Significance
New material from the Chengjiang fossil Lagerstätte clarifies the identity of three early Cambrian problematica. The presumed earliest hemichordate Galeaplumosus abilus and the putative ancient sea pen Chengjiangopenna wangii are in fact fragments of Xianguangia sinica. Here we demonstrate that X. sinica possessed a polypoid body, a blind gastric cavity partitioned by septum-like structures, a holdfast that contained an additional cavity functioning as a hydroskeleton, a basal pit used for anchorage, and a radial whorl of feather-like tentacles for ciliary suspension feeding. Phylogenetic analyses based on the new findings suggest that X. sinica represents an extinct body plan most closely allied to cnidarians and thus sheds light on their early evolution.
Keywords: Xianguangia sinica, Chengjiangopenna wangii, Galeaplumosus abilus, ciliary suspension feeder, cnidarian stem group
Abstract
The early Cambrian problematica Xianguangia sinica, Chengjiangopenna wangii, and Galeaplumosus abilus from the Chengjiang biota (Yunnan, China) have caused much controversy in the past and their phylogenetic placements remain unresolved. Here we show, based on exceptionally preserved material (85 new specimens plus type material), that specimens previously assigned to these three species are in fact parts of the same organism and propose that C. wangii and G. abilus are junior synonyms of X. sinica. Our reconstruction of the complete animal reveals an extinct body plan that combines the characteristics of the three described species and is distinct from all known fossil and living taxa. This animal resembled a cnidarian polyp in overall morphology and having a gastric cavity partitioned by septum-like structures. However, it possessed an additional body cavity within its holdfast, an anchoring pit on the basal disk, and feather-like tentacles with densely ciliated pinnules arranged in an alternating pattern, indicating that it was a suspension feeder rather than a predatory actiniarian. Phylogenetic analyses using Bayesian inference and maximum parsimony suggest that X. sinica is a stem-group cnidarian. This relationship implies that the last common ancestor of X. sinica and crown cnidarians was probably a benthic, polypoid animal with a partitioned gastric cavity and a single mouth/anus opening. This extinct body plan suggests that feeding strategies of stem cnidarians may have been drastically different from that of their crown relatives, which are almost exclusively predators, and reveals that the morphological disparity of total-group Cnidaria is greater than previously assumed.
Fossil problematica are extinct taxa that have defied unambiguous phylogenetic interpretations. They are enigmatic weirdos that have caused taxonomic headaches or have been unsatisfactorily shoehorned into one or another extant group. Problematica commonly occur in the fossil record of the Ediacaran and Cambrian (635–484 Mya), during which metazoans underwent a dramatic diversification. Therefore, deciphering fossil problematica from this crucial interval of evolution might provide pivotal insights into the origin and early radiation of metazoan body plans (1). Clarifying the anatomy and phylogenetic placement of some of these problematica has greatly improved our understanding of the evolution of major animal groups such as cnidarians (e.g., refs. 2 and 3), ctenophores (e.g., ref. 4), molluscs (5, 6), annelids (7), panarthropods (e.g., refs. 8 and 9), lophophorates (e.g., ref. 10), hemichordates (e.g., ref. 11), and chordates (12–15).
Here we examine three fossil problematica: Xianguangia sinica [Chen and Erdtmann (16)], Chengjiangopenna wangii [Shu and Conway Morris (17)], and Galeaplumosus abilus [Hou et al. (18)] from the early Cambrian Chengjiang biota (∼520 Mya) in southern China. These putative species have been assigned to three different animal groups: hexacorals (16, 19–24), octocorals (17, 25), and hemichordates (18), respectively. However, alternative views suggest that X. sinica was a ctenophore (23, 26), a lophophorate (27), an Ediacaran survivor (28), or a metazoan of unknown affinity (29, 30). Moreover, the phylogenetic placement of C. wangii as an ancient sea pen remains ambiguous (31) and the assignment of G. abilus to hemichordates has also been questioned (32). In this study, we demonstrate that these three problematica are in fact conspecifics. The assemblage of this Cambrian puzzle revealed the existence of a “feathered polyp,” most likely representing an offshoot of the cnidarian stem lineage that diverged early during the Cambrian radiation of animal body plans.
Systematic Paleontology
We examined 88 specimens of X. sinica from the lower Cambrian (series 2, stage 3) Chengjiang fossil deposit, which revealed a suite of distinctive morphological features, including sophisticated, feather-like tentacles, septum-like structures subdividing a central cavity into confluent compartments, a holdfast with a separate cavity, and a basal disk with a spacious pit (Figs. 1–4 and SI Appendix, Text S1, Figs. S1–S4 and Tables S1 and S2).
Xianguangia sinica Chen and Erdtmann 1991
(Figs. 1–3 and SI Appendix, Figs. S1–S4)
Fig. 1.
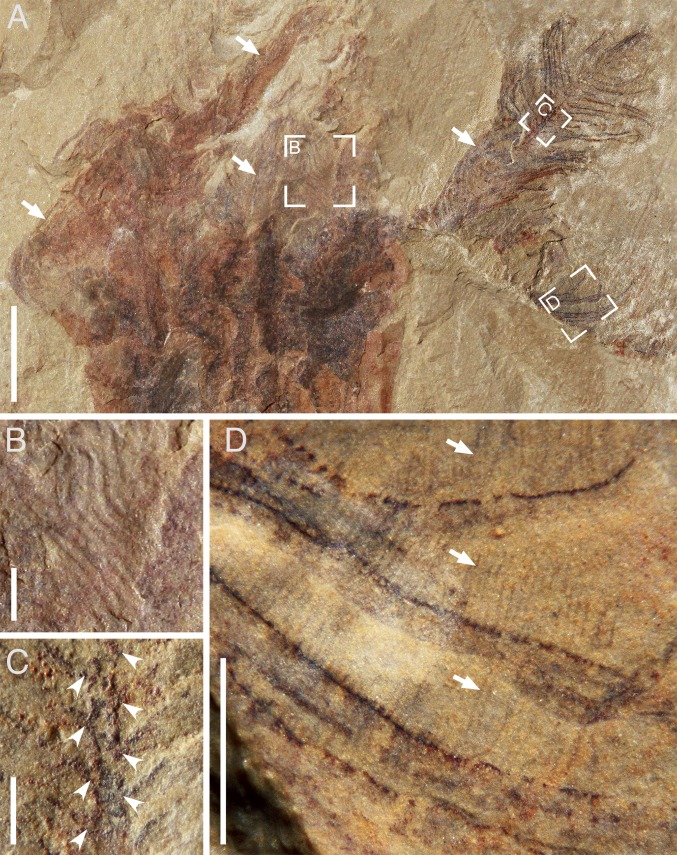
Xianguangia sinica, lower Cambrian, South China. (A) Complete specimen ELEL-SJ120376A showing feather-like tentacles, column, and holdfast. (B) Close-up of a pinnate tentacle (arrowed) in A. (C) Close-up of focus area in B showing long cilia fringing the pinnules. [Scale bars: (A) 5 mm; (B) 2 mm; (C) 1 mm.] Cc, circumferential constriction; Ci, cilia; Co, column; Ho, holdfast; Pi, pinnule; Ra, rachis; Te, tentacle.
Fig. 4.
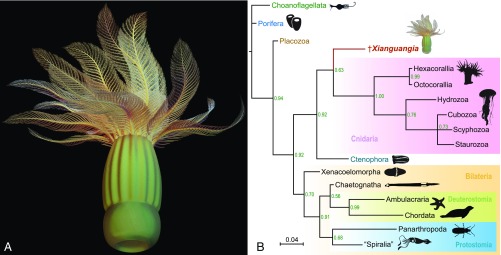
Reconstruction and phylogenetic position of X. sinica. (A) Three-dimensional model generated with 3ds Max. (B) Summary of metazoan relationships inferred from Bayesian analyses based on 111 characters and 37 taxa under Mkv + Γ model (see SI Appendix, Figs. S5–S7 and Text S2 and S3 for details). Numbers at nodes indicate posterior probabilities. X. sinica is resolved as a stem-group cnidarian. Eumetazoa, Neuralia, Bilateria, Nephrozoa, Protostomia, and Deuterostomia are monophyletic, whereas the monophyly of Spiralia is unresolved. Animal silhouettes are by courtesy of PhyloPic (www.phylopic.org).
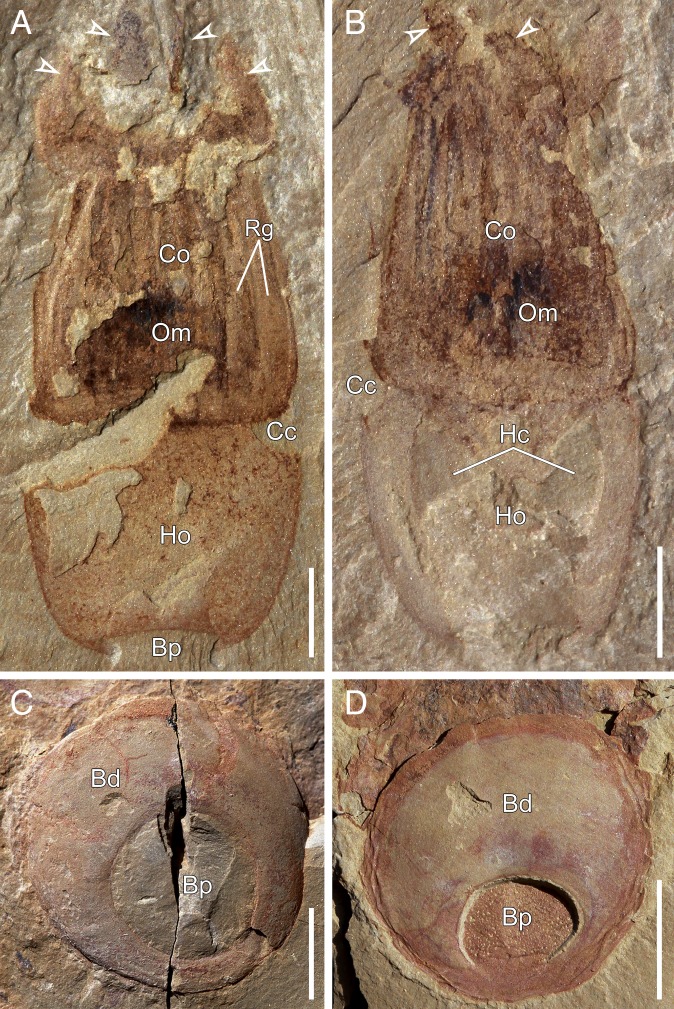
Fig. 3.
Anatomy of X. sinica. (A and B) Specimens ELEL-SJ120379 and SJ120380, respectively, showing column with external ridges and internal dark remains, circumferential constriction, and tentacles (arrowheads). (C) Aborally compacted specimen SJ101880 showing basal disk bearing a prominent pit filled with sediment. (D) Specimen SJ080827 showing upturned holdfast and basal pit (sediment removed). [Scale bars: 2 mm (A and B); 5 mm (C and D).] Bd, basal disk; Bp, basal pit; Cc, circumferential constriction; Co, column; Hc, holdfast cavity; Ho, holdfast; Om, organic material.
Emended Diagnosis.
Solitary, polyp-like in gross morphology, with a whorl of ∼18 tentacles surrounding an oral region, a column (trunk) with longitudinal ridges, and a basal holdfast. Tentacles feather-like, each with a rachis biserially flanked by up to 80 flexible, alternating pinnules. Arrays of long, fine filaments densely distributed along both sides of each pinnule. Column with a central cavity partitioned by radiating septum-like structures. Column surface rough, with parallel longitudinal ridges. A circumferential constriction separates the column from the holdfast. Holdfast with a separate cavity. Underside of holdfast smooth and convex with a prominent, circular pit.
Description.
A crown of feather-like tentacles projects from the marginal edge atop the column (Figs. 1A and 2A and SI Appendix, Figs. S1 and S2A) in a radial pattern (SI Appendix, Figs. S2F and S4A). The tentacles are straight proximally, but variably flexed distally (Fig. 1A and SI Appendix, Figs. S1D and S2A), ∼18 in number (SI Appendix, Fig. S4 A and B). Approximately 80 slender pinnules biserially branch off from the rachis of each tentacle at a variable angle of 30°–120° in an alternating pattern (Figs. 1B and 2 A and C and SI Appendix, Figs. S1D and S3 A and B). The pinnules appear flexible in shape (Figs. 1 B and C and 2B and SI Appendix, Fig. S3). In exceptionally well-preserved specimens, long filaments are observed densely arrayed along both sides of each pinnule (Figs. 1C and 2D and SI Appendix, Fig. S3 C–F). The filaments project nearly at the right angle from the surface of the pinnule, thus forming a comb-like arrangement (Figs. 1C and 2D and SI Appendix, Fig. S3 C–F). In the middle portion of the tentacles of our largest specimens (e.g., Fig. 2C), the rachis is ∼0.2 mm wide (SI Appendix, Table S2). The pinnules are spaced ∼0.4 mm apart from each other; they reach a maximum length of 4.3 mm in the middle of the tentacle, and gradually decrease in length toward the distal tip (Figs. 1B and 2A). The filaments range from 540 to 630 μm in length and are spaced ∼50 μm apart.
Fig. 2.
Tentacle architecture of X. sinica (ELEL-SJ080827B; SI Appendix, Fig. S1). (A) Upper part showing numerous pinnate tentacles (arrowed). (B–D) Close-up of focus areas in A showing sinuous pinnules (B), attachment sites (arrowheads) of pinnules branching from the rachis (C), and closely spaced cilia (arrows) fringing the pinnules (D), respectively. [Scale bars: 10 mm (A), 1 mm (B–D).]
The ring of tentacles surrounds a disk that bears a central oral opening leading into a central cavity filled with sediment (SI Appendix, Fig. S4A). Radiating bands of dark remains, which alternate with tentacle bases, extend from the wall of the column into the cavity (SI Appendix, Fig. S4B). The column is a robust cylindrical structure characterized by external longitudinal ridges and an internal central cavity preserved with dark remains of organic material (Fig. 3 A and B and SI Appendix, Fig. S4A). A circumferential constriction delineates the column from the holdfast (Figs. 1A and 3 A and B and SI Appendix, Fig. S2 A, D, and E). In the lateral view, the holdfast tapers slightly toward the base (Fig. 3 A and B) and is variable in length (compare Fig. 3 A and B to SI Appendix, Fig. S2D and Table S2). A voluminous cavity occurs within the holdfast, which, in contrast to the central cavity of the column, does not show any dark remains (Fig. 3B). The holdfast has a smooth, convex, disk-shaped underside referred to as the basal disk (Fig. 3 C and D and SI Appendix, Figs. S1A and S2 F and G). The basal disk bears a prominent, bowl-shaped pit, which in most specimens occurs in an eccentric position and is filled with sediment (Fig. 3 A, C, and D and SI Appendix, Fig. S2 E–G). The pit varies in size, with a diameter accounting for ∼40–60% of the basal disk (SI Appendix, Table S2). There is no evidence of any opening either on the basal disk or within the pit (Fig. 3D and SI Appendix, Fig. S4G). In some specimens, the holdfast is bent at the constriction, with the basal disk parallel to the bedding surface of the matrix rock (SI Appendix, Figs. S1 A–C and S2G).
Discussion
C. wangii and G. abilus Are Junior Synonyms of X. sinica.
Our results revealed that specimens, which were previously assigned to three different taxa including X. sinica, C. wangii, and G. abilus, are in fact parts of the same species. Reexamination of specimen ELI-Seapen-05–001 (SI Appendix, Fig. S4 C and D), originally named C. wangii and assigned to crown-group octocorals (17, 25), suggests that it is in fact an isolated tentacle of X. sinica. First, this specimen corresponds to the individual tentacles of X. sinica in overall shape and morphological details, both having a gradually tapering rachis flanked by alternating pinnules with filaments. Second, they show a similar size with the same proportions (SI Appendix, Table S2). Morphometric data revealed that the length of tentacles and the length and spacing of pinnules of X. sinica are proportional to those of C. wangii (SI Appendix, Table S2). The dark spots occurring along the pinnules in ELI-Seapen-05–001 (SI Appendix, Fig. S4D) were originally interpreted as autozooids diagnostic of octocorals (17, 25). However, these spots are most likely preservation artifacts because they are irregularly shaped, unevenly spaced, and variable in size (40–70 µm across). Moreover, the spots are one to two orders of magnitude smaller than typical autozooids of modern octocorals (31). We therefore suggest that C. wangii is not an ancient sea pen but a junior synonym of X. sinica.
Similarly, we propose that the type and only specimen of the putative Cambrian hemichordate G. abilus (18) (SI Appendix, Fig. S4E), recovered from the same fossil Lagerstätte, is most likely a fragment of X. sinica. This fragment, originally interpreted as a pterobranch zooid (18), consists of a complete tentacle with pinnules, a proximal portion of a second tentacle, and an associated piece of the column. The following lines of evidence support the interpretation of G. abilus as a fragment of X. sinica: (i) The tentacles of X. sinica and the purported “arms” of G. abilus show the same proportions, shape, and orientation; (ii) the number of pinnules [interpreted as “tentacles” in ref 18.] is consistent (∼80 on each tentacle) in X. sinica and G. abilus, whereas it is lower in extant pterobranchs [up to 40 on each arm (33)]; (iii) the width and spacing of the tentacles of G. abilus correspond to the pinnules of X. sinica; and (iv) the size of G. abilus (∼40 mm long) matches the tentacles of X. sinica rather than the pterobranch zooids, which typically do not exceed 1–2 mm (Rhabdopleura sp.) or 5 mm in length (Cephalodiscus sp.) (33). As has been pointed out previously (32), the putative evidence of tubes (coenecia) with fuselli—characteristic growth bands in tubes of pterobranchs and graptolites (32, 33)—is unconvincing in G. abilus. Given that the tubes are recalcitrant organic secretions of zooids that are expected to be better preserved than the zooids themselves, their absence in G. abilus is puzzling. It is also curious that the “feathered zooid arms” attach directly to the “cone-shaped zooid tube,” without any sign of the typical pterobranch body consisting of cephalic shield, collar, and trunk (33). Moreover, should the interpretation of the zooid tube be correct, this structure would be too narrow to accommodate the retracted zooid (32). Hence, the cone-shaped tube, together with the “putative contractile stalk,” in the presumed pterobranch can be better interpreted as a fragment of the column of X. sinica.
Taken together, we suggest that G. abilus and C. wangii are junior synonyms of X. sinica. This suggestion leaves the middle Cambrian tubicolous enteropneusts from the Burgess Shale (11, 34) and the alga-like pterobranchs (graptolites) from British Columbia and Utah (35, 36) among the oldest fossil record of hemichordates, and the rhopalium-bearing medusa (3) as the most convincing, fossil crown-group cnidarian (Medusozoa: Scyphozoa) from the Chengjiang biota.
X. sinica Was a Suspension Feeder with an Extinct Body Plan.
In addition to a polypoid body with a holdfast and numerous tentacles demonstrated previously (e.g., refs. 16, 23, 26, and 27), our study revealed previously unknown features of X. sinica (Fig. 4A), not least, a feather-like organization of tentacles with alternating pinnules that bear dense, biserial filaments. These filaments are interpreted here as cilia involved in suspension feeding. Fossilized cilia of similar size were reported from the tentacles of the brachiopod Heliomedusa orienta from the Chengjiang biota (37). Apart from brachiopods, dense arrays of cilia occur in suspension feeding apparatuses of various other aquatic invertebrates, including phoronids, entoprocts, bryozoans, polychaetes (sabellids and serpulids), pterobranchs, and rotifers (e.g., Stephanoceros fimbriatus) (38, 39). Compared with typical cilia of extant metazoans [axoneme diameter ∼0.25 μm (40)], the cilia of X. sinica appear rather thick (19–23 μm; SI Appendix, Table S2). This might be a result of secondary thickening during preservation or diagenesis. Alternatively, these filamentous structures might have been modified for filter feeding, with numerous long cilia bound together in a functional unit reminiscent of macrocilia of living ctenophores, measuring 15 µm in diameter and ∼100 μm in length (41). The alternate branching of pinnules in X. sinica (Fig. 4A) might have facilitated the uptake of food by preventing direct confluence of transported particles from opposite pinnules. A similar staggered arrangement of branches occurs in the tentacles of some living suspension feeders, such as sabellid polychaetes (42) and feather stars (43). Thus, the distinctive architecture of tentacles in X. sinica strongly implicates a suspension-feeding behavior using a ciliary system.
We further identified a spacious cavity within the holdfast, which most likely did not have a connection to the central cavity in the column of X. sinica because in contrast to the latter, it does not show dark organic remains (Fig. 3B). Reexamination of the specimen RCCBYU 10216 (23, 26) revealed that X. sinica most likely possessed septum-like structures within the central cavity (SI Appendix, Fig. S4A), corresponding in position to indentations between longitudinal ridges of the column (Fig. 3 A and B and SI Appendix). Like in anthozoan polyps, the septum-like structures might have served for stabilizing the column and increasing the surface area of the central cavity, which might have been part of the gastrovascular system.
In all specimens of X. sinica with a preserved holdfast, the basal disk appears as a convex structure, which bears a circular pit filled with sediment. This anatomy suggests that the animal was most likely able to anchor itself in the soft sediment of the Cambrian seafloor using its holdfast. The holdfast might have acted as a burrowing physa, embedded in the sediment in living condition. The holdfast cavity might have served as a hydrostatic skeleton and antagonist to the body wall musculature, whereas the basal pit might have been used for engulfing mud or sand—an anchoring strategy possibly also used by some Ediacaran (635–542 Mya) organisms (44).
In summary, our data revealed a unique combination of characters for X. sinica, including (i) a polypoid body bearing feather-like tentacles with densely ciliated, alternating pinnules used for suspension feeding; (ii) a blind gastrovascular cavity partitioned by septum-like structures into confluent compartments; (iii) a holdfast harboring a separate cavity that might have served as a hydroskeleton; and (iv) a basal disk with a circular pit probably used for anchoring the animal in the soft substrate. Furthermore, X. sinica was most likely a radially symmetric animal, as inferred from the radial arrangement of tentacles and septum-like structures and the absence of any features that would indicate bilateral symmetry. This combination of features reveals an extinct body plan that is unknown from any other fossil or living animal group but might have emerged in the Cambrian “explosion” of evolutionary innovations, along with some other unusual body plans that have since become extinct (4, 10, 45–47).
X. sinica Represents a Stem-Group Cnidarian.
X. sinica has been alternatively interpreted as a crown-group cnidarian (16, 19–22), a ctenophore (23, 26), a lophophorate (27), or a survivor of vendobionts (28). We have shown here that X. sinica had a relatively simple anatomy with a radial symmetry, a polypoid body, and a blind gut containing septum-like structures. This simple anatomy suggests that the animal was most likely composed of two germ layers corresponding to the organizational level of cnidarians and ctenophores. The overall external and internal anatomy also corresponds well to coelenterates rather than to the more complexly organized lophophorates with a U-shaped through-gut (48) or to the Ediacaran frondose vendobionts, which commonly had fractal branches (e.g., refs. 31 and 49).
An assignment of X. sinica to Ctenophora was based on the putative resemblance of the longitudinal ridges on the column to the comb rows of ctenophores (23, 26). However, the radial rather than biradial body symmetry and the presence of feather-like tentacles, septum-like structures, and a holdfast in X. sinica are inconsistent with the anatomy of fossil (4, 50) and extant ctenophores (e.g., ref. 51). X. sinica bears some resemblance to sabellid (e.g., Sabellastarte) and serpulid annelids (e.g., Spirobranchus) in its tentacle architecture. These polychaetes also possess a crown of feathery tentacles (radioles) with ciliated, alternating pinnules (e.g., ref. 42), but in contrast to the radiating tentacles of X. sinica, these are organized into two fan-shaped or spiral clusters projecting from their tubes. In addition, sabellids and serpulids are tube-dwelling worms with parapodia and, thus, distinct from a sessile polyp with a holdfast. Pterobranch hemichordates have one (Rhabdopleura) or several (Cephalodiscus and Atubaria) pairs of suspension-feeding arms (48) with similar architecture as the tentacles of X. sinica, but their paired arms are dorsally arranged and do not encircle the mouth; moreover, a careful inspection of all specimens of X. sinica (SI Appendix, Table S1) did not reveal any structure that might indicate an anal opening, thus fundamentally differing from the U-shaped gut of pterobranchs. Lophophorates [Phoronida, Bryozoa, Brachiopoda, and probably also the Hyolitha (10)] have ciliary filter-feeding structures in common with X. sinica. However, their lophophoral tentacles are ciliated rather than pinnate and typically are arranged in a coiled or horseshoe-shaped pattern (except for some bryozoans) (52). Also, the U-shaped alimentary tract of lophophorates clearly sets them apart from X. sinica.
The polypoid body shape of X. sinica has led some authors to assume that it might represent an early lineage of crown cnidarians, with previous reconstructions resembling a modern sea anemone (21–24). However, the feather-like architecture of tentacles described herein is unknown from extant actiniarians or possible Cambrian cnidarian candidates such as Eolympia pediculata (28), Archisaccophyllia kunmingensis (29), Thaumaptilon walcotti (53), Mackenzia costalis (53), and Echmatocrinus brachiatus (54). Typical actiniarian tentacles are unbranched and heavily laden with cnidocytes—specialized cells largely used for prey capture (51)—whereas we have shown here that the feather-like tentacles of X. sinica were most likely used for suspension feeding. It would be tempting to interpret the dense arrays of cilia on the pinnules as everted tubules of cnidae (nematocyst threads), given that they have similar length [∼200–600 µm for the latter (55, 56)]. However, discharged tubules of cnidae typically appear in irregular clusters and point in various directions (e.g., refs. 56 and 57), whereas the cilia of X. sinica show a regular, parallel arrangement. We reason that X. sinica most likely did not harbor cnidocytes because the architecture of its tentacles is analogous to that of modern suspension feeders mentioned above and because the densely ciliated pinnules of this animal would leave no space for cnidocytes.
Taken together, although X. sinica exhibits a peculiar suite of characters, some of the key features (including radial symmetry, polypoid body, and a single opening leading into a partitioned gastric cavity) indicate a close affinity of this animal to cnidarians. Our phylogenetic analyses using Bayesian inference and maximum parsimony based on 111 characters and 37 major eukaryote terminal taxa (SI Appendix, Table S3 and Text S2) indeed suggest that the “feathered polyp” was a stem-group cnidarian (Fig. 4B and SI Appendix, Figs. S5–S7). This phylogenetic placement implies that the last common ancestor of X. sinica and crown-group cnidarians was a benthic, diploblastic, polypoid animal that possessed a partitioned gastrovascular cavity with a single opening and a radial whorl of tentacles. This observation supports the “polyp theory” (e.g., refs. 54 and 55), which states that cnidarians originated from a sessile polypoid rather than a pelagic medusoid ancestor (e.g., refs. 58 and 59). This finding also implies that the radial symmetry displayed by X. sinica might be an ancestral trait of Cnidaria, which persisted in hydrozoans (60, 61) but was modified to bilateral or biradial symmetry in anthozoans (62, 63) and tetraradial symmetry in most medusozoans (52, 64). The evolution of the alternating, densely ciliated pinnules (“filtering combs”) on the tentacles adapted for suspension feeding can be interpreted by two equally parsimonious scenarios. In the first scenario, the ciliated pinnules evolved before the divergence of X. sinica from the cnidarian stem group. This scenario would suggest that suspension feeding is an ancestral feature (plesiomorphy) of total-group cnidarians and that the pinnules were lost in crown cnidarians, which instead coopted the raptorial feeding strategy using unbranched, cnida-laden tentacles. In the second scenario, the last common ancestor of X. sinica and crown cnidarians was a predator armed with cnidocytes, suggesting that suspension feeding is a derived feature (autapomorphy) of X. sinica. Whichever of the two scenarios is favored, the revealed body plan embodied by X. sinica suggests that the feeding behavior of stem-group cnidarians may have been strikingly different from that of their crown-group descendants. The distinctive suite of characters revealed in X. sinica significantly augments the morphological disparity of total-group Cnidaria.
Materials and Methods
All fossil specimens (n = 88) of X. sinica examined in this study were collected from the Cambrian Chengjiang Lagerstätte and deposited in four institutes in Beijing, Xi’an, Nanjing, and Kunming (SI Appendix, Table S1 and Text S1). Mechanical preparation of fossils was performed using a Zeiss Stemi 2000 stereomicroscope under various lighting conditions. Specimens were photographed using a Canon EOS 5D Mark II optical camera under sunlight and a Zeiss Stemi 2000-C microscope equipped with a digital camera under LED light. Backscattered electron (BSE) imaging and energy-dispersive X-ray spectroscopy (EDS) analysis of uncoated specimens were conducted on an FEI Quanta 650 FEG field emission scanning electron microscope in low-vacuum mode. Morphological measurements were conducted with Carl Zeiss AxioVision 4.1. Three-dimensional reconstruction of X. sinica was performed using 3ds Max 9.0. Phylogenetic analyses were conducted using MrBayes 3.2, TNT 1.1, and PAUP* 4.0. Application of phylogenetic terminology, including stem, crown, and total group, follows Xiao et al. (65).
Supplementary Material
Acknowledgments
This research has especially benefitted from invaluable comments from Shuhai Xiao (Virginia Tech). Jörg Maletz (Freie Universität Berlin), Robert Scott (University of Tulsa), Ivo de Sena Oliveira, and Vladimir Gross (University of Kassel), and three anonymous reviewers provided constructive suggestions. We thank Rong Wang, Qianping Lei, Manyan Wang, Yajing Wang, Haocheng Yu, Sunxuezi Cheng (China University of Geosciences, Beijing), and Meirong Cheng (Northwest University) for field/laboratory work; Huanzhen Li for constructing the 3D model; Bo Zhang, Congyuan Yin, Kai Liu, Yang Wang, and Shiran Liu (Peking University) for assistance with FEG-SEM analyses; Xianguang Hou, Xiaoya Ma, Peiyun Cong (Yunnan University), and Derek Siveter (University of Oxford) for providing images of X. sinica and G. abilus; and Lars Hering (University of Kassel) for help with Bayesian analyses. This research was supported by Grants 41572017, 41672009, and 41621003 from the National Natural Science Foundation of China (to Q.O., J.H., and D.S.), the Program for New Century Excellent Talents, Ministry of Education of China (NCET-13-1008 to Q.O.), the German Research Foundation (DFG; Ma 4147/3-1 to G.M.), and the Alexander von Humboldt Foundation (1164230 to Q.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.X. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701650114/-/DCSupplemental.
References
- 1.Jenner RA, Littlewood DTJ. Problematica old and new. Philos Trans R Soc Lond B Biol Sci. 2008;363:1503–1512. doi: 10.1098/rstb.2007.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Iten H, Leme JD, Simoes MG, Marques AC, Collins AG. Reassessment of the phylogenetic position of conulariids (?Ediacaran-Triassic) within the subphylum Medusozoa (Phylum Cnidaria) J Syst Palaeontol. 2006;4:109–118. [Google Scholar]
- 3.Han J, et al. The earliest pelagic jellyfish with rhopalia from Cambrian Chengjiang Lagerstätte. Palaeogeogr Palaeoclimatol Palaeoecol. 2016;449:166–173. [Google Scholar]
- 4.Ou Q, et al. A vanished history of skeletonization in Cambrian comb jellies. Sci Adv. 2015;1:e1500092. doi: 10.1126/sciadv.1500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caron J-B, Scheltema A, Schander C, Rudkin D. A soft-bodied mollusc with radula from the Middle Cambrian Burgess Shale. Nature. 2006;442:159–163. doi: 10.1038/nature04894. [DOI] [PubMed] [Google Scholar]
- 6.Vinther J, Parry L, Briggs DE, Van Roy P. Ancestral morphology of crown-group molluscs revealed by a new Ordovician stem aculiferan. Nature. 2017;542:471–474. doi: 10.1038/nature21055. [DOI] [PubMed] [Google Scholar]
- 7.Vinther J, Van Roy P, Briggs DE. Machaeridians are Palaeozoic armoured annelids. Nature. 2008;451:185–188. doi: 10.1038/nature06474. [DOI] [PubMed] [Google Scholar]
- 8.Chen JY, Hou XG, Lu HZ. Early Cambrian netted scale-bearing worm-like sea animal. Acta Palaeontologica Sin. 1989;28:12–27. [Google Scholar]
- 9.Ramsköld L. The second leg row of Hallucigenia discovered. Lethaia. 1992;25:221–224. [Google Scholar]
- 10.Moysiuk J, Smith MR, Caron J-B. Hyoliths are Palaeozoic lophophorates. Nature. 2017;541:394–397. doi: 10.1038/nature20804. [DOI] [PubMed] [Google Scholar]
- 11.Caron JB, Morris SC, Cameron CB. Tubicolous enteropneusts from the Cambrian period. Nature. 2013;495:503–506. doi: 10.1038/nature12017. [DOI] [PubMed] [Google Scholar]
- 12.Morris SC, Caron J-B. Pikaia gracilens Walcott, a stem-group chordate from the Middle Cambrian of British Columbia. Biol Rev Camb Philos Soc. 2012;87:480–512. doi: 10.1111/j.1469-185X.2012.00220.x. [DOI] [PubMed] [Google Scholar]
- 13.Morris SC, Caron J-B. A primitive fish from the Cambrian of North America. Nature. 2014;512:419–422. doi: 10.1038/nature13414. [DOI] [PubMed] [Google Scholar]
- 14.McCoy VE, et al. The ‘Tully monster’ is a vertebrate. Nature. 2016;532:496–499. doi: 10.1038/nature16992. [DOI] [PubMed] [Google Scholar]
- 15.Clements T, et al. The eyes of Tullimonstrum reveal a vertebrate affinity. Nature. 2016;532:500–503. doi: 10.1038/nature17647. [DOI] [PubMed] [Google Scholar]
- 16.Chen JY, Erdtmann BD. Lower Cambrian fossil Lagerstätte from Chengjiang, Yunnan, China: Insights for reconstructing early metazoan life. In: Simonetta AM, Morris SC, editors. The Early Evolution of Metazoa and the Significance of Problematic Taxa. Cambridge Univ Press; Cambridge, United Kingdom: 1991. pp. 57–76. [Google Scholar]
- 17.Shu DG, Conway Morris S. New diploblasts from Chengjiang fossil Lagerstätte. Earth Sci Front. 2006;13:227–233. [Google Scholar]
- 18.Hou XG, et al. An early Cambrian hemichordate zooid. Curr Biol. 2011;21:612–616. doi: 10.1016/j.cub.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Seilacher A. Early multicellular life: Late Proterozoic fossils and the Cambrian explosion. In: Bengtson S, editor. Early Life on Earth (Nobel Symposium No. 84) Columbia Univ Press; New York: 1994. pp. 389–400. [Google Scholar]
- 20.Chen JY, Zhou GQ, Zhu MY, Ye GY. The Chengjiang Biota: A Unique Window on the Cambrian Explosion. National Museum of Natural Science; Taiwan: 1996. [Google Scholar]
- 21.Chen JY, Zhou GQ. Biology of the Chengjiang fauna. Bull Natl Mus Nat Sci. 1997;10:11–106. [Google Scholar]
- 22.Chen JY. The Dawn of Animal World. Jiangsu Science and Technology Press; Nanjing, China: 2004. p. 366. [Google Scholar]
- 23.Hou XG, et al. The Cambrian Fossils of Chengjiang, China: The Flowering of Early Animal Life. Blackwell Publishing; Oxford: 2004. p. 248. [Google Scholar]
- 24.Hou XG, et al. The Cambrian Fossils of Chengjiang, China: The Flowering of Early Animal Life. 2nd Ed. John Wiley & Sons; Oxford: 2017. p. 328. [Google Scholar]
- 25.Shu DG, et al. Lower Cambrian vendobionts from China and early diploblast evolution. Science. 2006;312:731–734. doi: 10.1126/science.1124565. [DOI] [PubMed] [Google Scholar]
- 26.Hou XG, Bergström J, Wang HF, Feng XH, Chen AL. The Chengjiang Fauna: Exceptionally Well-Preserved Animals from 530 Million Years Ago. Yunnan Science and Technology Press; Kunming, China: 1999. [Google Scholar]
- 27.Luo HL, Hu SX, Chen LZ. Early Cambrian Chengjiang Fauna from Kunming Region, China. Yunnan Science and Technology Press; Kunming, China: 1999. [Google Scholar]
- 28.Han J, et al. Tiny sea anemone from the Lower Cambrian of China. PLoS One. 2010;5:e13276. doi: 10.1371/journal.pone.0013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou XG, Stanley GD, Zhao J, Ma XY. Cambrian anemones with preserved soft tissue from the Chengjiang biota, China. Lethaia. 2005;38:193–203. [Google Scholar]
- 30.Lei QP, Han J, Ou Q, Wan XQ. Sedentary habits of anthozoa-like animals in the Chengjiang Lagerstätte: Adaptive strategies for Phanerozoic-style soft substrates. Gondwana Res. 2014;25:966–974. [Google Scholar]
- 31.Laflamme M, Narbonne GM. Ediacaran fronds. Palaeogeogr Palaeoclimatol Palaeoecol. 2008;258:162–179. [Google Scholar]
- 32.Maletz J. Hemichordata (Pterobranchia, Enteropneusta) and the fossil record. Palaeogeogr Palaeoclimatol Palaeoecol. 2014;398:16–27. [Google Scholar]
- 33.Benito J, Pardos F. Microscopic Anatomy of Invertebrates. Vol 15. Wiley-Liss; New York: 1997. Hemichordata; pp. 15–101. [Google Scholar]
- 34.Nanglu K, Caron JB, Conway Morris S, Cameron CB. Cambrian suspension-feeding tubicolous hemichordates. BMC Biol. 2016;14:56. doi: 10.1186/s12915-016-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LoDuca ST, Caron J-B, Schiffbauer JD, Xiao S, Kramer A. A reexamination of Yuknessia from the Cambrian of British Columbia and Utah. J Paleontol. 2015;89:82–95. [Google Scholar]
- 36.Maletz J, Steiner M. Graptolite (Hemichordata, Pterobranchia) preservation and identification in the Cambrian Series 3. Palaeontology. 2015;58:1073–1107. [Google Scholar]
- 37.Zhang ZF, et al. Architecture and function of the lophophore in the problematic brachiopod Heliomedusa orienta (Early Cambrian, South China) Geobios. 2009;42:649–661. [Google Scholar]
- 38.Riisgård HU, Larsen PS. Particle capture mechanisms in suspension-feeding invertebrates. Mar Ecol Prog Ser. 2010;418:255–293. [Google Scholar]
- 39.Jørgensen CB, Kiørboe T, Møhlenberg F, Riisgård HU. Ciliary and mucus-net filter feeding, with special reference to fluid mechanical characteristics. Mar Ecol Prog Ser. 1984;15:283–292. [Google Scholar]
- 40.Lodish H, et al. Molecular Cell Biology. 4th Ed. W. H. Freeman; New York: 2000. p. 1184. [Google Scholar]
- 41.Tamm S, Tamm S. Macrociliary tooth patterns in beroid ctenophores. Bio Bull. 1991;181:355–356. doi: 10.1086/BBLv181n2p355. [DOI] [PubMed] [Google Scholar]
- 42.Riisgård HU, Ivarsson NM. The crown-filament pump of the suspension feeding polychaete Sabella penicillus: Effects of temperature, and energy cost. Mar Ecol Prog Ser. 1990;62:249–257. [Google Scholar]
- 43.Holland ND, Strickler JR, Leonard AB. Particle interception, transport and rejection by the feather star Oligometra serripinna (Echinodermata: Crinoidea), studied by frame analysis of videotapes. Mar Biol. 1986;93:111–126. [Google Scholar]
- 44.Jenkins RJF. Functional and ecological aspects of Ediacarian assemblages. In: Lipps JH, Signor P, editors. Origin and Early Evolution of the Metazoa. Plenum; New York: 1992. pp. 131–176. [Google Scholar]
- 45.Shu DG, et al. Primitive deuterostomes from the Chengjiang Lagerstätte (Lower Cambrian, China) Nature. 2001;414:419–424. doi: 10.1038/35106514. [DOI] [PubMed] [Google Scholar]
- 46.Caron J-B, Conway Morris S, Shu D. Tentaculate fossils from the Cambrian of Canada (British Columbia) and China (Yunnan) interpreted as primitive deuterostomes. PLoS One. 2010;5:e9586. doi: 10.1371/journal.pone.0009586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Brien LJ, Caron JB. A new stalked filter-feeder from the middle Cambrian Burgess Shale, British Columbia, Canada. PLoS One. 2012;7:e29233. doi: 10.1371/journal.pone.0029233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nielsen C. Animal Evolution: Interrelationships of the Living Phyla. 3rd Ed. Oxford Univ Press; Oxford: 2012. p. 402. [Google Scholar]
- 49.Narbonne GM. Modular construction of early Ediacaran complex life forms. Science. 2004;305:1141–1144. doi: 10.1126/science.1099727. [DOI] [PubMed] [Google Scholar]
- 50.Conway Morris S, Collins DH. Middle Cambrian ctenophores from the Stephen Formation, British Columbia, Canada. Philos Trans R Soc B Biol Sci. 1996;351:279–308. [Google Scholar]
- 51.Hyman LH. The Invertebrates. McGraw Hill; New York: 1940. p. 726. [Google Scholar]
- 52.Brusca RC, Moore W, Shuster SM. Invertebrates. 3rd Ed. Sinauer Associates; Sunderland, MA: 2016. p. 1052. [Google Scholar]
- 53.Conway Morris S. Ediacaran-like fossils in Cambrian Burgess Shale-type faunas of North-America. Palaeontology. 1993;36:593–635. [Google Scholar]
- 54.Ausich WI, Babcock LE. The phylogenetic position of Echmatocrinus brachiatus, a probable octocoral from the Burgess Shale. Palaeontology. 1998;41:193–202. [Google Scholar]
- 55.Kass-Simon G, Scappaticci JAA., Jr The behavioral and developmental physiology of nematocysts. Can J Zool. 2002;80:1772–1794. [Google Scholar]
- 56.Colin SP, Costello JH. Functional characteristics of nematocysts found on the scyphomedusa Cyanea capillata. J Exp Mar Biol Ecol. 2007;351:114–120. [Google Scholar]
- 57.Yanagihara AA, Kuroiwa JM, Oliver LM, Chung JJ, Kunkel DD. Ultrastructure of a novel eurytele nematocyst of Carybdea alata Reynaud (Cubozoa, Cnidaria) Cell Tissue Res. 2002;308:307–318. doi: 10.1007/s00441-002-0545-8. [DOI] [PubMed] [Google Scholar]
- 58.Bridge D, Cunningham CW, DeSalle R, Buss LW. Class-level relationships in the phylum Cnidaria: Molecular and morphological evidence. Mol Biol Evol. 1995;12:679–689. [Google Scholar]
- 59.Collins AG. Recent insights into cnidarian phylogeny. Smithson Contrib Mar Sci. 2009;38:139–149. [Google Scholar]
- 60.Reinhardt B, Broun M, Blitz IL, Bode HR. HyBMP5-8b, a BMP5-8 orthologue, acts during axial patterning and tentacle formation in hydra. Dev Biol. 2004;267:43–59. doi: 10.1016/j.ydbio.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 61.Marques AC, Collins AG. Cladistic analysis of Medusozoa and cnidarian evolution. Invertebr Biol. 2004;123:23–42. [Google Scholar]
- 62.Matus DQ, et al. Molecular evidence for deep evolutionary roots of bilaterality in animal development. Proc Natl Acad Sci USA. 2006;103:11195–11200. doi: 10.1073/pnas.0601257103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finnerty JR, Pang K, Burton P, Paulson D, Martindale MQ. Origins of bilateral symmetry: Hox and dpp expression in a sea anemone. Science. 2004;304:1335–1337. doi: 10.1126/science.1091946. [DOI] [PubMed] [Google Scholar]
- 64.Dzik J, Baliński A, Sun Y. The origin of tetraradial symmetry in cnidarians. Lethaia. 2017;50:306–321. [Google Scholar]
- 65.Xiao S, et al. The Weng’an biota and the Ediacaran radiation of multicellular eukaryotes. Natl Sci Rev. 2014;1:498–520. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.