Abstract
The use of opioid medications on both an acute and chronic basis is ubiquitous in the U.S. As opioid receptors densely populate the gastrointestinal tract, symptoms and side effects can be expected in these patients. In the esophagus, dysmotility may result manifesting with dysphagia and a syndrome indistinguishable from primary achalasia. In the stomach, a marked delay in gastric emptying may ensue with postprandial nausea and early satiety. Postoperatively, particularly with abdominal surgery, opioid induced ileus may ensue. In the colon, opioid induced constipation (OIC) is common. A unique syndrome termed narcotic bowel syndrome is characterized by chronic abdominal pain often accompanied by nausea and vomiting in the absence of other identifiable causes. With the recognition of the important role of opioids on gastrointestinal function, novel drugs have been developed which utilize this physiology. These medications include peripheral acting opioid agonists to treat OIC and combination agonist and antagonists used for diarrhea predominant irritable bowel syndrome. This review summarizes the most recent data in these areas.
Introduction
Pain as the Fifth Vital Sign and the WHO Ladder of Analgesics Use for Chronic Non-Cancer Pain
The concept of pain as the fifth vital sign was introduced to draw attention to the need to provide adequate pain relief to patients.1 The World Health Organization (WHO) recommended the use of opioids for management of moderate to severe chronic cancer pain; this strategy has been adopted for patients with chronic non-cancer pain in recent years.2
The following order of analgesic use was recommended in the WHO three-step ladder for treatment of cancer pain:3
STEP 1: non-opioids (aspirin, acetaminophen, diclofenac, ibuprofen); then, as necessary,
STEP 2: mild opioids (codeine, tramadol);
STEP 3: strong opioids such as morphine, buprenorphine, fentanyl, hydromorphone, methadone and oxycodone, administered until the patient is free of pain.
Non-opioids can be added to opioids for moderate to severe pain.
Opioid Use in Chronic Non-Cancer Pain
Chronic pain, defined as persistent pain for more than 3 months4 affects 10%–15% of the general population.5 In patients with chronic non-cancer pain, 80% of patients experience at least one adverse event, with constipation (41%), nausea (32%) and somnolence (29%) being most common6. Overall, the prevalence of OIC varies from 41 to 81%.6,7 In the United States, 4% of adults are taking chronic opioid therapy, chiefly for non-cancer pain, including 4.1% in population studies in Olmsted County, Minnesota.8 This study demonstrated a weak association with age, but not with gender, and the most common indications for opioid use in non-cancer pain were musculoskeletal (e.g. back, degenerative joint disease, fibromyalgia), post-surgery, and vascular pain.8 Opioids are more effective than non-opioid analgesics in controlling moderate to severe pain. Thus, ∼90% of patients with moderate to severe pain are treated with opioids.9 An estimated 20% of patients presenting to physicians' offices with pain symptoms in the United States were prescribed opioids.10 The Center for Disease Control (CDC) estimates that the reason for opioid-related overdosing is over-prescription of opioids by healthcare providers.11
Given that ∼4% of adults in the United States are on opioids for at least 3 months for chronic non-cancer indications, there are several public health initiatives12 aimed at reversing the epidemic of opioid use for different pain indications and the rising tide of deaths from opiates.13
Types of Opioid Receptors: μ, δ, κ
Opioid receptors are G(i/o) G-protein coupled receptors that regulate several functions, including pain, reward, mood, stress, gastrointestinal functions and respiration. The three main opioid receptors (μ, δ, κ)14 show a high degree of sequence homology, and a common opioid receptor binding pocket within a helical transmembrane core.15,16 The main differences in sequence between the receptors occur in the extracellular domains which contributes to ligand selectivity.17 The opioid peptides reduce intracellular cAMP by inhibiting adenylate cyclase. At the membrane level, they reduce neuronal excitability (by hyperpolarization resulting from increased potassium permeability of the membrane) and neurotransmitter release (by inhibition of voltage-gated calcium channels).18 The overall effect at the cellular level is thus inhibitory, resulting in a reduction in acetylcholine release, with overall inhibitory effect on the neuron.
Opioid receptors are widely distributed in the central and peripheral nervous system, the intestinal musculature and other tissues including the dorsal horn of the spinal cord where they process and relay afferent nociceptive signals to the central nervous system.19 In the brain, opioid receptors are mainly in areas involved in pain transmission: the thalamolimbic system, the periaqueductal grey matter, the rostral ventral medulla, the nucleus paragigantocellularis lateralis and the locus ceruleus.20
The pharmacologic and potential clinical effects of organ specific opioid stimulation are summarized in Table 1.
Table 1. Pharmacological effects of opiates in different regions of the gastrointestinal tract and clinical correlates.
| Site | Pharmacological effect | Potential Clinical effect |
|---|---|---|
| LES | Inhibition LOS relaxation | |
| Esophagus | Simultaneous contractions | “achalasia” |
| Gallbladder and biliary tract | Contraction | Biliary pain |
| Spasm sphincter of Oddi | Delayed digestion | |
| Decreased secretion | ||
| Gastroduodenum | Inhibition gastric emptying | Anorexia, nausea and vomiting, gastroparesis, postoperative ileus |
| Increase duodenal motility followed by quiescence | ||
| Increase pyloric tone | ||
| Small bowel | Increase tone/segmentation | Indigestion, Bloating, distension Constipation, postoperative ileus |
| Increase transit time | ||
| Increase absorption | ||
| Decrease secretion | ||
| Colon | Increase tone/segmentation | Bloating and distension |
| Increase transit time | Spasm, cramps, pain | |
| Increase absorption | Constipation | |
| Decrease secretion | Hard, dry stools | |
| Anorectum | Decrease rectal sensitivity | Incomplete evacuation |
| Increase internal sphincter tone | Straining constipation |
The μ-receptors are the principal mediators of analgesic action of endogenous and exogenous opioids, as well as the major side effects of sedation, bowel dysfunction, respiratory depression and dependence.21 The κ-receptors also mediate analgesia; other effects include bowel dysfunction, increased diuresis and sedation,22 κ agonists may relieve hyperalgesia produced by chronic use of μ opioid receptor therapies.23 However, the central activation of κ opioid receptors produces dose-dependent dysphoria and some agonists, such as salvinorin A, produce hallucinations.24 The δ-receptors are predominantly in the CNS where they produce analgesia, but they are also found in myenteric and submucosal neurons of the gut; their action results in inhibition of motility and secretion.19 μ and δ receptors are the principal opioid receptors in the gastrointestinal tract, and they are expressed predominantly in the submucosal and myenteric plexuses, respectively.25
Table 2 summarizes endogenous and exogenous mediators (focused on currently available medications) of the three major opioid receptor types, and their general effects on gastrointestinal motor and sensory functions.
Table 2. Three major opioid receptor types in gastrointestinal tract.
| δ-receptors | κ-receptors | μ-receptors | |
|---|---|---|---|
| Preferred endogenous ligand | Enkephalin | Dynorphin | β-endorphin |
| Location | Myenteric plexus | Myenteric plexus | Myenteric and submucosal plexuses |
| CNS | Afferent neurons | CNS and spinal cord | |
| Pharmacological Agonists | Morphine Trimebutine Loperamide |
||
| Eluxadoline | |||
| Pharmacological Antagonists | Eluxadoline, Alvimopan | Naloxone, Naltrexone | |
| - PAMORA | Alvimopan Methylnaltrexone Naloxegol |
||
| Gastrointestinal effects | Delayed transit | Delayed transit Visceral anti-nociception |
|
Since the clinically relevant opioids are μ opioids, this update focuses on μ-opioid receptors.
Gastrointestinal Effects of μ Opioids
Opioids have pharmacological effects throughout the gastrointestinal tract. They decrease gastric emptying and stimulate pyloric tone,26 inhibit propulsion, increase amplitude of nonpropulsive segmental contractions, increase fluid absorption in the small and large intestine, increase anal sphincter tone,27 and impair reflex relaxation of the anal sphincter in response to rectal distention. The acute effects of opioids on esophageal function are not as clear. Two studies have demonstrated an acute decrease in lower esophageal sphincter pressure by μ opioid receptor stimulation.28,29 Nevertheless, the sum of these motor and secretory effects result in anorexia, nausea, emesis, impaired ability to evacuate the bowel, as well as abdominal spasm, cramps, and pain.19,30 Decreased gastric, biliary, pancreatic and intestinal secretions interfere with digestion.
Differences in Acute vs. Chronic μ Opioid Effects in the GI Tract
One of the characteristics of μ opioid effects is the development of tolerance, which results in the need for increasing doses of opioids to achieve the same effects, such as on analgesia or euphoria. This tolerance develops from a desensitization in which opioid receptor stimulation by an agonist leads to lower signal transduction and effector response.31 The effects of chronic opioids on the gut similarly derive from the effects of generalized tolerance, but also differ due to the differential tolerance of gut regions to μ-opioids. For example, tolerance to the effects of μ opioids32,33 occurs in all gastrointestinal organs, except in the colon.34 Therefore, effects such as constipation do not abate over time. These differences may also reflect varying densities of opiate receptors that are organ specific in the gut.35 The exact mechanism of tolerance in humans is unclear, although the prevailing hypotheses are either de-phosphorylation which leads to activation of the receptor to bind an agonist,36 or binding of β-arrestin-2 which causes receptor internalization in the endosome, prolonging the desensitization of the receptor.37
Internalization of the opioid receptors may also occur. The down regulation of β-arrestin-2 does not occur in the ileum, thus causing tolerance to morphine, but this is not observed in the colon, which leads to receptor recycling to the plasma membrane and, hence, lack of tolerance in the colon and development of opiate-induced constipation as discussed.17 Finally, the different downstream effects of stimulating an opioid receptor may follow different time courses of dependence, also leading to a variation of gut effects.31
μ Opioids, GI Symptoms and Syndromes
Esophageal Motility Disorders
μ opioids may be associated with dysphagia or heartburn that may reflect intrinsic neural or sphincter dysfunction.
In patients with gastroesophageal reflux disease (GERD),38 acute morphine administration significantly decreases the rate of transient LES relaxations in patients vs controls, resulting in less reflux episodes and a decrease of the time at pH <4. Resting LES pressure is decreased both in health and in achalasia; swallow-induced LES relaxation is also significantly decreased by morphine in the healthy subjects.39 A range of manometric abnormalities have been reported in patients with dysphagia using chronic opioids, such as impaired LES relaxation, high amplitude/velocity and simultaneous esophageal waves,40 as well as esophagogastric junction outflow obstruction, higher integrated relaxation pressure, and lower distal latency on esophageal pressure topography.41 Some of these findings are in contrast to the acute opioid effects cited previously. This suggests a different esophageal physiology with acute and chronic usage. There may also be clinical and manometric features that mimic type 3 more commonly, or type 2 achalasia, as many of these patients cannot stop and/or require chronic opioid treatment, similar to primary achalasia, though there is some evidence that treatment outcome is worse in those with opioid-induced achalasia.42
Nausea and Emesis
Opioid administration can induce nausea or vomiting, and this is commonly seen in the postoperative period, with opioids being one factor in the multifactorial etiology.43 The pathophysiology involves, in part, peripheral inhibitory effects of opioids on gastrointestinal transit or the stimulation of the pyloric sphincter, which delays gastric emptying or causes gastroparesis. However, the primary mechanism of opioid-induced nausea and emesis is central, with direct stimulation of the chemoreceptor trigger zone in the area postrema in the floor of the fourth ventricle.44 NK-1 receptors in the area postrema are involved in the mechanism of emesis induced by morphine in ferrets,45 but there are no reports of efficacy of aprepitant on nausea, even though it reverses other effects of oxycodone.46 The clinical efficacy of 5-HT3 antagonists for opioid-induced emesis supports the hypothesis that stimulation of the area postrema may also be relevant in morphine-induced emesis in humans.47 Adding a prokinetic (e.g. metoclopramide), prochlorperazine, or a 5-HT3 antagonist to the opiate regimen is beneficial, especially in a postoperative pain control setting.48-50
Gastroparesis
Peripheral inhibitory effects of opioids on antral motility or the stimulation of the pyloric sphincter25 result in delayed gastric emptying or cause gastroparesis. Among patients evaluated for gastroparesis by the NIH Gastroparesis Consortium, 42% overall51 and 48% of those with abdominal discomfort score ≥3 and 33% of those with score <3 were on opiates/narcotics.52 It is important to note that even novel opioid agents that appear to induce less constipation when used chronically may retard gastric emptying. Thus, tapentadol (a mu-opioid receptor agonist and norepinephrine reuptake inhibitor) was associated with delayed gastric emptying comparable to the effect of oxycodone.53 Although tramadol was reported not to retard gastric emptying of solids or liquids in a crossover study of 12 healthy participants; however the same study showed 40% slower orocecal transit and significant delay in colonic transit,54 and tramadol induced dose-related inhibition of gastrointestinal transit in mice.55
Sphincter of Oddi
Opiates may have important effects on sphincter of Oddi function. The effects of opioids on the sphincter are predominantly myogenic, as evidenced by preservation of their effect on sphincter pressure in the presence of the neurotoxin tetrodotoxin.56 Furthermore, different opioid receptors may modulate sphincter of Oddi function. This is suggested by a specific increase in tonic pressure by morphine, but an increase in phasic sphincter pressure by naloxone.57 Nevertheless, the predominant effect of clinically used opioids appears to be an increase in sphincter of Oddi phasic pressure.58 In a retrospective study of post-cholecystectomy patients with suspected sphincter of Oddi dysfunction (SOD), 30% had taken opiate-containing drugs 15 to 120 minutes before the onset of pain, suggesting that opiates may have been the cause of the SOD.59 Likewise, eluxadoline, a mixed μ-opioid receptor agonist–δ-opioid receptor antagonist and δ-opioid receptor agonist recently approved for the treatment of IBS-D, has been associated with SOD in a small percentage (∼0.5%) of patients without a gallbladder.60 This was confirmed in a more recent study of nearly 2000 patients with IBS-D with 10 patients developing sphincter of Oddi spasm, most at the higher eluxadoline dose of 100mg.61 In a recent communication (https://www.fda.gov/Drugs/DrugSafety/ucm546154.htm), the U.S. Food and Drug Administration (FDA) issued a warning that eluxadoline should not be used in patients who do not have a gallbladder. An FDA review found these patients have an increased risk of developing serious pancreatitis that could result in hospitalization or death. In the communication dated March 15, 2017, the review showed that as of February 2017, two deaths in patients who did not have a gallbladder were considered to be associated with eluxadoline had been reported to FDA. One death was associated with pancreatitis with symptom onset within 1 hour of taking a single dose of eluxadoline, and the other death being associated with sphincter of Oddi spasm, manifested as severe abdominal pain and vomiting shortly after taking the first dose of the drug.
Post-Operative Ileus and Opioids
Opioids are a mainstay of pain relief following abdominal surgery and they inhibit gastrointestinal and colonic motility. Post-operative ileus is a complex disorder, and major intrinsic contributing factors include surgical stress (i.e., from handling the bowel), secretion of inflammatory mediators and endogenous opioids in the GI tract, and changes in hormone levels and electrolyte and fluid balance.19,62
Opioid-Induced Constipation
The μ opioids increase fluid absorption and inhibit motility in the colon. Opioid-induced constipation (OIC) is generally defined as a change from baseline in bowel habits and change in defecation patterns after initiating opioid therapy, which is characterized by any of the following: reduced frequency of spontaneous BM (SBM, <3 bowel movements /week); worsening of straining to pass BM; sense of incomplete evacuation; and harder stool consistency.63 These features have been recently adopted in Rome IV criteria (Table 3).64 OIC can occur even at low dosages of opioids65 and at any time after initiation of opioid therapy.66 Nausea, vomiting and gastroesophageal reflux are commonly associated with OIC.67
Table 3. Diagnostic Criteria for Opioid-Induced Constipation.
| 1. New, or worsening, symptoms of constipation when initiating, changing, or increasing opioid therapy that must include 2 or more of the following: |
|
| 2. Loose stools are rarely present without the use of laxatives |
The bowel function index (BFI) is a clinician assessment tool to appraise severity and responsiveness to current treatment. It includes ease of defecation, feeling of incomplete bowel evacuation, and personal judgment of constipation (Figure 1). Each variable is rated by the patient from 0 to 100, based on the experience in 7 days.68 The reference range of BFI scores for non-constipated patients from 0 to 28.8 provides a simple discrimination between constipated and non-constipated patients on opioid therapy.69 A BFI ≥30 is recommended as a criterion to identify patients on laxatives for whom prescriptions of FDA-approved therapies for OIC are justified.70
Figure 1.
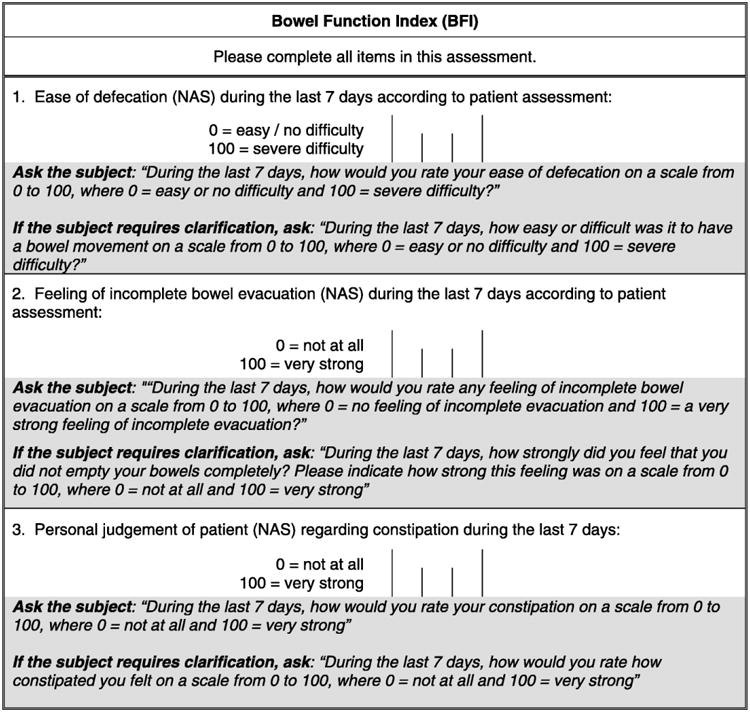
The BFI assessment tool and instructions for use.
Abbreviation: BFI, Bowel Function Index. Reproduced with permission from ref. 68, Rentz AM, et al. J Med Econ 2009;12:371–83.
Narcotic Bowel Syndrome
Narcotic bowel syndrome (NBS) is described as persistent moderate to severe daily abdominal pain of more than 3 months duration occurring in patients requiring more than 100 mg of morphine equivalent per day; the abdominal pain does not respond and may actually increase in response to escalating doses of narcotics.71 It is to be differentiated from the general causes of pain in patients on opiates as it is a disease of nociception independent of the opioid effects on gut motility and secretion (for review, see ref. 72). It is likely a more common syndrome than realized, but a 2009 epidemiologic study in Olmsted County estimated the prevalence at 4%.8
There are at least five postulated mechanisms or hypotheses to explain this paradoxical increase in pain.73 First, the normal pain control function of descending inhibition from the medulla of pain signals rising up the spinal cord becomes a facilitator of those pain signals through neuroplastic change. Second, chronic narcotic use causes inflammation of spinal glial cells through activation of toll-like receptors, and the inflammation leads to increased neuropathic pain. Third, chronic opiate exposure results in abnormal function of the N-methyl-D aspartate receptor (NMDAR) at the level of the spinal cord. Fourth, activation of G-protein coupled receptors by opioids may excite dorsal root ganglia leading to increased pain signals. Fifth, abnormalities of central processing of pain potentiate NBS. For example, in a study of 39 patients with NBS, Drossman et al. demonstrated a high prevalence of psychologic traits and traumatic exposures which have been linked to altered brain processing of painful signals.74
The precise interaction of these diverse mechanisms in production of pain in NBS is unclear. The diagnosis of NBS is suggested by the presentation of a patient on chronic opiates with chronic generalized, colicky abdominal pain, despite escalating doses of opiates, and worsening of pain with tapering of the dose. The pain may be associated with nausea and vomiting, and patients may present to emergency departments. An extensive negative evaluation usually ensues. Emergency evaluation is commonly sought for pain control and to evaluate for causes of abdominal pain that may be found in these patients, such as kidney stones and bowel obstruction.75
Treatment of NBS is difficult, requiring detoxification with substitution of opioids with non-opiate medications to control pain, anxiety and opiate withdrawal symptoms, including the use of clonidine. This is best handled through specialists and/or centers with expertise in opiate dependence.71 Although there is a high recidivism rate (approximately 50%), those who remain off of narcotics report improvement in pain.74
Acute Abdominal Pain in Patients on Chronic μ Opioid Treatment
Recent data show that, among the non-cancer patients attending the Mayo Clinic Emergency Department with acute abdominal pain, ∼19% (442/2354) were on μ opioid agonists for over 3 months for chronic pain. The indication for the opioids was abdominal pain in 21% (93/442) of these patients, suggesting that, despite the lack of evidence of efficacy or safety, μ opioid agonists are being prescribed for patients with non-cancer-related abdominal pain, which likely includes IBS.75
Therapeutic Uses of Opioid Receptor Agonists and Antagonists in Gastroenterology
Opioid Agents in Treatment of Functional GI Diseases
μ opioid agonists
Loperamide, a synthetic peripheral mu-opioid receptor agonist, is efficacious in the treatment of diarrhea in IBS patients, delaying intestinal transit,76 significantly decreasing stool frequency, increasing water and ion absorption, and improving stool consistency and urgency.77 Its advantage over other μ opioids, such as codeine or diphenoxylate, is that it does not cross the blood-brain barrier. Loperamide may also result in improvement in anal sphincter tone.78 Generally, loperamide compared with placebo does not have a significant effect on the perception of pain in IBS patients although pain associated with attacks of diarrhea may be reduced.79,80 The typical doses of loperamide are 2 mg after each loose bowel movement (usually <8 mg per day) or preprandial 2–4 mg in IBS patients with a prominent diarrhea after feeding.
As a group, the μ-opioid agonists are used for pain relief during acute exacerbations of pain in patients with IBS in a combined European and U.S. study, which documented use of opioids in 35% of attacks either alone or in combination with other drugs.81 In this retrospective study, IBS-D patients were more likely to use opioids during pain attacks (32% of attacks) than patients with IBS-C (20% of attacks) or IBS-M (19% of attacks). There are no randomized, controlled trials of the use of μopioid agonists in the treatment of chronic pain in patients with IBS. It is important to reiterate that there is no evidence for use of μ-opioid agonists for the pain of IBS.
A mixed opioid agent, eluxadoline
Eluxadoline is a μ- and κ-opioid receptor agonist and δ-opioid receptor antagonist with minimal oral bioavailability. Eluxadoline, at 100 mg and 200 mg, resulted in greater improvements in bowel movement frequency and urgency, global symptoms, IBS Symptom Severity Score, IBS quality of life, and adequate relief.82 Results on the primary efficacy endpoint (combined bowel function and pain) were generally confirmed in pivotal trials with a NNT of ∼860, though abdominal pain scores were not significant for the 75 or l00mg doses. The adverse events of pancreatitis and sphincter of Oddi spasm (SOS), each in 0.3% of patients in the controlled trials, led to exclusions from treatment of patients with a history of bile duct obstruction, pancreatitis, severe liver impairment, or severe constipation, and intake of more than three alcoholic beverages per day. An updated analysis of safety of eluxadoline in the phase 2 and 3 trials shows that clinically apparent SOS events were observed in eluxadoline-treated patients without a gallbladder and the majority were observed in with the higher (l00mg) dose of eluxadoline.61 The FDA Adverse Event Reporting System received information of 99 cases of pancreatitis, and 39 cases of SOS within 10 months of the availability of eluxadoline in the U.S.83
Treatment of Opioid-Associated Postoperative Ileus: Alvimopan
Alvimopan is a peripherally acting mu-opioid receptor antagonist (PAMORA) approved in the United States for management of postoperative ileus in patients after bowel resection. Alvimopan can accelerate recovery of GI function (especially for the lower GI tract), shorten the length of hospital stay, and reduce postoperative ileus-related morbidity without compromising opioid analgesia in an enhanced recovery setting.81 A recent meta-analysis of nine randomized controlled trials involving 4075 patients demonstrated that alvimopan significantly decreased the time to first passage of stool post operatively and lowered the chance of serious side effects.84
Prevention and Treatment of OIC
Choice of medication to prevent QIC
Oxycodone and naloxone
Naloxone is a relatively nonselective opioid antagonist used intravenously to treat opioid overdosing. When administered orally, standard formulation naloxone acts locally on μ opioid receptors in the gastrointestinal tract.85 Naloxone improved symptoms of OIC and reduced laxative use with only mild opioid withdrawal symptoms such as yawning, sweating and shivering.86
Prolonged release (PR) naloxone has extensive first pass metabolism (hepatic glucuronidation) which reduces its bioavailability for systematic action to <2%. Naloxone PR reduced mean colonic transit time by 2.1 hours when used in combination with oxycodone PR (20mg oxycodone/1 Omg naloxone) compared to oxycodone PR alone (20mg).87
Oxycodone PR and naloxone PR in combination (fixed ratio 2:1, approved at maximum dose of 40 and 20mg respectively) is superior to prolonged release oral oxycodone alone to treat OIC. Naloxone displaces oxycodone from the gastrointestinal μ opioid receptors with negligible action in the systemic circulation due to high first pass metabolism. In contrast, the bioavailability of oxycodone is 80%, and therefore its analgesic action mediated in the CNS is preserved. The combination of oxycodone PR and naloxone PR decreased BFI scores by 48.5 units (on 0-100 scale), increased the median number of complete SBM (CSBM)/week three-fold when compared to oxycodone alone and improved constipation-related quality of life.90-92 The most common adverse effects were nausea, vomiting, headache, constipation, and diarrhea,93 with 13% incidence of severe adverse events.
Tapentadol
Tapentadol is a μ opioid agonist and norepinephrine reuptake inhibitor;94 the latter function adds to the analgesic potential of the μ opioid agonism, predominantly through stimulation of α2 adrenergic receptors.95 The combined effects of tapentadol on pain sensation can be achieved with a relatively lower level of μ-opioid agonist to achieve analgesia equal to that of oxycodone with reduced gastrointestinal adverse effects such as constipation in chronic painful conditions such as moderate-to-severe chronic osteoarthritis-related knee pain96 or moderate-to-severe chronic low back pain.97 However, acutely administered tapentadol slows gastric emptying similarly to oxycodone, though it does not retard colonic transit.53
OTC laxatives and when to move to specific prescription treatments
Since laxatives were proven to be effective in some patients,98 they should be the first line treatment in patients with diagnosis of OIC. Guidelines from the European Association for Palliative Care (EAPC) recommend laxatives for the prophylaxis or management of OIC in patients with cancer.99 Unfortunately, prophylactic treatment to prevent constipation is seldom prescribed to outpatients who receive prescription opioids.100,101 A prospective, open-label study suggests that polyethylene glycol (13.81 grams daily) and sodium picosulphate (10 mg daily) are more efficacious than lactulose102 for outpatients with cancer on opioid therapy.
Recent studies have documented inadequate response of OIC to laxative treatment.
If there is insufficient clinical benefit with laxatives, as evidenced by a BFI score of >30 points, treatment with medications approved for OIC should be considered (PAMORA, combination of oxycodone and naloxone, lubiprostone). Reassessment of the BFI score is useful to monitor improvement in OIC.104
Treatment of OIC
Intestinal secretagogue: lubiprostone
Lubiprostone is a bicyclic fatty acid derived from PGE1 metabolite which increases fluid secretion in the gastrointestinal tract105 by stimulating the cystic fibrosis transmembrane regulator (CFTR) and type 2 chloride channels (C1C2) in the apical membrane to secrete chloride and water into the lumen, resulting laxation and acceleration of small intestinal and colonic transit.106
Lubiprostone compared to placebo increased the overall frequency of SBM/week, and reduced by 50% the time to first bowel movement in patients with OIC.107,108 In these same trials, lubiprostone significantly improved constipation symptoms such as abdominal discomfort, degree of straining, stool consistency, and constipation severity. Nausea, diarrhea, and abdominal pain were the most common side effects.
Lubiprostone-stimulated secretion of CI ions via C1C2 channels was inhibited in vitro in T84 cell lines by methadone. As a result of these studies, lubiprostone is contraindicated in OIC associated with methadone use. Lubiprostone, 24μg, twice daily (b.i.d.), was approved by the FDA for OIC in patients with non-cancer pain.
PAMORAs : Methyl naltrexone and naloxegol
Methylnaltrexone is a quarternary N-methyl derivative of naltrexone;110 the methyl group decreases the lipid solubility and increases polarity, preventing it from crossing into the brain.111 Peripherally administered methylnaltrexone decreased morphine-induced delay in orocecal transit time.112 In a 4-week trial of methylnaltrexone, 12mg once daily or every other day, compared to placebo in patients with OIC, there was significantly shortened time to first rescue-free bowel movements (RFBM), increased the number of weekly RFBM, improved degree of straining, decreased sense of incomplete evacuation, and improved PAC-QOL;113 an early response suggested excellent outcome. Another trial with same doses improved PAC-SYM scores, specifically the stool and rectal symptoms, with no effect on pain scores.114 Abdominal pain and nausea were the most common adverse events reported; other adverse effects were diarrhea, hyperhidrosis, and vomiting. The FDA has approved 12mg subcutaneous injection of methylnaltrexone for the treatment of OIC in patients taking opioids for chronic, non-cancer pain. There have been 7 cases of gastric or intestinal perforation reported in the FDA Adverse Event Reporting System in patients association with methylnaltrexone therapy for OIC during the first 18 months after approval.115 The causative relationship has not been established, since patients either had coincidental gastric ulcer or severe constipation which is itself a risk factor for perforation.
Naloxegol is a PEGylated derivative of naloxone116 that does not cross the blood brain barrier. In addition, P-glycoprotein transporter (PGP) transports naloxegol from the central nervous system.117 Thus, only negligible amounts of naloxegol reach the central nervous system and, therefore, it does not reduce pain relief from opioids. Naloxegol antagonized morphine-induced reduced orocecal transit, but it had no effects on miosis or opioid withdrawal symptoms, suggesting exclusively peripheral action.116,118
Large 12-week, phase II and phase III studies all showed naloxegol improved SBM from the first week of treatment,118,119 even in patients with inadequate response to laxatives. In these trials, naloxegol improved stool consistency, CSBM, percentage of days with straining, PAC-SYM and PAC-QOL and was well tolerated and safe.
In patients with OIC, an improvement in the frequency of SBMs by ≥3 per week was associated with consistent improvements in patient response outcomes, that is Patient Assessment of Constipation-Quality of Life (PAC-QOL) and PAC-Symptoms (PAC-SYM) at each study visit, and the Straining Scale and stool consistency or form (Bristol Stool Scale) with each bowel movement.120 From the United Kingdom's National Health Service and Personal Social Service perspective, a recent analysis suggests that naloxegol treatment is cost-effective in patients with OIC who are not responding to laxatives.121
When administered for 52 weeks in OIC patients with non-cancer pain,122 the most common side effects were abdominal pain, diarrhea, nausea, headache, and flatulence; no QT/QTc interval prolongation or serious adverse events occurred. The FDA approved naloxegol, 12.5 or 25mg, q.d., orally, for OIC in adults with chronic non-cancer pain, and requires surveillance of cardiovascular events in patients treated with naloxegol. A summary of medication trials used for OIC is given in Table 4.
Table 4. Clinical trials of drugs approved for OIC (pla=placebo; PAC-SYM= Patient assessment of constipation symptoms; # = size of study cohort; weeks refers to duration of trial in weeks; RFBM=Rescue free BM).
Updated with recent advances from ref. 123, Nelson A, Camilleri M. Ther Adv Gastroenterol 2015;8:206-20.
| DRUG | DESIGN | Weeks | # | STUDY ENDPOINTS | SPECIFIC OUTCOMES | REFERENCE |
|---|---|---|---|---|---|---|
| LUBIPROSTONE | 1. RCT | 12 | 431 | ≥1 SBM improvement over baseline frequency and ≥3 SBM/week for at least 9 weeks | 27.1% vs. 18.9% with p value <0.030 | Jamal et al 2015 |
| 2. RCT | 12 | 418 | Δ from baseline in SBM # at week 8 and overall | At 8 weeks, SBMs/week mean 3.3 vs. 2.4 (pla), P =0.005; Overall mean SBMs/week 2.2 vs. 1.6 (pla), P = 0.004 | Cryer et al 2014 | |
|
| ||||||
| OXYCODONE AND NALOXONE (OXY PR) | 1. RCT + open-label for 52 weeks | 12 | 278 | a. Change in BFI at week 4 compared to baseline b. CSBM |
40.9 BFI score at week 4 and 34.01 at week 12 compared to a baseline of 67.4 51% achieved CSBM in OXY PR compared to 26% in only oxycodone group at 4 weeks |
Lowestein et al 2009 |
| 2. RCT | 12 | 35 | Δ from baseline in BFI during treatment | BFI Score change of 23.3 compared to baseline of 61.3 (P< 0.0002) | Koopmans et al 2014 | |
|
| ||||||
| METHYL NALTREXONE (MNTX) | 1. RCT | 4 | 460 | Rescue free BM (RFBM) within 4 hours of first dose Time to BM within first 24 hours |
34.2% had RFBM with MNTX compared to 9.9% (pla) 46% had RFBM within 24 hours with MNTX compared to 25.3% (pla) |
Michna et al 2011 |
| 2. RCT | 4 | 460 | PAC SYM: Rectal symptoms Stool symptoms |
At 4 weeks, MNTX compared to placebo: -0.56 vs -0.30 (p<0.05) -0.76 vs -0.43 (p<0.001) |
Iyer et al 2011 | |
|
| ||||||
| NALOXEGOL | 1. RCT | 4 | 207 | Median Δ from baseline in SBM/wk after 4 weeks | 25 mg naloxegol (3.0 vs 0.8 [pla]; P = 0.0022); 50 mg naloxegol (3.5 vs 1.0 [pla]; P < 0.0001); Response rates higher 25 vs. 12.5mg | Webster et al 2013 |
| 2. RCT (two studies: 04 and 05) | 12 | 641 696 |
≥3 SBM/week and increase of ≥1 SBM compared to baseline for ≥ 9 of 12 weeks Δ Severity of straining Δ Stool consistency |
study 04: 44.4% vs. 29.4% [pla], P=0.001 study 05: 39.7% vs. 29.3% [pla], P=0.02 study 04: -0.73±0.05; study 05: -0.80±0.06; study 04: 0.66±0.07; study 05: 0.71+0.07 |
Chey et al 2014 | |
Conclusion
Opioid medications are commonly used in clinical practice and have acute or chronic effects on diverse regions of the gastrointestinal tract. Given their widespread use, it is imperative to consider whether any presentation with gastrointestinal symptoms may be related to the intake of opioids. Acute administration of opioids should be accompanied by symptomatic remedies to counter the acute pharmacological effects, and these include antiemetics and laxatives. The Bowel Function Index is a useful clinical tool to identify chronic OIC that is not responding satisfactorily to first-line therapies and to select patients for treatment with prescription medications approved for the treatment of OIC.
Acknowledgments
We thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding: Dr. Camilleri is funded by R01-DK92179 from National Institutes of Health.
Footnotes
Declaration of Conflicting Interests: Dr. Camilleri has served as a consultant (remuneration to Mayo Clinic) to AstraZeneca regarding naloxegol and is conducting research on pharmacodynamic effects of naloxegol. He received personal remuneration for one advisory board meeting with Shionogi on naldemedine for the treatment of opioid-induced constipation.
Dr. Lembo has served on an advisory committee, as a board member, and as a consultant for Forest Research Labs, Ironwood Pharmaceuticals, Prometheus, and Salix.
Dr. Katzka has research funding from Covidien and has consulted for Shire and Adare.
Authors' contributions: Dr. Camilleri: concept, mechanism of action, clinical and pharmacological effects of opioids
Dr. Lembo: treatment of opioid-induced constipation and manuscript review
Dr. Katzka: clinical and pharmacological effects of opioids
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walid MS, Donahue SN, Darmohray DM, et al. The fifth vital sign--What does it mean? Pain Pract. 2008;8:417–422. doi: 10.1111/j.1533-2500.2008.00222.x. [DOI] [PubMed] [Google Scholar]
- 2.Fine PG, Mahajan G, Mcpherson ML. Long-acting opioids and short-acting opioids: appropriate use in chronic pain management. Pain Med. 2009;10:S79–S88. doi: 10.1111/j.1526-4637.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 3.Jadad AR, Browman GP. The WHO analgesic ladder for cancer pain management: stepping up the quality of its evaluation. JAMA. 1995;274:1870–1873. [PubMed] [Google Scholar]
- 4.Verhaak PF, Kerssens JJ, Dekker J, et al. Prevalence of chronic benign pain disorder among adults: a review of the literature. Pain. 1998;77:231–239. doi: 10.1016/S0304-3959(98)00117-1. [DOI] [PubMed] [Google Scholar]
- 5.Mansfield KE, Sim J, Jordan JL, et al. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain. 2016;157:55–64. doi: 10.1097/j.pain.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European patient survey (Probe 1) Pain Med. 2009;10:35–42. doi: 10.1111/j.1526-4637.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 8.Choung RS, Locke GR, 3rd, Zinsmeister AR, et al. Opioid bowel dysfunction and narcotic bowel syndrome: a population-based study. Am J Gastroenterol. 2009;104:1199–1204. doi: 10.1038/ajg.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 10.http://www.cdc.gov/homeandrecreationalsafety/pdf/Common_Elements_in_Guidelines_for_Prescribing_Opioids-a.pdf
- 11.http://www.cdc.gov/primarycare/materials/opoidabuse/docs/pdaphperspective508.pdf
- 12.Murthy VH. Ending the opioid epidemic - a call to action. N Engl J Med. 2016 Nov 9; doi: 10.1056/NEJMp1612578. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 14.Williams JT, Ingram SL, Henderson G, et al. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65:223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metzger TG, Ferguson DM. On the role of extracellular loops of opioid receptors in conferring ligand selectivity. FEBS Lett. 1995;375:1–4. doi: 10.1016/0014-5793(95)01185-h. [DOI] [PubMed] [Google Scholar]
- 16.Paterlini MG. The function of the extracellular regions in opioid receptor binding: insights from computational biology. Curr Top Med Chem. 2005;5:357–367. doi: 10.2174/1568026053544579. [DOI] [PubMed] [Google Scholar]
- 17.Kane BE, Svensson B, Ferguson DM. Molecular recognition of opioid receptor ligands. AAPS J. 2006;8:E126–E137. doi: 10.1208/aapsj080115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galligan JJ, Akbarali HI. Molecular physiology of enteric opioid receptors. Am J Gastroenterol. 2014;2:17–21. doi: 10.1038/ajgsup.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–671. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 20.Stefano GB, Goumon Y, Casares F, et al. Endogenous morphine. Trends Neurosci. 2000;23:436–442. doi: 10.1016/s0166-2236(00)01611-8. [DOI] [PubMed] [Google Scholar]
- 21.Pasternak GW. Pharmacological mechanisms of opioid analgesics. Clin Neuropharmacol. 1993;16:1–18. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Greenwald MK, Stitzer ML, Haberny KA. Human pharmacology of the opioid neuropeptide dynorphin A (1-13) J Pharmacol Exp Ther. 1997;281:1154–1163. [PubMed] [Google Scholar]
- 23.Kivell B, Prisinzano TE. Kappa opioids and the modulation of pain. Psychopharmacology (Berl) 2010;210:109–119. doi: 10.1007/s00213-010-1819-6. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez D, Riba J, Bouso JC, et al. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006;85:157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Bagnol D, Mansour A, Akil H, et al. Cellular localization and distribution of the cloned mu and kappa opioid receptors in rat gastrointestinal tract. Neuroscience. 1997;81:579–591. doi: 10.1016/s0306-4522(97)00227-3. [DOI] [PubMed] [Google Scholar]
- 26.Camilleri M, Malagelada JR, Stanghellini V, et al. Dose-related effects of synthetic human beta-endorphin and naloxone of fed gastrointestinal motility. Am J Physiol. 1986;251:G147–G154. doi: 10.1152/ajpgi.1986.251.1.G147. [DOI] [PubMed] [Google Scholar]
- 27.Musial F, Enck P, Kalveram KT, et al. The effect of loperamide on anorectal function in normal healthy men. J Clin Gastroenterol. 1992;15:321–324. doi: 10.1097/00004836-199212000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Mittal RK, Frank EB, Lange RC, et al. Effects of morphine and naloxone on esophageal motility and gastric emptying in man. Dig Dis Sci. 1986;31:936–942. doi: 10.1007/BF01303214. [DOI] [PubMed] [Google Scholar]
- 29.Penagini R, Bartesaghi B, Zannini P, et al. Lower oesophageal sphincter hypersensitivity to opioid receptor stimulation in patients with idiopathic achalasia. Gut. 1993;34:16–20. doi: 10.1136/gut.34.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182:S11–S18. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 31.Allouche S, Noble F, Marie N. Opioid receptor densensitization: mechanisms and its link to tolerance. Front Pharmacol. 2014;5:280. doi: 10.3389/fphar.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharmacol Sci. 2001;22:67–70. doi: 10.1016/s0165-6147(00)01616-3. [DOI] [PubMed] [Google Scholar]
- 33.Pan YX. Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promoters. DNA Cell Biol. 2005;24:736–750. doi: 10.1089/dna.2005.24.736. [DOI] [PubMed] [Google Scholar]
- 34.Ling GS, Paul D, Simantov R, et al. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45:1627–1636. doi: 10.1016/0024-3205(89)90272-5. [DOI] [PubMed] [Google Scholar]
- 35.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;(2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 36.Williams JT, Ingram SL, Henderson G, et al. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65:223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claing A, Laporte SA, Caron MG, et al. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and ß-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 38.Penagini R, Bianchi PA. Effect of morphine on gastroesophageal reflux and transient lower esophageal sphincter relaxation. Gastroenterology. 1997;113:409–414. doi: 10.1053/gast.1997.v113.pm9247457. [DOI] [PubMed] [Google Scholar]
- 39.Penagini R, Bartesaghi B, Zannini P, et al. Lower oesophageal sphincter hypersensitivity to opioid receptor stimulation in patients with idiopathic achalasia. Gut. 1993;34:16–20. doi: 10.1136/gut.34.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther. 2010;31:601–606. doi: 10.1111/j.1365-2036.2009.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratuapli SK, Crowell MD, DiBaise JK, et al. Opioid-induced esophageal dysfunction (OIED) in patients on chronic opioids. Am J Gastroenterol. 2015;110:979–984. doi: 10.1038/ajg.2015.154. [DOI] [PubMed] [Google Scholar]
- 42.Ravi K, Murray JA, Geno DM, et al. Achalasia and chronic opiate use: innocent bystanders or associated conditions? Dis Esophagus. 2016;29:15–21. doi: 10.1111/dote.12291. [DOI] [PubMed] [Google Scholar]
- 43.Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992;77:162–184. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 44.Costello DJ, Borison HL. Naloxone antagonizes narcotic self blockade of emesis in the cat. J Pharmacol Exp Ther. 1977;203:222–230. [PubMed] [Google Scholar]
- 45.Ariumi H, Saito R, Nago S, et al. The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett. 2000;286:123–126. doi: 10.1016/s0304-3940(00)01113-7. [DOI] [PubMed] [Google Scholar]
- 46.Walsh SL, Heilig M, Nuzzo PA, et al. Effects of the NK1 antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusers. Addict Biol. 2013;18:332–343. doi: 10.1111/j.1369-1600.2011.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sussman G, Shurman J, Creed MR, et al. Intravenous ondansetron for the control of opioid-induced nausea and vomiting. Clin Ther. 1999;21:1216–1227. doi: 10.1016/s0149-2918(00)80024-7. [DOI] [PubMed] [Google Scholar]
- 48.Hirayama T, Ishii F, Yago K, et al. Evaluation of the effective drugs for the prevention of nausea and vomiting induced by morphine used for postoperative pain: a quantitative systematic review. J Pharm Soc Jpn. 2001;121:179–185. doi: 10.1248/yakushi.121.179. [DOI] [PubMed] [Google Scholar]
- 49.Chung F, Lane R, Spraggs C, et al. Ondansetron is more effective than metoclopramide for the treatment of opioid-induced emesis in post-surgical adult patients. Eur J Anaesthesiol. 1999;16:669–677. doi: 10.1046/j.1365-2346.1999.00547.x. [DOI] [PubMed] [Google Scholar]
- 50.Williams PI, Smith M. An assessment of prochlorperazine buccal for the prevention of nausea and vomiting during intravenous patient-controlled analgesia with morphine following abdominal hysterectomy. Eur J Anaesthesiol. 1999;16:638–645. doi: 10.1046/j.1365-2346.1999.00561.x. [DOI] [PubMed] [Google Scholar]
- 51.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–115. doi: 10.1053/j.gastro.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427–438. doi: 10.1111/nmo.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeong ID, Camilleri M, Shin A, et al. A randomised, placebo-controlled trial comparing the effects of tapentadol and oxycodone on gastrointestinal and colonic transit in healthy humans. Aliment Pharmacol Ther. 2012;35:1088–1096. doi: 10.1111/j.1365-2036.2012.05040.x. [DOI] [PubMed] [Google Scholar]
- 54.Maurer AH, Krevsky B, Knight LC, et al. Opioid and opioid-like drug effects on whole-gut transit measured by scintigraphy. J Nucl Med. 1996;37:818–822. [PubMed] [Google Scholar]
- 55.Dürsteler C, Mases A, Fernandez V, et al. Interaction between tramadol and two anti-emetics on nociception and gastrointestinal transit in mice. Eur J Pain. 2006;10:629–638. doi: 10.1016/j.ejpain.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Helm JF, Dodds WJ, Christensen J, Sarna SK. Control mechanism of spontaneous in vitro contractions of the opossum sphincter of Oddi. Am J Physiol. 1985;249:G572–G579. doi: 10.1152/ajpgi.1985.249.5.G572. [DOI] [PubMed] [Google Scholar]
- 57.Helm JF, Venu RP, Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Arndorfer RC. Effects of morphine on the human sphincter of Oddi. Gut. 1988;29:1402–1407. doi: 10.1136/gut.29.10.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donald R, Thompson M. Narcotic analgesic effects on the sphincter of Oddi: a review of the data and therapeutic implications in treating pancreatitis. Am J Gastroenterol. 2001;96:1266–1272. doi: 10.1111/j.1572-0241.2001.03536.x. [DOI] [PubMed] [Google Scholar]
- 59.Sherman S, Gottlieb K, Uzer MF, et al. Effects of meperidine on the pancreatic and biliary sphincter. Gastrointest Endosc. 1996;44:239–242. doi: 10.1016/s0016-5107(96)70158-x. [DOI] [PubMed] [Google Scholar]
- 60.Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242–253. doi: 10.1056/NEJMoa1505180. [DOI] [PubMed] [Google Scholar]
- 61.Cash BD, Lacy BE, Schoenfeld PS, et al. Safety of eluxadoline in patients with irritable bowel syndrome with diarrhea. Am J Gastroenterol. 2017;112:365–374. doi: 10.1038/ajg.2016.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luckey A, Livingston E, Taché Y. Mechanisms and treatment of postoperative ileus. Arch Surg. 2003;138:206–214. doi: 10.1001/archsurg.138.2.206. [DOI] [PubMed] [Google Scholar]
- 63.Gaertner J, Siemens W, Camilleri M, et al. Definitions and outcome measures of clinical trials regarding opioid-induced constipation: a systematic review. J Clin Gastroenterol. 2015;49:9–16. doi: 10.1097/MCG.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 64.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel disorders. Gastroenterology. 2016 Feb 18; doi: 10.1053/j.gastro.2016.02.031. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 65.Shook JE, Pelton JT, Hruby VJ, et al. Peptide opioid antagonist separates peripheral and central opioid antitransit effects. J Pharmacol Exp Ther. 1987;243:492–500. [PubMed] [Google Scholar]
- 66.Choi YS, Billings JA. Opioid antagonists: a review of their role in palliative care, focusing on use in opioid-related constipation. J Pain Symptom Manage. 2002;24:71–90. doi: 10.1016/s0885-3924(02)00424-4. [DOI] [PubMed] [Google Scholar]
- 67.Tuteja AK, Biskupiak J, Stoddard GJ, et al. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil. 2010;22:424–e496. doi: 10.1111/j.1365-2982.2009.01458.x. [DOI] [PubMed] [Google Scholar]
- 68.Rentz AM, Yu R, Muller-Lissner S, et al. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ. 2009;12:371–383. doi: 10.3111/13696990903430481. [DOI] [PubMed] [Google Scholar]
- 69.Ueberall MA, Muller-Lissner S, Buschmann-Kramm C, et al. The Bowel Function Index for evaluating constipation in pain patients: definition of a reference range for a non-constipated population of pain patients. J Int Med Res. 2011;39:41–50. doi: 10.1177/147323001103900106. [DOI] [PubMed] [Google Scholar]
- 70.Argoff CE, Brennan MJ, Camilleri M, et al. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med. 2015;16:2324–2337. doi: 10.1111/pme.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grunkemeier DMS, Cassara JE, Dalton CB, et al. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol. 2007;5:1126–1139. doi: 10.1016/j.cgh.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurlander JE, Drossman DA. Diagnosis and treatment of narcotic bowel syndrome. Nat Rev Gastroenterol Hepatol. 2014;11:410–418. doi: 10.1038/nrgastro.2014.53. [DOI] [PubMed] [Google Scholar]
- 73.Drossman D, Szigethy E. The narcotic bowel syndrome: a recent update. Am J Gastroenterol Suppl. 2014;2:22–30. doi: 10.1038/ajgsup.2014.6. [DOI] [PubMed] [Google Scholar]
- 74.Drossman DA, Morris CB, Edwards H, et al. Diagnosis, characterization, and 3-month outcome after detoxification of 39 patients with narcotic bowel syndrome. Am J Gastroenterol. 2012;107:1426–1440. doi: 10.1038/ajg.2012.142. [DOI] [PubMed] [Google Scholar]
- 75.Khemani D, Camilleri M, Roldan A, et al. Opioid analgesic use among patients presenting with acute abdominal pain and factors associated with surgical diagnoses. Neurogastroenterol Motil. 2016 Dec 25; doi: 10.1111/nmo.13000. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cann PA, Read NW, Holdsworth CD, et al. Role of loperamide and placebo in management of irritable bowel syndrome (IBS) Dig Dis Sci. 1984;29:239–247. doi: 10.1007/BF01296258. [DOI] [PubMed] [Google Scholar]
- 77.Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomised controlled trials. Gut. 2001;48:272–282. doi: 10.1136/gut.48.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun WM, Read NW, Verlinden M. Effects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinence. Scand J Gastroenterol. 1997;32:34–38. doi: 10.3109/00365529709025060. [DOI] [PubMed] [Google Scholar]
- 79.Lavö B, Stenstam M, Nielsen AL. Loperamide in treatment of irritable bowel syndrome--a double-blind placebo controlled study. Scand J Gastroenterol Suppl. 1987;130:77–80. doi: 10.3109/00365528709091003. [DOI] [PubMed] [Google Scholar]
- 80.Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scand J Gastroenterol Suppl. 1987;130:81–84. doi: 10.3109/00365528709091004. [DOI] [PubMed] [Google Scholar]
- 81.Hellström PM, Saito YA, Bytzer P, et al. Characteristics of acute pain attacks in patients with irritable bowel syndrome meeting Rome III criteria. Am J Gastroenterol. 2011;106:1299–1307. doi: 10.1038/ajg.2011.78. [DOI] [PubMed] [Google Scholar]
- 82.Dove LS, Lembo A, Randall CW, et al. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology. 2013;145:329–338. doi: 10.1053/j.gastro.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 83.FDA Adverse Event Reporting System Detailed Report from 2 Jan 2016 to 18 Oct 2016 under Freedom of Information Act. Eluxadoline/Viberzi. Issued 21 October 2016. [Google Scholar]
- 84.Xu LL, Zhou XQ, Yi PS, et al. Alvimopan combined with enhanced recovery strategy for managing postoperative ileus after open abdominal surgery: a systematic review and meta-analysis. J Surg Res. 2016;203:211–221. doi: 10.1016/j.jss.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 85.Liu M, Wittbrodt E. Low-dose oral naloxone reverses opioid-induced constipation and analgesia. J Pain Symptom Manage. 2002;23:48–53. doi: 10.1016/s0885-3924(01)00369-4. [DOI] [PubMed] [Google Scholar]
- 86.Meissner W, Schmidt U, Hartmann M, et al. Oral naloxone reverses opioid-associated constipation. Pain. 2000;84:105–109. doi: 10.1016/S0304-3959(99)00185-2. [DOI] [PubMed] [Google Scholar]
- 87.Smith K, Hopp M, Mundin G, et al. Naloxone as part of a prolonged release oxycodone/naloxone combination reduces oxycodone-induced slowing of gastrointestinal transit in healthy volunteers. Expert Opin Investig Drugs. 2011;20:427–439. doi: 10.1517/13543784.2011.563236. [DOI] [PubMed] [Google Scholar]
- 88.Meissner W, Leyendecker P, Mueller-Lissner S, et al. A randomised controlled trial with prolonged-release oral oxycodone and naloxone to prevent and reverse opioid-induced constipation. Eur J Pain. 2009;13:56–64. doi: 10.1016/j.ejpain.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 89.Burness CB, Keating GM. Oxycodone/naloxone prolonged-release: a review of its use in the management of chronic pain while counteracting opioid-induced constipation. Drugs. 2014;74:353–375. doi: 10.1007/s40265-014-0177-9. [DOI] [PubMed] [Google Scholar]
- 90.Lowenstein O, Leyendecker P, Hopp M, et al. Combined prolonged-release oxycodone and naloxone improves bowel function in patients receiving opioids for moderate-to-severe non-malignant chronic pain: a randomised controlled trial. Exp Opin Pharmacother. 2009;10:531–543. doi: 10.1517/14656560902796798. [DOI] [PubMed] [Google Scholar]
- 91.Mehta V, Alaward S, Kuravinakop S, et al. Effect of a fixed-dose opioid agonist/antagonist on constipation in patients on long-term opioids for non-malignant pain unable to tolerate laxatives. Pain Physician. 2014;17:415–442. [PubMed] [Google Scholar]
- 92.Poelaert J, Koopmans-Klein G, Dioh A, et al. Treatment with prolonged-release oxycodone/naloxone improves pain relief and opioid-induced constipation compared with prolonged-release oxycodone in patients with chronic severe pain and laxative-refractory constipation. Clin Ther. 2015;37:784–792. doi: 10.1016/j.clinthera.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 93.Sandner-Kiesling A, Leyendecker P, Hopp M, et al. Long-term efficacy and safety of combined prolonged-release oxycodone and naloxone in the management of non-cancer chronic pain. Int J Clin Pract. 2010;64:763–774. doi: 10.1111/j.1742-1241.2010.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kress HG. Tapentadol and its two mechanisms of action: is there a new pharmacological class of centrally-acting analgesics on the horizon? Eur J Pain. 2010;14:781–783. doi: 10.1016/j.ejpain.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 95.Bee LA, Bannister K, Rahman W, et al. Mu-opioid and noradrenergic α(2)-adrenoceptor contributions to the effects of tapentadol on spinal electrophysiological measures of nociception in nerve-injured rats. Pain. 2011;152:131–139. doi: 10.1016/j.pain.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 96.Afilalo M, Etropolski MS, Kuperwasser B, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: results of a randomized, double-blind, placebo-and active-controlled phase 3 study. Clin Drug Invest. 2010;30:489–505. doi: 10.2165/11533440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 97.Buynak R, Shapiro DY, Okamoto A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled phase III study. Expert Opin Pharmacother. 2010;11:1787–1804. doi: 10.1517/14656566.2010.497720. [DOI] [PubMed] [Google Scholar]
- 98.Ruston T, Hunter K, Cummings G, et al. Efficacy and side-effect profiles of lactulose, docusate sodium, and sennosides compared to PEG in opioid-induced constipation: a systematic review. Can Oncol Nurs J. 2013;23:236–246. doi: 10.5737/1181912x234236240. [DOI] [PubMed] [Google Scholar]
- 99.Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13:e58–e68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 100.Skollerud LM, Fredheim OM, Svendsen K, et al. Laxative prescriptions to cancer outpatients receiving opioids: a study from the Norwegian prescription database. Support Care Cancer. 2013;21:67–73. doi: 10.1007/s00520-012-1494-8. [DOI] [PubMed] [Google Scholar]
- 101.Hunold KM, Smith SA, Platts-Mills TF. Constipation prophylaxis is rare for adults prescribed outpatient opioid therapy from U.S. emergency departments. Acad Emerg Med. 2015;22:1118–1122. doi: 10.1111/acem.12745. [DOI] [PubMed] [Google Scholar]
- 102.Wirz S, Nadstawek J, Elsen C, et al. Laxative management in ambulatory cancer patients on opioid therapy: a prospective, open-label investigation of polyethylene glycol, sodium picosulphate and lactulose. Eur J Cancer Care (Engl) 2012;21:131–140. doi: 10.1111/j.1365-2354.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- 103.Coyne KS, Margolis MK, Yeomans K, et al. Opioid-induced constipation among patients with chronic noncancer pain in the United States, Canada, Germany, and the United Kingdom: laxative use, response, and symptom burden over time. Pain Med. 2015;16:1551–1565. doi: 10.1111/pme.12724. [DOI] [PubMed] [Google Scholar]
- 104.Argoff CE, Brennan MJ, Camilleri M, et al. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med. 2015;16:2324–2337. doi: 10.1111/pme.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cuppoletti J, Chakrabarti J, Tewari KP, et al. Differentiation between human Clc-2 and Cftr Cl- channels with pharmacological agents. Am J Physiol Cell Physiol. 2014;307:C479–C492. doi: 10.1152/ajpcell.00077.2014. [DOI] [PubMed] [Google Scholar]
- 106.Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G942–G947. doi: 10.1152/ajpgi.00264.2005. [DOI] [PubMed] [Google Scholar]
- 107.Jamal MM, Adams AB, Jansen JP, et al. A randomized, placebo-controlled trial of lubiprostone for opioid-induced constipation in chronic noncancer pain. Am J Gastroenterol. 2015;110:725–732. doi: 10.1038/ajg.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cryer B, Katz S, Vallejo R, et al. A randomized study of lubiprostone for opioid-induced constipation in patients with chronic noncancer pain. Pain Med. 2014;15:1825–1834. doi: 10.1111/pme.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cuppoletti J, Chakrabarti J, Tewari K, et al. Methadone but not morphine inhibits lubiprostone-stimulated Cl− currents in T84 intestinal cells and recombinant human Clc-2, but not Cftr Cl− currents. Cell Biochem Biophys. 2013;66:53–63. doi: 10.1007/s12013-012-9406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown DR, Goldberg LI. The use of quaternary narcotic antagonists in opiate research. Neuropharmacol. 1985;24:181–191. doi: 10.1016/0028-3908(85)90072-3. [DOI] [PubMed] [Google Scholar]
- 111.Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett. 2004;361:192–195. doi: 10.1016/j.neulet.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 112.Yuan CS, Wei G, Foss JF, et al. Effects of subcutaneous methylnaltrexone on morphine-induced peripherally mediated side effects: a double-blind randomized placebo-controlled trial. J Pharmacol Exp Ther. 2002;300:118–123. doi: 10.1124/jpet.300.1.118. [DOI] [PubMed] [Google Scholar]
- 113.Michna E, Blonsky ER, Schulman S, et al. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J Pain. 2011;12:554–562. doi: 10.1016/j.jpain.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 114.Iyer SS, Randazzo BP, Tzanis EL, et al. Effect of subcutaneous methylnaltrexone on patient-reported constipation symptoms. Value Health. 2011;14:177–183. doi: 10.1016/j.jval.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 115.Mackey AC, Green L, Greene P, et al. Methylnaltrexone and gastrointestinal perforation. J Pain Symptom Manage. 2010;40:e1–e3. doi: 10.1016/j.jpainsymman.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 116.Neumann TA, Van Patischen H, Marcantonio A, et al. Evaluation of single oral doses of Nktr118 (Peg-Naloxol) as a peripheral opioid antagonist (Poa): a double-blind placebo-controlled study in healthy male subjects. J Clin Pharmacol. 2007;47:1210. [Google Scholar]
- 117.Faassen F, Vogel G, Spanings H, et al. Caco-2 permeability, P-glycoprotein transport ratios and brain penetration of heterocyclic drugs. Int J Pharm. 2003;263:113–122. doi: 10.1016/s0378-5173(03)00372-7. [DOI] [PubMed] [Google Scholar]
- 118.Webster L, Dhar S, Eldon M, et al. A phase 2, double-blind, randomized, placebo-controlled, dose-escalation study to evaluate the efficacy, safety, and tolerability of naloxegol in patients with opioid-induced constipation. Pain. 2013;154:1542–1550. doi: 10.1016/j.pain.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 119.Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387–2396. doi: 10.1056/NEJMoa1310246. [DOI] [PubMed] [Google Scholar]
- 120.Coyne KS, Poon JL, Thompson C, et al. Translating clinical findings into the patient's perspective: post-hoc pooled analysis of bowel movement changes as a predictor of improvement in patients' opioid-induced constipation symptoms and outcomes. Clin Ther. 2016 Dec 6; doi: 10.1016/j.clinthera.2016.11.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 121.Lawson R, Ryan J, King F, et al. Cost effectiveness of naloxegol for opioid-induced constipation in the UK. Pharmacoeconomics. 2017;35:225–235. doi: 10.1007/s40273-016-0454-4. [DOI] [PubMed] [Google Scholar]
- 122.Webster L, Chey WD, Tack J, et al. Randomised clinical trial: the long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation. Aliment Pharmacol Ther. 2014;40:771–779. doi: 10.1111/apt.12899. [DOI] [PubMed] [Google Scholar]
- 123.Nelson AD, Camilleri M. Chronic opioid induced constipation in patients with non-malignant pain: challenges and opportunities. Ther Adv Gastroenterol. 2015;8:206–220. doi: 10.1177/1756283X15578608. [DOI] [PMC free article] [PubMed] [Google Scholar]


