner R. D. Molecular cloning of the zeta chain of the T cell antigen receptor. Science. 1988 Feb 26;239(4843):1018–1021. doi: 10.1126/science.3278377. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Hou D., Orloff D. G., Modi W. S., Seuanez H., O'Brien S. J., Klausner R. D. Molecular cloning and chromosomal localization of the human T-cell receptor zeta chain: distinction from the molecular CD3 complex. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9709–9713. doi: 10.1073/pnas.85.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A. M., Ross P., Luong E. T., Garcia-Morales P., Jelachich M. L., Biddison W. E., Klausner R. D., Samelson L. E. Tyrosine phosphorylation of the human T cell antigen receptor zeta-chain: activation via CD3 but not CD2. J Immunol. 1988 Nov 15;141(10):3532–3536. [PubMed] [Google Scholar]