Abstract
An ultrasonic-assisted technique was employed to extract crude polysaccharide from Tricholoma matsutake fruiting bodies. Single-factor tests and orthogonal experimental design (L 9(33)) were used to obtain the optimal extraction conditions. Results showed that the optimal parameters were as follows: ultrasonic temperature, 40 °C; ultrasonic time, 50 min; water to raw material ratio, 25 ml/g; ultrasonic frequency, 45 kHz; and ultrasonic power, 100 W. Three novel T. matsutake polysaccharide (TMP) fractions (TMP30, TMP60, and TMP80) were isolated and purified from TMP by stepwise alcohol precipitation. Their preliminary structural features were determined by high-performance anion-exchange chromatography with pulsed-amperometric detection (HPAEC-PAD) and Fourier transform infrared spectrophotometer (FT-IR) analyses. Furthermore, their in vitro antioxidant activity was investigated in terms of a reducing power assay and the scavenging rates of 2,2-diphenyl-1-picrylhydrazyl (DPPH) and hydroxyl radicals. The order of the various fractions based on their antioxidant activity was TMP80>TMP>TMP60>TMP30. These findings suggested that novel polysaccharide fractions from T. matsutake, especially TMP80, could be promising active macromolecules for biomedical use.
Keywords: Tricholoma matsutake polysaccharide, Orthogonal test design, Ultrasound-assisted extraction, Monosaccharide composition, Antioxidant activity
1. Introduction
Tricholoma matsutake, known in China as Songrong, is a kind of ectomycorrhizal symbiotic fungus belonging to the subgenus Tricholoma. It contains a variety of nutrients, such as polysaccharides, proteins, volatile flavoring substances, and minerals. It is one of the most precious mushrooms in the world due to its unique taste and flavor, and its pharmacological properties (Kim et al., 2010a). The fruiting bodies of T. matsutake have been used for the prevention and treatment of diseases for 1000 years in Asian countries, including Korea, Japan, and China (Yin et al., 2011; You et al., 2013).
Recent studies have shown that water-soluble T. matsutake polysaccharides (TMPs) from its fruiting bodies are one of its main active components, and have a range of pharmacological effects such as antitumour (Yang et al., 2010; You et al., 2013), immunobiological (Hoshi et al., 2008), antioxidative (Kim et al., 2010b; You et al., 2013), hematopoietic, and anti-mutagenic activity (Bohn and BeMiller, 1995). However, until now, no detailed studies have been carried out on the physicochemical characteristics and antioxidative capacities of the three polysaccharide fractions fractionated by stepwise alcohol precipitation from T. matsutake fruiting bodies.
Compared with conventional extraction methods, the ultrasound-assisted extraction (UAE) technique has many benefits, such as increased extraction yield, accelerated speed of extraction, shortened extraction duration, economical power consumption, and reduced use of energy and solvents (You et al., 2014; Chemat et al., 2017). UAE has proved recently to be a green and economically viable alternative to conventional extraction techniques for natural products and foods (You et al., 2014; Chemat et al., 2017). Also, compared with other test-design methods, orthogonal designs not only need fewer experiments, but also provide more information. Thus, an orthogonal design has been widely adopted in the optimization of experimental factors involved in extraction (Xu et al., 2005; Ye et al., 2013).
In the present study, UAE was employed to extract the crude TMP from T. matsutake fruiting bodies. Based on single-factor tests, an orthogonal experiment (L 9(33)) was used to optimize extraction conditions for UAE. We also focused on the preliminary determination of the structural features of three novel polysaccharides TMP30, TMP60, and TMP80, isolated and fractionated from TMP using different final concentrations of alcohol precipitation. In addition, the in vitro antioxidant capabilities of the fractions were estimated so as to seek new natural functional ingredients for use in the food and pharmaceutical industries.
2. Materials and methods
2.1. Materials and reagent equipment
T. matsutake fruiting bodies were purchased from the Kunming Edible Fungi Institute of General National Supply and Marketing Cooperative of the People’s Republic of China Company (Kunming, China). Monosaccharide standards D-glucose (D-Glc), D-galactose (D-Gal), D-arabinose (D-Ara), L-fucose (L-Fuc), D-mannose (D-Man), L-rhamnose (L-Rha), D-fructose (D-Fru), D-xylose (D-Xyl), D-GlcA, and D-GalA were purchased from Sigma-Aldrich (St. Louis, MO, USA). 2,2-Diphenyl-1-picrylhydrazyl free radical (DPPH•) was purchased from the Sigma Chemical Co. (St. Louis, MO, USA). All other reagents were bought from Sinopharm Chemical Reagent Co., Ltd., (Shanghai, China) and were of analytical reagent (AR) grade.
2.2. Extraction optimization and fractionation of polysaccharide TMP
2.2.1 Extraction of TMP and yield determination
Dried raw material (460 g) was ground into a powder and then pretreated with 85% ethanol at room temperature for 24 h to degrease and remove some small molecular materials, oligosaccharides, and some colored materials. The solid residue was separated from the solvent by centrifugation (1776g, 10 min) and dried in air.
Each pretreated sample (1.0 g) was extracted in an ultrasonic cleaner (KQ-100VDB double frequency numerical control ultrasonic cleaner, Kunshan Ultrasound Instrument Co., China) with distilled water. The extraction procedure was repeated once. The combined supernatant was concentrated to 1/5 of the original volume with a rotary evaporator. Dehydrated ethanol was slowly added to the concentrated supernatant to 80% (v/v) of the final alcohol content, then kept at 4 °C overnight. After centrifugation, dialysis, and lyophilization, the crude polysaccharide was obtained and named TMP.
Single-factor experiments were performed under the following conditions: ultrasonic frequency (45 and 80 kHz), ultrasonic power (from 60 to 100 W), extraction temperature (from 40 to 80 °C), ultrasonic time (from 20 to 60 min), and ratio of water to raw material (from 15 to 35 ml/g).
The yield (%) of TMP was calculated using the following formula: yield=M 1/M 0×100%, where M 1 was the weight (g) of crude TMP, and M 0 was the weight (g) of pretreated material.
2.2.2 Optimization for UAE parameters of the polysaccharide TMP
Based on the results of the single-factor experiments, two parameters (ultrasonic power and ultrasonic frequency) were selected at 100 W and 45 kHz, respectively. An orthogonal L 9(33) test design in the extraction mode was applied to optimize the other extraction parameters. The independent variables, including the water to raw material ratio (A), ultrasonic time (B), and extraction temperature (C) at three levels in the extraction process, are shown in Table 1. Nine-extraction tests (Table 2) were carried out and the crude TMPs were obtained according to the method mentioned above in Section 2.2.1, weighed, and then their extraction yields were calculated.
Table 1.
Factors and levels for orthogonal test design
| Level | Water to raw material ratio (A, ml/g) | Ultrasonic temperature (B, °C) | Ultrasonic time (C, min) |
| 1 | 20 | 50 | 30 |
| 2 | 25 | 60 | 40 |
| 3 | 30 | 70 | 50 |
Table 2.
Analysis of L 9(33) test results
| No. | Level |
Yield of TMP (%) | ||
| Water to raw material ratio (A) | Ultrasonic temperature (B) | Ultrasonic time (C) | ||
| 1 | 1 | 1 | 1 | 6.13 |
| 2 | 1 | 2 | 2 | 6.42 |
| 3 | 1 | 3 | 3 | 7.87 |
| 4 | 2 | 1 | 2 | 6.51 |
| 5 | 2 | 2 | 3 | 7.53 |
| 6 | 2 | 3 | 1 | 7.60 |
| 7 | 3 | 1 | 3 | 6.33 |
| 8 | 3 | 2 | 1 | 6.73 |
| 9 | 3 | 3 | 2 | 7.22 |
|
| ||||
| k 1 | 6.81 | 6.32 | 6.82 | |
| k 2 | 7.21 | 6.89 | 6.72 | |
| k 3 | 6.76 | 7.56 | 7.24 | |
| R | 0.45 | 1.24 | 0.52 | |
k 1, k 2, and k 3 refer to the sums of the test results (extraction yield) corresponding to the numbers in the 1st, 2nd, and 3rd columns, respectively. R refers to the result of extreme difference analysis. TMP: T. matsutake polysaccharide
2.2.3 Preparation and fractionation of three novel polysaccharides TMP30, TMP60, and TMP80
Under the optimal conditions, crude TMP was obtained from the pretreated dry powder of T. matsutake. A stepwise ethanol precipitation assay was used to fractionate the TMP (Du et al., 2013; Zhao et al., 2013). Briefly, dehydrated ethanol was slowly added to the concentrated supernatant to final alcohol concentrations of 30%, 60%, and 80%. Accordingly, three purified fractions termed TMP30, TMP60, and TMP80 were obtained.
2.3. Monosaccharide compositions of three polysaccharide fractions
Each sample of polysaccharide (2 mg) was hydrolyzed with 2 mol/L trifluoroacetic acid (TFA) at 110 °C for 5 h. Ten kinds of monosaccharides, D-Glc, D-Gal, D-Ara, L-Fuc, L-Rha, D-Fru, D-Xyl, D-Man, D-GlcA, and D-GalA were used as standards. The column was eluted with 2 mol/L NaOH at a flow rate of 0.45 ml/min. The monosaccharide composition and the percentages of various polysaccharides were determined by high-performance anion-exchange chromatography with pulsed-amperometric detection (HPAEC-PAD), using a CarboPac™ PA20 column (3 mm×150 mm) eluted with 2 mmol/L NaOH at a flow rate of 0.45 ml/min (Du et al., 2009; 2013).
2.4. Fourier transform infrared analysis
Infrared (IR) analysis was performed using a Fourier transform infrared spectrophotometer (FT-IR, Scimitor 800, Varian, USA). Two milligrams of each polysaccharide sample and 100 mg of KBr were mixed, ground, and pressed into a tablet in an agate mortar. IR spectra were recorded in the frequency range of 4000–400 cm−1 (Ye et al., 2009).
2.5. Antioxidant activity assays
The reducing power and DPPH and hydroxyl radical-scavenging activity of the crude TMP and its fractions (TMP30, TMP60, and TMP80) were determined according to our previous report (Du et al., 2013). Briefly, the reducing power assay was performed using the K3Fe(CN)6 reduction method (Wang et al., 2012), DPPH radical-scavenging activity was determined following Ardestani and Yazdanparast (2007) with minor modifications, and the hydroxyl radical-scavenging activity was measured by the salicylic acid method (Smironff and Cumbes, 1989).
2.6. General methods
The total sugar content was measured using anthrone-sulfonic acid with glucose as a standard (Zhang, 1999). The protein content was determined by Coomassie brilliant blue method Bradford using bovine serum albumin as the standard (Murphey et al., 1989). The reducing sugar content was estimated by the 3,5-dinitrosalicylic acid (DNS) colorimetry method (Zhang, 1999).
2.7. Statistical analysis
All data are presented as mean±standard deviation (SD) and were analyzed by the analysis of variance (ANOVA) method. The significance of any differences between groups was evaluated using Student’s t-test. All computations were performed using STST (Nanjing Agricultural University, Nanjing, China). All experiments were carried out three times.
3. Results and discussion
3.1. Effect of ultrasonic-assisted extraction conditions on the yield of TMP
3.1.1 Ultrasonic frequency
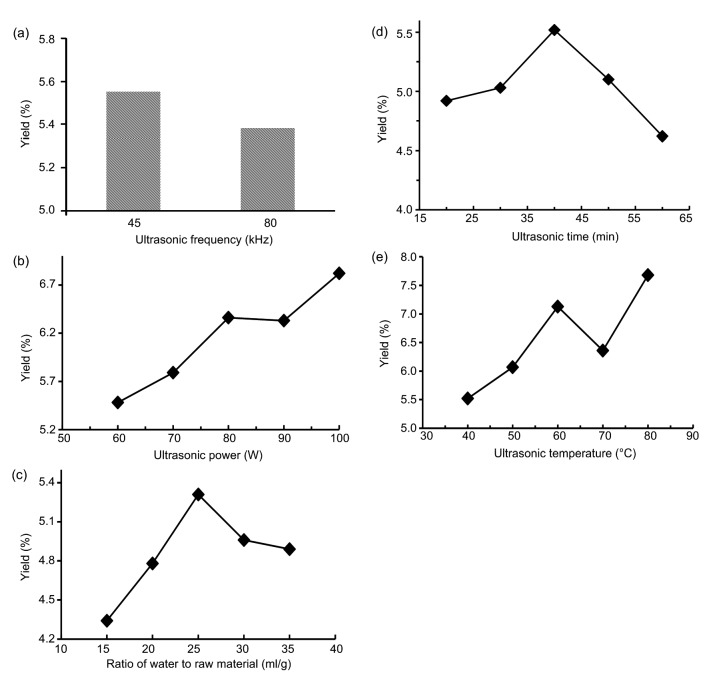
Firstly, to assess the influence of ultrasonic frequency on the yield of TMP, different ultrasonic frequencies (45 and 80 kHz) were set. Other parameters were set as follows: ultrasonic power, 80 W; ultrasonic time, 30 min; ultrasonic temperature, 70 °C; water to raw material ratio, 25 ml/g. The results are shown in Fig. 1a. When the ultrasonic frequency was set at 45 or 80 kHz, the yields of TMP were 5.55% and 5.38%, respectively, indicating that different ultrasonic frequencies affected the yield of TMP. So the optimal ultrasonic frequency was selected at 45 kHz for the orthogonal test.
Fig. 1.
Effects of different extraction parameters ultrasonic frequency(a), ultrasonic power (b), water to raw material ratio (c), ultrasonic time (d), and ultrasonic temperature (e) on the yields of polysaccharides TMP
3.1.2 Ultrasonic power
Different ultrasonic powers have been shown to affect the yield of polysaccharides (Hu et al., 2016). To investigate the influence of ultrasonic power on the extraction yield of TMP, in this study, the ultrasonic power was set at 60, 70, 80, 90, or 100 W while other parameters were set as follows: ultrasonic frequency, 45 kHz; ultrasonic time, 30 min; ultrasonic temperature, 70 °C; water to raw material ratio, 25 ml/g. The yield of TMP increased with increasing ultrasonic power from 60 to 100 W, increased only slowly from 80 to 90 W, and then reached a peak at 100 W (Fig. 1b). Thus, the optimal ultrasonic power was chosen as 100 W in orthogonal tests.
3.1.3 Water to raw material ratio
It is reported that the extraction yield of polysaccharides is influenced by the ratio of water to raw material (Huang and Ning, 2010). In this study, different ratios of water to raw material (15, 20, 25, 30, and 35 ml/g) were used to investigate their effects on the extraction yield of TMP. The yield of TMP rose clearly with an increasing ratio of water to material from 15 to 25 ml/g and reached a maximum at a ratio of 25 ml/g (Fig. 1c). However, when the ratio rose above 25 ml/g, the yield started to decline slowly. Thus, the optimal ratio of water to raw material was chosen as 25 ml/g.
3.1.4 Ultrasonic time
The ultrasonic time usually significantly affects the extraction yield of polysaccharide (Zhao et al., 2013). To assess its effect on the extraction yield of TMP, the ultrasonic time was set at 20, 30, 40, 50, or 60 min, while other extraction conditions were set as follows: ultrasonic frequency, 45 kHz; ultrasonic temperature, 70 °C; ultrasonic power, 80 W; water to raw material ratio, 25 ml/g. The extraction yield of TMP obviously increased within the initial 40 min, reached the maximum, and then decreased slightly from 40 to 60 min (Fig. 1d). Thus, the optimal ultrasonic time was chosen as 40 min.
3.1.5 Ultrasonic temperature
Increasingly, studies have reported that ultrasonic temperature plays an important role in the extraction yield of polysaccharides (Hu et al., 2016). In the present study, the effect of different temperatures (40, 50, 60, 70, and 80 °C) on the extraction yield of TMP was tested. The results indicated that the extraction yield significantly increased when the temperature increased from 40 to 60 °C, suddenly decreased at 70 °C, then increased at 80 °C (Fig. 1e). Therefore, the preferred ultrasonic temperature was selected as 60 °C.
3.2. Optimization for extraction of TMP
The results of orthogonal tests and extreme difference analysis are presented in Table 2. Here, the k and R values were calculated according to nine-extraction yields of TMP. The R values of the three factors were 0.45 (A), 1.24 (B), and 0.52 (C). Accordingly, the influence of extraction factors on yield decreased in the order B>C>A (ultrasonic temperature>ultrasonic time>ratio of water to raw material). That is to say, the ultrasonic temperature was found to be the most important determinant of the extraction yield. According to the k values of the three extraction factors, the optimal parameters would be the combination A 2 B 3 C 3, where 1‒3 are levels.
Integrating the results of the single-factor experiments with those of orthogonal tests, the optimal UAE conditions for TMP were obtained as follows: ratio of water to raw material, 25 ml/g; ultrasonic temperature, 40 °C; ultrasonic time, 50 min; ultrasonic frequency, 45 kHz; and ultrasonic power, 100 W. Moreover, through verified tests, the highest extraction yield of polysaccharide TMP was 8.06%.
The UAE technique in recent years has been widely applied due to its high efficiency and extraction rate (Li et al., 2013). Compared with the traditional hot-water extraction (HWE) method, the optimization results in this paper showed that UAE had increased TMP yield by 164%, and reduced the operation time and temperature by 320% and 137%, respectively (Yin et al., 2009). Furthermore, determination of the optimal conditions for UAE may help the industrialization or up-scaling of the UAE process. So pilot-plant testing of extraction of TMP would be our next step (Achat et al., 2012).
However, it has been reported that ultrasound might lead to degradation of natural products (Pingret et al., 2012). The main focus of this paper was screening for more bioactive TMPs. Moreover, our unpublished results showed that the TMPs extracted by the UAE technique had stronger bioactivity than those obtained by the HWE method. Whether TMPs extracted by UAE are degraded will be the subject of a future study.
3.3. Characterization of the three polysaccharide fractions
3.3.1 Composition of the three polysaccharides
The crude TMP extracted from T. matsutake fruiting bodies was obtained by a series of purification procedures such as ethanol infusion, ultrasonic extraction, and ethanol sedimentation. The total sugar, reducing sugar, polysaccharide, and protein content of the TMP were 35.81%, 4.54%, 31.27%, and 7.48%, respectively.
After fractionation from the crude TMP using a sequence of different final concentrations of alcohol precipitation, three different polysaccharides (TMP30, TMP60, and TMP80) were successfully obtained. They accounted for 45.38%, 30.51%, and 24.11% of the TMP, respectively (Table 3). Their physicochemical properties, including their total sugar and protein content, and monosaccharide component, are summarized in Table 3. The properties of polysaccharides, such as their composition and molar ratio, make an important contribution to their bioactivity (Lv et al., 2009). In the present study, HPAEC-PAD was applied to identify and quantify the monosaccharide composition of various polysaccharides under the same analytical conditions.
Table 3.
Monosaccharide components and properties of various polysaccharides from T. matsutake
| Component | Content (%)a
|
|||
| TMP30 | TMP60 | TMP80 | TMP | |
| Yield | 45.38 | 30.51 | 24.11 | 8.06 |
| Total sugar | 41.71 | 57.49 | 20.55 | 35.81 |
| Reducing sugar | 4.12 | 4.41 | 4.58 | 4.54 |
| Polysaccharideb | 37.59 | 53.08 | 15.97 | 31.27 |
| Protein content | 5.73 | 3.52 | 11.63 | 7.48 |
| Monosaccharide | ||||
| L-Fucose | 9.3 | 6.6 | 8.1 | |
| D-Galactose | 26.8 | 17.6 | 21.2 | |
| D-Glucose | 40.1 | 42.3 | 43.0 | |
| D-Xylose | 2.6 | 12.1 | 4.2 | |
| D-Mannose | 16.4 | 21.1 | 23.6 | |
The HPAEC-PAD results of 10 monosaccharide standards are shown in Fig. 2a, and the monosaccharide compositions of TMP30, TMP60, and TMP80 in Figs. 2b–2d, respectively. By matching the retention time with those of monosaccharide standards, five peaks were identified in the three fractions in the order L-Fuc, D-Gal, D-Glc, D-Xyl, and D-Man. That is to say, TMP30, TMP60, and TMP80 had the same monosaccharide composition, but different molar ratios. Comparing Fig. 2b with Fig. 2a, TMP30 was found to consist of L-Fuc, D-Gal, D-Glc, D-Xyl, and D-Man in a molar ratio of 9.3:26.8:40.1:2.6:16.4 (Table 3 and Fig. 2). Similarly, TMP60 and TMP80 contained the same monosaccharide components in a molar ratio of 6.6:17.6:42.3:12.1:21.1 and 8.1:21.2:43.0: 4.2:23.6, respectively (Figs. 2c and 2d, and Table 3). Obviously, glucose was the primary monosaccharide in the three polysaccharide fractions, with molar ratios of 40.1%, 42.3%, and 43.0%, respectively (Table 3 and Fig. 2). However, one unknown peak was detected (Fig. 2d), associated with a retention time at 9.51 min, which might imply the presence of a new compound in TMP80.
Fig. 2.
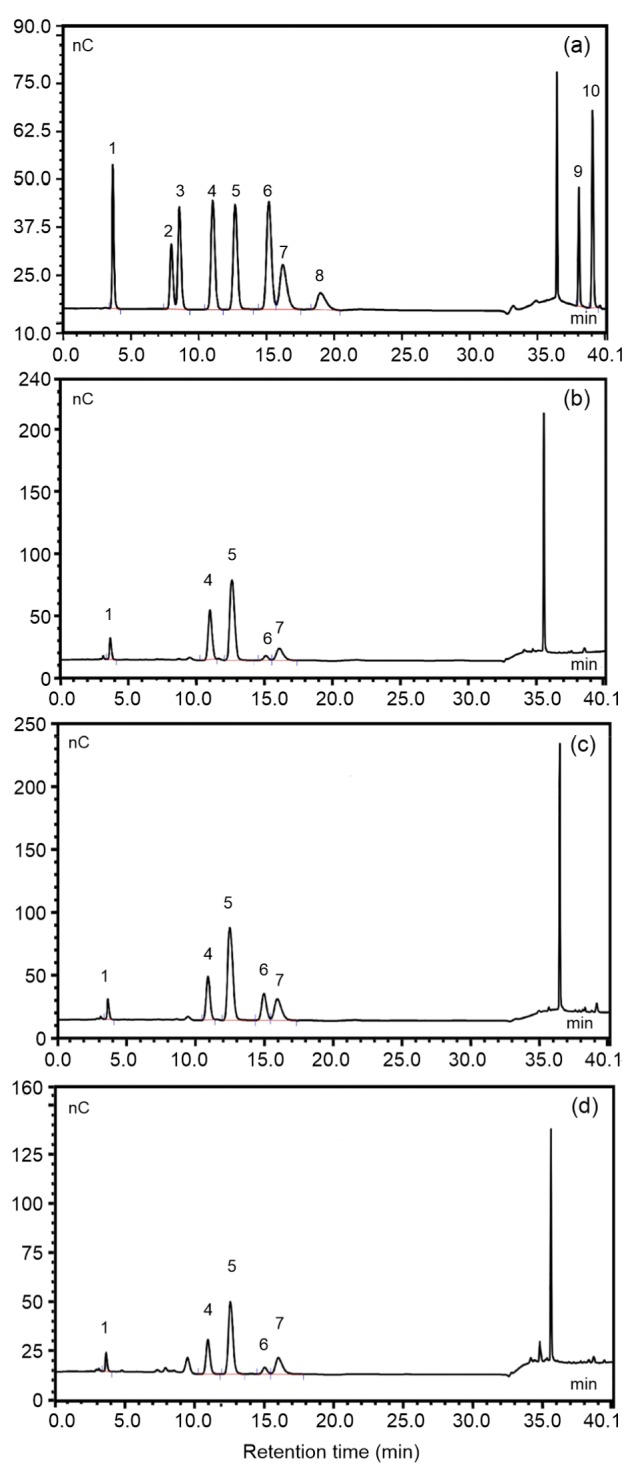
Spectra of HPAEC-PAD of 10 standard monosaccharides (a) and monosaccharide components released from TMP30 (b), TMP60 (c), and TMP80 (d)
Peaks: 1, L-fucose; 2, L-rhamnose; 3, L-arabinose; 4, D-galactose; 5, D-glucose; 6, D-xylose; 7, D-mannose; 8, D-fructose; 9, D-GalA; 10, D-GluA. HPAEC-PAD: high-performance anion-exchange chromatography with pulsed-amperometric detection. nC refers to the unit “nano-Coulomb” that represents electrical signal value
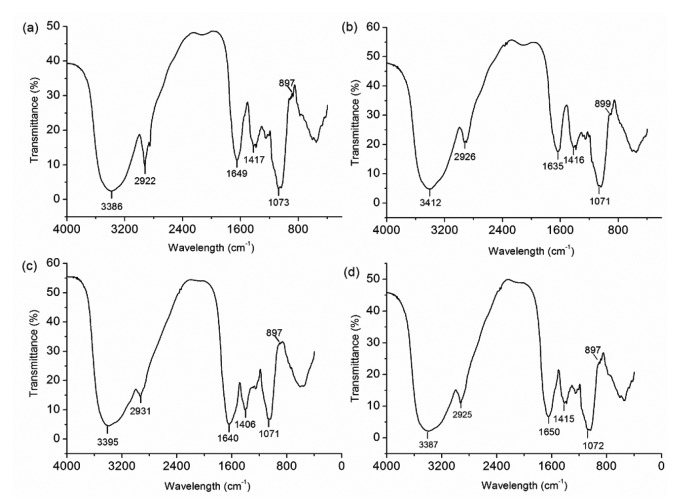
3.3.2 FT-IR analysis
The structural features of the four polysaccharide fractions (TMP30, TMP60, TMP80, and TMP) were further analyzed by FT-IR spectra (Fig. 3). The FT-IR spectra of the four fractions were similar (Fig. 3), indicating that they had a similar structure, which was in accordance with the monosaccharide composition analysis by HPAEC-PAD. The broad and strong bands at 3395‒3386 cm−1 were assigned to the O–H stretching vibration. The small peaks at 2931‒ 2922 cm−1 were due to the stretching and bending vibrations of C–H (Hu et al., 2016). Bands at both 1650‒1635 cm−1 and 1417‒1406 cm−1 were observed, corresponding to symmetric and asymmetric stretching, respectively, of C=O (Afshari et al., 2015). In addition, the absorptions at 1073‒1071 cm−1 (Fig. 3) indicated a pyranose form of sugar (Wang et al., 2016). A weak absorption at 897 cm−1 was observed in spectra of the four polysaccharide fractions, suggesting that pyranoses existed in the β-configuration in TMP30, TMP60, TMP80, and TMP (Luo et al., 2011).
Fig. 3.
FT-IR spectra of TMP30 (a), TMP60 (b), TMP80 (c), and TMP (d)
3.4. Antioxidant activity
3.4.1 Reducing power
The reducing power is generally used to evaluate the potential antioxidant activity of polysaccharides (Devasagayam et al., 2004). Reducing power was determined by measuring the formation of Prussian blue at 700 nm. A higher absorbance value at 700 nm indicated a relative stronger reduction force. It was clear that the reducing power of the four polysaccharide fractions increased with increasing concentration in the range of 62.5‒1200 µg/ml (Fig. 4a). The RP0.5AU values (defined as the effective concentration at which the absorbance was 0.5 for reducing power) of TMP30, TMP60, TMP80, and TMP were 4.33, 4.30, 1.39, and 2.15 mg/ml, respectively (Table 4). Therefore, the reducing power of the four fractions could be listed in the following order: TMP80>TMP>TMP60>TMP30. To some extent, this represents the order of potential antioxidant activity of the four fractions.
Fig. 4.
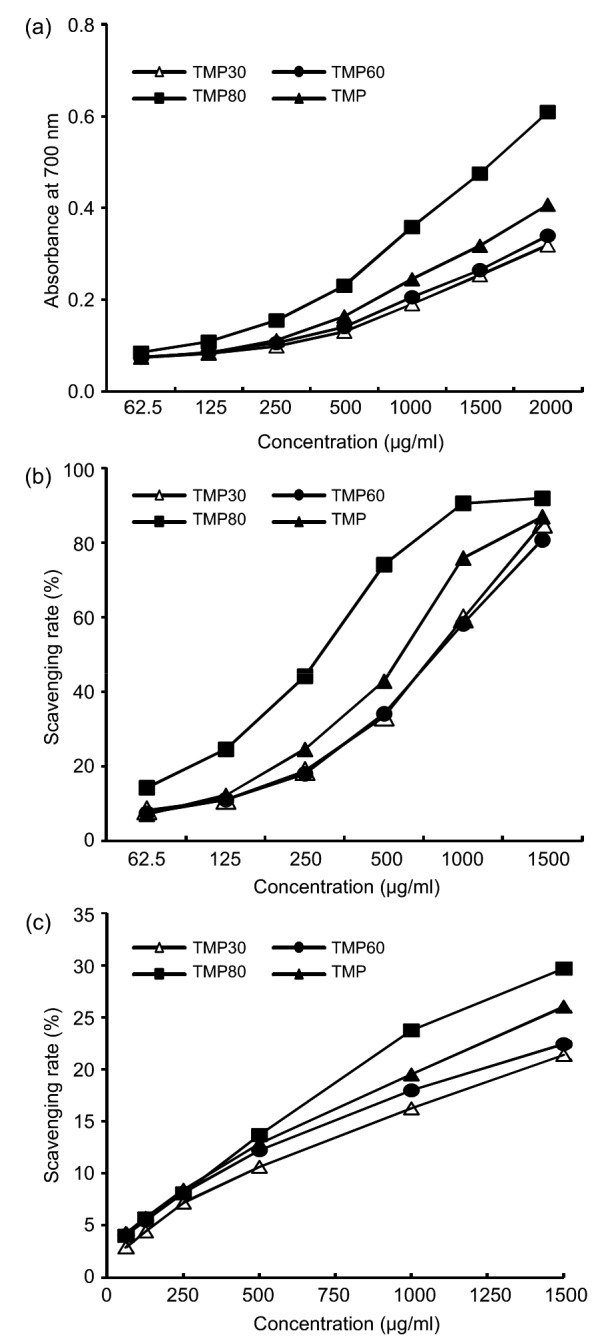
Antioxidant activity of various polysaccharide fractions from T. mastsutake
(a) Reducing power; (b) Scavenging activity to DPPH radical; (c) Scavenging activity to hydroxyl radical. Data are presented as the mean values (n=3)
Table 4.
EC50 values of various polysaccharide fractions from T. matsutake in antioxidant properties
| Fraction | EC50 value (mg/ml) |
RP0.5AU value (mg/ml) | |
| DPPH-scavenging activity assay | Hydroxyl-scavenging activity assay | ||
| TMP30 | 0.86 | 3.73 | 4.33 |
| TMP60 | 0.83 | 3.58 | 4.30 |
| TMP80 | 0.26 | 2.53 | 1.39 |
| TMP | 0.72 | 3.06 | 2.15 |
EC50 value: the effective concentration at which the antioxidant activity was 50%. RP0.5AU value: the effective concentration at which the absorbance was 0.5 for reducing power
3.4.2 DPPH radical-scavenging activity
DPPH radical-scavenging activity is also commonly used to represent a compound’s antioxidant activity. A higher scavenging rate of DPPH radicals indicates that the antioxidant ability of a material is stronger (Brand-Williams et al., 1995). Various fractions showed a dose-dependent scavenging effect at the tested dosage (62.5‒1500 µg/ml) (Fig. 4b). EC50 values, defined as the effective concentration at which the antioxidant activity is 50%, generally serve as important indicators of radical-scavenging activity. Lower EC50 values indicate stronger radical-scavenging activity. The EC50 values of TMP30, TMP60, TMP80, and TMP were 0.86, 0.83, 0.26, and 0.72 mg/ml, respectively (Table 4). Thus, the order of the four fractions in terms of their DPPH-scavenging activity was TMP80>TMP>TMP60>TMP30, which was the same as that of the reducing power.
3.4.3 Hydroxyl radical-scavenging activity
A hydroxyl radical-scavenging assay is also generally applied to assess the antioxidant effect of polysaccharides because hydroxyl free radicals may directly or indirectly cause tissue damage and induce many human diseases (Tursun et al., 2010). The results for TMP30, TMP60, TMP80, and TMP are shown in Fig. 4c. TMP80 exhibited the strongest hydroxyl radical-scavenging ability. The EC50 of TMP80 was 2.53 mg/ml, which was significantly lower than that of TMP30 (3.73 mg/ml), TMP60 (3.58 mg/ml), and TMP (3.06 mg/ml). That is to say, the order of the four fractions based on their hydroxyl radical-scavenging activity was TMP80>TMP>TMP60>TMP30 (Fig. 4c and Table 4), which was similar to that of reducing power and DPPH radical-scavenging effects.
4. Conclusions
In this study, UAE technology was employed to prepare crude TMP from T. matsutake fruiting bodies. Single-factor tests and orthogonal experimental design (L 9(33)) were used to obtain the optimal extraction conditions. Results showed that the extraction time, extraction temperature, and water to raw material ratio were the main factors influencing the yield of TMP. The optimal extraction conditions consisted of an ultrasonic temperature of 40 °C, an ultrasonic time of 50 min, and a water to raw material ratio of 25 ml/g. Under the optimal conditions, the yield of TMP was 8.06%. Three novel polysaccharide fractions, TMP30, TMP60, and TMP80, were isolated and purified from the crude TMP fraction using the final concentration of alcohol precipitation at 30%, 60%, and 80%, respectively. Their physicochemical properties, including total sugar and protein content, monosaccharide composition, and molar ratio, were studied because of their possible association with antioxidant capacities. Their chemical constituent and structural characteristics were determined using HPAEC-PAD and FT-IR analyses. The results showed that TMP30, TMP60, and TMP80 had almost the same monosaccharide composition (they all consisted mainly of L-Fuc, D-Gal, D-Glc, D-Xyl, and D-Man) but in different ratios. FT-IR spectra showed that pyranoses might exist in the β-configuration in TMP30, TMP60, TMP80, and TMP. Furthermore, compared with other polysaccharides, one unknown peak in the HPAEC-PAD spectrum of TMP80 was detected, which might imply the presence of a new compound in this fraction, worthy of further investigation.
The in vitro antioxidant activity of the polysaccharides was investigated based on a reducing power assay and scavenging of DPPH and hydroxyl radicals. The results of antioxidant activity assays showed that the various polysaccharide fractions exhibited increasing antioxidant activity with increasing dosage within the range tested. The order of reducing power was TMP80>TMP>TMP60>TMP30, which was the same as that of the DPPH and hydroxyl radical-scavenging capacities. In other words, TMP80 exhibited the strongest antioxidant activity among the fractions tested. These results indicated that fractionation by stepwise ethanol precipitation is an effective way to select polysaccharides with enhanced antioxidant activity. The differences in antioxidant activity might have been due to the physicochemical properties of the fractions, such as their total sugar and protein content, monosaccharide composition, and molar ratios.
Antioxidants can protect against excessive free radicals that have been implicated in the etiology of many diseases (Devasagayam et al., 2004). The findings reported here suggested that three polysaccharide fractions extracted from T. matsutake, especially TMP80, could be developed as ingredients in healthy and functional food to prevent or alleviate oxidative stress. The structural features of TMP80, especially their structure-function relationships, will be the focus of our continuing work.
Footnotes
Project supported by the Natural Science Foundation of Shandong Province of China (No. ZR2015CL002) and the Doctoral Research Startup Foundation of Liaocheng University (No. 31805), China
Compliance with ethics guidelines: Yun CHEN, Xiu-ju DU, Yang ZHANG, Xin-hua LIU, and Xuan-dong WANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Achat S, Tomao V, Madani K, et al. Direct enrichment of olive oil in oleuropein by ultrasound-assisted maceration at laboratory and pilot plant scale. Ultrason Sonochem. 2012;19(4):777–786. doi: 10.1016/j.ultsonch.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Afshari K, Samavati V, Shahidi SA. Ultrasonic-assisted extraction and in-vitro antioxidant activity of polysaccharide from Hibiscus leaf. Int J Biol Macromol. 2015;74:558–567. doi: 10.1016/j.ijbiomac.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Ardestani A, Yazdanparast R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007;104(1):21–29. doi: 10.1016/j.foodchem.2006.10.066. [DOI] [Google Scholar]
- 4.Bohn JA, BeMiller JN. (1→3)-β-D-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr Polym. 1995;28(1):3–14. doi: 10.1016/0144-8617(95)00076-3. [DOI] [Google Scholar]
- 5.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 6.Chemat F, Rombaut N, Sicaire A, et al. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 7.Devasagayam TPA, Tilak JC, Boloor KK, et al. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Phys India. 2004;52:794–804. [PubMed] [Google Scholar]
- 8.Du XJ, Yang Y, Ye LB, et al. Structural elucidation and immuno-stimulating property of an acidic heteropolysaccharide (TAPA1) from Tremella aurantialba . Carbohydr Res. 2009;344(5):672–678. doi: 10.1016/j.carres.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Du XJ, Mu HM, Zhou S, et al. Chemical analysis and antioxidant activity of polysaccharides extracted from Inonotus obliquus sclerotia. Int J Biol Macromol. 2013;62(11):691–696. doi: 10.1016/j.ijbiomac.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Hoshi H, Iijima B, Ishihara Y, et al. Absorption and tissue distribution of an immunomodulatory α-D-glucan after oral administration of Tricholoma matsutake . J Agric Food Chem. 2008;56(17):7715–7720. doi: 10.1021/jf801123k. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Jia XJ, Fang XB, et al. Ultrasonic extraction, antioxidant and anticancer activities of nove lpolysaccharides from Chuanxiong rhizome. Int J Biol Macromol. 2016;85:277–284. doi: 10.1016/j.ijbiomac.2015.12.046. [DOI] [PubMed] [Google Scholar]
- 12.Huang SQ, Ning ZX. Extraction of polysaccharide from Ganoderma lucidum and its immune enhancement activity. Int J Biol Macromol. 2010;47(3):336–341. doi: 10.1016/j.ijbiomac.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Kim SS, Lee JS, Cho JY, et al. Effects of C/N ratio and trace elements on mycelial growth and exo-polysaccharide production of Tricholoma matsutake . Biotechnol Bioproc E. 2010;15(2):293–298. doi: 10.1007/s12257-008-0226-x. [DOI] [Google Scholar]
- 14.Kim SS, Lee JS, Cho JY, et al. Process development for mycelial growth and polysaccharide production in Tricholoma matsutake liquid culture. J Biosci Bioeng. 2010;109(4):351–355. doi: 10.1016/j.jbiosc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Fabiano-Tixier AS, Tomao V, et al. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason Sonochem. 2013;20(1):12–18. doi: 10.1016/j.ultsonch.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Luo Q, Zhang J, Yan L, et al. Composition and antioxidant activity of water-soluble polysaccharides from Tuber indicum . J Med Food. 2011;14(12):1609–1616. doi: 10.1089/jmf.2011.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv Y, Yang XB, Zhao Y, et al. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009;112(3):742–746. doi: 10.1016/j.foodchem.2008.06.042. [DOI] [Google Scholar]
- 18.Murphey JM, Spayd SE, Powers JR. Effect of grape maturation on soluble protein characteristics of Gewurztraminer and White Riesling juice and wine. Am J Enol Viticult. 1989;40(3):199–207. [Google Scholar]
- 19.Pingret D, Durand G, Fabiano-Tixier A, et al. Degradation of edible oil during food processing by ultrasound: electron paramagnetic resonance, physicochemical, and sensory appreciation. J Agric Food Chem. 2012;60(31):7761–7768. doi: 10.1021/jf301286f. [DOI] [PubMed] [Google Scholar]
- 20.Smironff N, Cumbes QJ. Hyroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28(4):1051–1060. doi: 10.1016/0031-9422(89)80182-7. [DOI] [Google Scholar]
- 21.Tursun K, Zhan R, Zhang H, et al. Study on antioxidant activity of Acroptilon repens . Lett Biol. 2010;21(3):406–411. doi: 10.3969/j.issn.1009-0002.2010.03.025. (in Chinese) [DOI] [Google Scholar]
- 22.Wang NN, Zhang Y, Wang XP, et al. Antioxidant property of water-soluble polysaccharides from Poria cocos Wolf using different extraction methods. Int J Biol Macromol. 2016;83:103–110. doi: 10.1016/j.ijbiomac.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 23.Wang YF, Yang ZW, Wei XL. Antioxidant activities potential of tea polysaccharide fractions obtained by ultrafiltration. Int J Biol Macromol. 2012;50(3):558–564. doi: 10.1016/j.ijbiomac.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Xu F, Jabasini M, Baba Y. Screening of mixed poly (ethylene oxide) solutions for microchip separation of double-stranded DNA using an orthogonal design approach. Electrophoresis. 2005;26(15):3013–3020. doi: 10.1002/elps.200410434. [DOI] [PubMed] [Google Scholar]
- 25.Yang S, Ren XD, Sheng JX, et al. Preparation and the antitumor activity in vitro of polysaccharides from Tricholoma matsutake . W J Microbiol Biotechnol. 2010;26(3):497–503. doi: 10.1007/s11274-009-0196-y. [DOI] [Google Scholar]
- 26.Ye C, Han N, Teng FK, et al. Extraction optimization of polysaccharides of Schisandrae Fructus and evaluation of their analgesic activity. Int J Biol Macromol. 2013;57(6):291–296. doi: 10.1016/j.ijbiomac.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Ye LB, Zhang JS, Yang Y, et al. Structural characterisation of a heteropolysaccharide by NMR spectra. Food Chem. 2009;112(4):962–966. doi: 10.1016/j.foodchem.2008.07.017. [DOI] [Google Scholar]
- 28.Yin XL, You QH, Jiang ZH. Extraction and purification of Tricholoma matsutake polysaccharides. China Brewing. 2009;211(10):171–173. [Google Scholar]
- 29.Yin XL, You QH, Jiang ZH. Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr Polym. 2011;86(3):1358–1364. doi: 10.1016/j.carbpol.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 30.You LJ, Gao Q, Feng MY, et al. Structural characterisation of polysaccharides from Tricholoma matsutake and their antioxidant and antitumour activities. Food Chem. 2013;138(4):2242–2249. doi: 10.1016/j.foodchem.2012.11.140. [DOI] [PubMed] [Google Scholar]
- 31.You QH, Yin XL, Ji CW. Pulsed counter-current ultrasound-assisted extraction and characterization of polysaccharides from Boletus edulis . Carbohydr Polym. 2014;101(1):379–385. doi: 10.1016/j.carbpol.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 32.Zhang WJ. Biochemical Research Technology on Glycoconjugates, 2nd Ed. Zhejiang University Press, Hangzhou; 1999. (in Chinese) [Google Scholar]
- 33.Zhao Z, Xu X, Ye Q, et al. Ultrasound extraction optimization of Acanthopanax senticosus polysaccharides and its antioxidant activity. Int J Biol Macromol. 2013;59(4):290–294. doi: 10.1016/j.ijbiomac.2013.04.067. [DOI] [PubMed] [Google Scholar]