Abstract
Extracellular vesicles (EVs) represent a class of cell secreted organelles which naturally contain biomolecular cargo such as miRNA, mRNA and proteins. EVs mediate intercellular communication, enabling the transfer of functional nucleic acids from the cell of origin to the recipient cells. In addition, EVs make an attractive delivery vehicle for therapeutics owing to their increased stability in circulation, biocompatibility, low immunogenicity and toxicity profiles. EVs can also be engineered to display targeting moieties on their surfaces which enables targeting to desired tissues, organs or cells. While much has been learned on the role of EVs as cell communicators, the field of therapeutic EV application is currently under development. Critical to the future success of EV delivery system is the description of methods by which therapeutics can be successfully and efficiently loaded within the EVs. Two methods of loading of EVs with therapeutic cargo exist, endogenous and exogenous loading. We have therefore focused this review on describing the various published approaches for loading EVs with therapeutics.
Keywords: Exosomes, microvesicles, extracellular vesicles, EV therapeutics, drug loading
1. INTRODUCTION
Extracellular vesicles (EVs), comprising both exosomes and microvesicles are small spherical organelles that are derived from intracellular lipid compartments and are constantly being shed into the extracellular space and systemic circulation. The presence of the lipid bilayer protects the EV cargo from enzymatic degradation while the EVs move from donor to recipient cells. First reported by Pan and Johnstone in 1983 (1), EVs were disregarded as cellular junk and considered to be part of a disposal mechanism. However, EVs have gained increasing attention since the discovery that they serve as vehicles for communication and transfer of cellular material between different tissues and cell types (2, 3). Cargo found within EVs includes miRNA (2, 4), lncRNA (5, 6), mRNA (2), proteins (7) and DNA (3, 8, 9). Similar to early beliefs about EVs, non-coding RNA was also originally thought to be cellular waste and was disregarded for its assumed lack of a biological role (10). Ironically, both EVs and non-coding RNAs are now implicated in various disease states including cancer (11, 12), cardiovascular (13) and neuronal diseases (14). We refer the reader to the many excellent reviews on therapeutic EVs (15–18). This review will focus on the various methods to load therapeutic cargo into EVs and discuss some of the implications of loading including barriers that will need to be overcome as therapeutic EVs make their way to the clinic.
2. EV BIOGENESIS PATHWAY
A recent editorial review mentions that “extracellular vesicle” is a broad term designated to all the secreted membrane vesicles which include exosomes, microvesicles, ectosomes, matrix/calcifying vesicles, prostasomes and oncosomes (19). EVs are heterogeneous organelles in nature and could also be broadly classified as exosomes and microvesicles based upon the biogenesis pathway through which they arise (Figure 1). Other than the manner in which the exosomes and microvesicles are formed, there is no precise distinction between the two as they both have overlapping characteristics with each other in terms of their vesicle diameter (20) and the markers presented on their extracellular surfaces (21).
Figure 1.
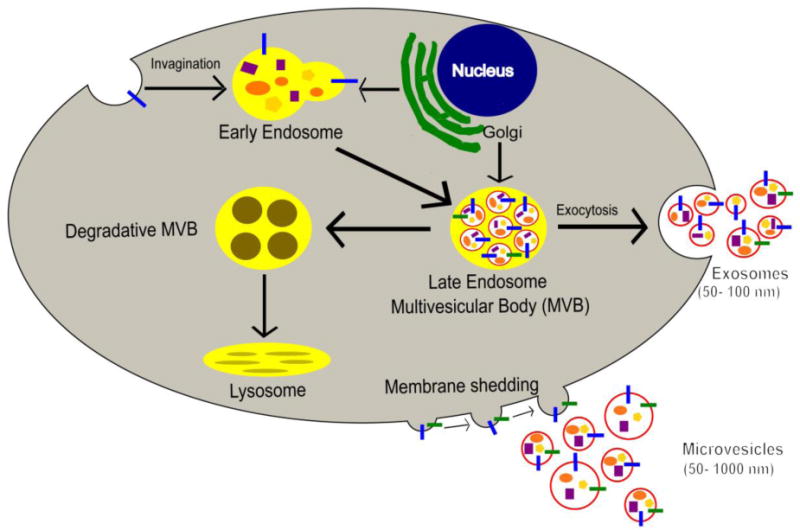
Biogenesis of Extracellular vesicles. Exosome biogenesis begins with the invagination of the plasma membrane followed by the inward budding of the endosome to form multivesicular bodies (MVBs) which then fuse with the plasma membrane to release these smaller types of EVs. Microvesicles on the other hand are formed directly by the outward budding of the plasma membrane.
Exosomes are small, homogenously distributed vesicles which range mostly from 50–100 nm in diameter (22). Exosome development progresses through a 3 step process of the endosomal pathway: early endosome, late endosome and recycling/degradation or exocytosis. The first step is the formation of the early endosome which involves the invagination of the plasma membrane. Next steps involve the inward budding of the early endosomal membrane which replaces the already existing endosomal luminal space with exosomes or small luminal vesicles (23, 24). This vesicle filled body is also referred to as the multivesicular body (MVBs). MVBs then fuse with the plasma membrane to release exosomes through the process of exocytosis (25) or MVBs could alternatively fuse to the lysosomes for degradation (26).
Compared to exosomes, microvesicles are larger in diameter and are more heterogeneously distributed in size ranging from 50–1000 nm (27). Microvesicles are formed from the outward budding process and fission of the plasma membrane when compared to the inward budding process of the exosomes (28). Apart from size and the process through which they are formed, microvesicles differ from exosomes not only by lipid content but also by protein content. Exosomes contain the ceramide lipid, which is produced within the endosome by the hydrolysis of sphingomyelin. Ceramide production is reported to be an essential step in exosome biogenesis (22).
3. EVs AS THERAPEUTICS
There are numerous ongoing efforts to develop biomolecular therapeutics to replace or to alter expression levels of dysfunctional genes and proteins. Since nucleic acids have been reported to rescue diseased conditions, a major focus has been made towards their successful delivery. Delivery of oligonucleotide therapies, however, remains a major hurdle due to rapid clearance, lack of tissue specific distribution, and poor cellular uptake. Several strategies have been deployed to effectively deliver oligonucleotides, such as viral vectors, liposome formulations, peptide conjugation and other approaches; some of which have been evaluated clinically (29–31). Nonetheless, significant obstacles remain, including immune recognition (32), random integration for viral vectors (33, 34), inflammatory toxicity and rapid clearance for liposomes (35, 36). Efforts therefore continue to develop and explore newer delivery methods that can overcome these hurdles. One such alternative strategy lies within the use of EVs which can be targeted for gene therapy. The therapeutics that primarily could benefit from EV delivery are RNA therapeutics as delivery of RNA molecules is restricted due to its hydrophilicity and negative charges (37). Since, EVs naturally contain and are shown to transfer this RNA cargo to the recipient cells via cell-cell communication, delivery through EVs could theoretically achieve the desired phenotypic and gene expression changes in the target cells, as has been previously demonstrated (38–40).
EVs are cellular components secreted by cells into the extracellular compartments by different organisms including humans (41), plants (42), fungi (43) and bacteria (44). EVs isolated from various sources consist of different biological composition and thus, depending upon its particular cargo moiety, regulate gene expression in the recipient cells. Unmodified EVs isolated from plants, animal milk, mesenchymal stem cells (MSCs), endothelial progenitors, dendritic cells (DC) and various other cell types have exhibited potential as therapeutics. Zhang and coworkers studied the effects of EVs (referred in the article as nanovesicles) that were isolated from grapefruit juice on different stem cells and reported that the nanovesicles are involved in protecting the mouse intestine from DSS-induced colitis (45). Another group reported that EVs isolated from bovine milk could be developed as a cost effective and scalable source of therapeutic EVs (46). MSCs have long been in the spotlight for their effectiveness in replacement therapies, wherein they have been utilized to replace damaged cells by becoming integrated into the affected tissues and restoring normal tissue and organ functions (47). It was recently reported that the therapeutic effects of MSCs are in fact attributed to the EVs that are secreted by the cells (48, 49). EVs isolated from induced pluripotent stem cell derived MSCs (iPSCs) have also been reported to attenuate limb ischemia in mice (50) and to promote bone regeneration in osteoporotic rats (51). In addition to the plant, animal and human stem cells, EVs could also be harvested from human embryonic kidney cells for therapeutic development. HEK293/HEK293T (human embryonic kidney) cells have been used extensively to produce recombinant protein (52). Because these cells grow quickly and exhibit high transfection efficiency and protein production, they have also been adapted to develop therapeutic EVs for RNA and protein delivery (53, 54). A recent study conducted by Zhang et al., further verified that HEK293T cells EVs have minimal negative effects as its cargo is not enriched by cancer or disease related pathways (55).
EVs are also reported to be secreted by both gram positive and gram negative bacteria (56). The EVs secreted from bacteria are referred to as the outer membrane vesicles (OMVs). OMVs are released by the bacteria as part of its survival mechanism, allowing interaction with its environment and assist in the development of resistance to antibiotics (57). As bacteria and other pathogens secrete EVs as part of a defense mechanism, studies were conducted to evaluate if antigen presenting cells when infected with pathogens could be used to develop EVs as vaccines. One such study involved isolating EVs from dendritic cells that were pulsed with Toxoplasma gondii antigens. These EVs containing pathogen specific antigens elicited both systemic and humoral response against toxoplasmosis when injected into the mice, (58, 59). This and several other ongoing studies have exhibited the potential of developing EVs into therapeutic vaccines against infectious diseases in humans and animals (60–62).
To be used as carriers for specific cargo, successful application of EV therapeutics is entirely dependent upon the extent of cargo loading. Several methods have been briefly addressed in the next section which describes loading of therapeutic cargo within the EVs.
4. LOADING MECHANISMS
There are two general processes for loading therapeutic cargo within EVs: exogenous (or direct loading) and endogenous. Exogenous methods include the loading of therapeutics within EVs once they are isolated. Exogenous loading can be further subdivided into passive and active loading (16). Passive loading refers to an incubation of the EVs with the therapeutic cargo so that it is passively loaded into the EVs. Active loading requires some type of disruption to the EV membrane, typically electroporation or the addition of surfactants. Endogenous loading refers to systems comprised of a donor cell that deposits the therapeutic cargo directly into the EV prior to its shedding. The most commonly used type of endogenous loading includes RNA loading into the EV following expression from a vector. Another form of endogenous loading is the cell extrusion method that is discussed in more detail below. In the following section we summarize some of the published exogenous and endogenous EV loading methods
4.1. Exogenous loading method (active loading)
4.1.1. Electroporation
Electroporation is a process by which transient pores are made into the membrane of the EVs to facilitate cargo loading. It is perhaps the most widely used method to load therapeutic cargo into EVs. In this method, purified EVs and therapeutic cargo are mixed together in an electroporation buffer. The mixture is then electroporated to disrupt the EVs’ structure leading to spontaneous pore formation and allowing the cargo to become incorporated into the EVs. This is usually followed by incubation at 37° C to allow time for the EVs to fully recover (63–65). Once loaded, EVs are washed with PBS to remove unloaded cargo, and then purified by ultracentrifugation. Loaded EVs could then be used for downstream in vitro or in vivo experiments. Several studies have implemented electroporation to load the exosomes with different types of cargo. Alvarez-Erviti et al., were the first to successfully load and deliver functional genetic material in vivo using exosomes (66). In this study primary dendritic cells from murine bone marrow were harvested and engineered to express the neuron specific Rabies Virus Glycoprotein peptide that was fused to an EV membrane expressing protein Lamp2b. Exosomes from the engineered cells were harvested and electroporated with GAPDH siRNA. Upon intravenous injection of the RVG-targeted exosomes in mice, neuron specific gene silencing was achieved. This was further demonstrated by Shtam, et al., (65),Banizs, et al., (67) and Walhgreen, et al., where RAD51 siRNA,luciferase siRNA and MAPK1 siRNA were loaded into exosomes by electroporation and successfully delivered to HeLa cells,endothelial cells, monocytes and lymphocytes respectively. Walhgreen, et al., carried out a thorough characterization study using fluorescent microscopy, northern blotting, and flow cytometry to confirm that the electroporated siRNA was encapsulation within the EVs. Lamichhane, et al., studied the possibility of delivering dsDNA via EVs (64). Linear dsDNA (750 bp) of the S. cerevisiae tRNA Ser (CGA) gene was electroporated into EVs derived from HEK293T cells. Loaded exosomes were incubated with HEK293T cells, and the 750 bp DNA fragment was detected by PCR in the recipient cells. Electroporation can also be used to load chemotherapeutic agents into EVs (63). Tian, et al., used electroporation to load doxorubicin into exosomes engineered to express iRGD peptide designed to target αv integrin subunit on the surface of cancer cells (63). The drug loaded exosomes were tested in vitro across different breast cancer cell lines. Similar to the free drug, loaded exosomes produced a decrease in viability in all cell lines evaluated. Unlike blank exosomes containing doxorubicin which showed no specific accumulation, iRGD-exosomes containing doxorubicin accumulated at the tumor site in vivo within 30 minutes and peaked at 2 hours. Doxorubicin loaded iRGD exosomes resulted in a remarkable inhibition in tumor growth compared to PBS, blank exosomes and free doxorubicin suggesting that exosomes could serve as an excellent delivery system to target and deliver chemotherapeutic agents to tumors.
Several EV and cargo characteristics are important for loading of therapeutic cargo into the EVs by electroporation. Lamichhane, et al., studied various parameters that may affect the loading of DNA fragments into EVs by electroporation (64). In this study, it was demonstrated that greater loading could be achieved by optimizing electroporation parameters as well as DNA quantity to obtain an optimal DNA:exosome ratio. The size of the DNA fragment was also critical for loading, as loading decreased with increasing fragment size, with a great reduction in loading occurring between the 750 and 1000 bp fragments. Vesicle size was also shown to be important for DNA loading with larger populations of microvesicles exhibiting better loading than smaller exosome-like vesicles (64). Similar factors may be important for other types of cargo, but those studies have not yet been published.
In summary, electroporation has shown to be useful for loading different types of therapeutic cargo into EVs including siRNA, DNA and chemotherapeutic agents. Similarly, it may also be used to deliver miRNA, mRNA and proteins. One of the main advantages of electroporation is that it has a minimal effect on exosomal components such as ligands and receptors present on its membrane surface. The heat generated due to electrical resistance is minimal (1° C/pulse) and does not damage the EV membrane (68). However, electroporation may trigger the aggregation of EVs, and change their morphological characteristics. This has been reported by Hood, et al., (69) and Johnsen, et al., (70). Electroporation may also promote aggregation of therapeutic siRNA as reported by Kooijmans, et al., (71). Kooijmans and coworkers investigated siRNA loading into EVs by electroporating siRNA cargo with and without EVs. They found that electroporating siRNA in the absence of EVs, led to large siRNA aggregate formation. This opens up a debate on the accuracy of the loading efficiency by electroporation. Thus, careful consideration needs to be taken while interpreting the loading using the electroporation method. In addition to cargo aggregation, EVs could also aggregate by fusing together as reported in the case of liposomes (72). Since the reports showing siRNA aggregate formation, several groups have employed citric acid buffer or the trehalose pulse media to reduce aggregation (68, 70, 71). Trehalose did not appear to affect EVs’ size without electroporation, nor did it hinder pore formation upon electroporation (70). In spite of the fact that aggregation could be prevented, electroporation relies upon the passive loading of the cargo. Thus this method will only be effective when the EVs are highly concentrated, and the ratio of the EV particles to the cargo molecules is thoroughly optimized. Finally, while electroporation may be useful for smaller studies, its lack of scalability in terms of supplying loaded EVs for clinical evaluation is a major disadvantage.
4.1.2 Saponin permeabilization
Another method that involves drug loading via permeabilization of the EV’s membrane is the use of saponin. Saponin is a detergent like molecule that interacts and removes cholesterol from the membrane forming pores without destroying the membrane (73, 74). This technique was used to introduce membrane impermeable dyes and other cargo within vesicles. This technique was utilized to load the protein catalase into exosomes derived from Raw 264.7 macrophages to be used as a drug delivery system in Parkinson’s disease therapy (75). Catalase is an antioxidant enzyme that functions to protect neuronal tissue from oxidative stress and neurodegradation and has been reported to be reduced in Parkinson’s disease (76, 77). In this study, the ability of catalase-loaded EVs to protect against oxidative stress was evaluated (75). These authors also compared different loading techniques (direct incubation, sonication, freeze thaw cycles and size extrusion). EVs loaded by direct incubation or saponin permeabilization showed no change in EVs size and morphology. Particles undergoing freeze thaw cycles were larger in size due to aggregation, whereas sonicated EVs appeared non-spherical. As far as catalase loading, EVs loaded using saponin had higher loading that those obtained by incubation or freeze thaw, but lower than sonication and extrusion. Moreover, EVs loaded using saponin, sonication or extrusion, showed a prolonged release of catalase as measured by retained catalase activity over time. In vitro, PC12 cells pretreated with 6-OHDA were used to mimic neurodegeneration. EVs loaded by saponin permeabilization and sonication showed the highest neuronal survival among all formulations. The formulations were also tested in vivo in C57BL/6 mice with acute brain inflammation. Catalase loaded EVs introduced via the intranasal route were able to reduce brain inflammation as seen by reduced Reactive Mac 1+, and higher TH-expressing DA neurons (75). Noteworthy, EVs loaded using saponin permeabilization showed better therapeutic effects in vivo than those obtained by sonication in spite of lower catalase loading. This might be due to disruption of the exosomes integrity due to sonication, making them more vulnerable to degradation via the reticuloendothelial system. Fuhrmann, et al., utilized saponin permeabilization to load porphyrins into EVs (78). Using saponin at 0.01% w/v for permeabilization resulted in significantly higher loading of porphyrins into EVs when compared other loading methods including direct incubation, electroporation and extrusion. Saponin permeabilization did not alter size distribution or surface charge of the EVs. Loaded EVs were taken up by MDA cells, and functional porphyrins were delivered as seen by cell viability changes. While, saponin permeabilization is a simple and easy way to load exosomes with therapeutic proteins and effectively deliver them to target areas; this method is only demonstrated in a handful of studies. Further work needs to be carried out to determine whether the use of saponin detergents are likely to disrupt the EV integrity thereby affecting their immunogenicity. Also, saponin like detergents are difficult to remove from EV preparations and could thus affect EV morphology, uptake and stability.
4.1.3 Hypotonic dialysis
Hypotonic dialysis relies on osmosis to load vesicles with therapeutic agents. EVs are dispersed in a hypotonic solution which causes the EVs to swell and form pores. This pore formation renders the membrane permeable for soluble therapeutic agents allowing drug loading. The loaded vesicles are then dispersed in an isotonic solution which restores the integrity of the EVs and results in drug encapsulation (79). Fuhrmann, et al., explored loading of porphyrins into EVs using hypotonic dialysis (78). EVs and porphyrin were loaded into a cellulose ester dialysis membrane which was placed in 10 mM phosphate buffer, wherein the mixture was continuously stirred at room temperature for four hours. Drug loaded EVs were then separated from the mixture by size-exclusion chromatography. As with saponin loading discussed in the previous section, EVs loaded by hypotonic dialysis had exhibited a higher drug content compared to direct incubation, electroporation and extrusion loaded vesicles (briefly discussed in section 4.2.3). One of the drawbacks with this technique is that it alters the size and charge of the loaded EVs. Thus, despite this significantly higher loading, the change in charge and morphology, and potentially other EV characteristics that were not evaluated resulted in diminished cellular uptake and failed to induce any photo toxicity in MDA-MB-231 breast cancer cells.
4.2 Exogenous loading methods (Passive loading)
4.2.1 Cholesterol conjugation
This technique involves enhancement of EV loading by covalently bonding cholesterol to the therapeutic oligonucleotide. Didiot, et al., explored the use of cholesterol modified huntingtin gene (Htt) siRNA to improve loading into EVs (40). A chemically modified Htt siRNA with a phosphorothioated tail, and 2′-fluoro or 2′-O-methyl pyrimidine modifications were used to protect against nuclease degradation. Cholesterol was then conjugated to the 3′ end of the passenger strand. The hydrophobicity imparted by the cholesterol allowed for enhanced membrane association, while the charge of the phosphorothioated tail promoted cellular uptake. EVs derived from U87 glioblastoma cells were simply loaded with the cholesterol tagged siRNA by incubation at 37° C for 90 minutes with shaking. The loaded EVs were then separated from the mixture by ultracentrifugation. This method produced encapsulation efficiencies in the range of 10–50%, with 1000–3000 copies of siRNA loaded per EV. Cholesterol conjugation was essential for loading as unconjugated siRNA did not associate with the EVs. Electron microscopy revealed that the majority of the siRNA were bound to the surface of the EVs while a portion was internalized into the vesicles. This finding was also confirmed by zeta potential measurements where loaded EVs had a lower surface charge than unloaded EVs. Loaded EVs exposed to primary neurons in vitro or unilateral infusion into the striatum in vivo decreased both mRNA/protein and mRNA, respectively. These findings indicate that the loaded EVs successfully delivered functional siRNA.
Another study by Stremersch, et al., involved loading of cholesterol labeled CD45 siRNA to dendritic and lung epithelial cell line EVs (80). They showed that even though the cholesterol siRNA was loaded effectively onto the EVs, and were taken up by dendritic JAWS11 and lung epithelial B16F10 cells, functional siRNA could not be delivered in vitro. The cause of the inconsistency in efficacy between these two studies is unclear (40, 80). It may be due to the type of EV and cells used since both studies used different EV and recipient cell combinations. In short, cholesterol conjugation is a straightforward method to load therapeutic oligonucleotides into EVs (40).
4.2.2 Simple drug incubation
Research has focused on developing various drug formulations with a unified goal to improve their therapeutic and pharmacokinetic profiles, especially that of the hydrophobic drugs. Liposomes have been on the forefront of delivering therapeutic drugs, improving their pharmacokinetic profile, increasing oral bioavailability and retention in the target tissues. However, opsonization and rapid clearance presents a potential problem for some of these nanoparticulate systems (35). Several strategies have been employed, including PEGylation of the liposomes, to help overcome some of these problems (81). EVs resemble liposomes with their similar particle sizes and lipid bilayer. Several studies have shown that drugs such as curcumin, doxorubicin and paclitaxel could be passively loaded within the EVs (63, 82–84). Curcumin interacted with the lipid membrane of the EV to form a curcumin-EV complex (82). Upon administration to the macrophages, curcumin-EV complexes exhibited greater anti-inflammatory activity compared to the free curcumin. When injected into a lipopolysaccharide (LPS) induced shock mouse model, improved survival was observed for the complex compared to the free drug. Curcumin-EV and Stat 3 inhibitor-EV complexes administered to the brain via the intranasal route protected the mice from LPS-induced brain inflammation, myelin oligodendrocyte glycoprotein peptide induced experimental autoimmune encephalomyelitis and delayed tumor growth (83). As a result of these preliminary successes, a phase I clinical trial (NCT01294072) is currently ongoing to evaluate if plant exosomes could effectively deliver the poorly soluble curcumin drug to colon cancer patients. A study was also conducted to evaluate the ability of EV complexes with doxorubicin and paclitaxel to cross the blood brain barrier in zebrafish (84). Pascucci, et al., reported strong anti-tumorigenic effects can be achieved by exposing MSCs with paclitaxel which is then released into the EVs. This was the first study to report that MSCs could be used to package and deliver drugs (85, 86).
4.3 Endogenous loading methods
4.3.1 Transfection/Overexpression
One strategy for loading therapeutic nucleic acid cargo within EVs is by transfecting oligonucleotides (miRNA/siRNAs/mRNAs) or a plasmid that will express the mRNA/miRNA/shRNA directly into the cells. Simple cell transfection could cause a passive loading of the cargo in the EVs which could then be used for therapeutic purposes. The transfections could be carried out by calcium phosphate method or by commercially available lipid reagents such as Lipofectamine, HiPerFect or Exofect transfection reagents. Since RNA and protein sequences are easily transfected as synthetic oligonucleotides or expressed from a plasmid backbone, this approach could be effectively used to package miRNA, siRNA, mRNA and protein within the EVs. Mizrak, et al., reported that overexpressing prodrug converting enzyme CD-URPT protein causes CD-URPT mRNA and protein to be sufficiently loaded into the EVs (54). When directly injected into the schwannomas, mRNA/protein loaded EVs along with systemic administration of 5-fluorocytosine was effective in reducing tumor growth. Another incidence of mRNA transfer by EVs was reported by Ridder, et al., who engineered glioma and carcinoma tumor cells to overexpress Cre mRNA/protein (12). Successful mRNA transfer was reported as Cre mediated recombination was observed in mice with Cre reporter background. Zomer, et al., and Ridder, et al., both reported that the observed recombination events resulted from Cre mRNA translation in the recipient cells since protein was not detected in the EVs (12, 87). Furthermore, several studies reported that miRNAs could be efficiently loaded into the EVs either by using miRNA expression backbones or by precursor or miRNA mimic/antimiR oligonucleotides transfections (53, 88–94). Akao, et al., reported that transfecting chemically modified miR-143 in THP-1 macrophages cells causes the modified miRNA to be loaded into the EVs (95). This work exhibited that manipulating cells by overexpressing a miRNA could be effectively used to load miRNA passively into the EVs. Ohno, et al., exhibited that the miRNA loaded EVs could also be targeted effectively to recipient cells by engineering their surface with targeting peptides (53). Ohno and colleagues observed that intravenously injected EVs accumulated within the tumor and reduced tumor burden. Another study by Liu, et al., exhibited that modifying the membrane surface of EV to express the rabies viral glycoprotein (RVG) peptide effectively delivers opioid receptor mu siRNA into the brain (96). The ease of application for this method makes it highly popular among the EV community to package cargo inside of the EVs. However, careful consideration needs to be given to the selection of the donor cells as some EV cargo may induce adverse effects in vivo. For example, overexpressing a miRNA within the donor cells could result in gene expression changes thus causing a change in the contents that are packaged into the EVs. Also, this technique compared to electroporation or cholesterol conjugation could show lower loading efficiency and requires optimization depending upon the donor cell line and its cargo.
4.3.2 Targeted and Modular EV loading approach
As addressed in the previous section, RNA molecules have been passively loaded within the EVs either by simply transfecting the cells with RNA expression vectors or RNA oligonucleotides (88, 97). However, until now, very little work has been done to understand if RNA cargo could be actively loaded into the EVs. The targeted and modular EV loading (TAMEL) approach is a unique way of actively loading RNA cargo into the EVs by engineering EV-enriched protein and a cargo RNA (98). The EV-enriched protein is a fusion protein which consists of a transmembrane protein domain and an RNA-binding domain. Hung and Leonard developed the TAMEL platform by fusing the MS2 bacteriophage coat protein to EV-enriched protein such as Human lamp2b and CD63 which could bind with the cognate MS2 stem loop that was engineered onto the cargo RNA (98). HEK293FT cells were used to stably express the plasmids that encoded for the proteins along with the cargo RNA which contained the mutated and wild type MS2 loops. The EV loading of mRNA molecules greater than 1.5 kb was lower compared to the loading of small RNA molecules (less than 0.5 kb). Thus, while the TAMEL approach exhibited a unique method of loading cargo in the EVs and achieved substantial high loading efficiencies, a failure of any phenotypic changes in the recipient cell-lines was observed. This was primarily due to the rapid degradation of the EV cargo upon internalization in the recipient cells, highlighting the inefficient endosomal escape when taken up by the recipient cell lines. These findings highlight one of the major concerns that limit engineered cells to produce therapeutic EVs. Since the Hung and Leonard study was carried out using the HEK293T derived EVs, the possibility exists that the choice of cell line by which these therapeutic EVs are made could be responsible for the lack of efficacy due to endosomal entrapment. It would be informative to explore engineering platforms in other donor cells such as mesenchymal stem cells or dendritic cells. Nevertheless, the TAMEL approach provides a unique way of loading RNA molecules within the EVs and could serve as preliminary work to build upon.
4.3.3 Cell extrusion generated exosome mimetic nanovesicles
Exosome mimetics are cell-derived vesicles that are produced by extruding the donor cells through filters of reducing pore sizes (99). Essentially, the vesicles are produced artificially by breaking up the cells and then reforming the contents into exosome mimetics as they retain some of the physical and biological characteristics of EVs. The exosome mimetic technique is shown to produce higher quantities of EVs (approximately 100 fold) when compared to the EVs released by the cells (99–101). Furthermore, since cells could be genetically engineered to express a specific targeting peptide on the cell surface, and since these mimetics maintain the exact topology of the plasma membrane proteins, this method could be developed to specifically target intended cells/tissues. Su Chul Jang, et al., developed the exosome mimetic nanovesicles to effectively deliver chemotherapeutics such as doxorubicin, 5-fluorouracil, gemcitabine and carboplatin and study their effects on tumor growth (99). Exosome mimetics were developed from human U937 monocytic cells and mouse Raw264.7 macrophages incubated with or without the chemotherapeutics. Serial extrusion was performed on the cells, followed by iodixanol gradient ultracentrifugation to obtain the therapeutic nanovesicles. EVs were also harvested from the same cells to compare the efficacy of EV to that of exosome mimetic nanovesicles. Both of the vesicles were similarly effective in reducing tumor growth in vivo. When compared to the free drug, 20 fold lower amount of exosome mimetics were needed to reduce tumor growth to the same extent. Another interesting finding was that when the exosome mimetics were isolated from the two cell lines containing the cancer drugs, and injected into an immunocompetent mouse tumor model, they both exhibited similar anti-tumor effects with no reported systemic side effects. Another study was conducted by Lunavat and colleagues which focused on loading an RNAi therapeutic within the exosome-mimetic nanovesicles (101). In this study, two methods of loading shRNA cargo into the mimetics were evaluated: firstly by exogenously electroporating the siRNA into the cell extruded vesicles and secondly by overexpressing the shRNA into the donor cells and then extruding the cells to collect the endogenously expressed shRNA nanovesicles. The results from this study were very similar to the previous one, showing that the nanovesicles produced using both exogenous and endogenous loading methods were both internalized within the recipient cells and caused reduction in the expression levels of c-Myc. The positive findings of the study imply that the exosome-mimetic nanovesicles could in fact be used to overcome some of the scale up issues that are currently associated with development of EV therapeutics.
4.3.4 Vexosome hybrid vesicles
Adeno-associated virus (AAV) vector delivery has been used in gene therapy studies for the treatment of several genetic diseases including central nervous system (CNS) disorders (102, 103). The efficacies of gene transfer and gene expression are limited by the humoral immune responses that produce neutralizing antibodies against these wild type AAV vectors (104). Some preliminary work is reported on loading the EVs with viral capsids (AAV vectors) which could serve as another method of overcoming some of the shortcomings of AAV vector delivery (105–107). These hybrid vesicles are referred to as “vexosomes” as they comprise of viral AAV vectors associated with EVs. Vexosomes combine the desirable features of both EV and AAV vector systems, providing enhanced transfection efficiencies in the recipient cells and EVs protects the vector from neutralizing antibodies in vivo. Vexosomes are produced by transfecting the AAV vectors into HEK293T cells and conditioned media containing the EVs associated with AAV vectors are isolated by the iodixanol gradient centrifugation method. Initial characterization of these vexosomes by transmission electron microscopy exhibited AAV/EV association with the size range of ~50 to 200 nm (105). A subsequent in vivo study showed that vexosomes outperform AAV vectors without EV association when administered in the presence of neutralizing antibodies (106). Vexosomes could also be engineered to display targeting peptides on their surface to enable enhanced delivery to target tissue. When injected systemically in mice, vexosomes crossed the blood brain barrier and enabled efficient transduction of central CNS cells (107). This series of studies conducted in the last few years highlights the potential of using vexosomes for gene delivery, particularly to the difficult to reach CNS cells.
4.3.5 EV sequence sorting mechanism
It is poorly understood how certain RNA molecules are preferentially sorted into the EVs. Villarroya-Beltri, et al., identified two short sequences which enabled the miRNA containing those sequences to be actively loaded within the EVs. These sequences were referred to as EXOmotifs. EXOmotif sequences bind to the heterogeneous nuclear riboprotein A2B1 (hnRNPA2B1) which are then loaded inside of the EVs (108). Mutating these motifs decreased the expression of miRNAs that were sorted in the EVs through protein binding. HNRNPA2B1 is a ubiquitous RNA binding protein, shown to regulate mRNA trafficking to axons in neural cells (109) and is also involved in regulating mRNAs by binding to the lncRNA (110). HNRNPA2B1 regulates mRNA trafficking via a 21-nucleotide sequence called as the RNA trafficking sequence. This sequence overlapped with both the EXOmotif sequences that were found to guide the miRNA loading into the EVs. Furthermore, the HNRNPA2B1 protein in exosomes is sumolyated, meaning it undergoes post-transcriptional modification which controls the binding of the protein to the miRNAs and is important for EXOmiRNAs loading into the EVs. Another group identified a stem loop forming sequence of 25 nucleotides within the 3′ UTR region of mRNA that promoted its accumulation in EVs (111). This sequence was called the zipcode sequence which contained miR-1289 binding region and a CUGCC sequence which increased the packaging of the mRNA transcript by two-fold when compared to the mRNA transcript without the zipcode sequence. As these EV sorting sequences could be engineered onto an endogenous gene to increase its loading within the EVs these EXOmotif and zipcode sequences provide a unique tool for loading the EVs with desired mRNA/miRNA therapeutics for gene therapy.
5. SUMMARY/PROSPECTUS
We review here various methods and techniques to load therapeutic cargo into EVs, including small molecule compounds and nucleic acid drugs. There has recently been much anticipation in the development of therapeutic EV delivery systems as they are derived from natural cellular processes and may reduce some of the toxicity that is associated with nanoparticle delivery systems (112, 113). In addition, with therapeutic EVs, it may be possible to deliver their payload to difficult to target tissues such as the CNS (66, 83). However, for the promise of therapeutic EVs to be achieved clinically, there must be the means to load the EVs with therapeutic cargo that is efficient, cost effective and scalable.
Various methods and techniques have been reviewed to load therapeutic cargo into EVs, including small molecule compounds and nucleic acid drugs. In addition to the classes of EV loading outlined herein, several recent reports have been published that describe novel methods to load EVs with therapeutic cargo. Kim et al., exploited the sonication method to load EVs with paclitaxel in order to overcome P-glycoprotein transporter mediated multiple drug resistance in cancer cells (114). Drug encapsulated within the EVs increased efficacy 50 times compared to that of the free drug. To overcome the conventional low efficiency loading of proteins, by Yim, et al., reported a new loading approach called exosome for protein loading via optically reversible protein-protein interaction (EXPLORs) (115). They demonstrated the ability to load intracellular proteins into EVs by successfully delivering Cre recombinase into the target cells both in vitro and in vivo.
We review two broad categories of therapeutic EV loading: endogenous and exogenous (Figure 2). Endogenous loading implies addition of therapeutic cargo to the EV directly from the donor cell while exogenous loading refers to the depositing of cargo into purified EVs. Endogenous loading is typically reserved for therapeutic cargo that is expressed from a vector or through engineering of the donor cell. The primary advantages of endogenous loading include having a complete cellular system that is scalable and that the therapeutic cargo is directly loaded into the drug delivery system (i.e. EV). If the therapeutic cargo (i.e. siRNA, miRNA, mRNA) is expressed from the donor cell, this obviates the need for introducing oligonucleotides, thus offering the potential for substantial cost savings. The primary disadvantage of endogenous loading is low loading efficiencies, while the loading efficiency for exogenous methods can be quite high, e.g. 3,000 siRNA copies per EV (40) and 2.9 μg of drug per μg of EV (82). The disadvantage of exogenous loading is the introduction of additional steps to the manufacturing process and in the case of nucleic acid delivery, the need for expensive chemically modified oligonucleotides.
Figure 2.
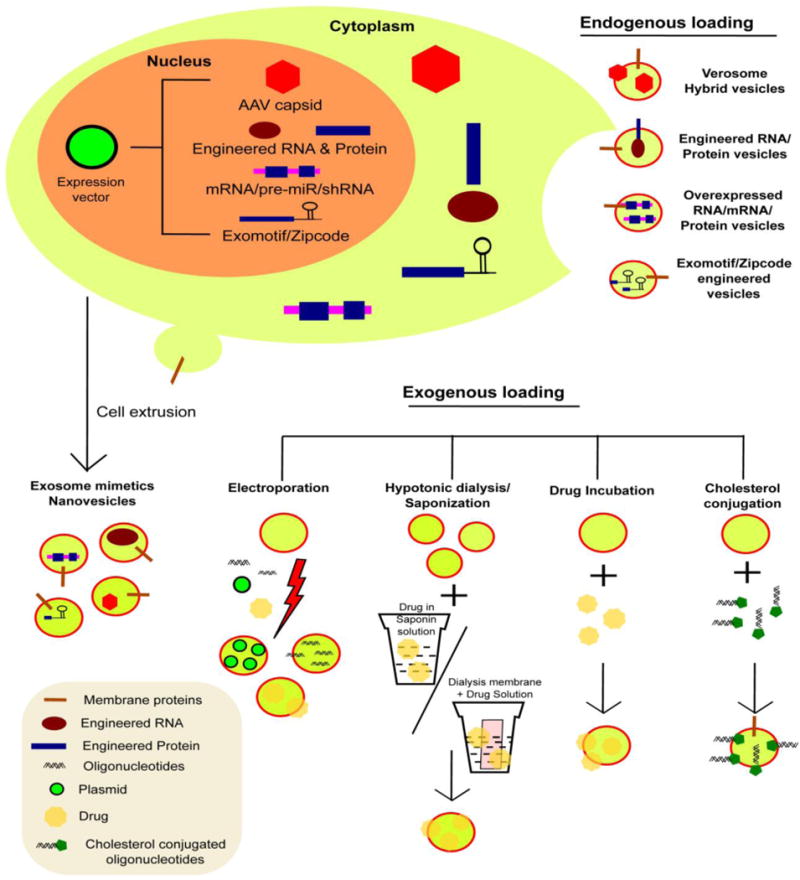
Different approaches to load EV therapeutics. EVs could be therapeutically loaded using endogenous and exogenous loading techniques. Endogenous approach involves overexpressing therapeutic cargo within the donor cells, which eventually gets loaded within the EVs. Exogenous approach includes the loading of therapeutic cargo within EVs once they are isolated. Depending upon the specific loading technique through which the cargo is loaded inside of the EVs, they could be further sub-classified into active and passive loading.
In order to compare the findings across different studies, it is important that the authors report the loading efficiency as both a percentage (i.e. amount of cargo loaded into EVs divided by the amount of cargo exposed to the EVs) and as the number of therapeutic molecules/copies of loaded EV. Secondly, the reported dose in mice should be presented as the amount of therapeutic cargo injected into mice in addition to the quantity of EVs (e.g. total EV number or μg of EV protein). To illustrate this point, we compared the loading efficiency and administered dose from published therapeutic EV studies (Table I). Thirteen of the 17 studies do not mention the loading efficiency percentage (Table I) therefore it is difficult to directly compare these methods. Granted it would not be helpful to compare a technique to load small molecules to one that loads nucleic acid, but it would be useful to compare those methods within each class of cargo. The loading percentage among these studies is quite large ranging from 0.2 – 80%. Of the 11 in vivo studies presented in Table I, 7 calculated the dose based upon the amount of EV particles or μg protein administered and not the active therapeutic.
Table I.
Overview of different loading methods:
| Loading method | Loading technique | Cargo loaded | Loading % | Amount of cargo/EV | In vivo testing | Dose | Ref. |
|---|---|---|---|---|---|---|---|
| Exogenous loading | Electroporation | Doxorubicin | Not reported | Not reported | Yes | 3 mg/kg doxorubicin | (63) |
| Electroporation | ds DNA | 50 ng | ~600 copies/EV | No | NA | (64) | |
| Electroporation | GAPDH siRNA | ~25% | NA | Yes | 150 μg of RNA encapsulate d in 150 ug of exosomes | (66) | |
| Sonication | Catalase | 26% | 940 molecules/EV | Yes | 1.2 × 10ˆ9 EV particles/10 μL 10 times every other day | (75) | |
| Saponin Permeabilzation | Catalase | Not reported | Not reported | Yes | 1.2 × 10ˆ9 exosome particles/10 μL 10 times every other day | (75) | |
| Saponin Permeabilization | Porphyrin | Not reported | ~7eˆ4 molecules/EV | No | NA | (78) | |
| Hypotonic Dialysis | Porphyrin | Not reported | ~7eˆ4 molecules/EV | No | NA | (78) | |
| Cholesterol Conjugation | Htt shRNA | 10–50% | 1000–3000 copies/EV | Yes | 20–30 × 10ˆ9 exosome particles/day | (40) | |
| Cholesterol Conjugation | GFP siRNA/CD45 siRNA | 80% | 73 copies/EV | No | NA | (80) | |
| Drug Incubation | Curcumin | Not reported | 2.9 ug/1μg EVs | Yes | 4 mg/kg curcumin | (82) | |
| Drug Incubation | Doxorubicin/Paclitaxel | 7.3 ng and 132 ng/1μg EVs | Yes | 0.2 mg/ml drug in 200 μg/L total EV protein | (84) | ||
| Endogeno us loading | miRNA overexpression | miR-143/143BP | Not reported | Not reported | No | NA | (88) |
| miRNA transfection | Let-7a | Not reported | Not reported | Yes | 4 μg exosome protein | (53) | |
| siRNA transfection | Opioid receptor Mu siRNA | Not reported | 0.14 pmol/μg EV | Yes (C57BL/6 mice) | 200 μg exosome protein | (96) | |
| mRNA/Protein transfection | CD-URPT | Not reported | Not reported | Yes (Orthotopic nude mouse) | Not mentioned | (54) | |
| Cell extrusion | Doxorubicin | Not reported | ~350 ug Drug/1μg NV | Yes (BALB/C mice bearing CT26 tumor) | 10 μg of total EV protein | (99) | |
| Cell extrusion & electroporation | GFP siRNA | Not reported | 3.7 × 1012 siRNA molecules/1μ g NV | No | NA | (101) | |
| Cell extrusion | c-myc siRNA | Not reported | Not reported | No | NA | (101) | |
| Targeted & Modular EV loading | Cas9 mRNA | Not reported | Not reported | No | NA | (98) | |
| Virus Exosome hybrid | adeno-associated virus | Not reported | <0.2% virus association with EVs | Yes (BALB/C mice) | Not mentioned | (107) |
It is difficult to speculate at this point in time what might be considered an optimal loading efficiency or predict loading efficiencies as data to support this point are sparse. In terms of percentage loading (amount loaded/amount added), the higher the better to maximize loading efficiency. However, in terms of cargo loaded per EV, it becomes more difficult to predict as there will be a saturation point. While Didiot, et al., estimate that the saturation point of EVs that are associated with the cholesterol-labeled shRNA is about 3,000 copies per EV (40), this topic has not been thoroughly addressed in the literature. For exogenous loading, an additional point to consider is the degree to which cargo is attached to the EV membrane rather than being internalized within the EV. The stability of the therapeutic agent could be compromised if attached to the EV and the ability of the loaded EVs to target specific cells or tissues adversely affected.
6. CONCLUSIONS
EVs are of great interest due to their involvement in pathophysiological processes and also to their apparent intrinsic ability to transport a wide range of biomolecules across different tissues, organs and cells. As several studies have highlighted, successful loading of therapeutics, ranging from synthetic oligonucleotides to small molecule compounds to viral vectors, can be achieved. Optimization of the techniques to load EVs will reduce cost and increase productivity, important factors as therapeutic EVs progress to the clinic.
Acknowledgments
This work was supported by the NIH UH2-UH3 award (1UH2TR000914-01) to T.D.S and M.A.P.
Abbreviations
- EV
extracellular vesicle
- MVB
multivesicular body
- MSC
mesenchymal stem cells
- DC
dendritic cells
- iPSCs
induced pluripotent stem cell derived MSCs
- OMVs
outer membrane vesicles
- Htt
huntingtin geneTAMEL: targeted and modular EV loading
- AAV
Adeno-associated virus
- CNS
central nervous system
- hnRNPA2B1
heterogeneous nuclear riboprotein A2B1
References
- 1.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–78. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 2.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 3.Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE. 2008;3(11):e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kogure T, Yan IK, Lin WL, Patel T. Extracellular Vesicle-Mediated Transfer of a Novel Long Noncoding RNA TUC339: A Mechanism of Intercellular Signaling in Human Hepatocellular Cancer. Genes & cancer. 2013;4(7–8):261–72. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gezer U, Ozgur E, Cetinkaya M, Isin M, Dalay N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int. 2014;38(9):1076–9. doi: 10.1002/cbin.10301. [DOI] [PubMed] [Google Scholar]
- 7.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262(19):9412–20. [PubMed] [Google Scholar]
- 8.Jin Y, Chen K, Wang Z, Wang Y, Liu J, Lin L, et al. DNA in serum extracellular vesicles is stable under different storage conditions. BMC Cancer. 2016;16(1):753. doi: 10.1186/s12885-016-2783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai J, Han Y, Ren H, Chen C, He D, Zhou L, et al. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol. 2013;5(4):227–38. doi: 10.1093/jmcb/mjt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowak R. Mining treasures from ‘junk DNA’. Science. 1994;263(5147):608–10. doi: 10.1126/science.7508142. [DOI] [PubMed] [Google Scholar]
- 11.Jiang L, Shen Y, Guo D, Yang D, Liu J, Fei X, et al. EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nat Commun. 2016;7:13045. doi: 10.1038/ncomms13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridder K, Sevko A, Heide J, Dams M, Rupp AK, Macas J, et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology. 2015;4(6):e1008371. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giricz Z, Varga ZV, Baranyai T, Sipos P, Pálóczi K, Kittel Á, et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75–8. doi: 10.1016/j.yjmcc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Abels ER, Redzic JS, Margulis J, Finkbeiner S, Breakefield XO. Potential Transfer of Polyglutamine and CAG-Repeat RNA in Extracellular Vesicles in Huntington’s Disease: Background and Evaluation in Cell Culture. Cell Mol Neurobiol. 2016;36(3):459–70. doi: 10.1007/s10571-016-0350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015 doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingato D, Lee JU, Sim SJ, Kwon YJ. Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery. J Control Release. 2016;241:174–85. doi: 10.1016/j.jconrel.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Marcus ME, Leonard JN. FedExosomes: Engineering Therapeutic Biological Nanoparticles that Truly Deliver. Pharmaceuticals (Basel) 2013;6(5):659–80. doi: 10.3390/ph6050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stremersch S, De Smedt SC, Raemdonck K. Therapeutic and diagnostic applications of extracellular vesicles. J Control Release. 2016 doi: 10.1016/j.jconrel.2016.07.054. [DOI] [PubMed] [Google Scholar]
- 19.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–9. [PubMed] [Google Scholar]
- 22.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 23.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329–39. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL. Late endosomes derive from early endosomes by maturation. Cell. 1991;65(3):417–27. doi: 10.1016/0092-8674(91)90459-c. [DOI] [PubMed] [Google Scholar]
- 25.Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278(22):20083–90. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 26.Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7(4):552–63. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- 27.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172(6):923–35. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8(13):2014–8. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- 29.Mavroudis D, Kouroussis C, Kakolyris S, Agelaki S, Kalbakis K, Androulakis N, et al. Phase I study of paclitaxel (taxol) and pegylated liposomal doxorubicin (caelyx) administered every 2 weeks in patients with advanced solid tumors. Oncology. 2002;62(3):216–22. doi: 10.1159/000059568. [DOI] [PubMed] [Google Scholar]
- 30.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286(5448):2244–5. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 31.Munyendo WL, Lv H, Benza-Ingoula H, Baraza LD, Zhou J. Cell penetrating peptides in the delivery of biopharmaceuticals. Biomolecules. 2012;2(2):187–202. doi: 10.3390/biom2020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowenstein PR, Mandel RJ, Xiong WD, Kroeger K, Castro MG. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther. 2007;7(5):347–60. doi: 10.2174/156652307782151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniel R, Smith JA. Integration site selection by retroviral vectors: molecular mechanism and clinical consequences. Hum Gene Ther. 2008;19(6):557–68. doi: 10.1089/hum.2007.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–77. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 35.Zhang JS, Liu F, Huang L. Implications of pharmacokinetic behavior of lipoplex for its inflammatory toxicity. Adv Drug Deliv Rev. 2005;57(5):689–98. doi: 10.1016/j.addr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, et al. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release. 2006;112(1):15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta. 2014;1846(1):75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107(14):6328–33. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–56. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 40.Didiot MC, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K, et al. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol ther. 2016 doi: 10.1038/mt.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–4. doi: 10.1093/nar/gkr828. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An Q, van Bel AJ, Hückelhoven R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal Behav. 2007;2(1):4–7. doi: 10.4161/psb.2.1.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16(1):24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol. 2015;40:97–104. doi: 10.1016/j.semcdb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21(7):1345–57. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.García-Castro J, Trigueros C, Madrenas J, Pérez-Simón JA, Rodriguez R, Menendez P. Mesenchymal stem cells and their use as cell replacement therapy and disease modelling tool. J Cell Mol Med. 2008;12(6B):2552–65. doi: 10.1111/j.1582-4934.2008.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, et al. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int J Proteomics. 2012;2012:971907. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6:10. doi: 10.1186/scrt546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, et al. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int J Biol Sci. 2016;12(7):836–49. doi: 10.7150/ijbs.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas P, Smart TG. HEK293 cell line: a vehicle for the expression of recombinant proteins. J Pharmacol Toxicol Methods. 2005;51(3):187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 53.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol ther. 2013;21(1):185–91. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, et al. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther. 2013;21(1):101–8. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Chen X, Yi J, Liu Y, Li D, Wang J, et al. Identification and Characterization of 293T Cell-Derived Exosomes by Profiling the Protein, mRNA and MicroRNA Components. PLoS One. 2016;11(9):e0163043. doi: 10.1371/journal.pone.0163043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–84. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13(10):605–19. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier-Poisson I. A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice. Microbes Infect. 2007;9(14–15):1614–22. doi: 10.1016/j.micinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Beauvillain C, Juste MO, Dion S, Pierre J, Dimier-Poisson I. Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine. 2009;27(11):1750–7. doi: 10.1016/j.vaccine.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 60.Zhu L, Song H, Zhang X, Xia X, Sun H. Inhibition of porcine reproductive and respiratory syndrome virus infection by recombinant adenovirus- and/or exosome-delivered the artificial microRNAs targeting sialoadhesin and CD163 receptors. Virol J. 2014;11:225. doi: 10.1186/s12985-014-0225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim OY, Hong BS, Park KS, Yoon YJ, Choi SJ, Lee WH, et al. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J Immunol. 2013;190(8):4092–102. doi: 10.4049/jimmunol.1200742. [DOI] [PubMed] [Google Scholar]
- 62.Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179(11):7692–701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- 63.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–90. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 64.Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol Pharm. 2015;12(10):3650–7. doi: 10.1021/acs.molpharmaceut.5b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 67.Banizs AB, Huang T, Dryden K, Berr SS, Stone JR, Nakamoto RK, et al. In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int J Nanomedicine. 2014;9:4223–30. doi: 10.2147/IJN.S64267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weaver JC. Electroporation: a general phenomenon for manipulating cells and tissues. J Cell Biochem. 1993;51(4):426–35. doi: 10.1002/jcb.2400510407. [DOI] [PubMed] [Google Scholar]
- 69.Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation. Anal Biochem. 2014;448:41–9. doi: 10.1016/j.ab.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnsen KB, Gudbergsson JM, Skov MN, Christiansen G, Gurevich L, Moos T, et al. Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology. 2016 doi: 10.1007/s10616-016-9952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kooijmans SA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJ, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release. 2013;172(1):229–38. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 72.Stoicheva NG, Hui SW. Electrofusion of cell-size liposomes. Biochim Biophys Acta. 1994;1195(1):31–8. doi: 10.1016/0005-2736(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 73.Jamur MC, Oliver C. Permeabilization of cell membranes. Methods Mol Biol. 2010;588:63–6. doi: 10.1007/978-1-59745-324-0_9. [DOI] [PubMed] [Google Scholar]
- 74.Jacob MC, Favre M, Bensa JC. Membrane cell permeabilization with saponin and multiparametric analysis by flow cytometry. Cytometry. 1991;12(6):550–8. doi: 10.1002/cyto.990120612. [DOI] [PubMed] [Google Scholar]
- 75.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ambani LM, Van Woert MH, Murphy S. Brain peroxidase and catalase in Parkinson disease. Arch Neurol. 1975;32(2):114–8. doi: 10.1001/archneur.1975.00490440064010. [DOI] [PubMed] [Google Scholar]
- 77.Abraham S, Soundararajan CC, Vivekanandhan S, Behari M. Erythrocyte antioxidant enzymes in Parkinson’s disease. Indian J Med Res. 2005;121(2):111–5. [PubMed] [Google Scholar]
- 78.Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 79.Tan S, Wu T, Zhang D, Zhang Z. Cell or cell membrane-based drug delivery systems. Theranostics. 2015;5(8):863–81. doi: 10.7150/thno.11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stremersch S, Vandenbroucke RE, Van Wonterghem E, Hendrix A, De Smedt SC, Raemdonck K. Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J Control Release. 2016;232:51–61. doi: 10.1016/j.jconrel.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 81.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1(3):297–315. [PMC free article] [PubMed] [Google Scholar]
- 82.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol ther. 2010;18(9):1606–14. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol ther. 2011;19(10):1769–79. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32(6):2003–14. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–70. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 86.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23(5):812–23. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046–57. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akao Y, Iio A, Itoh T, Noguchi S, Itoh Y, Ohtsuki Y, et al. Microvesicle-mediated RNA molecule delivery system using monocytes/macrophages. Mol Ther. 2011;19(2):395–9. doi: 10.1038/mt.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Su MJ, Aldawsari H, Amiji M. Pancreatic Cancer Cell Exosome-Mediated Macrophage Reprogramming and the Role of MicroRNAs 155 and 125b2 Transfection using Nanoparticle Delivery Systems. Sci Rep. 2016;6:30110. doi: 10.1038/srep30110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, et al. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol Ther. 2016;24(7):1290–301. doi: 10.1038/mt.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol Ther Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shimbo K, Miyaki S, Ishitobi H, Kato Y, Kubo T, Shimose S, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun. 2014;445(2):381–7. doi: 10.1016/j.bbrc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 95.Akao Y, Nakagawa Y, Hirata I, Iio A, Itoh T, Kojima K, et al. Role of anti-oncomirs miR-143 and -145 in human colorectal tumors. Cancer Gene Ther. 2010;17(6):398–408. doi: 10.1038/cgt.2009.88. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi: 10.1038/srep17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohno SI, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol ther. 2012 doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hung ME, Leonard JN. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J Extracell Vesicles. 2016;5:31027. doi: 10.3402/jev.v5.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 100.Jang SC, Gho YS. Could bioengineered exosome-mimetic nanovesicles be an efficient strategy for the delivery of chemotherapeutics? Nanomedicine (Lond) 2014;9(2):177–80. doi: 10.2217/nnm.13.206. [DOI] [PubMed] [Google Scholar]
- 101.Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lässer C, et al. RNAi delivery by exosome-mimetic nanovesicles - Implications for targeting c-Myc in cancer. Biomaterials. 2016;102:231–8. doi: 10.1016/j.biomaterials.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 102.Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K, et al. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol Ther. 2010;18(9):1731–5. doi: 10.1038/mt.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4(120):120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108(10):3321–8. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maguire CA, Balaj L, Sivaraman S, Crommentuijn MH, Ericsson M, Mincheva-Nilsson L, et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther. 2012;20(5):960–71. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.György B, Fitzpatrick Z, Crommentuijn MH, Mu D, Maguire CA. Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials. 2014;35(26):7598–609. doi: 10.1016/j.biomaterials.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hudry E, Martin C, Gandhi S, György B, Scheffer DI, Mu D, et al. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 2016;23(4):380–92. doi: 10.1038/gt.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Munro TP, Magee RJ, Kidd GJ, Carson JH, Barbarese E, Smith LM, et al. Mutational analysis of a heterogeneous nuclear ribonucleoprotein A2 response element for RNA trafficking. J Biol Chem. 1999;274(48):34389–95. doi: 10.1074/jbc.274.48.34389. [DOI] [PubMed] [Google Scholar]
- 110.Lan X, Yan J, Ren J, Zhong B, Li J, Li Y, et al. A novel long noncoding RNA Lnc-HC binds hnRNPA2B1 to regulate expressions of Cyp7a1 and Abca1 in hepatocytic cholesterol metabolism. Hepatology. 2016;64(1):58–72. doi: 10.1002/hep.28391. [DOI] [PubMed] [Google Scholar]
- 111.Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Ströbel T, Erkan EP, et al. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Silva AH, Locatelli C, Filippin-Monteiro FB, Martin P, Liptrott NJ, Zanetti-Ramos BG, et al. Toxicity and inflammatory response in Swiss albino mice after intraperitoneal and oral administration of polyurethane nanoparticles. Toxicol Lett. 2016;246:17–27. doi: 10.1016/j.toxlet.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 113.Silva AH, Locatelli C, Filippin-Monteiro FB, Zanetti-Ramos BG, Conte A, Creczynski-Pasa TB. Solid lipid nanoparticles induced hematological changes and inflammatory response in mice. Nanotoxicology. 2014;8(2):212–9. doi: 10.3109/17435390.2013.782076. [DOI] [PubMed] [Google Scholar]
- 114.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–64. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]


