Significance
Proper transcription regulation is necessary for timely and accurate gene expression underlying growth and development. Transcription is regulated at each stage of the transcription cycle—initiation, elongation, and termination—and it is critical to define the factors and sequences regulating RNA polymerase activity. Many studies have investigated the mechanisms used by transcription factors involved in regulation of transcription initiation and elongation, but a mechanistic understanding of transcription termination has been slower to emerge. Here we characterize the first archaeal transcription termination factor, termed euryarchaeal termination activity (Eta). The mechanisms of Eta-mediated termination provide the first understanding of archaeal factor-dependent termination and provide insight into and contrast with the mechanisms used for factor-dependent termination in extant life.
Keywords: RNA polymerase, factor-dependent transcription termination, Archaea, Eta, transcription
Abstract
RNA polymerase activity is regulated by nascent RNA sequences, DNA template sequences, and conserved transcription factors. Transcription factors promoting initiation and elongation have been characterized in each domain, but transcription termination factors have been identified only in bacteria and eukarya. Here we describe euryarchaeal termination activity (Eta), the first archaeal termination factor capable of disrupting the transcription elongation complex (TEC), detail the rate of and requirements for Eta-mediated transcription termination, and describe a role for Eta in transcription termination in vivo. Eta-mediated transcription termination is energy-dependent, requires upstream DNA sequences, and disrupts TECs to release the nascent RNA to solution. Deletion of TK0566 (encoding Eta) is possible, but results in slow growth and renders cells sensitive to DNA damaging agents. Our results suggest that the mechanisms used by termination factors in archaea, eukarya, and bacteria to disrupt the TEC may be conserved, and that Eta stimulates release of stalled or arrested TECs.
Each stage of transcription offers regulatory potential, and increasing evidence supports the idea that postinitiation transcription regulation may dominate in many instances (1–5). Although processive, transcription elongation is not uniform, and regulated pausing through interactions with DNA or nascent RNA sequences or through the action of conserved global and gene-specific regulators influences the elongation of RNA polymerase (RNAP) (5–11). Archaeal transcription is reliant on initiation factors with eukaryotic homology, but their access to promoter sequences is often limited or facilitated by bacterial-like transcription factors. In contrast, archaeal transcription elongation is seemingly regulated by universal or archaea-eukaryotic specific homologous factors (2, 12–18).
The ultimate control of transcription elongation is provided by factors and sequences that can disrupt the normally extremely stable transcription elongation complex (TEC) to terminate transcription and release the nascent transcript and RNAP from the DNA template (3, 19–27). The archaeal RNAP is sensitive to intrinsic transcription termination. DNA sequences encoding poly-U–rich sequences are sufficient to disrupt the archaeal TEC both in vivo and in vitro, and although sequence context can influence intrinsic transcription termination efficiency, there is no requirement for RNA structure for intrinsic termination (22, 26, 28). Bioinformatic analyses of archaeal genomes reveals that many genes are organized into operons. and that approximately one-half of genes and operons have sequences near their 3′ ends that are consistent with intrinsic termination signals (26, 29–31). The genome of Thermococcus kodakarensis is >92% coding, and the average intergenic space (after accounting for genes in operons) is only ∼50 bp (32). In the absence of an intrinsic termination sequence, the stability of the TEC would be predicted to easily permit continued elongation from one gene to the next and thereby remove the normal regulation imposed on expression of downstream sequences.
To date, protein factors that can disrupt the TEC have been characterized only for bacteria and eukarya. Insufficient intrinsic termination sequences for each gene and operon, polar repression of gene expression in the absence of coupled transcription and translation (i.e., polarity), and the recent description of transcription-coupled DNA repair in euryarchaea argue strongly that factors capable of disrupting TECs are encoded in archaeal genomes (32, 33). Bioinformatic analyses of archaeal genomes have identified some genes with limited homology to eukaryotic factors involved in RNA 3′-end formation (e.g., cleavage and polyadenylation specificity factor subunits), but to date, no biochemical activities have been described from archaeal cells that can disrupt the archaeal TEC. Importantly, these analyses have not identified any obvious homologs of the well-characterized bacterial termination factors rho (34) or Mfd (35).
We used a robust in vitro transcription system dependent on purified RNAP and basal initiation factors from the model archaea T. kodakarensis to purify the first archaeal-encoded activity that can disrupt the TEC (22, 36). Our assay is dependent on the disruption of stalled archaeal TECs that are normally extremely stable and remain intact even when challenged with the strong replicative minichromosome maintenance (MCM) helicase (37). The factor so purified, in native or recombinant form, requires access to upstream DNA and ATP hydrolysis to disrupt TECs. The encoding gene, TK0566, is universally conserved in known euryarchaea and thus was named euryarchaeal termination activity (Eta) (38, 39).
Eta was annotated as a DEAD-box RNA helicase (38, 40), but our analyses demonstrate that Eta does not require access to the nascent transcript to disrupt the archaeal TEC. Eta-mediated termination is not competitive with standard elongation rates, arguing that Eta targets stalled or arrested TECs. Although conserved, deletion of TK0566 (encoding Eta) is possible. Deletion of Eta does not influence polarity, suggestive of at least one additional termination factor in T. kodakarensis; however, deletion of Eta does render cells sensitive to DNA-damaging agents. The nucleic acid requirements, slow rate of termination, and sensitivity of strains lacking Eta to mutagens suggest that Eta may function analogously to the bacterial transcription-repair coupling factor Mfd. The combined in vitro and in vivo characterization of Eta demonstrates that factor-dependent transcription termination is used in all extant life and reveals similarities in TEC stability and susceptibility to factor-dependent termination.
Results
Identification of Eta.
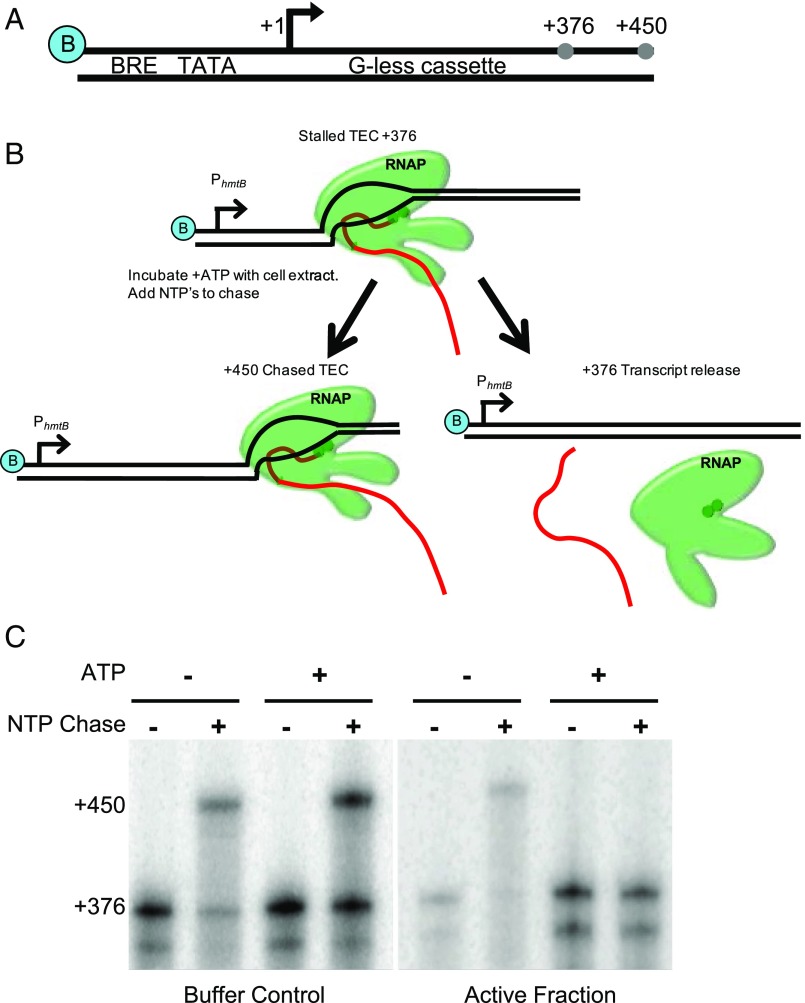
DNA templates attached to a solid support permit the generation of stable TECs at defined template positions by nucleotide deprivation (36, 41) (Fig. 1; experimental details in SI Materials and Methods). Stalled TECs are resistant to repeated washing, and we made use of the ability of washed TECs to resume elongation to assess TEC stability. Stalled TECs were incubated with partially purified T. kodakarensis lysates to provide any termination factors present with an opportunity to act on the stalled TECs. Reactions were then supplemented with NTPs to determine whether the TECs could resume transcription and generate a full-length transcript of 450 nt (Fig. 1B). Fractionated lysates were defined as “active” if the TECs incubated with these fractions were unable to resume elongation upon NTP addition (Fig. 1C). Known termination factors are energy-dependent; thus, a constraint on ATP dependence of presumptive termination activity was added to define active fractions.
Fig. 1.
Identification of an archaeal termination factor. (A) DNA templates contain a biotin moiety (blue B), a strong promoter, PhmtB, a 376bp G-less cassette, and permit elongation to produce a full-length transcript of +450. (B) Stalled TECs at the end of a G-less cassette (TEC+376) were incubated with cell lysate to identify termination factors. (C) Active fractions were identified as those that did not produce +450 transcripts when supplemented with lysate and ATP.
Several active fractions were chromatographically identified from T. kodakarensis lysates, implying the presence of multiple termination factors or the differential association of a single termination factor in separate complexes. The complexity of one active fraction was refined by repeated chromatographic separations until only a few proteins were present. The dominant proteins present in this purified fraction were identified by mass spectrometry (MS) (Table S1). Of the four abundant proteins, the ∼96-kDa product of TK0566 was deemed likely responsible for the ATP-dependent transcription termination activity.
Table S1.
Proteins identified by multidimensional protein identification technology in highly purified termination active fractions
| Gene | Annotation | MASCOT | Molecular weight, kDa |
| TK0566 | DEAH box RNA helicase | 645 | 96 |
| TK0752 | Acylamino acid-releasing enzyme | 1,120 | 72 |
| TK1626 | Hypothetical protein | 726 | 32 |
| TK2151 | Archaeal transcription regulator | 725 | 17 |
Eta Is an Energy-Dependent Transcription Termination Factor.
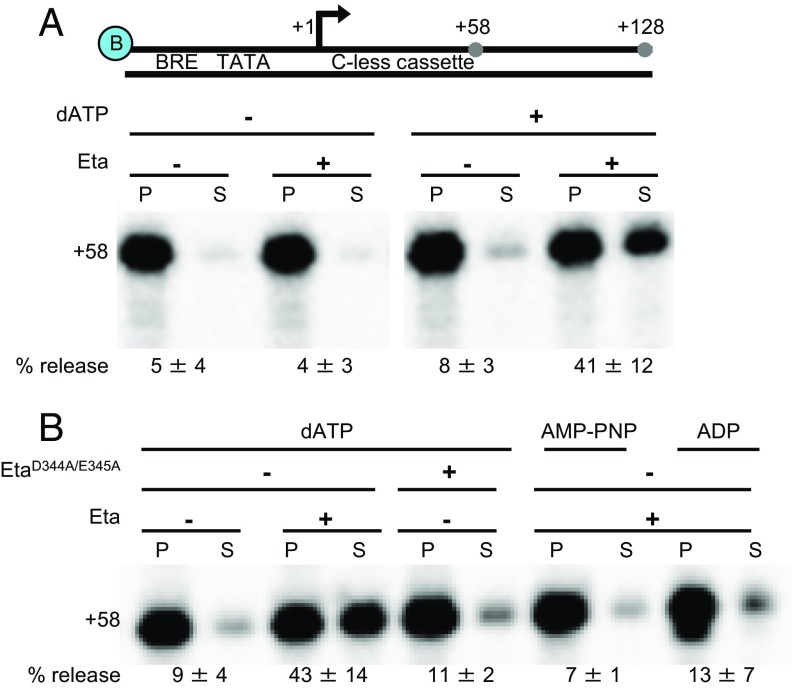
The failure of TECs to resume elongation on NTP addition (Fig. 1C) is suggestive of, but not definitive evidence of, transcription termination activity. To eliminate concerns of any unidentified factor being responsible for disrupting TECs, the protein product of TK0566 (832 aa; termed Eta) was recombinantly expressed and purified (Fig. S1). To demonstrate that Eta is a bona fide termination factor, we added Eta to in vitro transcription assays containing stalled TEC+58. TECs were stalled on a solid support that permits washing and separation of pellet and supernatant fractions to monitor dissociation of the TEC. Eta was capable of disrupting stalled TECs to release transcripts to solution (Fig. 2A). In the absence of Eta, or in the presence of Eta but in the absence of an energy source, just 4–8% of TEC+58 dissociate and release transcripts to solution (Fig. 2A). In contrast, the addition of Eta resulted in ATP-dependent dissociation of nearly one-half of all TECs. Both ATP and dATP support Eta activity, and dATP is used to avoid supplying RNAP with ATP.
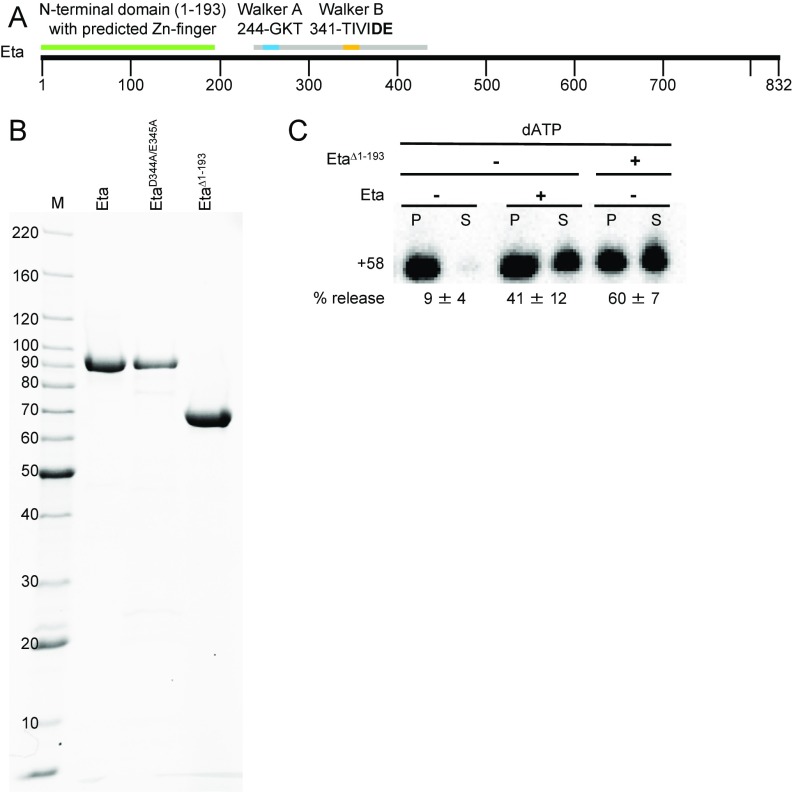
Fig. S1.
Eta purification and in vitro activity of EtaΔ1–193. (A) Linear representation of Eta highlighting the N-terminal domain (green), the P-loop NTPase domain (gray), and the Walker A and Walker B motifs (blue and orange, respectively). (B) Recombinant Eta and Eta variants (EtaD344A/E345A and Eta ∆1–193) were purified for use in vitro. Mass standards (M) are shown in kDa. (C) Eta ∆1–193 retains termination activity in vitro.
Fig. 2.
Eta is an energy-dependent transcription termination factor. (A) DNA templates contain a biotin moiety, PhmtB, a 58 bp C-less cassette, and permit elongation to produce full-length +128 transcripts. (B) Eta requires ATP or dATP hydrolysis to mediate transcription termination.
Eta-Mediated Termination Is Dependent on ATP Hydrolysis.
Eta is a superfamily II helicase (39), and conserved Walker A and B motifs are readily identified in a central P-loop NTPase domain (Fig. S1A). The Walker B consensus sequence hhhhDE contains an aspartate that coordinates Mg2+ and a glutamate that is essential for NTP hydrolysis (42, 43). The N terminus of Eta (residues 1–193) is less conserved and appears to contain a Zn-finger motif.
When ATP was replaced with ADP or the nonhydrolyzable analog AMP-PNP, Eta could not stimulate RNA release (Fig. 2B). An Eta variant, in which two Walker B residues were replaced by alanine (D344A + E345A), could not stimulate transcription termination above background levels (Fig. 2B). Near-homogenous preparations of Eta and EtaD344A+E345A were possible, but laborious. Deletion of the N terminus (amino acids 1–193) resulted in a protein that chromatographed more uniformly and retained full termination activity in vitro (Fig. S1C).
Eta-Mediated Termination Is Not Competitive with Elongation.
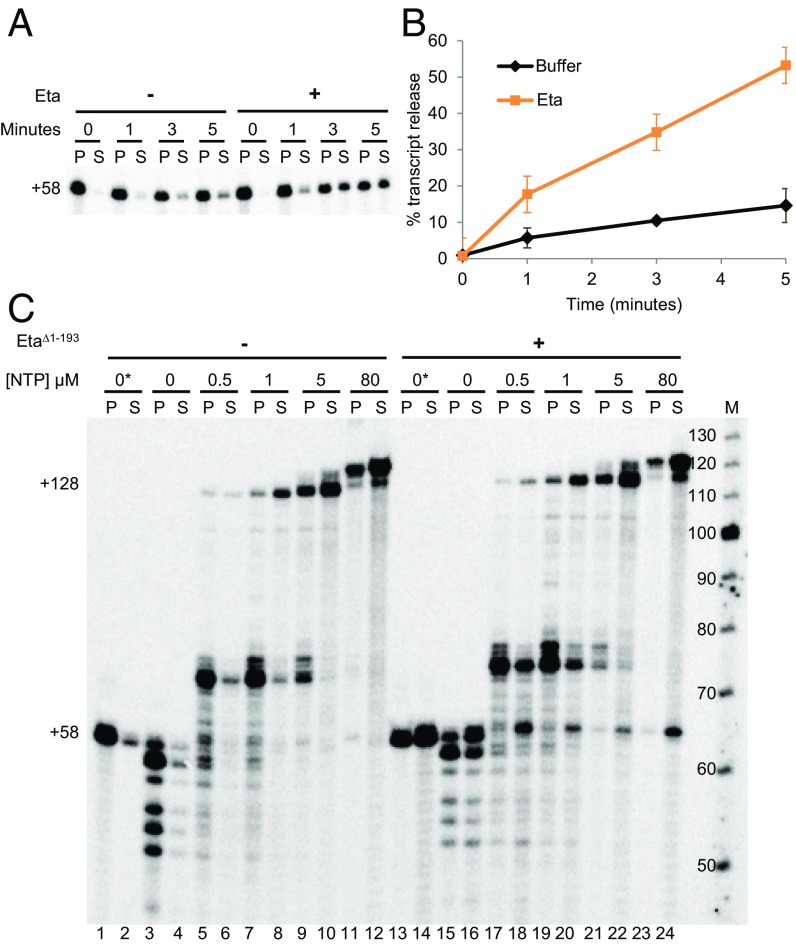
The addition of Eta to stalled TECs results in a slow and near-linear dissociation rate (Fig. 3 A and B). Transcription elongation and known mechanisms of factor-dependent transcription termination are in competition. Eta was added to stalled or slowly elongating TECs to monitor the ability of Eta to release transcripts from active TECs (Fig. 3C). When TECs were stalled without NTP, most remained intact but exhibited shortened transcripts, presumably by endonucleolytic cleavage and/or reverse catalysis (Fig. 3C, lanes 3 and 4). TEC+58 could be maintained by supplementing buffers with low concentrations of ATP, GTP, and UTP (Fig. 3C, lanes 1 and 2). The addition of all NTPs released TEC+58 to generate full-length +128-nt transcripts. Elongation was limited by sequences that direct pausing near ∼+70 nt at low NTP concentrations, but almost all TECs generated +128-nt transcripts without spontaneously dissociating. In contrast, the addition of Eta to stalled or slowly elongating TECs resulted in substantial transcript release to solution (Fig. 3C, lanes 13–20). Eta released most stalled TECs to solution (Fig. 3C, lanes 13 and 14), and Eta limited the percentage of TECs with shortened transcripts (Fig. 3C, lanes 15 and 16), suggesting that Eta keeps TECs in the forward configuration and/or pushes forward backtracked complexes. Eta disrupted TECs at the NTP-dependent pause at ∼+70 (Fig. 3C, lanes 17–22), but as the average duration of the pause was shortened by increasing NTP concentrations, Eta directed less termination. At NTP concentrations well below physiological concentrations (i.e., just 5 or 80 µM), Eta-mediated termination was noncompetitive with elongation, with the exception of a small percentage of TEC+58 that likely failed to elongate quickly (Fig. 3C, lanes 21–24).
Fig. 3.
Eta mediates termination of stalled or slowly elongating TECs. (A) Eta mediates the slow release of transcripts from stalled TEC+58. (B) Quantification from A. (C) Eta-mediated termination limits backtracking and is only competitive with transcription elongation at low NTP concentrations. M, labeled ssDNA maker to provide an approximation of RNA lengths.
Eta Interacts with RNAP in Vivo.
A strain of T. kodakarensis (Table S2) in which sequences encoding affinity and epitope tags were appended to the N terminus of TK0566 permitted purification of Eta via single-step chromatography under gentle conditions (44). The identity of copurifying partners was revealed by MS (Table S3), and several subunits of RNAP were identified, supporting Eta–RNAP interactions in vivo. Several large helicases, an ATPase, and NusA also were identified as Eta partners, suggesting that Eta may participate in activities besides termination.
Table S2.
Plasmids and T. kodakarensis strains used in this study
| Plasmid or strain | Key features | Source |
| Plasmids | ||
| Transcription templates | ||
| pTS236 | PhmtB with 376 nt G-less cassette (450 nt run off transcript) | Ref. 22 |
| pJW21 | PhmtB with 58 nt C-less cassette (128 nt run off transcript) | This work |
| pJW32 | PhmtB with 20 nt C-less cassette (128 nt run off transcript) | This work |
| pJW29 | PhmtB with 58 nt C-less cassette and Ssp1 cut site at position +18 (128 nt run off transcript) | This work |
| pJW30 | PhmtB with 58 nt C-less cassette and Ssp1 cut site at position +37 (128 nt run off transcript) | This work |
| Protein expression vectors | ||
| pTS317 | pQE-80L-TK0566 | This work |
| pJW26 | pQE-80L-TK0566D344A/E345A | This work |
| pJW28 | pQE-80L-TK0566∆1–193 | This work |
| Strain construction | ||
| pCSU-TK0566B | pTS700 containing TK0565:TK0567 | Ref. 45, this work |
| pOSU-TK0566NT | pTS700 containing TK0565-(his6-HA)TK0566-TK0567 | This work |
| pCSU-TK0566D344A/E345A | pCSU-TK0566B with D344A and E345A | This work |
| pTS429 | PhmtB (PF1848–TK1761, TK1762, TK1763) with adjacent trpE selectable marker | Ref. 32 |
| pTS435 | pTS429 with a stop codon at amino acid 381 of PF1848 | Ref. 32 |
| T. kodakarensis strains | ||
| TS559 | ΔTK2276, ΔTK0254::TK2276, ΔTK0664, ΔTK0149 | Ref. 45 |
| ∆TK0566 | TS559 ΔTK0566 | This work |
| TK0566-NT | TS559 TK0566-N-terminal his6-HA tag | This work |
| TK0566D344A/E345A | TS559 TK0566D344A/E345A | This work |
Table S3.
Proteins copurifying with His6-Eta from cellular lysates
| Gene | Annotation | MASCOT | Molecular weight, kDa |
| TK0566 | DEAD/DEAH box RNA helicase | 47,546 | 96 |
| TK1314 | ATPase | 6,035 | 50 |
| TK1015 | Large-helicase related protein | 1,093 | 106 |
| TK1332 | RNA helicase Ski2-like protein | 5,483 | 129 |
| TK1083 | RNAP subunit beta | 1,268 | 127 |
| TK1081 | RNAP subunit A′′ | 216 | 44 |
| TK1079 | NusA | 192 | 16 |
| TK1503 | RNAP subunit D | 144 | 29 |
| TK1499 | RNAP subunit N | 123 | 8 |
| TK1082 | RNAP subunit alpha | 111 | 103 |
Eta-Mediated Termination Requires Access to Upstream DNA Sequences.
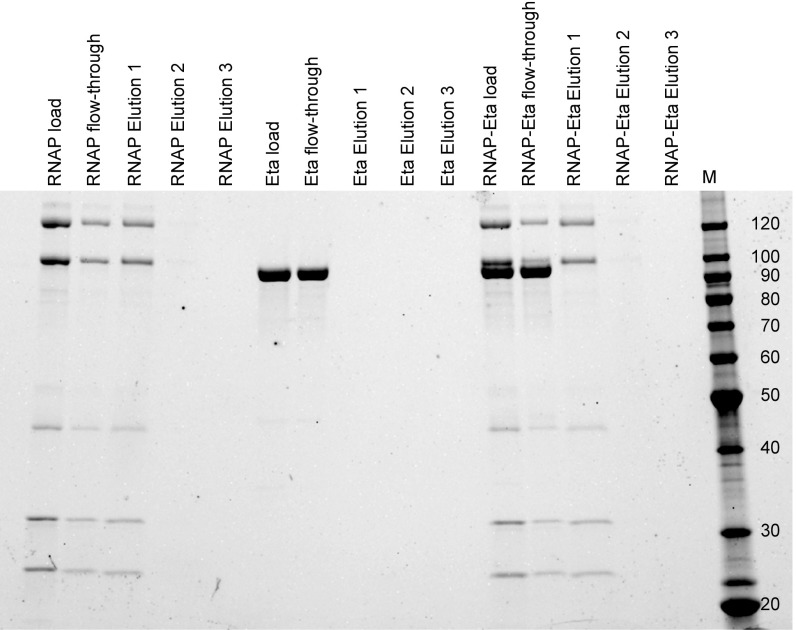
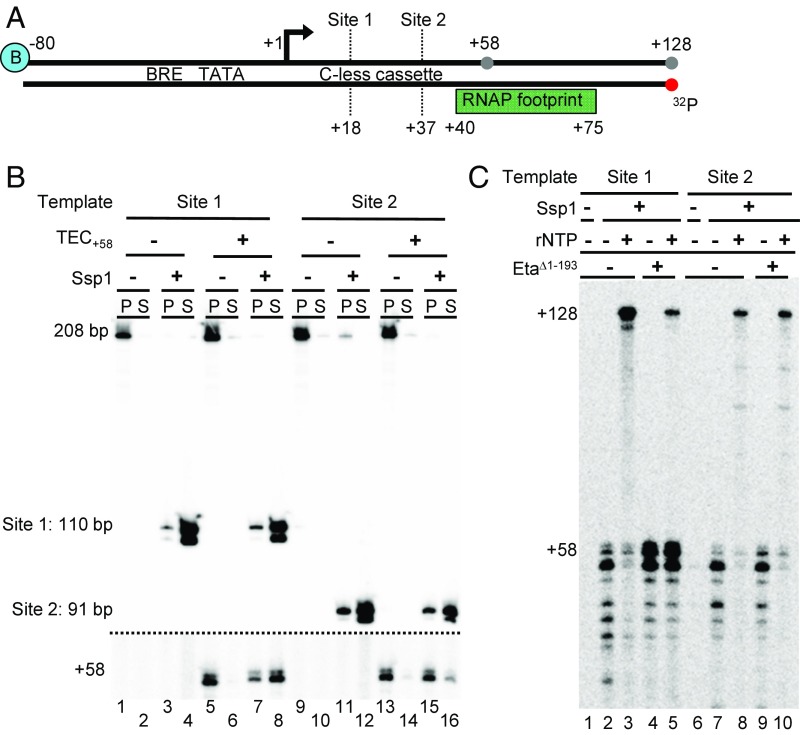
Despite the ability to identify RNAP in purifications of Eta from T. kodakarensis lysates, Eta does not retain long-lived associations with RNAP in solution (Fig. S2). Eta likely targets TECs through association with upstream or downstream DNA sequences or through the nascent transcript. To address the requirements for upstream DNA sequences, stalled TECs+58 were generated on templates that could be cleaved with Ssp1 to remove some or most upstream sequences (Fig. 4A). TECs were stable during the Ssp1 digestions, and monitoring radiolabeled DNA before and after cleavage demonstrated digestion of each template and release of the TEC+58 to solution (Fig. 4B). Digestion of templates containing the +37 Ssp1 site was less efficient at releasing TECs to solution compared with digestion at the +18 site in the presence of TEC+58 (Fig. 4B, lanes 7, 8, 15, and 16). The decrease in digestion efficiency is likely due to RNAP obstructing access of Ssp1 to this RNAP-proximal site.
Fig. S2.
Purified Eta and RNAP do not retain long-lived interactions in vivo. A pull-down experiment was performed using a nickel resin and RNAP-his6 only (positive control), Eta only (negative control), and RNAP-his6 + Eta. The portion of the sample that did not stick to the resin is indicated as flow-through, and the portion of the sample that eluted when a high imidazole buffer was added is indicated as elution. Samples were resolved on an SDS/PAGE with a marker shown in kDa.
Fig. 4.
Eta-mediated termination requires upstream DNA sequences. (A) DNA templates are identical except for the Ssp1-recognition sequences at positions +18 (site 1) and +37 (site 2) respectively. (B) Ssp1 digestion releases stalled TEC+58 to solution. DNA templates were radiolabeled at the 5’ position of the template strand (red dot). Radiolabeled DNA (above dashed line) and radiolabeled RNA (below dashed line) were visualized on a single gel, shown at two different contrast levels. (C) Upstream DNA is required for Eta-mediated transcription termination in vitro.
Radiolabeling of the nascent transcripts in Ssp1-released TECs allowed the ability of Eta to disrupt the TECs to be inferred by the ability of the TECs to elongate on the addition of NTP. Templates permitting Ssp1 digestion at +18 resulted in the release of TECs with at least a full turn of accessible upstream DNA and, based on the inability of the majority of released TECs to resume elongation on NTP addition, Eta could disrupt these complexes (Fig. 4C, lanes 4 and 5). In contrast, templates permitting digestion at +37 resulted in the release of TECs with essentially all upstream sequences removed and generated TECs that were largely resistant to Eta activity and produced full-length transcripts on NTP addition (Fig. 4C, lanes 9 and 10). No such requirement for downstream DNA or nascent RNA sequences was found for Eta-mediated termination of TECs (Fig. S3).
Fig. S3.
Enzyme-accessible downstream DNA nor nascent RNA is required for effective Eta-mediated termination. (A) Downstream DNA is dispensable for Eta-mediated termination. (B) Enzyme-accessible RNA sequences are not required for effective Eta-mediated termination. (C) RNase If digestion of stable TEC+58 eliminates enzyme accessible RNA but does not block Eta-mediated termination.
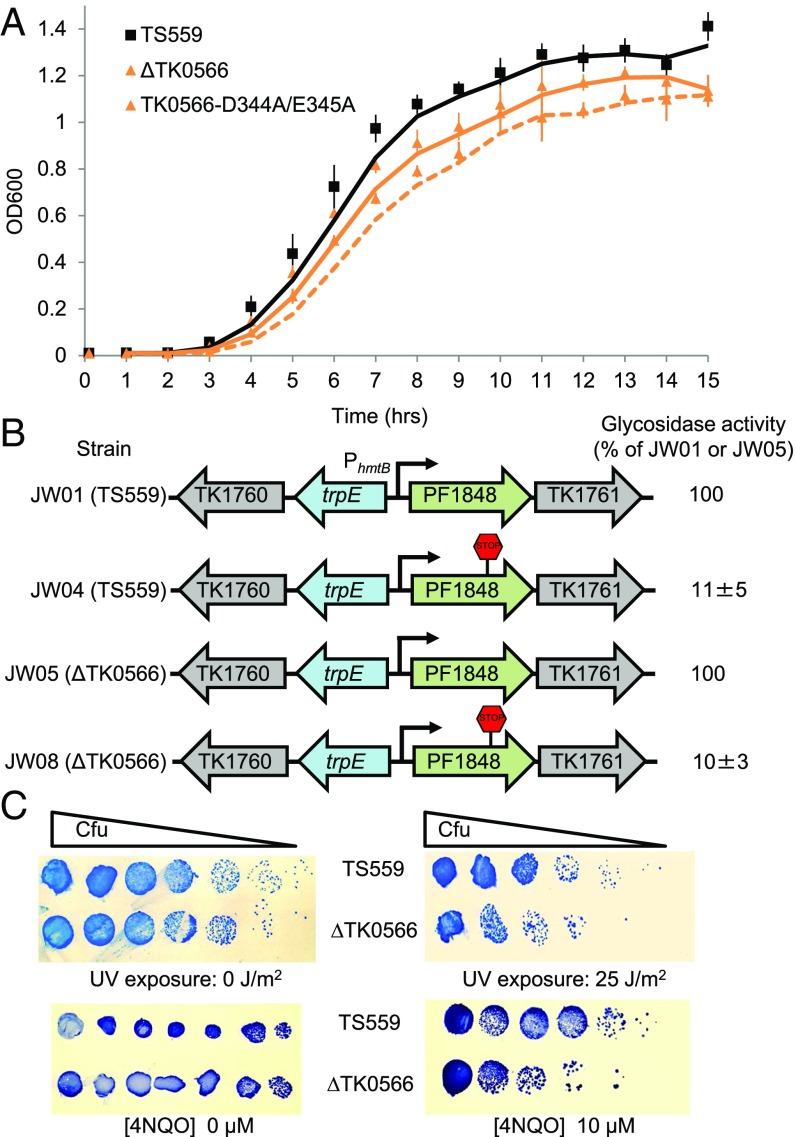
Eta Is Nonessential, but Deletion Impacts Growth of T. kodakarensis.
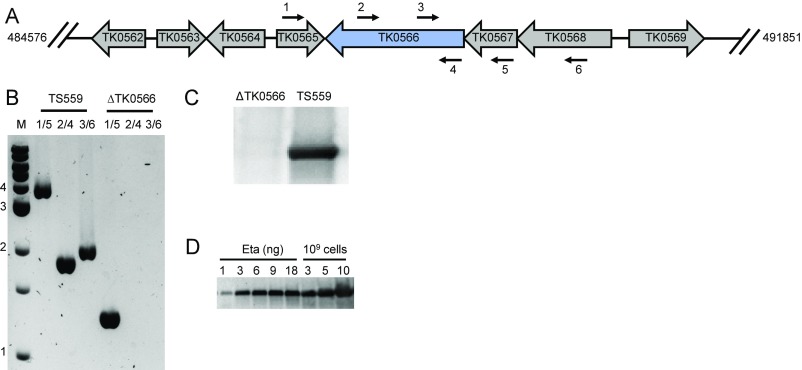
The entirety of the TK0566 coding sequence was markerlessly deleted from the T. kodakarensis genome (45, 46) (Fig. S4 and Table S2). Deletion or inactivation of Eta through changes to TK0566, such that a D344A + E345A variant Eta was encoded, slowed and limited growth (Fig. 5A). Western blot analysis using anti-Eta antibodies confirmed the loss of Eta in ΔTK0566 strains (Fig. S4C). Quantitative Western blot analysis suggested that Eta is normally present at low levels (∼50 copies per cell) compared with RNAP levels (∼2,000–3,000 copies per cell) (Fig. S4D).
Fig. S4.
TK0566 (encoding Eta) can be deleted from the T. kodakarensis chromosome and Eta is present at low concentration in vivo. (A) A map of the T. kodakarensis genome highlighting TK0566 and surrounding sequences. Primer binding positions 1–6 are designated by labeled arrows. (B) Diagnostic PCR confirming deletion of TK0566 sequences. (C) Western blots using anti-Eta antibodies confirming that deletion of TK0566 eliminates production of Eta in vivo. (D) Semiquantitative Western blots using anti-Eta antibodies suggesting that Eta is present in low concentrations in vivo.
Fig. 5.
Eta is nonessential, is not responsible for polarity in vivo, but loss of Eta limits growth and increases DNA damage susceptibility. (A) Deletion or inactivation of Eta hinders growth rate and final cell densities. The curves and errors shown represent means and standard averages of triplicate technical repeats of triplicate biological samples. (B) Eta is not responsible for polarity in vivo. The presence or absence of the nonsense codon in PF1848 is noted, and the percentage of β-glycosidase activity is reported as the mean and standard average of triplicate technical repeats of triplicate biological samples. (C) Deletion of Eta increases sensitivity to DNA damaging agents.
Eta Is Not Responsible for Polarity.
To determine whether Eta is the factor responsible for polarity in T. kodakarensis, we compared repression of a previously characterized polarity-influenced operon (32) in strains with and without Eta (Fig. 5B and Table S2). Activity from the downstream reporter gene in both strains was substantially reduced by the introduction of nonsense codons into the coding region of the upstream gene, but this reduction was not changed by the absence of Eta.
Deletion of Eta Increases Cellular Sensitivity to DNA-Damaging Agents.
The requirements for Eta-mediated transcription termination—energy dependence, a static or slowly elongating TEC, access to upstream DNA sequences, and no requirement for downstream DNA or RNA sequences—are reminiscent of the only other prokaryotic factor capable of disrupting the TEC, namely Mfd (47–49). Mfd mediates RNAP removal and initiates transcription-coupled DNA repair (TCR) in bacteria, and cells deleted for mfd exhibit a mild phenotype to some DNA-damaging agents (50, 51). Although TCR has not yet been demonstrated in T. kodakarensis, the archaeal RNAP does arrest at template strand DNA lesions (52), and recent support for TCR in related euryarchaea has been reported (33). We accessed the potential for Eta to influence DNA repair by challenging parental and Eta-deleted strains of T. kodakarensis to common DNA-damaging agents. Eta-deleted strains are at least an order of magnitude more sensitive to UV exposure than the parental strain (Fig. 5C, Top), and introduction of the heterocyclic mutagen 4-nitroquinoline 1-oxide (4NQO) (50) limits the growth of Eta-deleted strains more severely (Fig. 5C, Bottom).
SI Materials and Methods
Biochemical Identification of Transcription Termination Activities in T. kodakarensis Lysates.
Biomass was recovered from T. kodakarensis strain TS413 (rpoL-HA-his6) (62) grown at 85 °C to midexponential phase (OD600 ≅ 0.5). Cells were resuspended and sonicated in lysis buffer (25 mM Tris⋅HCl pH 8.0, 1 M NaCl, and 10% glycerol). Clarified cell lysate was loaded onto a Ni2+-charged matrix. Flow-through was collected; diluted with 3 volumes of 25 mM Tris⋅HCl pH 8.0, 10 mM MgCl2, and 0.1 mM EDTA; and then loaded onto a heparin column. The column was washed with the same buffer containing 160 mM NaCl, and a linear gradient over 50 mL to a final concentration of 1 M NaCl was used, followed by an isocratic gradient to a final concentration of 2 M NaCl. Fractions collected were used during the in vitro transcription termination assay and deemed active or inactive (see below). Active fractions were pooled and diluted with 25 mM Tris⋅HCl pH 8.8 and 10 mM MgCl2 to reduce conductivity, and then loaded onto a Q-Sepharose column. Fractions were eluted with a linear gradient over 80 mL to a final concentration of 400 mM NaCl, followed by an isocratic gradient to a concentration of 1 M NaCl. Then active fractions were identified, pooled, concentrated, and loaded onto a Superdex 200 column in 25 mM Tris⋅HCl pH 6.8 and 10% glycerol. Active fractions were identified, pooled, and loaded onto a MonoS column. The sample was eluted over 25 mL to a final concentration of 1 M NaCl, and active fractions were identified, pooled and loaded onto a MonoQ column. The column was washed with 5 mM NaCl, and a linear gradient over 35 mL to a concentration of 500 mM NaCl was used, followed by a 10-mL linear gradient to a concentration of 1 M NaCl. Active fractions were identified and resolved on a 15% discontinuous SDS/PAGE, and four abundant proteins were identified using MS.
Downstream DNA and Nascent RNA Sequences Are Not Required for Eta-Mediated Transcription Termination in Vitro.
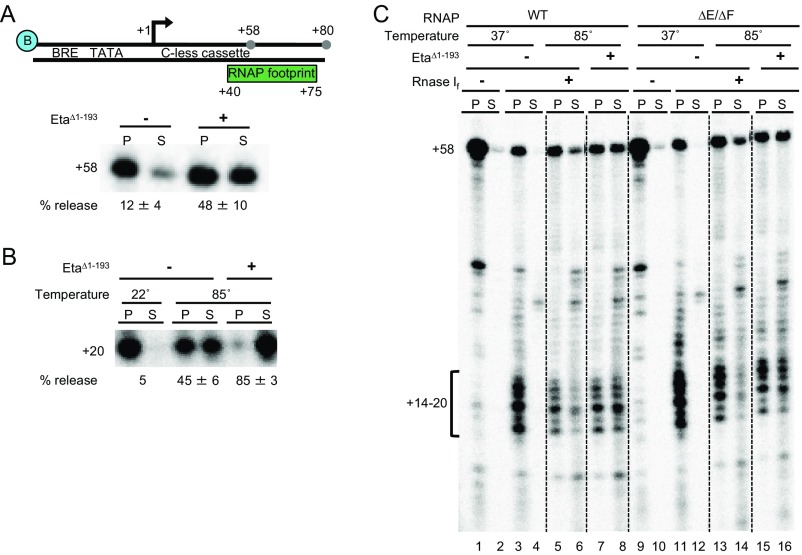
To address requirements for downstream DNA for Eta-mediated transcription termination, we assembled TEC+58 on templates that contained minimal accessible downstream DNA (Fig. S3A, Top). Using short templates that permit elongation only to generate TEC+80, essentially all downstream DNA sequences would be predicted to be enveloped by the TEC and not solvent-accessible. Eta-mediated termination was efficient on such templates (Fig. S3A, Bottom), suggesting that Eta-mediated transcription termination does not require downstream DNA sequences.
Any requirements for use of RNA transcripts to target TECs for Eta-mediated transcription termination were tested in two complementary formats. First, (Fig. S3B), TEC+20 were generated by NTP deprivation to limit the extent of enzyme-accessible transcript sequences. Approximately one-half of such TECs were unstable at high temperature, but Eta-mediated transcription termination was still active, releasing nearly all transcripts to solution under such conditions (Fig. S3B).
Given the spontaneous instability of TECs with short transcripts, we were concerned that such complexes might not represent TECs that have fully transitioned from initiation to elongation. To more accurately determine the requirements for any enzyme-accessible transcript sequences for Eta-mediated transcription termination, we generated TEC+58, then added RNase If (an RNA endonuclease that cleaves at all RNA dinucleotide bonds) to cleave enzyme-accessible RNA to a minimum (Fig. S3C). Generating stable TEC+58 (lanes 1 and 2) permitted RNase If digestion at 37 °C (lanes 3 and 4), which yielded stable TECs that contained a range of shortened RNAP-associated transcripts of just ∼14–20 nt on average. Challenging such complexes at physiological temperatures (85 °C, lanes 5 and 6) resulted in minimal disassociation of TECs containing ∼14–20 nt transcripts; however, the addition of Eta resulted in release of the bulk of these complexes to solution.
Previous studies demonstrated that subunits E and F (i.e., the stalk domain, homologous to eukaryotic Rpb7 and Rpb4, respectively) of RNAP can interact with the nascent RNA (62–66). Deletion of the stalk domain has no effect on promoter-dependent transcription initiation, abortive transcript synthesis, transcription elongation rate, stability of stalled TECs, or susceptibility of TECs to intrinsic termination (62). We hypothesized that TECs formed with the ΔE/ΔF RNAP variant might leave more RNA exposed to solution and thus accessible to RNase If. Surprisingly, repeating the experiment using a RNAPΔE/ΔF variant (Fig. S3C, lanes 9–16) showed no differences in RNase If cleavage patterns or RNAs retained in stable TECs. Challenging TEC+58 at physiological temperatures (85 °C, lanes 13 and 14) resulted in minimal disassociation of TECs containing ∼14–20 nt transcripts; however, the addition of Eta resulted in release of the bulk of these complexes to solution. The ability of Eta to drive the release of transcripts from TECs with minimal enzyme-accessible RNA in the presence and absence of the RNAP stalk domain suggests that Eta-mediated termination is not reliant on RNA transcript sequences or on the stalk domain of RNAP to mediate termination.
Protein Purifications.
rEta, rEtaD344A/E345A, and rEta∆1–193 were purified (Fig. S1) from Rosetta2 (DE3) cells carrying pQE-80L-Eta, pQE-80L-EtaD344A/E345A, and pQE-80L-Eta∆1–193, respectively, grown in LB medium with 34 µg/mL chloramphenicol and 40 µg/mL ampicillin. Eta expression was induced with 0.5 mM isopropyl β-D-1-thiogalactopyranoside, and cultures were grown overnight (∼16 h) at 17 °C. Biomass was harvested, resuspended, and sonicated in lysis buffer (25 mM Tris⋅HCl pH 6.8, 10% glycerol, 0.1 mM EDTA, 100 mM NaCl, and 1 mM β-ME). Eta was partially purified from clarified cell lysate by heat treatment at 85 °C for 20 min, followed by passage and fractionation of the cleared supernatant through three separate chromatographic columns (S-Sepharose, heparin, and MonoQ; GE Healthcare). Eta preparations were dialyzed into storage buffer (20 mM Tris⋅HCl pH 8.0, 0.1 mM EDTA, 100 mM KCl, and 50% glycerol) and quantified by Bradford assays (67).
DNA Template Construction.
Plasmids pJW21, pJW29, pJW30, and pJW32 were generated by inserting a gblock (IDT) containing the PhmtB promoter and a 128-bp full-length cassette into the pQE-80L vector. The DNA templates used during in vitro transcription were amplified from plasmids using standard molecular biology followed by purification of the linear, biotinylated templates (Agencourt AMPure XP; Beckman Coulter).
In Vitro Transcription.
TEC+58 were captured with NCMBs and washed three times in WB, then resuspended in 20 mM Tris⋅HCl pH 8, 250 mM KCl, 10 mM MgCl2, 2 mM DTT, 10 µM ATP, 10 µM GTP, and 10 µM UTP (Fig. S3A). Then 10-µL aliquots were combined with equal-volume reactions containing 15 mM Tris⋅HCl pH 8, 5 mM MgCl2, and 2 mM DTT, ± 4 mM dATP and ± 500 nM purified EtaΔ1–193. Reactions were incubated at 85 °C for 5 min, after which SCMBs were used to separate reactions into pellet and supernatant fractions. Pellet and supernatant fractions were incubated with STOP buffer and extracted, and RNA transcripts were purified as described above. Release was calculated by quantifying transcripts in the supernatant divided by transcripts quantified in the supernatant and pellet.
To obtain TEC+20, starting NTPs (200 µM ATP, 200 µM GTP, 10 µM UTP, and 10 µCi [α-32P]-UTP) were added to the reactions, followed by incubation at 85 °C for 5 min (Fig. S3B). TEC+20 were captured with NCMBs and washed three times in WB, then resuspended in 20 mM Tris⋅HCl pH 8, 250 mM KCl, 10 mM MgCl2, 2 mM DTT, 10 µM ATP, 10 µM GTP, and 10 µM UTP. Then 10-µL aliquots were combined with equal-volume reactions containing 15 mM Tris⋅HCl pH 8, 5 mM MgCl2, 2 mM DTT, and 4 mM dATP, ± 500 nM purified Eta. Reactions were incubated at 85 °C for 5 min, and then SCMBs were used to separate reactions into pellet and supernatant fractions. Pellet and supernatant fractions were incubated with STOP buffer and extracted, and RNA transcripts were purified and analyzed as above.
To obtain stalled TECs, starting NTPs (200 µM ATP, 200 µM GTP, 10 µM UTP, and 10 µCi [α-32P]-UTP) were added to the reactions, followed by incubating at 85 °C for 5 min with RNAPWt or RNAPΔE/ΔF (Fig. S3C). TEC+58 were captured with NCMBs, washed three times in WB, and then resuspended in RNase buffer (1× NEB Buffer 3 ± 8 U RNase If). Reactions were incubated at 37 °C for 10 min and washed twice in WB and resuspended in TB (20 mM Tris⋅HCl pH 8, 250 mM KCl, 10 mM MgCl2, 2 mM DTT, and 4 mM dATP, ± 500 nM Eta). Some of the reactions were incubated at 85 °C for 7 min, and then SCMBs were used to separate all reactions into pellet and supernatant fractions. Pellet and supernatant fractions were incubated with STOP buffer and extracted, and RNA transcripts were purified and analyzed as above.
Western Blot Analysis.
rEta was used as antigen to prepare polyclonal antibodies in rabbits at Cocalico Biologicals. Here 15 µg of midlog clarified cell lysate from T. kodakarensis strains TS559 and ΔTK0566 were resolved via SDS/PAGE, transferred to PVDF, and probed with primary anti-Eta antibodies followed by secondary anti-rabbit IgG-alkaline phosphatase conjugates (Promega) (Fig. S4C). The specified amounts of purified Eta or extracts from lysed strain TS559 T. kodakarensis are listed in Fig. S4D. These proteins were resolved via SDS/PAGE, transferred to PVDF, and probed with primary anti-Eta antibodies followed by secondary anti-rabbit IgG-HRP conjugate (Invitrogen).
Isolation of His6-Eta Containing Complexes and MS Identification of Binding Partners.
T. kodakarensis cells were harvested by centrifugation from 5-L cultures grown to mid-exponential phase at 85 °C in ASW-YT medium supplemented with 5 g of sodium pyruvate/L. The cells were resuspended in 30 mL of Buffer A (25 mM Tris⋅HCl pH 8, 500 mM NaCl, 10 mM imidazole, and 10% glycerol) and lysed by sonication. Clarified lysate was loaded onto a 1-mL HiTRAP chelating column (GE Healthcare) preequilibrated with NiSO4. The column was washed with Buffer A, and proteins were eluted using a linear imidazole gradient from Buffer A to 60% Buffer B (25 mM Tris⋅HCl pH 8, 100 mM NaCl, 150 mM imidazole, and 10% glycerol). Fractions that contained the tagged protein were identified by Western blot analysis, pooled, and dialyzed against Buffer C (10 mM Tris⋅HCl pH 8). The pooled samples were lyophilized and resuspended in 10 mM Tris⋅HCl pH 8.
The proteins were identified by 1D SDS/PAGE fractionation followed by LC/MSMS at the Ohio State University MS facility. A MASCOT score of >100 was considered meaningful. To obtain such a score, a minimum of two unique peptide fragments usually had to be identified from the same protein. Protein isolation and MS analyses of lysates from T. kodakarensis TS559 were also undertaken. From these controls, several T. kodakarensis proteins that bound and eluted from the Ni2+-charged matrix in the absence of a His6-tagged protein were identified. The proteins identified in the Eta sample that had a MASCOT score >100 and were not also present in the control samples are listed in Table S1.
In Vitro RNAP-Eta Binding Study.
Here 8 µg each of purified RNAP, recombinant Eta, or RNAP and Eta were resuspended in Buffer A (25 mM Tris⋅HCl pH 8, 250 mM NaCl, and 10% glycerol) to a final volume of 50 µL. The proteins were incubated at 85 °C for 10 min and loaded to Ni-NTA magnetic beads (Qiagen). Samples were washed three times with 250 µL of Buffer A and then eluted three times with 50 µL Buffer B (25 mM Tris⋅HCl pH 8, 100 mM NaCl, 10% glycerol, and 500 mM imidiazole). Samples were resolved on a 4–20% SDS/PAGE (Bio-Rad).
Discussion
Processive transcription requires extremely stable TECs (53). Biochemical and structural studies demonstrate that the overall stability of the TECs is composite, with inputs from RNAP–DNA, DNA–RNA, and RNAP–RNA interactions collectively stabilizing the TECs (10). DNA sequences, the encoded RNA sequences, and structures that form within or adjacent to RNAP can disrupt these contacts and destabilize the TECs, driving transcription termination (54). Intrinsic termination sequences often suffice to separate independent genes and operons by blocking continued downstream transcription, but not all termination events can be initiated via DNA sequences alone. Scenarios arise in all life where the stable TECs may halt transcription at any position, most likely in response to protein roadblocks or DNA damage, and these arrested TECs must be removed to maintain genome stability (55). In bacteria and archaea, transcription and translation are normally coupled (56, 57), and the uncoupling of these apparatuses offers regulatory potential that is exploited by factors (e.g., rho in bacteria), that disrupt the TECs and thus limit downstream expression.
We have demonstrated that factor-dependent transcription termination occurs in archaea and have characterized a bona fide archaeal transcription termination factor in vivo and in vitro. We purified this biochemical activity directly from cell lysates and then demonstrated that a single protein, Eta, drives TEC disassembly and release of RNA to solution. Our results confirm that factor-dependent transcription termination is conserved in all life, and that the factors capable of disrupting the TECs are all energy-dependent (4, 47, 58–60).
Factor-mediated disruption of the TECs in bacteria and eukarya is discriminatory to ensure that functional TECs generally are not terminated prematurely. Eta mediates transcript release from stalled or slow elongating TECs, but does so slowly. Eta-mediated termination is not competitive with standard RNAP elongation rates, and thus functional archaeal TECs are unlikely to be targeted for disruption. The rate of Eta-mediated termination is not supportive of Eta directing the 3′-end formation of many genes or operons, or of Eta governing polarity; direct assays of polarity have confirmed this.
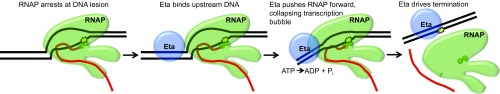
What then is the biological role of Eta? Eta likely targets RNAPs that are translocationally blocked at sites of DNA damage or are arrested owing to chromatin or other protein roadblocks. In support of such a role, deletion of TK0566 (encoding Eta) or introduction of mutations that encode inactive variants of Eta is possible, resulting in strains with slow growth and DNA-damage–sensitive phenotypes. Our results suggest that Eta follows a similar mechanism of termination as Mfd (Fig. 6) (47, 48). Eta likely binds to the DNA upstream of stalled TECs and, through ATP hydrolysis, translocates along the DNA, pushing RNAP forward. In the absence of continued synthesis, RNAP is hypertranslocated and/or the transcription bubble collapses, resulting in disassociation of the TEC and transcription termination. In addition, the increased sensitivity to DNA damage of strains lacking Eta is suggestive of a potential role for Eta in the recognition and removal of TECs stalled at DNA lesions. The presence of TCR in T. kodakarensis and any role for Eta in archaeal transcription-coupled DNA repair remains to be determined.
Fig. 6.
Model of Eta-mediated transcription termination. Eta recognizes RNAP arrested at the site of a DNA lesion (yellow). Eta binds to the upstream DNA and uses ATP hydrolysis to push RNAP forward, causing transcription bubble collapse and promoting transcription termination.
The conservation of Eta in most archaeal lineages (38, 39) argues that factor-mediated termination is commonplace in archaeal regulatory strategies. Continued probing of the mechanism of Eta-mediated transcription termination should provide insight into shared aspects of TEC stability and highlight susceptibilities of the TECs that can be exploited for regulatory control.
Materials and Methods
Strains, Plasmids, and Oligonucleotides.
Strains and plasmids are listed in Table S2, and oligonucleotides are listed in Table S4. All T. kodakarensis strains were constructed as described previously (45).
Table S4.
Oligonucleotides used in this study
| Name | Sequence | Application |
| T7-big_F | [biotin]-CGCGCGTAATACGACTCA CTATAGGG | Amplify DNA templates pTS326 and pJW21 |
| T3-big_R | CCCTTTAGTGAGGGTTAATTGCGG | Amplify DNA templates pTS326 and pJW21 |
| T3-big80_R | GGTCCAACGGCGACGAAC | Amplify DNA template pJW21 with downstream DNA of +80 (Fig. S1) |
| 016-TK0566B_F | AGCGAAAACTTCCCTTCGAGGCGGTGAAAGCTAACGCTCAGTCTCCCAGAGCCAGAGGTT | Site-directed mutagenesis for construction of pCSU-TK0566B |
| 017-TK0566B_R | AACCTCTGGCTCTGGGAGACTGAGCGTTAGCTTTCACCGCCTCGAAGGGAAGTTTTCGCT | Site-directed mutagenesis for construction of pCSU-TK0566B |
| TK0566D344A/E345A_F | GGAACCATAGTGATAGCCGCCATACACACGCTCGAC | Site-directed mutagenesis for construction of pJW26 and pCSU-TK0566D344A/E345A |
| TK0566D344A/E345A_R | GTCGAGCGTGTGTATGGCGGCTATCACTATGGTTCC | Site-directed mutagenesis for construction of pJW26 and pCSU-TK0566D344A/E345A |
| TK0566Δ1–193_F | AATTCATTAAAGAGGAGAAATTAAACTATGATCAAGGTGGACGAGCTTCCAGTCCCCGAG | Site-directed mutagenesis for construction of pJW28 |
| TK0566Δ1–193_R | CTCGGGGACTGGAAGCTCGTCCACCTTGATCATAGTTTAATTTCTCCTCTTTAATGAATT | Site-directed mutagenesis for construction of pJW28 |
| 1 | GTAGCGGATGAGGAGGTACTGAAGG | PCR diagnostics to confirm ∆TK0566 strain construction (Fig. 5B) |
| 2 | CCTTGATAAAGTCCTCTACGCCTTCGACCC | PCR diagnostics to confirm ∆TK0566 strain construction (Fig. 5B) |
| 3 | TACGCCCTCAGACTTCAGAACTTCC | PCR diagnostics to confirm ∆TK0566 strain construction (Fig. 5B) |
| 4 | TTAGCGTATCATCCTCCCTTCTTCTATCTCCCTCTTCAGTATCTTCGCC | PCR diagnostics to confirm ∆TK0566 strain construction (Fig. 5B) |
| 5 | ACTGATGAGGGAGATAAAGATAACC | PCR diagnostics to confirm ∆TK0566 strain construction (Fig. 5B) |
| 6 | ATAGACTTCGCCAAGCTCATATACC | PCR diagnostics to confirm ∆TK0566 strain construction (Fig. 5B) |
Protein Purifications.
RNAP, TBP, and TFB were purified as described previously (36). rEta, rEtaD344A/E345A, and rEta∆1–193 were purified from Rosetta2 (DE3) cells carrying pQE-80L-Eta, pQE-80L-EtaD344A/E345A, and pQE-80L-Eta∆1–193, respectively, as described in SI Materials and Methods.
In Vitro Transcription.
Assembly of preinitiation complexes and elongation via NTP deprivation were generally carried out as described previously (36). TEC+376 were captured with streptavidin-coated magnetic beads (SCMBs) and washed three times in wash buffer (WB) (20 mM Tris⋅HCl pH 8.0, 0.1 mM EDTA, 250 mM KCl, 4 mM MgCl2, and 20 μg/mL BSA). TEC+376 were resuspended in transcription buffer (TB) (250 mM KCl, 20 mM Tris⋅HCl pH 8.0, 10 mM MgCl2, and 2 mM DTT), then incubated at 85 °C ± 5 mM ATP in the presence or absence of partially purified lysates from T. kodakarensis. After 5 min, 200 µM rNTPs were added, and incubation at 85 °C was continued for 10 min. Then 5 volumes of 1.2× STOP buffer (0.6 M Tris⋅HCl pH 8.0 and 12 mM EDTA) containing 7 μg of tRNA (total) were added, the reaction was subjected to an equal volume phenol/chloroform/isoamyl alcohol (25:24:1, by vol) extraction, and radiolabeled RNA transcripts were precipitated from the aqueous phase with alcohol. Templates and RNA transcripts were separated on denaturing polyacrylamide gels, and radiolabeled RNA/DNA was detected using phosphorimaging (GE Healthcare) and analyzed using GE Imagequant 5.2.
TEC+58 were captured with Ni2+-coated magnetic beads (NCMBs), washed three times in WB, then resuspended in TB with 10 µM ATP, 10 µM GTP, and 10 µM UTP. Then 10-µL aliquots were combined with equal-volume reactions containing 15 mM Tris⋅HCl pH 8, 5 mM MgCl2, and 2 mM DTT, ± 4 mM energy source (dATP, AMP-PNP, or ADP) and ± 500 nM purified Eta or Eta variant. Reactions were incubated at 85 °C for 5 min, and then SCMBs were used to separate reactions into pellet and supernatant fractions. The pellet and supernatant fractions were incubated with STOP buffer and extracted, and RNA transcripts were purified as above. Release was calculated by quantifying transcripts in the S divided by transcripts quantified in the pellet and supernatant.
Washed TECs+58 were generated as above, then 10-µL aliquots were combined with equal-volume reactions containing 15 mM Tris⋅HCl pH 8, 5 mM MgCl2, 2 mM DTT, and 4 mM dATP, ± 500 nM Eta. Reactions in lanes 1 and 2 and lanes 13 and 14 were supplemented with 10 µM each of UTP, GTP, and ATP (indicated by 0*) (Fig. 3C). The concentrations of NTPs that allowed elongation to +128 during 7 min of incubation at 85 °C are listed in the figure. SCMBs were used to separate reactions into pellet and supernatant fractions.
Washed TECs+58 were generated on 32P-labeled, biotinylated templates as above, then TEC+58 were resuspended in digestion buffer (1× NEB Buffer 2.1 ± 8 U Ssp1-HF) and incubated at 37 °C for 30 min. SCMBs were used to separate reactions into pellet and supernatant fractions, and the pellet fraction was discarded. TEC+58 retained in the supernatant were captured with NCMBs and washed twice in WB then resuspended in TB with 10 µM ATP, 10 µM GTP, and 10 µM UTP, and 4 mM dATP ± 500 nM Eta. Reactions were incubated at 85 °C for 7 min, followed by the addition of 200 µM rNTPs, and incubation was extended at 85 °C for 5 min. Then 5 volumes of 1.2× STOP buffer containing 7 μg tRNA (total) were added, the reactions were extracted, and RNA transcripts purified and analyzed as above.
Polarity Assay.
Plasmids and strains (Table S2) generated for the polarity assay were constructed and β-glycosidase activity was measured as described previously, except that the parental strains were TS559 or ΔTK0566 (32). Percent activity was calculated by comparing activities of identical strains (TS559 or ΔTK0566) with and without stop codons in PF1848.
DNA Damage Assays.
UV irradiation assays were carried out as described previously (61). For 4NQO sensitivity assays, T. kodakarensis strains TS559 (parental) and ΔTK0566 were grown to an OD600 nm = 0.6 in ASW-YT-pyruvate media containing agmatine (45). ∼1 × 108 cells were anaerobically harvested, collected via centrifugation, and resuspended in 100 µL of 0.8× ASW. Cells were serially diluted with 0.8× ASW and spotted (in duplicate) onto ASW-YT plates lacking or containing 10 µM 4NQO. Plates were incubated at 85 °C for 18 h, after which the cells were transferred to a PVDF membrane, followed by staining with Coomassie Brilliant Blue.
Acknowledgments
We thank members of the T.J.S. laboratory. This work is supported by National Institutes of Health Grant GM100329 (to T.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704028114/-/DCSupplemental.
References
- 1.Li W, Giles C, Li S. Insights into how Spt5 functions in transcription elongation and repressing transcription coupled DNA repair. Nucleic Acids Res. 2014;42:7069–7083. doi: 10.1093/nar/gku333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirtreiter A, et al. Spt4/5 stimulates transcription elongation through the RNA polymerase clamp coiled-coil motif. Nucleic Acids Res. 2010;38:4040–4051. doi: 10.1093/nar/gkq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Y, Liu M, Spencer CA, Price DH. Involvement of transcription termination factor 2 in mitotic repression of transcription elongation. Mol Cell. 2004;14:375–385. doi: 10.1016/s1097-2765(04)00234-5. [DOI] [PubMed] [Google Scholar]
- 4.Fong N, et al. Effects of transcription elongation rate and Xrn2 exonuclease activity on RNA polymerase II termination suggest widespread kinetic competition. Mol Cell. 2015;60:256–267. doi: 10.1016/j.molcel.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artsimovitch I, Landick R. Pausing by bacterial RNA polymerase is mediated by mechanistically distinct classes of signals. Proc Natl Acad Sci USA. 2000;97:7090–7095. doi: 10.1073/pnas.97.13.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landick R. The regulatory roles and mechanism of transcriptional pausing. Biochem Soc Trans. 2006;34:1062–1066. doi: 10.1042/BST0341062. [DOI] [PubMed] [Google Scholar]
- 7.Hein PP, et al. RNA polymerase pausing and nascent-RNA structure formation are linked through clamp-domain movement. Nat Struct Mol Biol. 2014;21:794–802. doi: 10.1038/nsmb.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yakhnin AV, Murakami KS, Babitzke P. NusG is a sequence-specific RNA polymerase pause factor that binds to the non-template DNA within the paused transcription bubble. J Biol Chem. 2016;291:5299–5308. doi: 10.1074/jbc.M115.704189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulmage KA, Todor H, Schmid AK. Growth-phase-specific modulation of cell morphology and gene expression by an archaeal histone protein. MBio. 2015;6:e00649-15. doi: 10.1128/mBio.00649-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tadigotla VR, et al. Thermodynamic and kinetic modeling of transcriptional pausing. Proc Natl Acad Sci USA. 2006;103:4439–4444. doi: 10.1073/pnas.0600508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee TI, et al. Transcriptional regulatory networks in Saccharomyces cerevisiae. Science. 2002;298:799–804. doi: 10.1126/science.1075090. [DOI] [PubMed] [Google Scholar]
- 12.Ouhammouch M, Werner F, Weinzierl RO, Geiduschek EP. A fully recombinant system for activator-dependent archaeal transcription. J Biol Chem. 2004;279:51719–51721. doi: 10.1074/jbc.C400446200. [DOI] [PubMed] [Google Scholar]
- 13.Pritchett MA, Wilkinson SP, Geiduschek EP, Ouhammouch M. Hybrid Ptr2-like activators of archaeal transcription. Mol Microbiol. 2009;74:582–593. doi: 10.1111/j.1365-2958.2009.06884.x. [DOI] [PubMed] [Google Scholar]
- 14.Blombach F, et al. Archaeal TFEα/β is a hybrid of TFIIE and the RNA polymerase III subcomplex hRPC62/39. eLife. 2015;4:e08378. doi: 10.7554/eLife.08378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langer D, Hain J, Thuriaux P, Zillig W. Transcription in archaea: Similarity to that in eucarya. Proc Natl Acad Sci USA. 1995;92:5768–5772. doi: 10.1073/pnas.92.13.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Rucobo FW, Sainsbury S, Cheung ACM, Cramer P. Architecture of the RNA polymerase-Spt4/5 complex and basis of universal transcription processivity. EMBO J. 2011;30:1302–1310. doi: 10.1038/emboj.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gehring AM, Walker JE, Santangelo TJ. Transcription regulation in Archaea. J Bacteriol. 2016;198:1906–1917. doi: 10.1128/JB.00255-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker JE, Santangelo TJ. Analyses of in vivo interactions between transcription factors and the archaeal RNA polymerase. Methods. 2015;86:73–79. doi: 10.1016/j.ymeth.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J-S, Roberts JW. Role of DNA bubble rewinding in enzymatic transcription termination. Proc Natl Acad Sci USA. 2006;103:4870–4875. doi: 10.1073/pnas.0600145103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cambray G, et al. Measurement and modeling of intrinsic transcription terminators. Nucleic Acids Res. 2013;41:5139–5148. doi: 10.1093/nar/gkt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epshtein V, Dutta D, Wade J, Nudler E. An allosteric mechanism of Rho-dependent transcription termination. Nature. 2010;463:245–249. doi: 10.1038/nature08669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santangelo TJ, Reeve JN. Archaeal RNA polymerase is sensitive to intrinsic termination directed by transcribed and remote sequences. J Mol Biol. 2006;355:196–210. doi: 10.1016/j.jmb.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 23.Arimbasseri AG, Rijal K, Maraia RJ. Transcription termination by the eukaryotic RNA polymerase III. Biochim Biophys Acta. 2013;1829:318–330. doi: 10.1016/j.bbagrm.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kireeva ML, Komissarova N, Waugh DS, Kashlev M. The 8-nucleotide-long RNA:DNA hybrid is a primary stability determinant of the RNA polymerase II elongation complex. J Biol Chem. 2000;275:6530–6536. doi: 10.1074/jbc.275.9.6530. [DOI] [PubMed] [Google Scholar]
- 25.Komissarova N, Becker J, Solter S, Kireeva M, Kashlev M. Shortening of RNA:DNA hybrid in the elongation complex of RNA polymerase is a prerequisite for transcription termination. Mol Cell. 2002;10:1151–1162. doi: 10.1016/s1097-2765(02)00738-4. [DOI] [PubMed] [Google Scholar]
- 26.Dar D, Prasse D, Schmitz RA, Sorek R. Widespread formation of alternative 3′ UTR isoforms via transcription termination in archaea. Nat Microbiol. 2016;1:16143. doi: 10.1038/nmicrobiol.2016.143. [DOI] [PubMed] [Google Scholar]
- 27.Guo J, Turek ME, Price DH. Regulation of RNA polymerase II termination by phosphorylation of Gdown1. J Biol Chem. 2014;289:12657–12665. doi: 10.1074/jbc.M113.537662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santangelo TJ, Cubonová L, Skinner KM, Reeve JN. Archaeal intrinsic transcription termination in vivo. J Bacteriol. 2009;191:7102–7108. doi: 10.1128/JB.00982-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf YI, Rogozin IB, Kondrashov AS, Koonin EV. Genome alignment, evolution of prokaryotic genome organization, and prediction of gene function using genomic context. Genome Res. 2001;11:356–372. doi: 10.1101/gr.gr-1619r. [DOI] [PubMed] [Google Scholar]
- 30.Ermolaeva MD, White O, Salzberg SL. Prediction of operons in microbial genomes. Nucleic Acids Res. 2001;29:1216–1221. doi: 10.1093/nar/29.5.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price MN, Huang KH, Alm EJ, Arkin AP. A novel method for accurate operon predictions in all sequenced prokaryotes. Nucleic Acids Res. 2005;33:880–892. doi: 10.1093/nar/gki232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santangelo TJ, et al. Polarity in archaeal operon transcription in Thermococcus kodakaraensis. J Bacteriol. 2008;190:2244–2248. doi: 10.1128/JB.01811-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stantial N, Dumpe J, Pietrosimone K, Baltazar F, Crowley DJ. Transcription-coupled repair of UV damage in the halophilic archaea. DNA Repair (Amst) 2016;41:63–68. doi: 10.1016/j.dnarep.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Roberts JW. Termination factor for RNA synthesis. Nature. 1969;224:1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- 35.Selby CP, Witkin EM, Sancar A. Escherichia coli mfd mutant deficient in “mutation frequency decline” lacks strand-specific repair: In vitro complementation with purified coupling factor. Proc Natl Acad Sci USA. 1991;88:11574–11578. doi: 10.1073/pnas.88.24.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gehring AM, Santangelo TJ. Manipulating archaeal systems to permit analyses of transcription elongation-termination decisions in vitro. Methods Mol Biol. 2015;1276:263–279. doi: 10.1007/978-1-4939-2392-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakakibara N, Kelman LM, Kelman Z. Unwinding the structure and function of the archaeal MCM helicase. Mol Microbiol. 2009;72:286–296. doi: 10.1111/j.1365-2958.2009.06663.x. [DOI] [PubMed] [Google Scholar]
- 38.Fujiwara A, et al. Application of a Euryarchaeota-specific helicase from Thermococcus kodakarensis for noise reduction in PCR. Appl Environ Microbiol. 2016;82:3022–3031. doi: 10.1128/AEM.04116-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamieh H, Ibrahim H, Kozah J. Genome-wide identification of SF1 and SF2 helicases from archaea. Gene. 2016;576:214–228. doi: 10.1016/j.gene.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Atomi H, Fukui T, Kanai T, Morikawa M, Imanaka T. Description of Thermococcus kodakaraensis sp. nov., a well-studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea. 2004;1:263–267. doi: 10.1155/2004/204953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santangelo TJ, Cubonová L, James CL, Reeve JN. TFB1 or TFB2 is sufficient for Thermococcus kodakaraensis viability and for basal transcription in vitro. J Mol Biol. 2007;367:344–357. doi: 10.1016/j.jmb.2006.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Santangelo TJ, Cuboňová L, Reeve JN, Kelman Z. Affinity purification of an archaeal DNA replication protein network. MBio. 2010;1:e00221-10. doi: 10.1128/mBio.00221-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hileman TH, Santangelo TJ. Genetics techniques for Thermococcus kodakarensis. Front Microbiol. 2012;3:195. doi: 10.3389/fmicb.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato T, Fukui T, Atomi H, Imanaka T. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J Bacteriol. 2003;185:210–220. doi: 10.1128/JB.185.1.210-220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J-S, Marr MT, Roberts JW. E. coli transcription repair coupling factor (Mfd protein) rescues arrested complexes by promoting forward translocation. Cell. 2002;109:757–767. doi: 10.1016/s0092-8674(02)00769-9. [DOI] [PubMed] [Google Scholar]
- 48.Roberts J, Park J-S. Mfd, the bacterial transcription repair coupling factor: Translocation, repair and termination. Curr Opin Microbiol. 2004;7:120–125. doi: 10.1016/j.mib.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Chambers AL, Smith AJ, Savery NJ. A DNA translocation motif in the bacterial transcription-repair coupling factor, Mfd. Nucleic Acids Res. 2003;31:6409–6418. doi: 10.1093/nar/gkg868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen SE, et al. Roles for the transcription elongation factor NusA in both DNA repair and damage tolerance pathways in Escherichia coli. Proc Natl Acad Sci USA. 2010;107:15517–15522. doi: 10.1073/pnas.1005203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darrigo C, Guillemet E, Dervyn R, Ramarao N. The bacterial Mfd protein prevents DNA damage induced by the host nitrogen immune response in a NER-independent but RecBC-dependent pathway. PLoS One. 2016;11:e0163321. doi: 10.1371/journal.pone.0163321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gehring AM, Santangelo TJ. 2017. Archaeal RNA polymerase arrests transcription at DNA lesions. Transcription0.
- 53.Santangelo TJ, Artsimovitch I. Termination and antitermination: RNA polymerase runs a stop sign. Nat Rev Microbiol. 2011;9:319–329. doi: 10.1038/nrmicro2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ray-Soni A, Bellecourt MJ, Landick R. Mechanisms of bacterial transcription termination: All good things must end. Annu Rev Biochem. 2016;85:319–347. doi: 10.1146/annurev-biochem-060815-014844. [DOI] [PubMed] [Google Scholar]
- 55.Hanawalt PC, Spivak G. Transcription-coupled DNA repair: Two decades of progress and surprises. Nat Rev Mol Cell Biol. 2008;9:958–970. doi: 10.1038/nrm2549. [DOI] [PubMed] [Google Scholar]
- 56.French SL, Santangelo TJ, Beyer AL, Reeve JN. Transcription and translation are coupled in Archaea. Mol Biol Evol. 2007;24:893–895. doi: 10.1093/molbev/msm007. [DOI] [PubMed] [Google Scholar]
- 57.Adhya S, Gottesman M, De Crombrugghe B. Release of polarity in Escherichia coli by gene N of phage lambda: Termination and antitermination of transcription. Proc Natl Acad Sci USA. 1974;71:2534–2538. doi: 10.1073/pnas.71.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proudfoot NJ. Transcriptional termination in mammals: Stopping the RNA polymerase II juggernaut. Science. 2016;352:aad9926. doi: 10.1126/science.aad9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Heygère F, Schwartz A, Coste F, Castaing B, Boudvillain M. ATP-dependent motor activity of the transcription termination factor Rho from Mycobacterium tuberculosis. Nucleic Acids Res. 2015;43:6099–6111. doi: 10.1093/nar/gkv505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park J, Kang M, Kim M. Unraveling the mechanistic features of RNA polymerase II termination by the 5′-3′ exoribonuclease Rat1. Nucleic Acids Res. 2015;43:2625–2637. doi: 10.1093/nar/gkv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuba Y, et al. Comparative analyses of the two proliferating cell nuclear antigens from the hyperthermophilic archaeon, Thermococcus kodakarensis. Genes Cells. 2012;17:923–937. doi: 10.1111/gtc.12007. [DOI] [PubMed] [Google Scholar]
- 62.Hirata A, et al. Archaeal RNA polymerase subunits E and F are not required for transcription in vitro, but a Thermococcus kodakarensis mutant lacking subunit F is temperature-sensitive. Mol Microbiol. 2008;70:623–633. doi: 10.1111/j.1365-2958.2008.06430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grohmann D, Hirtreiter A, Werner F. RNAP subunits F/E (RPB4/7) are stably associated with archaeal RNA polymerase: Using fluorescence anisotropy to monitor RNAP assembly in vitro. Biochem J. 2009;421:339–343. doi: 10.1042/BJ20090782. [DOI] [PubMed] [Google Scholar]
- 64.Meka H, Werner F, Cordell SC, Onesti S, Brick P. Crystal structure and RNA binding of the Rpb4/Rpb7 subunits of human RNA polymerase II. Nucleic Acids Res. 2005;33:6435–6444. doi: 10.1093/nar/gki945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ujvári A, Luse DS. RNA emerging from the active site of RNA polymerase II interacts with the Rpb7 subunit. Nat Struct Mol Biol. 2006;13:49–54. doi: 10.1038/nsmb1026. [DOI] [PubMed] [Google Scholar]
- 66.Grohmann D, Hirtreiter A, Werner F. Molecular mechanisms of archaeal RNA polymerase. Biochem Soc Trans. 2009;37:12–17. doi: 10.1042/BST0370012. [DOI] [PubMed] [Google Scholar]
- 67.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]