Significance
Human and animals store fat in the form of multiple small intracellular structures, called lipid droplets, in brown adipocytes, and in a single large lipid droplet in white adipocytes. When environmental temperature decreases, white adipocytes can convert into brown adipocytes, in which fat is more accessible for burning and thermogenesis, and vice versa. The mechanism underlying the interconversion between multiple and single lipid droplets is not clear. Here, we report that a nuclear hormone signaling pathway regulates similar conversions in the nematode Caenorhabditis elegans, and that components of this pathway have counterparts in humans.
Keywords: nuclear receptor, thermosensitive lipid droplet fusion, CYP-37A1, EMB-8, DAF-12
Abstract
Nuclear receptors play important roles in regulating fat metabolism and energy production in humans. The regulatory functions and endogenous ligands of many nuclear receptors are still unidentified, however. Here, we report that CYP-37A1 (ortholog of human cytochrome P450 CYP4V2), EMB-8 (ortholog of human P450 oxidoreductase POR), and DAF-12 (homolog of human nuclear receptors VDR/LXR) constitute a hormone synthesis and nuclear receptor pathway in Caenorhabditis elegans. This pathway specifically regulates the thermosensitive fusion of fat-storing lipid droplets. CYP-37A1, together with EMB-8, synthesizes a lipophilic hormone not identical to Δ7-dafachronic acid, which represses the fusion-promoting function of DAF-12. CYP-37A1 also negatively regulates thermotolerance and lifespan at high temperature in a DAF-12–dependent manner. Human CYP4V2 can substitute for CYP-37A1 in C. elegans. This finding suggests the existence of a conserved CYP4V2-POR–nuclear receptor pathway that functions in converting multilocular lipid droplets to unilocular ones in human cells; misregulation of this pathway may lead to pathogenic fat storage.
A major challenge in the understanding of nuclear receptor signaling is to identify the specific ligands and physiological functions of orphan receptors at the molecular and subcellular level. In human, essentially all known endogenous steroid and nonsteroid hormones for nuclear receptors are synthesized by members of a large family of 50 microsomal cytochrome P450 monooxgenases, which require a common P450 oxidoreductase (POR) to carry out their catalysis. Many of these putative hormone-synthesizing enzymes are also of unknown function (1). Typically, the binding of hormone ligands to cognate nuclear receptors leads to both down-regulation of the expression of P450s and inactivation of the ligands, maintaining signaling homeostasis. For this reason, P450s are acclaimed as the “unsung heroes” of the P450–hormone–nuclear receptor signaling axis (2). Research on orphan P450s will likely lead to important discoveries of human nuclear receptor signaling and physiology.
The genome of the nematode Caenorhabditis elegans encodes 284 nuclear receptors, 82 P450s, and a single POR (EMB-8) (3–5), suggestive of a large repertoire of nuclear hormone receptor signaling. Among the 284 nuclear receptors, only one, DAF-12, has so far been linked to endogenous hormone ligands, the bile acid-like steroids Δ7 and Δ1,7-dafachronic acids (DAs) (6–8). DAs are synthesized by a P450 DAF-9 (i.e., CYP-22A1) with help from EMB-8/POR in addition to a Rieske-like oxygenase DAF-36 and a 3-hydroxysteroid dehydrogenase DHS-16 (9–11). When DAs are absent, DAF-12 recruits a transcriptional coregulator, DIN-1, to regulate genes that promote the formation of dauer, an alternative life form resistant to harsh environments. In contrast, DA-bound DAF-12 regulates the transcription of a different set of genes to promote reproductive growth and accelerate aging (12, 13). Thus, DAF-9–DAs–DAF-12–DIN-1 represents the only delineated P450–hormone–nuclear receptor pathway with clear physiological functions in C. elegans. This pathway is similar to the synthesis of bile acids that serve as ligands for farnesoid X receptor (FXR) in mammals (14).
Upon binding to DAs, DAF-12 also activates genes involved in fatty acid oxidation for energy production (15). However, it is not understood how DAF-12 signaling and metabolic flux converge on the subcellular fat-storing structures to regulate fat deposition and mobilization. In eukaryotes, fat is stored in a class of newly defined cell organelles called lipid droplets (LDs), whose morphological and metabolic status plays active roles in fat metabolism (16). Previously, we identified that the subcellular fat-storing structures of C. elegans are LDs (17, 18). By applying reliable and effective LD labeling methods to a systematic and saturated forward genetic screen, we isolated four classes of drop (lipid droplet abnormal) mutants that form supersized LDs in intestine cells (19). Here, we cloned two class III mutants, drop-1 and drop-8, and their suppressor mutant drop-31. We show that the three genes constitute a P450–nuclear receptor pathway.
Results
CYP-37A1 and EMB-8 Negatively Regulate Thermosensitive Supersized LD Formation.
When fed ad libitum, wild-type (WT) C. elegans produces triacylglycerol (TAG)-storing LDs with an upper size limit of 3 μm in diameter (17, 18). We previously isolated two class III supersized LD mutants, drop-1 and drop-8 (19), which upon a shift from 15/20 °C to 30 °C, quickly form supersized LDs ≥ 3 μm. We have now cloned drop-1 and drop-8 as cyp-37A1 and emb-8, respectively (Fig. S1 A and B). CYP-37A1 is orthologous to human CYP4V2; as mentioned above, EMB-8 is orthologous to POR.
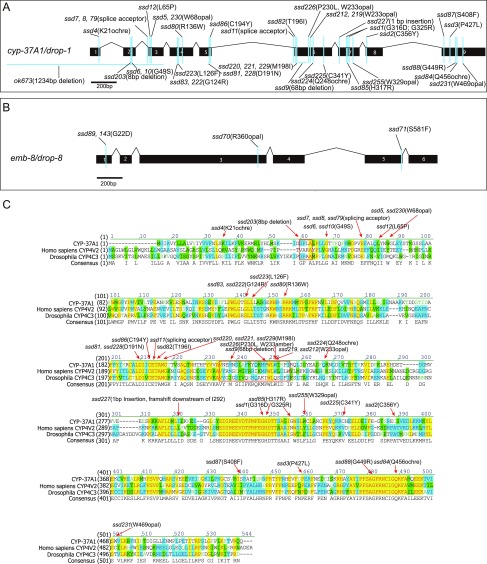
Fig. S1.
drop-1 and drop-8 are mutated in cyp-37A1 and emb-8. (A) The 38 alleles, in addition to ok673 of cyp-37A1/drop-1. (B) The four alleles of emb-8/drop-8. (C) CYP-37A1 is orthologous to human CYP4V2 and Drosophila CYP4C3. The three proteins’ sequences are aligned in Vector NTI (Invitrogen). Conserved amino acids are colored with identical residues in yellow. Mutant alleles are annotated and most missense mutations are after conserved amino acid residues.
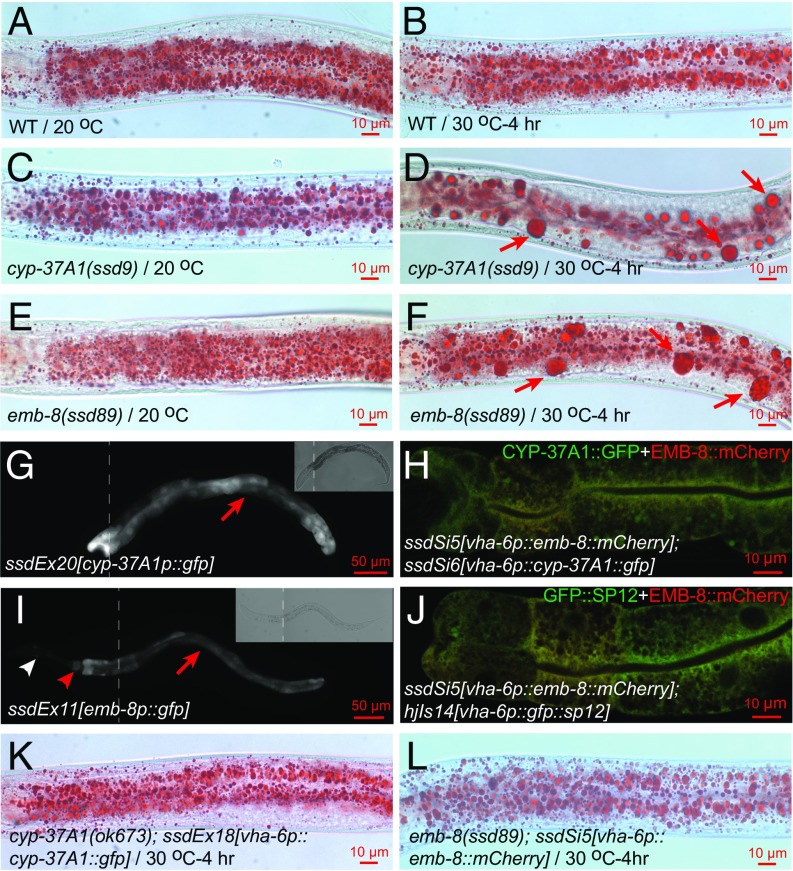
The 38 alleles of cyp-37A1 that we isolated include deletion, splicing, nonsense, and missense mutations (Fig. S1 A and C). Together with a 1,234-bp deletion allele, ok673, all alleles display a fully penetrant and strict temperature-sensitive supersized LD phenotype as revealed by postfix Oil Red O staining (Fig. 1 A–D), indicating null mutations. Only one emb-8 allele, ssd89(G22D), was previously isolated (19). ssd89(G22D) displays a fully penetrant supersized LD phenotype (Fig. 1 E and F). We have now used a noncomplementation screen strategy to isolate three additional alleles with a similar supersized LD phenotype (Fig. S1B). We constructed two transgenic lines, ssdEx20 and ssdEx21, in which a 1,259-bp cyp-37A1 promoter drives GFP reporter expression. The result shows that cyp-37A1 is specifically expressed in intestine cells (Fig. 1G). In another experiment, we constructed two transgenic lines, ssdEx17 and ssdEx18, in which a CYP-37A1::GFP fusion protein is driven by an intestine-specific promoter vha-6p. Both ssdEx17 and ssdEx18 rescued the supersized LD phenotype of cyp-37A1(ok673) (Fig. 1K). To investigate the subcellular localization of CYP-37A1 protein, we created a single-copy transgenic line ssdSi6 to express CYP-37A1::GFP driven by vha-6p using the MosSCI technology (20). The result shows that CYP-37A1::GFP localizes in a pattern characteristic of the endoplasmic reticulum (ER) (Fig. 1H). With similar experiments, we found that: emb-8 is expressed in the intestine, pharynx, and neurons surrounding the first pharyngeal bulb (Fig. 1I); transgenic EMB-8::mCherry protein colocalizes with CYP-37A1::GFP and an ER marker GFP::SP12; and EMB-8::mCherry rescues emb-8(ssd89) when expressed in the intestine (Fig. 1 H, J, and L).
Fig. 1.
Mutations of CYP-37A1 and EMB-8 cause supersized LD formation. (A and B) WT animals grown continuously at 20 °C to mid-L4 stage, or shifted from 20 °C to 30 °C for 4 h before reaching the mid-L4 stage, have no supersized LDs (≥3 μm in diameter). Images are of postfix Oil Red O staining. (C and D) When shifted to 30 °C for 4 h, cyp-37A1(ssd9) forms supersized LDs (arrows) and reduces the number of LDs smaller than 3 μm; (E and F) as in C and D, so does emb-8(ssd89). (G) cyp-37A1 (ssdEx20) is specifically expressed in intestine cells (arrow). (Inset) Bright field. (H) CYP-37A1::GFP protein (ssdSi6) colocalizes with EMB-8::mCherry (ssdSi5). (I) emb-8 (ssdEx11) is expressed in intestine (arrow), pharynx (red arrowhead), and neurons surrounding the first pharyngeal bulb (white arrowhead). (J) EMB-8::mCherry (ssdSi5) colocalizes with an ER marker GFP::SP12 (hjIs14). (K) Intestinal expression of CYP-37A1::GFP (ssdEx18) completely rescues cyp-37A1(ok673). (L) Intestinal expression of EMB-8::mCherry (ssdSi5) completely rescues emb-8(ssd89). For (G) and (I), GFP and bright field images are montages of two images (dashed lines indicate boundaries) covering the anterior and posterior parts of the same animal sample. For each Oil Red O experiment, more than 50 mid-L4–stage animals were stained and examined and at least 20 were imaged. Images are of the anterior half of the animal with head to the left. The same applies to other Oil Red O experiments, if not otherwise indicated.
CYP-37A1 and EMB-8 Mutations Derepress a Nonasymmetric and Thermosensitive LD Fusion Process.
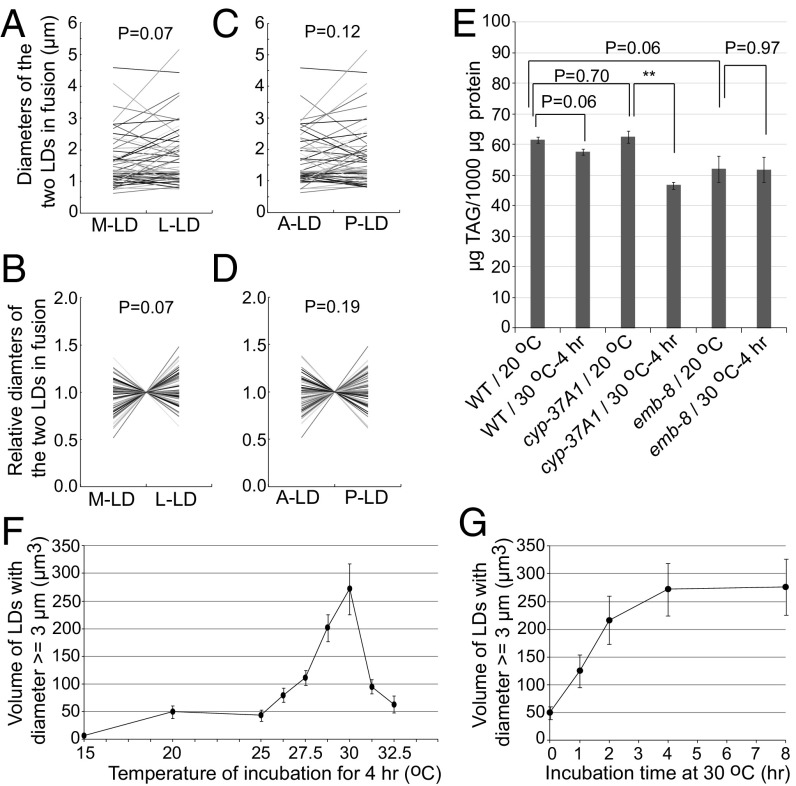
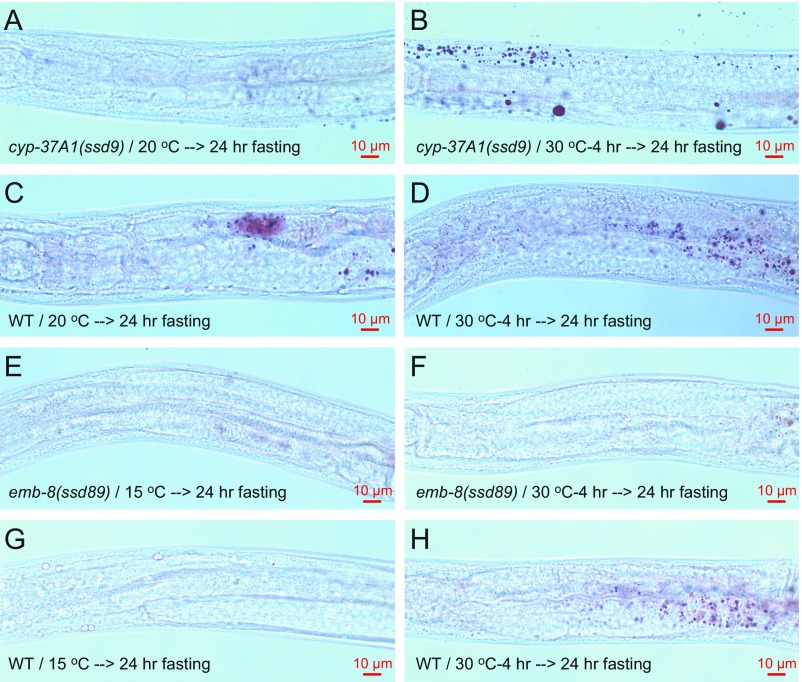
To test the mechanism of supersized LD formation, we time-lapse–imaged GFP::DGAT-2-labeled LDs in live cyp-37A1(ssd2) animals shifted to 30 °C. In 21 time-lapse movies, 57 LD fusion events but no single LD growth event was found. Similar to what was reported before (19), all fusion events happened in a time window shorter than 30 s and displayed no asymmetric partnership (Movie S1 and S2). The sizes of the two fusing LDs displayed no asymmetry either along the mediolateral or along the anteroposterior polarity axis of intestine cells (Fig. 2 A–D). To test whether the supersized LD phenotype of the mutants can be attributed to increased LD growth, we used lipid extraction and quantitative lipid analytical chemistry to measure the TAG levels. As shown by the results, at 20 °C, cyp-37A1(ssd9), emb-8(ssd89), and WT animals stored similar amounts of TAG. After being shifted to 30 °C for 4 h, emb-8(ssd89) had no increase in TAG, while cyp-37A1(ssd9) and WT had a modest decrease (Fig. 2E). Furthermore, fasting experiments showed that small and supersized LDs formed before and after temperature shift in the mutants are liable to hydrolysis, with no obvious difference to LDs in WT (Fig. S2), ruling out the possibility that the supersized LDs are a result of decreased LD hydrolysis.
Fig. 2.
Mutation of cyp-37A1 derepresses a nonasymmetric and thermosensitive LD fusion process. (A–D) The two fusing LDs are designated as medial (M) vs. lateral (L), or anterior (A) vs. posterior (P). The absolute diameters of the two LDs (A and C) and relative diameters normalized to the mean (B and D) are plotted as connecting lines. No significant size asymmetry is found between the two LDs. Paired two-sample t test. n = 57. (E) There is no significant difference of TAG level between WT and cyp-37A1(ssd9)/emb-8(ssd89), between before and after fusion for WT and emb-8(ssd89). There is a small but significant decrease after fusion for cyp-37A1(ssd9). Data are mean ± SEM. n = 3 for WT and cyp-37A1(ssd9); n = 7 for emb-8(ssd89). **P ≤ 0.01. Unpaired two-sample t test. (F and G) The volume of supersized LDs formed in the second intestine in cyp-37A1(ssd2); glo-4(ok623) is used to quantitate the fusion rate upon different temperature treatment or with different treatment time. Fusion starts at 25 °C, peaks at 30 °C, and declines beyond 30 °C (F). Fusion in cyp-37A1(ssd2); glo-4(ok623) increases over time and plateaus at 4 h, displaying a first-order kinetics (G). Data are mean ± SEM. n ≥ 10.
Fig. S2.
The fasting phenotype of cyp-37A1 and emb-8 mutants. (A and B) Small LDs before fusion at 20 °C and supersized LDs after fusion at 30 °C in cyp-37A1(ssd9) are liable to a 24 h fasting at 20 °C. (C and D) WT controls. (E and F) Small LDs before fusion at 15 °C and supersized LDs after fusion at 30 °C in emb-8(ssd89) are liable to a 24 h fasting at 20 °C. (G and H) WT controls.
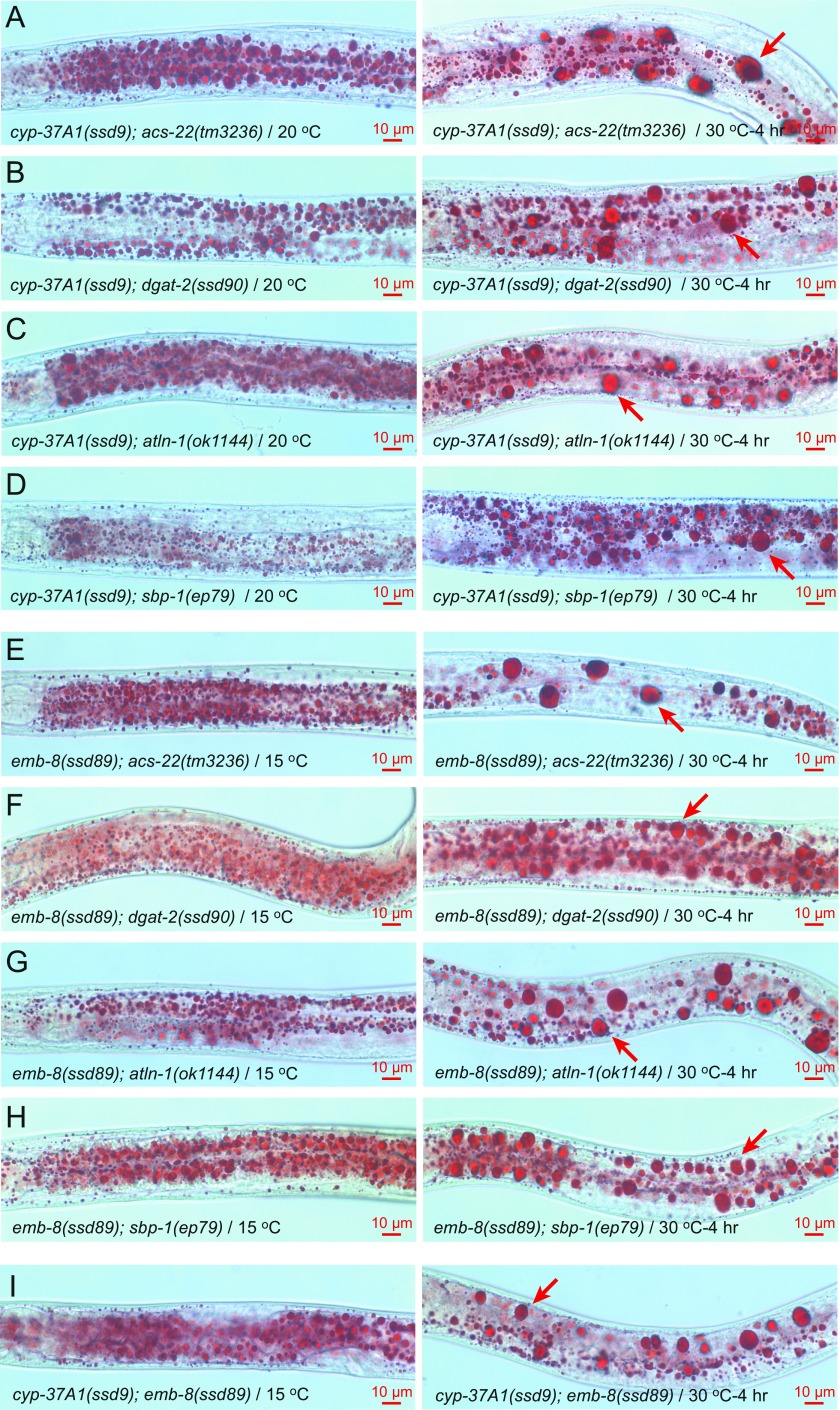
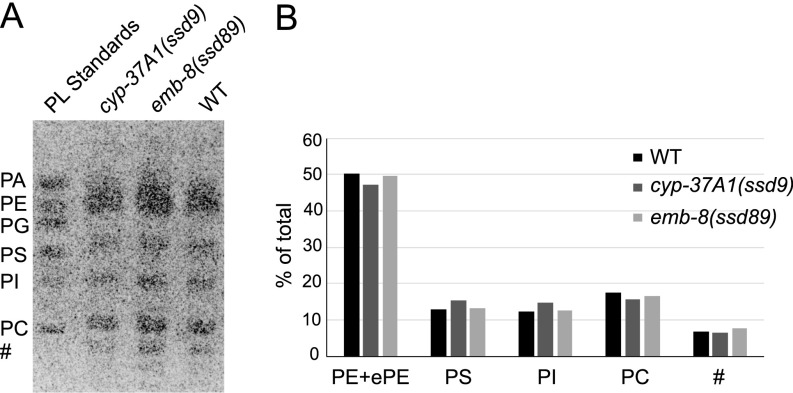
We used genetic analyses to test whether the supersized LD phenotype is dependent on TAG synthesis, lipogenesis, or other factors. As shown by postfix Oil Red O experiments on double mutants, the formation of supersized LDs of cyp-37A1(ssd9) and emb-8(ssd89) is not dependent on the LD growth-mediating ACS-22-DGAT-2 enzyme complex (17, 21), the lipogenic transcription factor SBP-1 (22), or the ER GTPase ATLN-1 (23) (Fig. S3 A–H). We also measured the phospholipid compositions of WT and mutants. The results showed that, compared with WT, cyp-37A1(ssd9) and emb-8(ssd89) do not show any obvious alteration in the relative amount of phosphatidylcholine or other phospholipids (Fig. S4).
Fig. S3.
Double mutant analysis of cyp-37A1 and emb-8. (A–D) The LD fusion phenotype of cyp-37A1(ssd9) is not abolished by acs-22(tm3236), dgat-2(ssd90), atln-1(ok1144), or sbp-1(ep79). (E–H) Same as A–D for emb-8(ssd89). (I) cyp-37A1(ssd9); emb-8(ssd89) is similar to each single mutant in LD fusion phenotype. Arrows point to supersized LDs.
Fig. S4.
No detectable difference of phospholipid composition between LD fusion mutants and wild type. (A) Total lipids of cyp-37A1(ssd9), emb-8(ssd89), and WT were separated along with mixed phospholipid standards on a TLC plate. The TLC plate was stained with Primuline and imaged under a UV imager. (B) The band intensity of each major phospholipid species was measured and plotted as percentage of the total phospholipids. No detectable difference of any major phospholipid species is found between WT and cyp-37A1(ssd9)/emb-8(ssd89). PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine; #, unidentified species. In the biological samples, no significant amount of PA was detected. An ether PE (ePE) species was not separated well from PE and was included in the PE data.
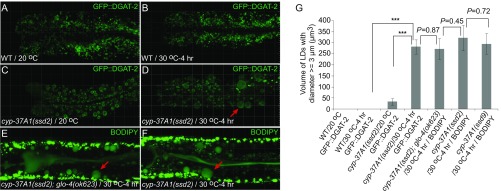
The null nature of cyp-37A1 alleles indicates that LD fusion is not a result of thermosensitive loss of CYP-37A1 enzyme function, but rather an up-regulation of a thermosensitive process. We used the LD marker GFP::DGAT-2, vital BODIPY staining, and vital BODIPY staining combined with a lysosome-related organelle-negative glo-4(ok623) mutant background to label LDs in WT and mutants (18, 19). We confocal-imaged these animals in three dimensions and quantified the volume of supersized LDs as an index of fusion process. The different labeling methods yielded the same result. In WT, no supersized LDs (diameters ≥ 3 μm) formed. In both cyp-37A1(ssd2) and cyp-37A1(ssd9) mutants, supersized LDs formed; the total volume of all supersized LDs formed in the two cells of the second intestine segment reaches ∼300 μm3 (Fig. S5). This volume can be approximated as 10 fusion events of LDs 3 μm in diameter in each cell, certainly an underestimation since small LDs (diameters < 3 μm) also fuse (Fig. 2A). We then used confocal imaging to quantify temperature dependence and reaction time course of the fusion process. As shown by the LD volume data, the fusion is temperature-dependent; it is first evident at 25 °C, peaks at 30 °C, and decreases beyond 30 °C (Fig. 2F). The fusion process also exhibits first order kinetics: the number of fusion events increases over time and plateaus at 4 h (Fig. 2G), when most small LDs are consumed (Fig. 1 D and F and Fig. S5 D–F).
Fig. S5.
Confocal imaging and quantification of supersized LDs formed in cyp-37A1 mutants. (A–F) LDs in WT and cyp-37A1(ssd2) were labeled with GFP::DGAT-2, BODIPY, or with BODIPY in a glo-4(ok623) background. Upon a shift to 30 °C for 4 h, supersized LDs (arrows) formed through fusion only in cyp-37A1 mutants. (Grid, 10 μm.) (G) The total volume of supersized LDs in the second intestine of cyp-37A1 mutant reaches ∼300 μm3 after fusion, with no significant difference between labeling methods or between cyp-37A1 alleles. n ≥ 10. ***P < 0.001, unpaired two-sample t test.
All of these results demonstrate that cyp-37A1 and emb-8 mutants form supersized LDs not through LD growth or lipogenesis but through a direct, thermosensitive, fast, and nonasymmetrical fusion process. The function of WT CYP-37A1 and EMB-8 is to repress this thermosensitive LD fusion process.
CYP-37A1 Is a Functional Ortholog of Human CYP4V2 and Represses Thermosensitive LD Fusion Without Affecting the Bulk Fatty Acid Composition.
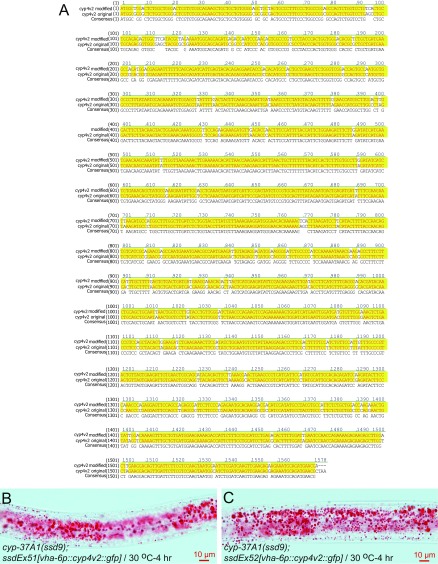
The LD fusion repression function must lie in the specificity of CYP-37A1, which should function at the same biochemical and genetic level as EMB-8, the general electron donor for all microsomal P450s. Consistent with this notion, the double-mutant cyp-37A1(ssd9); emb-8(ssd89) has a similar phenotype to each single mutant (Fig. S3I). At the protein sequence level, C. elegans CYP-37A1 is orthologus to human CYP4V2 and most of the missense mutations of CYP-37A1 are after amino acid residues that are conserved in CYP4V2 (Fig. S1C). We synthesized a synonymous CYP4V2 cDNA optimized for C. elegans codon use (24), and made two transgenic lines, ssdEx51 and ssdEx52, that express CYP4V2::GFP protein in intestine (Fig. S6A). In both lines, human CYP4V2 rescues the LD fusion phenotype of cyp-37A1(ssd9) (Fig. S6 B and C). Thus, CYP-37A1 and CYP4V2 are functional orthologs.
Fig. S6.
The human CYP4V2 rescues C. elegans cyp-37A1 mutant. (A) WT CYP4V2 cDNA sequence was custom-synthesized with modifications (cyp4v2 modified) to optimize codon use in C. elegans. The cDNA was introduced into transgenic lines to express CYP4V2::GFP fusion protein under the intestine-specific promoter vha-6p. (B and C) Two such transgenic lines, ssdEx51 and ssdEx52, rescue the LD fusion phenotype of cyp-37A1(ssd9).
CYP4V2 mutation is a risk factor for human Biette crystalline corneoretinal dystrophy (BCD), a chronic, severe eye disease that is marked by the pathological formation of glistening lipid inclusions with an unknown etiology in retinal pigment epithelium cells (RPEs) (25, 26). CYP4V2 ω-hydroxylates fatty acids in vitro and in a heterologous cell system and is thought to degrade bulk fatty acids in RPEs (27–30). Thus, to test the possibility that CYP-37A1 mutants affect the fatty acid composition in C. elegans, we used lipid analytical chemistry to quantitate fatty acid compositions of WT and the LD fusion mutants cyp-37A1(ssd9) and emb-8(ssd89). WT and cyp-37A1(ssd9) grown in parallel under the same food condition were not obviously different in TAG fatty acid composition, before or after LD fusion (Table S1). Changing the growth time of the OP50 Escherichia coli food on nematode growth medium (NGM) affects the fatty acid compositions of OP50, and then of C. elegans. This change does not affect the LD fusion phenotype of cyp-37A1(ssd9) and emb-8(ssd89). On such a different batch of food, WT and emb-8(ssd89) grown in parallel were both different to WT grown on the other batch of food in TAG fatty acid composition; but WT and emb-8(ssd89) were not different from each other (Table S1). Finally, when grown under the same food condition in parallel, WT, cyp-37A1(ssd9), and emb-8(ssd89) showed no consistent difference in total fatty acid composition (Table S1). Thus, we conclude that the role of CYP-37A1 and EMB-8 is not for the degradation of bulk fatty acids.
Table S1.
TAG fatty acid compositions and total fatty acid compositions
| Fatty acid | WT* 20 °C, % | WT* 30 °C, % | cyp-37A1* 20 °C, % | cyp-37A1* 30 °C, % | WT† 20 °C, % | WT† 30 °C, % | emb-8† 20 °C, % | emb-8† 30 °C, % | WT‡ 20 °C, % | WT‡ 30 °C %, | cyp-37A1‡ 20 °C, % | cyp-37A1‡ 30 °C, % | emb-8‡ 20 °C, % | emb-8‡ 30 °C, % |
| C14:0 | 1.21 | 1.12 | 0.95 | 0.88 | 2.55 | 2.71 | 3.32 | 3.63 | 0.76 | 0.73 | 0.68 | 0.56 | 0.75 | 0.59 |
| C15ISO | 6.24 | 5.18 | 4.48 | 4.30 | 6.47 | 5.81 | 5.26 | 4.96 | 3.04 | 2.56 | 2.73 | 2.78 | 2.81 | 2.77 |
| C16:0 | 4.14 | 4.42 | 4.32 | 3.98 | 5.76 | 7.17 | 7.14 | 6.20 | 3.74 | 3.48 | 3.72 | 2.75 | 3.52 | 3.00 |
| C16:1n7 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.09 | 0.87 | 1.25 | 0.89 | 1.10 | 0.90 |
| C17ISO | 5.61 | 6.51 | 6.25 | 7.93 | 5.26 | 6.66 | 5.79 | 6.80 | 4.00 | 4.45 | 4.59 | 5.86 | 5.06 | 5.80 |
| C17: Δ | 21.53 | 20.54 | 20.56 | 19.42 | 30.62 | 26.85 | 31.33 | 31.34 | 19.94 | 17.78 | 18.02 | 16.22 | 18.68 | 17.35 |
| C18:0 | 3.84 | 4.43 | 2.78 | 2.86 | 3.73 | 4.81 | 3.28 | 3.29 | 5.07 | 4.83 | 5.27 | 5.15 | 4.27 | 4.37 |
| C18:1n9 | 6.33 | 4.73 | 5.22 | 3.84 | 6.45 | 5.74 | 6.86 | 5.58 | 3.82 | 2.68 | 3.53 | 2.88 | 3.92 | 2.77 |
| C18:1n7 | 34.3 | 35.59 | 37.20 | 39.50 | 21.10 | 21.35 | 17.17 | 15.95 | 17.18 | 17.59 | 18.35 | 17.13 | 16.33 | 16.98 |
| # | 5.07 | 4.58 | 5.64 | 4.30 | 3.60 | 3.79 | 3.21 | 3.27 | 1.38 | 1.61 | 1.44 | 1.26 | 1.40 | 1.43 |
| C18:2n6 | 3.63 | 4.38 | 3.90 | 4.18 | 3.73 | 4.57 | 3.97 | 5.08 | 6.14 | 7.68 | 5.36 | 5.93 | 5.52 | 5.99 |
| C19: Δ | 8.03 | 8.53 | 8.71 | 8.81 | 10.74 | 10.53 | 12.67 | 13.91 | 9.73 | 8.91 | 9.25 | 9.68 | 11.23 | 10.55 |
| # | ND | ND | ND | ND | ND | ND | ND | ND | 0.74 | 0.91 | 0.81 | 0.76 | 0.88 | 0.88 |
| C20:2n6 | ND | ND | ND | ND | ND | ND | ND | ND | 0.69 | 1.20 | 1.21 | 1.70 | 1.13 | 1.55 |
| C20:3n6 | ND | ND | ND | ND | ND | ND | ND | ND | 3.47 | 3.25 | 3.60 | 3.49 | 3.56 | 3.39 |
| C20:4n6 | ND | ND | ND | ND | ND | ND | ND | ND | 1.48 | 1.30 | 1.66 | 1.53 | 1.67 | 1.44 |
| C20:4n3 | ND | ND | ND | ND | ND | ND | ND | ND | 4.81 | 6.26 | 5.00 | 6.24 | 4.60 | 5.53 |
| C20:5n3 | ND | ND | ND | ND | ND | ND | ND | ND | 12.93 | 13.92 | 13.53 | 15.17 | 13.57 | 14.71 |
Data are percentage of total and are mean ± SEM; #, fatty acids of unknown identity; ND, fatty acids with variable trace amounts are not included.
WT and cyp-37A1(ssd9) grown and processed in parallel have no significant difference in TAG fatty acid composition before (20 °C) or after (30 °C) LD fusion. n = 3 for each sample.
WT and emb-8(ssd89) grown and processed in parallel have no significant difference in TAG fatty acid composition before or after LD fusion. n = 7 for each sample.
WT, cyp-37A1(ssd9), and emb-8(ssd89) grown and processed in parallel have no significant difference in total fatty acid composition before or after LD fusion. n = 3 for each sample.
CYP-37A1 and EMB-8 Synthesize a Lipophilic Hormone to Repress DAF-12–Mediated LD Fusion.
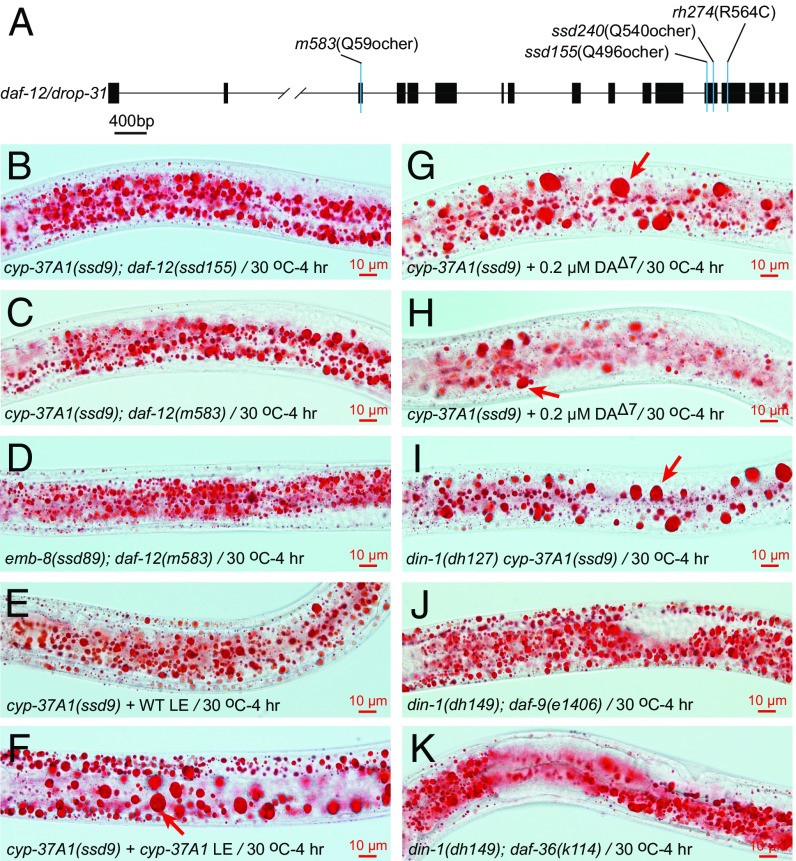
It is possible that CYP-37A1, together with EMB-8, modifies fatty acids or other lipid molecules to generate trace amount of bioactive metabolites that exert a signaling role. To investigate this possibility, we conducted cyp-37A1(ssd9) suppressor screens. We identified a suppressor group drop-31 with two alleles. Molecular cloning showed that drop-31 is mutated in the nuclear receptor gene daf-12 (Materials and Methods). daf-12(ssd155) and daf-12(ssd240) are nonsense mutations that abolish both the ligand binding domain and the transactivation domain (6), and thus are likely to be null mutants. daf-12(ssd155), daf-12(ssd240), and a known nonsense null mutation daf-12(m583), completely suppress LD fusion in cyp-37A1(ssd9) and emb-8(ssd89) (Fig. 3 A–D and Table 1). This result suggests that DAF-12 mediates the LD fusion activity that is derepressed in cyp-37A1(ssd9) and emb-8(ssd89), and that WT CYP-37A1 synthesizes a lipophilic hormones to repress DAF-12. To test this idea, we prepared a total lipid extract equivalent to 6 mg total protein (defined as 1X) from WT animals and found that applying the 1X lipid extract to intact cyp-37A1(ssd9) animals suppressed the LD fusion phenotype by 50.8%. In contrast, the 1X lipid extract from cyp-37A1(ssd9) suppressed cyp-37A1(ssd9) by only 5.3%. Delipidated extracts showed no activity in this assay (Fig. 3 E and F and Table 1).
Fig. 3.
DAF-12 mediates LD fusion in cyp-37A1 and emb-8 mutants. (A) The two drop-31 alleles, ssd155 and ssd240, are nonsense mutants of daf-12. m583 and rh274, two other daf-12 mutants, are null and gain-of-function (dauer-constitutive), respectively. (B–D) Null mutations of daf-12 completely suppress LD fusion of cyp-37A1(ssd9) and emb-8(ssd89). (E and F) A total lipid extract (LE) of WT but not of cyp-37A1(ssd9) suppresses LD fusion in cyp-37A1(ssd9) animals. (G) A supplementation of 0.2 μM Δ7-DA does not suppress LD fusion but occasionally decreases the amount of LDs in cyp-37A1(ssd9) (H). (I) din-1(dh127) does not suppress cyp-37A1(ssd9). (J and K) din-1(dh149) completely suppresses the supersized LD phenotype of daf-9(e1406) and daf-36(k114).
Table 1.
Epistasis analysis of the supersized LD phenotype
| Genotype and treatment | % SLD+ | n |
| cyp-37A1(ssd9) | 100 | 109 |
| cyp-37A1(ssd2) | 100 | 100 |
| cyp-37A1(ok673) | 100 | 97 |
| emb-8(ssd89) | 98.8 | 83 |
| cyp-37A1(ssd9); daf-12(ssd155) | 0 | 62 |
| cyp-37A1(ssd9); daf-12(m583) | 1.0 | 97 |
| emb-8(ssd89); daf-12(m583) | 0 | 102 |
| cyp-37A1(ssd9) + WT LE(1X) | 49.2 | 61 |
| cyp-37A1(ssd9) + WT LE(1X)-Del | 100 | 73 |
| cyp-37A1(ssd9) + cyp-37A1(ssd9) LE(1X) | 94.7 | 95 |
| cyp-37A1(ssd9) + cyp-37A1(ssd9) LE(1X)-Del | 100 | 69 |
| cyp-37A1(ssd9)+ DAΔ7(0.02 µM) | 79.3 | 58 |
| cyp-37A1(ssd9)+ DAΔ7(0.2 µM) | 87.7 | 57 |
| cyp-37A1(ssd9)+ DAΔ7(2 µM) | 55.9 | 102 |
| cyp-37A1(ssd9)+ DAΔ7(0.02 µM)* | 98.4 | 63 |
| cyp-37A1(ssd9)+ DAΔ7(0.2 µM)* | 95.5 | 66 |
| cyp-37A1(ssd9)+ DAΔ7(2 µM)* | 90.2 | 61 |
| daf-9(e1406)† | 87 | 54 |
| daf-9(e1406)+ DAΔ7(0.2 µM)† | 3.4 | 58 |
| daf-9(e1406)+ DAΔ7(2 µM)† | 0 | 58 |
| daf-36(k114)† | 21.4 | 56 |
| daf-36(k114) + DAΔ7(0.2 µM)† | 14 | 57 |
| daf-36(k114) + DAΔ7(2 µM)† | 0 | 56 |
| daf-12(rh274) | 100 | 54 |
| din-1(dh149); daf-12(rh274) | 0 | 81 |
| din-1(dh149); daf-9(e1406) | 0 | 81 |
| din-1(dh149); daf-36(k114) | 0 | 87 |
| din-1(dh127) cyp-37A1(ssd9) | 94.2 | 104 |
| din-1(dh127) cyp-37A1(ssd9); daf-9(e1406) | 11.1 | 45 |
| din-1(dh149) cyp-37A1(ssd9); daf-36(k114) | 98.5 | 66 |
All data are of L4 stage animals shifted to 30 °C for 4 h unless otherwise indicated. % SLD+, percentage of animals with supersized LDs. LE(1X), total lipid extract equivalent to 6 mg total protein. LE-Del, total lipid extract delipidated.
Scored at L3 stage.
Scored at 20 °C.
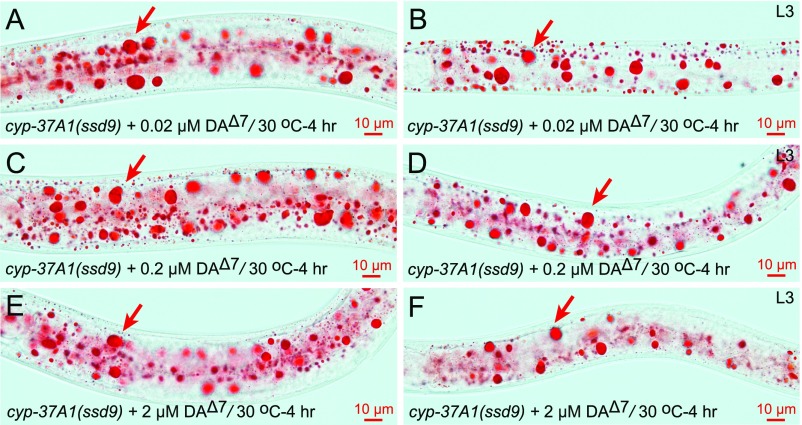
DAF-12 mediates the decision between reproductive development and dauer diapause; reproductive development is promoted by the presence of hormone ligands, known as DAs, whereas dauer formation is promoted by the absence of DAs (7, 8). Is DA the putative hormone synthesized by CYP-37A1? To address this question, we supplemented populations of cyp-37A1(ssd9) mutants with the classic DAF-12 ligand Δ7-DA (at concentrations of 0.02, 0.2, 2 μM) to test its effect on LD fusion. The results showed that Δ7-DA does not suppress LD fusion in cyp-37A1(ssd9) at the L3 stage but partially suppresses it at the L4 stage. However, the partial suppression at L4 is associated with a depletion of LDs rather than a block of LD fusion (Fig. 3 G and H). Moreover, the effect is nonlinear with Δ7-DA concentration (Table 1 and Fig. S7).
Fig. S7.
Δ7-DA has no effect on LD fusion. The addition of 0.02 μM (A and B), 0.2 μM (C and D), or 2 μM (E and F) Δ7-DA does not suppress the LD fusion phenotype of cyp-37A1(ssd9) at either the L4 stage or L3 stage. Arrows point to supersized LDs.
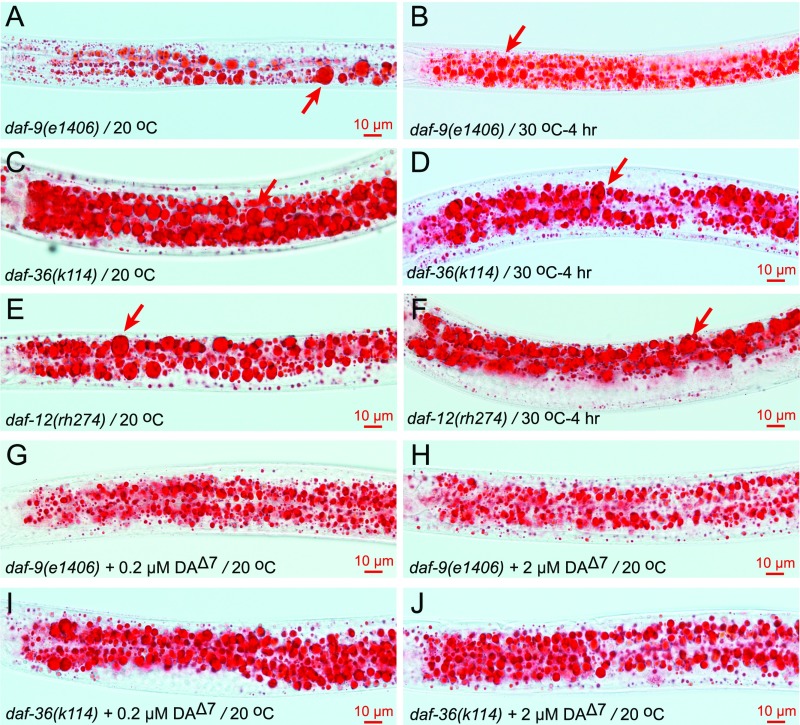
One explanation for these observations is that DAF-12 is acted upon by two functionally distinct ligand types: (i) the previously described DAs that inhibit dauer formation and promote lipolysis (15); and (ii) a novel hormone ligand that inhibits thermosensitive LD fusion. Consistent with this reasoning, the DA-defective mutants, daf-9(e1406), daf-36(k114), and the DA binding-defective mutant, daf-12(rh274), already form supersized LDs at 20 °C. However, in these three mutants, the formation of supersized LDs at 20 °C is not coincident with a decrease of small LDs (i.e., it is characteristic of LD growth instead of LD fusion). Moreover, supersized LDs or small LDs do not fuse when the three mutants are shifted to 30 °C (Fig. S8 A–F). Finally, the LD growth/lipogensis phenotype of daf-9(e1406) and daf-36(k114) is largely suppressed by the supplementation of 0.2 μM Δ7-DA and is completely suppressed by 2 μM Δ7-DA (Table 1 and Fig. S8 G–J). Thus, the role of DA in LD regulation differs from that of the postulated hormone synthesized by CYP-37A1.
Fig. S8.
The DA synthesis-defective and DA binding-defective mutants form supersized LDs that can be suppressed by Δ7-DA supplementation. (A) At 20 °C, the DA synthesis-defective daf-9(e1406) mutant forms dauer-like larva. The larva form supersized LDs (arrow) already at 20 °C. (B) When shifted from 20 °C to 30 °C for 4 h, the supersized or small LDs in daf-9(e1406) do not fuse. (C–F) Such is the case for the DA synthesis-defective mutant daf-36(k114) and the DA binding-defective mutant daf-12(rh274). Arrows point to supersized LDs. (G–J) The supersized LD phenotype of daf-9(e1406) and daf-36(k114) at 20 °C is suppressed by the supplementation of 0.2 μM or 2 μM Δ7-DA.
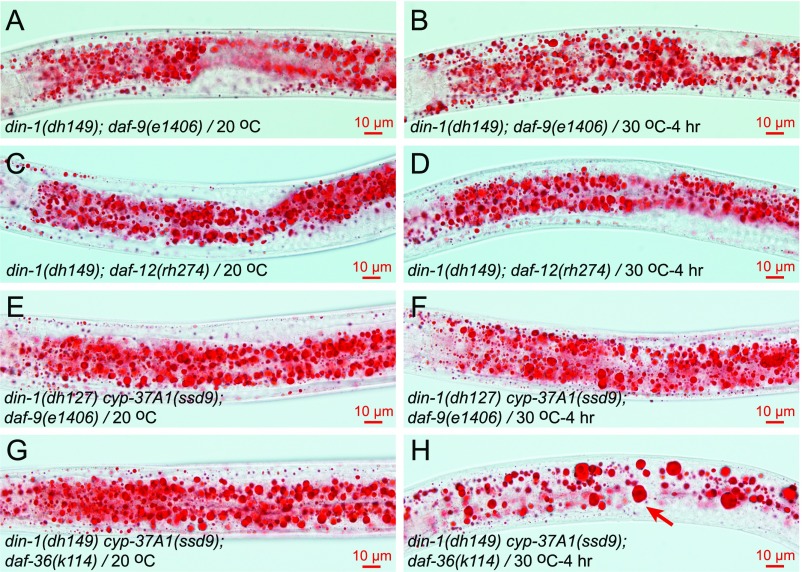
In the DA-defective mutants daf-9(e1406) and daf-36(k114), the prodauer function of DAF-12 requires the transcription cofactor DIN-1 (12). If DA synthesized by DAF-9/DAF-36 and the hormone synthesized by CYP-37A1 differentially regulate LD growth/lipogenesis and LD fusion, daf-9/daf-36 and cyp-37A1 mutants may differ in their DIN-1 dependence. Our results showed that this indeed is the case: when DIN-1 is dysfunctional, daf-9(e1406), daf-36(k114), and daf-12(rh274) do not form supersized LDs at 20 °C or when shifted to 30 °C (Fig. 3 J and K, Table 1, and Fig. S9 A–D); in contrast, when DIN-1 is dysfunctional, cyp-37A1(ssd9) still forms supersized LDs via thermosensitive fusion with a nearly full penetrance (Fig. 3I and Table 1).
Fig. S9.
The supersized LD phenotype of DA synthesis-defective and DA binding-defective mutants is dependent on DIN-1. (A and B) The double-mutant din-1(dh149); daf-9(e1406) does not form supersized LDs either at 20 °C or when shifted 30 °C for 4 h. (C and D) The same as in A and B is true for din-1(dh149); daf-12(rh274). (E and F) When both din-1 and daf-9 are mutated, thermosensitive LD fusion in cyp-37A1(ssd9) is partially impaired. (G and H) When both din-1 and daf-36 are mutated, thermosensitive LD fusion in cyp-37A1(ssd9) is not affected. Arrow points to supersized LDs.
To further test the differential roles of the hormone synthesized by CYP-37A1 and of DA, we compared double-mutant din-1(dh127) cyp-37A1(ssd9) (DA-present) and triple mutants din-1(dh127) cyp-37A1(ssd9); daf-9(e1406) and din-1(dh149) cyp-37A1(ssd9); daf-36(k114) (DA-deficient). The results showed that, with or without DAF-36, LD fusion in din-1(dh127) cyp-37A1(ssd9) is normal. With the presence of DAF-9, LD fusion in din-1(dh127) cyp-37A1(ssd9) is normal; without DAF-9, LD fusion in din-1(dh127) cyp-37A1(ssd9) is not enhanced but is partially impaired, indicating an unexpected cross-talk between CYP-37A1 and DAF-9 at the level of DAF-12 (Fig. 3I, Table 1, and Fig. S9 E–H).
cyp-37A1 Mutant Tolerates Heat Stress in a DAF-12–Dependent Manner.
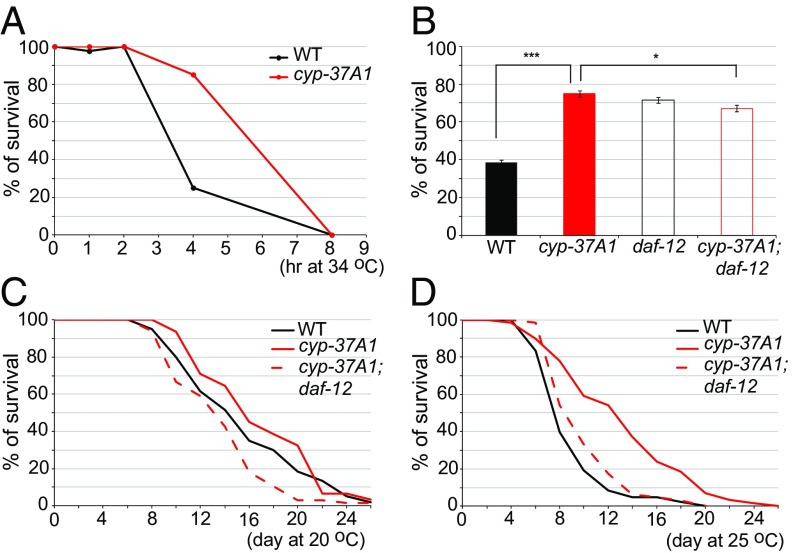
The thermosensitive LD fusion regulated by CYP-37A1 and DAF-12 may be linked to heat adaptation for C. elegans in its natural habitat. To test this hypothesis, we measured the survival rates of WT and cyp-37A1(ssd9) 1-d-adult animals upon exposure to 34 °C. The results showed that both WT and cyp-37A1(ssd9) start to die by 2-h exposure and that no animal survived an 8-h exposure (Fig. 4A). However, at the 4-h time point, the survival rate of cyp-37A1(ssd9) was much higher than WT. The increased survival of cyp-37A1(ssd9) is partially dependent on DAF-12 (Fig. 4B). We also measured the lifespans of WT and cyp-37A1(ssd9). At 20 °C, the mean lifespan of cyp-37A1(ssd9) is slightly longer (∼2 d longer) than that of WT. The extended lifespan of cyp-37A1(ssd9) is dependent on DAF-12 (Fig. 4C). At 25 °C, lifespans of both WT and cyp-37A1(ssd9) are shortened. However, the mean lifespan of cyp-37A1(ssd9) is much longer (∼5 d longer) than that of WT. The extended lifespan is completely dependent on DAF-12 (Fig. 4D). These results demonstrate that cyp-37A1 mutants have a better tolerance of acute heat stress and ambient heat stress in a DAF-12–dependent manner.
Fig. 4.
Thermotolerance and lifespan. (A) WT and cyp-37A1(ssd9) 1-d adult animals started to die upon 2-h treatment of 34 °C. All animals died upon 8-h treatment. (B) Under a 34 °C/4-h treatment, the survival rate of cyp-37A1(ssd9) is higher than that of WT. The better survival of cyp-37A1(ssd9) is partially impaired by daf-12(m583) mutation. Data are mean ± SEM of six replicates. ***P < 0.001, *P ≤ 0.05, unpaired two-sample t test. (C) At 20 °C, the lifespan of cyp-37A1(ssd9) is slightly increased compared with that of WT. The lifespan increase of cyp-37A1(ssd9) is abolished by daf-12(ssd155) mutation. (D) At 25 °C, the lifespan of cyp-37A1(ssd9) is much increased than that of WT. The lifespan increase of cyp-37A1(ssd9) is abolished by daf-12(ssd155) mutation. Lifespan results are typical of two independent experiments.
Discussion
In summary, we have identified a hormone synthesis and nuclear receptor pathway consisting of CYP-37A1, EMB-8, and DAF-12 that regulates environment temperature-dependent LD fusion in the nematode C. elegans. This pathway shares components with the DAF-9–DAs–DAF-12 pathway (6, 7, 12), but differs from that pathway in three respects: (i) the DAF-12 hormone ligand synthesized by CYP-37A1 is not DA; (ii) DAF-12 does not require DIN-1 to exert its LD fusion-promoting function; and (iii) the DAF-9–DAs–DAF-12 pathway regulates LD growth/lipogensis rather than LD fusion. These functional differences also correlate with the facts that CYP-37A1 is expressed and functions in intestine while the DA-synthesis enzymes DAF-9 and DAF-36 are mainly in hypodermis (9, 10).
To our knowledge, the CYP-37A1–hormone–DAF-12 pathway described here constitutes a second C. elegans nuclear receptor pathway for which both the hormone synthesis enzyme and cognate nuclear receptor are identified, and for which evidence of an endogenous hormone ligand is provided. Our results also suggest that this hormonal pathway may interact with the DA pathway at the DAF-12 level. It is possible but unlikely that CYP-37A1-EMB-8 and DAF-12 act in parallel and the metabolite synthesized by CYP-37A1-EMB-8 is not a hormone ligand for DAF-12. This alternative scenario still predicts a novel non-DA hormone DAF-12.
Among nuclear receptors, DAF-12 is evolutionarily basal (31). DAF-12 may have adapted to sense multiple hormone ligands and carry out pleiotropic physiological functions before the proliferation of nuclear receptors during evolution. This kind of adaptation may allow the poikilothermic C. elegans to survive diverse environmental stresses (e.g., temperature stress). Thus, it is interesting that dysfunction of the CYP-37A1–hormone–DAF-12 pathway results in not only thermosensitive LD fusion but also a better tolerance of a high temperature of 34 °C, a longer lifespan at a moderately high temperature of 25 °C, and a reduced reproduction rate (19). It suggests that C. elegans in the wild may use this pathway to balance individual survival and reproduction (population survival) according to environment temperature. Similar environment temperature-regulated adaptation responses include the DAF-9–DAs–DAF-12 pathway-regulated dauer formation and the PARQ-2 (adiponectin receptor)-regulated fatty acid unsaturation (6–13, 32, 33).
Perhaps what is more interesting is that the thermosensitive LD fusion process regulated by the CYP-37A1–hormone–DAF-12 pathway is analogous to the temperature-dependent interconversion of beige/brown adipocytes (with multilocular LDs and active lipolysis) and white adipocytes (with unilocular LD and reduced lipolysis) in mammals (34). In other eukaryotic systems, multilocular LDs convert to unilocular LD through direct fusion (typical fusion), which has been regarded as a purely biophysical coalescence process affected by LD surface phospholipid composition (35–37). Another mode of multilocular-to-unilocular conversion is the asymmetrical transfer of TAG from one LD to another (atypical fusion) through the pore formed by the Cidec protein complex (38, 39). Neither of these processes is known to be temperature-dependent.
In contrast, the LD fusion process uncovered by the cyp-37A1/emb-8 mutants differs in several respects to typical fusion and atypical fusion. First, it is dependent on environment temperature. Second, it has an optimal reaction temperature with a first-order kinetics typical of an enzymatic reaction, which is not predicted by a pure biophysical coalescence model (typical fusion). Third, it is not accompanied by an increase in TAG levels, although we cannot exclude the possibility of complementary changes in TAG synthesis and TAG hydrolysis in cyp-37A1/emb-8, that maintain TAG levels constant. In contrast, both typical fusion and atypical fusion were found to be accompanied by an increase of TAG levels (37, 40, 41). Fourth, it is not accompanied by detectable changes in phospholipids. Fifth, the C. elegans genome does not encode an apparent Cidea/Cidec homolog. These differences indicate that the thermosensitive LD fusion in C. elegans may involve a novel mechanism. However, it remains possible that the thermosensitive LD fusion shares some common mechanistic aspects with typical LD fusion, as both modes of fusion are fast and not asymmetric. For example, it could be that at 20 °C, cyp-37A1/emb-8 has already changed the LD membrane phospholipid or fatty acid composition so as to alter membrane fluidity, but that the higher temperature is required to facilitate fusion. We have not been able to obtain evidence to support this hypothesis, which was shown to be the basis of typical LD fusion (35, 37).
Given the conservation of the CYP-37A1–hormone–DAF-12 pathway components between nematode and human, it is tempting to make predictions for human physiology and pathophysiology. First, the in vivo function of CYP4V2, the human ortholog of CYP-37A1, may be for the synthesis of a hormone that acts on a nuclear receptor (VDR, LXR, or FXR), dysfunction of which results in LD fusion and supersized LD/“lipid inclusion” formation in RPEs. Second, potential BCD risk factors include eye temperature, nuclear receptor mutations not yet identified, and POR polymorphisms already identified in “normal” human subjects (42). Finally, because CYP4V2 is broadly expressed in many human organs (26), the DAF-12-equivalent nuclear receptor may also be expressed and function in these organs to negatively regulate LD fusion. Dysfunction of this regulatory pathway may be associated with as yet unidentified diseases.
Materials and Methods
C. elegans Strains and Culture Conditions.
The WT C. elegans was N2 Bristol. All strains were grown on OP50 E. coli-seeded NGM plates. The growth temperature was 20 °C if not otherwise indicated. Mid- to late-L4–stage animals were used for phenotype characterization. For temperature-shift experiments, animals were grown continuously at 15 °C or 20 °C and were then shifted to 30 °C for 4 h to reach the mid-L4 stage. Mid-L4–stage animals were picked out and assayed for LD phenotype. Strains used in this study can be found in SI Materials and Methods.
Molecular Cloning of drop-1 and drop-8.
drop-1(ssd2) and drop-8(ssd89) were previously mapped onto an LG II 21.39 ∼22.38 map unit and LG III −2.21 ∼ −0.75 map unit, respectively (19). Genomic DNAs of the two strains were subjected to whole-genome resequencing and single nucleotide variance (SNV) and insertion/deletion (Indel) analysis. drop-1 and drop-8 were found to be cyp-37A1 and emb-8 mutations, respectively (SI Materials and Methods).
Noncomplementation Screen of emb-8 Alleles.
N2 males were mutagenized with ethylmethanesulfonate (EMS) and were used to mate unc-32(e189) emb-8(ssd89) hermaphrodites. Non-Unc F1 progeny with supersized LD phenotype were isolated. The emb-8 locus of candidate isolates was PCR amplified and Sanger-sequenced for mutation (SI Materials and Methods).
cyp-37A1 Suppressor Screen and Molecular Cloning of drop-31.
cyp-37A1(ssd9) and cyp-37A1(ok673) were mutagenized with EMS. F2 progeny were screened for the absence of the supersized LD phenotype. A total of 35 mutants were obtained and were tested for complementation. Two isolates, ssd155 and ssd240, were found to belong to the same group drop-31. drop-31(ssd155) was subjected to SNP-based mapping and whole-genome resequencing, and was found to be a daf-12 mutation. See SI Materials and Methods for more details.
Additional methods can be found in SI Materials and Methods.
SI Materials and Methods
Caenorhabditis elegans Strains.
Mutant strains created or used in this study were: cyp-37A1(ssd1, ssd2, ssd3, ssd4, ssd5, ssd6, ssd7, ssd8, ssd9, ssd10, ssd11, ssd12, ssd79, ssd80, ssd81, ssd82, ssd83, ssd84, ssd85, ssd86, ssd87, ssd88, ssd203, ssd212, ssd219, ssd220, ssd221, ssd222, ssd223, ssd224, ssd225, ssd226, ssd227, ssd228, ssd229, ssd230, ssd231, ssd255, ok673) II, emb-8(ssd70, ssd71, ssd89, ssd143) III, daf-12(ssd155, ssd240, m583, rh274) X, din-1(dh127, dh149) II, sbp-1(ep79) III, atln-1(ok1144) IV, glo-4(ok623) V, daf-36(k114) V, dgat-2(ssd90) V, daf-9(e1406) X, acs-22(tm3236) X. Transgenic lines were: ssdEx20, 21[cyp-37A1p::gfp], ssdEx17, 18[vha-6p::cyp-37A1::3xflag::tev::gfp], ssdEx11, 12[emb-8p::3xflag::tev::gfp], ssdEx51, 52[vha-6p::cyp4v2::3xflag::tev::gfp], ssdSi5[vha-6p::emb-8::mCherry] II, ssdSi6[vha-6p::cyp-37A1::3xflag::tev::gfp] IV, hjIs14[vha-6p::gfp::sp12], hjSi56[vha-6p::3xflag::tev::gfp::dgat-2] IV. All strains and lines were out-crossed or back-crossed two to six times before analysis. Double mutants were made using standard techniques.
Molecular Cloning of drop-1 and drop-8.
drop-1(ssd2) and drop-8(ssd89) were previously mapped onto an LG II 21.39 ∼22.38 map unit (physical location: 13,930,530 ∼14,060,042 bp) and LG III −2.21 ∼ −0.75 map unit (physical location: 5,037,391 ∼7,319,832), respectively (19). Genomic DNAs of the two strains were extracted and sent to Novogene (Beijing) for whole-genome resequencing and SNV and Indel analysis with reference to the WBcel215.69 release of C. elegans genome sequence and gene model annotation. In the mapped region of drop-1(ssd2), a nonsynonymous mutation of cyp-37A1 (c.TGC→TAC, p.C356Y) was found. All other 37 alleles were Sanger-sequenced and were found to harbor nonsynonymous cyp-37A1 mutations. drop-8(ssd89) was found to harbor a nonsynonymous mutation of emb-8 (c.GGT→GAT, p.G22D).
Noncomplementation Screen of emb-8 Alleles.
N2 males were mutagenized with EMS. Healthy males were used to mate unc-32(e189) emb-8(ssd89) hermaphrodites. Non-Unc F1 progeny were grown to stage L3 to L4 and were then shifted to 30 °C for 4 h before being screened for supersized LD phenotype under a stereoscope at 180× magnification. A total of 7.4 × 104 F1s (i.e., 7.4 × 104 haploid genomes) were screened. Three F1s with supersized LDs, ssd70/ssd89, ssd71/ssd89, and ssd143/ssd89, were isolated. ssd70/ssd89 and ssd71/ssd89 survived to egg-laying adult stage but all eggs laid arrested at the embryonic stage. ssd143/ssd89 survived to adult stage and produced both Unc and non-Unc F2 progeny with supersized LD phenotype. ssd70/ssd89, ssd71/ssd89, and ssd143/ssd89 F1s were then lysed, subjected to PCR amplification of emb-8 genomic DNA, and sequenced by Sanger sequencing. Sequencing results confirmed ssd89 heterozygosity. ssd70 was found to be a nonsense mutation of c.CGA→TGA, p.R360opal; ssd71, c.TCC→TTC, p.S581F; and ssd143, the same as ssd89(c.GGT→GAT, p.G22D).
cyp-37A1 Suppressor Screen and Molecular Cloning of drop-31.
cyp-37A1(ssd9) and cyp-37A1(ok673) were mutagenized with EMS. F2 progeny derived from 1.06 × 105 haploid genomes were incubated at 20 °C and were then shifted to 30 °C for 4 h. F2 animals were screened for the absence of the supersized LD phenotype, which was readily identifiable in bright-field under a stereoscope at 180× magnification. Candidate suppressor mutants were incubated at 20 °C and were then shifted to 30 °C for 4 h to reach L4 stage. L4 animals were then postfix stained with Oil Red O and imaged on a compound microscope in bright field with a 60× oil objective, as described previously (19). Bona fide suppressors were identified as lacking LDs with diameters ≥ 3 μm. A total of 35 mutants were obtained. Through complementation tests, two isolates, ssd155 and ssd240, were found to belong to the same group drop-31. To map the suppressor mutants, we first created an SNP-based mapping strain for suppressor mutants by integrating cyp-37A1(ssd9) into ChrII (located at 21.0 ∼22.38 map unit) of the Hawaiian strain CB4856. The cyp-37A1(ssd9)-Hawaiian strain was used to map drop-31(ssd155). drop-31(ssd155) was mapped onto ChrX 1.88 ∼2.86 map unit (physical location: 10,252,658 ∼11,142,347 bp). drop-31(ssd155) was then whole-genome resequenced and analyzed for SNVs and Indels, as described above. A nonsense mutation in daf-12 (c.CAA→TAA, p.Q496ochre) was found. The daf-12 genomic DNA of ssd240 was then PCR-amplified and sequenced. A nonsense mutation (c.CAA→TAA, p.Q540ochre) was identified.
cyp-37A1 Constructs and Transgenic Lines.
A 1,259-bp DNA fragment just upstream of the translational start of cyp-37A1 was PCR-amplified with primers SOZ-140F/R [AGAAGCTT(HindIII) GGGAGTTGCTCAAGAGGTTCAGCG/AAGGA TCC(BamHI)CACCAAAGCAAGGAGGTACACAGCG]. The primer sequences underlined are recognition sites of restriction enzymes in parentheses (the same for others if not otherwise indicated). The DNA was cloned using restriction enzyme sites HindIII/BamHI to obtain plasmid pSOZ-52_cyp-37A1p::gfp. Plasmid pSOZ-52 was injected at 40 ng/μL into N2 animals. Two independent lines, ssdEx20 and ssdEx21, were obtained and yielded the same result. The cyp-37A1 cDNA was PCR amplified with primers SOZ-121F/R [ATCCTGCAGG(SbfI)ATGGGGATCGCTGTGTACCTC/AAGCGGCCGC(NotI)TCCCTGCTGCCGAACAGTAAAC]. The DNA was cloned using SbfI/NotI to obtain plasmid pSOZ-58_vha-6p::cyp-37A1::3xflag::tev::gfp. pSOZ-58 was injected at 40 ng/μL into cyp-37A1(ok673). Two independent lines ssdEx17 and ssdEx18 were obtained and both rescued cyp-37A1(ok673). A synthetic cDNA of human CYP4V2 with codons optimized for C. elegans (Ruibiotech) was cloned into pSOZ-58 using SbfI/NotI to obtain pSOZ-125_ vha-6p::cyp4v2::3xflag::tev::gfp. pSOZ-125 was injected at 30 ng/μL into cyp-37A1(ssd9). Two independent lines ssdEx51 and ssdEx52 were obtained and both rescued cyp-37A1(ssd9). For making single-copy integrated transgenic line, the cyp-37A1::3xflag::tev::gfp cassette of pSOZ-58 was PCR-amplified and subcloned into pSOZ-43 (contains pCFJ178 backbone) using PacI/NheI sites to obtain pSOZ-101_vha-6p::cyp-37A1::3xflag::tev::gfp. pSOZ-101 was injected into strain EG6250 at 30 ng/μL. Single-copy integrated line ssdSi6[vha-6p::cyp-37A1::3xflag::tev::gfp] was identified according to standard procedures and was outcrossed with N2 for four times before use.
emb-8 Constructs and Transgenic Lines.
A 1,500-bp promoter DNA just upstream of the translational start codon of emb-8 was PCR-amplified using primers SOZ-205F/R [ATACTAGT(SpeI)CCCGGTCTCGACACAACAAAT/GCAGATCT(BglII)GTTTAGAATGAGAGTTTCTG]. The PCR product was cloned using restriction enzyme sites SpeI/BglII to obtain plasmid pSOZ-79_emb-8p::3xflag::tev::gfp. pSOZ-79 was injected at 40 ng/μL into N2. Two independent lines ssdEx11 and ssdEx12 were obtained and yielded the same result. emb-8 cDNA was PCR-amplified using primers SOZ-204F/R [ATCCTGCAGG(SbfI)ATGCTGGCGTGGATTGTG/ATGCGGCCGC(NotI)TGACCACACATCAGC]. The cDNA was cloned into vectors through several steps to generate the final plasmid construct pSOZ-98_vha-6p::emb-8::mCherry (contains pCFJ151 backbone). pSOZ-98 was injected into strain EG6699 at 20 ng/μL. Single-copy integrated line ssdSi5[vha-6p::emb-8::mCherry] was identified according to standard procedures and was outcrossed with N2 two times before use.
LD Labeling, Imaging, and Size Measurement.
Postfix Oil Red O staining and bright-field imaging, BODIPY staining, GFP::DGAT-2 marking, and confocal imaging were essentially as previously described (19). LD size measurement was conducted essentially the same as previously described (17). Briefly, BODIPY, or GFP::DGAT-2–labeled stage-L4 animals were imaged with a Zeiss LSM780 confocal microscope. A z-stack of 20 × 0.45-μm depth was taken for each animal. Confocal z-stack files were then rendered 3D in Imaris 7.2 software (Bitplane). Labeled spherical structures in the two cells of the second intestine segment were then marked and diameters were measured. Volumes of supersized LDs (greater than 3-μm diameter) were calculated as π × (diameter/2)3 × 4/3.
Time-Lapse Imaging of LD Fusion.
The transgenic LD marker GFP::DGAT-2 (hjSi56) was introduced into cyp-37A1(ssd2). L4-stage cyp-37A1(ssd2); hjSi56 animals were immobilized with 0.2 mM levamisole on an agarose-padded slide. The slide was mounted on a Zeiss LSM 780 microscope with a temperature control chamber. The temperature was set at 30 °C. A z-stack of 20 × 0.45-μm depth was taken every 30 s. Time-lapse z-stacks were made into 3D movies in Zeiss ZEN software and were carefully inspected for fusion events. A total of 21 time-lapse movies were captured and 57 LD fusion events were identified. The two fusing LDs were measured for diameters in ZEN. LDs were assigned anterior (A-LD) vs. posterior (P-LD) and medial (M-LD) vs. lateral (L-LD) according to their relative positions in the intestine cells.
TAG Measurement and Fatty Acid Analysis.
TAG measurement and fatty acid analysis were conducted as previously described (17). Some changes were custom made. Each replicate sample for TAG measurement was derived from one 10-cm NGM/OP50 plate of 8,000 L4-stage animals, as was each sample for total fatty acid analysis. The sample for TAG measurement was extracted to obtain total lipid. Total lipid was separated on a thin-layer chromatography (TLC) plate, and TAG was then cut out and transmethylated to prepare fatty acid methyl esters (FAMEs). In contrast, the sample for total fatty acid analysis was homogenized and directly transmethylated without first being extracted for total lipid. The TAG FAMEs and total FAMEs were chromatographed on an Agilent HP7890A gas chromatography (GC) instrument coupled with a flame ionization detector. Each fatty acid peak was identified and integrated for area. Area percentage data were used to calculate TAG mass, TAG fatty acid composition, and total fatty acid composition. The animal sample subjected to a 30 °C/4 h treatment had a significant decrease of protein mass (heat-induced proteolysis) but no significant change of TAG mass. To normalize the TAG mass for the 30 °C/4 h sample, a companion sample of the same number of animals grown continuously at 20 °C to the same stage was measured for protein mass, and this protein mass was used to normalize the TAG masses of both the 20 °C sample and the 30 °C/4 h sample.
Phospholipid TLC and Quantification.
To quantify the relative levels of phospholipid species, total lipid was extracted as described above. Before TLC separation of the different phospholipid species, a silica gel G TLC plate (Fisher Scientific, #05-719-800) was baked at 110 °C in an oven for 1 h. The plate was washed by developing in a TLC tank with 50 mL/50 mL CHCl3/CH3OH until the solvent reached the top end. The plate was dried using a hairdryer and was then soaked in 100 mL 1.8% boric acid (Sigma #202878-50G; 99.999% purity) in 100% ethanol (HPLC grade). The plate was dried with a hairdryer and further dried at 100 °C in an oven for 15 min. Then, total lipid samples and phospholipid standards (Avanti Polar Lipids) were loaded onto the plate. The plate was developed in a TLC tank with 30 mL/35 mL/7 mL/35 mL CHCl3/CH3CH2OH/H2O/N(CH2CH3)3. The plate was then dried with a hairdryer and sprayed evenly with 0.05% lipid staining dye Primuline (Sigma-Aldrich, #206865) in 8/2 Acetone/H2O. The plate was dried with a hairdryer and was then imaged on a Bio-Rad Gel Doc XR+ imager with an excitation wavelength of 302 nm. The band intensity of each phospholipid species was measured and its percentage of total was calculated and presented as the relative level.
Lipophilic Hormone Extraction and Supplementation.
To test the lipophilic hormone activity, total lipid was extracted from mixed stages of WT or cyp-37-A1(ssd9) animals essentially in the same way as that described above with some changes. Briefly, animals were cultivated on four 15-cm NGM-20X OP50 plates to crowdedness. Animals were harvested with PBST (PBS, pH 7.2/0.001% Triton X-100) and washed four times with PBS. Animals were then homogenized in a 15-mL conical glass tube. One-tenth of the homogenate was taken out to extract total protein with 2× SDS buffer (4% SDS/0.1 M Tris-pH6.8). The concentration of the total protein sample was measured using a BCA Protein Assay Kit (Pierce). The rest nine-tenth of the homogenate was added with 2 mL cold Solution I (10:10:1 CHCl3:CH3OH:HCOOH, HCOOH added freshly) and was vortexed. Then 3 mL cold Solution II (10:10:2 CHCl3:CH3OH:H2O) was added and vortexed. The sample was kept at −20 °C overnight to allow sufficient lipid extraction. The next day, the sample was added with 0.8 mL 0.15 M KCl followed by vigorous vortexing, and then was shaken for 5 min on a rocker to allow mixing and extraction. The sample tube was spun at 300 × g for 5 min in a clinic centrifuge to separate the chloroform and water layers. The lower chloroform layer (in which the total lipid is) was transferred into a new glass tube and was completely dried under nitrogen flow at 37 °C on an evaporator. The dried lipid sample was dissolved in 100 μL ethanol, transferred into a 2-mL glass vial, sealed with nitrogen, and stored at −20 °C before use. A portion of the total lipid sample equivalent to 6 mg total protein (with volume adjusted to 10 μL in ethanol) was the crude 1× lipophilic hormone extract. The extract was mixed with 190 μL PBS and vortexed. The 200-μL mixture was added onto the 2-cm diameter OP50 Escherichia coli lawn of a 6-cm NGM plate. As a delipidated control, the 1× hormone extract was mixed with 190 μL PBS, vortexed, and centrifuged at 15,700 × g for 5 min in a microcentrifuge. After centrifugation, lipids were floated to the top layer. The lower aqueous fraction was collected as the delipidated control and was added onto the 2-cm diameter OP50 E. coli lawn of a 6-cm NGM plate. The supplemented plates were immediately blow dried in a laminar flow hood. Five to 10 L4-stage cyp-37A1(ssd9) animals were loaded onto each plate to produce F1s. F1s grown to L4 stage were postfix-stained with Oil Red O and scored for supersized LD phenotype. Supplementation and LD phenotype scoring were conducted two times.
DA Supplementation.
(25S)-Δ7-DA of HPLC grade (Cayman Chemical, #14101-100) was dissolved in 100% ethanol to prepare a stock solution of 2 mM. The stock was serially diluted in PBS to prepare working stocks of 2 μM, 0.2 μM, and 0.02 μM. A total volume of 200 μL working stock was added onto the 2-cm diameter OP50 E. coli lawn of a 6-cm NGM plate. The supplemented plates were immediately blow dried. Five to 10 L4 stage cyp-37-A1(ssd9) animals were loaded onto each DA-supplemented plate to produce F1s. F1s grown to L4 stage were postfix-stained with Oil Red O and scored for the supersized LD phenotype.
Thermotolerance Assay.
To test thermotolerance, 30 1-d adult-stage (24 h past L4) animals were incubated on an NGM/OP50 plate at 34 °C for designated time. Animals were then shifted back to 20 °C to recover for 24 h. Animals moving freely or moving upon gentle touch with a worm pick were counted as alive. One plate of animals was treated as an independent replicate.
Lifespan Assay.
To test lifespan at 20 °C, 90 synchronized animals that had just entered adulthood were incubated on three NGM/OP50 plates and were treated as a single replicate. Animals were counted every 2 d for survival rate. Animals were transferred onto fresh plates every 2 or 4 d. Animals moving freely or moving upon gentle touch with a worm pick were counted as alive. Animals forming “bag-of-worms” (bagged), dried (dried), or forming vulva with stuff coming out (p-vulva) were censored. To test lifespan at 25 °C, synchronized animals that grew continuously at 20 °C and had just entered adulthood were shifted to 25 °C. Other procedures were the same.
Supplementary Material
Acknowledgments
We thank Dr. David Weisblat for constructive comments on this manuscript; Drs. Gian Garriga, Yikun He, Legong Li, Peng Li, and Xiao Liu for useful discussions; Shuang Zhou for technical assistance; all members of our laboratory for discussion; Dr. Nengsheng Ye for helping us to use his gas chromatography instrument; Drs. X. Liu and G. Garriga for facilitating some of the imaging experiments; and Drs. E. Jorgensen and B. Liang for reagents and strains. Some other strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institute of Health Office of Research Infrastructure Programs (P40 OD010440). This work was supported by National Natural Science Foundation of China Grant 31370820 (to S.O.Z.) and Beijing Municipal Education Commission Grant KZ201410028032 (to S.O.Z.). S.L. was supported in part by an International Exchange and Joint Training Grant of the Graduate School of Capital Normal University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704277114/-/DCSupplemental.
References
- 1.Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120431. doi: 10.1098/rstb.2012.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans RM, Mangelsdorf DJ. Nuclear receptors, RXR, and the Big Bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gissendanner CR, Crossgrove K, Kraus KA, Maina CV, Sluder AE. Expression and function of conserved nuclear receptor genes in Caenorhabditis elegans. Dev Biol. 2004;266:399–416. doi: 10.1016/j.ydbio.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Menzel R, Bogaert T, Achazi R. A systematic gene expression screen of Caenorhabditis elegans cytochrome P450 genes reveals CYP35 as strongly xenobiotic inducible. Arch Biochem Biophys. 2001;395:158–168. doi: 10.1006/abbi.2001.2568. [DOI] [PubMed] [Google Scholar]
- 5.Rappleye CA, Tagawa A, Le Bot N, Ahringer J, Aroian RV. Involvement of fatty acid pathways and cortical interaction of the pronuclear complex in Caenorhabditis elegans embryonic polarity. BMC Dev Biol. 2003;3:8. doi: 10.1186/1471-213X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 2000;14:1512–1527. [PMC free article] [PubMed] [Google Scholar]
- 7.Motola DL, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 8.Mahanti P, et al. Comparative metabolomics reveals endogenous ligands of DAF-12, a nuclear hormone receptor, regulating C. elegans development and lifespan. Cell Metab. 2014;19:73–83. doi: 10.1016/j.cmet.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev Cell. 2001;1:841–851. doi: 10.1016/s1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- 10.Rottiers V, et al. Hormonal control of C. elegans dauer formation and life span by a Rieske-like oxygenase. Dev Cell. 2006;10:473–482. doi: 10.1016/j.devcel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Wollam J, et al. A novel 3-hydroxysteroid dehydrogenase that regulates reproductive development and longevity. PLoS Biol. 2012;10:e1001305. doi: 10.1371/journal.pbio.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludewig AH, et al. A novel nuclear receptor/coregulator complex controls C. elegans lipid metabolism, larval development, and aging. Genes Dev. 2004;18:2120–2133. doi: 10.1101/gad.312604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerisch B, et al. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci USA. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, et al. The nuclear receptor DAF-12 regulates nutrient metabolism and reproductive growth in nematodes. PLoS Genet. 2015;11:e1005027. doi: 10.1371/journal.pgen.1005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin S, Parton RG. Lipid droplets: A unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 17.Zhang SO, et al. Genetic and dietary regulation of lipid droplet expansion in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2010;107:4640–4645. doi: 10.1073/pnas.0912308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang SO, Trimble R, Guo F, Mak HY. Lipid droplets as ubiquitous fat storage organelles in C. elegans. BMC Cell Biol. 2010;11:96. doi: 10.1186/1471-2121-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, et al. A genetic screen for mutants with supersized lipid droplets in Caenorhabditis elegans. G3 (Bethesda) 2016;6:2407–2419. doi: 10.1534/g3.116.030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frøkjaer-Jensen C, et al. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu N, et al. The FATP1-DGAT2 complex facilitates lipid droplet expansion at the ER-lipid droplet interface. J Cell Biol. 2012;198:895–911. doi: 10.1083/jcb.201201139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker AK, et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–852. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klemm RW, et al. A conserved role for atlastin GTPases in regulating lipid droplet size. Cell Reports. 2013;3:1465–1475. doi: 10.1016/j.celrep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenico M, Lloyd AT, Sharp PM. Codon usage in Caenorhabditis elegans: Delineation of translational selection and mutational biases. Nucleic Acids Res. 1994;22:2437–2446. doi: 10.1093/nar/22.13.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bietti G. Ueber familiaeres vorkommen von “retinitispunctata albescens” (verbunden mit “dystrophia marginalis cristallinea corneae”), glitzern des glaskoerpers und anderen degenerativen augenveraenderungen. Klin Mbl Augenheilk. 1937;99:737–757. [Google Scholar]
- 26.Li A, et al. Bietti crystalline corneoretinal dystrophy is caused by mutations in the novel gene CYP4V2. Am J Hum Genet. 2004;74:817–826. doi: 10.1086/383228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano M, Kelly EJ, Rettie AE. Expression and characterization of CYP4V2 as a fatty acid omega-hydroxylase. Drug Metab Dispos. 2009;37:2119–2122. doi: 10.1124/dmd.109.028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano M, Kelly EJ, Wiek C, Hanenberg H, Rettie AE. CYP4V2 in Bietti’s crystalline dystrophy: Ocular localization, metabolism of ω-3-polyunsaturated fatty acids, and functional deficit of the p.H331P variant. Mol Pharmacol. 2012;82:679–686. doi: 10.1124/mol.112.080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly EJ, Nakano M, Rohatgi P, Yarov-Yarovoy V, Rettie AE. Finding homes for orphan cytochrome P450s: CYP4V2 and CYP4F22 in disease states. Mol Interv. 2011;11:124–132. doi: 10.1124/mi.11.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lockhart CM, Nakano M, Rettie AE, Kelly EJ. Generation and characterization of a murine model of Bietti crystalline dystrophy. Invest Ophthalmol Vis Sci. 2014;55:5572–5581. doi: 10.1167/iovs.13-13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson-Rechavi M, Maina CV, Gissendanner CR, Laudet V, Sluder A. Explosive lineage-specific expansion of the orphan nuclear receptor HNF4 in nematodes. J Mol Evol. 2005;60:577–586. doi: 10.1007/s00239-004-0175-8. [DOI] [PubMed] [Google Scholar]
- 32.Svensk E, et al. PAQR-2 regulates fatty acid desaturation during cold adaptation in C. elegans. PLoS Genet. 2013;9:e1003801. doi: 10.1371/journal.pgen.1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma DK, et al. Acyl-CoA dehydrogenase drives heat adaptation by sequestering fatty acids. Cell. 2015;161:1152–1163. doi: 10.1016/j.cell.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 35.Krahmer N, et al. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 2011;14:504–515. doi: 10.1016/j.cmet.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szymanski KM, et al. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci USA. 2007;104:20890–20895. doi: 10.1073/pnas.0704154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fei W, et al. A role for phosphatidic acid in the formation of “supersized” lipid droplets. PLoS Genet. 2011;7:e1002201. doi: 10.1371/journal.pgen.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong J, et al. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol. 2011;195:953–963. doi: 10.1083/jcb.201104142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puri V, et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci USA. 2008;105:7833–7838. doi: 10.1073/pnas.0802063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puri V, et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 41.Guo Y, et al. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey AV, Sproll P. Pharmacogenomics of human P450 oxidoreductase. Front Pharmacol. 2014;5:103. doi: 10.3389/fphar.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.