Abstract
DiGeorge/22q11.2 Deletion Syndrome (22q11DS) is a common genetic microdeletion syndrome that underlies several neurodevelopmental disorders including autism, attention deficit/hyperactivity disorder, and schizophrenia. In addition to cognitive impairments, those with 22q11DS have disrupted feeding and swallowing from birth onward. This perinatal dysphagia significantly compromises nutritional status, impairs appropriate weight gain, and can lead to life threatening aspiration-based infections. Appropriately timed excitation and inhibition of brainstem hypoglossal motor neurons, which innervate tongue muscles, is essential for proper feeding and swallowing. In this study we have examined changes in hypoglossal motor neuron function in the LgDel mouse model of 22q11DS. Hypoglossal motor neurons from LgDel mouse pups have action potentials with afterhyperpolarizations, mediated by a large conductance charybdotoxin-sensitive Ca activated K current, that are significantly shorter in duration and greater in magnitude than those in wild type pups. In addition, the amplitude, but not frequency, of glutamatergic EPSCs is diminished, and GABAergic, but not glycinergic, neurotransmission to hypoglossal motor neurons was reduced in LgDel animals. These observations provide a foundation for understanding the neurological changes in hypoglossal motor neuron function and their contribution to swallowing abnormalities that occur in DiGeorge/22q11.2 Deletion Syndrome.
Keywords: pediatric dysphagia, hypoglossal, brainstem circuitry, 22q11.2 Deletion/DiGeorge Syndrome
Introduction
Dysphagia, difficulties with feeding and swallowing, is a major complication in infants with a broad range of neurodevelopmental disorders (LaMantia et al., 2016). There is a substantially increased frequency of perinatal and pediatric dysphagia associated with DiGeorge/22q11.2 Deletion Syndrome (22q11DS), a common genetic microdeletion syndrome that includes autism, attention deficit/hyperactivity disorder, and schizophrenia. Disrupted feeding and swallowing from birth onward significantly compromises nutritional status, impairs appropriate weight gain, and can lead to life threatening aspiration-based infections of the nasal sinuses, middle ears, and lung in 22q11DS (LaMantia et al., 2016). Unfortunately, however, there are few, if any, therapeutic approaches to alleviate swallowing difficulties and the accompanying significant health problems. Discovery of potential new treatments for dysphagia in 22q11DS are hindered by the paucity of knowledge on the developmental, neuronal or circuit anomalies that contribute to feeding and swallowing dysfunction.
Feeding and swallowing require a complex series of movements that depends upon sequential activation of proximal motor neurons followed by inhibition of more distal motor neurons to ensure appropriately timed, unidirectional food ingestion and movement. Hypoglossal motor neurons, which innervate tongue muscles, are essential for proper sucking, mastication and deglutition/swallowing. These motor neurons receive both excitatory and inhibitory inputs that normally insure their activity is appropriately timed for tongue movements essential in feeding and swallowing. We have demonstrated that the patterning of the hindbrain, including regions that may contribute to the hypoglossal nucleus, and its inputs as well as the hypoglossal nerve are compromised during early development. These changes could result in divergent neuronal specification leading to altered intrinsic excitable properties or circuit modulation of hypoglossal neurons and the broader feeding and swallowing network.
We have examined changes in hypoglossal motor neuron function in the LgDel mouse model of 22q11DS. Infant mice with a heterozygous deletion of 28 contiguous genes, orthologous to the minimal critical deletion in human 22q11DS, apparently have feeding and swallowing difficulties. These include reduced weight compared to age and sex matched wild type (WT) littermates from shortly after birth through early maturity, delayed milk emptying from mouth to esophagus, and aspiration of milk into the nasal pharynx and lungs (LaMantia et al., 2016). Thus, we compared the electrophysiological properties, synaptic neurotransmission and activation of postsynaptic receptors in hypoglossal motor neurons in LgDel and WT mouse pups to determine whether compromised patterning and early cranial nerve development is reflected in altered excitability in hypoglossal motor neurons or neurotransmission to these neurons.
Experimental Procedures
All animal experimental procedures were performed in accordance with NIH and Institutional Animal Care and Use Guidelines. A total of 105 animals (64 wt, 41LD) were used to study hypoglossal motor neuron intrinsic firing characteristics (41 mice), the effect of Ca2+-dependent potassium channel antagonists on after-hyperpolarization potentials (27 mice), as well as spontaneous and miniature inhibitory and excitatory postsynaptic currents (54 mice). Only one neuron was recorded from each animal.
The minimal critical deletion associated with 22q11DS, a 1.5 MB heterozygous deletion on human Chr. 22, is paralleled in LgDel mice by an orthologous deletion on murine Chr. 16 (Maynard et al., 2013). Heterozygous adult LgDel males(C57BL6N background) were bred to C57BL6N WT females Thus, the deletion is inherited paternally in all mice analyzed. The day of birth was noted as postnatal day 0 (P0) based on the presence of a litter seen during a daily check of breeding cages. P7-14 pups were selected for electrophysiological experiments in a double-blinded manner. After experiments were performed and data were analyzed the identities of each animal was revealed based upon PCR genotyping (Meechan et al., 2009).
Acute brainstem slice preparation and electrophysiology patch clamp techniques
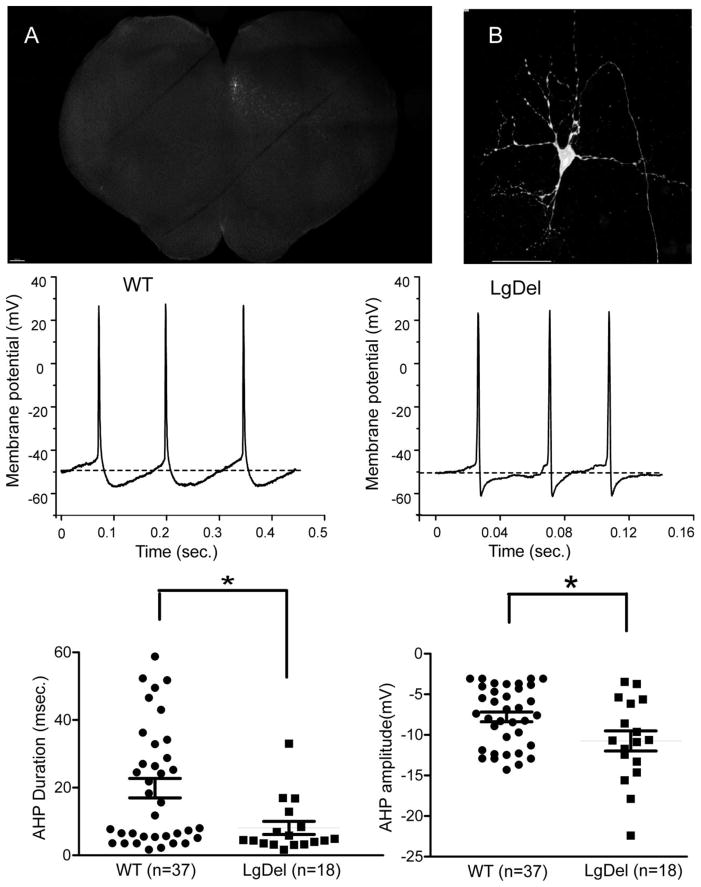
On the day of the experiment, pups were anesthetized with the short acting inhalation anesthetic isoflurane and sacrificed by cervical dislocation. The brains were quickly removed, and placed in cold (2°C) buffer (in mM): NaCl 140, KCl 5, CaCl2 2, D-Glucose 5, HEPES 10, pH 7.4, equilibrated with 100% O2. Slices were prepared by vibratome sectioning. The brains were oriented for sectioning with their caudal ends up and their rostral sides attached to an agar block perpendicular to the plane of the blade. The hypoglossal motor neurons in the dorsomedial brainstem were identified in the in vitro slice by their unique location and morphology in a transverse 350 microns thick section (as described previously (Wang et al., 2002); see also Figure 1).
Figure 1. The after-hyperpolarization potential (AHP) following action potential firing of hypoglossal neurons was significantly altered in LgDel P7-P14 mouse pups.
A brainstem slice with a single patched, biocytin filled neuron (arrow) within the hypoglossal nucleus is displayed in figure 1A and 1B. Figure 1B shows the biocytin-labeled patched hypoglossal neuron, with a large soma, multiple dorso-ventral oriented dendrites, and a genual axon characteristic of these cranial motor neurons. The middle two traces show typical examples of hypoglossal motor neurons (XII MNs) spontaneous firing spikes in wild type (left) and LgDel mice (right), the dashed line defining the resting Vm. In current clamp configuration XII MNs in LgDel mice possess a larger AHP amplitude but a shorter duration of AHP than wild type mice. Bottom scatter plots present statistic data. *p≤0.05. **p≤0.01, ***p≤0.001 for this and all following graphs. Scale bar: 200μm (fig. 1A), 20μm (fig. 1B).
Cut slices were then moved from the bath solution and incubated in the NMDG recovery solution (in mM) NMDG 93, HCl 93, KCl 2.5, NaH2PO4 1.2, NaHCO3 25, HEPES 20, D-Glucose 25, MgSO410, CaCl2 0.5 bubbled with 95% O2/5% CO2 at 34 °C in a water bath for 10 minutes. The brainstem slices were then moved to a recording chamber and perfused with standard aCSF solution contained (in mM) NaCl 125, KCl 3, NaHCO3 25, HEPES 5, D-glucose 5, MgSO4 1, CaCl2 2 and continuously bubbled with 95% O2/5% CO2 to maintain pH at 7.4 at room temperature (22–24°C). The electrodes were pulled from thin wall glass capillaries (World Precision Instruments, Inc. FL. USA) with a tip resistance of ~3–4 MΩ. Patch pipettes were filled with a pH 7.3 solution of either KCl (150 mM), MgCl2 (4 mM), EGTA (10 mM), Na-ATP (2 mM), HEPES (10 mM) or K-gluconic acid (150 mM), HEPES (10 mM), EGTA (10 mM), MgCl2 (1 mM), CaCl2 (1 mM) to isolate inhibitory or excitatory currents, respectively. Identified hypoglossal motor neurons were voltage-clamped at a holding potential of −80 mV to examine synaptic events. Gabazine (25 μM) was used to block GABAergic inhibitory neurotransmission, strychnine (1 μM) to block glycinergic inhibitory neurotransmission, and D(-)-2amino-5-phosphopentanoic acid (AP5; 20 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μM) to block glutamatergic excitatory neurotransmission in appropriate combinations to isolate glutamatergic EPSCs, GABAergic and/or glycinergic IPSCs. Focal drug application was performed using a PV830 Pneumatic PicoPump pressure delivery system (WPI, Sarasota, FL). Drugs were ejected from a patch pipette positioned within 30μm from the patched hypoglossal motor neurons. The maximum range of drug application has been previously determined to 100–120 μm from the tip the drug pipette forward, and considerably less behind the drug pipette (Wang et al., 2001). To study the effects of Ca activated potassium channel antagonists on the AHP, apamin (at doses of 100nM and 200nM) and charydbotoxin (at concentrations of 1nM, 10 nM, and 40nM) were added to the perfusate 15 mins after baseline measurements were recorded and were maintained in the perfusate for an additional 15 mins.
Visualization of XII MNs by biocytin injection
To visualize and confirm the identity of the recorded hypoglossal motor neurons (XII MNs), we added 0.5% biocytin into the patch pipette solution. At the end of each electrophysiology experiment the brainstem slices were soaked overnight in 4% paraformaldehyde and were then washed with PBS and incubated in streptavidin Alexa Fluor@647 in PBS containing 0.1% Triton X-100. Slices were mounted and cover slipped with prolong anti-fade mounting medium (Invitrogen, Eugene, OR). The brainstem slices containing biocytin-injected XII MNs were imaged by confocal microscopy (Zeiss LSM 710, Thornwood, NY, USA). Serial optical sections from the entire column of the slice were collected in combination with tile scans using a 25x oil objective in a Zeiss 710 confocal system. Imaris software was used to process the image data.
Data analyses
All electrophysiological data were collected and digitized via Clampex (10.2) and analyzed using Clampfit (10.2). Synaptic events were detected using MiniAnalysis version6.0.7 (Synaptosoft, Decatur, GA). All data have been tested for and found to be within a normal distribution with equal variances using frequency distribution and Bartlett’s corrected statistic test, respectively. All data are presented as mean ± SEM. Statistical comparisons were made using unpaired Student’s t tests for the WT and LgDel two group data comparisons, one-way ANOVA with repeated measures and Dunnett’s post-tests for Ca2+ dependent potassium channel experiments. P < 0.05 indicated significant differences. Software used for statistics was Graphpad Prism 5.0 (Graphpad Software, San Diego, CA), Microcal Origin 6.1 (OriginLabs Corp., Northhampton, MA), and Microsoft Excel (Microsoft Corp., Redmond, WA).
Results
We confirmed the identity of each recorded neuron in each slice by assessing its position within the hypoglossal nucleus (figure 1A). In these hypoglossal motor neurons, there were no significant differences in the resting membrane potential, action potential threshold or duration, membrane resistance, or membrane capacitance of the hypoglossal motor neurons between wild type (WT) mice and their LgDel siblings (table 1). Nevertheless, the after-hyperpolarization potential (AHP) that followed each action potential in hypoglossal neurons was significantly different in LgDel versus WT siblings. There is a significantly shorter AHP duration (8.1 ±1.9 msec. vs 21.2 ± 3.4 msec, p<0.05) which is also larger in amplitude in LgDel hypoglossal motor neurons (−11 ± 0.9mV vs −8.1 ± 0.7mV WT, p<0.05), compared to WT siblings (figure 1).
Table 1.
Hypoglossal motor neurons intrinsic firing characteristics in WT and LgDel mice
| RMP (mV) | AP threshold (mV) | AP duration (msec.) | Rm (GΩ) | Cm (pF/cm2) | |
|---|---|---|---|---|---|
| WT | −58.7±1.6 (n=37) | −52.3±1.9 (n=37) | 5.5±0.3 (n=37) | 123.3±16 (n=26) | 3.39±0.3 (n=24) |
| LgDel | −57.8±2 (n=18) | −50.3±2 (n=18) | 4.4±0.7 (n=18) | 100.2±18 (n=18) | 4.22±0.5 (n=15) |
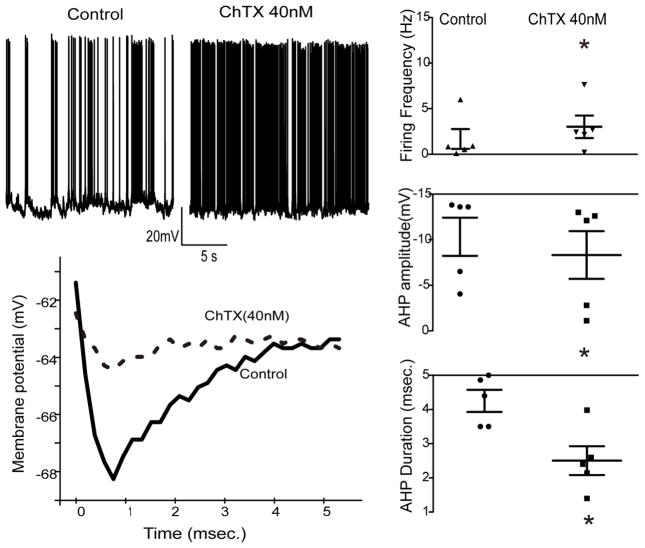
To further identify mechanisms that could be responsible for the altered AHP in hypoglossal motor neurons in LgDel animals we examined the changes in the AHP and firing frequency in these neurons upon application of different Ca2+-dependent potassium channel blockers apamin and charydbotoxin (ChTX). Under control conditions there was no significant difference (p>0.05) in spontaneous firing frequency in LgDel compared to WT animals. Apamin, at a concentration of either 100 or 200nM, had no effect on the AHP amplitude or duration in either LgDel or WT animals. In contrast, ChTX (40 nM), an antagonist for the high conductance Ca2+ activated (BK-type) channel significantly (p<0.05) increased the firing frequency of LgDel hypoglossal motor neurons but did not alter the AHP or firing rate of WT hypoglossal motor neurons (figure 2). In LgDel animals ChTX nearly doubled the firing frequency from 1.7 ± 1.1 Hz to 3.1 ± 1.2 Hz (p<0.05; figure 2, top traces). ChTX significantly (p<0.05) reduced the hypoglossal neuron AHP amplitude in LgDel animals (from −10.1 ± 2.1 mV to −8.3 ± 2.5 mV with ChTX), and significantly (p<0.05) shortened the AHP duration (from 4.3 ± 0.3 msec to 2.5 ± 0.4 msec with ChTX, figure 2, bottom trace).
Figure 2. The Ca2+-activated K+ channel antagonist charydbotoxin significantly increased hypoglossal motor neuron firing frequency in LgDel P7-P17 pups.
The XII MNs firing frequency was enhanced in LgDel mice after application of ChTX at the dose of 40nM (top trace). Charydbotoxin also reduced the hypoglossal neuron AHP amplitude and the AHP duration, bottom panel. Scatter plots graphs show all original data with statistic results.
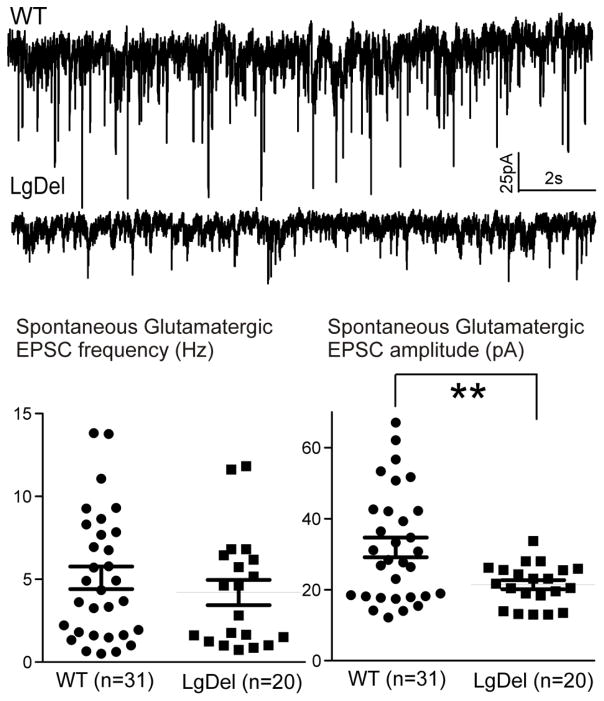
In addition to changes in the action potential characteristics in P7-14 LgDel hypoglossal motor neurons, we also evaluated differences in synaptic neurotransmission to LgDel versus WT hypoglossal neurons. There is no difference in the frequency of spontaneous excitatory glutamatergic postsynaptic currents (EPSCs) in hypoglossal neurons between LgDel and WT siblings. The amplitudes of EPSCs, however, are significantly (p<0.05) diminished in LgDel compared to WT siblings (LgDel 23 ± 1pA, WT 34 ± 3pA; figure 3). To determine whether this blunted excitation was likely due to a presynaptic or postsynaptic alteration we analyzed action potential-independent release of glutamate by recording miniature EPSCs (mEPSCs) in the presence of tetrodotoxin (TTX, 1 microM). In the presence of TTX there were no significant differences in mEPSC frequency or mEPSC amplitude between LgDel and WT siblings, indicating a potential site of dysfunction in the excitatory neurotransmission to hypoglossal neurons is likely changes in action potential dependent function in the preceding glutamatergic neuron, or neurons even further upstream from the hypoglossal motor neurons.
Figure 3. Glutamatergic EPSCs amplitude of XII MNs is diminished in LgDel P7-P17 pups.
The two representative traces show the amplitude of XII MNs EPSCs is diminished in LgDel mice compared to wild type sibling pups. Bottom scatter plots present original data with statistic results for glutamatergic EPSCs frequency (left) and amplitude (right).
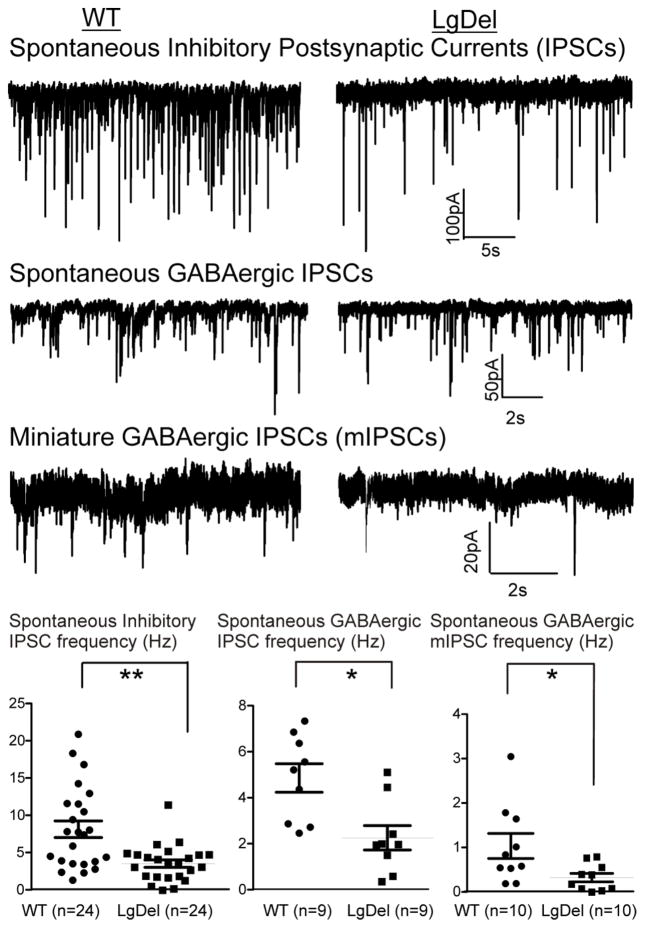
We next asked if inhibitory neurotransmission to hypoglossal motor neurons is disrupted, perhaps suggesting a change in the excitatory/inhibitory (E/I) balance in the circuit. The frequency, but not amplitude, of spontaneous inhibitory postsynaptic currents (IPSCs) in hypoglossal neurons is significantly decreased in LgDel hypoglossal neurons compared to WT (LgDel 4.4 ± 0.5 Hz, WT 8.1 ± 1.1 Hz, p<0.05; figure 4). We then distinguished whether one or both of the main inhibitory neurotransmitters used by brainstem interneurons were compromised. This attenuation of spontaneous inhibitory neurotransmission to hypoglossal motor neurons in LgDel P7-8 pups is due to a significant reduction of GABAergic IPSC frequency (2.6 ± 0.5Hz in LgDel compared to 5.5 ± 0.8 Hz in WT, p<0.05). In contrast, glycinergic IPSC frequency was not significantly different (p>0.05) in LgDel and WT siblings when examined independently. GABAergic mIPSC frequency in the presence of TTX was also diminished in LgDel versus WT siblings (LgDel 0.33 ± 0.1Hz, WT 1.0 ± 0.2 Hz, p=0.04; figure 4). There were no significant differences in the amplitudes of GABAergic IPSCs or mIPSCs in LgDel versus WT siblings.
Figure 4. The inhibitory postsynaptic currents (IPSCs) and GABAergic neurotransmission to the XII MNs are reduced in LgDel P7-P17 pups.
Typical examples are displayed the decrease in IPSCs frequency (top two traces, WT:left trace, LgDel: right trace), spontaneous GABAergic IPSCs frequency (middle traces), as well as miniature GABAergic IPSCs (bottom traces) of XII MNs in the LgDel mice. The IPSCs frequency is significantly (p=0.0049) lower in LgDel compared to WT mice. GABAergic IPSC frequency (middle traces), as well as GABA mIPSC frequency (lower traces) is also significantly diminished in 22q11DS mice compared to wild type siblings (p=0.01 and p=0.04, respectively). Bottom scatter plots showing statistical comparisons between WT and LgDel animals for the frequency of spontaneous IPSCs, GABAergic IPSCs as well as GABAergic miniature IPSCs.
Discussion
There are 3 major results from this study. First, the AHP is significantly shorter in duration and greater in magnitude in LgDel animals. Nevertheless, LgDel hypoglossal motor neurons have action potentials that are indistinguishable from WT littermates with the exception of the altered AHP. The AHP is diminished by charybdotoxin (ChTX), an antagonist for the high conductance Ca2+ activated (BK-type) channel in LgDel, but not WT siblings. Second, the amplitude of glutamatergic EPSCs is diminished, but the frequency of spontaneous excitatory glutamatergic neurotransmission to hypoglossal motor neurons does not differ in LgDel and WT siblings. In the presence of TTX, which isolates action potential-independent spontaneous glutamate release, there were no significant differences in mEPSC frequency or mEPSC amplitude. This indicates the most likely site of dysfunction in the excitatory neurotransmission to hypoglossal neurons is diminished action potential dependent function in the preceding glutamatergic neurons, or neurons even further upstream from the hypoglossal motor neurons. Third, GABAergic, but not glycinergic, neurotransmission to hypoglossal motor neurons was reduced, with reductions in both GABAergic IPSC and GABAergic mIPSC frequency. The blunted frequency of both GABAergic IPSCs and mIPSCs in hypoglossal neurons is indicative of presynaptic GABA neuron dysfunction or circuit disruption in LgDel animals.
The action potential properties, such as the AHP, in WT animals, as well as the excitatory and inhibitory neurotransmission in hypoglossal motor neurons we recorded are in general agreement with prior results from other groups (Viana et al., 1993; Sawczuk et al., 1997; Singer and Berger, 1999; Lape and Nistri, 2001; Horn and Solomon, 2014). Our results suggest that in the LgDel mouse model of 22q11DS, in contrast to WT a major substrate for dysfunction in the control of tongue muscles likely reflects altered hypoglossal motor neuron excitability, in particular the synaptic input to hypoglossal motor neurons and postsynaptic calcium dependent potassium currents intrinsic to hypoglossal motor neuron that manifest as changes in the AHP. There are two major subtypes of Ca dependent K channels; a small conductance (SK) channels that can be inhibited by apamin, and a large conductance (BK) charydbotoxin-sensitive channel. We show that ChTX, but not apamin, influence the AHP in LgDel pups. Apparently, large conductance Ca dependent K channels are most affected in LgDel siblings. BK channels are both voltage- and Ca- gated (Horn and Solomon, 2014). Our current data, however, does not distinguish whether the change in the AHP and function of BK channels in LgDel hypoglossal neurons is due to altered Ca sensitivity and/or voltage-dependent properties. Similar to the consequences of heterozygous 22q11 gene deletion we have found, other work has shown maternal nicotine exposure increases the excitability of hypoglossal motor neurons and also significantly increases transient and sustained potassium currents in these cells (Cholanian et al., 2017). A reduction in the duration of AHPs in LgDel animals might lead to an increased risk of exaggerated firing at the start of the reflex swallowing process when hypoglossal motor neurons are activated, whereas the larger amplitude of AHPs in LgDel animals might cause a larger accumulation of Ca and a blunted firing upon sustained activation, such as at the termination of prolonged swallowing, perhaps leading to an increased risk of aspirations. However these hypotheses need to be tested more directly, such as with in-vivo assessments of tongue muscle movements during swallowing in LgDel animals.
Our work also indicates both excitatory glutamatergic, as well as inhibitory GABAergic neurotransmission to hypoglossal motor neurons are altered in LgDel animals. Although the origins of GABAergic and glutamatergic synaptic inputs were not identified in this study, previous work by others has provided some potential sources of origin for these inputs (Ugolini, 1995). Neurons in the Nucleus of Roller may be the source of GABAergic neurotransmission to hypoglossal motor neurons, but this has not yet been tested directly (van Brederode et al., 2011). There is a glutamatergic input to hypoglossal neurons from the raphe pallidus which is primarily mediated by both NMDA and non-NMDA glutamate receptors (Bouryi and Lewis, 2003). Additional sources of glutamatergic input to hypoglossal neurons include the nucleus reticularis and nucleus tractus solitarius (NTS) (Fregosi and Ludlow, 2014). As the NTS receives sensory information involved in swallowing and feeding it is reasonable to postulate that the swallowing dysfunction in LgDel animals likely involves changes in the reflex and functional network circuit connection between glutamatergic NTS neurons and hypoglossal neurons. It is likely that the consequences of the reduced amplitude of excitatory EPSCs, in combination with decreased frequency of GABAergic inhibitory neurotransmission, leads to less phasic activity and reduced rhythmic reflex activity in hypoglossal motor neurons in LgDel animals upon swallowing during neuronal network activation.
In conclusion, several excitable properties of hypoglossal motor neurons are distinct in LgDel animals, a model of 22q11DS. LgDel mouse pups have hypoglossal motor neuron action potentials similar to WT littermates; however, afterhyperpolarization (AHP), mediated by a large conductance charybdotoxin-sensitive Ca activated K current, is significantly shorter in duration and greater in magnitude in LgDel animals. The amplitude, but not frequency, of glutamatergic EPSCs are diminished in LgDel siblings, and this dysfunction likely occurs due to diminished action potential dependent function in preceding glutamatergic neurons.
GABAergic, but not glycinergic, neurotransmission to hypoglossal motor neurons was reduced, and suggests presynaptic GABA neuron and circuits dysfunction in LgDel animals. Currently, it is known that the migration and final position of sub-populations of GABAergic interneurons is disrupted in the cerebral cortex of LgDel mice (Meechan et al., 2012). Similar disruptions in specification, differentiation and function of GABAergic interneurons, if present throughout the CNS due to diminished 22q11 gene dosage, may change the E/I balance of brainstem circuits. There is some indication that altered development of GABAergic circuitry due to mutations in key GABA synthetic or metabolic genes can influence hypoglossal neuronal differentiation and function (Fogarty et al., 2017). While it would be premature to attempt to target new therapies for swallowing difficulties in 22Q11DS patients based on these results, our work provides a foundation for understanding the neurological changes in hypoglossal motor neuron function and swallowing abnormalities that occur in this disease.
Highlights.
Hypoglossal neurons from LgDel animals have shorter duration and larger amplitude afterhyperpolarizations.
Glutamate amplitudes in hypoglossal neurons are diminished in LgDel siblings.
GABAergic events are less frequent in hypoglossal motor neurons in LgDel animals.
Acknowledgments
Grant support: NIH P01 HD 083157
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bouryi VA, Lewis DI. The modulation by 5-ht of glutamatergic inputs from the raphe pallidus to rat hypoglossal motoneurones, in vitro. J Physiol. 2003;553:1019–1031. doi: 10.1113/jphysiol.2003.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholanian M, Wealing J, Levine RB, Fregosi RF. Developmental nicotine exposure alters potassium currents in hypoglossal motoneurons of neonatal rat. J Neurophysiol. 2017 doi: 10.1152/jn.00774.2016. jn 00774 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Kanjhan R, Yanagawa Y, Noakes PG, Bellingham MC. Alterations in hypoglossal motor neurons due to gad67 and vgat deficiency in mice. Exp Neurol. 2017;289:117–127. doi: 10.1016/j.expneurol.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Ludlow CL. Activation of upper airway muscles during breathing and swallowing. J Appl Physiol (1985) 2014;116:291–301. doi: 10.1152/japplphysiol.00670.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn KG, Solomon IC. Effects of calcium (ca(2+)) extrusion mechanisms on electrophysiological properties in a hypoglossal motoneuron: Insight from a mathematical model. Progress in brain research. 2014;212:77–97. doi: 10.1016/B978-0-444-63488-7.00005-7. [DOI] [PubMed] [Google Scholar]
- LaMantia AS, Moody SA, Maynard TM, Karpinski BA, Zohn IE, Mendelowitz D, Lee NH, Popratiloff A. Hard to swallow: Developmental biological insights into pediatric dysphagia. Dev Biol. 2016;409:329–342. doi: 10.1016/j.ydbio.2015.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Nistri A. Characteristics of fast na(+) current of hypoglossal motoneurons in a rat brainstem slice preparation. Eur J Neurosci. 2001;13:763–772. doi: 10.1046/j.0953-816x.2000.01433.x. [DOI] [PubMed] [Google Scholar]
- Maynard TM, Gopalakrishna D, Meechan DW, Paronett EM, Newbern JM, LaMantia AS. 22q11 gene dosage establishes an adaptive range for sonic hedgehog and retinoic acid signaling during early development. Hum Mol Genet. 2013;22:300–312. doi: 10.1093/hmg/dds429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Cxcr4 regulation of interneuron migration is disrupted in 22q11.2 deletion syndrome. Proc Natl Acad Sci U S A. 2012;109:18601–18606. doi: 10.1073/pnas.1211507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/digeorge syndrome. Proc Natl Acad Sci U S A. 2009;106:16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Contribution of outward currents to spike-frequency adaptation in hypoglossal motoneurons of the rat. J Neurophysiol. 1997;78:2246–2253. doi: 10.1152/jn.1997.78.5.2246. [DOI] [PubMed] [Google Scholar]
- Singer JH, Berger AJ. Contribution of single-channel properties to the time course and amplitude variance of quantal glycine currents recorded in rat motoneurons. J Neurophysiol. 1999;81:1608–1616. doi: 10.1152/jn.1999.81.4.1608. [DOI] [PubMed] [Google Scholar]
- Ugolini G. Specificity of rabies virus as a transneuronal tracer of motor networks: Transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J Comp Neurol. 1995;356:457–480. doi: 10.1002/cne.903560312. [DOI] [PubMed] [Google Scholar]
- van Brederode JF, Yanagawa Y, Berger AJ. Gad67-gfp+ neurons in the nucleus of roller: A possible source of inhibitory input to hypoglossal motoneurons. I. Morphology and firing properties. J Neurophysiol. 2011;105:235–248. doi: 10.1152/jn.00493.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol. 1993;69:2150–2163. doi: 10.1152/jn.1993.69.6.2150. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann N Y Acad Sci. 2001;940:237–246. doi: 10.1111/j.1749-6632.2001.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Venkatesan P, Evans C, Baxi S, Mendelowitz D. Synaptic activation of hypoglossal respiratory motorneurons during inspiration in rats. Neurosci Lett. 2002;332:195–199. doi: 10.1016/s0304-3940(02)00957-6. [DOI] [PubMed] [Google Scholar]