Significance
Chlamydia trachomatis is the most common sexually transmitted bacterium, but most genital infections in women are asymptomatic. Consistent with this presentation, Chlamydia ascension into human endometrial tissue elicits robust innate and adaptive type 2 immunity. Herein, we genitally infected mice with C. trachomatis to explore the significance of type 2 innate immunity, finding that IL-4–secreting eosinophils promote endometrial stromal cell proliferation and prevent Chlamydia infection from triggering upper genital tract tissue destruction. Such results identify eosinophils as essential for repairing murine genital tissue repair after infectious insult. They also identify a need to define roles played by eosinophils in genital infections of women, and their role in other events associated with endometrial repair, including menstruation, endometritis, and endometriosis.
Keywords: Chlamydia trachomatis, endometrium, endometrial stromal cells, eosinophils, interleukin 4
Abstract
Genital Chlamydia trachomatis infections in women typically are asymptomatic and do not cause permanent upper genital tract (UGT) damage. Consistent with this presentation, type 2 innate and TH2 adaptive immune responses associated with dampened inflammation and tissue repair are elicited in the UGT of Chlamydia-infected women. Primary C. trachomatis infection of mice also causes no genital pathology, but unlike women, does not generate Chlamydia-specific TH2 immunity. Herein, we explored the significance of type 2 innate immunity for restricting UGT tissue damage in Chlamydia-infected mice, and in initial studies intravaginally infected wild-type, IL-10−/−, IL-4−/−, and IL-4Rα−/− mice with low-dose C. trachomatis inoculums. Whereas Chlamydia was comparably cleared in all groups, IL-4−/− and IL-4Rα−/− mice displayed endometrial damage not seen in wild-type or IL-10−/− mice. Congruent with the aberrant tissue repair in mice with deficient IL-4 signaling, we found that IL-4Rα and STAT6 signaling mediated IL-4–induced endometrial stromal cell (ESC) proliferation ex vivo, and that genital administration of an IL-4–expressing adenoviral vector greatly increased in vivo ESC proliferation. Studies with IL-4-IRES-eGFP (4get) reporter mice showed eosinophils were the main IL-4–producing endometrial leukocyte (constitutively and during Chlamydia infection), whereas studies with eosinophil-deficient mice identified this innate immune cell as essential for endometrial repair during Chlamydia infection. Together, our studies reveal IL-4–producing eosinophils stimulate ESC proliferation and prevent Chlamydia-induced endometrial damage. Based on these results, it seems possible that the robust type 2 immunity elicited by Chlamydia infection of human genital tissue may analogously promote repair processes that reduce phenotypic disease expression.
The obligate intracellular Gram-negative bacterium Chlamydia trachomatis is sexually transmitted to approximately 130 million individuals each year (1, 2). This high level of populational infectivity is facilitated by predilection of the bacterium to cause asymptomatic and persistent genital infection in women (3, 4). Although Chlamydia ascension into upper genital tract (UGT) tissue can elicit pelvic inflammatory disease (PID) and cause Fallopian tube damage that increases risk for ectopic pregnancy and infertility, the large majority of infections have no negative impact on genital tract structure or function (1).
The typical course of clinical disease suggests that C. trachomatis infection of the human female genital tract may promote formation of type 2 innate and adaptive immunity, a host defense system elicited in response to persistent foreign antigen stimulation that serves to dampen tissue inflammation and promote wound healing (5). Offering support for this possibility, we found that C. trachomatis endometrial infection in women elicited: differentiation of CD4+ T cells that expressed GATA3 and produced IL-4, alternative macrophage activation, enhanced activation of signaling pathways linked to tissue repair, and other host responses characteristic of type 2 immunity (6). Providing additional support for this possibility, an earlier longitudinal study found protection against incident genital Chlamydia infection was significantly enhanced among women whose peripheral blood mononuclear cells secreted IL-13 when stimulated ex vivo with chlamydial antigen (7).
Although asymptomatic infection and the scarcity of adverse reproductive sequelae (even after chronic infection) is consistent with a role for type 2 immune responses in women with genital C. trachomatis infection, it opposes long-held dogma that maintains type 1 immunity is the predominant and biologically relevant host response (8). However, this dogma was primarily developed in mouse models of genital Chlamydia muridarum infection where primary infection is rapidly cleared and no Chlamydia-specific TH2 immunity forms (9, 10). This model, therefore, may underestimate the role of Chlamydia-specific TH2 immunity in the human host response. Moreover, other experimental data implies type 1 immunity may have a two-sided effect—repetitive genital infection of mice and nonhuman primates elicited Chlamydia-specific TH1 immunity that cleared infection but triggered widespread reproductive tract damage (11, 12).
Although mouse model findings with C. muridarum were not consistent with typical clinical presentation in women, we posited that genitally infecting mice with low-dose inoculums of an oculogenital C. trachomatis serovar would more precisely recapitulate the human host response. Testing this hypothesis, we found instead that clearance of genital C. trachomatis infection in mice was also mediated by TH1 immunity and that infection did not generate C. trachomatis-specific TH2 immunity (12). However, this infection did not produce the extensive UGT damage typically seen in mice after primary C. muridarum infection (12). These studies thus offered preliminary, albeit indirect, indication that primary genital C. trachomatis infection of mice elicits host responses that dampen inflammation and prevent tissue damage.
Because mice did not form C. trachomatis-specific TH2 immunity (12), herein, we used a mouse model of primary C. trachomatis infection to delineate the role of type 2 innate immunity in regulating genital inflammation and tissue repair. Our line of investigation led us to increasingly focus on eosinophils, a type 2 innate immune cell shown to secrete preformed cytokines and other immunomodulatory substances that regulate tissue regeneration and wound healing at various tissue sites (13, 14). Although eosinophil function during genital infection was underexplored, these cells were previously shown prevalent in mouse endometrial tissue (15) and were more commonly seen in the endometrium of women with chronic endometritis than women with healthy endometrial tissue (16, 17). Utilization of a murine model of genital C. trachomatis infection in the current investigation allowed us to extend earlier findings, revealing that IL-4–secreting eosinophils promote endometrial stromal cell proliferation and are essential for endometrial tissue repair after infectious insult.
Results
IL-4 Signaling Prevents C. trachomatis Infection from Inducing UGT Pathology.
Although C. trachomatis is the most common sexually transmitted bacterium, only a small fraction of women with laparoscopically confirmed PID develop tubal factor infertility or other permanent damage in the reproductive tract (18). Likewise, primary intravaginal (ivag) C. trachomatis infection of wild-type (WT) mice elicited acute endometrial inflammation but no permanent UGT damage (12). These findings suggest genital C. trachomatis infection may trigger type 2 innate and adaptive immunity, host defenses that modulate tissue inflammation (6). These responses are regulated by cytokines that include IL-4 and IL-10, and are induced during persistent inflammatory states to diminish inflammation and stimulate tissue repair (5). Whereas tissue reparative roles for type 2 immune responses are well established in numerous other conditions (5, 13, 14, 19), their importance in the female genital tract during infectious insult was undefined.
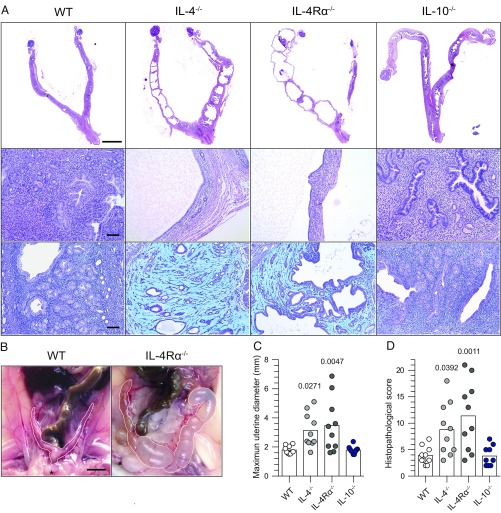
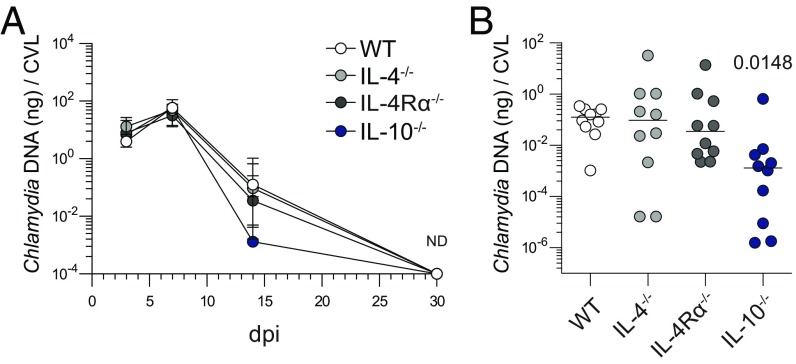
Because primary ivag C. trachomatis of WT mice is cleared without permanent tissue damage and without contribution from Chlamydia-specific TH2 immunity (12), it seemed ideally suited to dissect the role of type 2 innate immunity in the female genital tract. To begin, we compared genital pathology in WT, IL-10−/−, IL-4−/−, and IL-4Rα−/− mice 120 d after primary ivag infection with a low-dose C. trachomatis inoculum. Although no overt tissue damage was detected in WT and IL-10−/− mice, widespread endometrial pathology developed in IL-4−/− and IL-4Rα−/− mice (Fig. 1). Gross changes included hydrometra and prominent unilateral or bilateral uterine horn dilation (Fig. 1 A–C). UGT histological changes were characterized by endometrial atrophy and stromal myxomatous edema, with no significant inflammatory or fibrotic component (Fig. 1 A and D and Fig. S1). The tissue damage was confined to the uterine horns, as no oviduct pathology was detected. Because clearance of infection was comparable between WT, IL-4−/−, and IL-4Rα−/− mice (Fig. 2A), and slightly accelerated in IL-10−/− mice (Fig. 2B), it seemed unlikely the uterine pathology in mice with deficient IL-4 signaling was caused by impaired control of C. trachomatis replication.
Fig. 1.
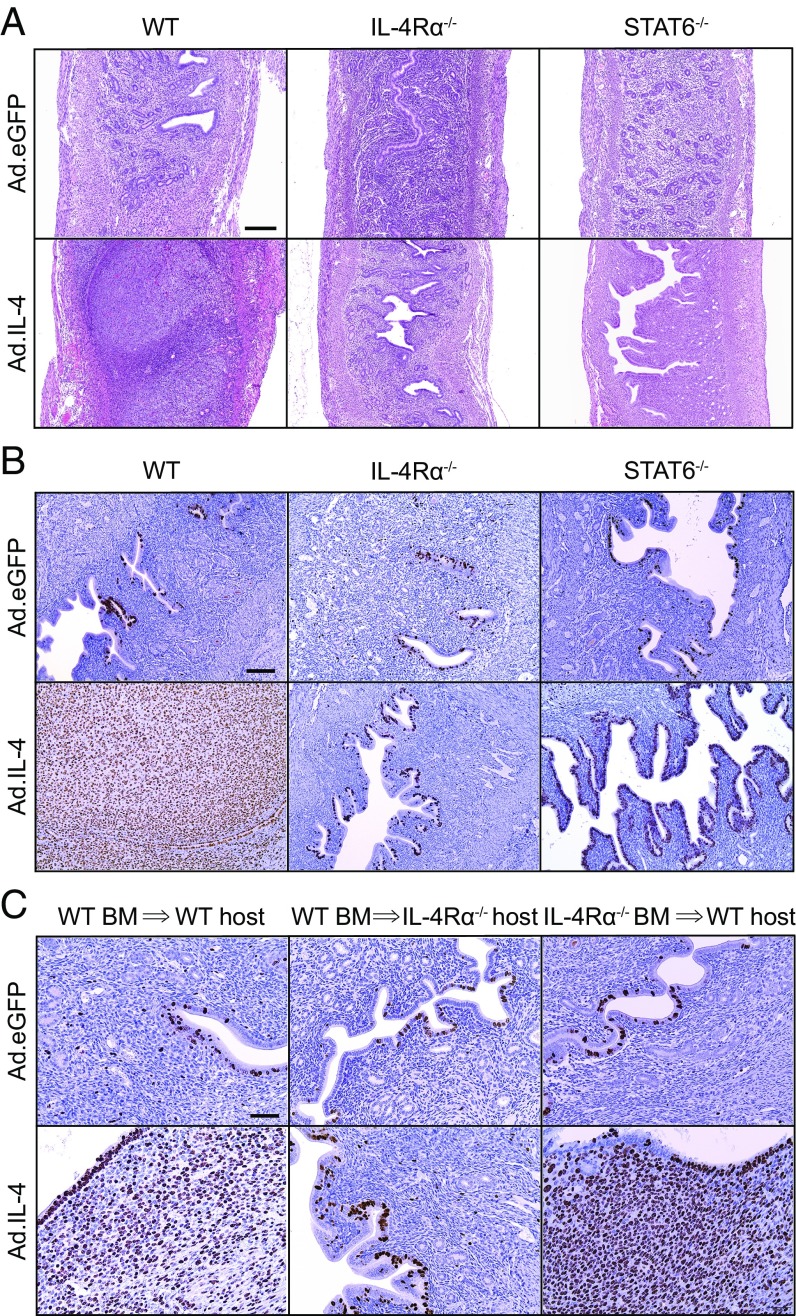
IL-4 signaling prevents UGT tissue damage after primary genital C. trachomatis infection. Six- to eight-week-old female WT, IL-4−/−, IL-4Rα−/−, and IL-10−/− mice were s.c. administered 1 mg of DMPA 5 d before ivag infection with 104 IFU of C. trachomatis serovar D (infection was daily for 3 consecutive days). Mice were euthanized on 120 dpi, and genital tracts removed en bloc for histopathological evaluation. (A) Top and Middle show representative H&E images of tissue damage detected only in mice with disrupted IL-4 signaling. Bottom displays representative images of Alcian blue and periodic acid–Schiff (PAS)-stained UGT sections that show the widespread endometrial stromal myxomatous edema elicited by Chlamydia infection of IL-4−/− and IL-4Rα−/− mice. (B) Representative macroscopic images show uterine horn dilation seen at 120 dpi only in those mice with disrupted IL-4 signaling (asterisks denote approximate location of pubic symphysis). Quantification of histopathological change was performed by determining the maximum uterine diameter in each animal (C) and the composite histopathological score (D) (as defined in Materials and Methods); (bars denote means, n = 10 per group); (all results shown are representative of three independent experiments). (Scale bars: A, Top, 4 mm; A, Middle and Bottom, 100 µm; B, 4.5 mm.)
Fig. S1.
Primary ivag C. trachomatis infection of mice with deficient IL-4 signaling does not induce endometrial fibrosis. UGTs from groups of mice described in Fig. 1 were stained with Masson’s trichrome stain. Representative images show that the areas of stromal edema (observed only in Chlamydia-infected IL-4−/− and IL-4Rα−/− mice at 120 dpi) were not rich in collagen fibers (results representative of three independent experiments). (Scale bar: 100 µm.)
Fig. 2.
Endometrial damage seen in mice with disrupted IL-4 signaling is not caused by a reduced ability to clear genital C. trachomatis infection. (A) Cervicovaginal lavages were collected at indicated dpi from groups of mice detailed in Fig. 1 to quantify Chlamydia DNA levels by quantitative real-time (qRT)-PCR (median ± range, n = 10); (ND, nondetectable). (B) Between-group comparison of Chlamydia DNA burden at dpi 14 shows clearance of infection was more rapid in IL-10−/− mice, whereas no differences in clearance were detectable among WT, IL-4−/−, and IL-4Rα−/− mice (bars denote medians, n = 10 per group; results shown are representative of three independently conducted experiments).
Differences in Chlamydia-Specific T-cell Immunity Do Not Promote Endometrial Pathology in IL-4−/− and IL-4Rα−/− Mice.
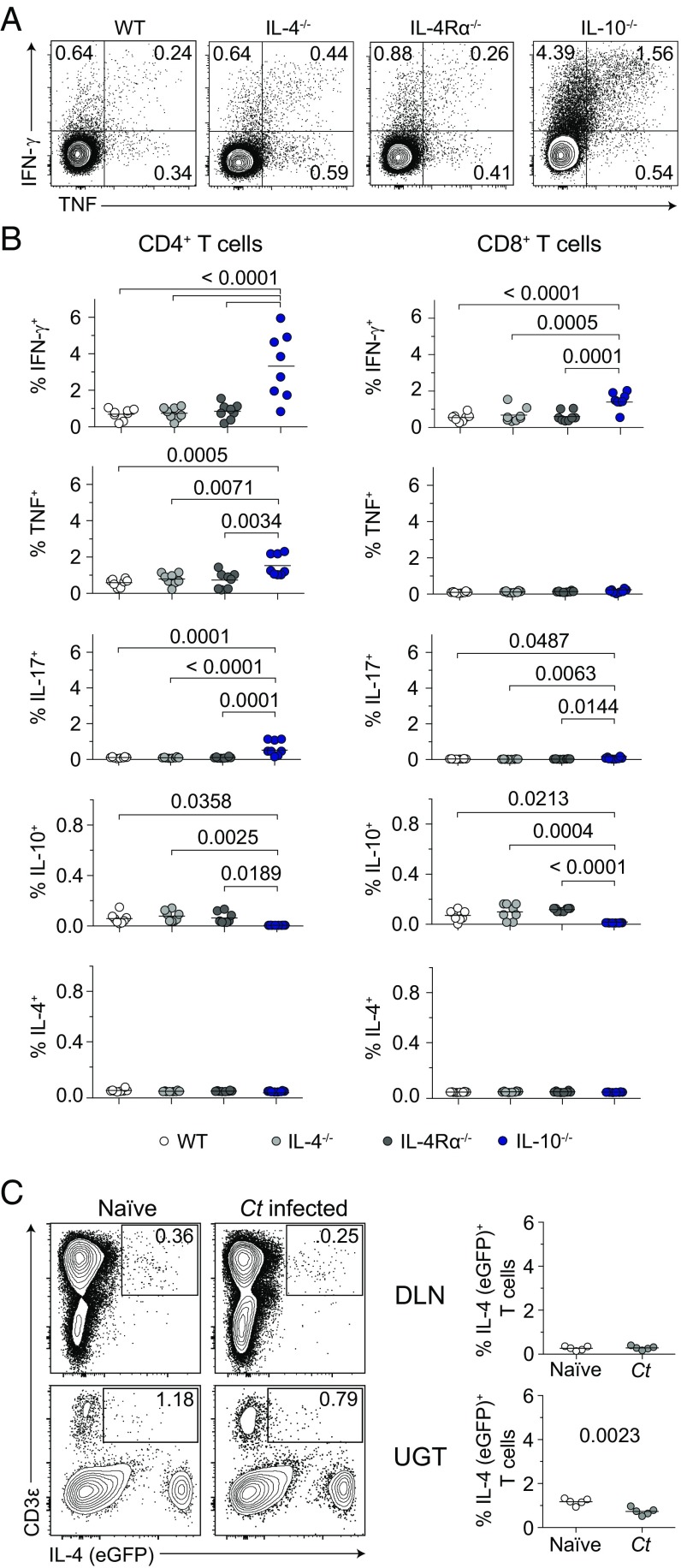
Although primary Chlamydia infection of WT mice caused no overt endometrial damage (Fig. 1), Chlamydia-specific TH1 and TH17 immune responses to repetitive genital challenge induced UGT destruction that diminished reproductive fertility (12). It therefore seemed possible that enhanced endometrial damage in IL-4−/− and IL-4Rα−/− mice was triggered by altered Chlamydia-specific T-cell immunity. However, when T cells from the draining lymph nodes (DLNs) of C. trachomatis-infected WT, IL-4−/−, and IL-4Rα−/− mice were stimulated ex vivo with chlamydial antigen, no significant differences in IFN-γ, TNF, IL-17, or IL-10 production were seen (Fig. 3 A and B). Conversely, T cells from IL-10−/− mice respectively displayed fivefold and sixfold higher frequencies of IFN-γ– and IL-17–secreting Chlamydia-specific CD4+ T cells (Fig. 3 A and B). Because no UGT pathology was seen in IL-10−/− mice (i.e., animals displaying highly robust Chlamydia-specific TH1 and TH17 immunity), this result indicated the tissue damage observed in IL-4−/− and IL-4Rα−/− mice was not triggered by immunopathological T-cell responses. In response to ex vivo stimulation with chlamydial antigen, T cells from all four mouse groups also failed to secrete detectable levels of IL-4 (Fig. 3B). The latter observation was confirmed by using DLNs and UGTs from Chlamydia-infected 4get reporter mice (a strain that allows identification of IL-4–producing cells by eGFP expression) (20) (Fig. 3C). Taken together, our studies thus far showed primary C. trachomatis infection of WT mice was cleared without causing UGT pathology, whereas altered Chlamydia-specific T-cell effector function did not cause the pervasive endometrial pathology seen in Chlamydia-infected IL-4−/− and IL-4Rα−/− mice.
Fig. 3.
Endometrial pathology in mice with disrupted IL-4 signaling is not caused by altered development of Chlamydia-specific T-cell immunity. Indicated strains of mice were infected as detailed in Fig. 1 and challenged with 106 IFU of C. trachomatis serovar D at 120 dpi. Mice were euthanized 5 d after challenge (dpc), and DLNs were processed into single-cell suspensions for incubation with inactivated Chlamydia EB or media alone. (A) Representative contour plots from intracellular cytokine staining that quantified IFN-γ and TNF production by CD4+ T cells stimulated with chlamydial antigen (quadrant numbers denote percentages of cytokine-producing cells). (B) Between-group comparisons of percentages of CD4+ and CD8+ T cells producing IFN-γ, TNF, IL-17, IL-10, and IL-4 in response to stimulation with chlamydial antigen (bars indicate means, n = 8 per group). (C) Using experimental conditions identical to those described in A, IL-4-IRES-eGFP (4get) reporter mice were euthanized at 5 dpc. DLN and UGT were excised and immediately processed for flow cytometric assays that identified IL-4+ T cells (bars denote means, n = 5 mice per group; results shown representative of two independently conducted experiments).
IL-4 Does Not Regulate Differentiation of Uterine Macrophages After Genital Chlamydia Infection.
Because enhanced UGT pathology in IL-4−/− and IL-4Rα−/− mice was not caused by altered Chlamydia-specific T-cell effector function, we used our mouse infection model to explore innate immune cells shown to have roles in tissue repair. These cells include macrophages (Mϕs) and eosinophils (5). Mϕs are abundant in endometrial stroma (21) and regulate embryo implantation (22), whereas their differentiation into M2 Mϕs is mediated by IL-4 signaling pathways (5). Likewise, eosinophils are resident in the endometrial stroma of mice (15). Both Mϕs and eosinophils were shown to mediate repair in a variety of tissues (5, 13, 14), but their role in repairing pathogen-induced genital mucosal tissue damage was uncertain.
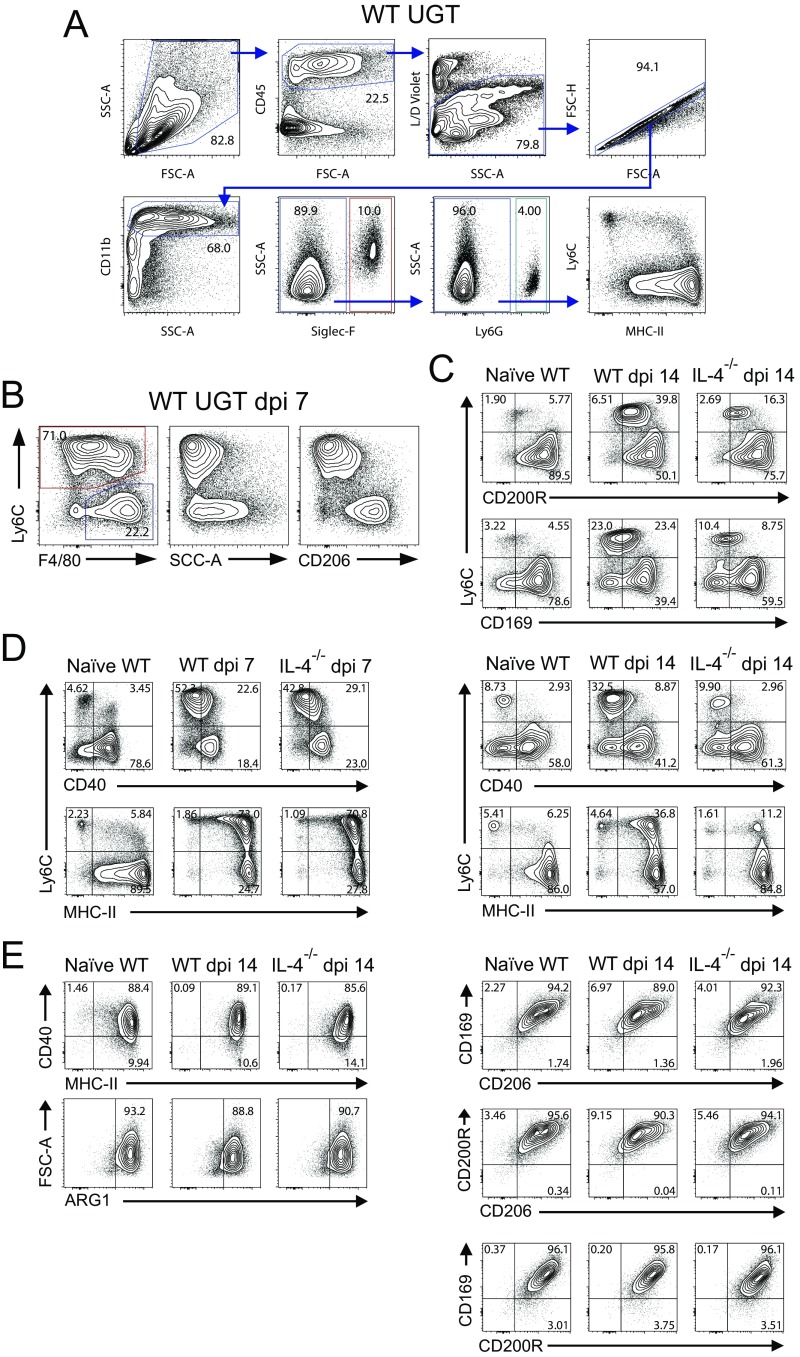
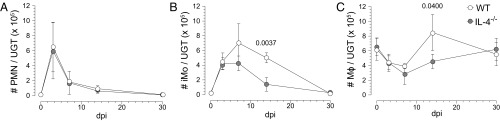
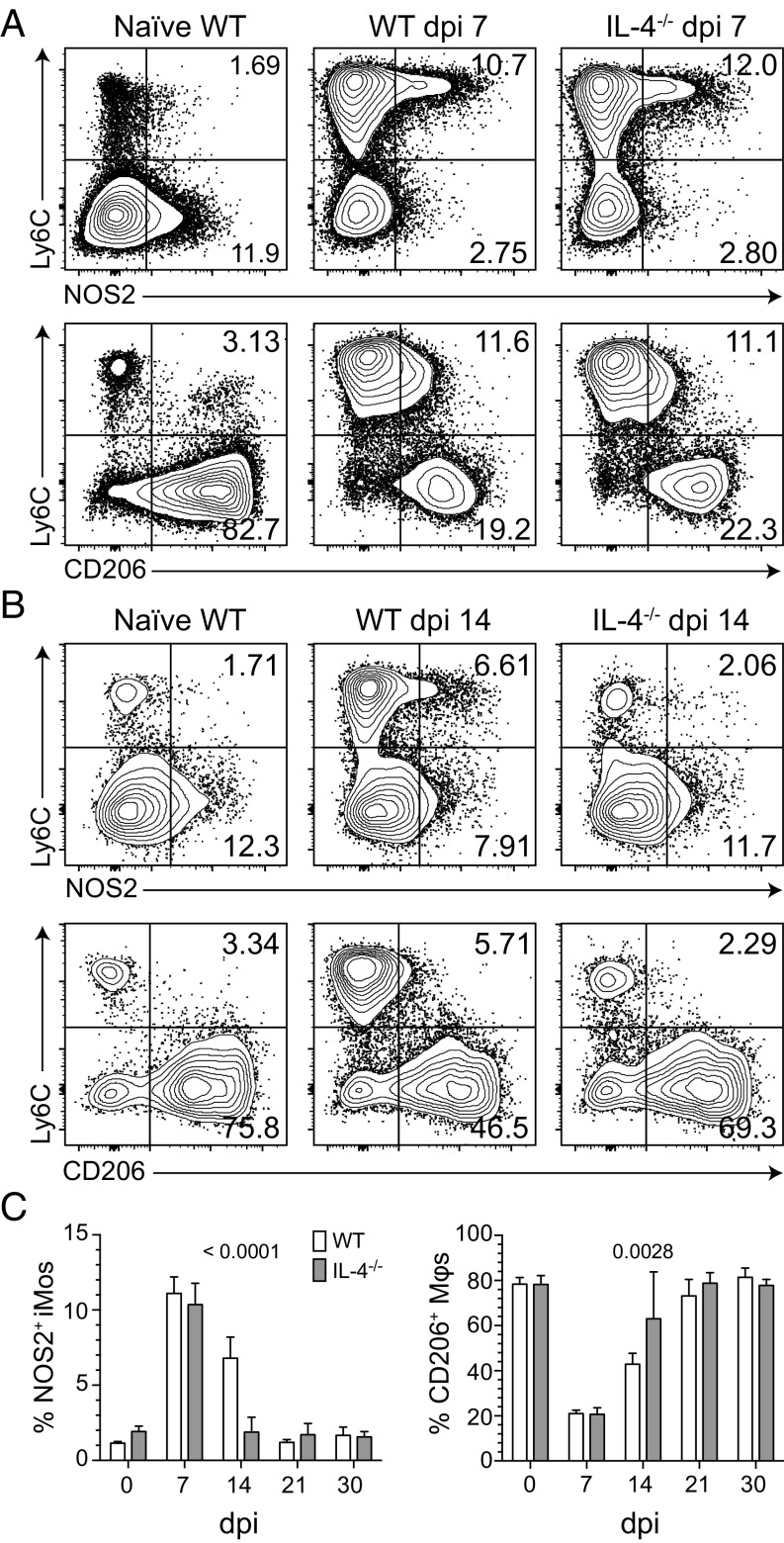
To begin our investigation of type 2 innate immune cells, we compared uterine myeloid cell recruitment and differentiation in WT and IL-4−/− mice after primary C. trachomatis infection (Fig. S2 A and B). In both, infection induced peak endometrial infiltrates of polymorphonuclear neutrophils (PMNs) and inflammatory monocytes (iMos) at 3 and 7 d after infection (dpi), respectively (Fig. 4). At 14 dpi, IL-4−/− mice displayed fewer uterine iMos and Mϕs than WT mice; however, this effect was not sustained at later time points (Fig. 4). Infection also elicited few substantive between-group differences in M1 and M2 marker expression by uterine iMos and Mϕs. At 7 dpi, WT and IL-4−/− mice displayed comparable percentages of NOS2+ iMos (M1), with uterine Mϕs from both groups of mice primarily exhibiting a M2 phenotype (as defined by CD206, CD200R, CD169, and arginase 1 expression patterns) (Fig. 5A and Fig. S2 C–E). At 14 dpi, IL-4−/− mice displayed a Mϕ polarization phenotype comparable to uninfected WT mice (i.e., a higher percentage of M2 uterine Mϕs and less iMos), whereas the UGT of WT mice contained a more prominent NOS2+ iMo population (Fig. 5 B and C). At 30 dpi (i.e., after resolution of primary infection), both WT and IL-4−/− mice displayed Mϕ polarization profiles comparable to uninfected mice (Fig. 5C). At each indicated time point after genital infection, we also saw no substantial between-group differences in uterine Mϕ expression of M2 markers, CD40, or MHC-II (Fig. 5 B and C and Fig. S2 C–E). Taken together, this examination of WT and IL-4−/− mice identified no significant differences in endometrial iMo and Mϕ differentiation after primary genital C. trachomatis infection.
Fig. S2.
Gating strategies used to characterize polarization status of uterine myeloid cell populations. Groups of mice described in Figs. 4 and 5 were euthanized, and UGT was processed for flow cytometric evaluation of uterine myeloid cells as detailed in Materials and Methods. (A) Representative contour plots show gating strategy that defined live CD45+CD11b+Siglec-F+ cells (i.e., eosinophils). Myeloid cells not defined as eosinophils were assessed for Ly6G expression to identify PMNs. (B) Myeloid cells that were not eosinophils or PMNs were evaluated for Ly6C and F4/80 expression. This procedure identified two uterine myeloid populations, iMos (red gate) and Mϕs (blue gate), with distinct patterns of granularity and CD206 expression (plots shown are from a WT mouse at 7 dpi). (C) Uterine myeloid populations also displayed differential expression of other M2 markers, such as CD200R and CD169 (plots shown are from a WT and IL-4−/− mice before infection and at 14 dpi). (D) There were differences in expression of CD40, but not of MHC-II, between iMos and Mϕs in infected mice of both strains (plots shown are from a WT and IL-4−/− mice before infection, at dpi 7, and at dpi 14). (E) Uterine Mϕs expressing high CD206 levels concomitantly expressed high levels of CD40, MHC-II, and the M2 polarization markers arginase 1 (ARG1), CD169, CD200R, and CD206. This expression pattern was unchanged after C. trachomatis infection and was unaffected by disrupted IL-4 signaling (plots shown are from indicated strains of mice before infection and at 14 dpi); (results are representative of two independent experiments).
Fig. 4.
Disrupted IL-4 signaling does not increase endometrial myeloid cell infiltration after C. trachomatis infection. WT and IL-4−/− mice were genitally infected with C. trachomatis as specified in Fig. 1. Animals were euthanized at indicated dpi, and UGT were processed for flow cytometric evaluation of myeloid cell infiltration. The absolute numbers of PMNs (A), iMos (B), and Mϕs (C) per UGT are shown, and identifies similar patterns of myeloid cell infiltration in WT and IL-4−/− mice (values are means ± SD, n = 3–6 mice per condition for each time point; results displayed are representative of two independently conducted experiments) (gates defining distinct myeloid cell populations are depicted in Fig. S2).
Fig. 5.
IL-4 signaling does not influence uterine iMo or Mϕ polarization after primary genital C. trachomatis infection. Uninfected WT and IL-4−/− mice remained uninfected or were infected with C. trachomatis as detailed in Fig. 1. Mice were euthanized at select dpi, and UGT were processed to evaluate myeloid mononuclear cell polarization by flow cytometry (as defined in Materials and Methods). Representative contour plots indicate NOS2 and CD206 expression patterns for iMos and Mϕs in mice at 7 (A) and 14 dpi (B) compared with uninfected mice. (C) Percentages of uterine NOS2+ iMos and CD0206+ Mϕs from live CD11b+ myeloid cells in WT vs. IL-4−/− mice at various dpi appeared distinct only at 14 dpi (bars denote means ± SD, n = 6 mice per condition for each time point; results displayed are representative of two independently conducted experiments).
IL-4-Producing Eosinophils Prevent Genital Tissue Damage After C. trachomatis Infection.
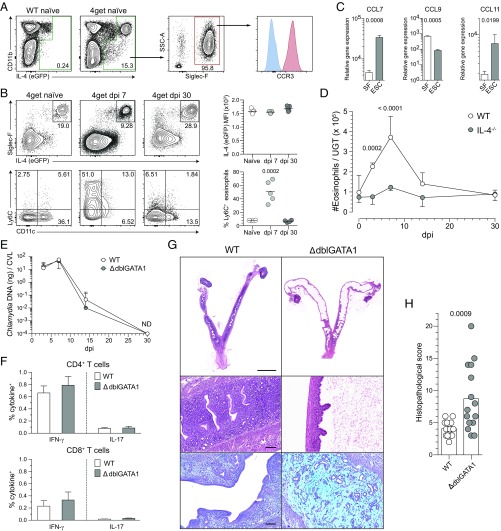
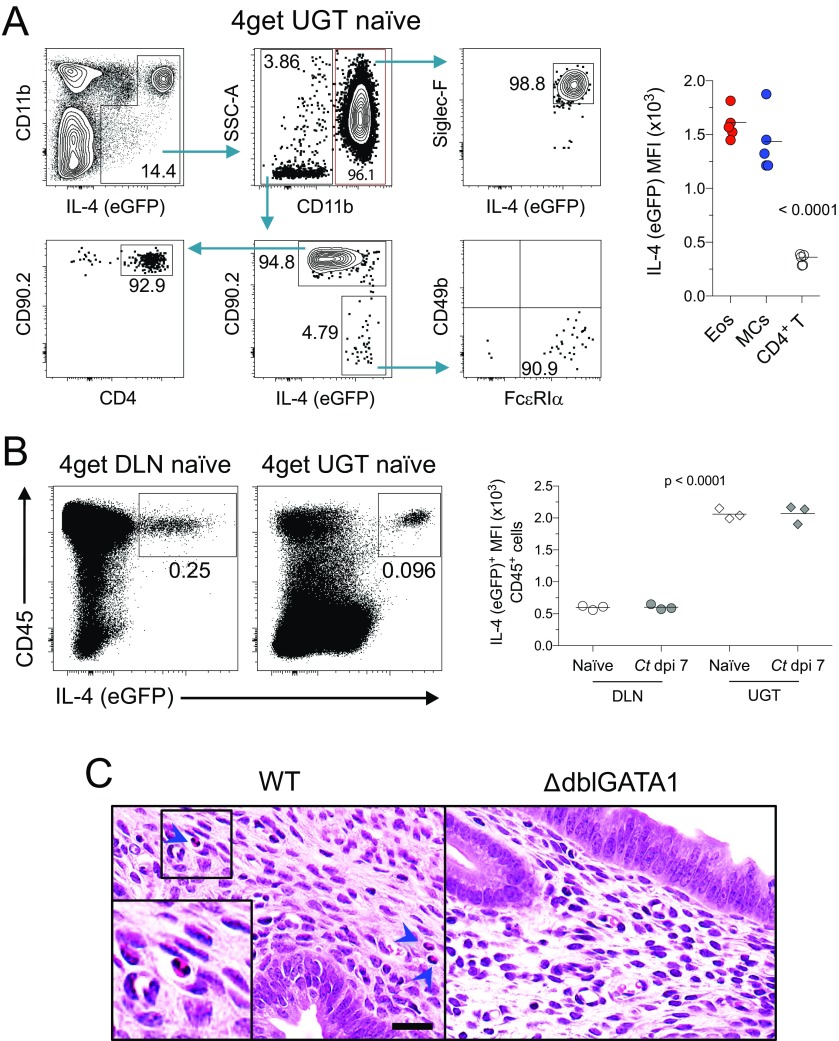
Because genital C. trachomatis infection of mice did not induce differentiation of IL-4–secreting T cells (Fig. 2), we alternatively explored whether innate immune cells were a source of IL-4 in the UGT. To facilitate this inquiry, we examined the UGTs from 4get reporter mice. These studies showed that at least 95% of IL-4–expressing cells in uninfected or Chlamydia-infected UGTs of mice were eosinophils (SSChi CD11b+ Siglec-F+ CCR3+) (Fig. 6 A and B). The remainder were CD4+ T cells and mast cells (SSChi CD49b− CD11c− FcεRI+) (Fig. S3A), whereas IL-4 was undetected in nonhematopoietic cells (Fig. S3B). Although relative IL-4 amounts produced by eosinophils were comparable among uninfected and Chlamydia-infected mice, we found acute infection increased endometrial eosinophil expression of Ly6C (Fig. 6B). We also found that compared with adult murine skin fibroblasts, endometrial stromal cells (ESCs) expressed significantly higher levels of CCL7 and CCL11 (Fig. 6C), chemokines regulating eosinophil infiltration of murine endometrial tissue (15). Likewise, we found endometrial eosinophil numbers were significantly increased by Chlamydia infection, but that these numbers returned to levels seen in uninfected mice after infection was resolved (Fig. 6D). Whereas infection increased endometrial eosinophil numbers in both WT and IL-4−/− mice, Chlamydia infection-induced tissue eosinophilia was blunted in IL-4−/− mice (Fig. 6D). These studies thus identified endometrial eosinophils as a major IL-4 source in the mouse UGT, and suggested that IL-4 secretion helps regulate the infiltration of endometrial eosinophils during Chlamydia infection.
Fig. 6.
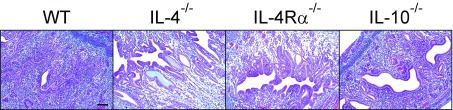
IL-4–producing eosinophils regulate repair of UGT tissue after C. trachomatis infection. Uterine horns from 4get reporter mice were processed as described in Fig. 4 to identify IL-4–producing cells by flow cytometry. (A) Representative contour plots show most IL-4–producing cells were of myeloid origin (gated on live total CD45+ cells from whole UGT), and at least 95% of these cells were eosinophils. Histogram displays representative CCR3 expression (blue line indicates FMO control). (B) Interestingly, eosinophils remained the primary cellular source of IL-4 throughout primary Chlamydia infection (Upper, gated on live CD11b+ myeloid cells), and displayed elevated levels of Ly6C during acute infection (Lower, gated on live SSChi CD11b+ Siglec-F+ cells; n = 5 in all conditions). (C) CCL7, CCL9, and CCL11 gene expression levels as assessed from isolated murine skin fibroblasts (SF) or ESCs (bars indicate means ± SD, n = 3 per group). (D) WT and IL-4−/− mice were infected as described in Fig. 4, and UGTs were collected and processed at indicated dpi to enumerate eosinophils via flow cytometry (n = 5 mice/condition/time point). (E) Six- to eight-week-old WT and ΔdblGATA1 mice were genitally infected with C. trachomatis as described in Fig. 1, and chlamydial clearance was defined as detailed in Fig. 2 (median ± range, n = 5 per group). (F) WT and ΔdblGATA1 mice were treated as indicated in Fig. 3, and DLNs were processed to evaluate Chlamydia-specific T-cell effector function (n = 7 per group; bars indicate means ± SD; each result displayed representative of 2–3 independent experiments). (G) Representative UGT histology from WT and ΔdblGATA1 mice 120 d after primary ivag C. trachomatis infection: Top and Middle display representative H&E images of tissue pathology detected in ΔdblGATA1 mice. Lower shows representative Alcian blue and PAS images of endometrial stromal myxomatous edema in infected ΔdblGATA1 mice that resembled pathology seen in Chlamydia-infected mice with disrupted IL-4 signaling. (Scale bars: Top, 4 mm; Middle and Bottom, 100 µm). (H) Semiquantitative scoring system defined in Materials and Methods showed significantly increased UGT pathology in ΔdblGATA1 mice (bars denote means, 15 mice per group) (results pooled from two independent experiments).
Fig. S3.
IL-4–producing cells include eosinophils, mast cells, and CD4+ T cells. DLNs and uterine horns from uninfected and infected 4get reporter mice were processed as described in Figs. 3 and 4 to identify IL-4–producing cells by flow cytometry (details in SI Materials and Methods). (A) Representative contour plots show gating strategy that defined the different IL-4 (eGFP) positive cellular populations in the UGT of naïve 4get mice. As shown in Right, eosinophils and mast cells express significantly higher levels of IL-4 than CD4+ T cells (n = 5). (B) In this strain of mice, only hematopoietic cells expressed IL-4, whereas IL-4+ cells in the UGT (mainly eosinophils) express higher levels of IL-4 than their counterparts in DLN (mostly CD4+ T cells) (n = 3 per group). (C) DMPA-treated uninfected female WT and ΔdblGATA1 mice were euthanized, and UGTs were processed as described in Fig. 6G. Representative H&E-stained images reveal the absence of eosinophils in the endometrial stroma of ΔdblGATA1 mice. Inset in Left shows a 2x digital amplification of the delineated area, whereas blue arrowheads denote endometrial eosinophils detected in naïve WT mice. (Scale bar: 25 µm.) All results shown are representative of 2–3 independent experiments.
Because eosinophils were the primary source of IL-4 in the mouse UGT, we used ΔdblGATA1 mice to define the role of eosinophils in endometrial tissue repair during C. trachomatis infection (the ΔdblGATA1 mouse contains a Gata1 promoter deletion that prevents eosinophil development) (Fig. S3C) (23). Comparable to Chlamydia eradication by WT, IL-4−/−, and IL-4Rα−/− mice (Fig. 1), we saw WT and ΔdblGATA1 mice similarly clear primary C. trachomatis infection (Fig. 6E) and display comparable Chlamydia-specific T-cell responses (Fig. 6F). However, only ΔdblGATA1 mice developed endometrial pathology, with these mice displaying histopathology that resembled tissue damage seen in mice with disrupted IL-4 signaling (Figs. 1 and 6 G and H). Together, these studies thus identified IL-4–producing eosinophils as essential for appropriate repair of endometrial tissue after infectious insult.
IL-4 Regulates ESC Proliferation.
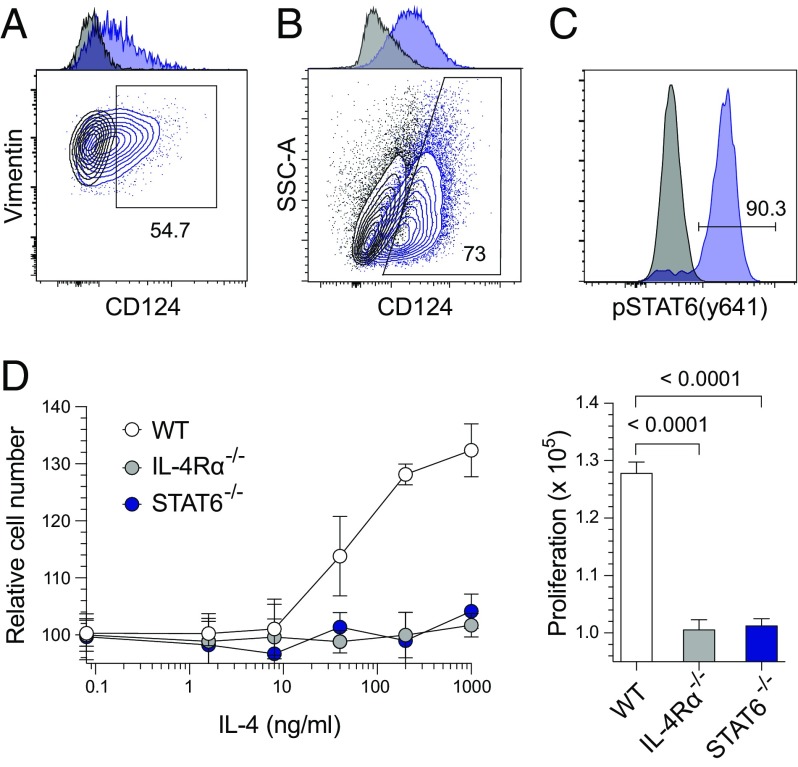
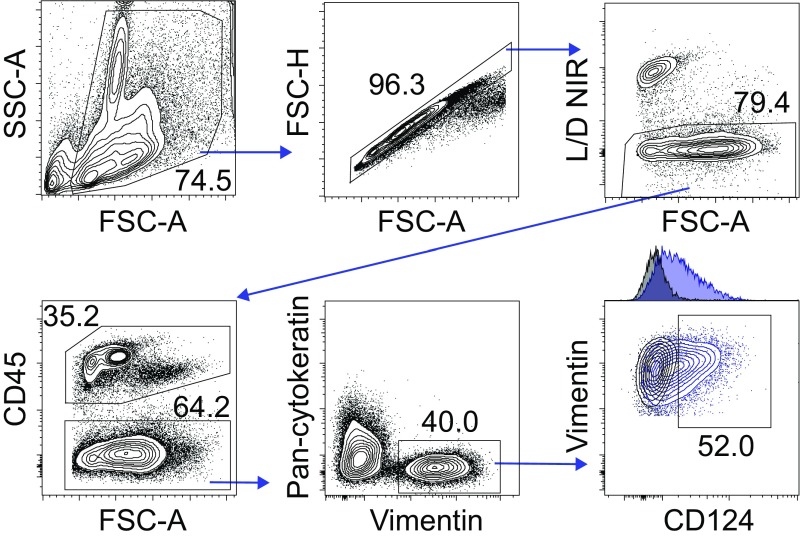
Earlier studies identified that tissue-resident stromal cells regulate repair by proliferating and differentiating in response to injury (13, 19). Based on an ability to proliferate and migrate through various endometrial layers, it was also proposed that human and murine ESCs similarly contribute to uterine tissue repair (24, 25). However, the exact mechanisms connecting host immune responses to ESC proliferation and endometrial regeneration were not well elucidated. Because we identified that IL-4 signaling was essential for repair of Chlamydia-infected endometrial tissue and that eosinophils were the primary source of IL-4 in the mouse endometrium, we performed in vitro and ex vivo experiments to define IL-4–mediated effects on ESC proliferation. Initial studies showed that ESCs express IL-4Rα (CD124) (Fig. 7 A and B and Figs. S4 and S5), and that isolated ESCs phosphorylate STAT6 in response to IL-4 (Fig. 7C). Additional in vitro assays demonstrated that IL-4 increased ESC proliferation (Fig. 7D), whereas IL-4 responsiveness was abrogated in ESCs isolated from IL-4Rα−/− or STAT6−/− mice (Fig. 7D). Combined, these studies identified that IL-4–mediated increases in ESC proliferation depend on STAT6 phosphorylation.
Fig. 7.
IL-4 induces STAT6-dependent in vitro proliferation of endometrial stromal cells (ESCs). (A) Uninfected WT (blue lines) and IL-4Rα−/− (black lines) mice were euthanized, and uteri were processed to evaluate CD124 (IL-4Rα) expression by ESCs directly ex vivo. (B) ESCs isolated from WT (blue lines) and IL-4Rα−/− (black lines) mice were similarly assessed for CD124 expression (ESCs were live CD45− vimentin+ CD90+ cells); A and B display representative contour plots and adjunct histograms. (C) Representative histograms of isolated ESCs from uninfected WT mice were treated with IL-4 (blue line) or vehicle (black line) for 15 min, fixed and permeabilized, and stained for pSTAT6 (Y641) expression. (D) ESCs isolated from WT, IL-4Rα−/−, and STAT6−/− mice were plated in serum-free media and treated with vehicle or specified IL-4 concentrations. Three days later, relative cell numbers were compared by using PrestoBlue cell viability reagent (Left). AUC analyses (Right) revealed that IL-4 stimulates ESC proliferation in an IL-4Rα and STAT6-dependent manner (values are means ± SD, n = 3 per group; results displayed are representative of 3–4 independent experiments).
Fig. S4.
Gating strategy used to determine CD124 expression in murine ESCs. As stated in SI Materials and Methods, uninfected WT mice were euthanized 5 d after receiving 1 mg of DMPA s.c., and UGTs were excised and processed into single-cell suspensions for flow cytometric analysis. Representative contour plots show gating strategy used to identify ESCs directly ex vivo (i.e., live vimentin+ cells not expressing CD45 or cytokeratin). CD124 (IL-4Rα) expression in ESCs from WT mice was quantified via flow cytometry by using gates defined using ESCs isolated from IL-4Rα−/− mice (results displayed are representative of three independent experiments).
Fig. S5.
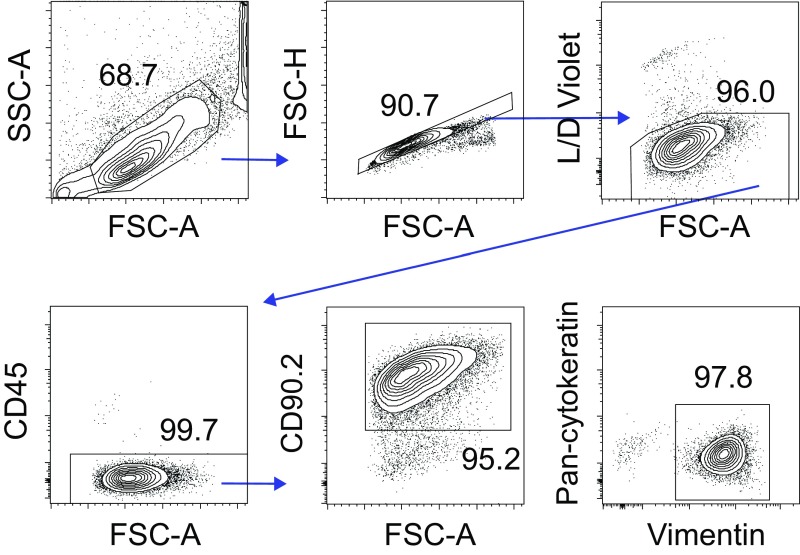
ESC isolation yielded high-purity ESC cultures. Murine ESCs were isolated from WT mice as described in Fig. S4 and SI Materials and Methods. Representative contour plots show gating strategy used to confirm that isolation procedures consistently yielded ≥95% ESC purity (as defined by CD90 and vimentin expression and no CD45 or cytokeratin expression). Of note, equivalent purity results were observed when ESCs were isolated from uterine horns of IL-4Rα−/− or STAT6−/− mice (results displayed are representative of four independent experiments).
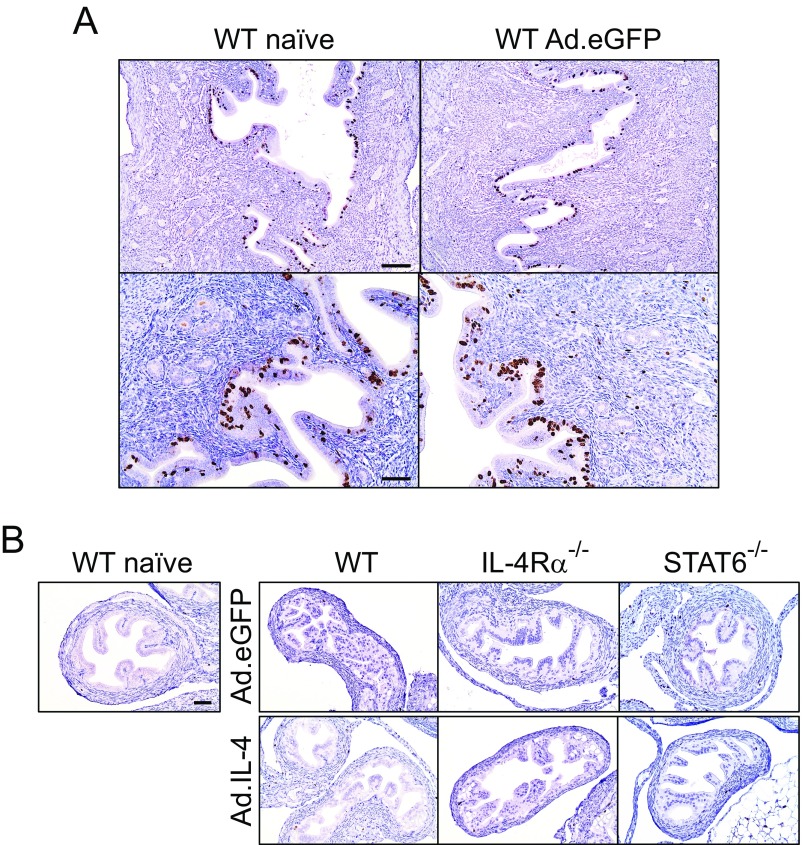
To resolve whether IL-4–mediated processes comparably enhance ESC proliferation in vivo, WT mice were intrauterinely administered an IL-4–expressing adenoviral vector (Ad.IL-4) (26). This localized IL-4 overexpression increased proliferation of ESCs and luminal endometrial epithelial cells and induced histologic changes that resembled endometrial decidual reaction (Fig. 8 A and B). It also suggested that IL-4–mediated stimulation of ESC proliferation did not depend on pathogen-induced tissue insult. Administering Ad.IL-4 to IL-4Rα−/− mice or an eGFP-expressing adenoviral vector (Ad.eGFP) to WT mice did not promote ESC proliferation (Fig. 8 and Fig. S6A), but Ad.IL-4 administration to STAT6−/− mice stimulated endometrial epithelial cell proliferation without affecting ESC proliferation (Fig. 8B). These results indicated IL-4–mediated signaling processes that promote in vivo ESC proliferation are IL-4Rα– and STAT6-dependent, whereas IL-4–mediated signaling processes that stimulate in vivo endometrial epithelial cell proliferation do not depend on STAT6. Finally, we saw no effect on oviduct epithelial or stromal cell proliferation in WT, IL-4Rα−/−, or STAT6−/− mice treated with Ad.IL-4 or Ad.eGFP (Fig. S6B), implying that IL-4–mediated effects on genital tract stromal cell proliferation were more relevant to endometrial repair processes.
Fig. 8.
IL-4 overexpression induces robust in vivo ESC proliferation that depends on IL-4Rα expression by nonhematopoietic cells. Uninfected WT, IL-4Rα−/−, or STAT6−/− mice were intrauterinely administered 1010 viral particles of Ad.eGFP or Ad.IL-4 via a nonsurgical embryo transfer device. Animals were euthanized 3 d later, and UGT excised and paraffin-embedded for histopathological evaluation (A) and immunohistochemical Ki-67 staining (B) (representative images shown; n = 3 per group). (C) As described in Materials and Methods, lethally irradiated WT or IL-4Rα−/− mice were reconstituted with BM cells from WT or IL-4Rα−/− mice (as indicated) 2 mo before treatment with Ad.eGFP or Ad.IL-4 (representative images displayed; n = 3 per group) (results representative of 2–3 independent experiments). (Scale bars: A, 400 µm; B = 100 µm; C, 50 µm.)
Fig. S6.
Ad.eGFP and Ad.IL-4 did not induce oviduct cell proliferation. (A) DMPA-treated female WT mice remained untreated (naïve) or intrauterinely administered Ad.eGFP. Mice were euthanized 3 d later, and UGT was processed as denoted in Fig. 8 A and B. Representative images indicate that Ki-67 expression was unaffected by Ad.eGFP administration (Scale bars: A, Upper, 100 µm; A, Lower and B, 50 µm.) (B) Oviducts of the same mice shown in Fig. 8 A and B and an untreated mouse (Left) were examined for Ki-67 expression (results are representative of 2–3 independent experiments).
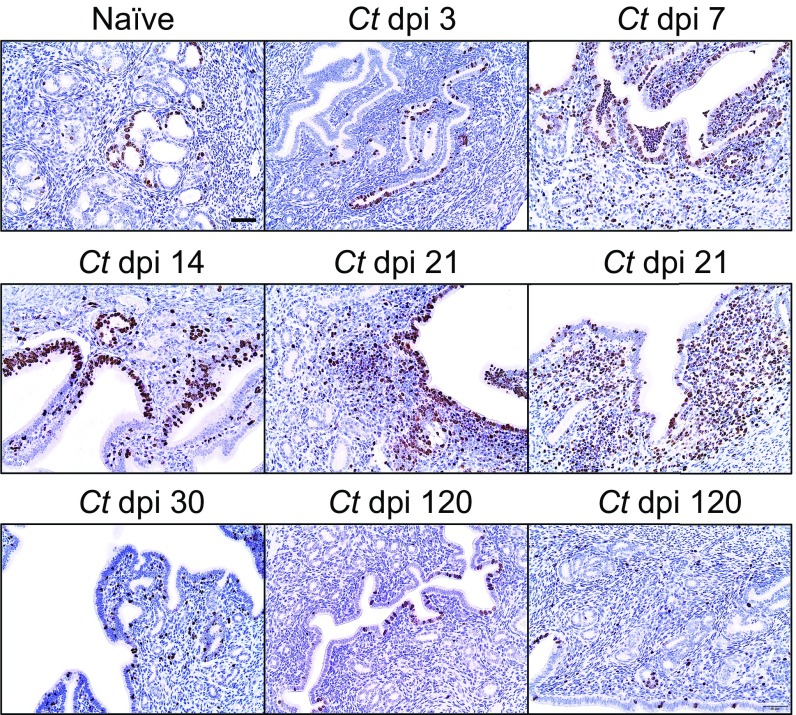
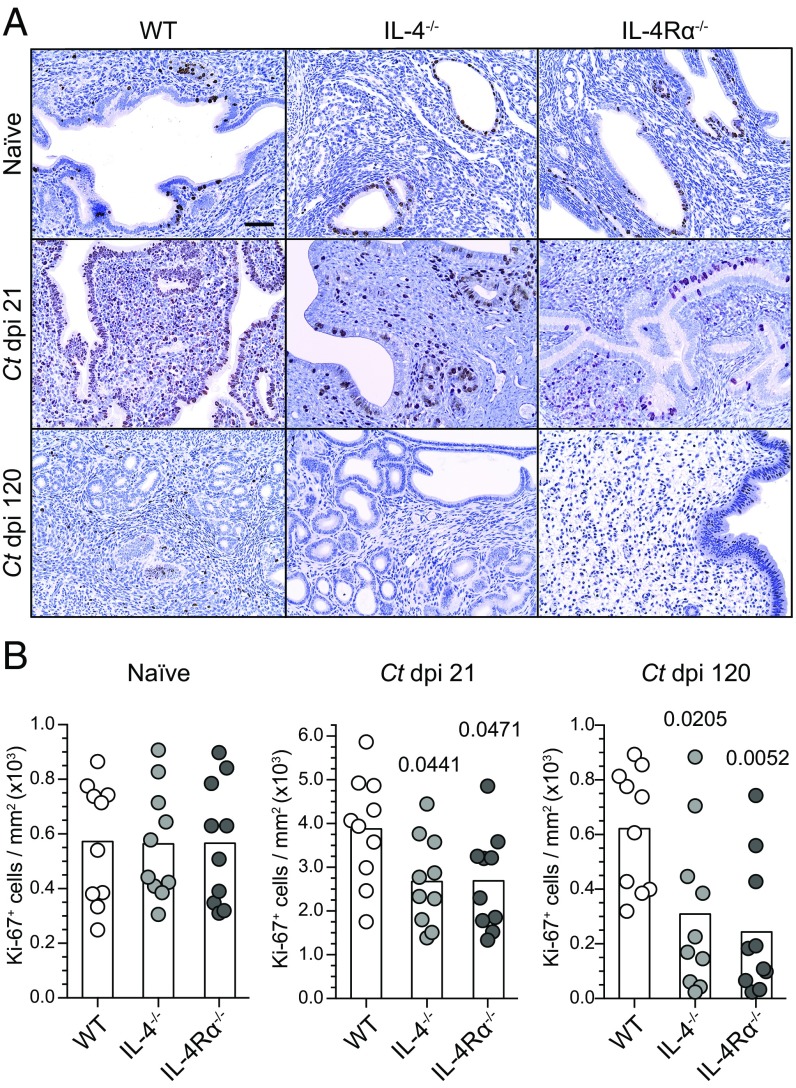
We concluded the current study by exploring whether IL-4 directly promotes in vivo ESC proliferation or whether the enhanced ESC proliferation occurs secondary to an IL-4–mediated effect on hematopoietic cells. To explore these possibilities, we intrauterinely administered Ad.IL-4 or Ad.eGFP to WT and IL-4Rα−/− mice reconstituted with WT bone marrow (BM) and WT mice reconstituted with IL-4Rα−/− BM. These BM chimera studies showed that in vivo ESC proliferation was increased in all groups of mice except those mice whose nonhematopoietic cells did not express IL-4Rα (Fig. 8C). As a follow-up to these results, we enumerated ESC proliferation at various time points after ivag infection of WT mice, and found highest ESC proliferation at 21 dpi (Fig. S7). Upon comparing levels of ESC proliferation in WT, IL-4−/−, or IL-4Rα−/− mice at this same time point after infection, we saw significantly lower ESC proliferation levels in the two groups of mice with defective IL-4 signaling (Fig. 9). Moreover, this difference in ESC proliferation became more profound at 120 dpi (Fig. 9). In combination with our earlier results, these studies showed that IL-4 acts directly on ESCs to increase their proliferation, and this effect helped preserve normal genital tract architecture during infectious insult.
Fig. S7.
Genital C. trachomatis infection induces robust ESC proliferation at 21 dpi. WT mice were genitally infected with C. trachomatis as indicated in Materials and Methods and euthanized at various dpi. Levels of Ki-67 expression in the endometrium were assessed in paraffin-embedded UGTs at the indicated dpi (all results are representative of two independent experiments).
Fig. 9.
IL-4 signaling promotes ESC proliferation after C. trachomatis infection. (A) Uninfected female WT, IL-4−/−, or IL-4Rα−/− mice remained uninfected or were ivag infected with C. trachomatis as detailed in Fig. 1. Animals were euthanized 21 and 120 d later to assess expression of Ki-67 by endometrial cells (representative images displayed). (Scale bar: 50 µm.) (B) Quantification of Ki-67+ endometrial cells from animals described in A (bars denote means, n = 10 per group); results representative of three independent experiments).
Discussion
To successfully resolve the system perturbations induced by physiologic change or infectious insult, endometrial tissue must display remarkable plasticity. During the human menstrual cycle, tissue homeostasis is restored by coordinated processes that include reepithelialization, ESC remodeling, and endothelial cell proliferation (24, 25). Whereas C. trachomatis ascension into endometrial tissue elicits an acute influx of PMNs and other proinflammatory leukocytes, only a small subset of women with laparoscopically confirmed PID develop permanent UGT damage (18). Although mice do not menstruate, their endometrium analogously undergoes cyclical rounds of cell growth and apoptosis during the estrus cycle (27, 28). Primary intravaginal C. trachomatis infection of mice likewise elicits robust endometrial inflammation, but causes no permanent UGT damage (12). Our study confirms and extends the latter observation, revealing IL-4–producing eosinophils mediate repair processes during primary Chlamydia infection that conserve normal endometrial structure.
Multiple results from our investigation support this conclusion. Our initial work identified IL-4−/− and IL-4Rα−/− mice as highly susceptible to endometrial damage after primary C. trachomatis infection. However, this susceptibility was unrelated to impaired Chlamydia control, because genital chlamydial clearance was equivalent among WT mice and mice with deficient IL-4 signaling. Likewise, altered T-cell function did not promote immunopathological tissue damage, because WT and IL-4−/− and IL-4Rα−/− mice showed comparable Chlamydia-specific TH1 and TH17 effector function. Moreover, and congruent with prior reports (29, 30), IL-10−/− mice cleared infection more rapidly and generated more robust Chlamydia-specific TH1 immunity than WT mice, but the exuberant TH1 response by IL-10−/− mice to Chlamydia infection did not stimulate damage to UGT tissue. Because impaired clearance or altered adaptive immunity did not explain the increased Chlamydia-induced endometrial pathology in IL-4−/− and IL-4Rα−/− mice, we explored the role of innate immune cells in modulating genital tissue repair after infectious insult. Although prior work identified the importance of M2 Mϕs in type 2 immunity and tissue repair (31), our study indicates that uterine Mϕs have a more negligible role in repair of endometrial tissue after primary C. trachomatis infection. Conversely, we uncovered an underappreciated significance for eosinophils as host defense against tissue damage during genital infection.
Although our study identifies that endometrial eosinophils provide an essential host defense during infectious insult, the capacity of IL-4–secreting eosinophils to promote ESC proliferation and tissue repair in the Chlamydia-infected endometrium is highly congruent with established roles for eosinophils in other tissues. To promote muscle regeneration, eosinophils stimulate fibro/adipogenic progenitors, bipotential cells capable of differentiating into fibroblasts and adipocytes (13, 14). IL-4–secreting eosinophils in mice are also rapidly recruited to sites of toxin-mediated liver damage, and stimulate tissue regeneration by directly promoting proliferation of quiescent hepatocytes (13, 14). In a mouse model of experimental colitis, resident mucosal eosinophils also exert protective effects by secreting antiinflammatory lipid mediators (32). Likewise, the detection of increased numbers of activated eosinophils in individuals with inactive ulcerative colitis suggests these cells may facilitate repair of colonic mucosal epithelium in humans (33). When pulmonary mucosal tissue of mice is repetitively challenged with a highly immunogenic allergen, airway hyperresponsiveness and lung inflammation is resolved by IL-10–secreting eosinophils (34). Consistent with the eosinophil-mediated repair of Chlamydia-infected endometrial tissue identified herein, eosinophils may also regulate noninfectious processes in the mouse endometrium. As an example, when mice are depleted of granulocytes with an anti–Gr-1 monoclonal antibody (an antibody that also binds and systemically depletes eosinophils) (35), altered patterns of uterine bleeding and tissue remodeling were detected (36, 37). Our study provides an important extension of these prior studies, demonstrating that IL-4–producing eosinophils are essential to prevent genital pathogen-induced destruction of UGT tissue.
To uncover this function for endometrial eosinophils, we used a murine model of genital C. trachomatis infection. Prior studies, including our own, failed to detect significant TH2 responses in the UGT of Chlamydia-infected mice (12, 29, 38), and we exploited this observation in the current investigation to interrogate the role of type 2 innate immunity in pathogen clearance and tissue repair. However, C. trachomatis is exclusively a human pathogen (12, 39), a factor that appears to limit the ability of murine C. trachomatis infection to fully model human host disease. Whereas phenotypic expression of disease in women typically involves Fallopian tube damage, we found that primary ivag C. trachomatis infection of IL-4−/− and IL-4Rα−/− mice elicits pervasive endometrial damage that is oviduct-sparing. Despite the dissimilar expression of C. trachomatis-induced UGT pathology, it seems likely our findings will inform processes of endometrial repair in women. In the first place, there is significant functional overlap between human and mouse eosinophils (40), including the production and secretion of preformed IL-4 (40-43). The recruitment of eosinophils to the endometrium also appears similar, because CCL11 and CCR3 expression by human and mice eosinophils regulates endometrial trafficking (44–48). Congruent with these observations, we found mouse ESCs express high levels of CCL11 (Fig. 6C), an eosinophil chemoattractant similarly secreted by IL-4–stimulated human endometriotic stromal cells (49). Unlike mice, eosinophils may not be a resident population in the human endometrium, but large numbers of these cells are known to infiltrate the genital tract of women during menstruation and cervical ripening and the endometrium of women with endometriosis and endometrial adenocarcinoma (50–53). Because these clinical conditions are all associated with tissue remodeling and activation of signaling pathways linked to tissue regeneration (54–57), it seems plausible that human and mouse endometrial eosinophils play analogous roles in repair of genital tissue. Further supporting the clinical relevance of our findings, retrospective studies found eosinophils more common in the endometrium of women with chronic endometritis vs. healthy controls (16, 17). Because increased eosinophil numbers appear less common in the endometrium of women with acute endometritis, and danger signals released from damaged genital epithelial cells are shown to active eosinophils (58), findings from these retrospective clinical studies imply that eosinophils also participate in endometrial tissue repair processes in humans.
In addition to revealing a role for eosinophils in endometrial tissue repair after infectious insult, our study identifies IL-4–mediated promotion of ESC proliferation as a mechanism by which eosinophils regulate this repair. The latter finding is congruent with prior experimental models that showed eosinophils and other type 2 innate immune cells regulate stromal cell-mediated tissue repair (13, 19). Earlier studies likewise showed human and mouse ESCs play key roles in the tissue repair necessitated by cyclic menstruation or parturition (24, 25). During endometrial regeneration, ESCs were also shown to provide a source of epithelial cells by undergoing mesenchymal-epithelial transition (28, 59). Although IL-4 was previously shown to promote in vitro proliferation of endometriotic stromal cells (60), our study establishes that IL-4 also promotes in vivo ESC proliferation, an essential reparative process in several animal models of endometrial tissue repair (24, 25). By identifying that no hematopoietic cell intermediaries are required to promote in vivo ESC proliferation (Fig. 8), our study also establishes that enhanced ESC proliferation is a direct effect of IL-4. Because genital C. trachomatis infection induced uterine pathology in IL-4−/− and IL-4Rα−/− mice (while uninfected mice of these same genotypes developed no pathology), our study provides strong evidence IL-4–secreting eosinophils promote ESC proliferation that repairs genital pathogen-induced endometrial tissue damage.
While establishing that eosinophils, a key type 2 innate immune cell, prevent UGT tissue damage in a mouse model of genital infection, we speculate that our study may also have implications for Chlamydia vaccine development. Genital C. trachomatis infection in women is often chronic, rarely causes phenotypic disease expression, and elicits robust type 2 innate and adaptive immunity (6, 7). This clinical presentation implies immune tolerance, not just immune-driven resistance mechanisms were selected by evolution to combat this ubiquitous human pathogen (61). Whereas tolerance exerts marginal influence on pathogen load, it is more capable than immune-driven resistance mechanisms alone to maintain immunological equilibrium and dampen immunopathological tissue damage (61). By demonstrating the significance of type 2 innate immunity during genital Chlamydia infection, our current findings support this possibility. Although inducing Chlamydia-specific type 1 immunity has been a long-term goal of Chlamydia vaccine research (62), candidate vaccines that elicit only TH1-type responses have potential to promote Chlamydia clearance and tissue immunopathology (11, 12). In other words, for a TH1-based Chlamydia vaccine to be both effective and safe, it may also need to induce TH2 responses that promote genital tissue repair and resolve the recurrent bouts of inflammation induced by repetitive exposure to the bacterium during sexual intercourse. Incorporating responses that promote tissue repair may be especially useful for Chlamydia vaccines, because memory TH2 responses are critical for preventing tissue damage induced by multicellular pathogens (63, 64). As another potential advantage of Chlamydia vaccines that induce TH2 immunity, permanent tissue damage is most effectively avoided when tissue repair is initiated shortly after inflammation onset (31). Whereas increasing the number of IL-4–secreting eosinophils in the UGT may not offer a mechanism by which Chlamydia vaccines minimize the risk of immunopathological tissue damage, our study highlights the significance of type 2 immunity during infectious insult in the female genital tract and the need to delineate the significance of type 2 immunity as human host defense against C. trachomatis and other genital pathogens.
Materials and Methods
Mice.
Mouse experiments were approved by Institutional Animal Care and Use Committees at The Ohio State University and the University of Pittsburgh. Experiments were performed per institutional guidelines and in accordance with the Guide for the Care and Use of Laboratory Animals (65). As indicated, 6- to 10-wk-old female BALB/cJ, BALB/c-Il4tm2Nnt/J (IL-4−/−), BALB/c-Il4ratm1Sz/J (IL-4Rα−/−), C.129P2(B6)-Il10tm1Cgn/J (IL-10−/−), C.129-Il4tm1Lky/J (4get), C.129S2-Stat6tm1Gru/J (STAT6−/−), and C.129S1(B6)-Gata1tm6Sho/J (ΔdblGATA1) mice (all from Jackson Laboratories) were housed in specific pathogen-free conditions before use. To generate bone marrow chimera mice, bone marrow was transferred from female WT BALB/cJ or IL-4Rα−/− mice into lethally irradiated female WT or IL-4Rα−/− mice as described (66), but with the modifications that mice were reconstituted with 5 × 106 bone marrow cells and used 2 mo after reconstitution.
C. trachomatis Infection.
Because mouse susceptibility to genital C. trachomatis infection is estrus cycle stage-dependent (67), mice were s.c. injected with 1 mg of depot-medroxyprogesterone acetate (DMPA) (Upjohn) 5 d before ivag infection. This dose produces pharmacologically relevant serum MPA levels (68) and ensures productive infection (12). When uninfected mice were used in experiments, they were similarly treated with DMPA. Murine ivag infection with 104 inclusion-forming units (IFUs) of oculogenital strain UW-3/Cx of C. trachomatis serovar D (ATCC VR885) (American Type Culture Collection) was performed as described (12). This infectious inoculum is consistent with C. trachomatis levels detected in human biological specimens (69). At various time points after infection, IFUs were measured as described (70).
Adenoviral Vector Administration and Confocal Microscopy.
The adenoviral type 5-derived E1- and E3-deleted replication-deficient vectors expressing eGFP (Ad.eGFP) or murine IL-4 (Ad.IL-4) used in our studies are described in detail (26). Briefly, as DMPA impairs genital mucosal barrier protection and increases susceptibility to genital viral infection (71), mice received 1 mg of this synthetic progestin 5 d before genital mucosal administration of any adenoviral vector. For in vivo transduction using adenoviral vectors, DMPA-treated mice were intrauterinely administered 1010 viral particles of indicated adenoviral vector via a nonsurgical embryo transfer device (NSET) (ParaTechs Corporation). Ivag administration of Ad.eGFP did not induce significant protein expression in the murine endometrium (Fig. S8). To define eGFP expression after Ad.eGFP ivag or intrauterine administration, genital tissues were harvested 1 d later, fixed with 4% methanol-free formaldehyde (Thermo Fisher Scientific), and embedded in 6% low melting point agarose (Life Technologies). Two hundred fifty-micrometer sections were permeabilized with 0.2% Triton X-100 (Sigma-Aldrich) solution in PBS, stained with 4,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich), embedded in mounting media (Vector Laboratories), and evaluated for reporter gene expression with a FV1000 spectral confocal microscope system (Olympus). As shown in Fig. S8, intrauterine Ad.eGFP was the only administration route that provided significant transduction of eGFP in the murine endometrium. To evaluate the effect of adenoviral-mediated transduction of eGFP or IL-4 on in vivo ESC proliferation, adenoviral vector-treated mice were killed 3 d after administration of recombinant adenovirus, and whole UGTs were excised for histopathological and immunohistochemical analysis (described in detail in SI Materials and Methods).
Fig. S8.
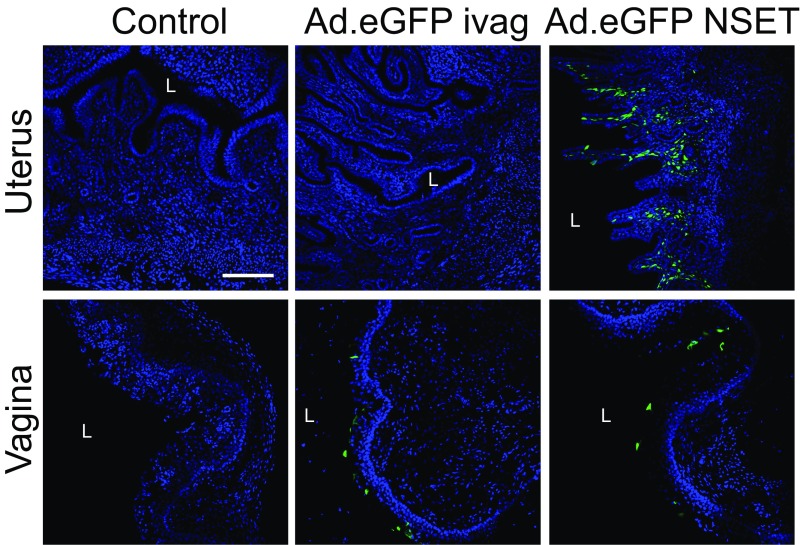
Intrauterine adenoviral vector administration induces significant endometrial expression of the transduced gene. To define the extent of eGFP expression in vaginal and endometrial mucosa after ivag or intrauterine Ad.eGFP administration, DMPA-treated WT mice remained untreated (control) or were ivag or intrauterinely administered 1010 viral particles of Ad.eGFP. Mice were euthanized 24 h later, and UGTs were processed for confocal microscopic evaluation as detailed in Materials and Methods. (Scale bar: 50 µm.) L, vaginal or uterine lumen (results are representative of two independent experiments).
Detailed descriptions of methods used for C. trachomatis quantification, histopathologic and immunohistochemical analysis, flow cytometric analysis, ESC isolation and evaluation of ESC function, RNA gene expression analysis, and statistical considerations are in the SI Materials and Methods.
SI Materials and Methods
C. trachomatis Quantification.
At indicated dpi, cervicovaginal lavages (CVLs) were obtained to quantify C. trachomatis DNA levels by qRT-PCR. For CVL collection, 30 µL of PBS was inserted into the vaginal vault and recovered. Total DNA was isolated from each sample with the DNeasy Blood & Tissue Kit (Qiagen) following manufacturer’s instructions. Using primers and methods described (12), Chlamydia (16S rRNA) DNA was quantified by using a TaqMan assay-based qRT-PCR. For graphical representation of Chlamydia clearance, nondetectable values were assigned the lowest value in the y axis (i.e., for purposes of log scale presentation), and denoted in figures as nondetectable (ND).
Histopathology and Immunohistochemistry.
Murine genital tracts were removed en bloc at indicated time points and immediately preserved in buffered 4% formaldehyde solution and paraffin-embedded. As indicated, 5-µm sections of cervix, uterine horns, and oviducts were stained with hematoxylin and eosin (H&E), Masson’s trichrome stain, or Alcian blue and periodic acid–Schiff (PAS) stain. Genital tract structures were assessed for acute inflammation (neutrophilic infiltrates), chronic inflammation (lymphocytic infiltrates), stromal edema, fibrosis, and luminal dilation by using the following semiquantitative histopathological aggregate scoring system: 0, no significant infiltration, edema, fibrosis, myxedema, or dilation; 1, infiltration at a single focus, minimal edema, fibrosis, myxedema, or dilation; 2, infiltration at two to four foci, mild edema, fibrosis, myxedema, or dilation; 3, infiltration at more than four foci, moderate edema, fibrosis, myxedema, or dilation; and 4, confluent infiltration, severe edema, fibrosis, myxedema, or dilation. Component scores for both uterine horns provided aggregate scores. All scoring was performed by investigators blinded to infection status, treatment group, and mouse genotype. To define maximum uterine diameter, slides were scanned at 400× resolution with an Aperio high-throughput digital scanner (Leica Biosystems) that acquired whole UGT images (Fig. 1A). Images were analyzed using ImageJ software (72) (diameters from multiple uterine horn areas were measured, and the largest uterine horn diameter selected for comparison). Where indicated, paraffin-embedded tissue sections were immunohistochemically stained for Ki-67 by using a rabbit monoclonal antibody (clone SP6, Thermo Fisher Scientific), a biotinylated polyclonal goat anti-rabbit IgG secondary antibody (Thermo Fisher Scientific), and the Vectastain Elite ABC HRP Reagent (Vector Laboratories). To quantify Ki-67+ endometrial cells in tissue sections, 10 separate fields were photographed using 200× magnification, and Ki-67+ cells were enumerated via ImageJ software. Images were separated into respective RGB channels and converted to 8-bit grayscale images. The threshold for the blue channel was adjusted to exclude non–Ki-67+ cells, and individual Ki-67+ cell numbers were calculated by using the analyze particles component of this software. The average of 10 separate fields was used in statistical comparisons.
Flow Cytometric Analyses.
For intracellular cytokine staining assays, DLNs were excised at 5 d after challenge and processed into single-cell suspension. Cells were resuspended at a density of 106 cells per mL in RPMI 1640 (Cellgro, Mediatech) supplemented with 10% FBS (Atlanta Biologicals), 2 mM l-glutamine, 1 mM sodium pyruvate, nonessential amino acids, 50 μM 2-ME, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 µg/mL gentamycin (all Cellgro) (hereafter termed complete media), and incubated for 36 h at 37 °C in 5% CO2 atmosphere with complete media alone, 5 μg/mL PHA (Sigma), or C. trachomatis serovar D EB (106 IFU/mL) previously inactivated by UV irradiation. For the last 6 h of incubation, cultures were administered manufacturer-recommended amounts of Brefeldin A (GolgiPlug, BD Biosciences). Cells were stained with LIVE/DEAD Fixable Near-IR or Aqua Dead Cell stain (Invitrogen), incubated with anti-CD16/CD32 mAb (2.4G2, BD Biosciences), and stained with combinations of the following antibodies: V450-conjugated anti-CD90.2 (53-2.1, BD Biosciences), BV510-conjugated anti-CD90.2 (clone 53–2.1, BioLegend), AF700- or PerCP-Cy5.5–conjugated anti-CD8α (53-6.7, BD Biosciences), BV605-conjugated anti-CD4 (clone RM4-5, BioLegend), APC-eF780–conjugated anti-CD11b (clone M1/70, eBioscience), BV510-conjugated anti-CD11b (M1/70, BioLegend), and PerCP-Cy5.5- or APC-eF780-conjugated anti-CD45R (clone RA3-6B2, eBioscience). After fixation and permeabilization with Cytofix/Cytoperm solution (BD Biosciences), cells were stained with combinations of the following antibodies: eF450- or AF488 conjugated anti–IFN-γ (XMG1.2, eBioscience), PE-Cy7- or AF700-conjugated anti-TNF (MP6-XT22, BioLegend), PE- or APC-conjugated anti–IL-17A (eBio17B7, eBioscience), AF488- or APC-conjugated anti–IL-10 (JES5-16E3, eBioscience) and PE- or PE-Cy7–conjugated anti-IL-4 (clone 11B11, eBioscience) diluted in Perm/Wash buffer (BD Biosciences). To characterize genital tract leukocyte subpopulations, entire UGTs were excised and processed into a single-cell suspension by using complete media that contained 1 mg/mL collagenase D (Roche), 1 mg/mL Dispase II (Roche), and 0.25 mg/mL DNase I (Sigma). Cells were incubated 1 h at 37 °C. Then EDTA (Lonza) was added (10 nM final concentration) to inhibit collagenase D and Dispase II digestion. Cell suspensions were strained by using 70-µm nylon cell strainers, and centrifuged at 300 × g for 10 min, washed with PBS, and suspended in PBS. Cells were incubated with LIVE/DEAD Fixable Violet or Near-IR or Aqua Dead Cell stain (Invitrogen), incubated with anti-CD16/32 mAb (93, BioLegend), and stained with combinations of the following monoclonal antibodies (mAbs): APC-eF780–conjugated anti-F4/80 (BM8), APC-eF780– or eF450–conjugated anti-CD3 (17A2), and APC-eF780–conjugated anti-CD11c (N418) from eBioscience; FITC-conjugated anti-CD40 (3/23), PE- or AF700- or eF450-conjugated anti-Ly6G (1A8), PE-CF596- or APC-conjugated anti-Siglec-F (E50-2440), PE- or PE-Cy7- or AF700-conjugated anti-Ly6C (AL-21), PerCP-conjugated anti-CD45 (30-F11), PE- or PE-Cy7- or V450-conjugated anti-CD90.2 (53-2.1) from BD Biosciences; FITC-conjugated anti-CD200R (OX-110), AF488-conjugated anti-CD40 (HM40-3), PE-conjugated anti-CD169 (3D6,112), PE-conjugated anti-FcεRIα (MAR-1), PE-conjugated anti-CCR3 (J07E5), PE-Cy7-conjugated CD4 (GK1.5), APC-conjugated anti-CD206 (C068C2), APC-conjugated CD49b (DX5), AF700-conjugated anti-Ly6C (HK1.4), BV510-conjugated anti-I-A/I-E (M5/114.15.2), and PE-Cy7 or BV605-conjugated anti-CD11c (N418) from BioLegend. In some instances, after fixation and permeabilization with Cytofix/Cytoperm solution, cells were stained with unconjugated anti-NOS2 polyclonal rabbit Ab or mouse anti-arginase 1 (both BD Biosciences), and incubated with PE-conjugated F(ab')2-goat anti-rabbit IgG (Invitrogen) or APC-conjugated rat anti-mouse Ig-G1 (BD Biosciences). Cells were collected on a LSR-II flow cytometer (BD Biosciences), and data was analyzed by using FlowJo software (Tree Star). Fluorescence minus one (FMO) controls, and biological comparison controls when available, established gates that defined intracellular cytokine production by live T cells and the myeloid cell population of interest (73). Cytokine-producing T-cell percentages were defined by subtracting cytokine percentage values defined in unstimulated samples.
ESC Isolation and Evaluation of Function.
Procedures were principally performed as described (60). Briefly, murine uteri were minced into small pieces, and incubated in RPMI 1640 containing 5% charcoal-treated FBS (Atlanta Biologicals), 1 mg/mL Collagenase D (Roche), 2 mg/mL Dispase II (Roche), and 0.25 mg/mL DNase I (Sigma) for 1 h at 37 °C. EDTA (Lonza) was added (10 nM final concentration) to inhibit protease digestion, and cell suspensions were strained by using 70-µm nylon cell strainers. As indicated, cell suspensions were stained with LIVE/DEAD Fixable Near-IR (Invitrogen), incubated with Fc Block (BD Biosciences), and stained with PE-conjugated anti-CD124 (mIL4R-M1, BD Biosciences), eF450-conjugated anti-CD45 (30-F11, eBioscience), and BV510-conjugated anti-CD11b (M1/70, BioLegend) mAbs. After staining, cells were fixed with Cytofix/Cytoperm solution, and stained with AF488-conjugated anti–pan-cytokeratin (AE1/AE3, eBiosciences) and AF647-conjugated anti-vimentin (D21H3, Cell Signaling Technology) mAbs diluted in Perm/Wash buffer. For ESC isolation, cell suspensions were centrifuged at 300 × g for 10 min, washed with PBS, suspended in DMEM/F12 medium (Life Technologies), plated into tissue culture flasks, and allowed to adhere for 30 min at 37 °C. Nonadherent cells were removed by gentle rinses with warm PBS, and ESCs cultured in Advanced DMEM/F-12 media (Life Technologies) supplemented with 5% charcoal-treated FCS, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (all Cellgro). When nearly confluent, ESC cultures were passaged by using 0.25% trypsin/EDTA solution (Sigma) (cells were passed at least four times before use). To determine ESC purity and CD124 expression, freshly detached ESCs were stained with LIVE/DEAD Violet (Invitrogen), PE-conjugated anti-CD124, PE-Cy7 anti-CD90.2 (53-2.1, eBioscience), and PerCP-conjugated CD45 (30-F11, BD Biosciences) mAbs. Cells were fixed with Cytofix/Cytoperm solution, and stained with AF488-conjugated anti–pan-cytokeratin (AE1/AE3, eBioscience) and AF647-conjugated anti-vimentin (D21H3, Cell Signaling Technology) mAbs diluted in Perm/Wash buffer. We routinely obtained ESCs with >95% purity as determined by characteristic fibroblast-like morphology, lack of CD45 and cytokeratin expression, and high expression of vimentin and CD90 (Fig. S5). ESCs were plated into six-well plates at 2 × 105 cells per well for qRT-PCR or phospho flow staining or 96-well plates at 5 × 103 cells per well for cell proliferation assays, and incubated at 37 °C in humidified 5% CO2/95% air environment for 24 h. Serum-containing media were removed and replaced with fresh serum-free DMEM/F-12 media containing antibiotics, and cells were cultured another 24 h. To evaluate dose-dependent IL-4 effects on ESC proliferation, wells were replenished with serum-free media containing various murine IL-4 concentrations (R&D Systems) or vehicle. After a 3-d incubation, relative cell numbers (% of vehicle-treated cells) per well were estimated by using PrestoBlue cell viability reagent (Life Technologies) (a 14-h incubation preceded defining of fluorescence). To evaluate STAT6 phosphorylation, ESCs were stimulated with murine IL-4 (1 µg/mL) for 10 min at 37 °C in a humidified 5% CO2/95% air environment. Cells were gently washed with warm PBS and incubated for 3 min with 0.25% trypsin/EDTA solution (total time 5 min). An equal volume of Fixation buffer (BD Biosciences) was added immediately, cells were resuspended vigorously, and incubated at room temperature for 10 min. Cells were washed with PBS twice and permeabilized on ice with ice cold Perm Buffer III (BD Biosciences) for 15 min. Cells were washed twice with Stain Buffer (BD Biosciences) and stained with PE-conjugated mouse anti-mouse pSTAT6 (Y641) (J71-773.58.11, BD Biosciences). ESCs were washed with Stain Buffer and immediately collected in a LSR-II flow cytometer. FMO controls, and internal negative controls when available, informed the setting of gates used to delineate ESC identity and expression of indicated markers. Murine skin fibroblast isolation was performed as described (74).
RNA Isolation and qRT-PCR.
Total RNA from UGTs or ESCs was isolated by using the RNeasy lipid tissue mini kit (Qiagen) according to manufacturer’s instructions. RNA concentration and purity were determined via a NanoDrop spectrophotometer (Thermo Fisher Scientific) (all samples displayed 260/280 ratios > 1.80). The TaqMan RNA-to-Ct 1-Step kit (Life Technologies) was used to determine relative gene expression with the Bio-Rad CFX96 qPCR System. Primers used for qRT-PCR were CCL7 (Mm00496525_m1), CCL9 (Mm_m1), CCL11 (Mm00809994_s1), and pyruvate carboxylase (Mm00500992_m1), the latter used as a housekeeping reference gene (all Life Technologies), and data was analyzed by using CFX Manager Software (Bio-Rad Laboratories).
Statistical Considerations.
All statistical analyses were performed by using Prism 6 software (GraphPad). Data normality was tested by using the D’Agostino–Pearson omnibus K2 test, the Shapiro–Wilk test, or evaluation of the residuals depending on sample size. Differences between two groups were compared by unpaired Student t tests or an unpaired Mann–Whitney u tests, depending on data distribution. For Chlamydia clearance and proliferation studies, area under the curves (AUCs) for each mouse or cell isolate/strain/condition were calculated (total Ct DNA/CVL × dpi; relative cell number × IL-4 concentration) and AUC values were compared. For multiple group comparisons, depending on data distribution, one-way ANOVA and Tukey’s or Dunnett’s multiple comparison post hoc tests or the Kruskal–Wallis test on ranks and Dunn’s multiple comparison post hoc test were used (P values <0.05 were considered statistically significant).
Acknowledgments
We thank The Ohio State University’s Comparative Pathology and Mouse Phenotyping Shared Resource (supported by NIH/National Cancer Institute Grant P30 CA016058) for support provided and Ann E. Thompson and Charles Lockwood for contributions made toward completion of this work. Financial support for this work was provided by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01HD072663, Stanford University School of Medicine, The Ohio State University College of Medicine, and University of Pittsburgh School of Medicine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621253114/-/DCSupplemental.
References
- 1.Gottlieb SL, Martin DH, Xu F, Byrne GI, Brunham RC. Summary: The natural history and immunobiology of Chlamydia trachomatis genital infection and implications for Chlamydia control. J Infect Dis. 2010;201:S190–S204. doi: 10.1086/652401. [DOI] [PubMed] [Google Scholar]
- 2.Newman L, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geisler WM. Duration of untreated, uncomplicated Chlamydia trachomatis genital infection and factors associated with chlamydia resolution: A review of human studies. J Infect Dis. 2010;201:S104–S113. doi: 10.1086/652402. [DOI] [PubMed] [Google Scholar]
- 4.Peipert JF. Clinical practice. Genital chlamydial infections. N Engl J Med. 2003;349:2424–2430. doi: 10.1056/NEJMcp030542. [DOI] [PubMed] [Google Scholar]
- 5.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: Evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vicetti Miguel RD, et al. Human female genital tract infection by the obligate intracellular bacterium Chlamydia trachomatis elicits robust Type 2 immunity. PLoS One. 2013;8:e58565. doi: 10.1371/journal.pone.0058565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen CR, et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: Importance of heat-shock protein 60 and interferon- gamma. J Infect Dis. 2005;192:591–599. doi: 10.1086/432070. [DOI] [PubMed] [Google Scholar]
- 8.Vicetti Miguel RD, Cherpes TL. Hypothesis: Chlamydia trachomatis infection of the female genital tract is controlled by Type 2 immunity. Med Hypotheses. 2012;79:713–716. doi: 10.1016/j.mehy.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 10.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun. 1997;65:2175–2182. doi: 10.1128/iai.65.6.2175-2182.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vicetti Miguel RD, Quispe Calla NE, Pavelko SD, Cherpes TL. Intravaginal Chlamydia trachomatis challenge infection elicits TH1 and TH17 immune responses in mice that promote pathogen clearance and genital tract damage. PLoS One. 2016;11:e0162445. doi: 10.1371/journal.pone.0162445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heredia JE, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goh YP, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci USA. 2013;110:9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 16.Adegboyega PA, Pei Y, McLarty J. Relationship between eosinophils and chronic endometritis. Hum Pathol. 2010;41:33–37. doi: 10.1016/j.humpath.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Perlman B, Goldsmith LQW, Heller DS. Are eosinophils a specific marker of chronic endometritis? J Gynecol Surg. 2016;32:345–347. [Google Scholar]
- 18.Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–192. [PubMed] [Google Scholar]
- 19.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 21.Pollard JW, Lin EY, Zhu L. Complexity in uterine macrophage responses to cytokines in mice. Biol Reprod. 1998;58:1469–1475. doi: 10.1095/biolreprod58.6.1469. [DOI] [PubMed] [Google Scholar]
- 22.Care AS, et al. Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest. 2013;123:3472–3487. doi: 10.1172/JCI60561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu C, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gargett CE, Nguyen HP, Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13:235–251. doi: 10.1007/s11154-012-9221-9. [DOI] [PubMed] [Google Scholar]
- 25.Weimar CH, Macklon NS, Post Uiterweer ED, Brosens JJ, Gellersen B. The motile and invasive capacity of human endometrial stromal cells: Implications for normal and impaired reproductive function. Hum Reprod Update. 2013;19:542–557. doi: 10.1093/humupd/dmt025. [DOI] [PubMed] [Google Scholar]
- 26.Feili-Hariri M, et al. Dendritic cells transduced to express interleukin-4 prevent diabetes in nonobese diabetic mice with advanced insulitis. Hum Gene Ther. 2003;14:13–23. doi: 10.1089/10430340360464679. [DOI] [PubMed] [Google Scholar]
- 27.Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16:818–834. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- 28.Cousins FL, et al. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS One. 2014;9:e86378. doi: 10.1371/journal.pone.0086378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marks E, Tam MA, Lycke NY. The female lower genital tract is a privileged compartment with IL-10 producing dendritic cells and poor Th1 immunity following Chlamydia trachomatis infection. PLoS Pathog. 2010;6:e1001179. doi: 10.1371/journal.ppat.1001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Igietseme JU, et al. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: Potential for cellular vaccine development. J Immunol. 2000;164:4212–4219. doi: 10.4049/jimmunol.164.8.4212. [DOI] [PubMed] [Google Scholar]
- 31.Allen JE, Wynn TA. Evolution of Th2 immunity: A rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masterson JC, et al. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut. 2015;64:1236–1247. doi: 10.1136/gutjnl-2014-306998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lampinen M, et al. Eosinophil granulocytes are activated during the remission phase of ulcerative colitis. Gut. 2005;54:1714–1720. doi: 10.1136/gut.2005.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda K, et al. Eosinophils contribute to the resolution of lung-allergic responses following repeated allergen challenge. J Allergy Clin Immunol. 2015;135:451–460. doi: 10.1016/j.jaci.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 36.Menning A, et al. Granulocytes and vascularization regulate uterine bleeding and tissue remodeling in a mouse menstruation model. PLoS One. 2012;7:e41800. doi: 10.1371/journal.pone.0041800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaitu’u-Lino TJ, Morison NB, Salamonsen LA. Neutrophil depletion retards endometrial repair in a mouse model. Cell Tissue Res. 2007;328:197–206. doi: 10.1007/s00441-006-0358-2. [DOI] [PubMed] [Google Scholar]
- 38.Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vicetti Miguel RD, Quispe Calla NE, Cherpes TL. Setting sights on chlamydia immunity’s central Paradigm: Can we hit a moving target? Infect Immun. 2017;85:e00129-17. doi: 10.1128/IAI.00129-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JJ, et al. Human versus mouse eosinophils: “That which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130:572–584. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: Eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol. 2001;166:4813–4817. doi: 10.4049/jimmunol.166.8.4813. [DOI] [PubMed] [Google Scholar]
- 42.Bjerke T, et al. Human blood eosinophils produce and secrete interleukin 4. Respir Med. 1996;90:271–277. doi: 10.1016/s0954-6111(96)90098-0. [DOI] [PubMed] [Google Scholar]
- 43.Travers J, Rothenberg ME. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015;8:464–475. doi: 10.1038/mi.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Lathbury LJ, Salamonsen LA. Expression of the chemokine eotaxin and its receptor, CCR3, in human endometrium. Biol Reprod. 2000;62:404–411. doi: 10.1095/biolreprod62.2.404. [DOI] [PubMed] [Google Scholar]
- 45.Hornung D, et al. Localization in tissues and secretion of eotaxin by cells from normal endometrium and endometriosis. J Clin Endocrinol Metab. 2000;85:2604–2608. doi: 10.1210/jcem.85.7.6665. [DOI] [PubMed] [Google Scholar]
- 46.Perez MC, Furth EE, Matzumura PD, Lyttle CR. Role of eosinophils in uterine responses to estrogen. Biol Reprod. 1996;54:249–254. doi: 10.1095/biolreprod54.1.249. [DOI] [PubMed] [Google Scholar]
- 47.Robertson SA, Mau VJ, Young IG, Matthaei KI. Uterine eosinophils and reproductive performance in interleukin 5-deficient mice. J Reprod Fertil. 2000;120:423–432. doi: 10.1530/jrf.0.1200423. [DOI] [PubMed] [Google Scholar]
- 48.Gouon-Evans V, Pollard JW. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology. 2001;142:4515–4521. doi: 10.1210/endo.142.10.8459. [DOI] [PubMed] [Google Scholar]
- 49.Ouyang Z, et al. Interleukin-4 induces expression of eotaxin in endometriotic stromal cells. Fertil Steril. 2010;94:58–62. doi: 10.1016/j.fertnstert.2009.01.129. [DOI] [PubMed] [Google Scholar]
- 50.Blumenthal RD, et al. Degranulating eosinophils in human endometriosis. Am J Pathol. 2000;156:1581–1588. doi: 10.1016/S0002-9440(10)65030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samoszuk M, Lin F, Rim P, Strathearn G. New marker for blood vessels in human ovarian and endometrial cancers. Clin Cancer Res. 1996;2:1867–1871. [PubMed] [Google Scholar]
- 52.Jeziorska M, Salamonsen LA, Woolley DE. Mast cell and eosinophil distribution and activation in human endometrium throughout the menstrual cycle. Biol Reprod. 1995;53:312–320. doi: 10.1095/biolreprod53.2.312. [DOI] [PubMed] [Google Scholar]
- 53.Knudsen UB, Uldbjerg N, Rechberger T, Fredens K. Eosinophils in human cervical ripening. Eur J Obstet Gynecol Reprod Biol. 1997;72:165–168. doi: 10.1016/s0301-2115(96)02686-3. [DOI] [PubMed] [Google Scholar]
- 54.Evans J, et al. Fertile ground: Human endometrial programming and lessons in health and disease. Nat Rev Endocrinol. 2016;12:654–667. doi: 10.1038/nrendo.2016.116. [DOI] [PubMed] [Google Scholar]
- 55.Maybin JA, Critchley HO. Menstrual physiology: Implications for endometrial pathology and beyond. Hum Reprod Update. 2015;21:748–761. doi: 10.1093/humupd/dmv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahendroo M. Cervical remodeling in term and preterm birth: Insights from an animal model. Reproduction. 2012;143:429–438. doi: 10.1530/REP-11-0466. [DOI] [PubMed] [Google Scholar]
- 57.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stenfeldt AL, Wennerås C. Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils. Immunology. 2004;112:605–614. doi: 10.1111/j.1365-2567.2004.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patterson AL, Zhang L, Arango NA, Teixeira J, Pru JK. Mesenchymal-to-epithelial transition contributes to endometrial regeneration following natural and artificial decidualization. Stem Cells Dev. 2013;22:964–974. doi: 10.1089/scd.2012.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.OuYang Z, et al. Interleukin-4 stimulates proliferation of endometriotic stromal cells. Am J Pathol. 2008;173:463–469. doi: 10.2353/ajpath.2008.071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eberl G. Immunity by equilibrium. Nat Rev Immunol. 2016;16:524–532. doi: 10.1038/nri.2016.75. [DOI] [PubMed] [Google Scholar]
- 62.de la Maza LM, Zhong G, Brunham RC. Update on Chlamydia trachomatis vaccinology. Clin Vaccine Immunol. 2017;24:e00543-16. doi: 10.1128/CVI.00543-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anthony RM, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen F, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
- 66.Jeon S, St Leger AJ, Cherpes TL, Sheridan BS, Hendricks RL. PD-L1/B7-H1 regulates the survival but not the function of CD8+ T cells in herpes simplex virus type 1 latently infected trigeminal ganglia. J Immunol. 2013;190:6277–6286. doi: 10.4049/jimmunol.1300582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ito JI, Jr, Harrison HR, Alexander ER, Billings LJ. Establishment of genital tract infection in the CF-1 mouse by intravaginal inoculation of a human oculogenital isolate of Chlamydia trachomatis. J Infect Dis. 1984;150:577–582. doi: 10.1093/infdis/150.4.577. [DOI] [PubMed] [Google Scholar]
- 68.Vicetti Miguel RD, et al. Dendritic cell activation and memory cell development are impaired among mice administered medroxyprogesterone acetate prior to mucosal herpes simplex virus type 1 infection. J Immunol. 2012;189:3449–3461. doi: 10.4049/jimmunol.1103054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dirks JA, et al. Chlamydia trachomatis load in population-based screening and STI-clinics: Implications for screening policy. PLoS One. 2015;10:e0121433. doi: 10.1371/journal.pone.0121433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vicetti Miguel RD, Henschel KJ, Dueñas Lopez FC, Quispe Calla NE, Cherpes TL. Fluorescent labeling reliably identifies Chlamydia trachomatis in living human endometrial cells and rapidly and accurately quantifies chlamydial inclusion forming units. J Microbiol Methods. 2015;119:79–82. doi: 10.1016/j.mimet.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quispe Calla NE, et al. Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection. Mucosal Immunol. 2016;9:1571–1583. doi: 10.1038/mi.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006;69:1037–1042. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- 74.Seluanov A, Vaidya A, Gorbunova V. Establishing primary adult fibroblast cultures from rodents. J Vis Exp. 2010;44:2033. doi: 10.3791/2033. [DOI] [PMC free article] [PubMed] [Google Scholar]