Significance
Hydrogen peroxide has been regarded exclusively as a hazard for bacteria; its sources and concentrations in natural habitats are uncertain. The cytochrome c peroxidase of Escherichia coli exhibits an expression pattern and flux rate that provides surprising insights into these issues. This periplasmic enzyme is induced only when H2O2 is present and molecular oxygen is absent. Intriguingly, it was ineffective as a defensive enzyme, but through its linkage to the quinone pool it did enable E. coli to respire using H2O2 as an anaerobic electron acceptor. We suggest that both chemical and biotic processes generate micromolar H2O2 at oxic/anoxic interfaces and that this scenario is common enough that microbes have evolved strategies to productively exploit the H2O2.
Keywords: oxidative stress, anaerobic respiration, OxyR
Abstract
Microbial cytochrome c peroxidases (Ccp) have been studied for 75 years, but their physiological roles are unclear. Ccps are located in the periplasms of bacteria and the mitochondrial intermembrane spaces of fungi. In this study, Ccp is demonstrated to be a significant degrader of hydrogen peroxide in anoxic Escherichia coli. Intriguingly, ccp transcription requires both the presence of H2O2 and the absence of O2. Experiments show that Ccp lacks enough activity to shield the cytoplasm from exogenous H2O2. However, it receives electrons from the quinone pool, and its flux rate approximates flow to other anaerobic electron acceptors. Indeed, Ccp enabled E. coli to grow on a nonfermentable carbon source when H2O2 was supplied. Salmonella behaved similarly. This role rationalizes ccp repression in oxic environments. We speculate that micromolar H2O2 is created both biologically and abiotically at natural oxic/anoxic interfaces. The OxyR response appears to exploit this H2O2 as a terminal oxidant while simultaneously defending the cell against its toxicity.
The facultative anaerobe Escherichia coli lives adjacent to the epithelial layer of the mammalian gut, where it can respire by scavenging trace oxygen that diffuses into the lumen from the epithelial cells. When oxygen levels decline, E. coli can ferment, although it is less successful than the coresident obligate anaerobes. However, upon excretion, E. coli can thrive, whereas the obligate anaerobes enter a period of stasis.
In fully oxic habitats, E. coli must cope with reactive oxygen species that are generated internally through the adventitious oxidation of redox enzymes (1). Superoxide (O2−) and hydrogen peroxide (H2O2) are potentially toxic, because they oxidatively inactivate cytoplasmic enzymes that use exposed [4Fe–4S] clusters or ferrous iron atoms as prosthetic cofactors (2–6). H2O2 also oxidizes the intracellular pool of unincorporated iron through the Fenton reaction, thereby generating hydroxyl radicals that can damage DNA (7). To protect itself from these oxidants, E. coli—like virtually all organisms—routinely synthesizes superoxide dismutases to keep O2− levels low and catalase and NADH peroxidase to minimize H2O2. As a consequence, wild-type E. coli grows well in oxic environments.
In addition to these basal protections, most bacteria have the capacity to ramp up their defenses when they encounter extracellular H2O2 (8, 9). Exogenous H2O2 moves freely through porins into the periplasm, and because it is small and uncharged, it can gradually diffuse across the cytoplasmic membrane into the cell interior, where it poses a hazard (10). When H2O2 levels rise in E. coli, the H2O2 directly oxidizes the sensory cysteine residue of the OxyR transcription factor, triggering the formation of a disulfide bond that locks the protein into an activated conformation (11, 12). OxyR then activates the expression of defensive genes (13). Catalase and NADH peroxidase activities are boosted more than 10-fold. Suf proteins improve the repair of iron–sulfur clusters (14, 15), the manganese importer MntH allows Mn to replace ferrous iron in mononuclear enzymes (16, 17), and the miniferritin Dps protects DNA from Fenton chemistry by sequestering loose iron (18–20). When H2O2 levels later fall, glutaredoxins deactivate OxyR by reducing its disulfide bond (21).
Data indicate that ∼200 nM steady-state intracellular H2O2 is sufficient to activate this response (10, 21). Because the cytoplasmic membrane is only semipermeable to H2O2 and the cytoplasm contains robust peroxidase and catalase activities, the internal H2O2 concentration is 5- to 10-fold lower than the external H2O2 concentration (10). Accordingly, measurements indicate that external H2O2 levels must exceed 2 μM to induce the intracellular OxyR response (21). Thus, one infers that despite dwelling in mostly hypoxic environments, E. coli must encounter micromolar levels of external H2O2 at some stage in its normal lifestyle. Strikingly, even obligate anaerobes manifest H2O2-inducible defenses, mediated either by OxyR or by PerR (22–24). The implication is that microbes may confront toxic doses of H2O2 even in low-oxygen environments.
Such a conclusion might resolve the conundrum of cytochrome c peroxidases. The biochemical activity of these enzymes was first described in 1940 (25), and although their reaction mechanism has been elaborated in detail (26), their physiological role continues to perplex. Notably, the bacterial enzymes are expressed only under anoxic conditions (27, 28). We now report that through YhjA, a cytochrome c peroxidase homolog, E. coli can exploit H2O2 as a terminal oxidant in a form of anaerobic respiration. This represents a surprising positive use of H2O2 in metabolism. YhjA is induced only when oxygen is scarce and H2O2 is present, implying that micromolar H2O2 can be found at oxic–anoxic interfaces. We suggest that YhjA be renamed Ccp, and we continue to use Ccp throughout this paper.
Results
Cytochrome c Peroxidase Is a Fourth Enzyme That Scavenges H2O2.
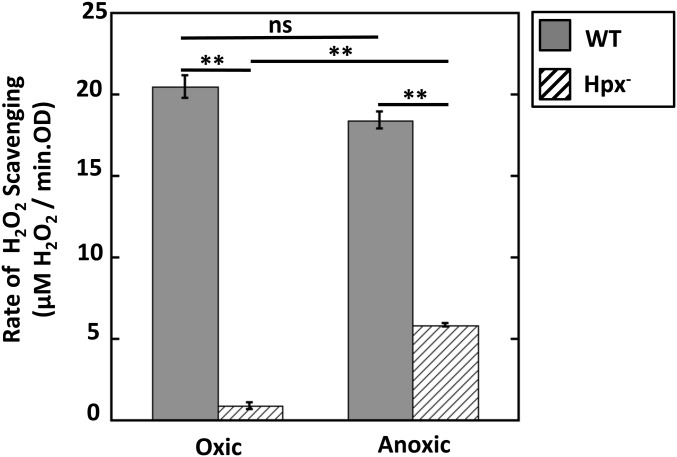
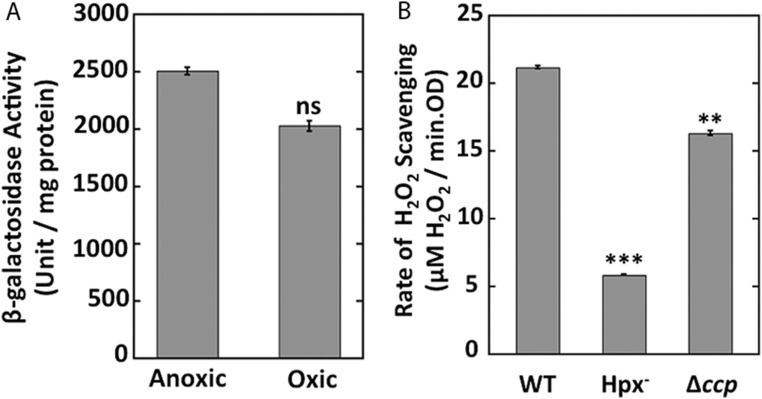
E. coli has three enzymes that are known to degrade H2O2 in vivo: the KatG and KatE catalases, plus the NADH peroxidase AhpCF. For convenience, mutants that lack all three enzymes (katG katE ahpCF) are termed hydroperoxidase-deficient (Hpx−) (29). When Hpx− mutants are grown aerobically, they do not clear micromolar H2O2 from medium at a significant rate (Fig. 1). However, we were surprised to find that when the same strain was grown and exposed to H2O2 under anoxic conditions, it displayed substantial ability to degrade H2O2 (Fig. 1, rightmost bar).
Fig. 1.
Under anoxic conditions, a new H2O2-degrading activity appears. Wild-type (MG1655) and Hpx− (LC106) cells were grown and assayed aerobically or anaerobically. Rates of H2O2 scavenging were measured as described. Error bars in this and subsequent figures represent SEM of three independent experiments. Asterisks represent statistical significance [*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; not significant (ns), P > 0.05].
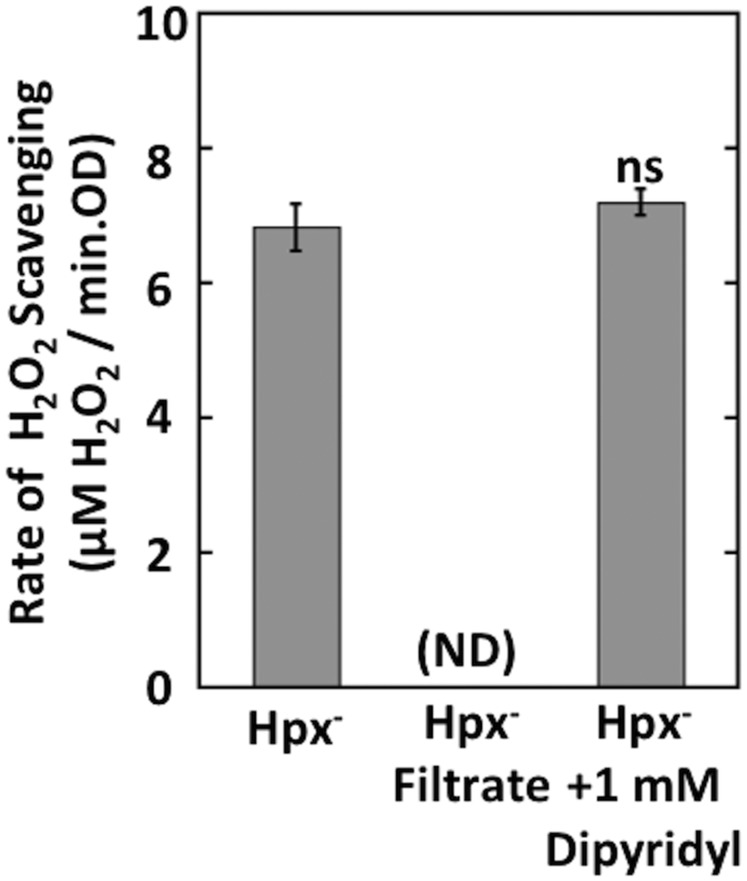
The culture filtrate did not degrade H2O2 (Fig. S1), indicating that the scavenging activity resided within the cells. We considered the possibility that H2O2 might be eliminated through Fenton reactions with the cytoplasmic pool of unincorporated iron. However, our calculations indicated that the cell was unlikely to contain enough iron to support the observed rate of H2O2 clearance (SI Materials and Methods), and indeed the scavenging activity persisted when cells were perfused with dipyridyl, a chelator that fully blocks intracellular Fenton chemistry (7) (Fig. S1).
Fig. S1.
H2O2 scavenging in Hpx− strains is not due to reactions with secreted metabolites or intracellular Fenton chemistry. The rate of H2O2 scavenging was measured with Hpx− cells, with the filtrate of anaerobically grown Hpx− (LC106) cells, or with cells treated with 1 mM dipyridyl (a cell-permeable iron chelator). ND, the rate was below the detection limit (0.1 μM H2O2/min-OD). ns, not significant compared with Hpx−.
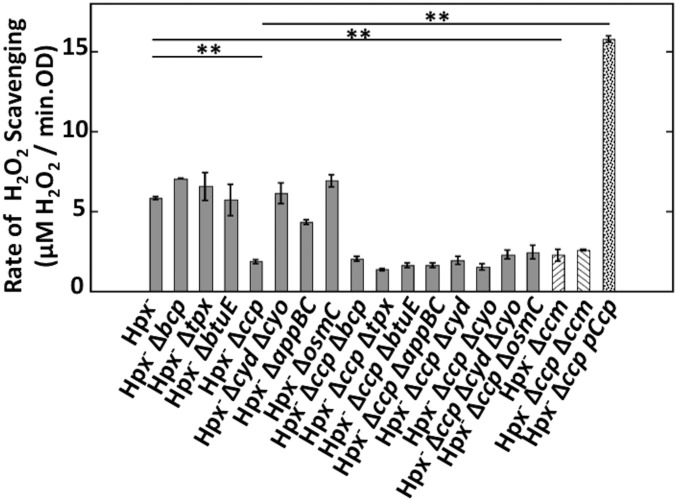
Therefore, we focused upon E. coli enzymes that have been reported to display peroxidase activities in vitro: thiol peroxidase (Tpx) (30), bacterioferritin comigratory protein (BCP) (31), a glutathione peroxidase homolog (BtuE) (32), a predicted cytochrome c peroxidase (YhjA, or Ccp) (28), and osmotically inducible peroxiredoxin (OsmC) (33). The physiological roles of these enzymes are uncertain, and they have not been reported to degrade H2O2 in vivo. We also considered the cytochrome bd-I, bd-II (AppBC), and bo terminal oxidases. These enzymes terminate aerobic respiration by transferring electrons from respiratory quinones to oxygen, but they also exhibit some peroxidase activity, because H2O2 is a formal intermediate in the oxygen-reduction process (34, 35). Each of the genes was deleted from the Hpx− parent strain, and the rate of H2O2 scavenging was then remeasured under anoxic conditions. The rate was substantially diminished only in the Δccp mutant, and this effect was reversed when ccp was overexpressed from a plasmid (Fig. 2). The other mutations had no further impact even when they were introduced into the Hpx− Δccp strain. We conclude that Ccp is an authentic scavenger, whereas the other proteins play little role in H2O2 clearance under these conditions. We do not know the source of the slight scavenging activity that persists in the Hpx− Δccp strain, and it is not considered further here.
Fig. 2.
Ccp scavenges H2O2 in anoxic Hpx− cells. The Hpx− parent strain and mutant derivatives were grown anaerobically, and the rate of H2O2 scavenging was measured. Cross-hatched bars: The cytochrome c maturation machinery is required for Ccp activity. Spotted bar, far right: the Hpx− Δccp mutant was genetically complemented using pACYC184-ccp under its own promoter. Asterisks represent statistical significance (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, P > 0.05). The single mutants other than Hpx− Δccp and Hpx− Δccm were not significantly different from Hpx−. None of the double mutants were significantly different from Hpx− Δccp. Strains used: Hpx− (LC106), Hpx− Δbcp (MK150), Hpx− Δtpx (MK154), Hpx− ΔbtuE (MK158), Hpx− Δccp (MK146), Hpx− Δcyd Δcyo (SSK53), Hpx− ΔappBC (MK180), Hpx− ΔosmC (MK208), Hpx− Δccp Δbcp (MK172), Hpx− Δccp Δtpx (MK174), Hpx− Δccp ΔbtuE (MK176), Hpx− Δccp ΔappBC (MK182), Hpx− Δccp Δcyd (MK164), Hpx− Δccp Δcyo (MK166), Hpx− Δccp Δcyd Δcyo (MK170), Hpx− Δccp ΔosmC (MK210), Hpx− Δccm (MK198), Hpx− Δccp Δccm (MK418), and Hpx− Δccp pAcyc184-ccp (MK430).
Ccp Is an Authentic Peroxidase That Derives Electrons from the Quinone Pool.
The yhjA (here, ccp) gene is annotated as a potential cytochrome c peroxidase. Well-studied Ccp enzymes contain two c-type hemes and localize in the mitochondrial intermembrane space or bacterial periplasm. In vitro, these enzymes can receive electrons from reduced respiratory cytochrome c protein; the Ccp can then transfer electrons to H2O2, reducing it to water (25, 36–38).
The E. coli respiratory chain does not use cytochrome c, and its Ccp belongs to a second class that has a third c-type heme, which serves as the electron entry port when artificial reductants are provided (26). E. coli has a single known pathway for synthesizing and exporting c-type heme to periplasmic proteins; this ccmABCDEFGH operon has been shown to provide c-type hemes to periplasmic nitrite reductase, which is encoded by the adjacent nir operon. We found that the Hpx− ΔccmABCDEFGH and the Hpx− Δccp ΔccmABCDEFGH mutants phenocopied the Hpx− Δccp mutant, confirming that the Ccm pathway is also the source of the c-type heme that activates Ccp (Fig. 2).
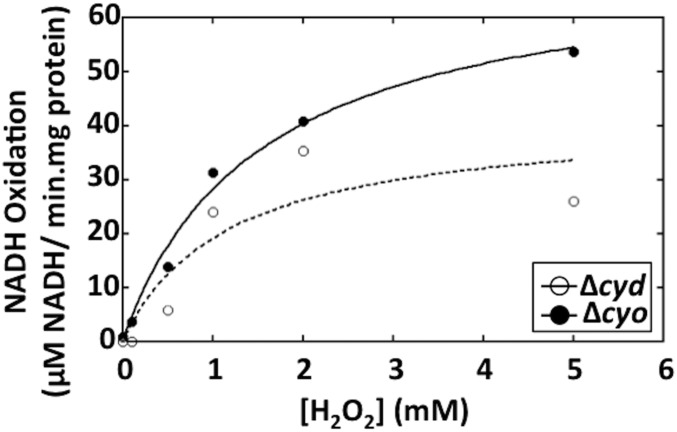
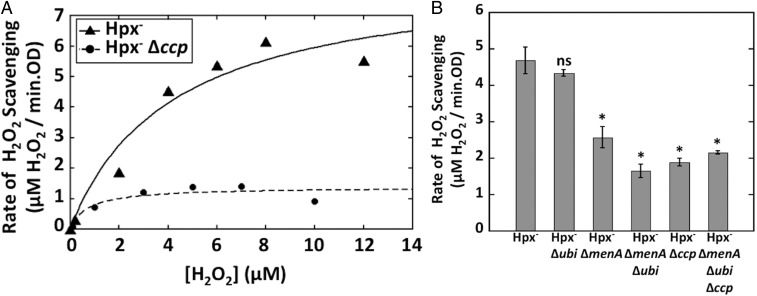
Because the Fenton reaction is chemically simple, it can be catalyzed at modest rates by many enzymes that contain solvent-exposed iron cofactors. However, such adventitious chemistry often requires high concentrations of H2O2. For example, although the bo and bd-I terminal oxidases can reduce H2O2 to water (35, 39), they do so effectively only at millimolar H2O2 concentrations that vastly exceed the low-micromolar concentrations of H2O2 that are likely to occur in nature (Fig. S2). In contrast, by measuring H2O2 clearance in an Hpx− background, we determined that the apparent Km of Ccp in intact cells was 5.2 ± 0.6 μM (Fig. 3A). We conclude that H2O2 is likely to be its physiological substrate.
Fig. S2.
Cytochrome bo and bd oxidases require millimolar doses of H2O2 for effective turnover. The rates of NADH oxidation by the bo and bd oxidases were measured using inverted membrane vesicles under anoxic conditions. The vesicles were prepared from Δcyd (AL454) or Δcyo (JEM1513) strains. The figure shows a representative curve from three independent experiments. The cytochrome bd-1 oxidase also exhibits some catalase activity, but this action similarly requires millimolar levels of H2O2 for rapid turnover (82).
Fig. 3.
(A) Ccp has a low effective Km for H2O2. Rates of anoxic H2O2 scavenging were measured with Hpx− (LC106) and Hpx− Δccp (MK146) cells. Triplicate measurements determined Km(app) to be 5.2 ± 0.6 μM. A single trial is shown here. (B) Respiratory quinones are required for Ccp function. The Hpx− strain (LC106) and its derivatives lacking ubiquinone (LC148); menaquinone (SSK6); both (LC160); ccp (MK146); or ubiquinone, menaquinone, and ccp (MK184) were grown anaerobically in LB medium, and the rates of H2O2 scavenging were measured. Asterisks represent statistical significance compared with the Hpx− strain (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, P > 0.05).
Ccp has been predicted to be a single-pass enzyme that is tethered to the cytoplasmic membrane, with the bulk of its polypeptide in the periplasm (40). The electron transport chain in the cytoplasmic membrane is an available source of electrons for periplasmic redox enzymes. In vitro, some quinone analogs were found to be capable of reducing some bacterial Ccp enzymes (41–43), although the activity was not detected with the Ccp of E. coli (42). We observed that Hpx− ubi mutants retained Ccp scavenging activity, but the activity was diminished in men mutants and was essentially absent from ubi men double mutants (Fig. 3B). Menaquinone is the primary quinone in membranes under the anoxic conditions of these experiments (44).
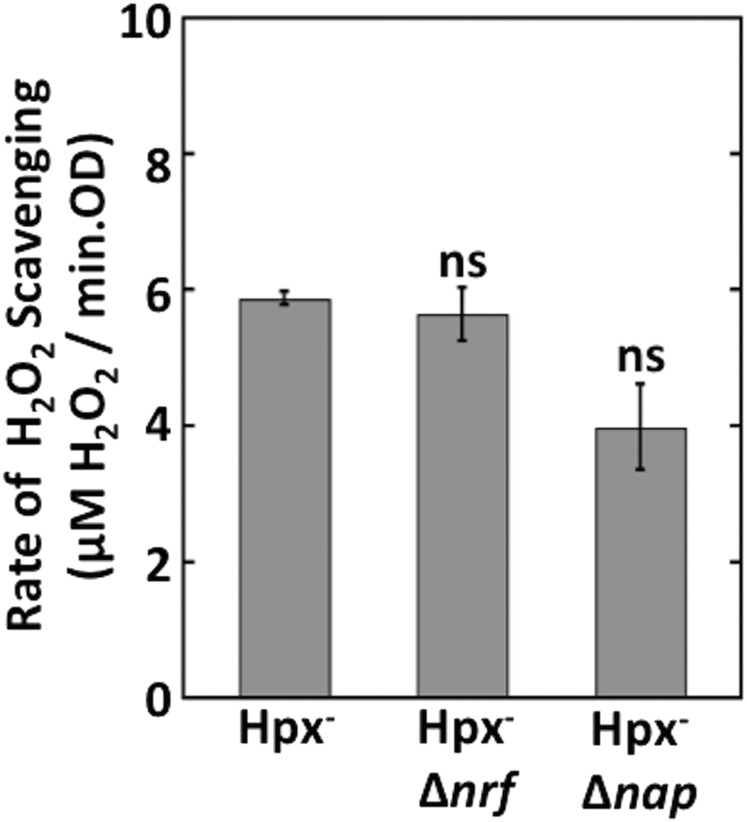
Periplasmic nitrite and nitrate reductases also acquire electrons from the quinone pool. In those cases, the structural genes for the catalytic enzymes sit alongside genes that encode bridging proteins that deliver electrons from the quinones to the catalytic enzymes. No analogous gene accompanies ccp. We verified that the Ccp scavenging activity persisted in mutants lacking the Nap and Nrf bridge proteins (Fig. S3); we infer that its association with the cytoplasmic membrane allows Ccp to receive electrons directly from the quinone pool.
Fig. S3.
Nap and Nrf are not required for H2O2 scavenging. The rate of H2O2 scavenging was measured using anoxic Hpx− Δnrf (MK218) and Hpx− Δnap (MK212) cells. The slight decrement in the Δnap mutant may be due to a polar effect upon downstream cytochrome c biosynthetic genes. ns, not significant compared with Hpx−.
Ccp enables anaerobic respiration with H2O2 as the terminal oxidant.
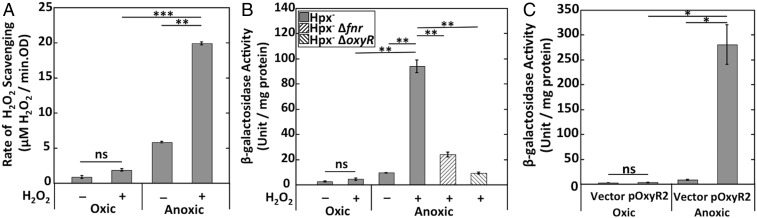
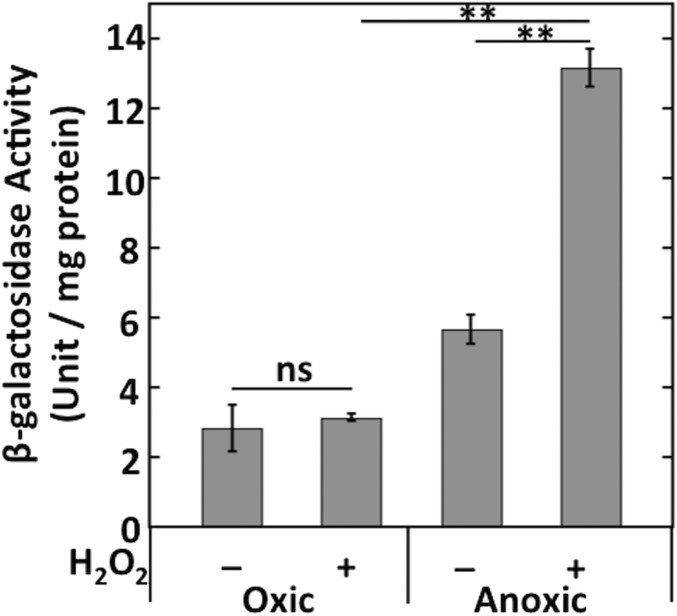
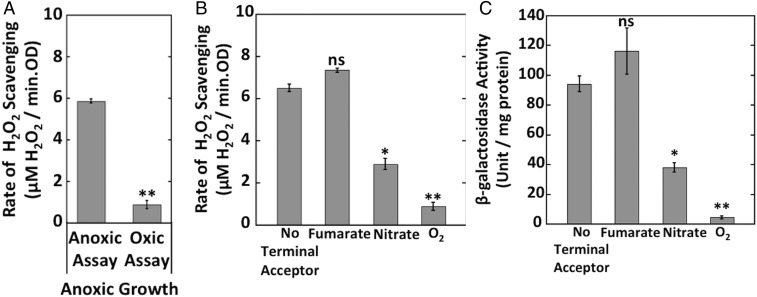
Partridge et al. (28) demonstrated previously that E. coli expresses ccp (called yhjA in their study) only under anoxic conditions, and mutants lacking either the Fnr or OxyR transcription factors failed to do so. They identified Fnr and OxyR binding sites upstream of the transcriptional start site. In their experiments, the involvement of OxyR appeared not to require the presence of H2O2. We found that the addition of H2O2 stimulated Ccp activity by threefold (Fig. 4A). We generated a ccp–lacZ transcriptional fusion and reexamined this pattern. Anoxia alone was insufficient to promote full expression; H2O2 was also required (Fig. 4B). This pattern was most easily observed with Hpx− strains that do not rapidly clear H2O2 from the medium, but H2O2 stimulated expression in wild-type cells as well (Fig. S4). Neither fnr nor oxyR mutants showed induction (Fig. 4B). The requirement for H2O2 was relieved by expression of OxyR2, a mutant form of OxyR that remains activated even in the absence of H2O2 (Fig. 4C). We suspect that the contamination of LB by H2O2 (45) may previously (28) have given the misleading impression that H2O2 was unnecessary for ccp induction. Collectively, the dual regulation by the OxyR and Fnr transcription factors ensures that E. coli expresses ccp only if molecular oxygen is absent and H2O2 is present. The requirement for H2O2 confirmed our view that it is the natural substrate of the enzyme.
Fig. 4.
Strong ccp expression requires both the absence of oxygen and the presence of H2O2. (A) The rates of H2O2 scavenging were measured in Hpx− cells grown in oxic or anoxic conditions. Where indicated, cells were incubated with 40 μM H2O2 for 1 h before assay. (B) β-galactosidase activity from the transcriptional ccp′–lacZ+ reporter fusion was measured in Hpx− (MK250), Hpx− Δfnr (MK274), and Hpx− ΔoxyR (MK278) mutants grown in oxic or anoxic media. Where indicated, cells were incubated with 40 μM H2O2 for 1 h before harvesting. (C) β-galactosidase activity from the fusion was measured in wild-type cells containing either a pACYC184-oxyR2 plasmid (expressing a constitutively active form of OxyR) (MK346) or empty vector (MK344), grown anaerobically. Asterisks represent statistical significance (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, P > 0.05).
Fig. S4.
ccp expression requires both the absence of oxygen and the presence of H2O2. β-galactosidase activity from the transcriptional ccp′–lacZ+ reporter fusion was measured in WT (MK246) cells grown in oxic or anoxic media. Where indicated, cells were incubated with 40 μM H2O2 for 1 h before harvesting. Asterisks represent statistical significance (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, P > 0.05).
Molecular oxygen is an obligatory precursor in all known routes of H2O2 formation, so the fact that Ccp is synthesized only in its absence was perplexing. However, we and others have observed that both facultative and obligately anaerobic bacteria continue to express oxidative defenses even when oxygen is absent, suggesting that H2O2 can find its way into anoxic habitats (Discussion). Therefore, we considered the possibility that in anoxic environments Ccp might replace AhpCF as the primary scavenger of H2O2. However, an ahpC–lacZ fusion demonstrated that AhpCF continues to be synthesized at full force in anaerobic cells (Fig. S5A). In fact, AhpCF and catalase remained the major enzymes involved in clearing H2O2 from the bulk medium, because Hpx− strains were defective at this activity. Ccp was a minor contributor (Fig. S5B).
Fig. S5.
Under anoxic conditions, cytoplasmic enzymes remain the primary scavengers of H2O2. (A) β-galactosidase activity of the transcriptional ahpCF′–lacZ+ reporter fusion was measured in Hpx− (MK188) cells grown anaerobically or aerobically. (B) The rate of H2O2 scavenging was measured in anoxic wild-type (MG1655) cells, the Hpx− (LC106) mutant, and the Δccp (MK416) mutant. Asterisks represent statistical significance (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, P > 0.05).
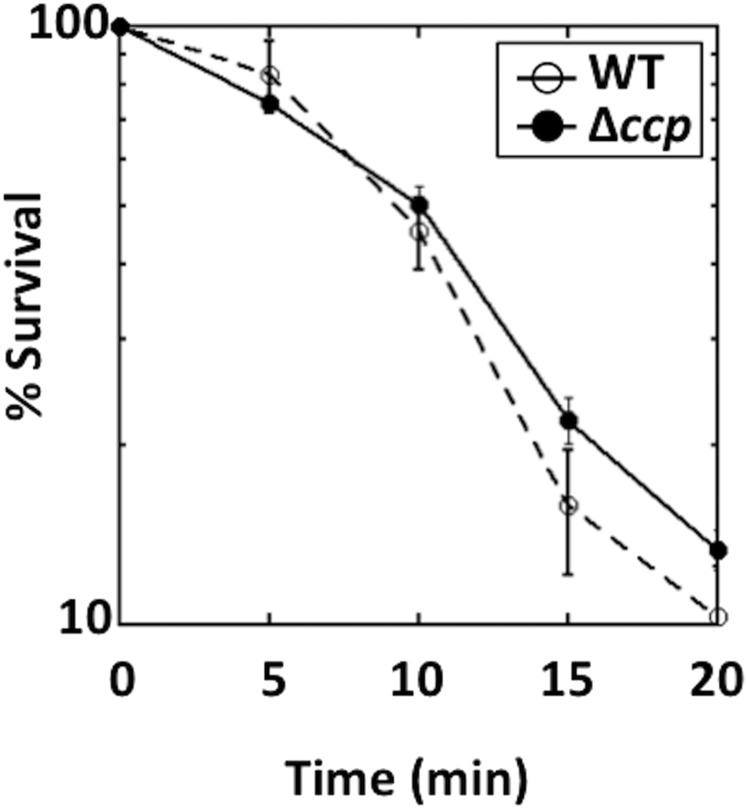
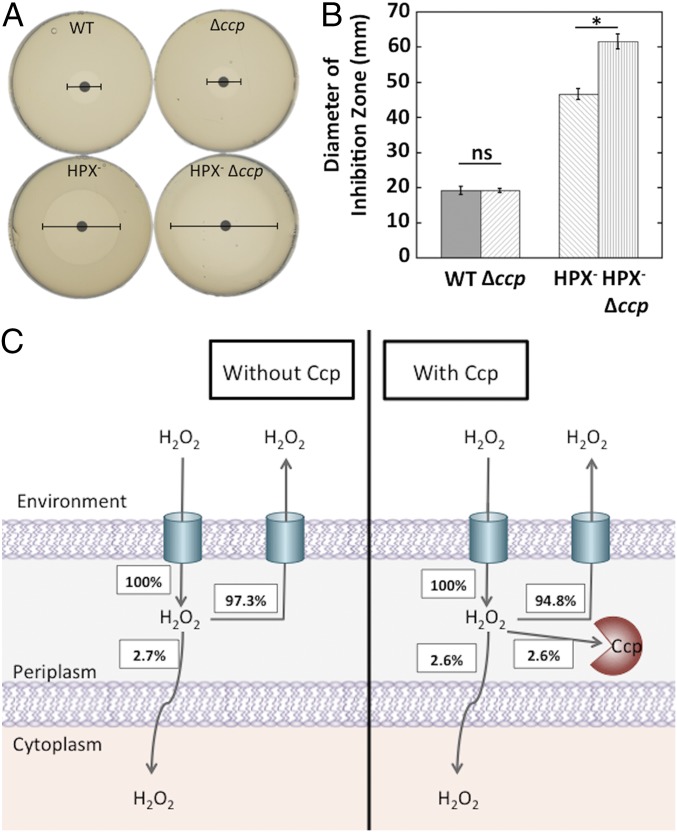
The established role of scavenging enzymes is to shield cells from the toxic effects of H2O2. It seemed plausible that Ccp might act as a new layer of defense that would degrade exogenous H2O2 before it could diffuse into the cytoplasm, where H2O2-sensitive enzymes are located. When wild-type cells and ccp mutants were challenged with millimolar H2O2, the rates of killing were indistinguishable (Fig. S6). However, that protocol might fail to elicit the protective effect of a peroxidase because the H2O2 dose grossly exceeds the effective Km of the enzyme, such that it can scavenge only a minute fraction of the H2O2. An alternative is to challenge cells with low doses of exogenous H2O2 that just barely poison metabolism. To do so, we tested the vulnerability of ccp mutants to a gradient of exogenous H2O2 through zone-of-inhibition experiments using a minimal glucose medium (Fig. 5 A and B). In this situation, the poisoning of amino acid biosynthetic enzymes sets the H2O2 sensitivity of the cell at 0.5–1 μM intracellular H2O2 (46); we thought this arrangement offered the best chance to detect any defensive impact of Ccp. However, the ccp mutants were again no more sensitive than wild-type cells. Only in the Hpx− background did Ccp exert an impact, which we attribute to its providing the sole route of clearance of H2O2 from the bulk medium. These data resemble zone-of-inhibition results that were previously obtained with other bacteria (43, 47–50) and or fungi (51, 52), in which mutants showed either only a marginal increase in sensitivity, or none at all. In contrast, the Hpx− strain was extremely sensitive, showing that in E. coli the cytoplasmic peroxidase and catalase activities play a much greater role in protecting the cell from H2O2.
Fig. S6.
A Δccp mutant is not more sensitive to millimolar doses of H2O2 than is the wild-type strain. Wild-type (MG1655) and Δccp mutant (MK416) cells were grown to OD600 ∼0.3 and challenged with 2.5 mM H2O2. Percent survival was calculated based on cfus. In contrast to an earlier report (28), we did not observe any difference in the sensitivities of these strains.
Fig. 5.
Ccp cannot protect the cytoplasm from exogenous H2O2. (A) Wild-type (MG1655), Δccp (MK416), Hpx− (LC106), and Hpx− Δccp (MK146) mutant cells were grown to OD600 ∼0.1 and spread on a plate. Disks soaked in H2O2 were put on the plate, and the diameter of zone of inhibition was measured after 24 h. (B) Data from A are shown as bar graphs. (C) Modeling (SI Materials and Methods) shows that H2O2 exchange between the external environment and the periplasm is too fast for Ccp to significantly diminish the periplasmic H2O2 level. Therefore, Ccp has minimal effect on H2O2 entry into the cytoplasm. Steady-state fluxes (in %) are calculated relative to the rate of H2O2 entry into the periplasm. Asterisks represent statistical significance (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, P > 0.05).
To understand why Ccp fails to shield the cytoplasm, we modeled H2O2 fluxes as H2O2 moves from the external environment into the periplasm. Outer membrane porins allow very rapid H2O2 movement into the periplasm (53), whereas the cytoplasmic membrane exhibits a membrane permeability coefficient that is lower by at least two orders of magnitude (10, 54). Calculations showed that the activity of Ccp could diminish the periplasmic concentration of H2O2 by only 0.1% in the face of its rapid exchange with the external environment (SI Materials and Methods). Accordingly, the rate of subsequent influx into the cytoplasm was effectively unchanged by Ccp (Fig. 5C). Thus, it seems implausible that the main role of Ccp is to protect the cell from exogenous H2O2.
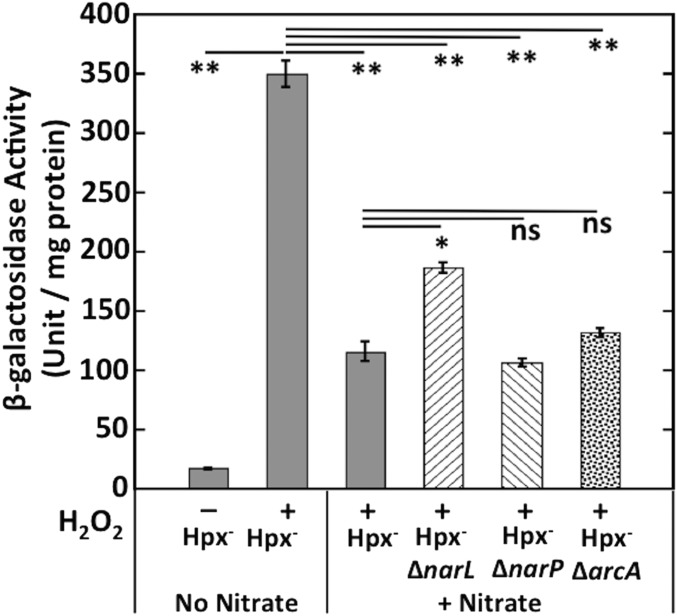
Because Ccp mediates electron flow from respiratory quinones to H2O2, its physiological role may be to exploit H2O2 as a terminal electron acceptor, an idea that was broached by Atack and Kelly (26). The regulatory data fit this model, as Ccp expression was blocked when oxygen, a superior acceptor, is available. Further, the presence of oxygen blocks Ccp turnover by competing for the electrons carried by the quinone pool (Fig. 6A). To probe further, we tested the effects upon ccp expression of added nitrate and fumarate, which are alternative anaerobic electron acceptors. Nitrate is a better respiratory substrate than fumarate, because nitrate reductase provides an additional coupling site in generating proton-motive force. Like oxygen, nitrate inhibited Ccp-mediated scavenging and ccp–lacZ expression (Fig. 6 B and C). E. coli has three transcription factors that respond to the presence of nitrate—NarL, NarP, and ArcA—but experiments failed to clarify which of these transcription factors might be involved in the repression (55) (Fig. S7).
Fig. 6.
Oxygen and nitrate block both Ccp activity and synthesis. (A) Hpx− (LC106) cells were grown anaerobically, and the rate of H2O2 scavenging was measured in absence or presence of oxygen. (B) Hpx− (LC106) cells were both grown and assayed for H2O2 scavenging either without an electron acceptor or in the presence of fumarate, nitrate, or oxygen. (C) Expression of the transcriptional ccp′–lacZ+ reporter fusion in Hpx− (MK250) cells grown either without an electron acceptor or in the presence of fumarate, nitrate, or oxygen. Cells were incubated with 40 μM H2O2 for 1 h before harvesting. Asterisks represent statistical significance compared with anoxic assay in A and no terminal acceptor in B and C (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, P > 0.05).
Fig. S7.
NarL, NarP, and ArcA do not mediate the inhibition of ccp transcription by nitrate. β-galactosidase activity of the transcriptional ccp′–lacZ+ reporter fusion was measured in Hpx− (MK250), Hpx− ΔnarL (MK404), Hpx− ΔnarP (MK412), and Hpx− ΔarcA (MK408) strains cultured with or without nitrate. If indicated, cells were incubated with 40 μM H2O2 for 1 h before harvesting. Asterisks represent statistical significance (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, P > 0.05).
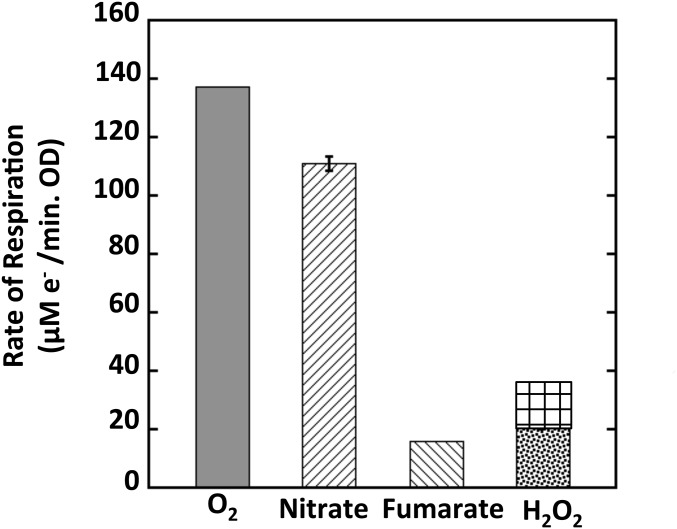
Fluxes through various E. coli metabolic pathways vary over five orders of magnitude. If the role of Ccp were to enable anaerobic respiration, the flux through Ccp should approximate fluxes to other electron acceptors. The rate of respiration through Ccp to H2O2 was measured under fully induced conditions; it was elevated threefold compared with the noninduced conditions that had been sampled in Fig. 1 (Fig. 7). This rate was found to be somewhat less than the respiratory rates with molecular oxygen and nitrate but comparable to the respiratory rate with fumarate. Thus, although the Ccp flux is insufficient to shield the cell from H2O2, it is sufficient to provide a respiratory benefit.
Fig. 7.
Rate of respiration of H2O2 is comparable to that of fumarate. Comparison of respiration rates by wild-type cells with different electron acceptors in glycerol medium. The grid pattern extension of the H2O2 bar represents calculated Vmax, because unlike the other acceptors, H2O2 was provided at a subsaturating (10 μM) concentration. See Materials and Methods for details. The rate of respiration by fumarate was calculated in SI Materials and Methods.
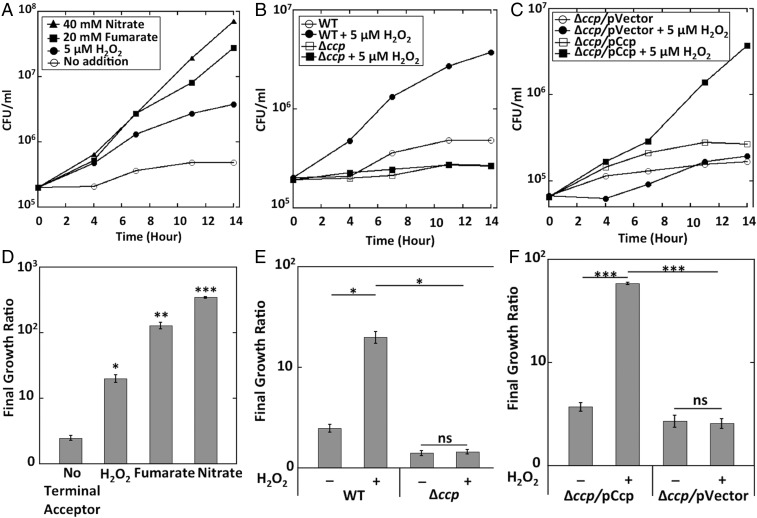
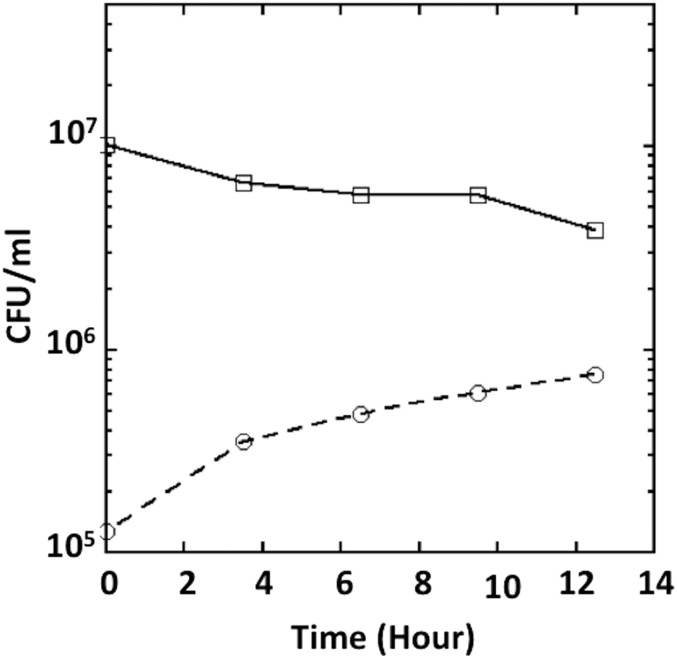
To test this idea more directly, we monitored anaerobic growth with glycerol as sole carbon source. Glycerol catabolism depends upon the oxidation of α-glycerolphosphate by the respiratory GlpABC dehydrogenase complex, which transfers electrons to the menaquinone pool. Thus, respiration can proceed only if an exogenous terminal oxidant is available. We tested whether H2O2 could support growth. Unlike other respiratory substrates, which can be added in millimolar concentrations, H2O2 must be maintained at micromolar levels to avoid toxicity; therefore, 5 μM H2O2 was supplied, and the H2O2 levels in the medium were monitored and replenished periodically. Dilute cells were used so they would not exhaust the H2O2 too quickly, and growth was tracked by viable cell counts rather than optical density. After the preculture electron acceptor (fumarate) was removed, cells divided twice more and then stopped. Fig. 8 shows that H2O2 enabled continued growth. Growth did not occur if ccp was deleted, and it was restored in the complemented strain.
Fig. 8.
Ccp allows respiratory growth using H2O2 as the final electron acceptor. (A) Wild-type cells were grown in anoxic glycerol medium without any electron acceptor (○) or in the presence of 40 mM nitrate (▲), 25 mM fumarate (▪), or 5 μM H2O2 (●). Viable cells (cfus) were determined at different time points. Residual growth in the absence of H2O2 appears to be due to trace oxygen in the anaerobic chamber (Fig. S8). (B) WT and Δccp (MK416) strains were grown in anoxic glycerol medium in the presence or absence of 5 μM H2O2. (C) Δccp mutants were complemented with the ccp gene under its own promoter in a plasmid. Strains with pACYC184-ccp (MK436) and the empty vector (MK432) were grown in presence or absence of 5 μM H2O2. (D) The initial (t = 0) and the final (14-h) time points from three biological replicates of A were used to calculate the growth ratio for each growth condition. (E) The initial (t = 0) and the final (14-h) time points from three biological replicates of B were used to calculate the growth ratio for each growth condition. (F) The initial (t = 0) and the final (14-h) time points from three biological replicates of C were used to calculate the growth ratio for each growth condition. Asterisks represent statistical significance (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, P > 0.05).
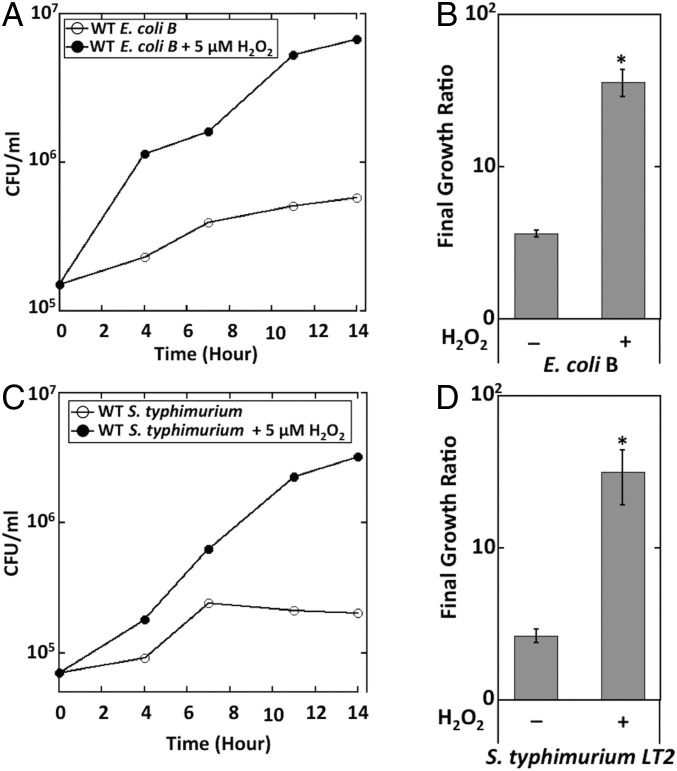
To rule out the possibility that the observed growth phenotype is specific to K12 strains, we confirmed that H2O2 also enabled the anaerobic respiration of an E. coli B strain and of Salmonella typhimurium (Fig. 9). In sum, the regulatory, flux, and growth data support the conclusion that the role of Ccp may be to exploit H2O2 as a respiratory substrate when better substrates are not available. Its location in the periplasm allows turnover even as AhpCF protects internal enzymes by keeping cytoplasmic H2O2 levels low.
Fig. 9.
E. coli B and S. typhimurium LT2 can grow using H2O2 as the final electron acceptor. (A) Wild-type E. coli B cells were grown in anoxic glycerol medium without any electron acceptor (○) or in the presence of 5 μM H2O2 (●). Viable cells (cfus) were determined at different time points. (B) The initial (t = 0) and the final (14-h) time points from three biological replicates of A were used to calculate the growth ratio for each growth condition. (C) Wild-type S. typhimurium LT2 was grown in anoxic glycerol medium without any electron acceptor (○) or in the presence of 5 μM H2O2 (●). Viable cells (cfus) were determined at different time points. (D) The initial (t = 0) and the final (14-h) time points from three biological replicates of A were used to calculate the growth ratio for each growth condition. Asterisks represent statistical significance compared with growth with no terminal acceptor (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, P > 0.05).
SI Materials and Methods
Calculating an Upper Limit for the Rate of Fenton Reaction in E. coli.
We wish to calculate the maximum contribution that intracellular Fenton reactions might make to the degradation of H2O2 inside E. coli. The Fenton reaction degrades H2O2:
The hydroxyl radical that is formed will subsequently oxidize organic molecules. The ferric iron can be reduced back to the ferrous form by various reductants, including cysteine and reduced flavins (76, 77). During steady exposure to 10 μM H2O2, as used in measurements of H2O2 clearance (Fig. 1), the iron will be partitioned between ferric and ferrous forms in a ratio that has not been determined. To maximize the potential rate of Fenton chemistry, we will overestimate the amount of ferrous iron by assuming that all of the iron is in its reduced form. EPR measurements indicate that when E. coli is cultured in the defined media used in this study, its cytoplasm contains ∼30 μM of unincorporated iron that can participate in Fenton reactions (78). Therefore, the rate of H2O2 degradation is
Previous studies reported the rate constants of the Fenton reaction for DNA-bound iron at 13 °C (k = 890 M−1⋅s−1) and 37 °C (k = 4,400 M−1⋅s−1). The reaction rate is faster for fully aqueous iron (k = 6,500 M−1⋅s−1 at 13 °C, and too fast to measure at 37 °C). If the activation energies for bound and aqueous iron as similar, we can estimate a rate of 32,000 M−1⋅s−1 for aqueous iron at 37 °C. Then, inside the cell,
One liter of E. coli cells contains 0.5 mL cytoplasm per OD600 (79). Therefore, the culture volume is 2,000× the cytoplasmic volume, and the rate of H2O2 clearance normalized to the full culture volume will be 0.29 μM⋅min−1⋅OD600. The actual measured rate of H2O2 clearance by Hpx− cells (Fig. 1) was 6 μM⋅min−1⋅OD600. Therefore, even when generously overestimated, the Fenton reaction could not be more than a very minor contributor.
Calculating the Impact of Ccm Upon Periplasmic H2O2 Concentrations.
It has been shown previously that the cytoplasmic volume of E. coli cells growing exponentially in LB medium is 3.23 × 10−18 m3 (79). The volume of periplasm (Volperi) is ∼25% of the cytoplasm, or 8.08 × 10−19 m3. If E. coli cells are modeled as cylinders with two half-spheres at both ends, and stipulating that the cell length is 6× the radius, the surface area of the inner (Ain) and outer (Aout) membrane would be 1.35 × 10−7 cm2 and 1.57 × 10−7 cm2, respectively. These statements are approximately true, and deviations can be shown to have little impact upon final conclusions.
The H2O2 concentration in the periplasm is determined by the balance between its rate of entry through the outer membrane (Vin) and the three processes that clear it from the periplasm: its rate of efflux back to the environment through the outer membrane (Vefflux), the rate at which it passes through the inner membrane into the cytoplasm (Vcyto), and the rate of its degradation by Ccp (Vccp). Reentry from the cytoplasm is essentially zero, due to efficient scavenging by cytoplasmic Ahp (10). Thus, in units of molarity/time,
The rate at which a solute crosses a membrane is proportional to solute concentration, membrane surface area (A), and the membrane permeability coefficient for that solute (P). Representing fluxes (J) in units of moles/time:
The permeability coefficient of the cytoplasmic membrane has been measured to be 1.6 × 10−3 cm/s (10, 54). Permeability across the outer membrane is primarily through porins; a lower limit of their permeability [determined for glycerol (53)] is 50 × 10−3 cm/s. The true value will be even higher for the smaller molecule H2O2, but the glycerol value will suffice to make the point. In this study, Ccp action upon H2O2 was measured with 10 μM H2O2.
The rate of peroxide clearance from media by Ccp when it is fully induced is 20 μM H2O2/min-OD or 0.33 μM H2O2/s-OD. One liter of E. coli cells at OD600 = 1 contains 0.125 mL periplasm (80); that is, at OD600 = 1, the ratio of medium volume to total periplasmic volume to media is 1,000/0.125. Therefore, the rate of H2O2 degradation within the periplasm will be 8,000× that in the total culture:
At the steady state:
By substitution the relative fluxes can be compared (% in parentheses):
Thus, the steady-state H2O2 concentration in the periplasm is primarily determined by the balance of H2O2 fluxes into and out of the outer membrane, with flux into the cytoplasm and consumption by Ccp having very minor effects. The impact of Ccp can be determined by reprising the calculations with the omission of Ccp flux:
The outcome is
Thus, Ccp reduces the periplasmic H2O2 concentration by only 2.5%, and it therefore lessens the flux into the cytoplasm by an equivalent amount. Recalculations with any plausible change in cell dimensions do not change this outcome; if a higher OM permeability coefficient is used, consistent with the small size of H2O2, then the impact of Ccp will only be further diminished. The upshot is that Ccp action cannot shield the cytoplasm of an isolated cell from environmental H2O2.
Rate of Anaerobic Respiration in Glucose Medium with Fumarate as Terminal Electron Acceptor.
Fumarate is generated endogenously during the anaerobic catabolism of glucose, and it is then used as an electron acceptor for the respiratory chain. Tran et al. (81) reported that E. coli cells generate 0.07 mol succinate per mol of glucose catabolized in a minimal medium lacking amino acids. Using the same medium we determined that the doubling times of cells was 70 min and the cell yield was 0.5 OD600 per 0.2% (11.1 mM) glucose provided. Therefore, the yield of succinate = 0.07 × 11.1 mM = 0.78 mM per 0.5 OD600, or 1.6 mM/OD600. The doubling time indicates a growth rate constant of 0.0099 min−1, which applies equally to the production of end products. Thus, the rate of succinate formation in this medium = 1.6 mM/OD600 × 0.0099 min−1 = 15 mM/OD600·min.
Discussion
Cytochrome c peroxidases are widely distributed among microbes. Previous studies demonstrated their activity in vitro, using artificial electron donors; here, experiments confirm that they degrade micromolar H2O2 at substantial rates in vivo. Ccps have generally been assumed to be defensive enzymes, but our analysis does not support this view, at least for E. coli Ccp. The enzyme cannot provide enough activity for isolated cells to lower the periplasmic H2O2 level below that of the surrounding medium, so Ccp does not shield the cytoplasm from H2O2. Further, because E. coli is a minor member of the intestinal flora, it is unlikely to assume the responsibility for clearing H2O2 from its environment. We do recognize that, in principle, if E. coli grew in a clonal biofilm with only a slow H2O2 influx from the surrounding environment, the scavenging activity that Ccp provides might help lower the H2O2 level within the biofilm itself. However, the scavenging activity provided by Ccp does not significantly exceed that which is already provided by cytoplasmic enzymes. Further, for Ccp to deplete H2O2 within a biofilm, the biofilm would have to be dense enough to limit H2O2 entry, but without simultaneously blocking the influx of the carbon sources that provide the respiratory electrons. Thus, Ccp could conceivably contribute as a defensive enzyme only in a very constrained set of circumstances.
However, our data do show that Ccp can allow E. coli to use H2O2 as a respiratory substrate. The regulatory controls upon ccp are more completely resolved for E. coli than for other bacterial or fungal Ccps, and they fit this model (Fig. 10). The transcriptional dependence upon OxyR dictates that the gene be transcribed only when H2O2 is present; Fnr collaborates to ensure that transcription only occurs when a better electron acceptor is not available. Apparent OxyR and Fnr binding sites have been identified (28). The details of promoter architecture and transcription factor interaction will be interesting, particularly because with other genes OxyR seems to operate independently of other transcription factors. This regulatory scheme is a nice demonstration that the two transcription factors are truly specific for their effector oxidants: in controlling ccp, Fnr is nonresponsive to H2O2, and OxyR is not activated by O2 per se.
Fig. 10.
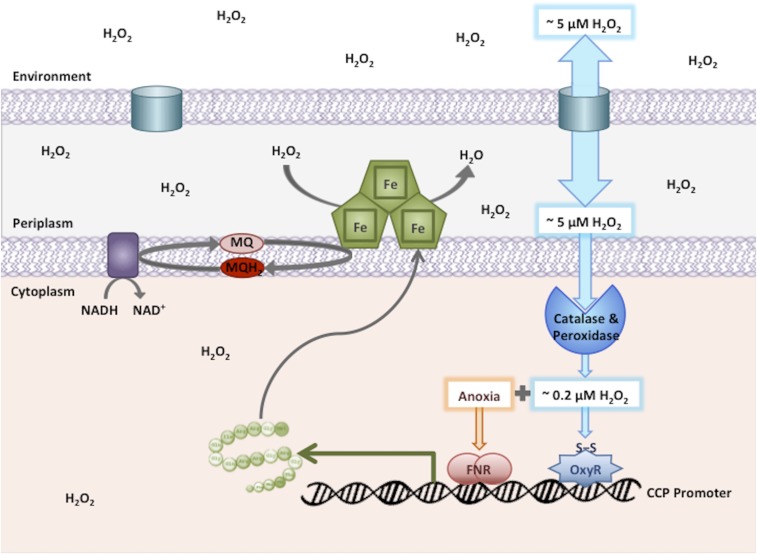
Proposed model for Ccp expression and function. H2O2 rapidly enters the periplasm through porins but penetrates the inner membrane at a lower rate. Activation of OxyR stimulates synthesis of catalase and NADH peroxidase, which keep the cytoplasmic H2O2 concentration low. In anoxic conditions, FNR is also activated, and together it and OxyR induce ccp. Ccp enters the periplasm and allows the respiratory chain to reduce H2O2 to H2O. In isolated cells, Ccp enables anaerobic respiration, but it does not degrade H2O2 quickly enough to reduce the periplasmic or cytoplasmic H2O2 concentrations.
But the bigger picture presents an obvious question: In what circumstances might E. coli encounter H2O2 but not O2? We suggest that at oxic–anoxic interfaces both abiotic and biotic processes may generate H2O2. Such interfaces occur along the margins of intestines and in static ground sediments, and their defining characteristic is that highly reducing environments containing reduced sulfur and metal species collide with an oxygenated one. In the intestinal lumen, sulfate-reducing bacteria generate millimolar amounts of sulfide (56, 57), which presumably diffuses to the margins where it will mix with oxygen. E. coli lives in those margins, as evidenced by the fact that to colonize a mouse intestine, it relies upon a specialized cytochrome oxidase with high oxygen affinity and the ability to function in the presence of sulfide (58–60). When reductants encounter oxygen, metal-catalyzed sulfide oxidation generates H2O2. Further, lactic acid bacteria commonly dwell in hypoxic environments. Although they are classic fermentative organisms, when oxygen becomes available many of them redirect substrate through pyruvate, lactate, or NADH oxidases. These enzymes produce H2O2 as a stoichiometric product (61, 62). Finally, Bacteroides species that predominate in the intestine generate substantial H2O2 when they encounter oxygen, presumably due to the adventitious autoxidation of their low-potential redox systems (63). Thus, at these interfaces, both chemical and biological processes are likely to diminish O2 levels and send H2O2 diffusing into anoxic zones. We suspect that this is the source of the H2O2 that E. coli exploits. Indeed, Campylobacter ccp mutants exhibit colonization defects that are consistent with the notion that H2O2 is an important component of the intestinal environment (64).
Other bacteria also express Ccp only in hypoxic environments, suggesting that it serves a common purpose in most organisms. Seib et al. (65) noted that Neisseria gonorrhea encodes a Ccp enzyme, whereas its close relative N. meningitidis does not. The authors speculated that the key difference is that N. meningitidis dwells in the oxygen-rich nasopharynx, whereas N. gonorrhea lives in the urogenital tract where oxygen is scant and lactic-acid bacteria may excrete H2O2. Recently, Stacy et al. (66) noted a shift in the gene set used by Aggregatibacter actinomycetemcomitans when it was coinfected with Streptococcus gordonii in a murine thigh abscess model; they inferred that Aggregatibacter respiration was stimulated by a terminal oxidant that S. gordonii released. The data would fit the idea that Aggregatibacter Ccp was exploiting H2O2 that S. gordonii generates as a fermentation product.
The apparent Km of E. coli Ccp is 5 μM, and the electron flux through Ccp would be enough to sustain growth only if H2O2 approached this level. We and others have modeled H2O2 flow through a porous outer membrane and a semipermeable inner membrane into a cytoplasm containing high titers of peroxidases and catalases (10, 54). The upshot is that micromolar H2O2 outside cells will generate an equivalent level in the periplasm but perhaps a 10-fold lower level in the cytoplasm. The set point of OxyR is submicromolar (10, 21), so this flux is enough to activate OxyR and to induce Ccp. This situation allows Ccp to use micromolar external H2O2 as a periplasmic oxidant while at the same time the threat to cytoplasmic biomolecules is minimal. The other activities comprising the OxyR response would presumably help to keep the cell fit even while it respired using H2O2.
Although catalase generates molecular oxygen when it degrades H2O2, the primary scavengers of H2O2 in enteric bacteria are NADH peroxidases (29, 67). Therefore, oxygen production by H2O2-fed cells was insufficient to enable cytochrome oxidase-dependent growth. In any case, in natural habitats any oxygen generated by cytoplasmic catalases would immediately escape the source bacterium before it could be employed for respiration, because oxygen crosses membranes at diffusion-limited rates.
The existence of H2O2 in microoxic environments may explain why many bacteria are programmed for robust anti-H2O2 responses—including obligate anaerobes, whose oxidative defenses otherwise seem counterintuitive. One might suppose that these systems are useful during the occasional entry of anaerobes into oxic environments—but some of their peroxidatic scavenging systems, including rubrerythrins, are ineffectual when oxygen levels are high enough to poison central metabolism (24). Implicitly, these peroxidases, like Ccp, must serve to degrade H2O2 in habitats that contain little oxygen. Interestingly, low-micromolar H2O2 levels are also predicted to obtain to the phagosomal vesicles. By chance, Salmonellae, which naturally move between intestinal and macrophage habitats, might be equipped by its OxyR regulon to handle both environments.
The employment of a toxin such as H2O2 as a respiratory substrate strikes an ironic note, but this evolutionary step recapitulates the adoption of O2. Life emerged in an anoxic world; when O2 accumulated 2 billion years later (68), cells were threatened by its propensity to deactivate enzymes that have radical or low-potential metal centers. Defensive enzymes arose. But the toxicity of oxygen did not preclude the simultaneous appearance of respiratory enzymes that exploit it as an electron acceptor. We infer that the story with H2O2 may be analogous.
Materials and Methods
Reagents.
All antibiotics (ampicillin, chloramphenicol, kanamycin, tetracycline, and spectinomycin), ortho-nitrophenyl-β-galactoside, 2,2′-bipyridyl, horseradish peroxidase, 30% hydrogen peroxide, casein acid hydrolysate, sulfanilamide, N-(1-naphthyl)ethylenediamine dihydrochloride, and NADH were purchased from Sigma-Aldrich. Amplex UltraRed reagent was obtained from Life Technologies.
Strains and Bacterial Growth.
Strains, primers, and plasmids used in this study are listed in Tables S1–S3. Deletion mutations were made using the λ-red recombinase (69) or ordered from the Keio collection (70). Mutant strains were assembled by P1 transduction (71), and the inheritance of mutant alleles was confirmed by PCR analysis. A malE::Tn10 mutation was used to cotransduce the ubiA420 allele; the malE mutation itself was inconsequential in these experiments because the media lacked maltose. Transcriptional lacZ fusions were created as described (72), and the fusions were recombined into the lambda attachment site so that the native alleles were retained.
Table S1.
Strains
| Strain | Genotype | Source |
| MG1655 | F− wild type | E. coli Genetic Stock Center |
| LC106 (Hpx−) | ΔahpF::kan Δ(katG::Tn10)1 Δ(katE12::Tn10) | 2 |
| BW25113 | lacI rrnB ΔlacZ hsdK ΔaraBAD ΔrhaBAD | 69 |
| E. coli B | F− wild type | E. coli Genetic Stock Center |
| Salmonella typhimurium LT2 | Wild type | Lab stock |
| AL427 | Δ(katG17::Tn10)1 Δ(ahpCF1::cat)1 Δ(katE12::Tn10)1 | Lab stock |
| Al443 | F- λ- lacIq rrnBT14 ΔlacZWJ16 hsdR514 ΔaraBADAH33 ΔrhaBADLD78 ΔcydAB1::cat | Lab stock |
| AL454 | As MG1655 ΔcydAB1::cat | Lab stock |
| JEM1513 | As MG1655 with Δ(cyoABCDE)456::kan | Lab stock |
| JW1212-1 | F− Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) ΔnarL732::kan rph-1 Δ(rhaD-rhaB)568 hsdR514 | E. coli Genetic Stock Center |
| JW2181-2 | F− Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) ΔnarP746::kan rph-1, Δ(rhaD-rhaB)568 hsdR514 | E. coli Genetic Stock Center |
| JW4364-1 | F- Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) ΔarcA726::kan rph-1 Δ(rhaD-rhaB)568 hsdR514 | E. coli Genetic Stock Center |
| KK220 | F− Δ(pncA-xthA) thr-1 leuB6 proA2 his-4 thi-1 argE2 lacY1 galK2 rpsL supE44 ara-14 xyl-15 mtl-1 tsx-33 fnr(Bsm-Mlu)::Ω (SpR) zcj::Tn10 | Lab stock |
| LC148 | As LC106 with ubiA420∼malE::Tn10* | Lab stock |
| LC160 | As LC148 with Δ(menA::cam) | Lab stock |
| MK144 | As BW25113 with Δccp::cam | This study |
| MK146 | As LC106 with Δccp::cam | P1(MK144) × LC106 |
| MK148 | As BW25113 with Δbcp::cam | This study |
| MK150 | As LC106 with Δbcp::cam | P1(MK148) × LC106 |
| MK152 | As BW25113 with Δtpx::cam | This study |
| MK154 | As LC106 with Δtpx::cam | P1(MK152) × LC106 |
| MK156 | As BW25113 with ΔbtuE::cam | This study |
| MK158 | As LC106 with ΔbtuE::cam | P1(MK156) × LC106 |
| MK160 | As MK146 with camR gene flipped out | This study |
| MK162 | As BW25113 with ΔcyoABCDE::cat | This study |
| MK164 | As MK160 with ΔcydAB1::cat | P1(Al443) × MK160 |
| MK166 | As MK160 with ΔcyoABCDE::cam | P1(MK162) × Mk160 |
| MK168 | As MK164 with camR gene flipped out | This study |
| MK170 | As MK168 with ΔcyoABCDE::cam | P1(MK162) × Mk168 |
| MK172 | As MK160 with Δbcp::cam | P1(MK148) × MK160 |
| MK174 | As MK160 with Δtpx::cam | P1(MK152) × MK160 |
| MK176 | As MK160 with ΔbtuE::cam | P1(MK156) × MK160 |
| MK178 | As BW25113 with ΔappBC::cam | This study |
| MK180 | As LC106 with ΔappBC::cam | P1(MK178) × LC106 |
| MK182 | As MK160 with ΔappBC::cam | P1(MK178) × MK160 |
| MK184 | As LC160 with Δccp::cam | P1(MK144) × LC160 |
| MK188 | As LC106 with attλ::[pSJ501::pahpC’-lacZ+]∼cat | P1(SRK57) × LC106 |
| Mk196 | As BW25113 with Δ(ccmABCDEFGH)::cam | This study |
| MK198 | As LC106 with Δ(ccmABCDEFGH)::cam | P1(MK196) × LC106 |
| MK204 | As BW25113 with ΔosmC::cam | This study |
| MK206 | As BW25113 with Δ(napABCDFGH)::cam | This study |
| Mk208 | As LC106 with ΔosmC::cam | P1(MK204) × LC106 |
| MK210 | As MK160 with ΔosmC::cam | P1(MK204) × MK160 |
| MK212 | As LC106 with Δ(napABCDFGH)::cam | P1(MK206) × LC106 |
| MK216 | As BW25113 with Δ(nrfABCDEFG)::cam | This study |
| MK218 | As LC106 with Δ(nrfABCDEFG)::cam | P1(MK216) × LC106 |
| MK240 | As SJ130 with attλ::[pSJ501::yhjA’-lacZ+] ∼camR | This study |
| MK246 | As MG1655 with attλ::[pSJ501::yhjA’-lacZ+] ∼camR | P1(MK204) × MG1655 |
| MK250 | As LC106 with attλ::[pSJ501::yhjA’-lacZ+] ∼camR | This study |
| MK274 | As MK250 with Δfnr(Bsm-Mlu)::Ω (SpR) zcj::Tn10 | P1(KK220) × MK250 |
| MK278 | As MK250 with ΔoxyR::spec | P1(XY20) × MK250 |
| MK336 | As MK246 with camR gene flipped out | This study |
| MK344 | As MK336 with pACYC184 | This study |
| MK346 | As MK336 with pGSO58 (poxyR2) | This study |
| MK400 | As AL427 with attλ::[pSJ501::yhjA’-lacZ+] ∼camR | This study |
| MK404 | As MK400 with ΔnarL732::kan | P1(JW1212-2) × MK400 |
| MK408 | As MK400 with ΔarcA726::kan | P1(JW4364-1) × MK400 |
| MK412 | As MK400 with ΔnarP746::kan | P1(JW2181-2) × MK400 |
| MK416 | As MG1655 with Δccp::cam | P1(MK144) × MG1655 |
| MK418 | As MK160 with Δ(ccmABCDEFGH)::cam | P1(MK196) × MK160 |
| MK420 | As MK416 with camR gene flipped out | This study |
| MK430 | As MK160 with pACYC184-ccp | This study |
| MK432 | As MK420 with pACYC184 | This study |
| MK436 | As MK420 with pACYC184-ccp | This study |
| SJ130 | As MG1655 with Δ(lacZ1::cat)1 | Lab stock |
| SRK57 | attλ::[pSJ501::PahpC’-lacZ+]∼cat | Lab stock |
| SSK53 | As LC106 with cyd::cam Δ(cyoABCDE)456::kan linked to zaj::Tn10 | Lab stock |
| SSK6 | As LC106 with ΔmenA::cam | Lab stock |
| XY20 | as MG1655 with ΔoxyR::spec | Lab stock |
The ubiA420 mutation in LC148 is linked to malE::Tn10. Because none of the growth or assay conditions in this paper contained maltose, the malE mutation has no effect on the observed phenotype.
Table S3.
Plasmids
| Plasmids | Characteristics | Source |
| pSJ501 | pAH125 derivative with cat flanked by flp sites | 15 |
| pKD3 | bla FRT cat FRT PS1 PS2 oriR6K | 69 |
| pKD46 | bla PBAD gam bet exo pSC101 oriTS | 69 |
| pCP20 | bla cat cI857 λPR flp pSC101 oriTS | 74 |
| pACYC184 | TetR CmR | 75 |
| pGS058 | pACYC184 containing oxyR2 [A233V] | 75 |
| pACYC184-ccp | pACYC184 containing ccp gene and promoter | This study |
Table S2.
Primers
| Primer | Sequence |
| ccp-deletion | 5′-GAAAATGGTCTCACGTATTACCGCGATCGGCCTGGCTGGCGTCGCGTGTAGGCTGGAGCTGCTTCG-3′ |
| 5′-CTGTGCAGGAAAGCTACGATATCATCCACATCCTCCTGCGGCAGCTCCATATGAATATCCTCCTTAG-3′ | |
| bcp-deletion | 5′-GCCCACAGCCCCGCATCTGCGGACGCAGCAAATATTGAGCAAGCGTGTAGGCTGGAGCTGCTTCG-3′ |
| 5′-GAAGTGAGGTATGCTGGCCGCAAGCGCAGCCAGCACGGAATGGAGCATATGAATATCCTCCTTAG-3′ | |
| btuE-deletion | 5′-CGACGGTGAAGTGACCACGCTGGAGAAGTTCGCCGGTAATGTGCTGTGTAGGCTGGAGCTGCTTCG-3′ |
| 5′-CGGCGTCATATCCGGGGAAAAACGCTGGATGACTTTTCCGTCCCTGCCCATATGAATATCCTCCTTAG-3′ | |
| tpx-deletion | 5′-CCCCGGGCAATCACAAACGGGGTATGTAAGGGCCAGGCTTCCTCGTGTAGGCTGGAGCTGCTTCG-3′ |
| 5′-GAGCTGCTTCGTAATCCGGCTCGGTGGTGATTTCATCCACCAGCTGGCCATATGAATATCCTCCTTAG-3′ | |
| appBC-deletion | 5′-GTGGGATGTCATTGATTTATCGCGCTGGCAGTTTGCTCTGACCGCGCTGTAGGCTGGAGCTGCTTCG-3′ |
| 5′-CTCGTTTTCGTTACGGCGGAGAGTTTCTGTTGTCATGCGCCCCCACCATATGAATATCCTCCTTAG-3′ | |
| osmC-deletion | 5′-GGTCAGGCACACTGGGAAGGCGATATCAAACGCGGGAAGGGAACAGTTGTAGGCTGGAGCTGCTTCG-3′ |
| 5′-GCATCAGGCATTCACGCGCCTGACGCGTCATCCGGCAATGCTTTACGCATATGAATATCCTCCTTAG-3′ | |
| cyoABCDE-deletion | 5′-GCCCGCCCAGACTCCTTCAGCACTCCCCTTTTGTTATAACGCCCTTGTAGGCTGGAGCTGCTTCG-3′ |
| 5′-GGTTTTGTTACCACACAGCAGCCAGCAGCGTATGCGAGTCCGGTACCATATGAATATCCTCCTTAG-3′ | |
| ccmABCDEFGH-deletion | 5′-CTGAACGCAGGAGAGTGGGTACAAATCACCGGTAGCAACGGCGCGGTGTAGGCTGGAGCTGCTTCG-3′ |
| 5′-ATCTCCCACGCGGCAACGGCTTCGCCAAATCGCTGCTGCTCAAAGGCATATGAATATCCTCCTTAG-3′ | |
| napABCDFGH-deletion | 5′-GATTGATGCATCCCGTCGGGGCATACTCACTGGTCGCTGGCGCAAATGTAGGCTGGAGCTGCTTCG-3′ |
| 5′-CCTGGCTCGACTTCACGCATATCGGGCAGCTTGTGCGCTATCCCTTTCATATGAATATCCTCCTTAG-3′ | |
| nrfABCDEFG-deletion | 5′-TTGCCCCGCAGCATCCCGATCAATATCTCTCCTGGAAAGCCACCTTGTAGGCTGGAGCTGCTTCG-3′ |
| 5′-CGACTCAACCAGCTGTGTTCGGTTAACTCGCGGTGAGTTGAGATCCACATATGAATATCCTCCTTAG-3′ | |
| ccp-promoter for lacZ fusion | 5′-TTTCTGCAGCCGCAATCATGATATGCC-3′ |
| 5′-TTTGAATTCCGGTGGTGAGTTATTGTG-3′ | |
| ccp gene for complementation | 5′-TTTTCTAGACCGCAATCATGATATGCC-3′ |
| 5′-TTTGGATCCGCCCAAACATTGCTGTTC-3′ |
Luria broth and base minimal A salts were of standard composition (71). Glucose–amino acid medium was minimal A salts supplemented with 0.2% casein acid hydrolysate, 0.2% glucose, 5 μg/mL thiamine, 0.02% MgSO4, and 0.5 mM tryptophan. Where indicated, 40 mM nitrate or 25 mM fumarate was added. Uracil (1 mM) was added to the media in experiments that involved quinone biosynthetic mutants, because dihydroorotate dehydrogenase requires a quinone substrate.
β-galactosidase activity was measured as described (71), using cells that were grown to OD600 of 0.1 and then challenged with H2O2 for 1 h if indicated. Protein concentrations were measured using Bradford assay (Coomassie protein assay reagent; Thermo Scientific) using BSA as the standard. All anoxic growth and assays were done using anoxic buffers, media, and reaction components in a Coy anaerobic chamber (Coy Laboratory Products, Inc.) under 85% N2, 10% H2, and 5% CO2.
Hydrogen Peroxide Scavenging Assay.
Overnight cultures were diluted to OD600 of 0.01 into fresh glucose–amino acids medium, and cells were grown to OD600 of 0.2. Cells were then diluted to OD600 of 0.02 in fresh medium containing 10 μM H2O2. The culture was maintained at 37 °C, and 1-mL samples were removed every 4 min for 20 min. The samples were centrifuged (1 min at 15,300 × g, 4 °C) and the supernatant was frozen on dry ice and stored at −80 °C freezer. The samples were subsequently thawed on ice, and the concentration of peroxide was measured using Amplex UltraRed/horseradish peroxidase (29) in a Shimadzu RF Mini-150 fluorometer.
Glycerol Growth Assays.
Cells were grown anaerobically overnight in minimal A medium supplemented with 40 mM glycerol, 25 mM fumarate, amino acids (histidine, tyrosine, isoleucine, leucine, valine, and phenylalanine, each at 0.5 mM), 0.02% magnesium sulfate, and 5 μg/mL thiamine. The branched-chain and aromatic amino acids were provided to avoid growth disruptions due to the potential inactivation of iron enzymes in their biosynthetic pathways. E. coli cannot catabolize these amino acids to use them as energy sources. Fumarate was included to enable anaerobic respiration. Cells were then precultured in the same medium to OD600 of 0.1 and then washed four times with minimal A salts to remove fumarate. Bacteria were diluted to OD600 of 0.0001 in the fresh 37 °C medium without fumarate. Respiratory oxidants (40 mM nitrate, 25 mM fumarate, or 5 μM H2O2) were added as indicated. The residual H2O2 levels were periodically determined by Amplex UltraRed analysis, and H2O2 was replenished as needed to restore the concentration to 5 μM. Samples were periodically removed, diluted, and plated on LB agar. Colonies were counted the next day, and the colony-forming units in the original culture were calculated. Slight residual growth in the absence of electron acceptors was apparent only at very low cell densities and probably indicates trace oxygen in the media (Fig. S8).
Fig. S8.
Trace oxygen is the probable source of slight residual anaerobic growth in the absence of added electron acceptors. Growth occurred only at very low cell densities (○); at higher cell densities that would quickly scavenge oxygen, no increase in biomass was perceptible (□). One sample culture is shown from two replicates.
NADH Oxidation Assay.
Inverted membrane vesicles were prepared as described (73) from ∆cyo and ∆cyd mutants that had been grown aerobically in LB medium to 0.25 OD600. The NADH oxidation reaction was performed using anoxic reagents in the Coy chamber with inverted membrane vesicles, 50 μM NADH, and different concentrations of H2O2. NADH oxidation was measured using the extinction coefficient for NADH of 6,220 cm−1⋅M−1 at 340 nm.
Nitrate Reduction and Oxygen Consumption Assays.
Wild-type cells were grown to 0.25 OD600 in glucose–amino acid medium supplemented with 40 mM nitrate. Cells were washed three times with minimal A salts to remove the residual nitrite and inoculated to 0.020 OD600 in glucose-amino acid medium. The concentration of nitrite was measured every 30 min using a modified version of the Griess assay. Sulfanilamide (250 μL of 2% wt/vol in 5% HCl), N-(1-naphthyl)ethylenediamine dihydrochloride (250 μL of 0.1% wt/vol in water) and 500 μL sample were mixed, and the absorbance at 540 nm was measured after 20 min of incubation in room temperature.
The rate of oxygen consumption was determined during aerobic growth in glycerol medium, using a Clark electrode (Micrometrix). Wild-type cells were grown anaerobically from 0.01 to 0.1 OD600. Cells were moved to the electrode chamber, and the level of oxygen was recorded every 10 s for 5 min. The rate of respiration with oxygen was then normalized to OD600.
The rate of anaerobic respiration using fumarate as an acceptor is calculated in SI Materials and Methods.
H2O2 Killing Assay.
Cells were grown anaerobically to OD600 = 0.3 in LB, and 2.5 mM H2O2 was added. At different time points, samples were diluted and plated on LB agar. Colonies were counted after a day, and percent survival was calculated based on colony-forming units.
Measuring Zones of Inhibition.
All steps were performed in the anaerobic chamber with anoxic materials. Cells were grown in minimal glucose medium to OD600 = 0.1; they were then mixed with 4 mL top agar (0.8%, 50 °C) and spread on plates of the same composition. Sterile disks (6 mm) were soaked in 15 μL 100 mM H2O2 and placed in the middle of the plate. The diameter of the inhibition zone was measured after 24 h.
Acknowledgments
We thank Patrick Degnan for his assistance with bioinformatics studies. This work was supported by NIH Grant GM049640.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.J.B. is a guest editor invited by the Editorial Board.
See Commentary on page 8678.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701587114/-/DCSupplemental.
References
- 1.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat Rev Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuo CF, Mashino T, Fridovich I. α, β-Dihydroxyisovalerate dehydratase. A superoxide-sensitive enzyme. J Biol Chem. 1987;262:4724–4727. [PubMed] [Google Scholar]
- 3.Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem. 1993;268:22369–22376. [PubMed] [Google Scholar]
- 4.Jang S, Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem. 2007;282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobota JM, Imlay JA. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc Natl Acad Sci USA. 2011;108:5402–5407. doi: 10.1073/pnas.1100410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu M, Imlay JA. Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol Microbiol. 2013;89:123–134. doi: 10.1111/mmi.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 8.Christman MF, Morgan RW, Jacobson FS, Ames BN. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 9.Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 10.Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol. 2001;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 12.Lee C, et al. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat Struct Mol Biol. 2004;11:1179–1185. doi: 10.1038/nsmb856. [DOI] [PubMed] [Google Scholar]
- 13.Zheng M, et al. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol. 2001;183:4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Yeo WS, Roe JH. Induction of the sufA operon encoding Fe-S assembly proteins by superoxide generators and hydrogen peroxide: Involvement of OxyR, IHF and an unidentified oxidant-responsive factor. Mol Microbiol. 2004;51:1745–1755. doi: 10.1111/j.1365-2958.2003.03946.x. [DOI] [PubMed] [Google Scholar]
- 15.Jang S, Imlay JA. Hydrogen peroxide inactivates the Escherichia coli Isc iron-sulphur assembly system, and OxyR induces the Suf system to compensate. Mol Microbiol. 2010;78:1448–1467. doi: 10.1111/j.1365-2958.2010.07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehres DG, Janakiraman A, Slauch JM, Maguire ME. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H2O2, Fe2+, and Mn2+ J Bacteriol. 2002;184:3151–3158. doi: 10.1128/JB.184.12.3151-3158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anjem A, Imlay JA. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem. 2012;287:15544–15556. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altuvia S, Almirón M, Huisman G, Kolter R, Storz G. The dps promoter is activated by OxyR during growth and by IHF and sigma S in stationary phase. Mol Microbiol. 1994;13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 19.Ilari A, Ceci P, Ferrari D, Rossi GL, Chiancone E. Iron incorporation into Escherichia coli Dps gives rise to a ferritin-like microcrystalline core. J Biol Chem. 2002;277:37619–37623. doi: 10.1074/jbc.M206186200. [DOI] [PubMed] [Google Scholar]
- 20.Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli. Proc Natl Acad Sci USA. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aslund F, Zheng M, Beckwith J, Storz G. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci USA. 1999;96:6161–6165. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocha ER, Herren CD, Smalley DJ, Smith CJ. The complex oxidative stress response of Bacteroides fragilis: The role of OxyR in control of gene expression. Anaerobe. 2003;9:165–173. doi: 10.1016/S1075-9964(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 23.Strand KR, et al. Oxidative stress protection and the repair response to hydrogen peroxide in the hyperthermophilic archaeon Pyrococcus furiosus and in related species. Arch Microbiol. 2010;192:447–459. doi: 10.1007/s00203-010-0570-z. [DOI] [PubMed] [Google Scholar]
- 24.Mishra S, Imlay JA. An anaerobic bacterium, Bacteroides thetaiotaomicron, uses a consortium of enzymes to scavenge hydrogen peroxide. Mol Microbiol. 2013;90:1356–1371. doi: 10.1111/mmi.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altschul AM, Abrams R, Hogness TR. Cytochrome c peroxidase. J Biol Chem. 1940;136:777–793. [Google Scholar]
- 26.Atack JM, Kelly DJ. Structure, mechanism and physiological roles of bacterial cytochrome c peroxidases. Adv Microb Physiol. 2007;52:73–106. doi: 10.1016/S0065-2911(06)52002-8. [DOI] [PubMed] [Google Scholar]
- 27.Van Spanning RJM, et al. FnrP and NNR of Paracoccus denitrificans are both members of the FNR family of transcriptional activators but have distinct roles in respiratory adaptation in response to oxygen limitation. Mol Microbiol. 1997;23:893–907. doi: 10.1046/j.1365-2958.1997.2801638.x. [DOI] [PubMed] [Google Scholar]
- 28.Partridge JD, Poole RK, Green J. The Escherichia coli yhjA gene, encoding a predicted cytochrome c peroxidase, is regulated by FNR and OxyR. Microbiology. 2007;153:1499–1507. doi: 10.1099/mic.0.2006/004838-0. [DOI] [PubMed] [Google Scholar]
- 29.Seaver LC, Imlay JA. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol. 2001;183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cha MK, Kim WC, Lim CJ, Kim K, Kim IH. Escherichia coli periplasmic thiol peroxidase acts as lipid hydroperoxide peroxidase and the principal antioxidative function during anaerobic growth. J Biol Chem. 2004;279:8769–8778. doi: 10.1074/jbc.M312388200. [DOI] [PubMed] [Google Scholar]
- 31.Jeong W, Cha MK, Kim IH. Thioredoxin-dependent hydroperoxide peroxidase activity of bacterioferritin comigratory protein (BCP) as a new member of the thiol-specific antioxidant protein (TSA)/Alkyl hydroperoxide peroxidase C (AhpC) family. J Biol Chem. 2000;275:2924–2930. doi: 10.1074/jbc.275.4.2924. [DOI] [PubMed] [Google Scholar]
- 32.Arenas FA, et al. The Escherichia coli btuE gene, encodes a glutathione peroxidase that is induced under oxidative stress conditions. Biochem Biophys Res Commun. 2010;398:690–694. doi: 10.1016/j.bbrc.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesniak J, Barton WA, Nikolov DB. Structural and functional features of the Escherichia coli hydroperoxide resistance protein OsmC. Protein Sci. 2003;12:2838–2843. doi: 10.1110/ps.03375603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma J, et al. Glutamate-89 in subunit II of cytochrome bo3 from Escherichia coli is required for the function of the heme-copper oxidase. Biochemistry. 1999;38:15150–15156. doi: 10.1021/bi991764y. [DOI] [PubMed] [Google Scholar]
- 35.Al-Attar S, et al. Cytochrome bd displays significant quinol peroxidase activity. Sci Rep. 2016;6:27631. doi: 10.1038/srep27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verduyn C, van Wijngaarden CJ, Scheffers WA, van Dijken JP. Hydrogen peroxide as an electron acceptor for mitochondrial respiration in the yeast Hansenula polymorpha. Yeast. 1991;7:137–146. doi: 10.1002/yea.320070207. [DOI] [PubMed] [Google Scholar]
- 37.Richardson DJ, Ferguson SJ. Competition between hydrogen peroxide and nitrate for electrons from the respiratory chains of Thiosphaera pantotropha and Rhodobacter capsulatus. FEMS Microbiol Lett. 1995;132(1-2):125–129. [Google Scholar]
- 38.Goodhew CF, elKurdi AB, Pettigrew GW. The microaerophilic respiration of Campylobacter mucosalis. Biochim Biophys Acta. 1988;933:114–123. doi: 10.1016/0005-2728(88)90061-8. [DOI] [PubMed] [Google Scholar]
- 39.Zaslavsky D, Gennis RB. Proton pumping by cytochrome oxidase: Progress, problems and postulates. Biochim Biophys Acta. 2000;1458:164–179. doi: 10.1016/s0005-2728(00)00066-9. [DOI] [PubMed] [Google Scholar]
- 40.Lomize AL, Lomize MA, Krolicki SR, Pogozheva ID. Membranome: A database for proteome-wide analysis of single-pass membrane proteins. Nucleic Acids Res. 2017;45:D250–D255. doi: 10.1093/nar/gkw712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada H, Takashima E, Konishi K. Molecular characterization of the membrane-bound quinol peroxidase functionally connected to the respiratory chain. FEBS J. 2007;274:853–866. doi: 10.1111/j.1742-4658.2006.05637.x. [DOI] [PubMed] [Google Scholar]
- 42.Takashima E, Yamada H, Yamashita T, Matsushita K, Konishi K. Recombinant expression and redox properties of triheme c membrane-bound quinol peroxidase. FEMS Microbiol Lett. 2010;302:52–57. doi: 10.1111/j.1574-6968.2009.01830.x. [DOI] [PubMed] [Google Scholar]
- 43.Charoensuk K, et al. Physiological importance of cytochrome c peroxidase in ethanologenic thermotolerant Zymomonas mobilis. J Mol Microbiol Biotechnol. 2011;20:70–82. doi: 10.1159/000324675. [DOI] [PubMed] [Google Scholar]
- 44.Wallace BJ, Young IG. Role of quinones in electron transport to oxygen and nitrate in Escherichia coli. Studies with a ubiA- menA- double quinone mutant. Biochim Biophys Acta. 1977;461:84–100. doi: 10.1016/0005-2728(77)90071-8. [DOI] [PubMed] [Google Scholar]
- 45.Ezraty B, Henry C, Hérisse M, Denamur E, Barras F. Commercial Lysogeny Broth culture media and oxidative stress: A cautious tale. Free Radic Biol Med. 2014;74:245–251. doi: 10.1016/j.freeradbiomed.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Sobota JM, Gu M, Imlay JA. Intracellular hydrogen peroxide and superoxide poison 3-deoxy-D-arabinoheptulosonate 7-phosphate synthase, the first committed enzyme in the aromatic biosynthetic pathway of Escherichia coli. J Bacteriol. 2014;196:1980–1991. doi: 10.1128/JB.01573-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herren CD, Rocha ER, Smith CJ. Genetic analysis of an important oxidative stress locus in the anaerobe Bacteroides fragilis. Gene. 2003;316:167–175. doi: 10.1016/s0378-1119(03)00759-5. [DOI] [PubMed] [Google Scholar]
- 48.Turner S, Reid E, Smith H, Cole J. A novel cytochrome c peroxidase from Neisseria gonorrhoeae: A lipoprotein from a Gram-negative bacterium. Biochem J. 2003;373:865–873. doi: 10.1042/BJ20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takashima E, Konishi K. Characterization of a quinol peroxidase mutant in Aggregatibacter actinomycetemcomitans. FEMS Microbiol Lett. 2008;286:66–70. doi: 10.1111/j.1574-6968.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- 50.Schütz B, Seidel J, Sturm G, Einsle O, Gescher J. Investigation of the electron transport chain to and the catalytic activity of the diheme cytochrome c peroxidase CcpA of Shewanella oneidensis. Appl Environ Microbiol. 2011;77:6172–6180. doi: 10.1128/AEM.00606-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charizanis C, Juhnke H, Krems B, Entian K-D. The mitochondrial cytochrome c peroxidase Ccp1 of Saccharomyces cerevisiae is involved in conveying an oxidative stress signal to the transcription factor Pos9 (Skn7) Mol Gen Genet. 1999;262:437–447. doi: 10.1007/s004380051103. [DOI] [PubMed] [Google Scholar]
- 52.Giles SS, Perfect JR, Cox GM. Cytochrome c peroxidase contributes to the antioxidant defense of Cryptococcus neoformans. Fungal Genet Biol. 2005;42:20–29. doi: 10.1016/j.fgb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Nikaido H, Rosenberg EY. Effect on solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J Gen Physiol. 1981;77:121–135. doi: 10.1085/jgp.77.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: Implications for microbial killing. J Biol Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 55.Unden G, Bongaerts J. Alternative respiratory pathways of Escherichia coli: Energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta. 1997;1320:217–234. doi: 10.1016/s0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 56.Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992;72:57–64. doi: 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 57.Pitcher MCL, Beatty ER, Cummings JH. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut. 2000;46:64–72. doi: 10.1136/gut.46.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones SA, et al. Respiration of Escherichia coli in the mouse intestine. Infect Immun. 2007;75:4891–4899. doi: 10.1128/IAI.00484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korshunov S, Imlay KRC, Imlay JA. The cytochrome bd oxidase of Escherichia coli prevents respiratory inhibition by endogenous and exogenous hydrogen sulfide. Mol Microbiol. 2016;101:62–77. doi: 10.1111/mmi.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forte E, et al. The terminal oxidase cytochrome bd promotes sulfide-resistant bacterial respiration and growth. Scientific Reports. 2016;6:23788. doi: 10.1038/srep23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pericone CD, Park S, Imlay JA, Weiser JN. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the fenton reaction. J Bacteriol. 2003;185:6815–6825. doi: 10.1128/JB.185.23.6815-6825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seki M, Iida K, Saito M, Nakayama H, Yoshida S. Hydrogen peroxide production in Streptococcus pyogenes: Involvement of lactate oxidase and coupling with aerobic utilization of lactate. J Bacteriol. 2004;186:2046–2051. doi: 10.1128/JB.186.7.2046-2051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu Z, Imlay JA. The fumarate reductase of Bacteroides thetaiotaomicron, unlike that of Escherichia coli, is configured so that it does not generate reactive oxygen species. MBio. 2017;8:e01873–e16. doi: 10.1128/mBio.01873-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bingham-Ramos LK, Hendrixson DR. Characterization of two putative cytochrome c peroxidases of Campylobacter jejuni involved in promoting commensal colonization of poultry. Infect Immun. 2008;76:1105–1114. doi: 10.1128/IAI.01430-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seib KL, Tseng HJ, McEwan AG, Apicella MA, Jennings MP. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: Distinctive systems for different lifestyles. J Infect Dis. 2004;190:136–147. doi: 10.1086/421299. [DOI] [PubMed] [Google Scholar]
- 66.Stacy A, Fleming D, Lamont RJ, Rumbaugh KP, Whiteley M. A commensal bacterium promotes virulence of an opportunistic pathogen via cross-respiration. MBio. 2016;7:e00782–e00716. doi: 10.1128/mBio.00782-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hébrard M, Viala JP, Méresse S, Barras F, Aussel L. Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J Bacteriol. 2009;191:4605–4614. doi: 10.1128/JB.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anbar AD. Oceans. Elements and evolution. Science. 2008;322:1481–1483. doi: 10.1126/science.1163100. [DOI] [PubMed] [Google Scholar]
- 69.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baba T, et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol 2:2006.0008.
- 71.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 1972. [Google Scholar]
- 72.Haldimann A, Wanner BL. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol. 2001;183:6384–6393. doi: 10.1128/JB.183.21.6384-6393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Messner KR, Imlay JA. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J Biol Chem. 1999;274:10119–10128. doi: 10.1074/jbc.274.15.10119. [DOI] [PubMed] [Google Scholar]
- 74.Cherepanov PP, Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158:9–14. doi: 10.1016/0378-1119(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 75.Kullik I, Stevens J, Toledano MB, Storz G. Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for DNA binding and multimerization. J Bacteriol. 1995;177:1285–1291. doi: 10.1128/jb.177.5.1285-1291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park S, Imlay JA. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J Bacteriol. 2003;185:1942–1950. doi: 10.1128/JB.185.6.1942-1950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woodmansee AN, Imlay JA. Reduced flavins promote oxidative DNA damage in non-respiring Escherichia coli by delivering electrons to intracellular free iron. J Biol Chem. 2002;277:34055–34066. doi: 10.1074/jbc.M203977200. [DOI] [PubMed] [Google Scholar]
- 78.Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci USA. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imlay JA, Fridovich I. Assay of metabolic superoxide production in Escherichia coli. J Biol Chem. 1991;266:6957–6965. [PubMed] [Google Scholar]
- 80.Ravindra Kumar S, Imlay JA. How Escherichia coli tolerates profuse hydrogen peroxide formation by a catabolic pathway. J Bacteriol. 2013;195:4569–4579. doi: 10.1128/JB.00737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tran QH, Bongaerts J, Vlad D, Unden G. Requirement for the proton-pumping NADH dehydrogenase I of Escherichia coli in respiration of NADH to fumarate and its bioenergetic implications. Eur J Biochem. 1997;244:155–160. doi: 10.1111/j.1432-1033.1997.00155.x. [DOI] [PubMed] [Google Scholar]
- 82.Borisov VB, et al. Cytochrome bd oxidase from Escherichia coli displays high catalase activity: An additional defense against oxidative stress. FEBS Lett. 2013;587:2214–2218. doi: 10.1016/j.febslet.2013.05.047. [DOI] [PubMed] [Google Scholar]