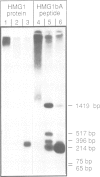
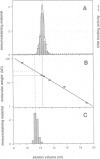
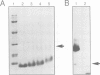
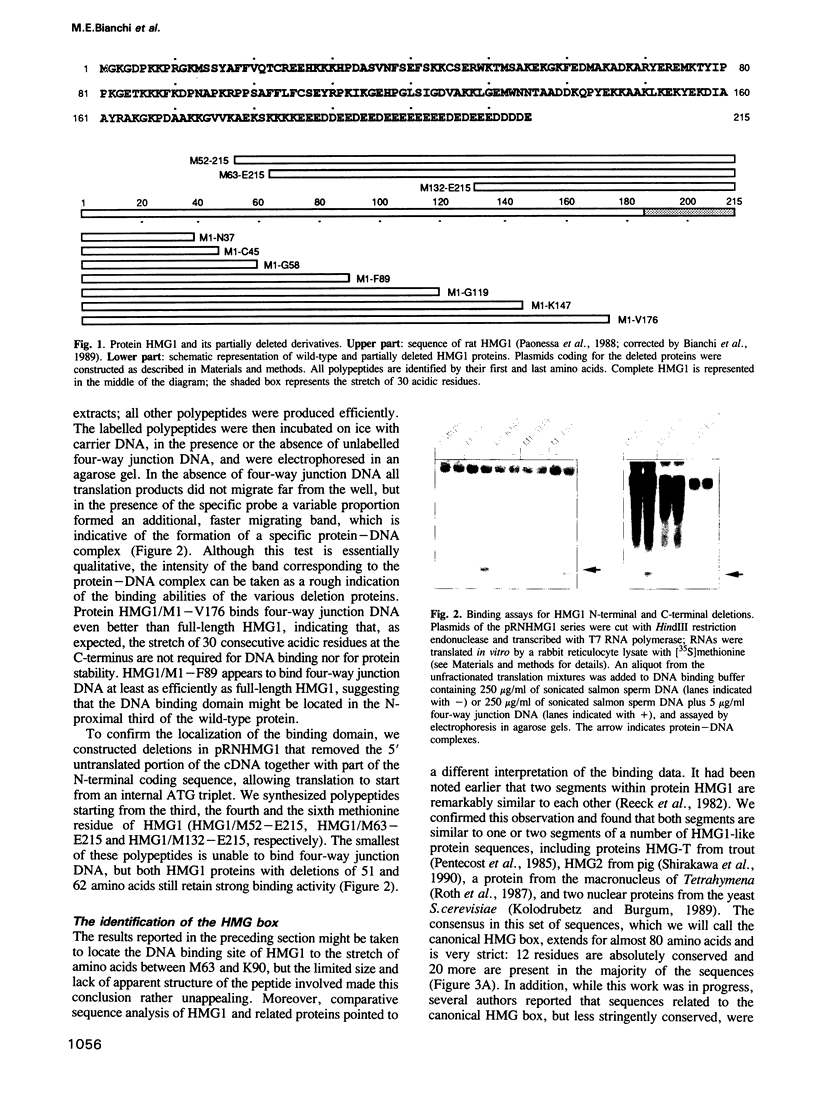
Abstract
The mammalian nuclear protein HMG1 contains two segments that show a high sequence similarity to each other. Each of the segments, produced separately from the rest of the protein in Escherichia coli, binds to DNA with high specificity: four-way junction DNA of various sequences is bound efficiently, but linear duplex DNA is not. Both isolated segments exists as dimers in solution, as shown by gel filtration and chemical crosslinking experiments. HMG1-like proteins are present in yeast and in protozoa: they consist of a single repetition of a motif extremely similar to the DNA binding segments of HMG1, suggesting that they too might form dimers with structural specificity in DNA binding. Sequences with recognizable similarity to either of the two DNA binding segments of HMG1, called HMG boxes, also occur in a few eukaryotic regulatory proteins. However, these proteins are reported to bind to specific sequences, suggesting that the HMG box of proteins distantly related to HMG1 might differ significantly from the HMG box of HMG1-like proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya A., Lilley D. M. The contrasting structures of mismatched DNA sequences containing looped-out bases (bulges) and multiple mismatches (bubbles). Nucleic Acids Res. 1989 Sep 12;17(17):6821–6840. doi: 10.1093/nar/17.17.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M. E., Beltrame M., Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 1989 Feb 24;243(4894 Pt 1):1056–1059. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- Bianchi M. E. Interaction of a protein from rat liver nuclei with cruciform DNA. EMBO J. 1988 Mar;7(3):843–849. doi: 10.1002/j.1460-2075.1988.tb02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M. E. Production of functional rat HMG1 protein in Escherichia coli. Gene. 1991 Aug 15;104(2):271–275. doi: 10.1016/0378-1119(91)90261-9. [DOI] [PubMed] [Google Scholar]
- Bonne-Andrea C., Harper F., Sobczak J., De Recondo A. M. Rat liver HMG1: a physiological nucleosome assembly factor. EMBO J. 1984 May;3(5):1193–1199. doi: 10.1002/j.1460-2075.1984.tb01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einck L., Bustin M. The intracellular distribution and function of the high mobility group chromosomal proteins. Exp Cell Res. 1985 Feb;156(2):295–310. doi: 10.1016/0014-4827(85)90539-7. [DOI] [PubMed] [Google Scholar]
- Finkel'shtein A. V. Odnomernaia model' Izinga dlia polipeptidnoi tsepi, obrazuiushchei lokal'nye vtorichnye struktury neskol'kikh tipov. Dokl Akad Nauk SSSR. 1975 Jul 21;223(3):744–747. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gribskov M., Burgess R. R., Devereux J. PEPPLOT, a protein secondary structure analysis program for the UWGCG sequence analysis software package. Nucleic Acids Res. 1986 Jan 10;14(1):327–334. doi: 10.1093/nar/14.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990 Jul 19;346(6281):245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Halazonetis T. D., Georgopoulos K., Greenberg M. E., Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988 Dec 2;55(5):917–924. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- Jantzen H. M., Admon A., Bell S. P., Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990 Apr 26;344(6269):830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- Jones N. Transcriptional regulation by dimerization: two sides to an incestuous relationship. Cell. 1990 Apr 6;61(1):9–11. doi: 10.1016/0092-8674(90)90207-u. [DOI] [PubMed] [Google Scholar]
- Kaplan D. J., Duncan C. H. Full length cDNA sequence for bovine high mobility group 1 (HMG1) protein. Nucleic Acids Res. 1988 Nov 11;16(21):10375–10375. doi: 10.1093/nar/16.21.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Burke J., Smith M., Klar A., Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988 May;7(5):1537–1547. doi: 10.1002/j.1460-2075.1988.tb02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Fos-Jun heterodimers and Jun homodimers bend DNA in opposite orientations: implications for transcription factor cooperativity. Cell. 1991 Jul 26;66(2):317–326. doi: 10.1016/0092-8674(91)90621-5. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Scheer U., Dabauvalle M. C., Bustin M., Franke W. W. High mobility group proteins of amphibian oocytes: a large storage pool of a soluble high mobility group-1-like protein and involvement in transcriptional events. J Cell Biol. 1983 Sep;97(3):838–848. doi: 10.1083/jcb.97.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlstaedt L. A., Sung E. C., Fujishige A., Cole R. D. Non-histone chromosomal protein HMG1 modulates the histone H1-induced condensation of DNA. J Biol Chem. 1987 Jan 15;262(2):524–526. [PubMed] [Google Scholar]
- Kolodrubetz D., Burgum A. Duplicated NHP6 genes of Saccharomyces cerevisiae encode proteins homologous to bovine high mobility group protein 1. J Biol Chem. 1990 Feb 25;265(6):3234–3239. [PubMed] [Google Scholar]
- Liu-Johnson H. N., Gartenberg M. R., Crothers D. M. The DNA binding domain and bending angle of E. coli CAP protein. Cell. 1986 Dec 26;47(6):995–1005. doi: 10.1016/0092-8674(86)90814-7. [DOI] [PubMed] [Google Scholar]
- Murchie A. I., Clegg R. M., von Kitzing E., Duckett D. R., Diekmann S., Lilley D. M. Fluorescence energy transfer shows that the four-way DNA junction is a right-handed cross of antiparallel molecules. Nature. 1989 Oct 26;341(6244):763–766. doi: 10.1038/341763a0. [DOI] [PubMed] [Google Scholar]
- Paonessa G., Frank R., Cortese R. Nucleotide sequence of rat liver HMG1 cDNA. Nucleic Acids Res. 1987 Nov 11;15(21):9077–9077. doi: 10.1093/nar/15.21.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentecost B. T., Wright J. M., Dixon G. H. Isolation and sequence of cDNA clones coding for a member of the family of high mobility group proteins (HMG-T) in trout and analysis of HMG-T-mRNA's in trout tissues. Nucleic Acids Res. 1985 Jul 11;13(13):4871–4888. doi: 10.1093/nar/13.13.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikaard C. S., Smith S. D., Reeder R. H., Rothblum L. rUBF, an RNA polymerase I transcription factor from rats, produces DNase I footprints identical to those produced by xUBF, its homolog from frogs. Mol Cell Biol. 1990 Jul;10(7):3810–3812. doi: 10.1128/mcb.10.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptitsyn O. B., Finkelstein A. V. Theory of protein secondary structure and algorithm of its prediction. Biopolymers. 1983 Jan;22(1):15–25. doi: 10.1002/bip.360220105. [DOI] [PubMed] [Google Scholar]
- Reeck G. R., Isackson P. J., Teller D. C. Domain structure in high molecular weight high mobility group nonhistone chromatin proteins. Nature. 1982 Nov 4;300(5887):76–78. doi: 10.1038/300076a0. [DOI] [PubMed] [Google Scholar]
- Roth S. Y., Schulman I. G., Cook R. G., Allis C. D. The complete amino acid sequence of an HMG-like protein isolated from the macronucleus of Tetrahymena. Nucleic Acids Res. 1987 Oct 12;15(19):8112–8112. doi: 10.1093/nar/15.19.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth G. P., Cook G. R., Bradbury E. M., Gottesfeld J. M. Transcription factor IIIA induced bending of the Xenopus somatic 5S gene promoter. Nature. 1989 Aug 10;340(6233):487–488. doi: 10.1038/340487a0. [DOI] [PubMed] [Google Scholar]
- Shirakawa H., Tsuda K., Yoshida M. Primary structure of non-histone chromosomal protein HMG2 revealed by the nucleotide sequence. Biochemistry. 1990 May 8;29(18):4419–4423. doi: 10.1021/bi00470a022. [DOI] [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L., Smith M. J., Foster J. W., Frischauf A. M., Lovell-Badge R., Goodfellow P. N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Singh J., Dixon G. H. High mobility group proteins 1 and 2 function as general class II transcription factors. Biochemistry. 1990 Jul 3;29(26):6295–6302. doi: 10.1021/bi00478a026. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tremethick D. J., Molloy P. L. High mobility group proteins 1 and 2 stimulate transcription in vitro by RNA polymerases II and III. J Biol Chem. 1986 May 25;261(15):6986–6992. [PubMed] [Google Scholar]
- Tsuda K., Kikuchi M., Mori K., Waga S., Yoshida M. Primary structure of non-histone protein HMG1 revealed by the nucleotide sequence. Biochemistry. 1988 Aug 9;27(16):6159–6163. doi: 10.1021/bi00416a050. [DOI] [PubMed] [Google Scholar]
- Watt F., Molloy P. L. High mobility group proteins 1 and 2 stimulate binding of a specific transcription factor to the adenovirus major late promoter. Nucleic Acids Res. 1988 Feb 25;16(4):1471–1486. doi: 10.1093/nar/16.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L., Huang J. K., Johnson B. H., Reeck G. R. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989 Feb 11;17(3):1197–1214. doi: 10.1093/nar/17.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. M., Dixon G. H. Induction by torsional stress of an altered DNA conformation 5' upstream of the gene for a high mobility group protein from trout and specific binding to flanking sequences by the gene product HMG-T. Biochemistry. 1988 Jan 26;27(2):576–581. doi: 10.1021/bi00402a012. [DOI] [PubMed] [Google Scholar]