Abstract
This study investigates paternal brain function with the hope of better understanding the neural basis for variation in caregiving involvement among men. The neuropeptides oxytocin (OT) and vasopressin (AVP) are implicated in paternal caregiving in humans and other species. In a double-blind, placebo-controlled, within-subject pharmaco-functional MRI experiment, we randomized 30 fathers of 1–2 year old children to receive either 24 IU intranasal OT before one scan and placebo before the other scan (n = 15) or 20 IU intranasal AVP before one scan and placebo before the other scan (n = 15). Brain function was measured with fMRI as the fathers viewed pictures of their children, unknown children and unknown adults, and as they listened to unknown infant cry stimuli. Intranasal OT, but not AVP, significantly increased the BOLD fMRI response to viewing pictures of own children within the caudate nucleus, a target of midbrain dopamine projections, as well as the dorsal anterior cingulate (dACC) and visual cortex, suggesting that intranasal oxytocin augments activation in brain regions involved in reward, empathy and attention in human fathers. OT effects also varied as a function of order of administration such that when OT was given before placebo, it increased activation within several reward-related structures (substantia nigra, ventral tegmental area, putamen) more than when it was given after placebo. Neither OT nor AVP had significant main effects on the neural response to cries. Our findings suggest that the hormonal changes associated with the transition to fatherhood are likely to facilitate increased approach motivation and empathy for children, and call for future research that evaluates the potential of OT to normalize deficits in paternal motivation, as might be found among men suffering from post-partum depression.
Keywords: Oxytocin, Vasopressin, Father, fMRI
1. Introduction
In contrast to most mammalian species, humans have been characterized as an alloparental species in which mothers typically receive help from other adults in raising their children (Hrdy, 2009). In some societies and cultures, the primary helper is the father, and in this situation, paternal involvement is associated with reduced child mortality and morbidity (Gaudino et al., 1999; Weitoft et al., 2003), as well as improved social, psychological, and educational outcomes (Cabrera et al., 2000; Sarkadi et al., 2008). Yet, despite these facts, there is remarkable variation in paternal involvement (Hrdy, 2009) and father absence has increased precipitously over the last half of the 20th century (Cabrera et al., 2000), highlighting the importance of understanding the neurobiological influences on paternal caregiving.
Oxytocin (OT) is a naturally occurring endogenous neuropeptide that is produced in the paraventricular and supraoptic nuclei of the hypothalamus and released into both the brain and the peripheral circulation (Meyer-Lindenberg et al., 2011). Peripherally, it promotes both uterine contractions during labor and milk letdown during nursing, while centrally, it promotes maternal caregiving (Rilling and Young, 2014) and other forms of attachment. In rats, OT acts on the mesolimbic dopamine (DA) system to facilitate the motivation to approach and nurture offspring (Numan, 2007). The mesolimbic DA system may also motivate caregiving in humans. For example, nucleus accumbens activation scales to the degree of “baby schema” (i.e., cuteness) of child pictures, as well as with the self-reported motivation to care for the children among women (Glocker et al., 2009a; Glocker et al., 2009b). Moreover, OT has been shown to augment activation within the mesolimbic DA system as women view pictures of unknown crying infants (Gregory et al., 2015). Thus, humans and rodents may share core neural mechanisms that motivate maternal behavior (Rilling and Young, 2014).
Recent work suggests that maternal and paternal behavior rely on similar neural substrates. For example, the medial preoptic area of the hypothalamus is now known to be a critical node for parental behavior in both sexes (Wu et al., 2014). Perhaps, like maternal caregiving, paternal caregiving is mediated by OT acting in the mesolimbic DA system. Married fathers have higher levels of plasma OT than unmarried non-fathers (Mascaro et al., 2014b), and plasma OT levels increase over the first 6 months of fatherhood (Gordon et al., 2010). Moreover, paternal plasma OT levels are correlated with father-infant affect synchrony and with stimulatory touch in father-infant play sessions (Feldman et al., 2011; Gordon et al., 2010). In addition, intranasal OT treatment increases paternal stimulatory and exploratory play with toddlers and increases the duration of episodes of father-infant touch and social reciprocity. These augmented paternal behaviors in turn alter the infant’s behavior, increasing the duration of infant gaze to the father and infant object manipulation, as well as infant salivary oxytocin (Weisman et al., 2012). Overall, OT appears to motivate paternal behaviors that facilitate father-infant bonding. Collectively, these findings lead to the prediction that intranasal OT will augment activation within the mesolimbic DA system as men view pictures of their children.
Infants solicit parental caregiving not only by their appearance but also through crying. In mice, OT in the left auditory cortex accelerates maternal retrieval of crying pups (Marlin et al., 2015). Infant crying can also be an aversive stimulus and parents must often regulate their initial negative emotional reaction to it in order to deliver appropriate care. In female rats, OT acts via the medial preoptic area (MPOA) to inhibit an avoidance system that includes the amygdala (Numan, 2007). Intranasal OT also attenuates the amygdala response to unknown infant cries among nulliparous women (Riem et al., 2011), consistent with inhibition of an avoidance pathway. The same study also found that intranasal OT augmented the anterior insula response to unknown infant cries, suggesting that OT may also enhance empathic responses to infant cries. The effect of OT on the neural response to infant cries in fathers has not yet been investigated.
Vasopressin (AVP), which differs from OT by only two amino acids, has also been implicated in paternal behavior. AVP injections into the lateral septum elicit paternal behavior in male prairie voles (Wang et al., 1994), and AVP-immunoreactive staining in bed nucleus of stria terminalis (BNST) terminals predicts paternal behavior in California mice (Bester-Meredith and Marler, 2003). In primates, marmoset monkey fathers have increased V1a vasopressin receptor density as well as increased dendritic spine density on neurons in prefrontal cortex (Kozorovitskiy et al., 2006). Finally, intranasal AVP administration increased attention to virtual baby-related avatars in human fathers-to-be (Cohen-Bendahan et al., 2015). However, the role of AVP in human parental behavior, as well as its neural mechanism, has not been investigated.
In the current double-blind, placebo-controlled, within-subject pharmaco-functional MRI experiment, we randomized 30 fathers of 1–2 year old children to receive either 24 IU intranasal OT on one scan and placebo on the other (n = 15) or 20 IU intranasal AVP on one scan and placebo on the other (n = 15). Brain function was measured with fMRI as the fathers viewed pictures of their children, unknown children and unknown adults, and as they listed to unknown infant cry stimuli. Fathers also rated their subjective reactions to cry stimuli. Our aim was to evaluate the effect of intranasal OT and AVP on paternal brain function and subjective ratings of cry stimuli. We also sought to determine if any such effects were modulated by attachment security or early life experience, as has been reported previously (Bakermans-Kranenburg and van IJzendoorn, 2013; Bartz et al., 2011). We hypothesized that intranasal OT would augment activation within the mesolimbic DA system as men viewed pictures of their children, and that it would also augment activation in auditory cortex and anterior insula in response to baby cries, while suppressing activation to baby cries in the amygdala. We further hypothesized that intranasal OT would attenuate negative subjective reactions to cry stimuli through inhibition of the avoidance pathway. Finally, we expected these effects of OT to be stronger among fathers with supportive family backgrounds (Bakermans-Kranenburg and van IJzendoorn, 2013) and high levels of attachment anxiety (De Dreu, 2012b). Given the much more limited body of research on AVP and paternal behavior, we did not have a-priori hypotheses as to how it would influence brain function in fathers.
2. Material and methods
2.1. Subjects
Thirty-one biological fathers of 1–2 year old children were recruited by posting flyers around the Emory University campus, at local parks and daycare centers. Fathers were required to be currently cohabitating with their committed partner and child. Enrollment in the study required participation by the father, his adult partner and their child. All enrolled fathers had female partners. The study was approved by the Emory Institutional Review Board, and all participants gave written informed consent (mothers signed on behalf of children). Fathers were screened and excluded for self-reported history of head trauma, seizures, or other neurological disorders, psychiatric illness, alcoholism, or any other substance abuse, serious medical illness, claustrophobia, and for ferrous metal in any part of body. Fathers were between the age of 22 and 43 years (M = 32.8, SD = 4.7) and had between one and five children, with one as the modal number (M = 1.71, SD = 0.97). Twenty-seven out of 31 fathers were married to their partner. The average amount of time married fathers had been married was 5.27 years (SD = 3.70). For the 4 fathers who were not married to their partner, the average amount of time they had lived with their partner was 4.75 years (SD = 2.36).
2.2. Study design
The mother and child came to the laboratory, and experimenters photographed children as described in Section 2.4. Fathers received two fMRI scans on two different occasions separated by 2–10 days (M = 5.71, SD = 2.12). 15 fathers were randomized to receive 24 IU intranasal OT prior to one scan and placebo prior to the other scan, and a separate group of 16 fathers were randomized to receive 20 IU intranasal AVP prior to one scan and placebo prior to the other scan (one father was excluded from image analysis due to excessive motion during the fMRI scan; another father had excessive motion during the picture stimuli under AVP treatment and was excluded from the relevant analyses). We choose these doses given the large number of published studies reporting prosocial effects at these doses (Guastella et al., 2010; Guastella et al., 2011; Hurlemann and Scheele, 2016; Tabak et al., 2015; Thompson et al., 2006). The order of neuropeptide and placebo administration was counterbalanced across subjects within each of the two groups (OT, AVP).
During fathers’ first visit, a study physician or nurse practitioner completed a history and physical examination on each father to determine their eligibility. Eligible fathers completed self-report parenting questionnaires (described in Section 2.3) before self-administering nasal sprays containing OT, AVP or placebo (details on the preparation of OT, AVP and placebo nasal sprays can be found in the supplementary material). Following intranasal administration of AVP, CSF concentrations begin rising within 10 min, continue to increase for up to 80 min, and remain above those of placebo-treated subjects at 100–120 min after administration (Born et al., 2002). Previous studies using intranasal OT and AVP in human subjects have observed neural and behavioral effects at 50 min post-administration (Kirsch et al., 2005; Kosfeld et al., 2005; Thompson et al., 2006). fMRI scans therefore began approximately 50 min (M = 51.29, SD = 4.69) after neuropeptide or placebo administration. During the fMRI scans, fathers viewed pictures of their own child as well as an unknown child and an unknown adult matched on sex and ethnicity with their own child. Afterwards, while still in the scanner, they listened to infant cry and auditory control stimuli (described in Section 2.5). After exiting the scanner, fathers again listened to cry stimuli and rated on a 7 point Likert scale how grating, urgent, piercing, aversive, compelling, manipulative, and spoiled they found the cries (1 = not at all, 7 = extremely); they also rated how irritated, sympathetic, alarmed, angry, upset, compassionate, distressed, annoyed, and tender the cries made them feel. The procedure for the fathers’ second visit was identical to the first except that they did not complete the history and physical examination or the questionnaires.
2.3. Questionnaires
To assess fathers’ early childhood experience, we administered the Parental Love Withdrawal Scale (van Ijzendoorn et al., 2011), the Parental Bonding Instrument (PBI) (Parker et al., 1979) and the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003). The Parental Love Withdrawal Scale asks subjects how well statements such as “My mother is a person who will not talk to me when I displease her” described their parents when they were children, ranging from 1 (not at all well) to 5 (very well). The PBI is a widely used measure of fundamental parental style as perceived by the child and assesses two parenting dimensions, namely “care” and “overprotection”. CTQ assesses participants’ maltreatment history in childhood and has five dimensions: sexual abuse, emotional abuse, physical abuse, emotional neglect and physical neglect. We also administered the Experience in Close Relationships-Revised (ECR-R) Adult Attachment Questionnaire (Fraley et al., 2000) to assess fathers’ attachment-related anxiety and attachment-related avoidance.
2.4. Photograph stimuli
Adult photographs were obtained from male and female trained actors who were asked to generate happy, sad, and neutral facial expressions. Unknown child photographs were obtained from male and female age-matched children. For both own and unknown children, we captured eight pictures of each facial expression during a play session. If the child did not make one of the facial expressions naturally, sad faces were elicited by the mother leaving the room or taking a favorite toy from the child, and happy faces were elicited with singing, dancing, or tickling. Fathers were scanned while viewing pictures of happy, sad, and neutral facial expressions of three different people: (1) their own child, (2) an unknown child, and (3) an unknown adult. Fathers were instructed to “please observe each picture and try to share the emotions of the person in the picture.” For each expression, fathers viewed eight different pictures of the person making that expression over the course of four blocks, and each picture was viewed twice (Fig. S1). During a single block, four different photographs of the same expression type were shown, each for 3 s. There was a 0.5-s fixation between each photograph. Thus, the duration of each block was 14 s. After every six blocks, subjects viewed a fixation block of equal duration. The total duration of the task was 9 min 48 s ((36 face blocks + 6 fixation blocks) × 14 s per block). Photographs were presented in pseudorandom order, and fathers always viewed own children at the end.
2.5. Cry stimuli
Cry stimuli (C) were obtained from two infants, aged 3 and 5 months. Stimuli were purchased from an online audio database (www.audionetwork.com), and edited to 10 s clips using online available software (www.audacity.com). Two types of control stimuli were synthesized for each cry using Praat 5.1 and Adobe Audition 3.0 software. For one control, referred to as Con, an emotionally neutral baby vocalization was created to match the duration, intensity, spectral content and amplitude envelope of the cry stimulus. The second control, TCon, was a pure tone that preserved the mean fundamental frequency and amplitude envelop of the cry. Participants listened to the audio stimuli through Pro Ears Ultra 28 MRI-compatible headphones and were told prior to the task: ‘You will now hear a series of sounds. You do not have to do anything except listen to them’. The six different sound files (C1, C2, Con1, Con2, TCon1 and TCon2) were presented in pseudorandom order over four blocks such that each sound was presented four times. There was an inter-trial interval of 6 s between each stimulus (Fig. S2). Each of the four blocks lasted 126 s and the total duration of the task was 8 min 24 s.
2.6. Anatomical image acquisition
Subjects were positioned in a Siemens MAGNETOM Prisma 3 T MRI scanner. Subjects lay motionless in a supine position in the scanner with padded head restraint to minimize head movement during scanning. High-resolution T1-weighted images were acquired using a 3D magnetization-prepared rapid gradient-echo (MPRAGE) sequence with a GRAPPA factor of 2. The T1 scan protocol, optimized for 3 Tesla, used the following imaging parameters: a repetition time/inversion time/echo time (TR/TI/TE) of 1900/900/3.67 ms, a flip angle of 8°, a volume of view of 256 × 256 × 176 mm3, a matrix of 256 × 256 × 176, and isotropic spatial resolution of 1.0 × 1.0 × 1.0 mm3. Total T1 scan time was approximately 5 min.
2.7. Functional image acquisition
T2-weighted images were collected using an Echo-Planar Imaging (EPI) sequence for BOLD fMRI. EPI images were collected in an interleaved fashion with the following imaging parameters selected to minimize susceptibility and distortion artifacts in the orbitofrontal cortex: TR = 2000 ms, TE = 28 ms, matrix = 64 × 64, FOV = 224 mm, in-plane resolution 3.5 mm, slice thickness = 2.5 mm, and 34 axial slices with a gap of 1.05 mm in between.
2.8. Cry rating analysis
After exiting the scanner, subjects listened to the two different cry stimuli a second time and rated them on a Likert scale. Data were analyzed with an ANOVA model with treatment (drug vs. placebo) and cry (C1 or C2) as within subject factors and the order in which the fathers received the drug (drug-placebo or placebo-drug) as a between subject factor. All statistical analyses were implemented in IBM SPSS statistics 23.0 (IBM Corp., Armonk, NY).
2.9. Neuroimaging data analysis
The analysis was conducted with the Oxford Center for Functional Magnetic Resonance Imaging of the Brain’s software library (FSL, http://www.fmrib.ox.ac.uk/fsl/). The preprocessing pipeline of the fMRI data involves (1) motion correction using the MCFLIRT (Jenkinson et al., 2002), (2) non-brain tissue removal using the BET (Smith, 2002), (3) slice timing correction, (4) high-pass temporal filtering with a cut-off of 200 s, (5) spatially smoothing with a Gaussian kernel of full-width at half maximum (FWHM) of 5 mm, and (6) normalizing to MNI space via corresponding extracted T1 brain using Boundary-Based-Registration (Greve and Fischl, 2009).
The preprocessed fMRI data were analyzed using the general linear model (GLM) for univariate statistical analysis. We defined 9 regressors for the picture run: own child’s happy face (OH), own child’s sad face (OS), own child’s neutral face (ON), unknown child’s happy face (UH), unknown child’s sad face (US), unknown child’s neutral face (UN), unknown adult’s happy face (AH), unknown adult’s sad face (AS), and unknown adult’s neutral face (AN). We collapsed across the different emotions and specified three different contrasts: own child vs. unknown child (O-U), own child vs. unknown adult (O-A) and unknown child vs. unknown adult (U-A). We also specified emotion-specific contrasts between own child and adult pictures (e.g., OH-AH, OS-AS, ON-AN). A separate GLM was defined for the cry run for each subject that modeled the neural response to infant cries (C) and the two auditory controls (Con, and TCon). We focused our analysis on the contrast C-TCon. The individual-level GLM was implemented using FILM (FMRIB’s Improved Linear Model).
At the group level, we performed whole brain analyses using random effect models that were designed to investigate the effects of OT and AVP compared to placebo in both the picture and the cry runs. We used paired t-tests to compare contrasts between neuropeptide and placebo treatments and an Ordinary Least Square (OLS) algorithm to estimate beta values in FEAT. The resultant Z statistics (Gaussianized t) images were thresholded using clusters determined by Z > 2.81 (voxel-wise 2-tailed p < 0.005), and a family-wise error (FWE)-corrected cluster significance threshold of p < 0.05 was applied to the suprathreshold clusters, unless otherwise noted.
We specified functional regions of interest (ROI) based on the contrast [(O-A)OT-(O-A)PL] for picture stimuli in the caudate nucleus, anterior cingulate cortex (ACC) and visual cortex. The functional ROIs were centered on the voxel of peak activation within each structure and extended 10 mm in the x, y and z direction. The average BOLD percent signal change was then plotted for each facial expression of emotion (happy, sad and neutral) for each stimulus type (own child, unknown child and adult). Outliers, defined as ±3 * IQR (Interquartile Range) were excluded from ROI plots and analyses. These included two subject’s visual cortex responses for the own child - adult contrast and one subject’s response to unknown sad faces in the caudate nucleus, ACC and visual cortex ROIs. Furthermore, to determine if early life experience or attachment style modulated the effect of OT on brain activation, we tested for correlations between the contrast [(O-A)OT–(O-A)PL] and scores on these measures within the three functional ROIs and in a whole brain covariate analysis.
3. Results
3.1. Main effects of child picture stimuli under placebo treatment
Viewing own children, compared with viewing adults, yielded widespread activation, including the caudate nucleus, putamen, substantia nigra, thalamus, medial prefrontal cortex (MPFC) and visual cortex, as well as deactivation in subgenual ACC (Fig. S3a, Supplementary Table 1). The contrast between own and unknown child pictures yielded a very similar pattern of activation (Fig. S3b; Supplementary Table 1). There was no significant activation for the contrast between unknown child and adult pictures.
3.2. Main effects of child cry stimuli in placebo group
The contrast between listening to infant cries and listening to the auditory tone control yielded activation in right auditory cortex, as well as deactivation in supplementary motor cortex, somatosensory cortex, insula and visual cortex (Fig. S4; Supplementary Table 1).
3.3. OT effects
3.3.1. Pictures
3.3.1.1. Own-adult
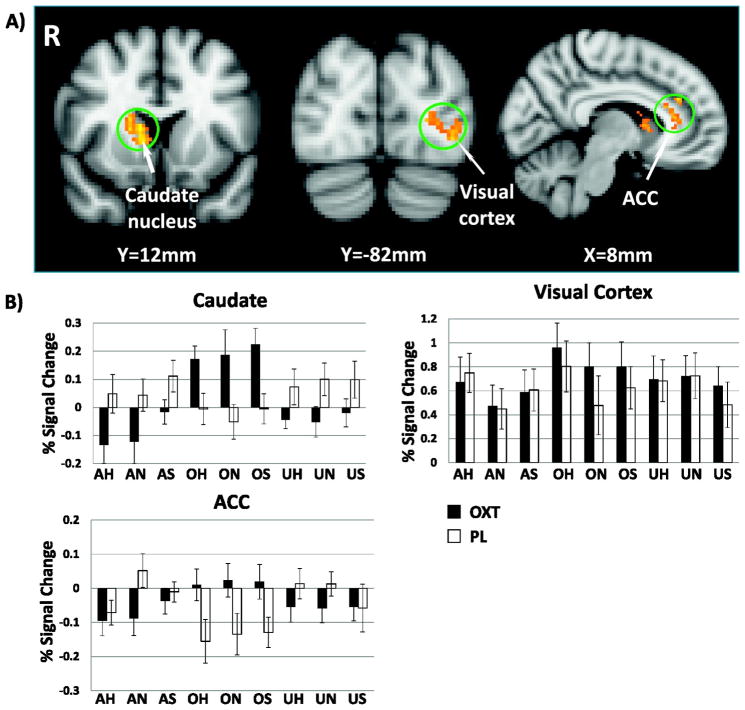
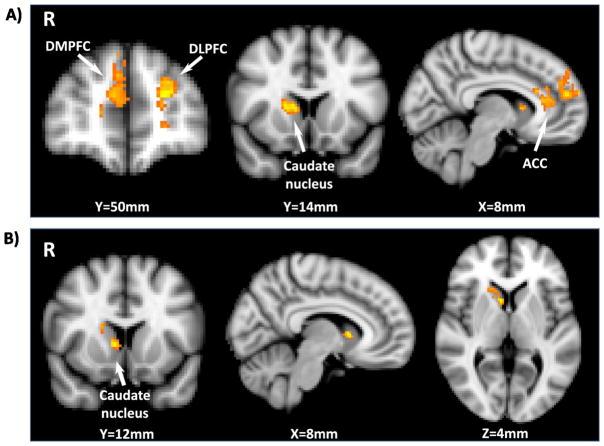
Compared with placebo, OT robustly augmented the BOLD response to viewing pictures of own children relative to adults in the caudate nucleus, the dACC, and visual cortex (Fig. 1a, Table 1). For the dACC, OT effects were negatively correlated with baseline activation under placebo treatment (Fig. S5). That is, OT-induced increases were larger for fathers with lower levels of activation under placebo. This was not true of the caudate nucleus or visual cortex. We also examined the effect of OT on the neural response to each emotion separately. Similar to the overall main effect, OT augmented the neural response to own child neutral faces in the caudate nucleus and dACC. However, OT additionally augmented activation in both dorsomedial and dorsolateral prefrontal cortex (DMPFC and DLPFC) (Fig. 2a). For happy faces, OT augmented the response to own child faces in the caudate nucleus only (Fig. 2b). There was no significant effect of OT on the neural response to own child sad faces.
Fig. 1.
OT effects on the neural response to own child picture stimuli. a) Brain regions where OT augmented the contrast between viewing own children and adult picture stimuli (O-A), b) average percent signal change (±1 SE) within three functional ROIs from a). Adult happy (AH), adult neutral (AN), adult sad (AS), own happy (OH), own neutral (ON), own sad (OS), unknown happy (UH), unknown neutral (UN), unknown sad (US).
Table 1.
Activation table for oxytocin effect. Voxels are 2 mm isotropic.
| Brain Regions | MNI coordination of local maxima (mm) | Local maxima Z | Cluster size (voxel) | ||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | |||
| Picture stimuli: own-adult, OT > PL | |||||
| Caudate nucleus | 18 | 16 | 10 | 4.55 | 390 |
| ACC | 8 | 34 | 26 | 3.90 | 192 |
| Visual cortex | −32 | −72 | −2 | 4.03 | 138 |
Fig. 2.
OT effects on the BOLD response to viewing different facial expressions of emotion in own children. Brain areas in which OT augments the contrast a) own child neutral faces - adult neutral faces and b) own child happy faces - adult happy faces.
To evaluate whether early life experience or attachment style modulated the effect of OT on activation in the regions where OT increased the response to own-adult across all facial expressions (caudate, dorsal ACC, visual cortex), we tested for correlations across fathers between OT effects on brain activation and scores on the Parental Love Withdrawal Scale, the Parental Bonding Instrument (PBI), the Childhood Trauma Questionnaire, and the Experience in Close Relationships-Revised (ECR-R) Adult Attachment Questionnaire. After correction for multiple comparisons, there were no significant correlations. A complementary whole brain analysis with PBI, ECR-R, CTQ and parental love withdrawal as covariates revealed a negative correlation between maternal care in the PBI and OT increases in the inferior frontal gyrus (IFG), anterior cingulate gyrus, precentral gyrus, precuneus, insula and visual cortex, among other areas (Supplementary Table 2). In other words, OT increased activation in these areas more-so in fathers reporting lower quality bonds with their mothers during childhood (Fig. S6). There were no significant correlations for the other measures.
3.3.1.2. Own-unknown, unknown-adult OT effect
OT had no significant effect on the contrast between own and unknown child pictures or the contrast between unknown child pictures and unknown adult pictures. However, the ROI plots in Fig. 1b suggest that OT increases the caudate nucleus and ACC responses to own child, but not unknown child or adult stimuli.
3.3.2. Cries
3.3.2.1. Subjective ratings
After correction for multiple comparisons, there were no significant main effects of OT on any of the 16 subjective ratings of the cry stimuli. Nor were there any significant OT by order of administration interaction effects.
3.3.2.2. Cry-tone control, OT effect
A whole brain analysis did not yield any significant main effect of OT on brain activation in response to infant cries. A whole brain analysis with PBI, ECR-R, CTQ and parental love withdrawal as covariates revealed a negative correlation between maternal care on PBI and OT augmentation of the temporo-parietal junction (TPJ) (Fig. S7; Supplementary Table 2), meaning that fathers reporting lower quality bonds with their mothers during childhood had larger OT-induced increases in TPJ activation to infant cries. There was also a significant positive correlation between maternal love withdrawal and OT effects on cerebellar activation (Fig. S8; Supplementary Table 2).
3.4. AVP effects
3.4.1. Pictures
3.4.1.1. Own-adult AVP effect
AVP did not significantly modulate the neural response to viewing own child pictures vs. adult pictures. This was also true when each emotional expression was examined separately. Neither was AVP effects modulated by our measures of early life experience or attachment security.
3.4.1.2. Own-unknown, unknown-adult AVP effect
AVP had no significant effect on the contrast between own and unknown child pictures or the contrast between unknown child pictures and unknown adult pictures.
3.4.2. Cries
3.4.2.1. Subjective ratings
There was no main effect of AVP on fathers’ subjective cry ratings. Nor was there a drug by order of administration (AVP-PL vs. PL-AVP) interaction.
3.4.2.2. Cry-tone control, AVP effect
AVP had no effect on the neural response to infant cries, nor were AVP effects modulated by our measures of early life experience or attachment style.
4. Discussion
As outlined in the introduction, considerable evidence suggests that OT facilitates multiple aspects of paternal caregiving (Feldman et al., 2011; Gordon et al., 2010; Mascaro et al., 2014b; Weisman et al., 2012). Our study was designed to investigate the neural mechanism for this effect. Our main finding is that intranasal OT increases the caudate nucleus, dACC and visual cortex response in fathers viewing pictures of their toddlers. In contrast, AVP had no effect on paternal neural responses to viewing pictures of their toddlers. Neither OT nor AVP significantly modulated the neural response of fathers to infant cries.
For the placebo treatment, the contrast between viewing own children and viewing sex and ethnicity-matched adults yielded activation in several areas that have been reported previously in imaging studies of parental brain function, such as the substantia nigra (SN), thalamus and medial prefrontal cortex (MPFC). These areas are implicated in reward (SN), empathy (thalamo-cingulate) and theory of mind (MPFC) (Rilling, 2013) (Fig. S3). These activations were specific to the fathers’ own children, as largely the same regions were activated for own-unknown, but not unknown-adult. Relative to an auditory tone control, infant cries activated only the right auditory cortex (Fig. S4). Multiple regions that are commonly activated in response to infant cries did not show activation in our study. This may have to do with the choice of control stimuli. We used a pure tone that preserved the mean fundamental frequency and amplitude envelop of the cry, whereas some other groups have used white noise controls. Interestingly, when we relax our voxel-level threshold to two-tailed p < 0.01 corrected, a threshold that may be vulnerable to false positives (Eklund et al., 2016), activation appears in the inferior frontal gyrus (IFG), a classic mirror neuron region involved in motor simulation and empathy (Rizzolatti and Fogassi, 2007) that has been reported in previous fMRI studies using infant cry stimuli (Mascaro et al., 2014a; Riem et al., 2011).
Administration of 24 IU intranasal OT augmented the caudate nucleus response to viewing pictures of own children compared with viewing sex and ethnicity-matched adults. This was a robust finding, with 14 of 15 fathers having a positive value for the contrast. The caudate nucleus is a target of midbrain dopamine projections that are known to be involved in reward processing. Our result therefore suggest that OT may increase the reward or salience of own child visual stimuli. Although the caudate nucleus is a target of midbrain DA projections, it is not part of the mesolimbic DA system, which is instead focused on the nucleus accumbens. Therefore, our results are not strictly consistent with predictions based on neurobiological models of parental caregiving in rats, in which OT activates the mesolimbic DA approach system to motivate caregiving behaviors (Numan, 2007). However, the caudate is part of another midbrain DA system, the nigrostriatal system, which is also involved in reward and motivation (Ikemoto et al., 2015) and commonly activated in neuroimaging studies of parental brain function (Rilling, 2013). This finding is also consistent with three studies showing that intranasal OT enhances reward-based activation of the striatum in men. One study showed that 24 IU intranasal OT augmented the caudate nucleus response to same-sex positive social interactions among men (Feng et al., 2015). Another study found that intranasal OT increased the nucleus accumbens response to viewing pictures of a female partner (Scheele et al., 2013). A third study showed that 40 IU intranasal OT increased striatum activation during reward and loss anticipation in both PTSD patients and controls (Nawijn et al., 2016).
OT also increased the dACC response to own child pictures compared with adults. The cingulate cortex is critically involved in parental caregiving. Remarkably, hamsters are able to parent effectively after removal of the neocortex as long as the limbic system is spared, however further damage to the thalamo-cingulate pathway severely disrupts maternal behavior (MacLean, 1990). Rat and hamster mothers with cingulate lesions often have problems nest building, retrieving pups, and actively allowing their pups to nurse (MacLean, 1990; Murphy et al., 1981; Slotnick, 1967; Stamm, 1955). Despite this, other behaviors such as mating, aggression and food getting remain intact suggesting that the damage does not result in global motor or motivational deficits (Murphy et al., 1981). The cingulate cortex is also commonly activated by child picture and/or cry stimuli in fMRI studies (Rilling, 2013). The anterior cingulate cortex may have a special role in parental empathy. fMRI meta-analyses implicate the anterior cingulate in empathy (Fan et al., 2011), and a recent review concluded that the anterior cingulate gyrus is central to the vicarious experience of both pain and reward (Lockwood, 2016). Moreover, a recent study in prairie voles showed that consolation of distressed, familiar conspecifics, a behavior likely to be motivated by empathy or related emotional contagion, is mediated by OT-induced dACC activation (Burkett et al., 2016). Our results therefore suggest that OT may be enhancing fathers’ empathic responses to their children. This interpretation is plausible given that we in fact prompted the fathers to empathize with the people they viewed in the pictures. However, the anterior cingulate is also implicated in social information processing (Apps et al., 2016) and an alternative interpretation is that OT is simply augmenting fathers’ processing of social information from their children. Finally, the dorsal ACC is also part of the brain’s reward system (Haber and Knutson, 2010) and may integrate reinforcement history to guide voluntary behavior (Holroyd and Coles, 2008). Therefore, another interpretation is that along with the caudate nucleus, this activation reflects OT’s ability to increase the salience or reward value of own child stimuli to fathers.
Using data from a previously published study (Feng et al., 2015), we also find that 24 IU intranasal OT significantly increased the dACC response to same-sex positive social interactions among men (Fig. S9). Thus, OT augmentation of dACC activation to positive social stimuli in men is a reproducible finding. Importantly, OT augmentation of activation within the dACC was greatest among fathers who had the lowest dACC response to their child’s picture at baseline in the placebo group (Fig. S5). Conceivably, these are fathers that have less empathy for their child at baseline and would most benefit from OT enhancement of empathy.
Separate analysis of the three different facial expressions of emotion in our study revealed that OT increased the caudate nucleus response to both neutral and happy faces, suggesting that OT augmentation of approach motivation generalizes to children with both of these expressions. It should be noted however that the functional ROI plots in Fig. 1 show that OT effects for sad faces trend in the same direction. Dorsal ACC activation was only found for neutral faces, suggesting that OT augmentation of empathic responses may be limited to neutral faces. However, OT effects for happy and sad faces trend in the same direction as for neutral. OT also augmented the neural response to neutral faces in DMPFC. DMPFC is strongly implicated in perspective-taking and theory of mind (Molenberghs et al., 2016). Thus, OT may be augmenting both emotional (dACC) and cognitive (DMPFC) empathy in fathers.
In addition to the caudate nucleus and the dACC, OT also increased activation in visual cortex in response to own child pictures. This is consistent with a recent study showing that OT enhanced visual cortex activity in response to faces more so than geometric shapes (Andari et al., 2016). Visual cortex activation is known to be modulated by attention (Lane et al., 1999), so OT may be enhancing the salience of one’s child relative to unknown adults. Indeed, primate OT receptors are concentrated in visual cortex as well as other areas involved in the allocation of attention to visual stimuli (Freeman and Young, 2016).
One previous study investigated the effect of intranasal OT on brain function in human fathers. Unlike our study, this study found 24 IU intranasal OT to attenuate the globus pallidus response to passively viewing own child pictures compared with viewing a familiar child. However, this same study also reported OT-induced increased activation in the body of the caudate nucleus for own child pictures compared with unknown child pictures (Wittfoth-Schardt et al., 2012). While our effects are in the head of the caudate, the caudate body may also be a target of midbrain DA projections involved in parental motivation.
Given that OT receptors are found in both the brain and the periphery, there is some uncertainty as to the mechanism underlying these effects (Churchland and Winkielman, 2012). AVP has been detected in human CSF following intranasal administration (Born et al., 2002), which most have interpreted as evidence that it is able to penetrate the blood-brain barrier. Recently, a small human study reported a 64% elevation in CSF OT following intranasal administration (Striepens et al., 2013). Studies with non-human animals also support the conclusion that intranasal OT increases brain OT levels. For example, Neumann et al. (2013) combined intranasal OT and microdialysis to show increases in amygdala and hippocampal OT 30–60 min after intranasal OT administration in rats. However, it remains possible that these effects are mediated by OT binding to peripheral OT receptors that in turn influence brain activity via the vagus nerve or some other mechanism.
Effects of OT are known to be modulated by early childhood experience (Bakermans-Kranenburg and van IJzendoorn, 2013) and attachment style (Bartz et al., 2011). We found that fathers scoring low on maternal care in the PBI had greater OT augmentation to child picture stimuli in several brain areas that have been linked with empathy. These included the IFG, anterior cingulate cortex and the insula. The IFG is part of the mirror neuron system and is believed to facilitate understanding others’ facial expressions through motor simulation (Rizzolatti and Fogassi, 2007). The anterior cingulate and insula are both strongly implicated in emotional empathy (Lockwood, 2016; Singer and Lamm, 2009). Thus, OT may be facilitating empathy for children to a greater degree in fathers reporting less care from their mother as a child. This finding seems to conflict with the evidence that OT effects on prosocial behavior are stronger for those with supportive family backgrounds (Bakermans-Kranenburg and van IJzendoorn, 2013).
OT augmentation of the neural response to own child pictures did not generalize to pictures of unknown children (Fig. 1b), a finding consistent with evidence that OT supports prosocial behavior with in-group but not out-group members (De Dreu, 2012a). That is, as in rodents, OT may channel prosocial tendencies toward family members as opposed to strangers (Bosch and Neumann, 2012).
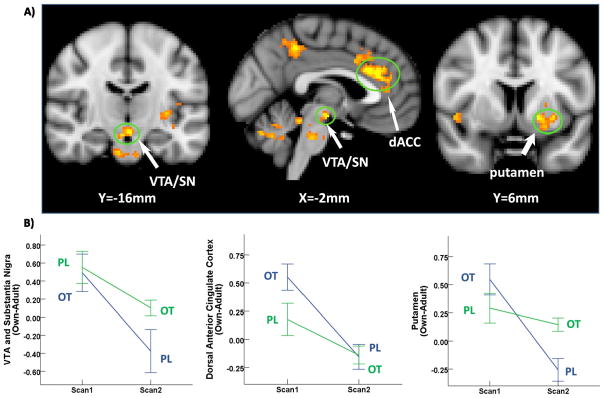
OT is known to influence both learning and conditioning (Kosaki and Watanabe, 2016; Modi and Young, 2012; Sarnyai and Kovacs, 2014). It is therefore possible that OT effects at time 1 could extend to time 2 when subjects received placebo. This would result in a significant drug (OT vs. PL) by order of administration (OT-PL, PL-OT) interaction effect in which the PL-OT effect was stronger than the OT-PL effect. Within the three ROIs in Fig. 1, only visual cortex showed a significant interaction effect. However, rather than a carryover effect, OT had a larger effect relative to placebo when administered first (OT-PL) rather than second (PL-OT) (Fig. S10). A complementary whole brain analysis revealed significant interaction effects across multiple brain regions, including brain areas involved in reward processing such as the ventral tegmental area and substantia nigra (VTA/SN), putamen and dorsal ACC (Fig. 3, Table 2). Within each of these ROI’s, however, we again observed stronger effects for the OT-PL group than the PL-OT group. This interaction effect does not include the caudate nucleus, where there was no difference in OT effects as a function of order of administration. Nonetheless, these results suggest that OT augments paternal reward from viewing their child’s picture to a greater extent if it is administered before the father views the picture for the first time. Given that dopamine and oxytocin are known to interact within the ventral striatum to facilitate social bonding (Skuse and Gallagher, 2009; Young et al., 2005), one possible explanation is that DA levels are higher when the stimulus is novel at first presentation and that combination of high DA and high OT drives the reward system to a greater extent than the combination of lower DA and high OT on second exposure. Importantly, these interaction effects could also reflect habituation from scan 1 to scan 2, irrespective of the treatment order.
Fig. 3.
Significant drug by order of administration (OT-PL vs. PL-OT) interaction. a) Brain regions where OT-PL group demonstrated a larger oxytocin effect than PL-OT group for own-adult contrast; b) plot of mean % signal change with ±1 SE error bars at each scan for both orders of administration. Note that the data are plotted such that main effects of OT would yield lines of differing slope, whereas interaction effects yield lines with similar slopes.
Table 2.
Brain regions showing significant interactions between drug treatment and order of administration. Voxels are 2 mm isotropic.
| Brain regions | MNI coordination of local maxima (mm) | Local maxima Z | Cluster size (voxel) | ||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | |||
| Oxytocin effects | |||||
| Pictures: own-adult OT-PL > PL-OT (drug order) | |||||
| VTA and SN extending into the pons, lingual gyrus and visual cortex | −2 | −16 | −14 | 4.54 | 1701 |
| L putamen and insula | −18 | 6 | −4 | 4.52 | 718 |
| Cerebellum | −32 | −60 | −28 | 4.59 | 650 |
| R supramarginal gyrus, angular gyrus | 58 | −36 | 38 | 4.17 | 511 |
| Cingulate | −4 | 30 | 26 | 4.75 | 500 |
| R visual cortex | 24 | −86 | 34 | 4.84 | 390 |
| L supramarginal gyrus, angular gyrus | −50 | −60 | 48 | 3.9 | 333 |
| Posterior cingulate extending into precuneus cortex | 0 | −44 | 46 | 4.39 | 219 |
| L visual cortex | −20 | −72 | 38 | 4.14 | 210 |
| R precuneus cortex | 20 | −54 | 12 | 4.54 | 180 |
| Frontal pole | −28 | 70 | 10 | 4.21 | 166 |
| R insula cortex | 38 | 20 | −4 | 4.23 | 139 |
| R middle frontal gyrus | −38 | 34 | 44 | 3.91 | 121 |
OT had no effects on fathers’ subjective ratings of the cry stimuli, and there was no significant main effect of OT on the neural response to cry stimuli. The amygdala is a key node within the offspring avoidance neural system of rats. Since baby cries can be aversive, we hypothesized that intranasal OT would attenuate fathers’ amygdala activation, rendering the cries less aversive. Moreover, a previous study found intranasal OT to attenuate the amygdala response to cries among women (Riem et al., 2011). We did not observe this predicted effect, however when we compare activation between OT and placebo groups at scan 1 rather than examining the within-subject effects of OT, we find that OT attenuates the amygdala response to the cries at a corrected threshold of p < 0.05 (Fig. S11). While this analysis is underpowered with only 7 OT and 8 PL subjects and should therefore be interpreted with caution, it suggests that OT effects may be strongest upon initial exposure to cry stimuli and merits further investigation with larger sample sizes. Similar to picture stimuli, OT-induced increases in activation to cry stimuli were stronger in fathers reporting less care from their mother as a child, and this effect was localized to another region implicated in empathy, the temporo-parietal junction (TPJ). In particular, the TPJ has been linked with theory-of-mind processing (Molenberghs et al., 2016). There was also a significant positive correlation between parental love withdrawal from the mother and OT modulation of cerebellar activation, such that OT increased cerebellar activation to a greater extent in fathers who had higher maternal love withdrawal as a child. While we interpret this finding cautiously, it could reflect an augmented emotional reaction to the cries, since the cerebellum is increasingly recognized for its role in emotion (Schutter and van Honk, 2005). Interestingly, testosterone administration to young women enhanced cerebellar activation to infant crying, perhaps via conversion to estradiol (Bos et al., 2010).
Although AVP is involved in paternal behavior in a variety of species, we observed no significant main effects of AVP on cry ratings or on the neural response to either picture or cry stimuli. On the surface, this would seem to imply that OT is more important than AVP for paternal motivation and empathy in humans. However, two qualifications are needed. First, we have only investigated a single dose of AVP (20 IU) and other doses could yield different results. Second, significant AVP effects might emerge with a larger sample and increased statistical power.
One important limitation of this study is that our interpretation of results is partially based on reverse inference (Poldrack, 2011). Because many individual brain structures have multiple functions, the functional interpretations we have offered, while plausible and we hope even compelling, are not the only possible interpretations. Wherever possible, we have attempted to interpret our results in light of non-human animal studies where evidence for causal relationships between brain systems and behavior are stronger. Another limitation is that the significant correlations plotted in Figs. S5 and S6 are based on a small sample size and could therefore be driven by a small number of data points. It will be important to attempt to replicate these correlations in future studies.
5. Conclusion
While it has long been known that pregnancy hormones prime mammalian females for parental caregiving (Rilling and Young, 2014), only recently has it become clear that males of some mammalian species, including our own, also experience hormonal changes that may prime them for parental caregiving. These changes include decreases in testosterone and increases in oxytocin (Gettler et al., 2011; Gordon et al., 2010; Gray et al., 2002; Mascaro et al., 2014b). Here, in a sample of fathers of 1–2 year old children, we show that intranasal oxytocin, but not vasopressin, augments activation in brain regions involved in empathy and in the motivation to approach offspring. Thus, the hormonal changes associated with the transition to fatherhood are also likely to promote male parental caregiving. Our results also call for future research that explores the potential of OT to normalize pathological deficits in paternal motivation, as might be found in men suffering from post-partum depression (Paulson et al., 2006).
Supplementary Material
Acknowledgments
This study was supported by National Institute of Child Health and Human Development [grant R21HD078778] and the National Center for Advancing Translational Sciences of the National Institutes of Health [award number UL1TR000454]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Lynnet Richey for assistance with making figures and Elissar Andari for many helpful comments on the manuscript.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yhbeh.2017.01.006.
References
- Andari E, Richard N, Leboyer M, Sirigu A. Adaptive coding of the value of social cues with oxytocin, an fMRI study in autism spectrum disorder. Cortex. 2016;76:79–88. doi: 10.1016/j.cortex.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Apps MA, Rushworth MF, Chang SW. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron. 2016;90:692–707. doi: 10.1016/j.neuron.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci. 2011;6:556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Marler CA. Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered Peromyscus mice. Behav Neurosci. 2003;117:455–463. doi: 10.1037/0735-7044.117.3.455. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bos PA, Hermans EJ, Montoya ER, Ramsey NF, van Honk J. Testosterone administration modulates neural responses to crying infants in young females. Psychoneuroendocrinology. 2010;35:114–121. doi: 10.1016/j.psyneuen.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera NJ, Tamis-LeMonda CS, Bradley RH, Hofferth S, Lamb ME. Fatherhood in the twenty-first century. Child Dev. 2000;71:127–136. doi: 10.1111/1467-8624.00126. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Horm Behav. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, Beijers R, van Doornen LJ, de Weerth C. Explicit and implicit caregiving interests in expectant fathers: do endogenous and exogenous oxytocin and vasopressin matter? Infant Behav Dev. 2015;41:26–37. doi: 10.1016/j.infbeh.2015.06.007. [DOI] [PubMed] [Google Scholar]
- De Dreu CK. Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Horm Behav. 2012a;61:419–428. doi: 10.1016/j.yhbeh.2011.12.009. [DOI] [PubMed] [Google Scholar]
- De Dreu CK. Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion. Psychoneuroendocrinology. 2012b;37:871–880. doi: 10.1016/j.psyneuen.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev. 2011;35:903–911. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Dev Sci. 2011;14:752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 2015;9:754–764. doi: 10.1007/s11682-014-9333-9. [DOI] [PubMed] [Google Scholar]
- Fraley RC, Waller NG, Brennan KA. An item response theory analysis of self-report measures of adult attachment. J Pers Soc Psychol. 2000;78:350–365. doi: 10.1037//0022-3514.78.2.350. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J Neuroendocrinol. 2016:28. doi: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudino JA, Jr, Jenkins B, Rochat RW. No fathers’ names: a risk factor for infant mortality in the State of Georgia, USA. Soc Sci Med. 1999;48:253–265. doi: 10.1016/s0277-9536(98)00342-6. [DOI] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Longitudinal evidence that fatherhood decreases testosterone in human males. Proc Natl Acad Sci U S A. 2011;108:16194–16199. doi: 10.1073/pnas.1105403108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Gur RC, Sachser N. Baby schema in infant faces induces cuteness perception and motivation for caretaking in adults. Ethology. 2009a;115:257–263. doi: 10.1111/j.1439-0310.2008.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, Sachser N, Gur RC. Baby schema modulates the brain reward system in nulliparous women. Proc Natl Acad Sci U S A. 2009b;106:9115–9119. doi: 10.1073/pnas.0811620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biol Psychiatry. 2010;68:377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PB, Kahlenberg SM, Barrett ES, Lipson SF, Ellison PT. Marriage and fatherhood are associated with lower testosterone in males. Evol Hum Behav. 2002;23:193–201. [Google Scholar]
- Gregory R, Cheng H, Rupp HA, Sengelaub DR, Heiman JR. Oxytocin increases VTA activation to infant and sexual stimuli in nulliparous and postpartum women. Horm Behav. 2015;69:82–88. doi: 10.1016/j.yhbeh.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB. Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol Psychiatry. 2010;67:1220–1222. doi: 10.1016/j.biopsych.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Unkelbach C, Alvares GA, Hickie IB. Arginine Vasopressin selectively enhances recognition of sexual cues in male humans. Psychoneuroendocrinology. 2011;36:294–297. doi: 10.1016/j.psyneuen.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. Dorsal anterior cingulate cortex integrates reinforcement history to guide voluntary behavior. Cortex. 2008;44:548–559. doi: 10.1016/j.cortex.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Hrdy SB. Mothers and Others. Harvard University Press; Cambridge, Massachusetts: 2009. [Google Scholar]
- Hurlemann R, Scheele D. Dissecting the role of oxytocin in the formation and loss of social relationships. Biol Psychiatry. 2016;79:185–193. doi: 10.1016/j.biopsych.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Yang C, Tan A. Basal ganglia circuit loops, dopamine and motivation: a review and enquiry. Behav Brain Res. 2015;290:17–31. doi: 10.1016/j.bbr.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaki Y, Watanabe S. Conditioned social preference, but not place preference, produced by intranasal oxytocin in female mice. Behav Neurosci. 2016;130:182–195. doi: 10.1037/bne0000139. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Lane RD, Chua PML, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Lockwood PL. The anatomy of empathy: vicarious experience and disorders of social cognition. Behav Brain Res. 2016;311:255–266. doi: 10.1016/j.bbr.2016.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD. The Triune Brain in Evolution: Role in Paleocerebral Functions. Plenum Press; New York: 1990. [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’Amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascaro JS, Hackett PD, Gouzoules H, Lori A, Rilling JK. Behavioral and genetic correlates of the neural response to infant crying among human fathers. Soc Cogn Affect Neurosci. 2014a;9:1704–1712. doi: 10.1093/scan/nst166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascaro JS, Hackett PD, Rilling JK. Differential neural responses to child and sexual stimuli in human fathers and non-fathers and their hormonal correlates. Psychoneuroendocrinology. 2014b;46:153–163. doi: 10.1016/j.psyneuen.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm Behav. 2012;61:340–350. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Johnson H, Henry JD, Mattingley JB. Understanding the minds of others: a neuroimaging meta-analysis. Neurosci Biobehav Rev. 2016;65:276–291. doi: 10.1016/j.neubiorev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Murphy MR, MacLean PD, Hamilton SC. Species-typical behavior of hamsters deprived from birth of the neocortex. Science. 1981;213:459–461. doi: 10.1126/science.7244642. [DOI] [PubMed] [Google Scholar]
- Nawijn L, van Zuiden M, Koch SB, Frijling JL, Veltman DJ, Olff M. Intranasal oxytocin enhances neural processing of monetary reward and loss in post-traumatic stress disorder and traumatized controls. Psychoneuroendocrinology. 2016;66:228–237. doi: 10.1016/j.psyneuen.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Parker G, Tupling H, Brown LB. Parental bonding instrument. Br J Med Psychol. 1979;52:1–10. [Google Scholar]
- Paulson JF, Dauber S, Leiferman JA. Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics. 2006;118:659–668. doi: 10.1542/peds.2005-2948. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, van Ijzendoorn MH, Rombouts SA. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol Psychiatry. 2011;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Rilling JK. The neural and hormonal bases of human parental care. Neuropsychologia. 2013;51:731–747. doi: 10.1016/j.neuropsychologia.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L. Mirror neurons and social cognition. In: Dunbar RIM, Barrett L, editors. The Oxford Handbook of Evolutionary Psychology. Oxford University Press; Oxford: 2007. pp. 179–196. [Google Scholar]
- Sarkadi A, Kristiansson R, Oberklaid F, Bremberg S. Fathers’ involvement and children’s developmental outcomes: a systematic review of longitudinal studies. Acta Paediatr. 2008;97:153–158. doi: 10.1111/j.1651-2227.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovacs GL. Oxytocin in learning and addiction: from early discoveries to the present. Pharmacol Biochem Behav. 2014;119:3–9. doi: 10.1016/j.pbb.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, Maier W, Hurlemann R. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci U S A. 2013;110:20308–20313. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J. The cerebellum on the rise in human emotion. Cerebellum. 2005;4:290–294. doi: 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Ann N Y Acad Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Slotnick BM. Disturbances of maternal behavior in the rat following lesions of the cingulate cortex. Behaviour. 1967;29:204–236. doi: 10.1163/156853967x00127. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm JS. The function of the median cerebral cortex in maternal behavior of rats. J Comp Physiol Psychol. 1955;48:347–356. doi: 10.1037/h0042977. [DOI] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wullner U, Maier W, Hurlemann R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA, Meyer ML, Castle E, Dutcher JM, Irwin MR, Han JH, Lieberman MD, Eisenberger NI. Vasopressin, but not oxytocin, increases empathic concern among individuals who received higher levels of paternal warmth: a randomized controlled trial. Psychoneuroendocrinology. 2015;51:253–261. doi: 10.1016/j.psyneuen.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci U S A. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ijzendoorn MH, Huffmeijer R, Alink LR, Bakermans-Kranenburg MJ, Tops M. The impact of oxytocin administration on charitable donating is moderated by experiences of parental love-withdrawal. Front Psychol. 2011;2:258. doi: 10.3389/fpsyg.2011.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci U S A. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Oxytocin administration to parent enhances infant physiological and behavioral readiness for social engagement. Biol Psychiatry. 2012;72:982–989. doi: 10.1016/j.biopsych.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Weitoft GR, Hjern A, Haglund B, Rosen M. Mortality, severe morbidity, and injury in children living with single parents in Sweden: a population-based study. Lancet. 2003;361:289–295. doi: 10.1016/S0140-6736(03)12324-0. [DOI] [PubMed] [Google Scholar]
- Wittfoth-Schardt D, Grunding J, Wittfoth M, Lanfermann H, Heinrichs M, Domes G, Buchheim A, Gundel H, Waller C. Oxytocin Modulates Neural Reactivity to Children’s Faces as a Function of Social Salience. Neuropsychopharmacology. 2012;37:1799–1807. doi: 10.1038/npp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–330. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.