Significance
Substance use disorders affect >8% of the US adult population. Mitigating this problem requires a better understanding of how these substances alter reward system structures such as the nucleus accumbens (NAc). Although classically studied in a neuron-centric manner, the immune system contributes to synaptic and behavioral changes associated with drug experience. We report that the pattern recognition molecule toll-like receptor 4 (TLR4) plays a role in NAc synaptic physiology and drug reward. We show NAc subregion-specific differences in basal synaptic properties consistent with alterations in induction of NMDA receptor-dependent synaptic plasticity in TLR4 knockout (TLR4.KO) animals. Furthermore, we show that TLR4.KO animals exhibit deficits in drug reward learning and confirm microglia as the primary cell type expressing TLR4.
Keywords: Toll-like receptor 4, neuroimmune, cocaine, nucleus accumbens, synapse
Abstract
Behavioral manifestations of drug-seeking behavior are causally linked to alterations of synaptic strength onto nucleus accumbens (NAc) medium spiny neurons (MSN). Although neuron-driven changes in physiology and behavior are well characterized, there is a lack of knowledge of the role of the immune system in mediating such effects. Toll-like receptor 4 (TLR4) is a pattern recognition molecule of the innate immune system, and evidence suggests that it modulates drug-related behavior. Using TLR4 knockout (TLR4.KO) mice, we show that TLR4 plays a role in NAc synaptic physiology and behavior. In addition to differences in the pharmacological profile of N-methyl-d-aspartate receptors (NMDAR) in the NAc core, TLR4.KO animals exhibit a deficit in low-frequency stimulation-induced NMDAR-dependent long-term depression (LTD). Interestingly, the synaptic difference is region specific as no differences were found in excitatory synaptic properties in the NAc shell. Consistent with altered NAc LTD, TLR4.KO animals exhibit an attenuation in drug reward learning. Finally, we show that TLR4 in the NAc core is primarily expressed on microglia. These results suggest that TLR4 influences NAc MSN synaptic physiology and drug reward learning and behavior.
The integration of dopaminergic and glutamatergic signals within the nucleus accumbens (NAc) is key to processing motivation, reward, and goal-directed behavior (1). Exposure to drugs of abuse leads to behavioral adaptations by recruiting molecular mechanisms of learning and memory within the reward system (2, 3). Adaptations in NAc synaptic properties following exposure to drugs of abuse have been extensively characterized in a circuit-specific manner (4–8). Although these studies revealed important insights into neuronal factors and alterations, they largely ignored the contribution of nonneuronal mechanisms to synaptic adaptations underlying drug-related behaviors. Recent studies have begun to elucidate the role of the innate immune system and, more specifically, microglia in drug reward behavior and physiology (9, 10). However, many questions remain regarding the role of the innate immune system in supporting synaptic reorganization within the reward circuitry.
Toll-like receptor 4 (TLR4) is a pattern recognition molecule of the innate immune system linked to alcohol (11), morphine (12), and cocaine (COC)-associated behaviors (13). However, the conclusions surrounding alcohol and COC have been disputed (14, 15). TLR4 recognizes gram-negative bacteria and “danger signals” released by damaged tissue (16). Beyond pathogen detection, TLR4 is associated with a wide range of behaviors including stress-induced depression (17), visceral pain (18), and opioid reward (12). Despite the growing number of studies pointing to TLR4’s involvement in various motivated behaviors, there has been no examination into its role in glutamatergic synaptic physiology. Additionally, the localization of TLR4 within NAc subregions is unknown. To address these questions, we performed cell-type–specific electrophysiology in the NAc core and shell subregions, field potential recordings, drug reward behavioral assays, and fluorescent in situ hybridization. Our findings suggest that TLR4 plays a role in basal NAc core synaptic physiology, plasticity, and drug reward behavior. We also confirm microglia as the primary cells expressing Tlr4 in the NAc core.
Results
TLR4.KO and Wild-Type Mice Exhibit Synaptic Differences in the NAc Core but Not Shell.
Within the NAc core and shell subregions, 90–95% of neurons are medium spiny neurons (MSN) expressing D1 or D2 dopamine receptors (1). Although similar in morphology, these MSNs differ in biochemistry, anatomical connectivity, and function (19–22). Furthermore, experience-dependent changes of glutamatergic synapses occur in a cell-type–specific manner (5, 6, 22–25), and activation of these NAc MSN subtypes differentially regulates drug reward behavior (3, 5, 23). Therefore, we addressed whether TLR4 influenced excitatory synaptic function within the NAc in a cell-type–specific manner.
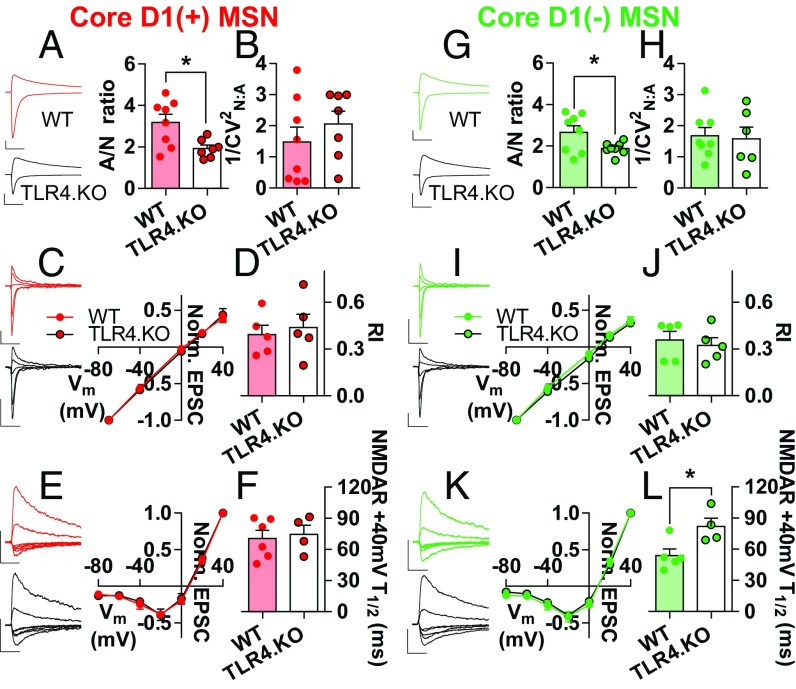
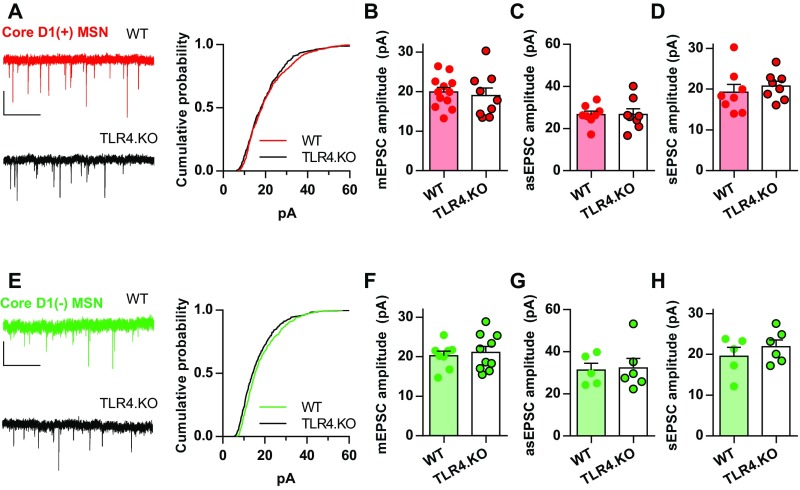
To assess cell-type–specific NAc MSN physiology, we bred wild-type (WT) and TLR4.KO mice to bacterial artificial chromosome transgenic mice expressing the tdTomato fluorophore driven by the D1 dopamine receptor promotor. Whole-cell voltage clamp recordings were made from MSNs that expressed [D1(+)] or lacked [D1(−)] tdTomato fluorescence. Presence or absence of fluorescence defines D1 and D2 MSNs as described previously (6, 26). To determine the impact of TLR4 expression on excitatory synaptic properties in the NAc core, we assessed pre- and postsynaptic properties. In NAc core D1(+) MSNs, TLR4.KO animals exhibit a significantly decreased AMPA receptor (AMPAR)/NMDA receptor [NMDAR; AMPAR/NMDAR (A/N)] ratio compared with WT (Fig. 1A). This suggests either a decrease in postsynaptic strength through reduced AMPAR transmission or an increase in NMDAR transmission. Altered AMPAR transmission may result from differential AMPAR stoichiometry or synaptic quantity. To test for differences in AMPAR stoichiometry, we assessed AMPAR current-voltage (I–V) relationships and rectification index (RI) (Fig. 1 C and D). We found no differences in AMPAR stoichiometry in D1(+) MSNs. To test for alterations in synaptic AMPAR function, we analyzed the amplitudes of miniature excitatory postsynaptic currents (mEPSC) and found no difference between WT and TLR4.KO D1(+) MSNs (Fig. S1 A and B). Not surprisingly, we also found no differences in spontaneous EPSC amplitudes (sEPSC; Fig. S1C). Whereas A/N ratios represent synaptic transmission from a subset of evoked afferents, mEPSCs and sEPSCs sample indiscriminately. To assess quantal events sampled from the evoked afferents used in A/N ratios, we used a Sr2+-based artificial cerebral spinal fluid (ACSF) to record and analyze electrically stimulated asynchronous EPSCs (asEPSC) (6, 27). We found no differences in asEPSC amplitudes on D1(+) MSNs (Fig. S1D). Together, these results suggest that AMPAR transmission is not altered in TLR4.KO D1(+) MSNs. Therefore, the decreased A/N ratios are likely caused by altered NMDAR transmission.
Fig. 1.
Altered synaptic properties in NAc core of TLR4.KO mice. (A, Left) Representative −70 mV and +40 mV evoked current traces from D1(+) MSNs of WT (red) and TLR4.KO (black) animals. The peak current at −70 mV and the current magnitude of 50 ms following current flow at +40 mV was used to calculate the A/N ratio (WT: n(cells)/N(mice) = 8/4; TLR4.KO: n/N = 7/4). (Right) Summary plot of D1(+) A/N ratio. (B) Summary ratio of 1/CV2NMDAR to 1/CV2AMPAR (1/CV2N:A) in D1(+) MSNs (WT: n/N = 8/4; TLR4.KO: n/N = 7/4). (C, Left) Representative isolated AMPAR current traces recorded between −70 to +40 mV from D1(+) MSNs. (Right) Mean AMPAR I–V plot from D1(+) MSNs. (D) RI: I+40 mV/I−70 mV (50 μM D-APV; n/N = 5/3 for both WT and TLR4.KO). (E, Left) Representative isolated NMDAR current traces recorded between −80 and +40 mV from D1(+) MSNs. (Right) Mean NMDAR I–V plot from D1(+) MSNs. (F) Time to half-peak of +40 mV NMDAR currents (10 µM NBQX; WT: n/N = 6/4; TLR4.KO: n/N = 4/3). (G–L) Representative traces and summary plots of D1(−) MSNs for A/N ratio (WT: green, n/N = 8/4; TLR4.KO: black, n/N = 8/4), 1/CV2N:A (WT: n/N = 8/4; TLR4.KO: n/N = 5/4), AMPAR I–V and RI (n/N = 5/3 for both WT and TLR4.KO), and NMDAR I–V and NMDAR time to half-peak (WT: n/N = 5/4; TLR4.KO: n/N = 4/3). All recordings were taken in the presence of picrotoxin (50 μM). (Scale bars: 100 pA; 50 ms.) *P < 0.05, unpaired t test.
Fig. S1.
No significant differences in quantal AMPAR transmission onto NAc core TLR4.KO MSNs. (A, Left) Representative traces of mEPSCs from of WT (red) and TLR4.KO (black) D1(+) MSNs. (Right) Cumulative probability plot of mEPSC amplitudes from D1(+) MSNs. (B) Summary plot of mEPSC amplitudes. (C) Summary plot of asEPSC amplitudes from D1(+) MSNs. (D) Summary plot of sEPSC amplitudes. (E–H) Representative traces, mEPSC amplitude cumulative probability and summary plot, asEPSC amplitude summary plot, and sEPSC amplitude summary plot for D1(−) MSNs (WT: green; TLR4.KO: black). All recordings were taken in the presence of picrotoxin (50 μM). mEPSCs were taken in the presence of tetrodotoxin (1 μM). asEPSCs and sEPSCs were recorded using low-Ca2+ ACSF with Sr2+. n/N = 5–12 cells from three to five animals per group. (Scale bars: 20 pA; 1 s.) P > 0.05 for all comparisons, unpaired t test.
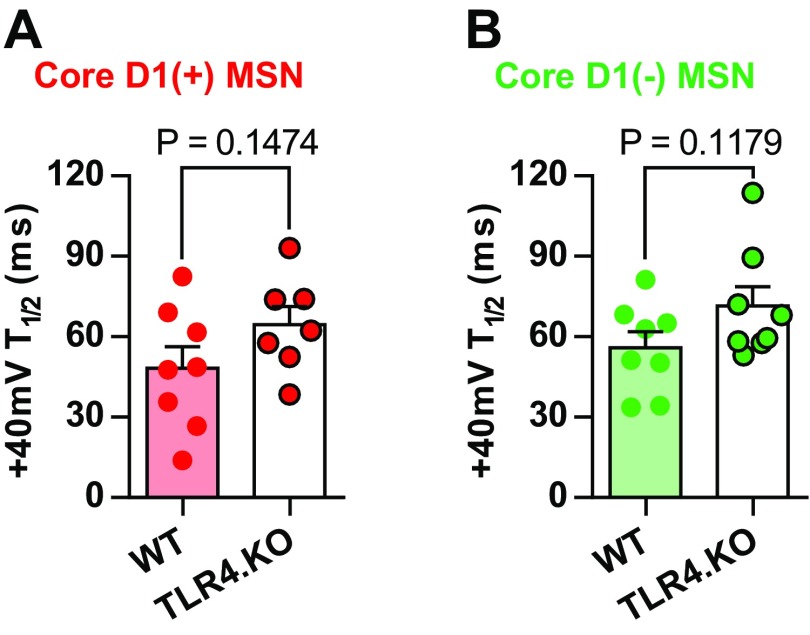
Differences in NMDAR transmission may stem from receptor number, function, stoichiometry, or expression of NMDAR-only synapses. The latter are known as “silent” synapses and are an important substrate for metaplasticity (24). To assess potential differences in silent synapses, we calculated the ratio of 1/CV2NMDAR to 1/CV2AMPAR (1/CV2N:A) as described previously (26). We found no evidence for differences in silent synapses between D1(+) MSNs from WT and TLR4.KO animals (Fig. 1B). Alterations in NMDAR stoichiometry are associated with experience-dependent changes in NAc MSN physiology (19). Therefore, we assessed NMDAR I–V relationships and decay kinetics for initial investigation into NMDAR stoichiometry as a potential cause of altered postsynaptic strength. We found no significant differences between WT and TLR4.KO animals in the NMDAR I–V relationship of D1(+) MSNs (Fig. 1E). However, we observed a trend toward increased time to half-peak of +40-mV dual component responses taken from the A/N ratios (Fig. S2; WT = 48.2 ± 8.010 ms; TLR4.KO = 64.56 ± 6.682 ms; P = 0.1474, unpaired t test); to our surprise, we found no differences in isolated NMDAR decay kinetics in TLR4.KO D1(+) MSNs (Fig. 1F).
Fig. S2.
TLR4.KO mice trend toward increased decay kinetics of dual-component (AMPAR and NMDAR) currents. (A) Time to half-peak of dual component currents at +40 mV from WT and TLR4.KO D1(+) MSNs (WT: n/N = 8/4, TLR4.KO: n/N = 7/4). (B) Time to half-peak of dual component currents at +40 mV from WT and TLR4.KO D1(−) MSNs (WT n/N = 8/4; TLR4.KO n/N = 8/4). P > 0.05 for all comparisons, unpaired t test.
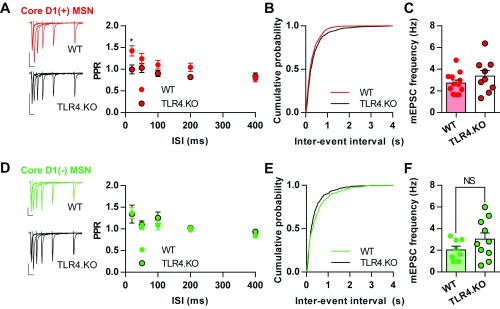
To characterize presynaptic properties of TLR4.KO animals, we examined glutamate release probability using paired-pulse ratios (PPR) and mEPSC frequency. PPR is inversely proportional to the presynaptic release probability. We observed that D1(+) MSNs from TLR4.KO animals have a decreased PPR at the 20-ms but not at the 50-, 100-, 200-, or 400-ms interstimulus intervals (ISI) (Fig. S3A). However, this result was not corroborated by mEPSC frequency (Fig. S3 B and C; WT = 2.727 ± 0.2791 Hz; TLR4.KO = 3.367 ± 0.5189 Hz; P = 0.2598, unpaired t test). Together, these data suggest that TLR4.KO animals have altered postsynaptic properties, possibly due to altered NMDAR transmission, in D1(+) MSNs in the NAc core.
Fig. S3.
Presynaptic properties of NAc core TLR4.KO MSNs. (A, Left) Representative paired pulse evoked EPSCs from WT (red; n/N = 15/6) and TLR4.KO (black; n/N = 7/4) MSNs. ISI included 20, 50, 100, 200, and 400 ms. The ratio of the second stimulus-evoked current over the first gives the PPR summarized. (Right) Summary plot of PPR data. (B) Cumulative probability plot of mEPSC frequency from D1(+) MSNs. (C) Summary plot of mEPSC frequency from D1(+) MSNs (WT: n/N = 12/5; TLR4.KO: n/N = 9/4). (D–F) Representative traces, summary plots, and cumulative probability plot for PPR (WT: green, n/N = 14/5; TLR4.KO: black, n/N = 7/4) and mEPSC frequency (WT: n/N = 8/5; TLR4.KO: n/N = 10/4) from D1(−) MSNs. (Scale bars: 100 pA, 50 ms.) *P < 0.05, NS: not significant, two-way ANOVA with Sidak post hoc test for PPR, unpaired t test for mEPSC frequency.
Cell-type–specific differences in NAc MSN synaptic physiology underlie behavioral differences in reward and motivation (5, 20, 22, 28). Thus, we also assessed synaptic properties in NAc core D1(−) MSNs of TLR4.KO animals. We found that, similar to the D1(+) cells, D1(−) MSNs exhibit a decreased A/N ratio (Fig. 1G). In this population of MSNs, no differences were found for 1/CV2N:A, AMPAR I–V, RI, mEPSC amplitude, sEPSC amplitude, asEPSC amplitude, PPR, mEPSC frequency, and NMDAR I–V (Fig. 1 G–L, Fig. S1 E–H, and Fig. S3 D–F). However, we found that TLR4.KO D1(−) MSNs exhibit significantly slower NMDAR decay kinetics compared with WT (Fig. 1L). These observations suggest that TLR4.KO MSNs exhibit altered NMDAR stoichiometry without alterations in AMPAR transmission or presynaptic release properties.
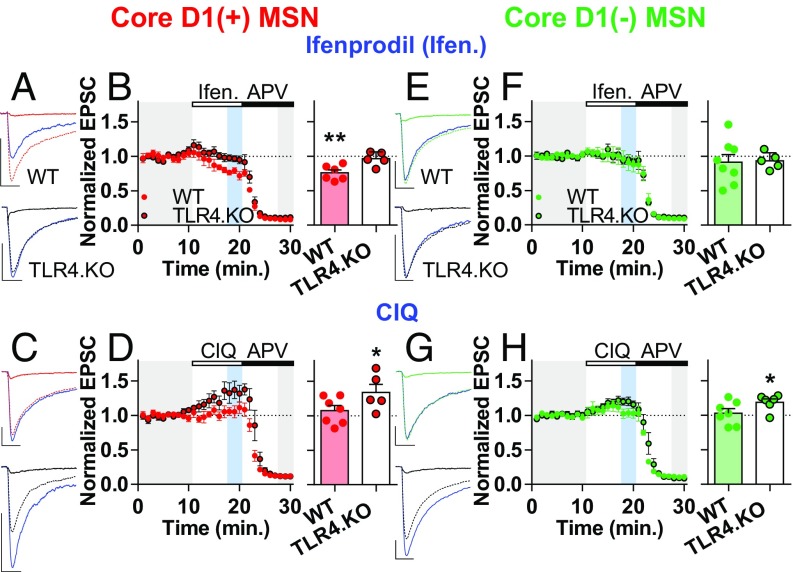
The specific GluN2 subunit greatly influences NMDAR deactivation kinetics. GluN2A subunits exhibit the fastest deactivation kinetics with the widest abundance in the adult synapse whereas GluN2D has the slowest deactivation kinetics with GluN2B and 2C in the middle (29). To determine the functional NMDAR profile, we applied the GluN2B antagonist Ifenprodil (3 μM) (23) to pharmacologically isolated NMDAR currents from NAc MSNs. This was followed by D-APV (50 μM) to confirm that recorded currents were from NMDARs. If TLR4.KO MSNs exhibit increased GluN2B function, then Ifenprodil will cause a greater depression of NDMAR transmission in these cells. Ifenprodil caused a significant decrease in NMDAR currents from WT D1(+) MSNs (Fig. 2 A and B). However, we found TLR4.KO D1(+) MSNs to be insensitive to Ifenprodil (Fig. 2 A and B). Although less common, increased NMDAR decay kinetics may also be caused by GluN2C or GluN2D subunits (29). To test for this, we assessed the effect of the GluN2C/D-positive allosteric modulator CIQ (30 μM) (6) on isolated NMDAR currents from D1(+) MSNs. Whereas CIQ did not cause a significant difference from baseline in WT MSNs, it caused a significant potentiation in TLR4.KO cells (Fig. 2 C and D). The Ifenprodil and CIQ experiments were also repeated on D1(−) MSNs. Ifenprodil did not cause any significant difference from baseline in either WT or TLR4.KO D1(−) MSNs (Fig. 2 E and F). CIQ did not significantly alter NMDAR transmission from WT D1(−) MSNs; however, the compound caused a modest yet significant increase in NMDAR currents in TLR4.KO D1(−) MSNs (Fig. 2 E–H). Taken together, these Ifenprodil/CIQ experiments provide evidence for decreased GluN2B function on TLR4.KO D1(+) MSNs along with increased GluN2C/D function in both D1(+) and D1(−) cells. Our finding that TLR4.KO animals express altered NMDAR properties on both subtypes of MSNs compared with WTs suggests a shared mechanism through which TLR4 affects synaptic physiology. Altered NMDARs in NAc MSNs are associated with behavioral adaptations affecting motivation, including chronic pain (23), COC experience (6), and chronic intermittent ethanol exposure (30). A basal difference in NMDAR transmission on both MSN types raises the possibility that TLR4.KO animals may exhibit altered learning mechanisms related to NAc core-dependent motivational and reward behavior.
Fig. 2.
Altered NAc core NMDAR pharmacological profile in TLR4.KO mice. (A) Representative D1(+) MSN NMDAR traces from WT (red) and TLR4.KO (black) animals overlaid with traces following Ifenprodil (3 μM; blue) and APV (50 μM; solid nonblue) application. (B, Left) Summary plot of D1(+) Ifenprodil experiments. (Right) Quantification of Ifenprodil response on the normalized EPSCs (WT: n/N = 6/3; TLR4.KO: n/N = 5/4). (C) Representative D1(+) MSN NMDAR traces from WT and TLR4.KO animals overlaid with traces following CIQ (30 μM; blue) and APV (solid nonblue) application. (D, Left) Summary plot of D1(+) CIQ experiments. (Right) Quantification of CIQ response on normalized EPSCs (WT: n/N = 7/4; TLR4.KO: n/N = 5/4). (E–H) Representative traces, summary plots, and quantification of D1(−) MSNs for Ifenprodil (WT: green, n/N = 8/5; TLR4.KO: black, n/N 5/4) and CIQ experiments (WT: n/N = 7/4; TLR4.KO: n/N = 6/4). All experiments were performed holding the cell at −50 mV using a low-Mg2+ solution with picrotoxin (50 μM) and NBQX (10 µM). (Scale bars: 100 pA; 50 ms.) *P < 0.05, **P < 0.01, one-sample t test vs. baseline value of 1.0.
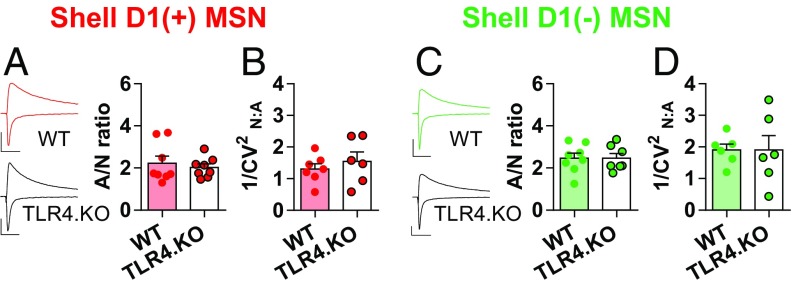
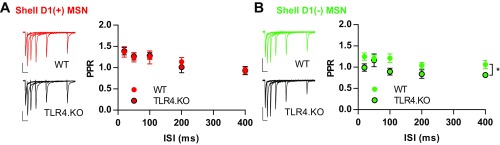
The anatomy and physiology of the NAc shell is distinct from the NAc core with different hippocampal, prefrontal cortical, and midbrain dopaminergic inputs (1, 8, 31). Thus, it is not surprising that experience-dependent changes in MSN synaptic physiology differ between the subregions (8, 26). Unlike the core subregion, we observed no postsynaptic differences between TLR4.KO and WT D1(+) and D1(−) NAc shell MSNs as assessed through A/N ratios and 1/CV2N:A (Fig. 3). We did, however, observe a reduction of PPR in TLR4.KO D1(−) MSNs [Fig. S4; genotype effect F(1,13) = 6.632, P = 0.0231, two-way repeated measures ANOVA] suggesting altered presynaptic release probability. We conclude that TLR4.KO animals exhibit an alteration in postsynaptic properties in both MSN subtypes of the NAc core but not shell subregions.
Fig. 3.
Lack of postsynaptic differences NAc shell. (A, Left) Representative evoked current traces recorded from −70 mV and +40 mV from D1(+) MSNs of WT (red) and TLR4.KO (black) animals. (Right) Summary plot of A/N ratios (WT: n/N = 8/5; TLR4.KO: n/N = 8/4). (B) Summary plot of 1/CV2N:A in D1(+) MSNs (WT: n/N = 7/4; TLR4.KO: n/N = 6/4). (C and D) Representative traces and summary plots of D1(−) MSNs for A/N ratio (WT: green, n/N = 8/5; TLR4.KO: black, n/N = 7/4), 1/CV2N:A (WT: n/N = 6/5; TLR4.KO: n/N = 6/4), All recordings taken in the presence of picrotoxin (50 μM). (Scale bars: 100 pA; 50 ms.) P > 0.05 for all comparisons, unpaired t test.
Fig. S4.
Presynaptic release probabilities of WT and TLR4.KO MSNs in the NAc shell. (A, Left) Representative paired pulse evoked EPSCs from D1(+) WT (red; n/N = 8/5) and TLR4.KO (black; n/N = 7/4) MSNs. (Right) Summarized PPRs. (B) Representative PPRs and summary from D1(−) MSNs (WT: green, n/N = 7/4; TLR4.KO: black, n/N = 8/4). All recordings taken in the presence of picrotoxin (50 μM). (Scale bars: 100 pA; 50 ms.) *P < 0.05, genotype effect, two-way repeated measures ANOVA.
TLR4.KO Mice Exhibit Long-Term Depression Deficits in the NAc Core.
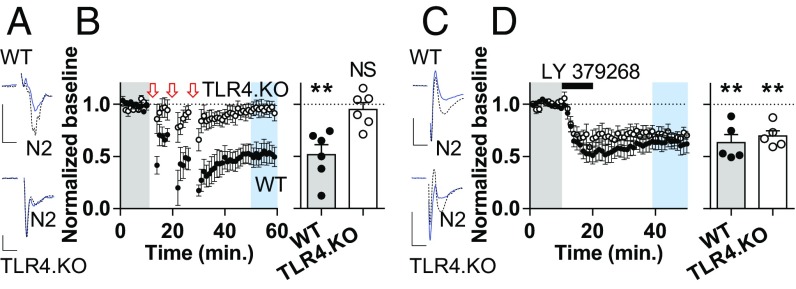
Synaptic plasticity is a substrate for learning and memory. Within the NAc, perturbations in plasticity mechanisms are associated with alterations in reward and motivation-related behaviors (20, 22, 23). In addition, behavioral experiences related to stress (22), pain (23), and drugs of abuse (6–8, 24, 27) alter plasticity mechanisms in the NAc. With evidence for altered NMDAR transmission on NAc core MSNs, we thought that TLR4.KO animals might exhibit changes in NMDAR-dependent synaptic long-term depression. To test this hypothesis, we performed extracellular field potential recordings from the NAc core. Using a well-established NMDAR-dependent low-frequency (LFS) stimulation protocol (3 × 3 min, 5 Hz stimulation of NAc afferents with 5 min between each LFS train) (22, 27, 32), we assessed long-term depression (LTD) in WT and TLR4.KO mice. In support of our hypothesis, this stimulation protocol induced a depression of evoked field potential responses in slices from WT animals but not TLR4.KO animals (Fig. 4 A and B). To confirm that this lack of LFS-LTD is not due to general lack of plasticity mechanisms in TLR4.KO animals, we assessed LTD dependent on group II metabotropic glutamate receptors (mGluR). Application of the group II mGluR agonist LY 379268 (200 nM, 10 min) (33) caused a significant reduction in field potentials in both WT and TLR4.KO animals (Fig. 4 C and D). Thus, the NAc core of TLR4.KO animals exhibit impairments in NMDAR-dependent LTD without deficits in group II mGluR-dependent LTD. In combination with our results showing differences in NMDAR subunit composition, these data suggest the lack of LTD in TLR4.KO is due to impairments in induction mechanisms. Loss of TLR4 function therefore hinders the ability of LFS to reduce synaptic strength in NAc core MSNs.
Fig. 4.
Impaired NMDAR-dependent LTD and intact group II mGluR LTD in the NAc core of TLR4.KO mice. (A) Representative traces from baseline (dashed black) and post LFS (blue) from WT and TLR4.KO field potential experiment. (B, Left) Summary plot of NMDAR LTD (LFS); 3 × 3 min, 5 Hz separated by 5 min) from NAc core. Arrows denote LFS. (Right) Quantification of LFS experiments (WT: nexperiments/N = 6/5; TLR4.KO: n/N = 6/4). (C and D) Representative traces, summary plot, and quantification of group II mGluR agonist application (LY 379268, 200 nM, 10 min) effect on N2 responses. (WT: n/N = 5/4; TLR4.KO: n/N = 5/3). All recordings taken in the presence of picrotoxin (50 μM). (Scale bars: 0.4 mV; 4 ms.) NS: not significant. **P < 0.01, one-sample t test of normalized N2 from last 10 min of experiment vs. baseline value of 1.0.
TLR4.KO Mice Exhibit Deficits in Drug Reward Learning.
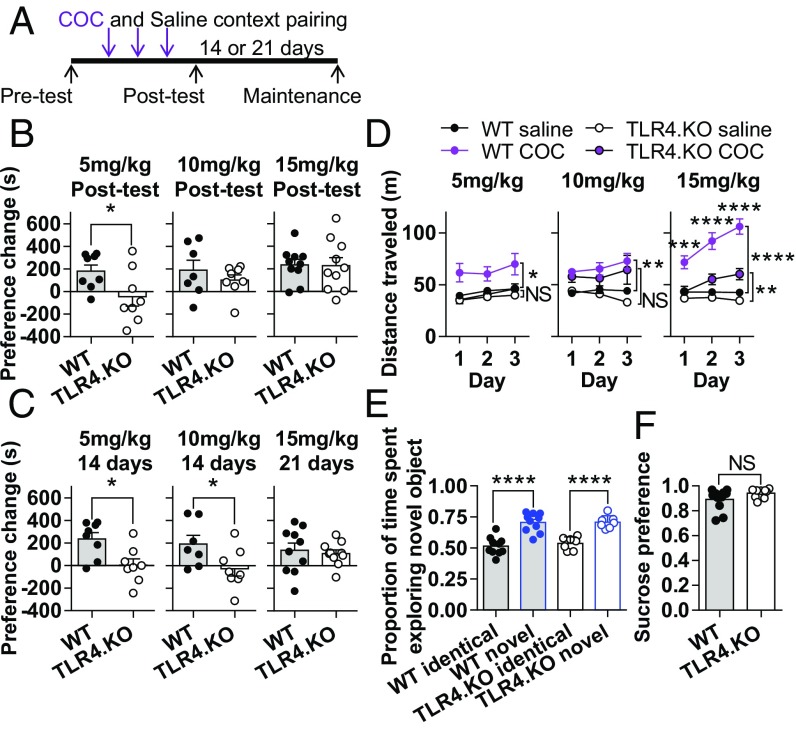
The NAc core is a nexus for drug-seeking and motivational behavior (1); therefore, the inability to regulate synaptic strength in this region has implications in associated learning. Deficits in NMDAR-dependent synaptic plasticity mechanisms are associated with altered drug reward behavior (33, 34). With evidence for a deficit in NAc core NMDAR-dependent LTD, we hypothesized that TLR4.KO animals exhibit altered drug reward learning. To test this, we performed a COC place conditioning (conditioned place preference, or CPP) assay as previously described (19) (Fig. 5A). In this assay, all mice were given three injections of COC and three injections of saline (one of each/context pairing day; Fig. 5A). We found that TLR4.KO mice have a significant attenuation in preference following conditioning with a 5-mg/kg dose (Fig. 5B). To test whether this result signifies an impairment or a complete loss of COC reward learning, we examined two higher doses of COC (10 mg/kg and 15 mg/kg). In support of TLR4.KO animals having decreased COC reward learning, we found no significant differences in CPP between WT and TLR4.KO animals for 10 mg/kg and 15 mg/kg COC (Fig. 5B).
Fig. 5.
TLR4.KO mice exhibit attenuated drug reward learning without deficits in episodic memory or expression of anhedonia. (A) Timeline of COC CPP at three doses of COC (5, 10, and 15 mg/kg). (B) Preference changes assessed at the posttest time point compared with pretest. *P < 0.05, unpaired t test. (C) Preference changes at the maintenance time point compared with pretest. *P < 0.05, unpaired t test. (D) Locomotor response to saline and COC during context pairing days: 5 mg/kg WT saline vs. COC F(1,14) = 5.851, P = 0.0298; 5 mg/kg TLR4.KO saline vs. COC F(1,14) = 0.4099, P = 0.5324; 10 mg/kg WT saline vs. COC F(1,12) = 14.55, P = 0.0025; 10 mg/kg TLR4.KO saline vs. COC F(1,14) = 4.178, P = 0.0602; 15 mg/kg WT saline vs. COC F(1,18) = 92.46, P < 0.0001; 15 mg/kg TLR4.KO saline vs. COC F(1,18) = 13.83, P = 0.0016, two-way repeated measures ANOVA. ***P < 0.001, ****P < 0.0001, Sidak post hoc test for TLR4.KO COC vs. WT COC. (E) Proportion of time(s) spent exploring objects in novel object recognition task. Genotype effect F(1,15) = 0.1981, P = 0.6626; object effect F(1,15) = 87.32, P < 0.0001; WT identical vs. novel, t = 7.658, P < 0.0001; TLR4.KO identical vs. novel, t = 5.777, P < 0.0001, two-way repeated measures ANOVA. ****P < 0.0001, Sidak post hoc test. (F) Summary of 18-h two-bottle choice sucrose preference test. NS: not significant, unpaired t test. n = 7–11 animals/group for all experiments.
Additionally, TLR4.KO animals did not maintain a change in preference for 10 mg/kg COC when assessed 14 d later (Fig. 5C), suggesting that TLR4.KO animals may have a decreased persistence of drug reward learning. Furthermore, TLR4.KO animals display less COC-induced hyperactivity than WT mice (Fig. 5D). The differences between genotypes are most evident at the highest COC dose tested.
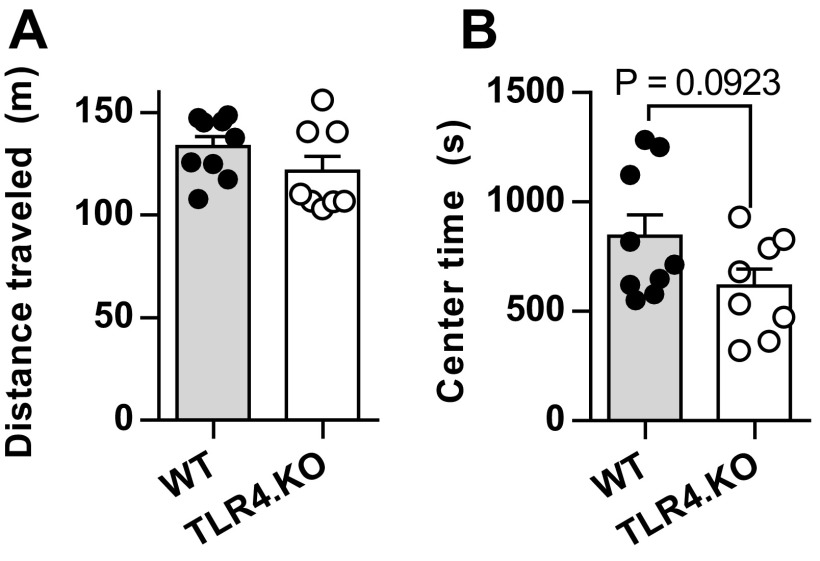
Importantly, these reductions in preference are not due to differences in episodic memory as TLR4.KO animals do not significantly differ in preference change from WT mice at 15 mg/kg COC (Fig. 5B) and show no deficits in novel object recognition, a hippocampus-associated task (Fig. 5E). In addition, anhedonia is not a likely cause for impaired drug reward learning as we found no differences between WT and TLR4.KO animals for the sucrose preference test (Fig. 5F). Finally, to control for basal behavioral states, we assessed open field locomotor activity and center time. No differences were observed for distance traveled. However, there was a trend toward decreased center time in the TLR4.KO animals (WT = 843.5 ± 98.52 s; TLR4.KO = 615.3 ± 79.72 s; P = 0.0923, unpaired t test) (Fig. S5 A and B). These results support our hypothesis that TLR4.KO animals exhibit specific alterations in drug reward learning.
Fig. S5.
No significant differences seen in distance traveled or center time as assessed by 60-min open field test. (A) Locomotor activity of WT and TLR4.KO animals. (B) Total center time of same assessed in same test. WT: n = 9; TLR4.KO: n = 8. P > 0.05 for both measurements, unpaired t test.
TLR4 in NAc Core Expressed Primarily on Microglia.
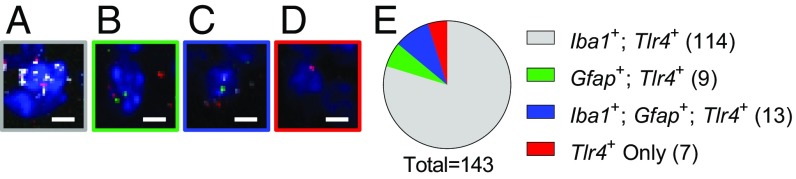
To determine where Tlr4 was expressed within the NAc core, we performed multiplex fluorescent in situ hybridization from frozen NAc core sections taken from naive WT mice. Prior studies looking into Tlr4 expression in the NAc used fluorescence activated cell sorting followed by qPCR but did not differentiate between the core and shell subregions (35). Consistent with results from the NAc as a whole (35), we found that the majority (∼80%) of TLR4-expressing cells in the NAc core could be classified as microglia (Fig. 6; Tlr4+, Iba1+, Gfap−; n(cells) = 114/143; N(animals) = 4) [where n is the number of cells counted and N is the number of mice]. The rest included astrocytes (Tlr4 +, Gfap+, Iba1−; n = 9/143), cells expressing both astrocytic and microglial markers (Tlr4+, Iba1+, Gfap+; n = 13/143), and some other cell populations (Tlr4+, Iba1−, Gfap−; n = 7/143). These results suggest the possibility of an interaction between NAc core MSNs and microglia mediating the synaptic and behavioral effects observed in TLR4.KO animals.
Fig. 6.
NAc core Tlr4 expression is primarily on microglia. Multiplex fluorescent in situ hybridization was performed using RNAscope to detect mRNA transcripts for Tlr4 (red), Iba1 (white), and Gfap (green) on a background of DAPI (blue). (A) Representative Iba1+, Tlr4+ cell. (B) Representative Gfap+, Tlr4+ cell. (C) Representative Iba1+, Gfap+, Tlr4+ cell. (D) Representative Tlr4+ cell. (E) Summary of cell counts (n/N = 143/4); 79.72% of quantified cells were Iba1+, Tlr4+. (Scale bars: 5 μm.)
Discussion
In the present study, we used TLR4.KO and cell-type–specific reporter mice to investigate the interaction between the innate immune system and key elements of the reward circuit. We provide evidence that TLR4 significantly influences NAc core NMDAR synaptic transmission, synaptic plasticity, and COC-induced behavioral plasticity. Whereas we observed altered NMDAR transmission and plasticity in the NAc core, we found no postsynaptic differences between WT and TLR4.KO animals in the neighboring NAc shell. Furthermore, we found that these mice exhibit blunted behavioral responding to COC and that NAc core Tlr4 is primarily expressed on microglia. These results suggest that TLR4, likely expressed on microglia, is a molecular regulator in the NAc associated with reward learning.
TLR4 and Drug Reward Behavior.
Although numerous neuron-centric studies have revealed important insights into how drugs of abuse alter behavior and NAc physiology, far less is known about the role of the innate immune system in this sequela. One intriguing molecular target is the pattern recognition molecule TLR4. Along with its function in innate immunity, research suggests that TLR4 may play a role in reward behaviors associated with alcohol (11), morphine (12), and COC (13). Northcutt et al. (13) demonstrated that TLR4 antagonists diminish COC self-administration in rats through an effect mediated by the ventral tegmental area. These investigators also found COC reward behavior diminished in the C3H/HeJ mouse line. Although the C3H/HeJ line is deficient in TLR4, these mice are also homozygous for an inversion spanning 20% of chromosome 6 (36), making it difficult to draw specific conclusions. A later study also found that the pharmacologic reduction in COC reward in rats may be due to nonspecific effects as the same doses TLR4 antagonists also caused decreased food reward behavior (14). Additionally, TLR4’s role in alcohol reward has also been disputed (15). These discrepancies prompt continued investigation of the nature of TLR4’s involvement in drug reward.
TLR4 and NAc Synaptic Physiology.
The NAc is a brain region that integrates information on motivation and reward to initiate goal-directed behavior (1). Virtually every drug of abuse causes changes within the NAc (2, 3), and reversal of synaptic changes leads to reversal of drug reward learning (5, 7, 8). These changes occur in a subregion (8, 37) and cell-type–specific (20, 24, 25) manner. We found that TLR4.KO mice had decreased A/N ratios in both D1(+) and D1(−) MSNs in the NAc core but not shell. With no observed differences in AMPAR function, we conclude that these differences are due to alterations in NMDARs.
Our results from TLR4.KO animals showed significantly slower isolated NMDAR decay kinetics in D1(−) MSNs and a trend toward slower dual component (AMPAR and NMDAR) kinetics in D1(+) MSNs. As increased NMDAR decay kinetics are commonly associated with up-regulation of GluN2B subunits (23) but could also be due to greater functional expression of GluN2C/D, we hypothesized an up-regulation of their function TLR4.KO MSNs. NMDAR pharmacology experiments showed decreased GluN2B function in D1(+) MSNs from TLR4.KOs along with increased GluN2C/D function in both MSN populations. As GluN2C and GluN2D NMDAR subunits exhibit deactivation kinetics even slower than that of GluN2B (38), their up-regulation in TLR4.KO MSNs provides an explanation for decreased A/N ratios.
Another possibility for decreased A/N ratios is an increase in NMDAR-only– containing silent synapses. In the neighboring NAc shell, silent synapses generated in the context of COC exposure are enriched with the GluN2B NMDAR subunit (24). We found no significant differences in 1/CV2N:A, an estimation of the number of silent synapses (26), along with reduced GluN2B function on D1(+) MSNs from TLR4.KOs. Together, these results argue against increased NMDAR transmission due to increased silent synapse number. Although we did not test for synaptic NMDAR density, our results point toward altered NMDAR subunit stoichiometry contributing to the differences observed for A/N ratios. Alterations of NAc NMDAR GluN2 subunits are associated with behavioral paradigms known to affect motivation and reward. In the NAc core, this includes increased GluN2B function in D1(−) MSNs following chronic pain (23), increased GluN2C/D function of thalamic inputs onto D1(+) MSNs following COC exposure and withdrawal (6), and aversion-resistant ethanol intake requiring GluN2C function on prefrontal-cortical and insular inputs (39). In addition, studies performed examining the neighboring shell subregion or the NAc as a whole provide additional evidence for the importance of GluN2 subunits on motivation/reward behaviors (24).
With basal differences in NMDAR subunit profiles, we hypothesized that TLR4.KO animals exhibit alterations in NMDAR-dependent plasticity. This idea was supported through extracellular field potential recordings revealing a lack of NMDAR-associated LFS-LTD in TLR4.KO mice. Much controversy remains on the role of the specific NMDAR subunits in scaling synaptic plasticity from long-term potentiation (LTP) to LTD where some suggest LTD is dependent on GluN2B whereas GluN2A is important for LTP (29). An intriguing possibility is that TLR4 regulates the NMDAR-dependent threshold for inducing LTP vs. LTD. Rather than prevent plasticity, reduced GluN2B and increased GluN2C/D function in TLR4.KO mice may shift the propensity for synaptic changes. Further experiments are necessary to test this possibility. In combination, our experiments suggest TLR4.KO animals exhibit a NAc core-specific alteration of NMDAR transmission and a deficit in NMDAR-dependent synaptic plasticity. Thus, TLR4 may play a role in the developmental profile of NMDARs at NAc core synapses.
A lack of LFS-LTD in the NAc core is associated with a range of behavioral manipulations affecting motivation and reward including COC experience (33), exposure to palatable foods (32), and chronic restraint stress (22). However, many of these plasticity assays were only examined following behavioral experiments, and distinctions between LTD induction and expression were not always clarified. Psychostimulants such as COC can depress excitatory synapses in the NAc core (34), and blocking NMDAR-dependent LTD expression through inhibiting AMPAR endocytosis prevents locomotor sensitization (40). On the other hand, loss of NMDAR-dependent LTD is associated with COC-exposed mice exhibiting signs of “addiction” (drug seeking, motivation, and continued use despite negative consequences) compared with COC-exposed “nonaddicted” mice (33). Given the range of behavioral adaptations associated with NMDAR-dependent plasticity, it is difficult to make a specific prediction for the behavioral effect of a presumed change in the NMDAR-dependent LTD induction mechanism in naive TLR4.KO animals. We found that TLR4.KO mice express attenuations in COC CPP and associated locomotor sensitization, both noncontingent drug reward learning processes.
To confirm the cell type(s) expressing TLR4 in the NAc, we performed fluorescent in situ hybridization on brain sections. We found that the majority of Tlr4-expressing cells in the NAc core are microglia, echoing fluorescent-assorted cell sorting/qPCR results from the NAc as a whole (35). Given the many differences seen in WT mice when examining NAc synaptic properties in an input-specific manner, it will be interesting to see whether TLR4 and/or microglia influence any part of this physiology and associated behaviors.
Microglia, Immune Signaling, and Drug Reward.
Throughout the brain, microglia and their associated cytokines play important roles in modulation of synapses via multiple mechanisms. These include complement-mediated pruning of spines during development of the reticulogeniculate system (41), regulation of synaptogenesis/elimination in the motor cortex through microglial BDNF (42), and the homeostatic scaling up of synapses in the hippocampus through TNFα (43). Microglia also exhibit brain-region–specific differences in cellular aging and transcriptional profiles (44), which may underlie observed synaptic differences between the NAc core and shell. Importantly, microglia have also been implicated in drug addiction. Methamphetamines induce activation of microglia in humans (9). Additionally, rodent models also support a role for microglial adaptation following COC administration (45).
In the NAc core, microglial TNFα scales down synaptic strength on D1(+) MSNs in response to COC, and lacking this cytokine exacerbates COC locomotor sensitization (10). This suggests that TNFα combats drug-induced increases in synaptic strength. TNFα is one of several known cytokines released in response to ligands binding TLR4 (46). TNFα from peripheral monocytes can also influence motor learning and cortical dendritic spine dynamics independently of microglia (47), suggesting a complex interplay between central and peripheral immune modulators. We showed that TLR4.KO animals express a basal difference in NAc NMDARs with an attenuation of COC reward learning and associated motor changes. This suggests that a mechanism independent of TNFα underlies the associated findings. Given the importance of microglia in shaping synaptic physiology and behavior along with TLR4’s role in detecting factors including damaged tissue signals in addition to pathogens (16), it is tempting to think about loss of a constitutively active signaling cascade or altered gut-brain communication (48) perturbing microglia to cause a basal change in NAc physiology.
In summary, we show that TLR4 influences NAc synaptic function and COC behavior. These results expand upon the spectrum of immunologic communications with the nervous system that modulates behavior. Given TLR4’s link to conditions affecting motivation and reward such as drug exposure and depression (12, 17), it is tantalizing to imagine the NAc as a nexus for neuroimmune interactions in such pathologies.
Materials and Methods
For more detailed descriptions of materials and methods, see SI Materials and Methods.
Mice.
TLR4.KO, WT, and Drd1a-tdTomato male mice aged 6–12 wk were used in accordance with policies approved by the Institutional Animal Care and Use Committee at Vanderbilt University. All mice were on a C57BL/6 background.
Electrophysiology.
Electrophysiological recordings from NAc sagittal slices (250 µm) were performed similar to previously described (6, 20, 49) (SI Materials and Methods).
Histology.
Fresh-frozen 16-µm mouse brain sections were used. All procedures for multiplex fluorescent in situ hybridization were performed per RNAscope fluorescent multiplex assay protocol (Advanced Cell Diagnostics Inc.) (SI Materials and Methods). Probes used included mouse Tlr4, Gfap, and Iba1 (Aif1).
Behavior.
Conditioned place preference (19), novel object recognition (50), sucrose preference (51), and open field tests (19) were performed similarly to previously described protocols (SI Materials and Methods).
Data Analysis.
All data are presented as a mean ± SEM. Individual data points represent individual cells for whole-cell physiology, slices for field potentials, and animals for behavioral assays. Sample sizes are presented as n/N, where n is the number of cells (whole cell) or slices (field potentials) and N is the number of mice. Statistical significance was tested using one-sample t tests, unpaired t tests, two-way, and repeated measures ANOVA with further comparisons made using Sidak post hoc tests. Representative traces have had stimulus artifacts removed.
SI Materials and Methods
Animals.
Male mice aged 6–12 wk were used. Animals were separated by sex and housed together in groups of two to five per cage on a 12-h/12-h light/dark cycle. All experiments were performed during the light cycle. WT C57/B6 mice used in the behavior experiments were purchased from the Jackson Laboratory. TLR4.KO animals on a C57/B6 background were generously donated by Luc Van Kaer, Vanderbilt University, Nashville, TN, and maintained in our colony. For all electrophysiology experiments, these mouse lines were also bred to carry a bacterial artificial chromosome carrying the tdTomato fluorophore under control of the Drd1a promoter (6). All mice are on a C57BL/6 background.
Histology.
RNAscope fluorescent in situ hybridization was performed on fresh-frozen tissue similar to previously described (52). Mice were anesthetized and decapitated, and the brains were rapidly extracted into an ice-cold sucrose solution (in mM: sucrose 182.6, NaCl 19.8, KCl 0.5, MgCl2 1.0, CaCl2 2.0, NaH2PO4 1.2, NaHCO3 25.9, glucose 10.0). Brains were blocked, covered with optimum cutting temperature compound (Sakura Finetek), and rapidly frozen with Super Friendly Freeze-It (Fisher Scientific). The 16-µm coronal sections were made using a HM 505N cryostat (Microm International).
Multiplex RNAscope probe binding, amplification, and mounting were performed per written instructions. Probes against the following mRNA were used: Aif1 (Iba1), Gfap, and Tlr4. Confocal microscopy was performed using a Fluoview FV-1000.
Electrophysiology.
Whole-cell voltage clamp recordings were performed from the NAc core and shell similar to as previously described (6, 20). Briefly, parasagittal slices (250 µm) were prepared from mouse brains using a Leica VT1200 vibratome submerged in an oxygenated (95% O2; 5%CO2) ice-cold sucrose solution (in mM: sucrose 182.6, NaCl 19.8, KCl 0.5, MgCl2 1.0, CaCl2 2.0, NaH2PO4 1.2, NaHCO3 25.9, glucose 10.0). Slices then sat undisturbed for >1 h in an oxygenated ACSF solution (NaCl 118.93, KCl 2.49, MgCl2 1.30, CaCl2 2.5, NaH2PO4 1.0, NaHCO3 12.21, glucose 23.57) in a holding chamber (∼25° C) before recording.
We performed whole-cell voltage clamp recordings from NAc core and shell MSNs using IR-DIC video microscopy. The NAc core and shell were identified using the anterior commissure as a landmark. The D1-tdTomato-positive (D1+) MSNs were identified through the presence of the tdTomato fluorophore. Cells lacking the tdTomato fluorophore [D1(−)] are defined as putative D2 MSNs as 90–95% of neurons in the NAc are MSNs roughly divided into those expressing D1 or D2 dopamine receptors (1). Whole-cell configuration was achieved using electrodes (3.0–7.0 MΩ) filled with a cesium-based internal solution (in mM: CsMeSO3 120, CsCl 15, NaCl 8, Hepes 10, EGTA 0.2, TEA-Cl 10, MgATP 4, NaGTP, Spermine 0.1, QX-314 Bromide 5). Oxygenated ACSF was continuously perfused into the recording chamber at a rate of ∼2 mL/min. Picrotoxin (50 μM) was added to block current through GABAA receptors. Afferent fibers were stimulated with a bipolar nichrome wire electrode placed near the border between the NAc and the cortex. Recordings were performed using a Multiclamp 700B amplifier (Molecular Devices), filtered at 2 kHz, and digitized at 10 kHz. EPSCs of 100–500 pA were evoked at a frequency of 0.05–0.2 Hz. Data acquisition and analysis were performed using ClampX (Molecular Devices).
A/N ratios for a given cell were calculated as its EPSC magnitude while held at −70 mV divided by the EPSC magnitude 50 ms after the start of current efflux while held at +40 mV. For each cell’s A/N ratio, a minimum of 10 consecutive responses at −70 mV and +40 mV were averaged together. Coefficient of variation (CV) was calculated by dividing the SD of 30–60 consecutive EPSCs by the mean. The 1/CV2N:A was calculated as (1/CV2NMDAR)/(1/CV2AMPAR) as described previously (26).
AMPAR current-voltage plots were performed in the presence of AP5 (10 μM). EPSC magnitudes were acquired at holding potentials of −70, −40, 0, +20, and +40 mV. A minimum of three responses were averaged for each holding potential and normalized to the EPSCs at −70 mV. The RI was calculated as the current magnitude at +40 mV/current magnitude at −70 mV.
NMDAR current-voltage plots were performed in the presence of NBQX (10 μM). EPSC magnitudes were acquired at holding potentials of −80, −40, −20, 0, +20, and +40 mV. A minimum of three responses were averaged for each holding potential. All responses were normalized to the EPSC magnitude at +40 mV. Time to half-peak was calculated using traces recorded at +40 mV.
The mEPSCs were recorded in the presence of tetrodotoxin (1 μM) and analyzed using a template search. The sEPSCs and asEPSCs were recorded in an ACSF solution where Sr2+ (2.5 mM SrCl2) replaced Ca2+. The 500 ms leading up to each afferent stimulation were used for sEPSC analysis. The time period of 200 ms following each initial electrically evoked release event was used for asEPSC analysis. sEPSC and asEPSCs were analyzed using a template search.
PPRs were acquired through application of two successive afferent stimuli of equal intensity. The ISIs examined were 20, 50, 100, 200, and 400 ms. The PPR was calculated as EPSC2/EPSC1. For each ISI of each cell, the ratios of six consecutive responses were averaged.
Ifenprodil and CIQ wash-on experiments were performed in a low-Mg2+ ACSF solution with NBQX and picrotoxin to block AMPAR and GABAA transmission (6). For these experiments, cells were clamped to −50 mV, and NMDAR EPSCs were recorded. After acquisition of a 10-min stable baseline, Ifenprodil (3 μM) or CIQ (30 μM) was washed on for 10 min. Responses from the final 3 min of drug wash-on were averaged and compared with the baseline. This was followed by D-APV to confirm NMDAR currents.
Extracellular field potentials from the NAc core were performed similarly to as previously described (49) using slices prepared similarly to that of whole-cell experiments. Recordings were performed using lower impedance electrodes (1.0–2.0 MΩ) filled with ACSF. Afferents were stimulated as described above at 0.05 Hz. Fiber volley (N1) and population spike (N2) were assessed. For plasticity experiments, a 10-min stable N2 baseline was acquired before either LFS (3 × 3 min; 5 Hz stimulation with 5 min between each bout) or a 10-min wash-on of the group II mGluR agonist LY 379268 (200 nM). Responses were recorded for 30 min after LFS induction or drug removal. Experiments with unstable N1 were discarded.
Cocaine-Conditioned Place Preference.
A biased conditioned place preference assay was performed as previously described (19). Each session (pretest, conditioning, posttest) was 20 min in duration. Overhead video recordings of mouse activity were tracked and analyzed with automated software (EthoVision XT; Noldus). During the pretest, animals were placed in an open field chamber (ENV-510; Med Associates) equally divided into two sections with contextually distinct wall patterns and floor textures for 20 min. The amount of time spent on each side was recorded. Conditioning sessions were performed daily for the following 3 d, separated by 4 h. All mice received an i.p. injection of cocaine (5, 10, or 15 mg/kg) before exposure to the initially less-preferred pattern/texture combination. The same day, all mice received an i.p. injection of saline before exposure to the context where more time was initially spent. Treatment order was varied for each day. Finally, on day 5, a posttest was performed where the animal was given 20 min with access to both pattern/texture combinations. A change in the amount of time spent on the initially less-preferred context was assessed. A second posttest was performed either 14 d or 21 d later to assess the persistence of the learned preference. No animals were discarded based on an exclusion criteria of >80% initial preference for either context during the prestest.
Novel Object Recognition.
Novel object recognition was performed similarly to as previously described (50). We performed a 5-min habituation session in an empty test chamber before the assay. During the familiarization session, animals were given 10 min to explore two identical objects. Following a 1-h intersession interval, a test session was performed. Here, the animals were given 10 min to explore the same chamber with one of the identical objects replaced with a novel one. Familiarization and test sessions were recorded with an overhead camera. We analyzed the first 20 s of total object exploration. Proportion of time spent exploring the novel object (test session) and the identical object that it replaced (familiarization session) was quantified over the 20-s total exploration. Animals were excluded if there was >70% preference for either identical object. Videos were manually analyzed blinded to genotype.
Sucrose Preference Test.
A two-bottle choice sucrose preference test was performed modified from previously described methods (51). A solution of 2% sucrose dissolved in water or drinking water alone was placed in 50-mL conical tubes fit with a rubber stopper and sipper tube (Fisher). Conical tube assemblies were weighed before and after acclimation and test sessions. Mice were initially acclimatized to two-bottle choice conditions for 18 h with water alone. The less-preferred bottle/side was then filled with the sucrose solution before the mouse was placed back in the cage for an 18-h test. Sucrose preference was calculated as the proportion of sucrose solution consumed during the test session [change in mass of sucrose tube/(change in mass of sucrose tube + water tube)]. All mice explored/drank from both water tubes during acclimation. One mouse from each genotype was discarded due to leaky stoppers.
Open Field Test.
Open field tests were performed similarly to as previously described (19). Mice were placed in an open field activity chamber (ENV-510; Med Associates) for 60 min. Overhead video recordings were analyzed for locomotor activity and center time using automated software (Ethovision XT; Noldus).
Drugs.
Cocaine HCl and picrotoxin were purchased from Sigma-Aldrich. NBQX, D-APV, Ifenprodil, CIQ, and LY 379268 were purchased from Tocris Bioscience.
Acknowledgments
We thank Dr. Luc van Kaer for providing us with TLR4 knockout mice and Brandon Turner, Kevin Manz, Kellie Wilson, Drs. Max Joffe, Carrie Grueter, and Dipanwita Ghose for comments on the manuscript. This work was supported by National Institutes of Drug Abuse Grant F30DA040343 (to D.T.K.); National Institute of General Medical Sciences Grant T32GM007347 (to D.T.K.); and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to B.A.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705974114/-/DCSupplemental.
References
- 1.Sesack SR, Grace AA. Cortico-basal ganglia reward network: Microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joffe ME, Grueter CA, Grueter BA. Biological substrates of addiction. Wiley Interdiscip Rev Cogn Sci. 2014;5:151–171. doi: 10.1002/wcs.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2012;22:545–551. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pascoli V, Turiault M, Lüscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2011;481:71–75. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- 5.Pascoli V, et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- 6.Joffe ME, Grueter BA. Cocaine experience enhances thalamo-accumbens N-methyl-D-aspartate receptor function. Biol Psychiatry. 2016;80:671–681. doi: 10.1016/j.biopsych.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee BR, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Y-Y, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekine Y, et al. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewitus GM, et al. Microglial TNF-α suppresses cocaine-induced plasticity and behavioral sensitization. Neuron. 2016;90:483–491. doi: 10.1016/j.neuron.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.June HL, et al. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology. 2015;40:1549–1559. doi: 10.1038/npp.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchinson MR, et al. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci. 2012;32:11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Northcutt AL, et al. DAT isn’t all that: Cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry. 2015;20:1525–1537. doi: 10.1038/mp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanda G, et al. Lack of specific involvement of (+)-naloxone and (+)-naltrexone on the reinforcing and neurochemical effects of cocaine and opioids. Neuropsychopharmacology. 2016;41:2772–2781. doi: 10.1038/npp.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris RA, et al. Genetic and pharmacologic manipulation of TLR4 has minimal impact on ethanol consumption in rodents. J Neurosci. 2017;37:1139–1155. doi: 10.1523/JNEUROSCI.2002-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neill LAJ. Primer: Toll-like receptor signaling pathways—What do rheumatologists need to know? Nat Clin Pract Rheumatol. 2008;4:319–327. doi: 10.1038/ncprheum0802. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, et al. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun. 2016;53:207–222. doi: 10.1016/j.bbi.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tramullas M, et al. Toll-like receptor 4 regulates chronic stress-induced visceral pain in mice. Biol Psychiatry. 2014;76:340–348. doi: 10.1016/j.biopsych.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Joffe ME, Vitter SR, Grueter BA. GluN1 deletions in D1- and A2A-expressing cell types reveal distinct modes of behavioral regulation. Neuropharmacology. 2017;112:172–180. doi: 10.1016/j.neuropharm.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tejeda HA, et al. Pathway- and cell-specific kappa-opioid receptor modulation of excitation-inhibition balance differentially gates D1 and D2 accumbens neuron activity. Neuron. 2017;93:147–163. doi: 10.1016/j.neuron.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz N, et al. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science. 2014;345:535–542. doi: 10.1126/science.1253994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graziane NM, et al. Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci. 2016;19:915–925. doi: 10.1038/nn.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hearing MC, et al. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci USA. 2016;113:757–762. doi: 10.1073/pnas.1519248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. ∆FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci USA. 2013;110:1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: A neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 28.Francis TC, et al. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry. 2015;77:212–222. doi: 10.1016/j.biopsych.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 30.Renteria R, Maier EY, Buske TR, Morrisett RA. Selective alterations of NMDAR function and plasticity in D1 and D2 medium spiny neurons in the nucleus accumbens shell following chronic intermittent ethanol exposure. Neuropharmacology. 2017;112:164–171. doi: 10.1016/j.neuropharm.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 32.Brown RM, et al. Addiction-like synaptic impairments in diet-induced obesity. Biol Psychiatry. 2017;81:797–806. doi: 10.1016/j.biopsych.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasanetz F, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 34.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz JM, Smith SH, Bilbo SD. FACS analysis of neuronal-glial interactions in the nucleus accumbens following morphine administration. Psychopharmacology (Berl) 2013;230:525–535. doi: 10.1007/s00213-013-3180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. The Jackson Laboratory 000659-C3H/HeJ. Available at: https://www.jax.org/strain/000659. Accessed March 14, 2017.
- 37.Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- 38.Wyllie DJA, Livesey MR, Hardingham GE. Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology. 2013;74:4–17. doi: 10.1016/j.neuropharm.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seif T, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brebner K, et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310:1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- 41.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parkhurst CN, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 44.Grabert K, et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z-J, et al. Activin A is increased in the nucleus accumbens following a cocaine binge. Sci Rep. 2017;7:43658. doi: 10.1038/srep43658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohannon JK, Hernandez A, Enkhbaatar P, Adams WL, Sherwood ER. The immunobiology of toll-like receptor 4 agonists: From endotoxin tolerance to immunoadjuvants. Shock. 2013;40:451–462. doi: 10.1097/SHK.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garré JM, Silva HM, Lafaille JJ, Yang G. CX3CR1(+) monocytes modulate learning and learning-dependent dendritic spine remodeling via TNF-α. Nat Med. 2017;23:714–722. doi: 10.1038/nm.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu P, et al. Double deletion of melanocortin 4 receptors and SAPAP3 corrects compulsive behavior and obesity in mice. Proc Natl Acad Sci USA. 2013;110:10759–10764. doi: 10.1073/pnas.1308195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leger M, et al. Object recognition test in mice. Nat Protoc. 2013;8:2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- 51.Christoffel DJ, et al. Effects of inhibitor of κB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology. 2012;37:2615–2623. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Kim J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience. 2015;311:253–267. doi: 10.1016/j.neuroscience.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]