Significance
The hippocampus with its dense reciprocal axonal projections to and from cortex is widely believed to mediate numerous cognitive functions. However, it is unknown whether and how specific hippocampal–cortical activity contributes to the brain-wide functional connectivity. Here, we use optogenetics and fMRI to examine how excitatory neural activity initiated in the dorsal dentate gyrus of the hippocampus propagates and modulates resting-state fMRI (rsfMRI) connectivity. We discover its robust propagation brain-wide at low frequency (1 Hz), which enhances interhemispheric rsfMRI connectivity and cortical and subcortical visual responses. Our findings highlight the important role of slow hippocampal–cortical oscillatory activity in driving brain-wide rsfMRI connectivity and mediating sensory processing.
Keywords: hippocampus, resting-state functional connectivity, optogenetic, fMRI, low frequency
Abstract
The hippocampus, including the dorsal dentate gyrus (dDG), and cortex engage in bidirectional communication. We propose that low-frequency activity in hippocampal–cortical pathways contributes to brain-wide resting-state connectivity to integrate sensory information. Using optogenetic stimulation and brain-wide fMRI and resting-state fMRI (rsfMRI), we determined the large-scale effects of spatiotemporal-specific downstream propagation of hippocampal activity. Low-frequency (1 Hz), but not high-frequency (40 Hz), stimulation of dDG excitatory neurons evoked robust cortical and subcortical brain-wide fMRI responses. More importantly, it enhanced interhemispheric rsfMRI connectivity in various cortices and hippocampus. Subsequent local field potential recordings revealed an increase in slow oscillations in dorsal hippocampus and visual cortex, interhemispheric visual cortical connectivity, and hippocampal–cortical connectivity. Meanwhile, pharmacological inactivation of dDG neurons decreased interhemispheric rsfMRI connectivity. Functionally, visually evoked fMRI responses in visual regions also increased during and after low-frequency dDG stimulation. Together, our results indicate that low-frequency activity robustly propagates in the dorsal hippocampal–cortical pathway, drives interhemispheric cortical rsfMRI connectivity, and mediates visual processing.
The hippocampus (HP) plays a prominent role in central nervous system functions, particularly in episodic memory (1, 2) and spatial navigation (3, 4). The HP, including the dentate gyrus (DG), can evoke large-scale influences on cortical activity, because the HP receives convergent information from sensory and limbic cortices before sending reciprocal divergent projections to create a highly interactive corticohippocampal–cortical network. The HP, including DG, CA3, and CA1, and neocortex, are connected via the entorhinal cortex (EC) (5, 6), such that the dorsolateral-to-ventromedial projection that originates in the EC corresponds to a dorsoventral axis of termination in the HP (5). This anatomical topography suggests that a functional gradient could exist along the HP dorsoventral axis. Previous studies demonstrated that dorsal HP (dHP) and cortex are functionally integrated during sensory processing and memory consolidation (7–9). Specifically, dHP can integrate multimodal sensory information and process memory operations (7) using excitatory long-range projections (10). This process occurs over multiple brain circuits, but the role of dHP in complex networks, particularly its influence on brain-wide functional connectivity, is not well understood.
Resting-state functional MRI (rsfMRI) (11–14) provides an invaluable, noninvasive imaging technique to map long-range, brain-wide functional connectivity networks based on the temporal coherence of infraslow (0.005–0.1 Hz) blood oxygen level dependent (BOLD) activity. The functional relevance of specific brain-wide networks in cognition can be inferred through rsfMRI connectivity. Numerous studies demonstrate that changes in rsfMRI connectivity are highly correlated to sensory, memory, and learning task performance, detailed in two comprehensive review papers (13, 15). These studies indicate that changes in brain-wide functional connectivity facilitate and modulate diverse cognitive functions. Despite the enormous potential inherent in this technique, the neural basis of rsfMRI connectivity remains unknown, which impedes further interpretation of neural activity and interactions within and between brain networks. Previous attempts to uncover the neural basis of rsfMRI in anesthetized rats and awake macaques implicated delta oscillations (16, 17) and alpha oscillations (18, 19), respectively. Furthermore, cortical slow oscillations (<1 Hz) (20, 21) resemble the spatiotemporal characteristics of infraslow coherent activity in brain-wide functional networks detected by rsfMRI. Previous studies suggest that these cortical oscillations may underlie rsfMRI connectivity (22–24). Further, a recent study indicates the coupling between resting-state hemodynamics and the oscillatory activity of excitatory neurons (25). However, the answer remains generally inconclusive due to the correlative nature of those studies without directly probing the effects of modulating the brain’s electrical activity during rsfMRI.
Brain-wide slow oscillations are a characteristic feature of the mammalian neocortex that occurs spontaneously in the virtual absence of sensory stimulation, such as during anesthesia (26, 27), natural sleep (9, 28), or quiescent waking (29). Previous studies also found similar slow oscillatory activities in the hippocampus (30, 31). In particular, DG granule cells modulate hippocampal slow oscillations phase locked to neocortical slow oscillations with a short delay (31). This dynamic property suggests that individual hippocampal neurons can form functional connections with other neurons to create synchronized networks, particularly in slow oscillation frequency ranges, to mediate complex cognitive functions. Such functional coupling may establish the temporal framework for coordinated information transfer within and between networks. Human rsfMRI studies also identified the hippocampus as a densely connected region that may coordinate brain functional connectivity (32–34). Furthermore, a recent study demonstrated the propagation of low-frequency activity from the hippocampus to the cortex in humans (34). Thus, directly examining hippocampal activity with respect to brain-wide propagation and connectivity is essential to uncover the fundamental neural basis of rsfMRI.
Currently, there is no direct evidence that demonstrates the influence of low-frequency activity or oscillations that propagate along the hippocampal–cortical axis on brain-wide rsfMRI connectivity. Because the DG primarily receives cortical terminal projections and acts as a bridge for corticohippocampal–cortical network communication, we examined how spatiotemporally specific activity initiated in the dorsal DG (dDG) influences cortical activity and subsequent brain-wide rsfMRI connectivity by combining optogenetic, cell-specific stimulation of Ca2+/calmodulin-dependent protein kinase IIa (CaMKIIα)-expressing excitatory neurons (i.e., dDG granule cells) and large-scale fMRI detection (35–38). We also examined the functional effects of such changes in brain-wide rsfMRI connectivity on sensory processing.
Results
fMRI Reveals Brain-Wide Frequency-Dependent Activity Propagation from Dorsal Hippocampus.
We used virally mediated optogenetics to selectively stimulate CaMKIIα-expressing excitatory neurons in dDG in normal adult male rats. Anatomical MRI scans confirmed the location of the virus injection and optical fiber implantation in dDG of all animals (Fig. S1A). Immunohistochemistry confirmed that CaMKIIα+ excitatory neurons of the dDG, but not the GABAergic inhibitory neurons, expressed ChR2-mCherry (Fig. S1 B and C). Whole-brain fMRI determined frequency-dependent spatiotemporal characteristics of brain-wide, long-range evoked responses driven by dDG stimulation using a block design. We performed optogenetic stimulation in lightly anesthetized rats (1% isoflurane). Blue light pulses at four frequencies (0.5 Hz, 1 Hz, 2 Hz, and 40 Hz with 5%, 10%, 20%, and 30% pulse width duty cycle, respectively; light intensity, 40 mW/mm2) were delivered to dDG neurons in a block design paradigm. We chose reduced duty cycles for 0.5-Hz, l-Hz, and 2-Hz stimulation to avoid excessively long stimulation pulse widths that may not be physiological.
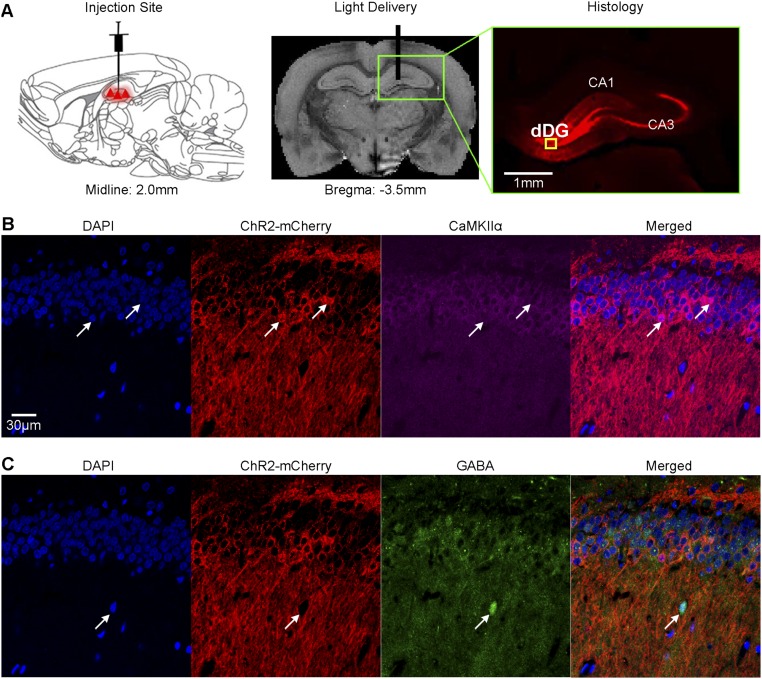
Fig. S1.
Experimental setup for optogenetic stimulation and histological characterization of ChR2::CaMKIIα viral expression in dorsal dentate gyrus (dDG) excitatory neurons. (A) Schematic (Left) and T2-weighted anatomical image (Middle) show the viral injection and fiber implantation sites, respectively. Histology image shows viral expression in dHP (Right, green box). The yellow box indicates the location of magnified confocal images shown in B and C. (B) Merged representative confocal images costained for the nuclear marker DAPI, mCherry, and excitatory marker CaMKIIα confirmed colocalization of ChR2-mCherry and CaMKIIα+ neurons of dDG (white arrows). (C) Confocal overlays of nuclear marker DAPI, ChR2-mCherry, and inhibitory marker GABA showed no colocalization of ChR2-mCherry and GABA+ neurons of dDG (white arrow).
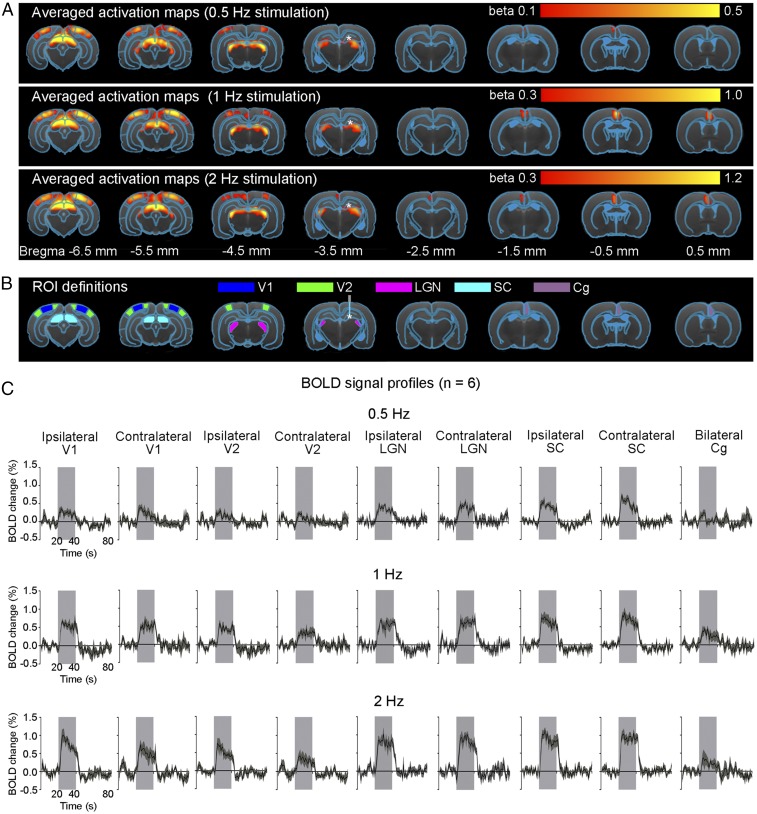
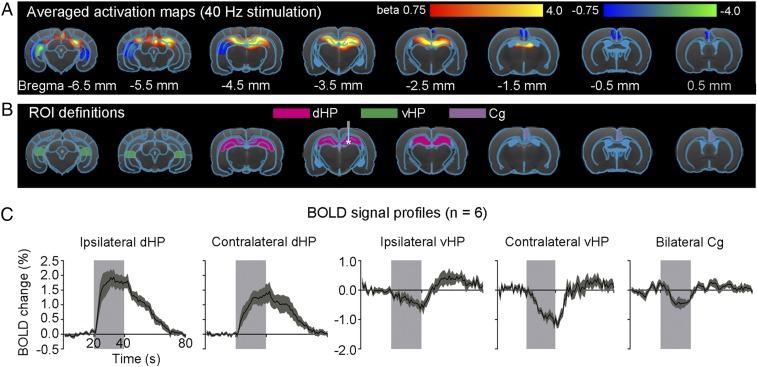
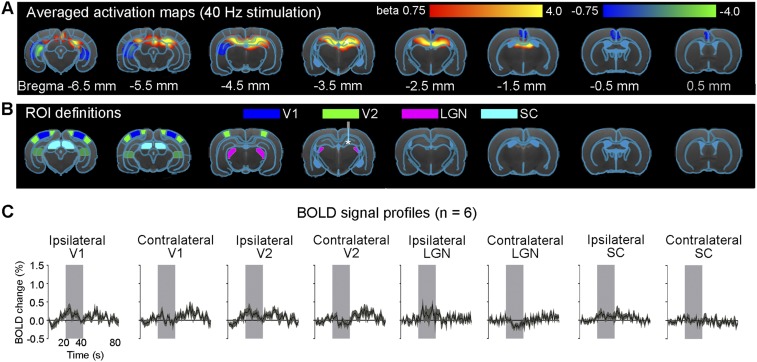
A 1-Hz optogenetic stimulation of dDG evoked positive and robust BOLD responses in bilateral primary visual cortex (V1), secondary visual cortex (V2), lateral geniculate nucleus (LGN) and superior colliculus (SC), as well as the cingulate cortex (Cg) (Fig. 1 A and C). We observed similar BOLD responses at 0.5- and 2-Hz stimulations (Fig. 1 A and C). We also stimulated dDG at a higher frequency (40 Hz) and observed strong positive responses in bilateral dHP and negative responses in bilateral ventral HP (vHP) and Cg (Fig. 2 A and C). However, we detected no responses in V1, V2, LGN, and SC (Fig. S2). The sequence of fMRI responsivity demonstrates spatiotemporal response properties from dHP shifting from regions actively involved in processing visual information and cognition at low frequencies (0.5–2 Hz) to local hippocampal regions (40 Hz). The absolute BOLD signal amplitude in bilateral V1 at 0.5 Hz was generally lower than those at 1 Hz and 2 Hz, mainly due to the reduced pulse width duty cycle of 5% used at 0.5 Hz.
Fig. 1.
Brain-wide activation of visually related regions during low-frequency optogenetic stimulation of dDG excitatory neurons in dHP. (A) Averaged activation maps at 0.5-Hz (Top), 1-Hz (Middle), and 2-Hz (Bottom) stimulation. * indicates the stimulation site. Robust positive BOLD responses detected in bilateral V1, V2, LGN, and SC, as well as Cg (n = 6; t > 3.1, corresponding to P < 0.001). We used a lower statistical threshold (t > 2.5, corresponding to P < 0.01) for 0.5-Hz stimulation to detect the low BOLD responses. (B) Regions of interest (ROIs) defined by the rat brain atlas to extract the BOLD signal profiles. Abbreviations: g, cingulate cortex; LGN, lateral geniculate nucleus; SC, superior colliculus; V1, primary visual cortex; V2, secondary visual cortex. (C) The respective BOLD signal profiles extracted from the ROIs. Error bars indicate ±SEM.
Fig. 2.
Local hippocampal activation and deactivation during 40-Hz optogenetic stimulation of dDG excitatory neurons in dHP. (A) Averaged activation maps at 40-Hz stimulation. * indicates the stimulation site. Robust positive BOLD responses are detected in bilateral dHP, whereas negative responses are detected in the bilateral vHP and Cg (n = 6; t > 3.1 or t < −3.1, corresponding to P < 0.001). (B) Regions of interest (ROIs) are based on the rat brain atlas to extract bold signal profiles. Abbreviations: Cg, cingulate cortex; dHP, dorsal hippocampus; vHP, ventral hippocampus. (C) BOLD signal profiles extracted from the ROIs. Error bars indicate ±SEM.
Fig. S2.
High-frequency optogenetic stimulation (40 Hz) of dDG excitatory neurons in dHP does not evoke brain-wide cortical and subcortical activation in V1, V2, LGN, and SC. (A) Averaged activation maps at 40-Hz stimulation. No robust positive BOLD responses are detected in bilateral V1, V2, LGN, and SC. (B) Regions of interest (ROIs) defined by the rat brain atlas to extract BOLD signal profiles. Abbreviations: LGN, lateral geniculate nucleus; SC, superior colliculus; V1, primary visual cortex; V2, secondary visual cortex. (C) The respective BOLD signal profiles extracted from the ROIs. Error bars indicate ±SEM.
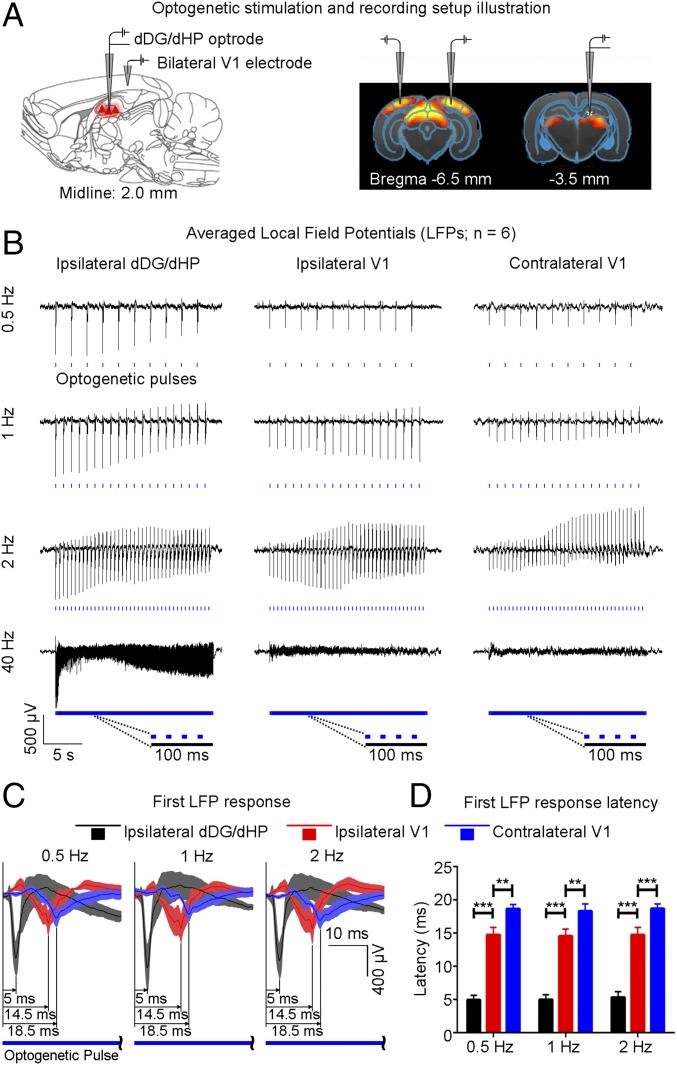
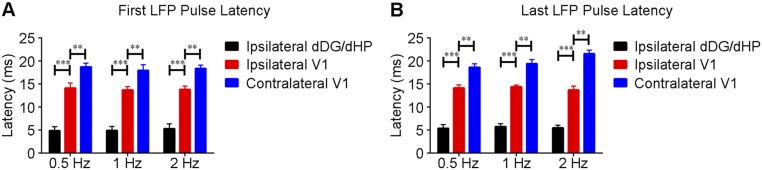
To gain insight into the fMRI findings, we performed extracellular electrophysiological recordings using low-impedance (1 MΩ) electrodes to characterize downstream signal propagation from dDG excitatory neurons in dHP to V1 using identical stimulation paradigms (Fig. 3A). Local field potentials (LFPs), which are highly correlated with BOLD signals (39), revealed that only low-frequency stimulation (0.5–2 Hz) evoked strong LFP responses in bilateral V1 (Fig. 3B), corroborating the fMRI results (Fig. 1), although all tested stimulation frequencies evoked LFP responses in dDG/dHP. We further investigated the response latencies to determine the dynamic properties within the hippocampal–cortical circuit. Following dDG stimulation, evoked responses first occurred in the ipsilateral dHP, followed by the ipsilateral V1, and finally the contralateral V1 (Fig. 3C). Latency was measured at the first prominent trough of the optogenetically evoked LFP response. Latency measurements were similar for 0.5-Hz, 1-Hz, and 2-Hz stimulation (0.5 Hz: ipsilateral dDG/dHP = 4.95 ± 0.71 ms, ipsilateral V1 = 14.77 ± 1.08 ms, contralateral V1 = 18.68 ± 0.65 ms; 1 Hz: ipsilateral dDG/dHP = 5.02 ± 0.71 ms, ipsilateral V1 = 14.57 ± 1.03 ms, contralateral V1 = 18.33 ± 1.06 ms; and 2 Hz: ipsilateral dDG/dHP = 5.35 ± 0.85 ms, ipsilateral V1 = 14.77 ± 1.09 ms, contralateral V1 = 18.72 ± 0.68 ms) (Fig. 3D). Although optogenetically evoked LFP profiles evolved throughout the stimulation period, the comparative latency measurements between the first and last response were similar (Fig. S3), demonstrating stable response latencies. These latency measurements indicate that the first evoked responses occurred in the ipsilateral dDG/dHP and propagated to the ipsilateral V1 polysynaptically (9.5 ± 1.3 ms, P < 0.001; one-way ANOVA followed by Bonferroni’s post hoc test), before reaching contralateral V1 via monosynaptic interhemispheric callosal connections (40) (4.0 ± 1.5 ms, P < 0.01; one-way ANOVA followed by Bonferroni’s post hoc test). However, we do not preclude that propagation to contralateral V1 can occur polysynaptically through contralateral dHP. No evoked responses were observed in the naïve animal (Fig. S4), indicating that the observed responses were a direct result of ChR2 stimulation, not photoelectrically induced artifacts or undesired light-induced activations of the visual pathway. In addition, multiunit activity (MUA) recordings in dDG showed all stimulation frequencies successfully elicited hippocampal spikes without failure (Fig. S5). We made the exposed optical fiber cannula (used to deliver blue light pulses) opaque using heat shrinkable sleeves in all MRI and electrophysiological recording experiments to prevent light leakage that may cause undesired visual stimulation to animals.
Fig. 3.
Local field potentials (LFPs) in ipsilateral dDG/dHP and bilateral V1 confirm neuronal activity underlies BOLD fMRI responses, and latency measurements indicate polysynaptic propagation of low-frequency activity from dHP. (A) Recording electrodes location in ipsilateral dDG/dHP and bilateral V1. (B) Averaged LFP traces in ipsilateral dDG/dHP and bilateral V1 for 0.5-Hz, 1-Hz, 2-Hz, and 40-Hz stimulation (n = 6). All frequencies evoked responses in ipsilateral dDG/dHP. Only low-frequency stimulation evoked strong responses in bilateral V1. (C) LFP responses from the first optogenetic pulse for 0.5-Hz, 1-Hz, and 2-Hz stimulation. Error bars indicate ±SEM. (D) Latency measurements of the first LFP response during low-frequency stimulation (0.5 Hz: ipsilateral dDG/dHP = 4.95 ± 0.71 ms, ipsilateral V1 = 14.77 ± 1.08 ms, contralateral V1 = 18.68 ± 0.65 ms; 1 Hz: ipsilateral dDG/dHP = 5.02 ± 0.71 ms, ipsilateral V1 = 14.57 ± 1.03 ms, contralateral V1 = 18.33 ± 1.06 ms; 2 Hz: ipsilateral dDG/dHP = 5.35 ± 0.85 ms, ipsilateral V1 = 14.77 ± 1.09 ms, contralateral V1 = 18.72 ± 0.68 ms; one-way ANOVA followed by Bonferroni’s post hoc test; **P < 0.01 and ***P < 0.001). Error bars indicate ±SEM.
Fig. S3.
Similarities in measured latencies between the first and last optogenetically evoked response during low-frequency (1 Hz) dDG stimulation. (A) First and (B) last responses used for latency measurements during low-frequency dDG stimulation (n = 6). Error bars indicate ±SEM. Abbreviations are as follows: dDG, dorsal dentate gyrus; dHP, dorsal hippocampus; V1, primary visual cortex.
Fig. S4.
Optogenetic stimulation in a naïve animal does not evoke LFP responses. Averaged LFP traces in ipsilateral dDG/dHP and bilateral V1 for 1-Hz and 40-Hz dDG stimulation in a control naïve animal. Results demonstrate that the observed optogenetically evoked LFP responses (Fig. 3) are a direct result of ChR2 stimulation, not photoelectrically induced artifacts or undesired light-induced activation of the visual pathway.
Fig. S5.
Multiunit activity (MUA) recording in local neurons of dDG reveals optogenetically evoked spikes at different stimulation frequencies. Traces of dDG MUA from a representative animal during optogenetic stimulation (Left) and the corresponding higher resolution dDG spike traces extracted at two locations after the onset of stimulation (Middle, red; Right, green). The recordings demonstrate that hippocampal spikes are successfully elicited at all frequencies with no indication of failure.
Low-Frequency Optogenetic Stimulation of dDG Excitatory Neurons in dHP Enhances Brain-Wide Resting-State Functional Connectivity.
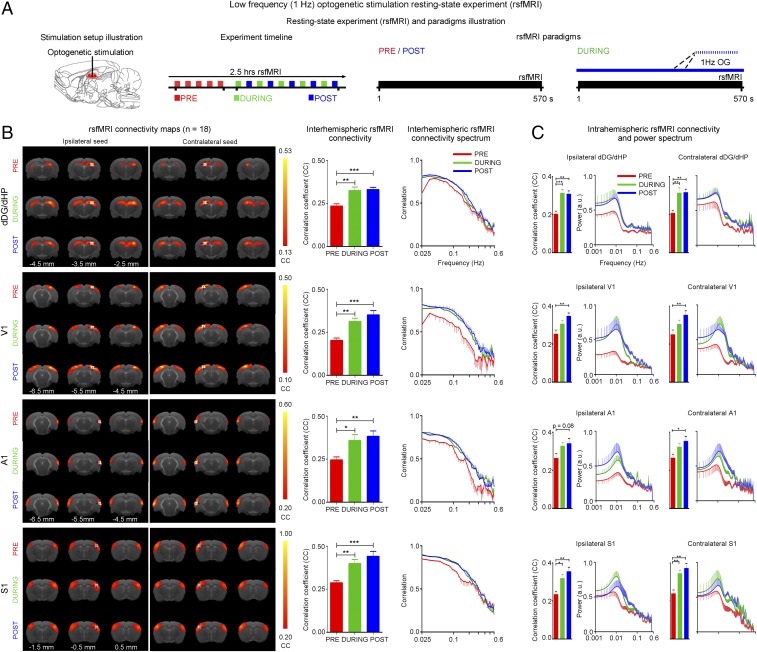
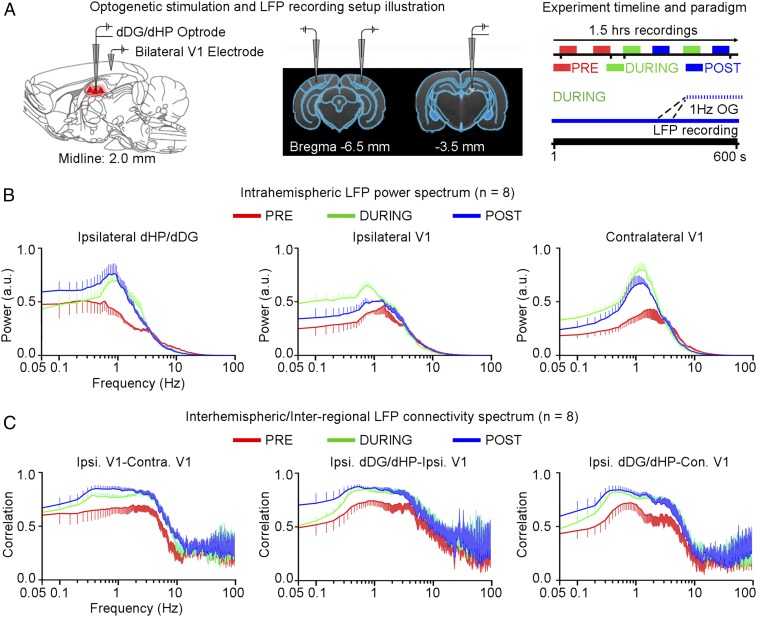
Next, we examined the effects of low-frequency activity propagating along the dorsal hippocampal–cortical pathway (Figs. 1 and 3) on interhemispheric or bilateral rsfMRI connectivity (Fig. 4A). Before the rsfMRI acquisition, we measured LFPs in dDG/dHP and V1 to ensure that sustained 1-Hz stimulation (400 s) did not evoke different LFP response characteristics observed during shorter stimulation durations (20 s) (Fig. S6 vs. Fig. 3B). LFPs showed steady evoked responses, which demonstrate the stability of 1-Hz evoked responses in both stimulated and activated regions. The strength of interhemispheric rsfMRI connectivity increased progressively in dHP, V1, primary auditory cortex (A1), and primary somatosensory cortex (S1) during (during) and after (post) 1-Hz dDG stimulation, which showed an increase in the spatial extent of connectivity maps (Fig. 4B, Left). The interhemispheric rsfMRI connectivity strengthened significantly during stimulation in dHP, V1, A1, and S1 (Fig. 4B, Middle; n = 18; dHP: 37.3 ± 7.4%, P < 0.01; V1: 55.6 ± 11.5%, P < 0.01; A1: 44.2 ± 6.9%, P < 0.05; and S1: 43.3 ± 9.0%, P < 0.01; one-way ANOVA followed by Bonferroni’s post hoc test). We also observed a significant enhancement of interhemispheric rsfMRI connectivity post stimulation (Fig. 4B, Middle; n = 18; dHP: 46.2 ± 8.6%, P < 0.001; V1: 72.9 ± 13.8%, P < 0.001; A1: 53.9 ± 7.6%, P < 0.01; and S1: 58.9 ± 10.9%, P < 0.001; one-way ANOVA followed by Bonferroni’s post hoc test). We then computed the connectivity spectrum of rsfMRI signals that were bilaterally correlated. We observed an increase in infraslow (<0.1 Hz) rsfMRI BOLD activity in dHP, V1, A1, and S1 during and post stimulation (Fig. 4B, Right). We further examined the intrahemispheric or local rsfMRI connectivity, which was increased during (Fig. 4C, Left; n = 18; ipsilateral dHP: 59.6 ± 8.6%, P < 0.001; contralateral dHP: 55.6 ± 8.2%, P < 0.01; ipsilateral S1: 38.3 ± 9.4%, P < 0.05; and contralateral S1: 45.6 ± 8.7%, P < 0.01; one-way ANOVA followed by Bonferroni’s post hoc test) and post stimulation (Fig. 4C; n = 18; ipsilateral dHP: 58.4 ± 6.9%, P < 0.01; contralateral dHP: 57.4 ± 6.8%, P < 0.01; ipsilateral V1: 43.8 ± 5.4%, P < 0.01; contralateral V1: 45.7 ± 11.3%, P < 0.01; contralateral A1: 33.7 ± 7.3%, P < 0.05; ipsilateral S1: 51.2 ± 7.7%, P < 0.01; and contralateral S1: 60.5 ± 13.0%, P < 0.01; one-way ANOVA followed by Bonferroni’s post hoc test).
Fig. 4.
Increased interhemispheric rsfMRI connectivity during (during) and after (post) low-frequency (1 Hz) optogenetic stimulation of dDG excitatory neurons in dHP. (A) Schematic of the optogenetic stimulation setup (Left), a typical rsfMRI experiment timeline with five baseline (pre) scans acquired before five “during” scans interleaved with five post scans (Middle), and the corresponding rsfMRI paradigms used (Right). (B) RsfMRI connectivity maps of dHP, V1, A1, and S1 (Left), corresponding quantification of interhemispheric connectivity (Middle), and their respective connectivity spectrum (Right), pre, during, and post low-frequency optogenetic stimulation. rsfMRI maps generated by correlation analysis of band-pass filtered (0.005–0.1 Hz) BOLD signals using a seed defined in the ipsilateral and contralateral side. Seed location is indicated by a blue crosshair. Quantification of the interhemispheric rsfMRI connectivity (n = 18; one-way ANOVA followed by Bonferroni’s post hoc test; *P < 0.05, **P < 0.01, and ***P < 0.001; error bars indicate ±SEM). (C) Quantification of intrahemispheric rsfMRI connectivity (Left) and the respective power spectrum (Right) of ipsilateral and contralateral dHP, V1, A1, and S1.
Fig. S6.
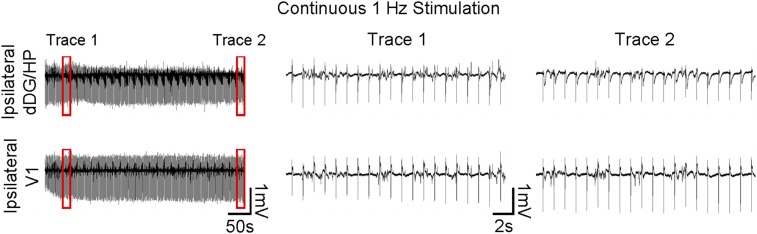
Continuous low-frequency (1 Hz) dDG stimulation (400 s) does not alter the characteristics of optogenetically evoked LFP responses. Trace 1 (Middle) is taken 50 s from the onset of stimulation and trace 2 (Right) at the end. Both traces show that evoked responses are steady throughout prolonged stimulation.
We then performed LFP recordings in bilateral V1 and ipsilateral dDG/dHP to determine the neuronal activity underlying enhanced V1 interhemispheric rsfMRI connectivity and examine possible interregional (i.e., dDG/dHP–V1) connectivity changes after low-frequency optogenetic stimulation under similar conditions to rsfMRI experiments (Fig. 5A). We computed the ipsilateral dDG/dHP and bilateral V1 intrahemispheric LFP frequency spectra (Fig. 5B), as well as the V1–V1 interhemispheric and dDG/dHP–V1 LFP interregional connectivity spectra (Fig. 5C). We observed a pronounced peak at ∼1 Hz in ipsilateral dDG/dHP and bilateral V1 intrahemispheric LFP spectrum during and post stimulation (Fig. 5A) and found a general increase of slow (0.1–1 Hz) and delta (1–4 Hz) oscillations (Fig. 5A), indicating a persistent modulation of LFP activity by low-frequency optogenetic stimulation of dDG excitatory neurons. The connectivity spectra demonstrate that the bilateral V1, ipsilateral dDG/dHP–ipsilateral V1, and ipsilateral dDG/dHP–contralateral V1 correlation of slow and delta oscillations strengthened during and post stimulation (Fig. 5C). These results indicate that persistent low-frequency activity propagating from dDG/dHP along the hippocampal–cortical pathway enhances interhemispheric rsfMRI connectivity. They demonstrate that increased slow and delta oscillations, in addition to the increased infraslow rsfMRI BOLD activity, contribute to enhanced brain-wide rsfMRI connectivity. These results further suggest significant effects on the integration of sensory information and memory operations, because such functions normally occur via cross-frequency coupling mechanisms between co-occurring brain oscillations (41).
Fig. 5.
Increased slow (0.1–1 Hz) and delta (1–4 Hz) neuronal oscillations during (during) and after (post) low-frequency (1 Hz) optogenetic stimulation of dDG excitatory neurons in dHP. (A) Illustration of LFP recording electrode location in ipsilateral dDG/dHP and bilateral V1 (Left) and a typical LFP recording experiment timeline with the corresponding paradigms (Right). (B) Intrahemispheric LFP power spectrum of ipsilateral dDG/dHP and bilateral V1. Error bars indicate ±SEM. (C) LFP connectivity spectra for interhemispheric V1–V1 and interregional dDG/dHP–V1. Error bars indicate ±SEM.
High-Frequency Stimulation of dDG Excitatory Neurons in dHP Does Not Enhance Brain-Wide Resting-State fMRI Connectivity.
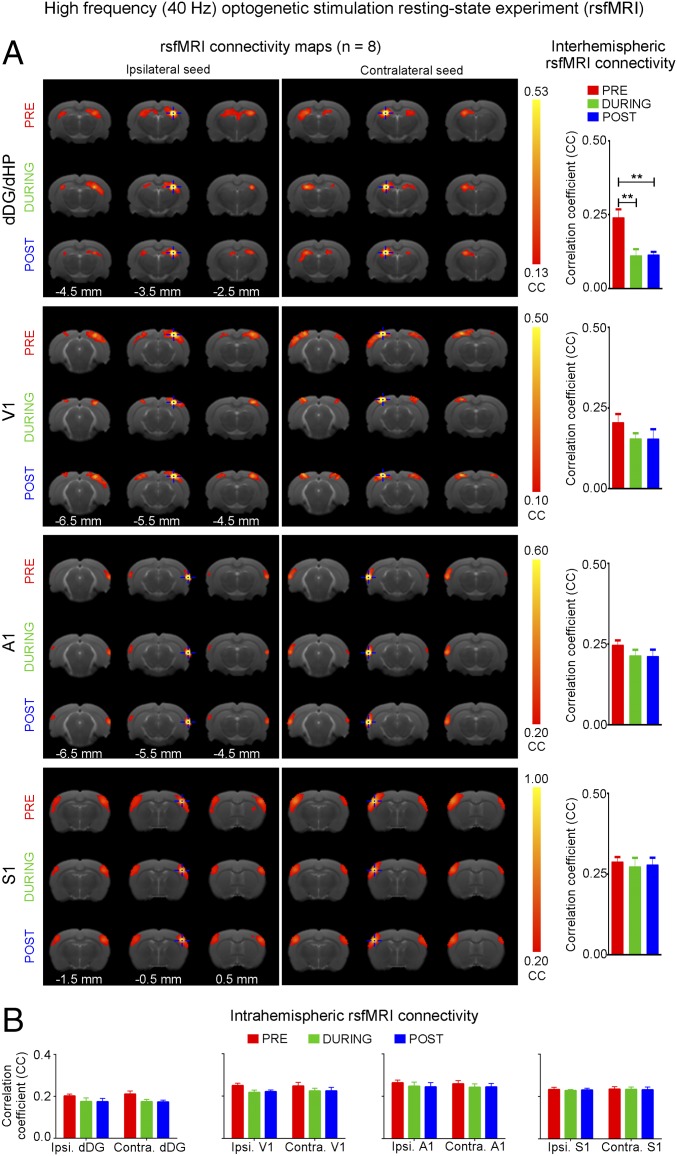
Separate rsfMRI experiments (n = 8) examined high-frequency stimulation (40 Hz) (Fig. 6A). No significant increase in inter- and intrahemispheric rsfMRI connectivity was observed during (during) and after (post) 40 Hz optogenetic stimulation of dDG (Fig. 6). Only interhemispheric rsfMRI connectivity in dHP exhibited a significant decrease in strength. These results demonstrate that only low-frequency activity enhances brain-wide functional connectivity.
Fig. 6.
High-frequency (40 Hz) optogenetic stimulation of dDG excitatory neurons in dHP does not enhance interhemispheric rsfMRI connectivity. (A) rsfMRI connectivity maps of dHP, V1, A1, and S1 before (pre), during (during), and after (post) high-frequency optogenetic stimulation (Left) and the corresponding quantification of interhemispheric rsfMRI connectivity (Right). (B) Quantification of intrahemispheric rsfMRI connectivity.
Pharmacologically Blocking dDG Neurons in dHP Decreases Brain-Wide Resting-State fMRI Connectivity.
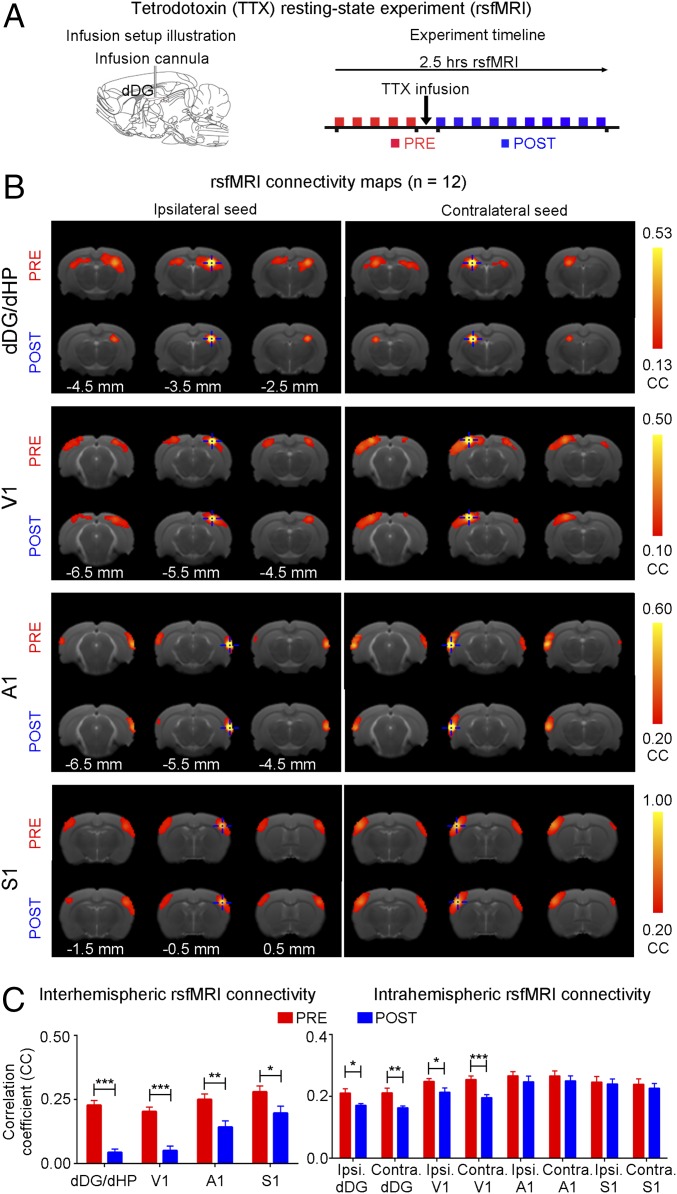
We examined the effects of pharmacologically inactivating dDG neurons in dHP on rsfMRI interhemispheric connectivity using tetrodotoxin (TTX). rsfMRI was acquired before (pre) and after (post) infusion of TTX (Fig. 7A). Pharmacological blockade significantly decreased rsfMRI interhemispheric connectivity in dHP, V1, A1, and S1, and intrahemispheric connectivity in dHP and V1 (Fig. 7 B and C). These results present additional evidence that dHP is a pivotal structure that coordinates brain-wide rsfMRI connectivity.
Fig. 7.
Pharmacological inactivation of dDG neurons in dHP decreases interhemispheric rsfMRI connectivity. (A) Illustration of the tetrodotoxin (TTX) infusion setup (Left) and a typical rsfMRI experiment timeline whereby TTX is infused into dHP a minute after the last baseline (pre) scan (Right). The first post scan is acquired a minute after the completion of TTX infusion. (B) rsfMRI connectivity maps of dHP, V1, A1, and S1, pre and post infusion of TTX. (C) Quantification of the interhemispheric rsfMRI connectivity (Left; n = 12; paired t test followed by Bonferroni’s post hoc test; *P < 0.05, **P < 0.01, and ***P < 0.001; error bars indicate ±SEM).
Low-Frequency Stimulation of dDG Excitatory Neurons in dHP Enhances Visually Evoked fMRI Responses.
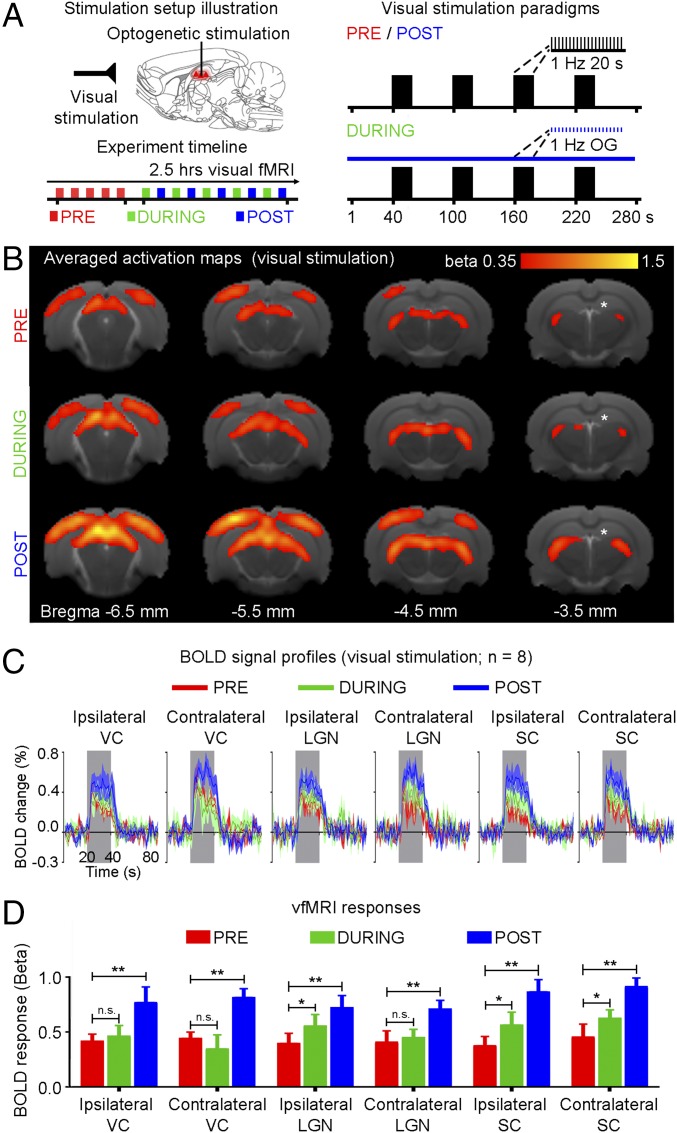
To investigate functional effects of low-frequency dorsal hippocampal–cortical activity on visual processing, visual fMRI (vfMRI) was performed before (pre), during (during), and after (post) continuous 1-Hz optogenetic stimulation (Fig. 8A). Visually evoked BOLD responses occurred along the visual pathway, including bilateral LGN, SC, and VC (Fig. 8 B and C). However, BOLD responses increased in ipsilateral LGN and bilateral SC during stimulation (Fig. 8D) (n = 8; ipsilateral LGN: 37.9 ± 19.8%, P < 0.05; ipsilateral SC: 49.2 ± 28.1%, P < 0.05; contralateral SC: 38.6 ± 14.5%, P < 0.05; one-way ANOVA followed by Bonferroni’s post hoc test). BOLD responses further increased in bilateral VC, LGN and SC post stimulation (Fig. 8D) (n = 8; ipsilateral VC: 93.4 ± 28.9%, P < 0.01; contralateral VC: 87.2 ± 14.2%, P < 0.01; ipsilateral LGN: 80.9 ± 18.5%, P < 0.01; contralateral LGN: 71.0 ± 25.3%, P < 0.01; ipsilateral SC: 136.5 ± 40.1%, P < 0.01; contralateral SC: 105.9 ± 22.0%, P < 0.01; one-way ANOVA followed by Bonferroni’s post hoc test). These results indicate that persistent low-frequency activity propagating along hippocampal–cortical pathways can enhance sensory responses that underlie sensory processing.
Fig. 8.
Low-frequency (1 Hz) optogenetic stimulation of dDG excitatory neurons in dHP enhances visually evoked fMRI responses. (A) Illustration of binocular visual and optogenetic fMRI stimulation setup (Top Left). Block design visual stimulation paradigm (20 s on, 60 s off) presented before (pre), during (during), and after (post) continuous low-frequency optogenetic stimulation (Right). Typically, five baseline visual fMRI scans were acquired before five “during” scans were interleaved with five post scans (Bottom Left). (B) Averaged activation maps for visual stimulation pre, during, and post low-frequency stimulation. BOLD responses are detected in bilateral V1, V2, LGN, and SC (n = 8; t > 3.1, corresponding to P < 0.001). (C) BOLD signal profiles in the bilateral V1, LGN, and SC. Error bars indicate ±SEM. (D) Comparison of vfMRI responses (one-way ANOVA followed by Bonferroni’s post hoc test; *P < 0.05 and **P < 0.01; error bars indicate ±SEM).
Discussion
Here, we examined large-scale effects of spatiotemporal-specific propagation of downstream hippocampal activity using optogenetic stimulation and brain-wide fMRI and rsfMRI. Stimulation of dDG excitatory neurons at low frequency, but not high frequency, evoked robust cortical and subcortical brain-wide responses. This low-frequency stimulation enhanced interhemispheric hippocampal and cortical rsfMRI connectivity. LFP recordings revealed an increase in slow oscillations in dHP and visual cortex (VC), interhemispheric visual cortical connectivity, and hippocampal–cortical connectivity. Meanwhile, pharmacological inactivation of dorsal hippocampus decreased brain-wide rsfMRI connectivity. Functionally, visually evoked fMRI responses in visual regions also increased during and after low-frequency dHP stimulation. Together, these experimental results highlight the role of low-frequency activity propagating along the hippocampal–cortical pathway, particularly its contribution to interhemispheric cortical rsfMRI connectivity.
Our fMRI results and electrophysiological recordings demonstrate that the anatomical topography within HP is spatiotemporally specific with low-frequency activity propagating from dDG/dHP downstream to VC and Cg. Note that a recent human rsfMRI study of low-frequency hippocampal–cortical propagation also showed characteristics of activity propagation between the hippocampus and visual cortex (34). Interestingly, the robust detection of BOLD responses in bilateral visual cortices observed in the present study suggests that low-frequency activity initiated in dDG may drive slow oscillatory activity to coordinate visual memory replay in the visual cortex and hippocampus during slow-wave sleep (9). Although LFP recordings in bilateral V1 only showed robust primary optogenetically evoked responses, we do not preclude that the presence of secondary responses like slow oscillations may contribute to BOLD responses. Moreover, Cg, a prefrontal cortical region implicated in memory (42) and learning (43), was also activated in our fMRI experiments. This result reinforces the role of dHP in cognitive functions.
We do not suggest that the presence of high-frequency activity could not be evoked by our low-frequency optogenetic stimulation. Previous studies showed features of diffuse brain-wide activity that were identified in relation to high-frequency hippocampal sharp wave ripple events (44–46). The relationship between slow oscillations and ripples in the hippocampus and/or cortex is well documented (30, 47–50). In particular, electrophysiological studies demonstrated that a large proportion of endogenous slow oscillations in the cortex and hippocampus precede the generation of hippocampal ripples (47–49). This finding indicates that slow oscillations and ripples are tightly coupled within the hippocampal–cortical pathways.
Low-frequency activity is a key contributor to integrate long-range interactions within thalamocortical–thalamic networks (21, 51–53). A recent study investigated the relationship between single neuron spiking activity and brain-wide cortical calcium dynamics (54). The authors found that thalamic spikes could produce various large-scale cortical activity patterns, which were underpinned by large-scale slow calcium activity (<1 Hz). Complementarily, earlier work using voltage-sensitive dye imaging also showed similar large-scale slow spontaneous cortical activity patterns (55). In fact, our recent study demonstrated brain-wide cortical activity propagation when the somatosensory thalamus was optogenetically stimulated at low frequency (36). Therefore, subcortical regions, such as thalamus, could initiate brain-wide cortical activity.
Our fMRI results and LFP latency analyses suggest that low-frequency activity propagates downstream from ipsilateral dDG/dHP to ipsilateral VC via the ipsilateral lateral EC (LEC) (5), before reaching contralateral VC through the corpus callosum (40, 56) to finally terminate in the LGN and SC via corticothalamic and corticocollicular descending projections. The medial EC (MEC) may not actively participate in our observed responses because dHP is functionally coupled with LEC, not MEC, to establish functional gradients along the dorsoventral hippocampal axis (8). In addition, our optogenetic stimulation of dDG reveals unique spatiotemporal response characteristics compared with fMRI studies using CA3 electrical stimulation (57) or optogenetic CA1 stimulation (58). We postulate that different stimulation locations elicit distinct spatiotemporal responses within hippocampal subfields. The hippocampal network encompasses multiple CaMKIIα-excitatory neurons, such as granule cells in DG and pyramidal cells in CA3/CA1, reciprocally connected to a network of GABAergic interneurons (5). Whereas we exclusively stimulated dDG excitatory neurons, orthodromic activation of various intrahippocampal circuits, and subsequent, monosynaptic excitation of interneurons and polysynaptic excitation of CA3/CA1 excitatory cells may occur. Based on our results, we propose that dHP has a frequency-dependent spatiotemporal gradient across hippocampal subfields, such that dDG facilitates downstream propagation of low-frequency activity brain-wide.
Importantly, our study demonstrates that interhemispheric cortical rsfMRI connectivity increases after low-frequency dDG stimulation (Fig. 4). This low-frequency activity propagates downstream from dDG/dHP and underlies changes in neuronal synchrony or neuronal excitability to substantially enhance brain-wide functional connectivity. Indeed, our LFP measurements show that hippocampal–cortical slow and delta oscillations enhance neuronal synchrony in bilateral V1 (Fig. 5). The parallel increase in both interhemispheric rsfMRI connectivity (at infraslow frequency 0.005–0.1 Hz) and slow- and delta-frequency neuronal oscillations (0.1–4 Hz) indicates a tight association between the two measures, because slow oscillations generate large, synchronous membrane-potential fluctuations in many neurons throughout brain-wide networks (21, 22, 59). Our results demonstrate the modulatory effects of increased neuronal slow oscillations on corticocortical and hippocampal–cortical connectivity. Numerous studies have demonstrated the importance of hippocampal and cortical theta oscillations (4–12 Hz) (60) to coordinate groups of neurons to integrate sensory information (61) and consolidate memory (62, 63). Comparatively, our results suggest that the hippocampus plays an even greater role in coordinating brain-wide neural activity, particularly at slow oscillations. Whereas we observed increased hippocampal delta oscillations, hippocampal delta activities have been observed so far only in primates and humans but not rodents (64, 65). Previous studies in rodents showed that theta-like oscillations in the hippocampus shifted to lower frequencies (i.e., delta range) during light anesthesia (∼1.0% isoflurane) (66, 67). This finding suggests that our increased delta oscillation results might arise from increased theta-like oscillations after low-frequency stimulation.
Whereas we exclusively focused on the dHP in this study, we do not preclude that the vHP could also affect rsfMRI connectivity. Given the differences in anatomical and functional organization (7, 8), the vHP may predominantly affect nonsensory-related regions through unknown mechanisms. Interhemispheric or homotopic rsfMRI connectivity networks are conserved across species (17, 68–70) and under distinct brain states (e.g., anesthesia, sleep, and wake) (71, 72) and consistent among different human subjects (73). We (14, 36) and others (16, 17, 68) have reliably measured interhemispheric rsfMRI connectivity in sensory cortices of rodents under light isoflurane (∼1.0%). However, the detection of more complex rsfMRI networks, such as default mode network, could be altered by anesthesia (74).
Brain-wide slow oscillations exhibit two distinct states, namely, up states with vigorous synaptic activity and down states of relative quiescence, that cycle between each other (26, 29). Up states, in particular, can increase neuronal sensitivity to synaptic inputs and enhance their responsiveness to weak, subthreshold inputs (75, 76). Our observed increase in neuronal synchrony of slow oscillatory activity, triggered by low-frequency stimulation of dDG, could modulate incoming signals, such as visual inputs, to promote robust evoked responses and significantly increase interhemispheric rsfMRI connectivity in V1. Studies investigating the dependence of evoked cortical responses on spontaneous network activity in up states demonstrated a linear relationship between spontaneous membrane potential levels and the magnitude of evoked responses in cortical neurons. Such a linear relationship between up-state membrane potential and evoked responses was described in anesthetized cat visual cortex (77, 78). However, our study indicates that this modulation extends beyond cortical regions, because responses to visual inputs increased not only in visual cortices but also in visual subcortical regions.
Hippocampal–cortical activity is state dependent, so the light anesthesia used here may alter their characteristics (34, 66, 67, 79). Nevertheless, prior work revealed synchronized interhemispheric delta oscillations as a major contributor to infraslow rsfMRI connectivity in anesthetized rodents (16, 17). A recent human rsfMRI study demonstrated that low-frequency oscillations, such as delta, propagate from the hippocampus toward cortex and slowly in the opposite direction during sleep (34). An EEG study in humans reported EEG differences under anesthesia and sleep/wake conditions, but the authors observed sleep oscillations (i.e., slow and delta dominant) under anesthesia (80). Such oscillations were also found in rodent studies during extracellular and/or intracellular recordings (67, 81, 82). Taken together, these studies highlight the robust presence of low-frequency activity in brain-wide interactions under various states, including anesthetized and sleep conditions.
In conclusion, our results demonstrate that slow oscillatory activity propagating along dorsal hippocampal–cortical pathways, contributes to interhemispheric cortical connectivity and mediates sensory functions. This finding advances our fundamental understanding of the neural basis and functional role of brain-wide rsfMRI connectivity. Further, we present an integrated optogenetic fMRI approach to interrogate rsfMRI mechanisms and to explore neuromodulation of brain connectivity.
Methods
Subjects.
Adult male Sprague Dawley rats (250–300 g) were used in all experiments. Animals were individually housed under a 12-h light/dark cycle with access to food and water ad libitum. All animal experiments were approved by the University of Hong Kong's Committee on the Use of Live Animals in Teaching and Research (CULATR). Group I underwent optogenetic fMRI experiments (n = 6), group II underwent LFP recording experiments (n = 6), group III underwent MUA recording experiments (n = 3), group IV underwent rsfMRI experiments (n = 18, low-frequency stimulation, n = 8, high-frequency stimulation and n = 12, TTX infusion), group V underwent LFP recording experiments at resting state (n = 8, low-frequency stimulation), and group VI underwent optogenetic vfMRI experiments (n = 8). Full details of animal surgical procedures, optogenetic stimulation paradigms, optogenetic fMRI/rsfMRI/vfMRI acquisition and analysis procedures, electrophysiological recordings and analysis protocols, and histology are provided in SI Methods.
Data and Code Availability.
The data that support the findings of this study and computer codes used are available from the corresponding author upon request.
SI Methods
Virus Packaging.
Recombinant AAV vectors were serotyped with AAV5 coat proteins and produced by the vector core at the University of North Carolina at Chapel Hill, Chapel Hill, NC. Viral titer (in particles per milliliter) was 4 × 1012 for AAV5-CaMKIIα-ChR2(H134R)-mCherry. Maps are available online from www.stanford.edu/group/dlab/optogenetics.
Stereotactic Surgery for Viral Injection.
All stereotactic surgeries were performed with rats at 6–7 wk of age. Rats were anesthetized with an i.p. bolus injection of a ketamine (90 mg/kg) and xylazine (40 mg/kg) mixture. The scalp was shaved, and rats were secured in a stereotactic apparatus with nonrupturing ear bars. A heating pad was used to prevent hypothermia, and buprenorphine (0.05 mg/kg, s.c.) was administered to minimize pain. Following a midline incision, a small craniotomy was made, and viral injection was performed at two depths in dDG (−3.5 mm posterior to Bregma, +2.0 mm medial-lateral right hemisphere, −3.5 and −4.0 mm from the surface of dura). Three microliters of viral constructs (1.5 μL at each depth) were delivered through a 5-μL syringe and 33-gauge beveled needle injected at 150 nL/min. Following injection, the injection needle was held in place for 10 min before slowly retracting it from the brain. Scalp incision was sutured, and animals were kept on a heating pad until recovery from anesthesia. Buprenorphine (0.05 mg/kg, s.c.) was administered postinjection twice daily for 72 h to minimize discomfort. Enrofloxacin was also administered orally for 72 h to minimize infection and inflammation postsurgery. Animals recovered for 6–7 wk before conducting MRI and electrophysiological experiments.
Optogenetic fMRI, Optogenetic rsfMRI, and Optogenetic vfMRI Experiments.
Before animals were placed in-magnet, surgery was performed to implant a custom-made plastic optical fiber cannula (POF), 450-μm core diameter, at the injection site. Before surgery, animals were anesthetized with isoflurane (induction 3% and maintenance 2%), and secured on a stereotactic frame. Following a midline incision, a craniotomy was performed at the same coordinates used for injection, because the previous craniotomy could be located for reference. Before the POF was inserted into the brain, it was first bent to create an arc with a 4-mm radius and secured permanently in place with an optical adhesive. The POF was then made opaque using heat shrinkable sleeves to prevent light leakage during stimulation to elicit undesired visual stimulation. The fiber tip of the POF was then scored to create a beveled edge to facilitate insertion of the fiber and minimize injury to brain tissue. Dental cement was then applied to the POF to affix it on the skull. Buprenorphine (0.05 mg/kg, s.c.) was administered postimplantation to minimize discomfort before MRI experiments.
Tetrodotoxin rsfMRI Experiments.
Before animals were placed in-magnet, surgery was performed to implant an MRI-compatible cannula, 250-μm internal diameter in dDG. Before surgery, animals were anesthetized with isoflurane (induction 3% and maintenance 2%) and secured on a stereotactic frame. Buprenorphine (0.05 mg/kg, s.c.) was administered postimplantation to minimize discomfort before MRI experiments. The concentration of tetrodotoxin (TTX) used was 5–10 ng/μL, similar to the values used in a previous in vivo study (83).
Animal Preparation for MRI Experiments.
All MRI experiments were carried out on a 7T MRI scanner (PharmaScan 70/16, Bruker Biospin) using a transmit-only birdcage coil in combination with an actively decoupled receive-only surface coil. After surgery, one to two drops of 2% lidocaine was applied to the chords to provide local anesthesia before endotracheal intubation. The animals were mechanically ventilated at a rate of 60 breaths per minute with 1–1.5% isoflurane in room-temperature air using a ventilator (TOPO, Kent Scientific). During all fMRI experiments, animals were placed on a plastic cradle with the head fixed with a tooth bar and plastic screws in the ear canals. Rectal temperature was maintained at ∼37.0 °C using a water circulation system. Continuous physiological monitoring was performed using an MRI-compatible system (SA Instruments). Vital signs were within normal physiological ranges (rectal temperature: 36.5–37.5 °C, heart rate: 350–420 beat/min, breathing: 60 breath/min, oxygen saturation: >95%) throughout the duration of the experiments (14, 36, 84).
Scanner-Synchronized Stimulation.
An Arduino programming board synchronized the scanner trigger and the lasers for optogenetic and visual stimulation. Computers and light delivery systems were kept outside the magnet and long optical patch cables (5–10 m) delivered light into the bore of the scanner. For optogenetic stimulation, blue light was delivered using a 473-nm DPSS laser measured before scanning as 8 mW at the fiber tip (450 μm, NA = 0.5) corresponding to a light intensity of 40 mW/mm2. For visual stimulation, blue light was delivered using a 473-nm DPSS laser via a separate optical patch cable. The emitted light was measured before the start of scanning as 0.5 mW at the fiber tip (400 μm, NA = 0.39). Laser stimulation protocols varied depending upon the fMRI experiment parameters.
To determine the frequency-dependent spatiotemporal characteristics of evoked dDG responses (optogenetic fMRI experiments), four frequencies were used (0.5 Hz, 1 Hz, 2 Hz, and 40 Hz) with a light intensity of 40 mW/mm2. A 100-ms pulse duration was used for all stimulation frequencies, except 40-Hz stimulation where a 7.5-ms pulse was used. Here, we chose a reduced duty cycle of 5%, 10%, and 20% for 0.5-Hz, 1-Hz, and 2-Hz stimulation, respectively (in contrast to 30% for 40 Hz) to avoid very long stimulation pulse widths (e.g., 300 ms). Long ChR2 optogenetic pulse width can cause neuronal output silencing and adversely affect neural activity efficacy (85). dDG excitatory neurons were stimulated with a standard block design paradigm that consisted of 20-s light-on and 60-s light-off periods. Three to four trials were recorded for each frequency in an interleaved manner in each animal.
To investigate the effects of low-frequency (1 Hz) stimulation in dDG for interhemispheric functional connectivity (rsfMRI experiments), a total of 15 scans were performed on each animal, 5 before (pre), 5 during (during), and 5 after (post) optogenetic stimulation. A 1-Hz optogenetic stimulation (light intensity = 8 mW: 40 mW/mm2; 10% duty cycle) was presented in a continuous manner for 10 min (total length of a typical rsfMRI scan).
To examine whether low-frequency stimulation could alter brain functional responses, vfMRI experiments were performed pre, during, and post 5-min continuous 1-Hz optogenetic stimulation (light intensity = 8 mW: 40 mW/mm2; 10% duty cycle). Blue light flashing at 1 Hz was delivered via an optical fiber placed 3 cm in front of the eyes for binocular visual stimulation in vfMRI. The vfMRI paradigm used consisted of four blocks of 20-s visual stimulation and 40-s rest. In total, 15 scans were acquired (5 scans at each time point) for each animal.
MRI Acquisition Procedure.
Scout T2-weighted (T2W) images were first obtained using a rapid acquisition with relaxation enhancement (RARE) sequence to determine the transverse, coronal, and sagittal planes of the brain. Sixteen contiguous 1.0-mm slices were then positioned in the transverse orientation according to the rat brain atlas to cover the majority of the brain. RARE T2W images were acquired for anatomical reference with field of view (FOV) = 32 × 32 mm2, matrix = 256 × 256, RARE factor = 8, echo time (TE) = 36 ms, repetition time (TR) = 4,200 ms. Optogenetic fMRI, rsfMRI, and vfMRI measurements were obtained for the same slices using a single-shot Gradient-Echo Echo-Planar-Imaging (GE-EPI) sequence with FOV = 32 × 32 mm2, matrix = 64 × 64, flip angle = 56° (optogenetic fMRI and vfMRI) or 50° (rsfMRI), TE = 20 ms, TR = 1,000 ms (optogenetic fMRI and vfMRI) or 750 ms (rsfMRI).
Optogenetic fMRI, rsfMRI, and vfMRI Data Analysis.
For each animal, all fMRI images were realigned to the mean image of the first fMRI session using SPM8 (Wellcome Department of Imaging Neuroscience, University College, London). Then, the T2W images from each animal were registered to a custom-made brain template acquired from a separate age-matched rat with the same settings. Registration was performed by affine transformation and Gaussian smoothing to maximize normalized mutual information (SPM8). Only then were the fMRI images resliced correspondingly (14, 36, 84).
For optogenetic fMRI and vfMRI, data from repeated fMRI sessions were averaged, in-plane smoothed [full width at half maximum (FWHM) = 1 pixel], and high-pass filtered (128 s). Then, a general linear model (GLM) was applied to calculate the response coefficient (β) maps for each stimulus. Student’s t test was performed to identify activated voxels using the threshold P < 0.001, except when mapping 0.5-Hz optogenetic fMRI BOLD responses, a t test threshold of P < 0.01 was used. The mapped responses were then compared between each animal to assess result quality before group level analysis. At the group level, realigned, registered, and resliced images corresponding to the same fMRI session were averaged across all animals. Similar smoothing, filtering, and GLM processes were applied to map responses. BOLD temporal profiles for each stimulation frequency were extracted from identical regions of interest (ROIs) delineated from the rat brain atlas (optogenetic fMRI) (36); ROIs for each experimental condition were generated from statistically thresholded voxels (vfMRI) (84).
For rsfMRI, voxelwise linear detrending with least-squares estimation was performed temporally to eliminate the drift caused by physiological noises and system instability. A temporal band-pass filter (0.005–0.1 Hz) was applied without spatial smoothing. To determine functional connectivity changes pre, during, and post low-frequency (1 Hz) optogenetic stimulation, seed-based analysis (SBA) was performed. For SBA, two 3 × 3 voxel regions were chosen as the ipsilateral and contralateral seed, respectively, in the dorsal hippocampus (dHP), primary visual cortex (V1), primary auditory cortex (A1), and primary somatosensory cortex (S1). Regionally averaged time course from the voxels within each seed served as the respective reference time course. Pearson’s correlation coefficients were calculated between the reference time course and the time course of every other voxel to generate two rsfMRI connectivity maps for each region. ROIs delineated from the rat brain atlas were then chosen to extract the correlation coefficients (CCs) from the connectivity maps (e.g., contralateral dHP ROI for maps generated using ipsilateral dHP seed). Interhemispheric functional connectivity for each region was then quantified by averaging the CC value of the corresponding ipsilateral and contralateral ROI. In addition, intrahemispheric power spectrum and interhemispheric connectivity spectrum for each region were calculated from nonfiltered rsfMRI data. Intrahemispheric power spectra were generated by taking the Fourier transformed time course extracted from the respective ROIs. For interhemispheric connectivity spectra, rsfMRI signals were first filtered into individual frequency bands using bandpass filters with a window size FWHM of 0.025 Hz. Pearson’s correlation coefficient was then calculated for each frequency band (86).
In Vivo Electrophysiological Experiments.
Electrophysiological recordings were performed under the same anesthesia protocols. Local field potential (LFP) (group II) recordings were performed at the stimulation site, ipsilateral dDG/dHP with an optrode and the bilateral primary visual cortex (−6.5 mm posterior to Bregma, ±3.8 mm lateral to midline, 1.0 mm from the surface of dura) with a tungsten microelectrode (Microprobes). Extracellular spike recordings (group III) were performed at the stimulation site, ipsilateral dDG with an optrode. The optogenetic stimulation paradigm in LFP experiments was the same as that used in optogenetic fMRI. LFP recordings at resting state (group V) were performed at ipsilateral dDG/dHP and bilateral V1 pre, during, and post 10-min continuous 1-Hz optogenetic stimulation. Note that the fiber outside the brain was made opaque using heat shrinkable sleeves to avoid undesired visual stimulation.
Multisite LFP Recordings.
To confirm that evoked BOLD responses were mediated by underlying neural activity and to delineate the involved downstream signal propagation pathway, simultaneous LFP recordings at three brain regions were performed on lightly anesthetized animals. Recordings were performed under the same anesthesia protocols (1.0% isoflurane) as in MRI experiments, with body temperature maintained using a heating pad. Electrodes placed in bilateral V1 were single tungsten microelectrodes (impedance = 1 MΩ, tip diameter = 10 μm). An optrode was placed in dDG/dHP. We constructed the optrode using a single tungsten microelectrode (impedance = 1 MΩ, tip diameter = 10 μm) coupled to a POF using protocols described previously (87). Electrophysiological data were acquired with a 32-channel OpenEphys data acquisition system (digitized at 30 kHz and bandpass filtered between 0.1 and 8 kHz). Laser stimulation was controlled using an Arduino programming board and light pulses were recorded simultaneously with the neural data. Laser stimulation protocols mirrored those used in optogenetic fMRI experiments (light intensity = 8 mW: 40 mW/mm2, 0.5–2 Hz: 10% duty cycle, 40 Hz: 30% duty cycle, interleaved block designed paradigm).
To investigate the neuronal origins of increased long-range interhemispheric rsfMRI functional connectivity strength after low-frequency stimulation, simultaneous LFP recordings at bilateral V1 and ipsilateral dDG/dHP were performed on anesthetized animals under similar protocols as described above. Laser stimulation protocols mirrored those used in rsfMRI experiments. Resting-state LFPs were recorded pre, during, and post low-frequency continuous optogenetic stimulation (1 Hz; light intensity = 8 mW: 40 mW/mm2; 10% duty cycle) for 10 min.
Single Optrode Recordings.
To confirm functional expression of ChR2(H134R) in dDG neurons and its response to blue light, optical stimulation with simultaneous electrophysiological recording using an optrode was performed on anesthetized animals. The homemade optrode consisted of one single tungsten microelectrode (impedance = 2 MΩ, tip diameter = 10 μm) coupled to our POF, using protocols described previously (87). Recordings were performed under the same anesthesia protocols (1.0% isoflurane) as in MRI experiments with body temperature maintained using a heating pad. Multiunit activity (MUA) recordings were acquired with a 32-channel OpenEphys data acquisition system (digitized at 30 kHz and bandpass filtered between 0.1–8 kHz). Laser stimulation was controlled using an Arduino programming board and light pulses were recorded simultaneously with the neural data. Laser stimulation protocols mirrored those used in optogenetic fMRI experiments (light intensity = 8 mW: 40 mW/mm2, 0.5–2 Hz: 10% duty cycle, 40 Hz: 30% duty cycle, interleaved block designed paradigm).
LFP and LFP at Resting-State Data Analysis.
Raw data were initially imported into custom-written Matlab software and bandpass filtered between 0.1 and 300 Hz with a sampling frequency of 10 kHz for analysis. A notch filter centered at 50 Hz was also applied to remove power-line noise. For LFP, individual animal LFPs were averaged across four stimulation blocks to generate a single LFP trace for each stimulation frequency, which was then averaged across animals. For low-frequency stimulation, the evoked responses to the first optogenetic stimulation pulse were extracted, and latency was measured from the most prominent trough. For comparison, the latency was also measured at the evoked responses to the last optogenetic stimulation pulse. For LFP at resting state, the signals at each recorded region were computed to generate their corresponding intrahemispheric power spectrum. Additionally, the interhemispheric/interregional connectivity spectrum for V1–V1 and dHP–V1 were also generated by first filtering the LFP signals into individual frequency bands using bandpass filters with a window size (full width at half maximum) of 0.05 Hz, before calculating the Pearson’s correlation coefficient for each frequency band.
MUA Data Analysis.
Raw data were imported into Offline Sorter software (Plexon) and high-pass filtered with a cutoff frequency of 300 Hz. Spikes were then sorted using waveform features (peak and valley heights) and principal components in which a negative and positive threshold crossing at twice the noise SD was imposed, and then this sorting was verified by visual inspection before spike frequency analysis. Spike sorted data were then imported into Neuroexplorer software (Nex Technologies) for further analysis. Using Neuroexplorer, neural spiking was assessed in the 5-ms preceding and following each light-stimulation pulse during 20-s stimulation.
Histology, Immunohistochemistry, and Confocal Imaging.
Upon completion of in vivo studies, animals were deeply anesthetized with pentobarbital and transcardially perfused with ice-cold 4% paraformaldehyde (PFA) in PBS. Brains were extracted and fixed in 4% PFA for 4 h at 4 °C. The brains were equilibrated in 20% sucrose in PBS at 4 °C overnight. Axial sections (40 μm) were prepared on a freezing microtome (model 860, AO Scientific Instruments). Consecutive sections (120 μm apart) were mounted and examined with a laser confocal microscope (Carl Zeiss LSM780). For immunohistochemistry, free-floating sections were processed with 5% normal goat serum and 0.3% Triton X-100 in PBS with primary antibodies against rabbit polyclonal to CaMKIIα (1:400; Abcam) and guinea pig polyclonal to GABA (1:200; Abcam) at 4 °C for 24 h. After washing with PBS, sections were then incubated for 2 h at room temperature with secondary antibodies Alexa Fluor 647 conjugate goat anti-rabbit IgG and Alexa Fluor 488 conjugate goat anti-guinea pig IgG (both 1:500; Molecular Probe). Slices were then washed and mounted using FluoroShield mounting medium with DAPI (Abcam). Double or triple immunofluorescence was assessed with a laser confocal microscope (Carl Zeiss LSM780).
Acknowledgments
We thank Drs. I. Timofeev, M. M. Poo, C. S. Poon, J. H. Lee, and X. Han for their insightful comments; Drs. W. H. Yung, K. K. Tsia, Y. I. Shih, Q. Li, S. J. Fan, A. To, and Misters A. Mok and Y. Qui for their technical assistance; and Dr. K. Deisseroth who provided us with the ChR2 viral construct. This work was supported by the Hong Kong Research Grant Council (Grants C7048-16G and HKU17103015 to E.X.W.) and the Lam Woo Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703309114/-/DCSupplemental.
References
- 1.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Maguire EA, et al. Knowing where and getting there: A human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 4.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 6.Yeckel MF, Berger TW. Feedforward excitation of the hippocampus by afferents from the entorhinal cortex: Redefinition of the role of the trisynaptic pathway. Proc Natl Acad Sci USA. 1990;87:5832–5836. doi: 10.1073/pnas.87.15.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 9.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 10.Buzsáki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 11.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 12.Smith SM, et al. WU-Minn HCP Consortium Resting-state fMRI in the Human Connectome Project. Neuroimage. 2013;80:144–168. doi: 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 14.Zhou IY, et al. Brain resting-state functional MRI connectivity: Morphological foundation and plasticity. Neuroimage. 2014;84:1–10. doi: 10.1016/j.neuroimage.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Park HJ, Friston K. Structural and functional brain networks: From connections to cognition. Science. 2013;342:1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- 16.Lu H, et al. Low- but not high-frequency LFP correlates with spontaneous BOLD fluctuations in rat whisker barrel cortex. Cereb Cortex. 2016;26:683–694. doi: 10.1093/cercor/bhu248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu H, et al. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci USA. 2007;104:18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Saalmann YB, Pinsk MA, Arcaro MJ, Kastner S. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron. 2012;76:1010–1020. doi: 10.1016/j.neuron.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schölvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci USA. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 21.Sheroziya M, Timofeev I. Global intracellular slow-wave dynamics of the thalamocortical system. J Neurosci. 2014;34:8875–8893. doi: 10.1523/JNEUROSCI.4460-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsui T, Murakami T, Ohki K. Transient neuronal coactivations embedded in globally propagating waves underlie resting-state functional connectivity. Proc Natl Acad Sci USA. 2016;113:6556–6561. doi: 10.1073/pnas.1521299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amemiya S, Takao H, Hanaoka S, Ohtomo K. Global and structured waves of rs-fMRI signal identified as putative propagation of spontaneous neural activity. Neuroimage. 2016;133:331–340. doi: 10.1016/j.neuroimage.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 24.Mitra A, Snyder AZ, Tagliazucchi E, Laufs H, Raichle ME. Propagated infra-slow intrinsic brain activity reorganizes across wake and slow wave sleep. Elife. 2015;4:e10781. doi: 10.7554/eLife.10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, et al. Resting-state hemodynamics are spatiotemporally coupled to synchronized and symmetric neural activity in excitatory neurons. Proc Natl Acad Sci USA. 2016;113:E8463–E8471. doi: 10.1073/pnas.1525369113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steriade M, Nuñez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations [corrected] J Neurosci. 2006;26:5665–5672, and erratum (2006) 26. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chauvette S, Crochet S, Volgushev M, Timofeev I. Properties of slow oscillation during slow-wave sleep and anesthesia in cats. J Neurosci. 2011;31:14998–15008. doi: 10.1523/JNEUROSCI.2339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neske GT. The slow oscillation in cortical and thalamic networks: Mechanisms and functions. Front Neural Circuits. 2016;9:88. doi: 10.3389/fncir.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolansky T, Clement EA, Peters SR, Palczak MA, Dickson CT. Hippocampal slow oscillation: A novel EEG state and its coordination with ongoing neocortical activity. J Neurosci. 2006;26:6213–6229. doi: 10.1523/JNEUROSCI.5594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn TT, Sakmann B, Mehta MR. Differential responses of hippocampal subfields to cortical up-down states. Proc Natl Acad Sci USA. 2007;104:5169–5174. doi: 10.1073/pnas.0700222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. Neuroimage. 2010;49:3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79:798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitra A, et al. Human cortical-hippocampal dialogue in wake and slow-wave sleep. Proc Natl Acad Sci USA. 2016;113:E6868–E6876. doi: 10.1073/pnas.1607289113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leong AT, et al. Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile. Proc Natl Acad Sci USA. 2016;113:E8306–E8315. doi: 10.1073/pnas.1616361113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferenczi EA, et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351:aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu X, et al. Sensory and optogenetically driven single-vessel fMRI. Nat Methods. 2016;13:337–340. doi: 10.1038/nmeth.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 40.Boudkkazi S, et al. Release-dependent variations in synaptic latency: A putative code for short- and long-term synaptic dynamics. Neuron. 2007;56:1048–1060. doi: 10.1016/j.neuron.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 41.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brincat SL, Miller EK. Frequency-specific hippocampal-prefrontal interactions during associative learning. Nat Neurosci. 2015;18:576–581. doi: 10.1038/nn.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Logothetis NK. Neural-event-triggered fMRI of large-scale neural networks. Curr Opin Neurobiol. 2015;31:214–222. doi: 10.1016/j.conb.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez-Villegas JF, Logothetis NK, Besserve M. Diversity of sharp-wave-ripple LFP signatures reveals differentiated brain-wide dynamical events. Proc Natl Acad Sci USA. 2015;112:E6379–E6387. doi: 10.1073/pnas.1518257112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Logothetis NK, et al. Hippocampal-cortical interaction during periods of subcortical silence. Nature. 2012;491:547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- 47.Staresina BP, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015;18:1679–1686. doi: 10.1038/nn.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mölle M, Yeshenko O, Marshall L, Sara SJ, Born J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol. 2006;96:62–70. doi: 10.1152/jn.00014.2006. [DOI] [PubMed] [Google Scholar]
- 49.Sirota A, Csicsvari J, Buhl D, Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 51.Crunelli V, David F, Lőrincz ML, Hughes SW. The thalamocortical network as a single slow wave-generating unit. Curr Opin Neurobiol. 2015;31:72–80. doi: 10.1016/j.conb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: A dialogue between three cardinal oscillators. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 54.Xiao D, et al. Mapping cortical mesoscopic networks of single spiking cortical or sub-cortical neurons. Elife. 2017;6:e19976. doi: 10.7554/eLife.19976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohajerani MH, et al. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat Neurosci. 2013;16:1426–1435. doi: 10.1038/nn.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martínez-García F, González-Hernández T, Martínez-Millán L. Pyramidal and nonpyramidal callosal cells in the striate cortex of the adult rat. J Comp Neurol. 1994;350:439–451. doi: 10.1002/cne.903500308. [DOI] [PubMed] [Google Scholar]
- 57.Moreno A, Morris RGM, Canals S. Frequency-dependent gating of hippocampal-neocortical interactions. Cereb Cortex. 2016;26:2105–2114. doi: 10.1093/cercor/bhv033. [DOI] [PubMed] [Google Scholar]
- 58.Weitz AJ, et al. Optogenetic fMRI reveals distinct, frequency-dependent networks recruited by dorsal and intermediate hippocampus stimulations. Neuroimage. 2015;107:229–241. doi: 10.1016/j.neuroimage.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci USA. 2008;105:16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colgin LL. Rhythms of the hippocampal network. Nat Rev Neurosci. 2016;17:239–249. doi: 10.1038/nrn.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jutras MJ, Fries P, Buffalo EA. Oscillatory activity in the monkey hippocampus during visual exploration and memory formation. Proc Natl Acad Sci USA. 2013;110:13144–13149. doi: 10.1073/pnas.1302351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Romani S, Lustig B, Leonardo A, Pastalkova E. Theta sequences are essential for internally generated hippocampal firing fields. Nat Neurosci. 2015;18:282–288. doi: 10.1038/nn.3904. [DOI] [PubMed] [Google Scholar]
- 63.Siegle JH, Wilson MA. Enhancement of encoding and retrieval functions through theta phase-specific manipulation of hippocampus. Elife. 2014;3:e03061. doi: 10.7554/eLife.03061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vass LK, et al. Oscillations go the distance: Low-frequency human hippocampal oscillations code spatial distance in the absence of sensory cues during teleportation. Neuron. 2016;89:1180–1186. doi: 10.1016/j.neuron.2016.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobs J. Hippocampal theta oscillations are slower in humans than in rodents: Implications for models of spatial navigation and memory. Philos Trans R Soc Lond B Biol Sci. 2013;369:20130304. doi: 10.1098/rstb.2013.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perouansky M, et al. Slowing of the hippocampal θ rhythm correlates with anesthetic-induced amnesia. Anesthesiology. 2010;113:1299–1309. doi: 10.1097/ALN.0b013e3181f90ccc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lustig B, Wang Y, Pastalkova E. Oscillatory patterns in hippocampus under light and deep isoflurane anesthesia closely mirror prominent brain states in awake animals. Hippocampus. 2016;26:102–109. doi: 10.1002/hipo.22494. [DOI] [PubMed] [Google Scholar]
- 68.Pawela CP, et al. Resting-state functional connectivity of the rat brain. Magn Reson Med. 2008;59:1021–1029. doi: 10.1002/mrm.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou ZC, et al. Resting state network topology of the ferret brain. Neuroimage. 2016;143:70–81. doi: 10.1016/j.neuroimage.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belcher AM, et al. Functional connectivity hubs and networks in the awake marmoset brain. Front Integr Nuerosci. 2016;10:9. doi: 10.3389/fnint.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 72.Larson-Prior LJ, et al. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci USA. 2009;106:4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu H, et al. Rat brains also have a default mode network. Proc Natl Acad Sci USA. 2012;109:3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCormick DA, et al. Persistent cortical activity: Mechanisms of generation and effects on neuronal excitability. Cereb Cortex. 2003;13:1219–1231. doi: 10.1093/cercor/bhg104. [DOI] [PubMed] [Google Scholar]
- 76.Shu Y, Hasenstaub A, Badoual M, Bal T, McCormick DA. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J Neurosci. 2003;23:10388–10401. doi: 10.1523/JNEUROSCI.23-32-10388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 78.Haider B, Duque A, Hasenstaub AR, Yu Y, McCormick DA. Enhancement of visual responsiveness by spontaneous local network activity in vivo. J Neurophysiol. 2007;97:4186–4202. doi: 10.1152/jn.01114.2006. [DOI] [PubMed] [Google Scholar]
- 79.Pilz GA, et al. Functional imaging of dentate granule cells in the adult mouse hippocampus. J Neurosci. 2016;36:7407–7414. doi: 10.1523/JNEUROSCI.3065-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li CY, Poo MM, Dan Y. Burst spiking of a single cortical neuron modifies global brain state. Science. 2009;324:643–646. doi: 10.1126/science.1169957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharma AV, Wolansky T, Dickson CT. A comparison of sleeplike slow oscillations in the hippocampus under ketamine and urethane anesthesia. J Neurophysiol. 2010;104:932–939. doi: 10.1152/jn.01065.2009. [DOI] [PubMed] [Google Scholar]
- 83.Telensky P, et al. Functional inactivation of the rat hippocampus disrupts avoidance of a moving object. Proc Natl Acad Sci USA. 2011;108:5414–5418. doi: 10.1073/pnas.1102525108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao PP, Zhang JW, Fan SJ, Sanes DH, Wu EX. Auditory midbrain processing is differentially modulated by auditory and visual cortices: An auditory fMRI study. Neuroimage. 2015;123:22–32. doi: 10.1016/j.neuroimage.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 85.Herman AM, Huang L, Murphey DK, Garcia I, Arenkiel BR. Cell type-specific and time-dependent light exposure contribute to silencing in neurons expressing Channelrhodopsin-2. Elife. 2014;3:e01481. doi: 10.7554/eLife.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li JM, Bentley WJ, Snyder LH. Functional connectivity arises from a slow rhythmic mechanism. Proc Natl Acad Sci USA. 2015;112:E2527–E2535, and correction (2015) 112:E5221. doi: 10.1073/pnas.1419837112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sparta DR, et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat Protoc. 2011;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study and computer codes used are available from the corresponding author upon request.