Abstract
Macropinocytosis, a ruffling-driven process that allows the capture of large material, is an essential aspect of normal cell function. It can be either constitutive, as in professional phagocytes where it ends with the digestion of captured material, or induced, as in epithelial cells stimulated by growth factors. In this case, the internalized material recycles back to the cell surface. We herein show that activation of Rho GTPases by a bacterial protein toxin, the Escherichia coli cytotoxic necrotizing factor 1 (CNF1), allowed epithelial cells to engulf and digest apoptotic cells in a manner similar to that of professional phagocytes. In particular, we have demonstrated that 1) the activation of all Rho, Rac, and Cdc42 by CNF1 was essential for the capture and internalization of apoptotic cells; and 2) such activation allowed the discharge of macropinosomal content into Rab7 and lysosomal associated membrane protein-1 acidic lysosomal vesicles where the ingested particles underwent degradation. Taken together, these findings indicate that CNF1-induced “switching on” of Rho GTPases may induce in epithelial cells a scavenging activity, comparable to that exerted by professional phagocytes. The activation of such activity in epithelial cells may be relevant, in mucosal tissues, in supporting or integrating the scavenging activity of resident macrophages.
INTRODUCTION
Macropinocytosis indicates a ruffling-driven phenomenon that leads to the ingestion of large particles into irregular primary endocytic vesicles called macropinosomes (Swanson and Watts, 1995). The formation of macropinosomes, which is the direct consequence of the closure of lamellipodia generated at ruffling membrane domains, occurs differently in different cell types. Whereas in macrophages or dendritic cells macropinosomes are constitutively formed (Swanson and Watts, 1995), in fibroblasts or epithelial cells their occurrence is dramatically but transiently stimulated by different stimuli, such as phorbol esters (Swanson, 1989) or growth factors (Racoosin and Swanson, 1992). The fate of macropinosomes also varies depending on the cell type, undergoing fusion with lysosomes in macrophages (Racoosin and Swanson, 1993) and recycling back to the cell surface in epidermal growth factor-stimulated A431 cells (Hewlett et al., 1994). From a functional point of view, macropinocytosis 1) may account for total nutrient supply in strains of the amoeba Dictyostelium discoideum (Hacker et al., 1997); 2) may play a key role in antigen presentation by class II or even class I major histocompatibility complexes in dendritic cells (Sallusto et al., 1995); 3) may be used by “professional phagocytes,” such as macrophages, to exert a “scavenging” activity (in addition to phagocytosis) (Swanson and Watts, 1995); and 4) can be exploited by several bacterial pathogens as a means of entry and survival in epithelial cells (Finlay and Cossart, 1997).
Among the different stimuli able to induce macropinocytosis in epithelial cells, we have previously described a protein toxin derived from pathogenic strains of Escherichia coli that favors the uptake of large material, such as latex beads or bacteria (Falzano et al., 1993). This toxin, the cytotoxic necrotizing factor type 1 (CNF1) is a 110-kDa monomeric protein that has been shown to permanently activate Rho GTPases (Flatau et al., 1997; Schmidt et al., 1997). These GTPases encompass three groups of proteins (Rho, Rac, and Cdc42) that are differently involved in the actin cytoskeleton organization. Rho induces stress fibers assembly and Rac membrane ruffling activity, whereas Cdc42 is involved in filopodia formation (Hall, 1998). Rho, Rac, and Cdc42 are all activated by CNF1 through the deamidation of a pivotal glutamine residue in the switch 2 domain, which is involved in GTP hydrolysis (glutamine 63 in Rho [Flatau et al., 1997; Schmidt et al., 1997] or 61 in Cdc42 and Rac [Lerm et al., 1999b]). Through the activation of Rho GTPases, CNF1 induces a number of actin-dependent phenomena, such as contractility, cell spreading, and the assembly of focal adhesion plaques (Fiorentini et al., 1997; Lacerda et al., 1997) as well as the formation of actin stress fibers and an intense and generalized ruffling activity (Falzano et al., 1993; reviewed in Boquet and Fiorentini, 2000). CNF1-induced membrane ruffling is reminiscent of the ruffling elicited by invasive bacteria (Francis et al., 1993; Hardt et al., 1998) and is consistent with the ability of epithelial cells to exert macropinocytosis.
In this study we investigated 1) the specific role of Rho GTPases in the macropinocytosis induced by CNF1, and 2) the events after the engulfment of apoptotic cells by CNF1-stimulated human epithelial cells. We showed that the engulfment process in CNF1-treated epithelial cells was driven by the activation of Rho, Rac, and Cdc42 and was followed by a proper degradative pathway. This suggested that epithelial cells may be induced to share or compete with professional phagocytes in a crucial pathophysiological activity, such as the removal of apoptotic cells.
MATERIALS AND METHODS
Cell Cultures
HEp-2 cells (human larynx carcinoma cell line) and U937 cells (a monomyelocytic human cell line) were grown as previously described (Falzano et al., 1993; Cossarizza et al., 1995, respectively). Human monocytes-derived macrophages were obtained by plastic adherence from peripheral blood mononuclear cells as reported (Fais et al., 1996). Apoptotic bodies were obtained from U937 as previously described (Cossarizza et al., 1995).
CNF1 and CNF1 Mutant Recombinant Protein
Wild-type and mutant CNF1 were purified as previously described (Falzano et al., 1993). To construct the CNF1 mutant, the cnf1 gene together with the promoter region of the toxin was amplified by the human uropathogenic E. coli strain J96 (O4:K6) (Blum et al., 1995). Plasmid pCR2.1 was used for cloning cnf1 into E. coli INVα F' (TA Cloning kit; Amersham Pharmacia Biotech, Les Ulis, France). The cnf1-C866S mutant gene was obtained by site-directed mutagenesis with the use of the QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). Mutation of cysteine 866 into a serine abolished the catalytic activity of the toxin (Schmidt et al., 1998). cnf1-C866S cloned in pCR2.1 plasmid was transformed into the E. coli XL1 blue strain from which the mutant protein was purified. The gel shift analysis was performed as previously described (Contamin et al., 2000).
Cell Treatments
HEp-2 cells seeded at a concentration of 2 × 104 cells/ml, either in 24-well plate or in 35-mm Petri dishes, were exposed for 6, 18, 24, and 48 h to 10−10 M CNF1 and 4 × 10−8 M mutant CNF1. The mutant CNF1 used was 400 times more concentrated than the wild-type toxin, to obtain a complete competition for the binding step. Apoptotic cells (5 × 104) were next added to untreated, CNF1-, and CNF1 mutant-treated cells as well as to 7-d culture monocyte-derived macrophages, for different time lengths (1, 5, 10, 20, 30 min, 3 h, and overnight). At the end of each treatment, cells were processed for light or electron microscopy. For inhibition of protein synthesis, HEp-2 cells were preincubated for 30 min with 50 μg/ml cycloheximide (CHX) before exposure to CNF1. After 48 h of incubation, apoptotic cells were added as described above. All the experiments were performed at least four times with triplicate samples for each point. In each experiment at least 500 cells (randomly chosen) were counted.
Assay of Macropinocytosis
To define how bound but not ingested cells are distinguished from ingested cells we have determined the plane at which the apoptotic cell is laying with respect to the plane of epithelial cell nuclei. In fact, by varying the focal plane, it is possible to define which apoptotic cell lies at the same plane of the epithelial cell nucleus. That apoptotic cell was considered as internalized.
Transfection of HEp-2 Cells
Control HEp-2 cells were transfected either with 1 μg/well of plasmid DNA encoding myc-tagged dominant positive forms of either Rho, Rac, or Cdc42 GTPases (RhoV14, RacV12, and Cdc42V12) or with 2.5 μg/35-mm Petri dish of plasmid encoding RhoV14-GFP. HEp-2 cells treated with CNF1 for 24 h were transfected with 1 μg of the negative forms of either Rho, Rac, or Cdc42 GTPases (RhoN19, RacN17, and Cdc42N17). Both control and CNF1-treated HEp-2 cells were transfected with 1 μg of plasmids encoding Rab5-GFP and Rab7-GFP. DOTAP Liposomal Transfection Reagent kit (Roche Molecular Biochemicals, Mannheim, Germany) was used to transfect cells according to the manufacturer's recommendations. Twenty-four hours after cell transfection, 5 × 104 apoptotic bodies were added to monolayers and, after different time points (1, 5, 10, 20, 30 min, 3 h, and overnight), cells were either fixed in paraformaldehyde and processed for fluorescence microscopy or observed with a phase contrast inverted microscope and monitored by microcinematography, as described below.
Rho-inhibiting Bacterial Protein Toxins
Clostridium difficile toxin B (CdB) and Clostridium sordellii lethal toxin (LT) were generously provided by M.R. Popoff (Paris, France). The chimeric toxin C3B, prepared as previously described (Aullo et al., 1993), was a kind gift from P. Boquet (Nice, France). Epithelial cells were exposed for 3 h to CdB (0.5 μg/ml) or LT (1 μg/ml) or C3B (1 μg/ml) before addition of CNF1. After further 48 h of incubation with CNF1, cells were overnight challenged with apoptotic cells, fixed, and processed for fluorescence microscopy as below described. All the experiments were performed at least four times with triplicate samples for each point.
Immunocytochemistry
HEp-2 cells were seeded on glass chamber slides (Nunc, Naperville, IL) at the concentration of 2 × 104 cells/ml. After treatment with CNF1 and the subsequent incubation with apoptotic bodies, chamber slides were fixed with ethanol (70%) or methanol (70%) for 5 min at 4°C for lysosomal associated membrane protein-1 (Lamp-1) detection. Cells were then stained by immunocytochemistry with the use of Dako EnVision System (Dako, Denmark) horseradish peroxidase, with the use of the peroxidase-antiperoxidase method. Rabbit polyclonal antibodies recognizing the cytosolic part of Lamp-1 were kindly provided by S. Méresse (Marseille, France). Terminal deoxynucleotidyl transferase dUTP nick-end labeling reaction (In Situ Cell Death Detection kit; Roche Molecular Biochemicals) (Negoescu et al., 1996) was performed with the use of the peroxidase-antiperoxidase method or alkaline phosphatase antialkaline phosphatase (Dako) method, in single and double staining, as appropriate (Fais et al., 1995).
Fluorescence Microscopy
Control and treated HEp-2 cells were fixed with 3.7% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.4) for 10 min at room temperature and then permeabilized with 0.5% Triton X-100 in PBS (pH 7.4). Cells were stained with 1) the nuclear dye Hoechst 33258 (Sigma, St. Louis, MO) alone (for Rab-GFP transfected cells and for nontransfected cells); 2) Hoechst and anti-c-myc monoclonal antibody 9E10.3 (Chemicon International, Temecula, CA) (for cells transfected with dominant positive or negative forms of the Rho GTPases); 3) Hoechst and anti-early endosome antigen 1 (EEA1) goat polyclonal antibody (Santa Cruz Biotechnologies, Santa Cruz, CA). After 30 min at 37°C, cells were washed and in the cases 1) and 3) incubated with a fluorescein isothiocyanate-conjugated anti-mouse and anti-goat antibodies, respectively. Coverslips were mounted in glycerol/PBS (2:1) and analyzed with a Nikon Microphot fluorescence microscope. For acidic vesicle detection, both untreated and CNF1-treated living cells were incubated at 37°C for 10 min with 2.5 μg/ml acridine orange (Sigma). Finally, after washings, coverslips were mounted and analyzed as described above.
Video Microscopy
Time-lapse cinematography was obtained with the use of a phase contrast Nikon inverted microscope equipped with a Zeiss charge-coupled device camera and a JVC time lapse videotape recorder. Living HEp-2 cells treated for 48 h with CNF1 10−10 M or transfected with RhoV14-GFP, and then challenged with apoptotic cells, have been studied. Films were recorded under standard condition (5% CO2 humid atmosphere at 37°C). The ingestion of apoptotic bodies by epithelial cells was clearly detected by decreasing tape speed at least 360 times (to obtain a quick-time movie after a 3-h recording).
Scanning Electron Microscopy
Control and treated cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at room temperature for 20 min. After postfixation in 1% OsO4 for 30 min, cells were dehydrated through graded ethanols, critical point dried in CO2, and gold coated by sputtering. The samples were examined with a Cambridge 360 scanning electron microscope.
Transmission Electron Microscopy (TEM)
Cells grown in monolayer were fixed with 2.5% glutaraldehyde in buffer 0.2 M cacodylate (pH 7.4) for 30 min, washed, and postfixed for 1 h with 1% OsO4 in the same buffer at 4°C. They were dehydrated in an alcohol gradient and embedded in epoxy resin (Agar 100 resin; Agar Scientific, Stansted, United Kingdom) by routine procedures. Ultrathin sections, obtained with an LKB Ultrotome Nova, were stained with uranyl acetate and lead citrate and examined with a Philips 208 transmission electron microscope.
Statistical Analysis
The values in Table 1 and Figures 1 and 3–6 are the means ± SDs from four separate experiments. Student's t test for correlated samples was used. A p value of <0.01 was considered significant. Correlation has been evaluated by with the use of Statistics program for Macintosh by a specific paired correlation test.
Table 1.
Percentage of HEp-2 cells treated with CNF1 (for 48 h) efforting macropinocytosis: dependence on the time of exposure to apoptotic bodies
| Time of exposure to apoptotic bodies (min) | Percentage of cells containing apoptotic bodies |
|---|---|
| 15 | 2 ± 0.4 |
| 30 | 7 ± 0.8 |
| 60 | 15 ± 1.5 |
| 90 | 19 ± 2.0 |
| 120 | 25 ± 1.0 |
| 180 | 45 ± 1.4 |
| Overnight | 47 ± 1.6 |
The long incubation time with apoptotic bodies did not affect HEp-2 cells viability as viewed by trypan blue exclusion test (data not shown).
Figure 1.
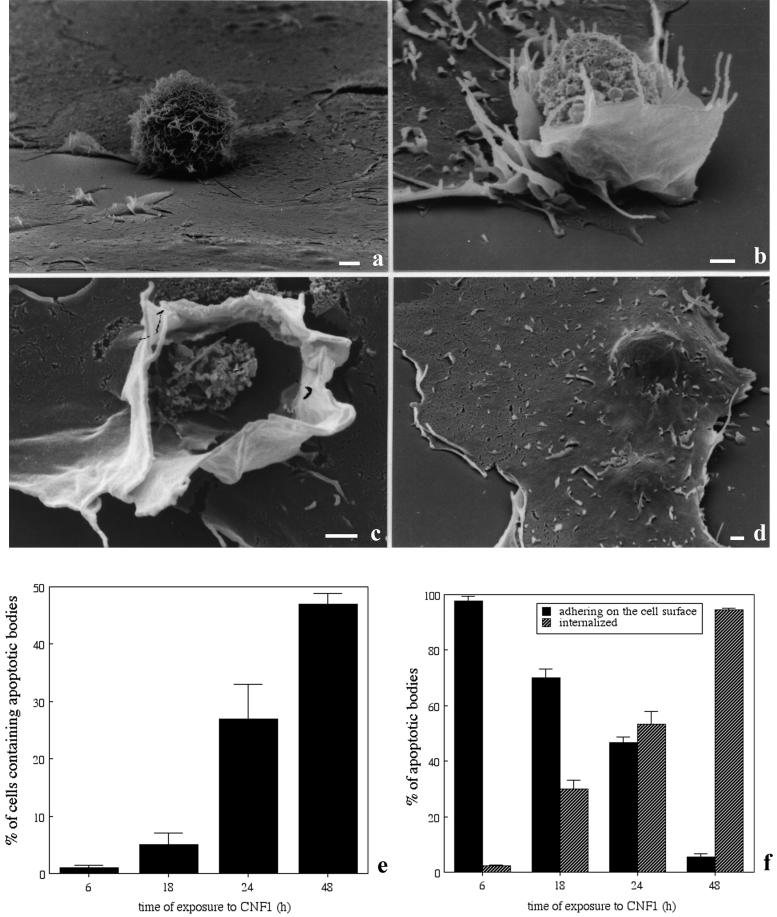
Macropinocytosis of apoptotic bodies induced by CNF1 in epithelial cells. Scanning electron microscopy analysis of unstimulated epithelial cells (a) and cells exposed to 10−10 M CNF1 for 48 h (b–d). After overnight interaction with epithelial cells apoptotic bodies are contacted by filopodia and small ruffles (b), surrounded and enveloped (c) by larger ruffles, and internalized into the cytoplasm (d). (e) Percentage of cells containing apoptotic bodies increases by time of treatment with 10−10 M CNF1. (f) Percentage of apoptotic cells present on the epithelial cell surface decreases over time, whereas the number of internalized apoptotic bodies concomitantly increases. The results, reported as percentages (±SDs), are from four different experiments in each of which at least 500 cells (randomly chosen) were counted. Bar in a–d, 1 μm.
Figure 3.
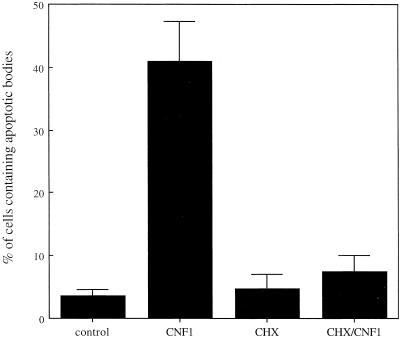
Graph showing the percentage of epithelial cells containing apoptotic bodies. Preexposure with 50 μg/ml the protein synthesis inhibitor CHX clearly reduced the induction of the macropinocytotic activity of CNF1-treated HEp-2 cells. The results, reported as percentages (±SDs), are from four different experiments in each of which at least 500 cells (randomly chosen) were counted.
Figure 6.
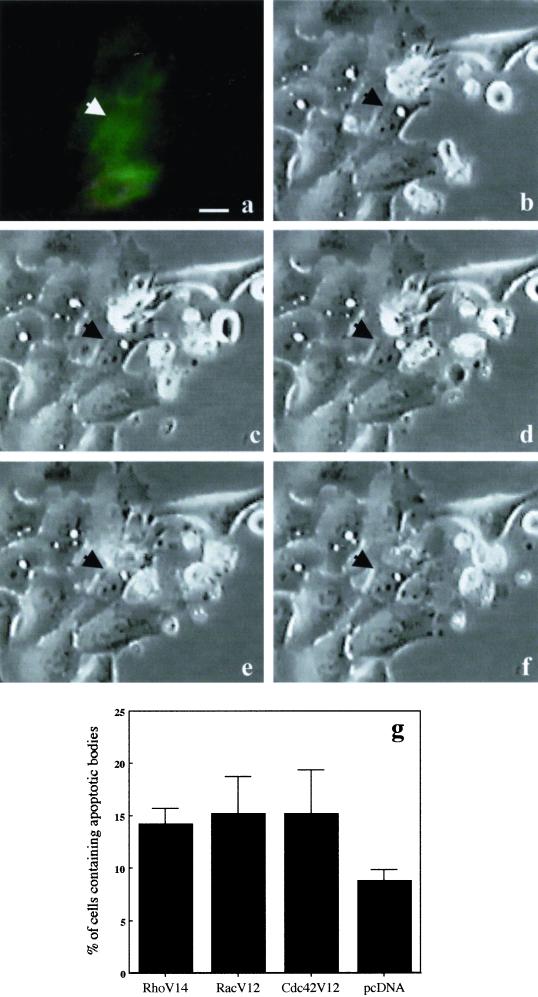
(a–f) Macropinocytosis of apoptotic bodies in HEp-2 cells transfected with RhoV14-GFP. Cells were transfected with 2.5 μg of RhoV14-GFP-encoding plasmid per Petri dish and then challenged with 5 × 104 apoptotic cells. The interaction between a HEp-2 transfected cell (a) and an apoptotic cell was monitored by microcinematography. A selected field showing the dynamics of the capture and internalization processes was chosen (b–f). The selected micrographs were taken 10 min (b), 20 min (c), 1 h (d), 2 h (e), and 3 h (f) after the addition of apoptotic cells to the culture medium. Arrowheads indicate a transfected epithelial cell, which contacts and progressively internalizes an apoptotic cell. (g) Graph showing the percentage of control HEp-2 cells able to macropinocytose apoptotic bodies once transfected with 1 μg of dominant positive forms of Rho GTPase (RhoV14-RacV12-Cdc42V12 myc-tagged). In these cells a slight but significant increase in the macropinocytotic activity is evident with respect to cells transfected with the control plasmid (pcDNA). The results, reported as percentages (±SDs) of transfected epithelial cells containing apoptotic bodies, are from four different experiments in each of which at least 100 transfected cells were counted. Bar, 10 μm.
RESULTS
CNF1 Induces Macropinocytosis of Apoptotic Cells by Human Epithelial Cells
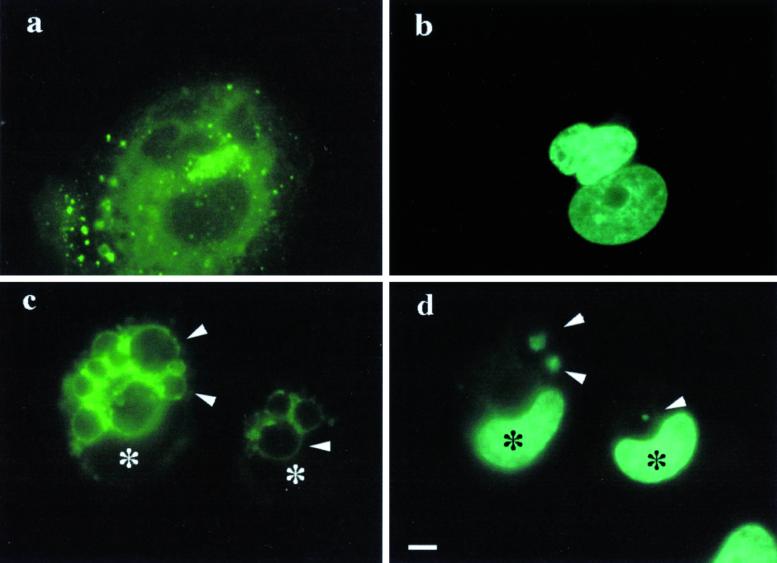
We first investigated the ability of CNF1-activated epithelial HEp-2 cells to engulf U937 cells triggered to apoptosis. This approach was chosen as representative of a specific cell scavenging activity. As observed by scanning electron microscopy, apoptotic cells did just adhere on the surface of unstimulated epithelial cells without being internalized (Figure 1a). In contrast, CNF1-activated epithelial cells first contacted the apoptotic cells via extension of filopodia and membrane ruffles (Figure 1b), which surrounded and subsequently wrapped around (Figure 1c) the apoptotic cells. These events led to the engulfment of apoptotic cells into the epithelial cells (Figure 1d). In cells exposed to CNF1, time course experiments showed that the percentage of cells containing apoptotic bodies increased with the length of exposure to the toxin, reaching a maximum at 48 h (Figure 1e). The internalization of apoptotic bodies did occur at different extents also depending on the time of their incubation with epithelial cells (Table 1). Moreover, while the percentage of internalized apoptotic bodies increased with the time of CNF1 treatment, apoptotic cells merely adhering to the epithelial cell surface progressively decreased (Figure 1f).
Macropinocytosis of apoptotic cells by CNF1-stimulated epithelial cells was also monitored by time-lapse video microscopy. A selected field describing the dynamics of such phenomenon is shown (Figure 2, a–d). The arrows point an apoptotic cell that was progressively internalized by a CNF1-stimulated epithelial cell. CNF1-activated epithelial cells were able to engulf apoptotic cells independently from the actual apoptotic state of the prey (because both early or late apoptotic cells were engulfed, as detected by annexin V staining) but were unable to ingest live cells (our unpublished results).
Figure 2.
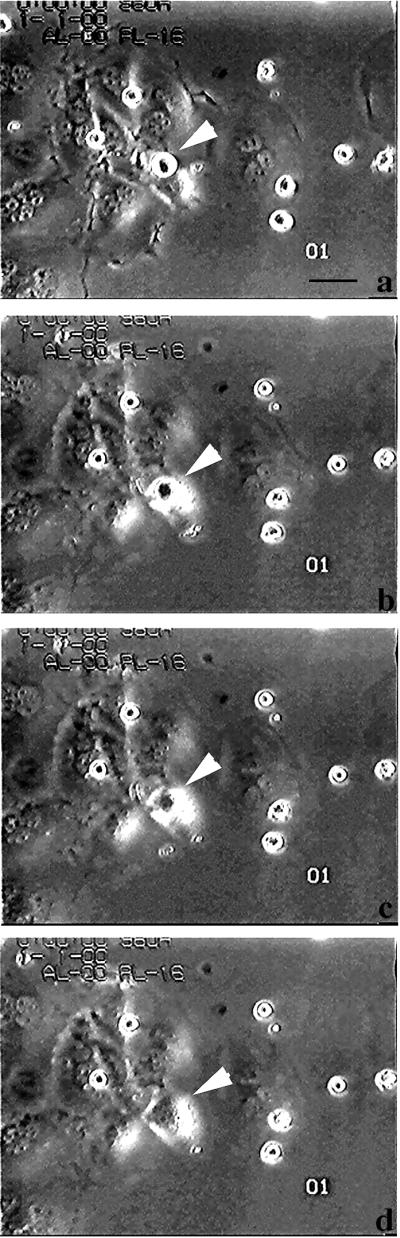
Microcinematography on apoptotic cell internalization by CNF1-treated epithelial cells. Cells were exposed to 10−10 M CNF1 for 48 h and then challenged with 5 × 104 apoptotic bodies. Such interaction was followed for 3 h and monitored by microcinematography. A selected field showing the dynamic of the capture and internalization processes was chosen (a–d). The selected micrographs were taken 0 min (a), 10 min (b), 20 min (c), and 1 h (d) after the addition of apoptotic cells to the culture medium. Arrowheads indicate an apoptotic cell that is progressively internalized by one epithelial cell. Bar, 10 μm.
On the other hand, the long incubation time necessary for CNF1 to increase the apoptotic cell uptake (Figure 1e) suggests the occurrence of transcriptional changes. We have therefore carried out experiments with the use of the protein synthesis inhibitor CHX to verify whether the ex novo protein synthesis was required for the engulfment of apoptotic bodies. The results obtained, reported in Figure 3, showed that CHX was able to block the CNF1-induced macropinocytosis of apoptotic bodies, suggesting that cells needed to synthesize new proteins to exploit such function.
Rho GTPases Are Required for Macropinocytotic Activity Induced by CNF1 in Epithelial Cells
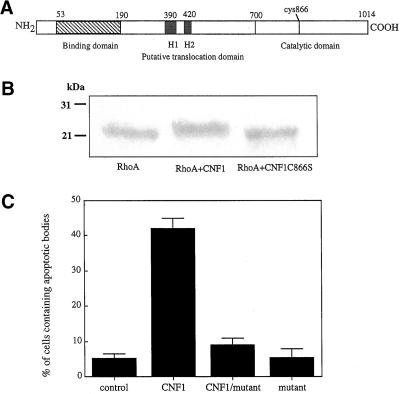
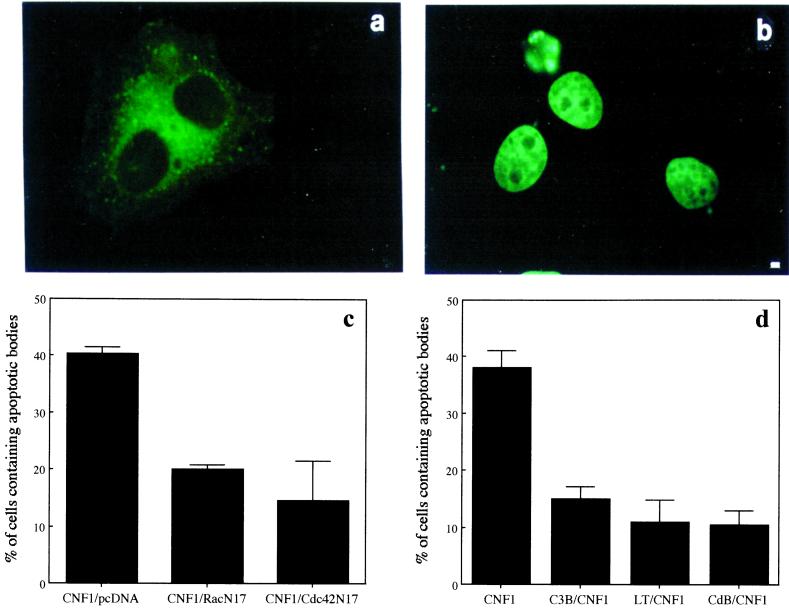
To analyze whether the enzymatic activity of CNF1 was necessary for the development of the toxin-induced macropinocytosis, a nontoxic mutant of CNF1 in which the catalytic cysteine residue (cys 866) (Figure 4a) was converted to serine was constructed. As already reported (Schmidt et al., 1998), the CNF1 C866S completely lacks its enzymatic activity as demonstrated by the inability to upshift the apparent molecular weight of Rho (Figure 4b). Exposure to CNF1 C866S alone for 48 h rendered HEp-2 cells unable to capture apoptotic cells (Figure 4c). The simultaneous addition of the mutant together with the wild-type CNF1 led to the inhibition of the CNF1-induced macropinocytotic activity (Figure 4c), indicating a competition at the level of receptor binding (Contamin et al., 2000). Together, these results demonstrate the need of the fully enzymatic activity of CNF1 to exert macropinocytosis.
Figure 4.
Rho deamidation is essential for macropinocytosis in epithelial cells. (a) Schematic representation of the CNF1 molecule. The position of the catalytic amino acid cys866, which has been changed into serine in the mutant CNF1, is evidenced. (b) Coomassie Brilliant Blue-stained gel of control recombinant Rho protein, Rho protein exposed to wild-type CNF1, or mutant CNF1 (CNF1 C866S). The molecular ratio of CNF1 to RhoA was 2:1. Note that the mutant CNF1 has lost the ability to cause the Rho upward shift typical of CNF1. (c) Graph showing the percentage of epithelial cells containing apoptotic bodies after 48 h of treatment with CNF1, CNF1 mutant, and CNF1 plus CNF1 mutant. The results, reported as percentages (±SDs) of epithelial cells containing apoptotic bodies after overnight exposure, are from four different experiments in each of which at least 500 cells (randomly chosen) were counted.
We further investigated whether the CNF1-dependent macropinocytotic activity in epithelial cells was triggered by activated Rho GTPases by 1) blocking CNF1 activity with transfection of cells with the dominant negative forms of Rho GTPases or with the use of “classical” bacterial protein toxins as inhibitors of Rho proteins; and 2) activating epithelial cells with dominant positive forms of the Rho GTPases. CNF1-treated epithelial cells transfected with the dominant negative forms of Rac (RacN17) or Cdc42 (Cdc42N17) showed a reduced ability to ingest apoptotic bodies (49 ± 3 and 57 ± 8%, respectively) with respect to CNF1-treated cells transfected with the control plasmid (pcDNA) (Figure 5c). Although being low the percentage of cells transfected with the dominant negative Rho N19 (our unpublished results), the results were consistent with those obtained after treatment with exoenzyme C3 (see below). We then used the after Rho inhibitors: 1) Clostridium botulinum exoenzyme C3, a selective inhibitor of Rho (Chardin et al., 1989); 2) CdB, which inactivates Rho, Rac, and Cdc42 (Just et al., 1995); and 3) LT, which inhibits Ras, Rap, and Rac but not Rho or CdC42 (Popoff et al., 1996). Besides blocking the typical CNF1-induced morphological changes, CdB and LT also impaired the engulfment of apoptotic bodies (Figure 5d). On the other hand, although unable to prevent the CNF1-induced phenotype (Fiorentini et al., 1995), C3 also significantly decreased macropinocytosis of apoptotic bodies (Figure 5d). Consistently, when control epithelial cells were transfected with each of the dominant active form of RhoA, Rac, and Cdc42, a slight but significant (p < 0.01) increase in the phagocytic activity was observed compared with cells transfected with the control plasmid (Figure 6g). It is evident that DNA transfection per se induced a very modest uptake of apoptotic cells, which is nearly doubled in cells transfected with the GTPases (Figure 6g). Although the role of Rac as inducer of macropinocytosis is well defined (Dharmawardhane et al., 2000; Nobes and Marsh, 2000), the finding that Rho may promote such process is still poorly investigated. Therefore, to further explore this last point, we have used time-lapse video microscopy to follow the internalization of an apoptotic cell by epithelial cells transfected with the dominant active form of the Rho-GTPase (RhoV14-GFP). The results obtained showed that, similarly to what occurs in CNF1-treated epithelial cells, Rho activation induced the ingestion of apoptotic cells. Figure 6, a–f, clearly shows the progressive engulfment of an apoptotic cell by an adhering cell with spike-like protrusions. Together, these findings suggest that a coordinate activity of the Rho GTPases is required for mimicking the macropinocytotic activity triggered by CNF1
Figure 5.
Rho GTPases drive macropinocytosis in epithelial cells. (a and b) As an example of a transfected cell with an apoptotic body inside we show the fluorescence micrograph of an epithelial cell exposed to 10−10 M CNF1 for 24 h and then transfected with 1 μg of RacN17 encoding plasmid. Apoptotic bodies were added for 3 h before fixation. Hoechst staining (b) clearly evidences the presence of an apoptotic body in a transfected cell (a). (c) Graph showing the percentage of CNF1-treated epithelial cells able to macropinocytose apoptotic bodies once transfected with dominant negative forms of the Rho GTPases. (d) Graph showing the inhibition of CNF1-induced macropinocytotic activity by exposure to the Rho-inhibiting bacterial toxins C3B (1 μg/ml), LT (1 μg/ml), and CdB (0.5 μg/ml) for 3 h. The results reported as percentages (±SDs) of epithelial cells containing apoptotic bodies are from four different experiments in each of which at least 100 cells were counted. Bar in a and b, 1 μm.
Macropinosomes in CNF1-activated Human Epithelial Cells May Discharge Their Contents into Lysosomal Degradative Compartments
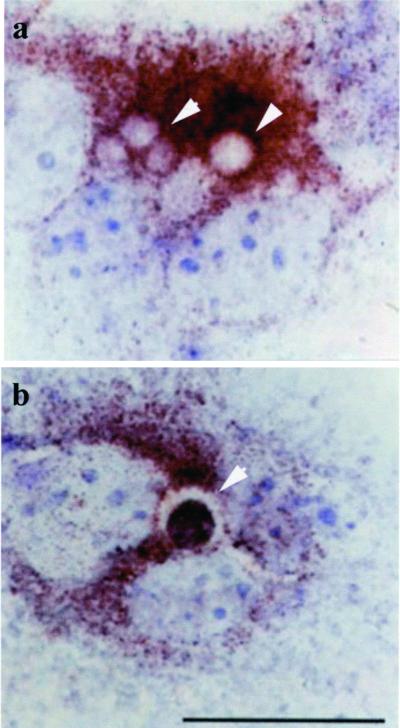
We next followed the route undertaken by the captured apoptotic bodies in CNF1-activated epithelial cells. In particular, we studied the association between apoptotic body-containing vacuoles and the small GTPases Rab5 and Rab7, which define early and late endosomes, respectively (Bucci et al., 1992; Vitelli et al., 1997), and the lysosomal transmembrane protein Lamp-1 (de Saint-Vis et al., 1998). Macropinosomes in CNF1-activated epithelial cells that contained the apoptotic bodies were always found negative for early endosomal markers such as Rab5 (Figure 7, a and b) or EEA1 (our unpublished results). In contrast, similarly to what occurs in late phagosomes of macrophages, apoptotic bodies were observed inside macropinosomal membranes expressing Rab7 (Figure 7, c and d). This finding strongly suggested that macropinosomes in human epithelial cells stimulated by CNF1 may undergo fusion with lysosomal-like structures. To explore this hypothesis, epithelial cells were stained with an antibody that recognizes the cytosolic component of Lamp-1, a transmembrane protein specifically associated with lysosomes (de Saint-Vis et al., 1998). As shown in Figure 8, apoptotic bodies captured by CNF1-activated cells could be observed in vacuoles stained by the Lamp-1 antibody, a finding that suggests the occurrence of a lytic enzyme activity inside such vesicles. In CNF1-treated epithelial cells, the vesicles positive for Rab7 and Lamp-1 appeared very large irrespective of the presence inside of apoptotic bodies (Figures 7, c and d, and 8). This suggests that CNF1 is capable of affecting vesicle trafficking in accordance with the nature of macropinocytosis that is characterized by the continuous formation of ruffling-driven macropinosomes.
Figure 7.
Intracellular trafficking of apoptotic bodies in Rho-activated epithelial cells. (a and c) Epithelial cells exposed to 10−10 M CNF1 for 24 h and then transfected with 1 μg of Rab5-GFP- (a) or Rab7-GFP (c)-encoding plasmid. (b and d) Hoechst staining of the same fields as in a and c, respectively. Apoptotic bodies are constantly absent in Rab5-positive vesicles, independently from the time of interaction with epithelial cells. Apoptotic bodies evidenced by Hoechst staining are observable inside some Rab7-positive vesicles (arrowheads) (d), indicating a passage through such endosomal compartment. The results obtained with Rab5 and Rab7 are each from four separate experiments in each of which at least 100 cells were counted. Approximately 8% of Rab7-positive vesicles was found to contain apoptotic bodies. Asterisks indicate the position of nuclei in c and d. Bar in a–d, 1 μm.
Figure 8.
Intracellular distribution of Lamp-1 in CNF1-treated epithelial cells. Immunocytochemistry analysis of epithelial cells exposed to 10−10 M CNF1 for 48 h (a and b). Arrowheads in a and b indicate vesicles stained with Lamp-1. In b is well evident an apoptotic body (stained with the terminal deoxynucleotidyl transferase dUTP nick-end labeling reaction) inside a Lamp-1-positive vesicle. The results obtained with Lamp-1 are from four separate experiments in each of which 100 cells were counted. Approximately 10% of Lamp-1-positive vesicles was found to contain apoptotic bodies. Bar, 10 μm.
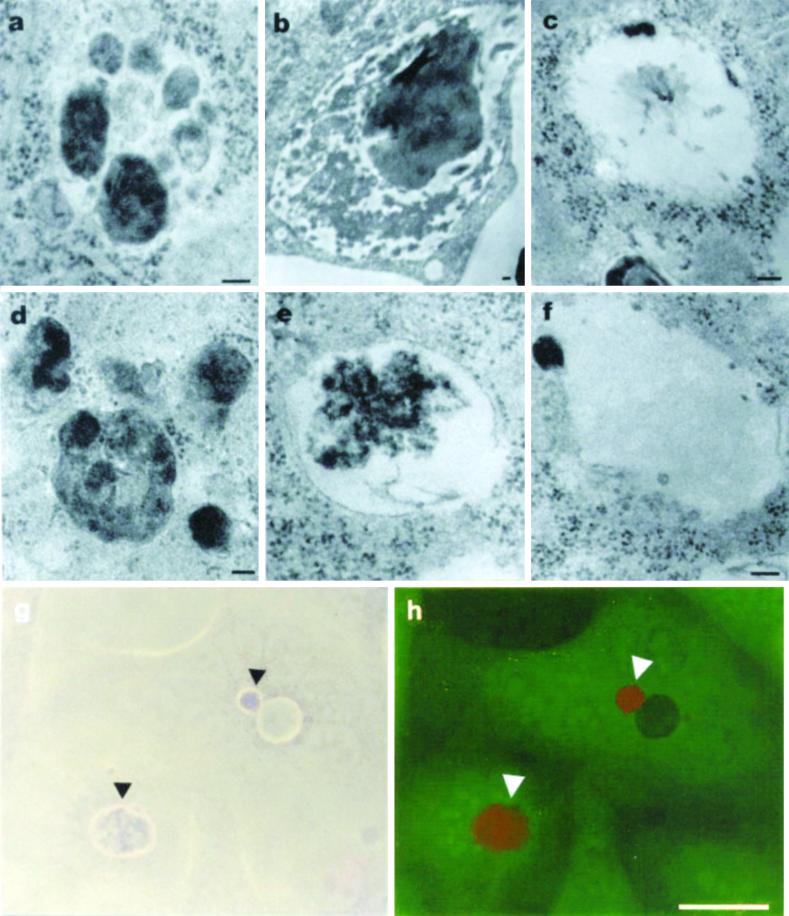
We therefore used TEM analysis to compare the degradative process efforded by CNF1-activated epithelial cells to that of classical primary human macrophages. Figure 9 clearly shows that the degradation of apoptotic bodies occurred progressively in a similar manner in macrophages (Figures 9, a–c) and CNF-1-activated epithelial cells (Figure 9, d–f). Experiments aimed at evaluating acidification of vacuoles containing apoptotic bodies showed that such vacuoles (Figure 9g; phase contrast) assumed a strong orange-red staining with acridine orange (Figure 9h). Acridine orange is a metachromatic dye that has been used extensively to assess phagosome-lysosome fusion. The red staining inside vacuoles indicates an acidic pH and, as a consequence, acidic digestion of apoptotic bodies, whereas the apoptotic corps outside vacuoles were yellow-green. This last set of observations strongly supports our finding that, in CNF1-activated epithelial cells, the degradation of apoptotic bodies occurs in macropinosomes fused with lysosomal-like structures.
Figure 9.
Phagocytic-like activity in CNF1-treated epithelial cells. (a–f) TEM micrographs showing the engulfment and degradation of apoptotic cells in macrophages (a–c) and epithelial cells (d–f) treated with 10−10 M CNF1 for 48 h. In macrophages (a) and epithelial cells (d), the chromatinic structures of apoptotic cells are clearly visible inside the intracytoplasmatic vacuoles. (b and e) Apoptotic bodies appear partially degraded in the vacuoles. (c and f) After overnight interaction of apoptotic bodies with macrophages (c) or epithelial cells (f), a small amount of chromatic debris is visible, suggesting a degradation activity inside the vacuoles. By acridine orange fluorescent staining, the acidic nature of the apoptotic body-containing vesicles (arrowheads) is well evident in h. (g) Respective brightfield image. Bar in a–f, 0.1 μm and in g and h, 10 μm.
DISCUSSION
We herein show that CNF1 enables epithelial cells to perform a scavenging activity toward apoptotic cells via a mechanism that combines macropinocytosis and degradation and therefore is novel for epithelial cells. Macropinocytosis triggered by CNF1 was clearly dependent on the activation of regulatory proteins of the Rho family and started with the promotion of an intense membrane ruffling. Such Rho-dependent membrane activity drove the capture of apoptotic cells into macropinosomes lacking the early endocytic compartment marker Rab5. Such macropinosomes in CNF1-activated epithelial cells may then discharge their contents into Rab7 and Lamp-1 acidic compartments where the degradation of the ingested particles occurs.
Macropinocytosis by CNF1 in epithelial cells was a de novo-induced phenomenon that required synthesis of new proteins. Moreover, to promote macropinocytosis, CNF1 had to be enzymatically active because a mutation in its catalytic site blocked such ability. This suggests that Rho proteins, the targets of CNF1, may be pivotal in driving the macropinocytotic activity. In fact, epithelial cells exposed to CNF1 and then transfected with dominant negative forms of Rho GTP-binding proteins or treated with toxins inhibiting these GTPases, demonstrated a decrease in macropinocytotic activity. Conversely, transfection with each dominant positive Rho GTPase increased the percentage of epithelial cells able to capture apoptotic cells, although such ability was significantly lower than that observed with treatment of cells with CNF1. Thus, to be fully exploited, the CNF1-induced macropinocytotic activity needs the activation of Rho, Rac, and Cdc42 GTPases, being each of them probably involved in different phases of the process. Rho GTPases have been reported to be pivotal for induction of phagocytosis (Caron and Hall, 1998; Massol et al., 1998) or macropinocytosis (Ridley et al., 1992), but not always all three groups of proteins have been involved in such endocytic phenomena. In fact, it has been shown that the mechanism triggered by Shigella to invade epithelial host cells relies on the activation of Rho, Rac, and Cdc42 (Mounier et al., 1999), whereas the mechanism induced by Salmonella requires the selective activation of Cdc42 and Rac but not Rho (Hardt et al., 1998). As regards phagocytosis, it has been reported that ingestion of particles bound by the Fcγ receptor on macrophages apparently requires Rac and Cdc42 activation (Caron and Hall, 1998), whereas phagocytosis of particles bound to the CR3 receptor involves Rho GTPase activation only (Caron and Hall, 1998). Note, the exoenzyme C3, although unable to prevent the morphological changes induced by CNF1 (Fiorentini et al., 1995), was found to block the macropinocytotic activity. This might be due to the ability of C3 to impair the tyrosine phosphorylation induced by CNF1 (Lacerda et al., 1997). Tyrosine phosphorylation is a critical step in the induction of phagocytosis by macrophages and, in fact, different bacterial pathogens exploit such signaling pathway to override phagocytosis and exert their pathogenicity (Goosney et al., 1999). Together, these findings suggest that Rho, Rac, and Cdc42 may act in a coordinate manner in CNF1-treated epithelial cells to organize actin filaments, to build a functional membrane ruffle, and to allow macropinocytosis.
In CNF1-activated epithelial cells, apoptotic bodies were always found in compartments negative for early endosomal markers such as Rab5 or EEA1. Rab5, a small GTPase that allows the mixing of endosome or early phagosome contents (Bucci et al., 1992), and EEA1, an early endosome-associated antigen recently shown to be a Rab5 effector (Millis et al., 1999), both function as regulators of early endosome homotypic fusions. Thus, particles taken up by CNF1-activated cells are most likely engulfed by vesicles formed by the membrane ruffling activity as described when cells are transfected with the dominant positive form of Rac (Ridley et al., 1992). Macropinocytosis stimulated by Rac-dependent ruffling in epithelial cells (as that induced by EGF [Hewlett et al., 1994]), allows the capture and internalization of the material that is, however, rapidly recycled back to the cell surface (Racoosin and Swanson, 1993; Hewlett et al., 1994), with a process called regurgitation (Veithen et al., 1998). In the case of CNF1-treated epithelial cells (i.e., Rho, Rac, and Cdc42 contemporary stimulation), macropinosomes may undergo fusion with Rab7 and Lamp-1 positive vesicles (acidic degradative vesicles) where apoptotic cells are progressively degraded. Recent evidence suggests that the Rho GTPase family of signaling proteins is also implicated in the control of the endocytic traffic (Ellis and Mellor, 2000). For instance, RhoD has been reported to regulate early endosome dynamics and distribution by directing the rate of vesicular traffic along cytoskeletal tracks (Murphy et al., 1996). RhoD, however, does not represent a target for CNF1. In fact, CNF1 specifically recognizes a short segment of the Rho, Rac, and Cdc42 switch 2 domain (Lerm et al., 1999a; Flatau et al., 2000) that is present but not strictly localized in the switch 2 domain in RhoD. One attractive alternative hypothesis to explain the fusion of macropinosomes with lysosomes in CNF1-treated cells is that RhoB (a substrate of CNF1), which localizes on the late endosomal/lysosomal compartment (Adamson et al., 1992), may upon activation by CNF1 facilitate this fusion. Indeed, RhoB, through activation of its downstream effectors (Ellis and Mellor, 2000), has been implicated in the control of vesicles fusions (Ellis and Mellor, 2000). As shown above, apoptotic bodies engulfed by CNF1-activated cells reached both Rab7-positive and Lamp-1-positive acidic compartments. In addition, apoptotic bodies within these compartments exhibited classical features of progressive degradation identical to those observed in phagolysosomes. This observation strongly suggests that CNF1-activated epithelial cells behave as bona fide phagocytes, particularly in the last part of the scavenging process.
In conclusion, we have demonstrated that after CNF1 activation human mucosal epithelial cells may share the job of macrophages, in developing the ability to remove apoptotic cells by a novel mechanism driven by the activation of Rho GTPases. We can therefore hypothesize that such activity may be normally activated in mucosal epithelial cells in supporting or integrating the scavenging activity of resident macrophages during bacterial overgrowth. The possibility that Rho-activated epithelial cells might also have a role in processing and presenting self and foreign antigens to the mucosal immune system is currently under investigation.
ACKNOWLEDGMENTS
We thank Dr. A. Galmiche for providing us with plasmids encoding Rab5-GFP and the Rab7-GFP proteins, Dr. R. Busca for plasmids encoding myc-tagged dominant positive or negative forms of the Rho GTPases, and Prof. I. Just for plasmid encoding RhoV14-GFP. We are grateful to Profs. P. Boquet, J.R. Murphy, and S.C. Chow for critical reading of the manuscript and to Dr. M. Falchi for useful suggestions.
REFERENCES
- Adamson P, Paterson HF, Hall A. Intracellular localization of the p21 rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aullo P, Giry M, Olsnes S, Popoff MR, Kocks C, Boquet P. A chimeric toxin to study the role of the 21 kDa GTP binding protein rho in the control of actin microfilament assembly. EMBO J. 1993;12:921–931. doi: 10.1002/j.1460-2075.1993.tb05733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum G, Falbo V, Caprioli A, Hacker J. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and alpha hemolysin from the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol Lett. 1995;126:189–195. doi: 10.1111/j.1574-6968.1995.tb07415.x. [DOI] [PubMed] [Google Scholar]
- Boquet P, Fiorentini C. The cytotoxic necrotizing factor 1 (CNF1) from Escherichia coli. In: Aktories K, Just I, editors. Handbook of Experimental Pharmacology: Bacterial Protein Toxin. Vol. 145. Berlin: Springer-Verlag; 2000. pp. 361–379. [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Chardin P, Boquet P, Madaule P, Popoff MR, Rubin EJ, Gill DM. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contamin S, Galmiche A, Flatau G, Benmerah A, Boquet P. The p21 Rho-activating toxin cytotoxic necrotizing factor 1 is endocytosed by a clathrin-independent mechanism and enters the cytosol by an acidic-dependent membrane translocation step. Mol Biol Cell. 2000;11:1775–1787. doi: 10.1091/mbc.11.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossarizza A, Franceschi C, Monti D, Salvioli S, Bellesia E, Rivabene R, Biondo L, Rainaldi G, Tinari A, Malorni W. Protective effect of N-acetylcysteine in tumor necrosis factor-α-induced apoptosis in U937 cells: the role of mitochondria. Exp Cell Res. 1995;220:232–240. doi: 10.1006/excr.1995.1311. [DOI] [PubMed] [Google Scholar]
- de Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin J-J, Aôt-Yahia S, Patel S, Mattei M-G, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S. A novel lysosome-associated membrane glycoprotein DC-Lamp, induced upon maturation, is transiently expressed in MHC Class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- Dharmawardhane S, Schürmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell. 2000;11:3341–3352. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S, Mellor H. Regulation of endocytic traffic by Rho family GTPase. Trends Cell Biol. 2000;10:85–88. doi: 10.1016/s0962-8924(99)01710-9. [DOI] [PubMed] [Google Scholar]
- Fais S, Borghi P, Gherardi G, Logozzi MA, Belardelli F, Gessani S. Human immunodeficiency virus type 1 induces cellular polarization, intercellular adhesion molecule-1 redistribution, and multinucleated giant cell generation in human primary monocytes but not in monocyte-derived macrophages. Lab Invest. 1996;75:783–790. [PubMed] [Google Scholar]
- Fais S, Capobianchi MR, Abbate I, Castilletti C, Gentile N, Cordiali Fei P, Ameglio F, Dianzani F. Unidirectional budding of HIV-1 at the site of cell-to-cell contact is associated with co-polarization of intercellular adhesion molecules and HIV-1 viral matrix protein. AIDS. 1995;9:329–335. [PubMed] [Google Scholar]
- Falzano L, Fiorentini C, Donelli G, Michel E, Kocks C, Cossart P, Cabanié L, Oswald E, Boquet P. Induction of phagocytic behavior in human epithelial cells by Escherichia coli cytotoxic necrotizing factor 1. Mol Microbiol. 1993;9:1247–1254. doi: 10.1111/j.1365-2958.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Finlay BB, Cossart P. Exploitation of mammalian cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Donelli G, Matarrese P, Fabbri A, Paradisi S, Boquet P. Escherichia coli cytotoxic necrotizing factor 1: evidence for induction of actin assembly by constitutive activation of the p21 Rho GTPase. Infect Immun. 1995;63:3936–3944. doi: 10.1128/iai.63.10.3936-3944.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini C, Fabbri A, Flatau G, Donelli G, Matarrese P, Lemichez E, Falzano L, Boquet P. Escherichia coli cytotoxic necrotizing factor 1 (CNF1): a toxin which activates the Rho GTPase. J Biol Chem. 1997;272:19532–19537. doi: 10.1074/jbc.272.31.19532. [DOI] [PubMed] [Google Scholar]
- Flatau G, Landraud L, Boquet P, Bruzzone M, Munro P. Deamidation of RhoA glutamine 63 by E. coli CNF1 toxin requires a short sequence of the GTPase switch 2 domain. Biochem Biophys Res Commun. 2000;267:588–592. doi: 10.1006/bbrc.1999.1904. [DOI] [PubMed] [Google Scholar]
- Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Rho GTPase activation by bacterial toxin-induced glutamine deamidation. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- Francis CL, Ryan TA, Jones BD, Smith SJ, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- Goosney DL, Celli J, Kenny B, Finlay BB. Enteropathogenic Escherichia coli inhibits phagocytosis. Infect Immun. 1999;67:490–495. doi: 10.1128/iai.67.2.490-495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J Cell Sci. 1997;110:105–112. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases, and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hardt WD, Chen LH, Schuebel KE, Bustelo XR, Galan JE. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear response in host cells. Cell. 1998;93:815–826. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytotic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- Lacerda HM, Pullinger GD, Lax AJ, Rozengurt E. Cytotoxic necrotizing factor 1 from Escherichia coli and dermonecrotic toxin from Bordetella bronchiseptica induce p21 Rho-dependent tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 cells. J Biol Chem. 1997;272:9587–9596. doi: 10.1074/jbc.272.14.9587. [DOI] [PubMed] [Google Scholar]
- Lerm M, Schmidt G, Goehring UM, Schirmer J, Aktories K. Identification of the region of rho involved in substrate recognition by Escherichia coli cytotoxic necrotizing factor 1 (CNF1) J Biol Chem. 1999a;274:28999–29004. doi: 10.1074/jbc.274.41.28999. [DOI] [PubMed] [Google Scholar]
- Lerm M, Selzer J, Hoffmeyer A, Rapp UR, Aktories K, Schmidt G. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1: activation of c-Jun N-terminal kinase in HeLa cells. Infect Immun. 1999b;67:496–503. doi: 10.1128/iai.67.2.496-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massol P, Montcourrier P, Guillemot JC, Chavrier P. Fc receptor-mediated phagocytosis requires Cdc42 and Rac1. EMBO J. 1998;17:6219–6229. doi: 10.1093/emboj/17.21.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis IG, Jones AT, Clague MJ. Regulation of endosome fusion. Mol Membr Biol. 1999;16:73–79. doi: 10.1080/096876899294788. [DOI] [PubMed] [Google Scholar]
- Mounier J, Laurent V, Hall A, Fort P, Carlier MF, Egile C. Rho family GTPases control entry of Shigella flexneri into epithelial cells but not intracellular mobility. J Cell Sci. 1999;112:2069–2080. doi: 10.1242/jcs.112.13.2069. [DOI] [PubMed] [Google Scholar]
- Murphy C, Saffrich R, Grummt M, Gournier H, Rybin V, Rubino M, Auvinen P, Lutcke A, Parton RG, Zerial M. Endosome dynamics regulated by a Rho protein. Nature. 1996;384:427–432. doi: 10.1038/384427a0. [DOI] [PubMed] [Google Scholar]
- Negoescu A, Lorimier P, Labat-Moleur F, Drouet C, Robert C, Guillermet C, Brambilla C, Brambilla E. In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J Histochem Cytochem. 1996;44:959–968. doi: 10.1177/44.9.8773561. [DOI] [PubMed] [Google Scholar]
- Nobes C, Marsh M. Dendritic cells: new roles for Cdc42 and Rac in antigen uptake? Curr Biol. 2000;10:739–741. doi: 10.1016/s0960-9822(00)00736-3. [DOI] [PubMed] [Google Scholar]
- Popoff MR, Chaves-Olarte E, Lemichez E, von Eichel-Streiber C, Thelestam M, Chardin P, Cussac D, Antonny B, Chavrier P, Flatau G, Giry M, De Gunzburg J, Boquet P. Ras, Rap, and Rac small GTP-binding proteins are targets for Clostridium sordellii lethal toxin glucosylation. J Biol Chem. 1996;271:10217–10224. doi: 10.1074/jbc.271.17.10217. [DOI] [PubMed] [Google Scholar]
- Racoosin EL, Swanson JA. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992;102:867–880. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121:1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing.factor 1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Selzer J, Lerm M, Aktories K. The Rho-deamidating cytotoxic necrotizing factor 1 from Escherichia coli possesses transglutaminase activity. J Biol Chem. 1998;273:13669–13674. doi: 10.1074/jbc.273.22.13669. [DOI] [PubMed] [Google Scholar]
- Swanson JA. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J Cell Sci. 1989;94:135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- Veithen A, Amyere M, Van Der Smissen P, Cupers P, Courtoy PJ. Regulation of macropinocytosis in v-Src-transformed fibroblast: cyclic AMP selectively promotes regurgitation of macropinosomes. J Cell Sci. 1998;111:2329–2335. doi: 10.1242/jcs.111.16.2329. [DOI] [PubMed] [Google Scholar]
- Vitelli R, Santillo M, Lattero D, Chiariello M, Bifulco M, Bruni CB, Bucci C. Role of the small GTPase rab7 in the late endocytic pathway. J Biol Chem. 1997;272:4391–4397. doi: 10.1074/jbc.272.7.4391. [DOI] [PubMed] [Google Scholar]