Abstract
Purpose
To investigate longitudinal temporal and spatial associations between disc hemorrhage (DH) and rates of local retinal nerve fiber layer (RNFL) thinning before and after DHs.
Design
Longitudinal observational cohort study.
Participants
Forty eyes of 37 participants (23 with glaucoma and 17 with suspect glaucoma at baseline) with DH episodes during follow-up from the Diagnostic Innovations in Glaucoma Study and the African Descent and Glaucoma Evaluation Study.
Methods
All subjects underwent optic disc photography annually and spectral-domain optical coherence tomography (OCT) RNFL thickness measurements every 6 months. The rates of RNFL thinning were calculated using multivariate linear mixed-effects models before and after DH.
Main Outcome Measures
Rates of global and local RNFL thinning.
Results
Thirty-six eyes of 33 participants with inferior or superior DHs were analyzed. The rates of RNFL thinning were significantly faster in DH quadrants than non-DH quadrants after DH (−2.25 and −0.69 μm/year, P < 0.001). In the 18 eyes with intensified treatment after DH, the mean rate of RNFL thinning significantly slowed after treatment compared to before treatment in the non-DH quadrants (−2.89 and −0.31μm/year, P < 0.001), but not in the DH quadrants (−2.64 and −2.12 μm/year, P = 0.19). In 18 eyes with unchanged treatment, the rate of RNFL thinning in DH quadrant was much faster after DH than before DH (P = 0.008). Moreover, compared to eyes without a treatment change, intensification of glaucoma treatment after DH significantly reduced the global, non-DH quadrants and DH quadrant rates of RNFL thinning after DH compared to before DH (global, P = 0.004; non-DH quadrant, P < 0.001; DH quadrant, P = 0.005). In the multiple linear regression analysis, treatment intensification (β, 1.007; P = 0.005), visual field MD (β, 0.066; P = 0.049), and difference in IOP before and after DH (β, −0.176; P = 0.034) were associated significantly with the difference of global RNFL slope values before and after DH.
Conclusions
Although there is a worsening of the rate of RNFL thinning in a DH quadrant after DH, glaucoma treatment intensification may have a beneficial effect in reducing this rate of thinning.
INTRODUCTION
Glaucoma is characterized by progressive loss of retinal ganglion cells (RGCs) and associated morphological changes to the optic nerve and retinal nerve fiber layer (RNFL).1 Disc hemorrhage (DH) is well known as an important risk factor for the development and progression of glaucoma.2–6 Previous studies have shown the association between DH and glaucoma progression in function4–6 and structure.7–11 The location of DH is also known to relate spatially to notches in the neuroretinal rim,12 progressive localized thinning of the RNFL,13 and focal visual field progression.2, 5, 14, 15 However, there has been less detailed information regarding the longitudinal temporal and spatial association between DH and glaucoma progression.
It has been reported previously that DH might be a result of progressive structural damage using visual field testing5 or optic disc photographs.10 However, it has not been clarified whether DH is only consequent to glaucoma progression or whether it could accelerate subsequent glaucoma progression. The ability to image the RNFL quantitatively provides an opportunity to investigate this.
Recent spectral-domain (SD-) optical coherence tomography (OCT) technology enables detection of local progressive RNFL thinning with high sensitivity and reproducibility.16–18 Longitudinal reports have evaluated glaucoma progression retrospectively after DH using time-domain (TD-) OCT or SD-OCT, but not before DH.7–9 It has been observed that the progressive RNFL thinning after DH was associated with the location of DH compared to the respective contralateral quadrants or the contralateral eyes without DH. However, the relationship between structural damage and subsequent DH still is not well understood.
DH is often used as an indicator for additional intraocular pressure lowering, and Medeiros et al.6 previously showed that intraocular pressure (IOP) lowering was associated with the reduction of rate of visual field progression after DH. Their results suggest that the influence of glaucoma treatments on glaucoma progression should also be considered in investigating glaucoma progression related to DH. In this study, we therefore investigated the differences in rates of local RNFL thinning before and after DH and the influence of glaucoma treatment after DH on these rates.
Methods
Participants
Participants were enrolled in the longitudinal Diagnostic Innovations in Glaucoma Study (DIGS) and the African Descent and Glaucoma Evaluation Study (ADAGES). DIGS is conducted at the Hamilton Glaucoma Center at the University of California, San Diego (UCSD) and ADAGES is a multicenter study conducted at UCSD, the University of Alabama at Birmingham, and the New York Eye and Ear Infirmary. The protocols of the two studies are identical, and the methodological details have been described previously.19
All patients from the DIGS and ADAGES who met the inclusion criteria described below were enrolled in the present study. Informed consent was obtained from all participants. This prospectively designed study received institutional review board approval at each of the involved sites. The methodology adhered to the tenets of the Declaration of Helsinki and to the Health Insurance Portability and Accountability Act.
Eligible participants had best-corrected visual acuity of 20/40 or better, spherical refraction within ±5.0 diopters (D), cylinder correction within ±3.0 D, and open angles on gonioscopy at study entry. Participants were excluded if they had a history of intraocular surgery (except for uncomplicated cataract or glaucoma surgery). Eyes with coexisting retinal disease, uveitis, or nonglaucomatous optic neuropathy were excluded from the investigation. Normal participants were excluded from this study. All participants who had at least 1 DH during follow-up and underwent at least 2 OCT examinations of sufficient quality both before and after the DH were analyzed in the current study. Eyes that did not have target DHs located in the inferior or superior quadrant were excluded from the analysis. When the eye had multiple DHs during OCT follow-up, the first disc hemorrhage during OCT follow-up period was determined as the target DH. If any additional DHs were observed in the contralateral quadrant of the target DH during OCT follow-up periods, the eye was excluded from the analysis.
Stereophotography
All patients had stereoscopic optic disc photographs repeated at least every 12 months during follow-up. The images were reviewed with a stereoscopic viewer (Screen-VU stereoscope; PS Manufacturing, Portland, OR) by 2 or more experienced graders masked to the subjects’ identity and to other test results. The location of the DH was classified into 4 90-degree sectors (superior, inferior, temporal, and nasal). The methodology used to grade optic disc photographs at the UCSD Optic Disc Reading Center has been provided elsewhere.19 Only photographs of adequate quality were included. Discrepancies between the 2 graders were resolved by consensus or adjudication by a third experienced grader. For this report, DHs were defined as located within 1/2 disc diameter from the optic disc border or within the RNFL as a splinter or flame-shaped hemorrhage and not associated with optic disc edema, papillitis, diabetic retinopathy, central or branch retinal vein occlusion, or any other retinal disease.20
Spectralis Spectral-Domain Optical Coherence Tomography
The RNFL thickness (RNFLT) was measured with the Spectralis SD-OCT parapapillary circle scan (software version 5.4.7.0). Details of the procedure have been described previously.17,21 Spectralis incorporates a real-time eye tracking system that couples confocal laser scanning ophthalmoscope and SD-OCT scanners to adjust for eye movements and to ensure that the same location of the retina is scanned over time. A total of 1536 A-scan points were acquired from a 3.45-mm circle centered on the optic disc. Spectralis SD-OCT images measured from April 2009 to August 2016 were included, and the RNFLT measurements were estimated in 4 sectors separated into 90-degree intervals (superior, inferior, temporal, and nasal) using the same criteria as DH on photographs. All images were processed and reviewed by the Imaging Data Evaluation and Assessment Center in the University of California, San Diego. Images with noncentered scans, inaccurate segmentation of the RNFL, or signal strength of 15 dB or less were excluded from the analysis.
Standard automated perimetry
All standard automated perimetry was performed using the 24–2 Swedish interactive thresholding algorithm (SITA) (Humphrey Field Analyzer; Carl Zeiss Meditec, Dubin, CA) strategies. Only repeatable and reliable tests (≤33% fixation losses and false-negative results and ≤15% false-positive results) were included.
Statistical Analysis
The rates of global or local RNFL thinning were compared between before and after DH globally or sectorally. Linear mixed effects modeling was used to estimate the rates of change of global or local RNFL thinning, to account for repeated measurements over time and correlations between the two eyes of an individual.17, 18, 22 A set of mixed-effects models were fit with RNFLT as the response, time as a fixed effect and random intercept and slope for each eye nested within each patient. The random slope values calculated using mixed effects modeling were compared with paired t-test between before and after DHs. We fitted models to evaluate rates of loss in global RNFL, in DH quadrants and non-DH quadrants separately. Comparisons between these groups for categorical variables were evaluated using the chi-square test. Univariate linear regression analysis and stepwise multiple linear regression analysis were performed to identify parameters associated with differences in global RNFL slope values before and after DH. All statistical analyses were performed with R version 3.3.1 (http://www.r-project.org) and SPSS Version 20 (IBM Corp., Armonk, NY). The mixed effects modeling was performed using the R package ‘lme4’. P values less than 0.05 were considered statistically significant.
Results
In 40 eyes of 37 subjects, at least one DH was detected in optic disc stereophotographs of the subjects during OCT follow-up. Table 1 shows the demographic and ophthalmic characteristics of the study participants. Four eyes of 4 subjects were excluded from the analysis, because the location of their target DHs was not inferior or superior. As a result, a total of 36 eyes of 33 participants were analyzed in this paper. Seven eyes (19%) of 7 subjects showed multiple DHs during the follow-up periods: 6 eyes had DHs only at one quadrant (inferior quadrant, 5 eyes; superior quadrant, 1 eye) and 1 eye at two different quadrants (inferior and temporal quadrants). None of the eyes required exclusion due to showing both superior and inferior DHs during the follow-up period. The location of the target DH for analysis was the inferior quadrant in 22 eyes and superior quadrant in 14 eyes.
Table 1.
Demographic and Ophthalmic Characteristics in Eyes with Inferior or Superior Optic Disc Hemorrhage during Spectraldomain Optical Coherence Tomography Follow-up (n = 40 Eyes of 37 Subjects)
| By subject (N=37) | |
| Age (years) | 66.0±11.9 |
| Sex (F/M), no | 27/10 |
| Race (white/black/asian), no. | 25/6/6 |
| By eye (N=40) | |
| Diagnosis (glaucoma/glaucoma suspect), no | 23/17 |
| IOP during OCT follow-up (mmHg) | 14.5±3.1 |
| IOP at the beginning of OCT follow-up (mmHg) | 15.0±4.2 |
| Axial length (mm) | 24.24±1.16 |
| CCT (μm) | 546.1±44.0 |
| SAP MD at DH (dB) | −3.75±5.12 |
| SAP PSD at DH (dB) | 4.38±3.39 |
| Baseline RNFL thickness (μm) | |
| Global | 80.63±14.69 |
| Inferior | 89.64±23.86 |
| Superior | 98.80±20.60 |
| Temporal | 70.43±15.65 |
| Nasal | 63.43±16.69 |
| Location of DH | |
| Inferior | 22 (55%) |
| Superior | 14 (35%) |
| Temporal | 3 (8%) |
| Nasal | 1 (3%) |
| Detected number of DH (multiple/single) | 9/31 |
| Number of glaucoma medications at the beginning of OCT follow-up | 1.9±1.1 |
CCT = central corneal thickness; DH = disc hemorrhage; MD = mean deviation; PSD = pattern standard deviation; RNFL = retinal nerve fiber layer.
Values are shown as mean ± standard deviation.
The follow-up period and number of OCT examination were similar before and after DH. The mean ± standard deviation of global RNFL thinning before and after DH was −1.28±0.98 μm/year and −1.03±0.69 μm/year, respectively, which were not significantly different (P = 0.21). The rates of RNFL thinning were significantly slower after DH in non-DH quadrants compared with before DH (−1.91±1.69 μm/year versus −0.69±1.14 μm/year, P = 0.002). No significant difference in the rates of RNFL thinning before and after DH were found in the DH quadrant (−2.01±1.49 μm/year versus −2.25±1.49 μm/year, P = 0.41).
The thirty-six eyes were divided into 2 groups according to the presence or absence of glaucoma treatment intensification upon commencement of target DH to investigate its effect on rates of RNFL thinning. The details of the groups are shown in Table 3. Treatment and management remained unchanged in 18 eyes (2.3±0.9 glaucoma medications), while 2 eyes underwent trabeculectomy, 1 eye underwent selective laser trabeculoplasty, and medications were intensified in 15 eyes after DH (number of glaucoma medications increased from 1.5±1.1 to 2.9±0.9). In both groups, the rates of RNFL thinning before DH were similar in DH and non-DH quadrants (Table 4). In the eyes receiving intensified treatment, the rate of RNFL thinning in non-DH quadrant became significantly slower after DH (Figure 1A–C). The rate of RNFL thinning in the DH quadrant was not significantly different before and after DH, but significantly faster than in the non-DH quadrant after DH. In the eyes of the unchanged treatment group, the rate of RNFL thinning in the DH quadrant was much faster after DH than before DH, while rates in the non-DH quadrant did not change significantly (Figure 1D–F).
Table 3.
Comparison of the Demographics and Test Results between Eyes with and without Treatment Intensification after Disc Hemorrhage
| Unchanged treatment (18 eyes, 18 subjects) |
Intensified treatment (18 eyes, 17 subjects) |
P | |
|---|---|---|---|
| Age (years) | 66.7 (60.4, 73.1) | 64.5 (59.0, 69.9) | 0.57* |
| Sex (F/M), no | 13/5 | 12/5 | 0.72† |
| Race (white/black/asian), no. | 12/2/4 | 13/2/2 | 0.66† |
| Diagnosis (glaucoma/glaucoma suspect), no | 10/8 | 12/6 | 0.49† |
| Axial length (mm) | 24.29 (23.75, 24.82) | 24.32 (23.69, 24.95) | 0.93* |
| CCT (μm) | 538.3 (513.5, 563.1) | 551.6 (530.7, 572.6) | 0.39* |
| SAP MD at DH (dB | −3.54 (−5.39, −1.68) | −4.24 (−7.56, −0.91) | 0.70* |
| SAP PSD at DH (dB) | 4.31 (2.42, 6.21) | 4.67 (3.04, 6.31) | 0.76* |
| Baseline RNFL thickness (μm) | |||
| Global | 79.6 (72.8, 86.3) | 81.3 (72.8, 89.7) | 0.74* |
| Inferior | 89.9 (77.1, 102.7) | 87.0 (75.4, 98.6) | 0.72* |
| Superior | 98.3 (90.6, 106.0) | 100.4 (87.1, 113.7) | 0.78* |
| Temporal | 68.9 (61.7, 76.1) | 73.6 (65.6, 81.5) | 0.37* |
| Nasal | 60.7 (52.9, 68.4) | 64.2 (55.2, 73.3) | 0.53* |
| IOP at entry of the prospective study (mmHg) | 18.1 (15.2, 20.9) | 17.6 (14.5, 20.7) | 0.83* |
| Maximum IOP during the prospective study (mmHg) | 22.9 (19.4, 26.5) | 21.0 (18.2, 23.8) | 0.37* |
| Mean IOP before DH (mmHg) | 14.0 (12.3, 15.8) | 16.0 (14.0, 18.0) | 0.13* |
| Mean IOP after DH (mmHg) | 13.4 (12.1, 14.7) | 14.6 (13.4, 15.7) | 0.17* |
| Number of glaucoma medications at entry of the prospective study | 0.9 (0.4, 1.4) | 1.1 (0.5, 1.6) | 0.62* |
| Number of glaucoma medications before DH | 2.3 (1.8, 2.7) | 1.8 (1.2, 2.4) | 0.17* |
| Number of glaucoma medications after DH | 2.3 (1.8, 2.7) | 2.9 (2.4, 3.4) | 0.041* |
CCT = central corneal thickness; DH = disc hemorrhage; IOP = intraocular pressure; MD = mean deviation; PSD = pattern standard deviation; RNFL = retinal nerve fiber layer.
Values are shown as mean (95% Confidence Interval) unless otherwise indicated.
Statistically significant values are shown in bold.
Comparison was performed using unpaired t-test* or Chi-square test†.
Table 4.
Influence of Intensified Treatment after disc hemorrhages on Rates of Retinal Nerve Fiber Layer Thinning in the Eyes with Superior or Inferior Disc Hemorrhage
| Unchanged treatment (n = 18 eyes) | Intensified treatment (n = 18 eyes) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Before DH | After DH | P* | Before DH | After DH | P* | |
| Mean IOP (mmHg) | 14.0 (12.3, 15.8) | 13.4 (12.1, 14.7) | 0.21 | 16.0 (14.0, 18.0) | 14.6 (14.0, 15.7) | 0.020 |
| Rate of RNFL thinning (μm/year) | ||||||
| Global | −0.83 (−1.34, −0.31) | −1.11 (−1.38, −0.84) | 0.29 | −1.73 (−2.06, −1.39) | −0.95 (−1.35, −0.54) | 0.004 |
| DH quadrant (μm/year) | −1.38 (−2.16, −0.60) | −2.38 (−3.08, −1.68) | 0.008 | −2.64 (−3.21, −2.08) | −2.12 (−2.91, −1.32) | 0.19 |
| Non-DH quadrant (μm/year) | −0.93 (−1.46, −0.39) | −1.07 (−1.43, −0.71) | 0.69 | −2.89 (−3.71, −2.08) | −0.31 (−0.98, 0.37) | <0.001 |
| P† | 0.14 | 0.004 | 0.57 | 0.001 | ||
| Change in rate of RNFL thinning after DH (μm/year)** | ||||||
| Unchanged treatment (n = 18 eyes) | Intensified treatment (n = 18 eyes) | P*** | ||||
| Global | −0.28 (−0.82, 0.26) | 0.78 (0.28, 1.28) | 0.004 | |||
| Inferior | −0.44 (−1.17, 0.30) | 1.00 (0.24, 1.77) | 0.007 | |||
| Superior | −0.70 (−1.43, 0.04) | 2.11 (0.90, 3.32) | <0.001 | |||
| Temporal | 0.22 (−0.81, 1.26) | −0.02 (−0.90, 0.87) | 0.71 | |||
| Nasal | −0.29 (−0.72, 0.14) | 0.32 (0.05, 0.60) | 0.017 | |||
| DH quadrant | −1.00 (−1.70, −0.30) | 0.53 (−0.28, 1.34) | 0.005 | |||
| Non-DH quadrant | −0.14 (−0.84, 0.57) | 2.59 (1.58, 3.59) | <0.001 | |||
DH = disc hemorrhage; IOP = intraocular pressure; RNFL = retinal nerve fiber layer.
Values are shown as mean (95% Confidence Interval). Statistically significant values are shown in bold.
Paired t-test comparing before and after DH.
Paired t-test comparing DH quadrants and non-DH quadrants.
Rate of change before DH minus rate of change after DH.
Unpaired t-test comparing the change in rate of RNFL thinning in the unchanged treatment group compared to intensified treatment group.
Figure 1.
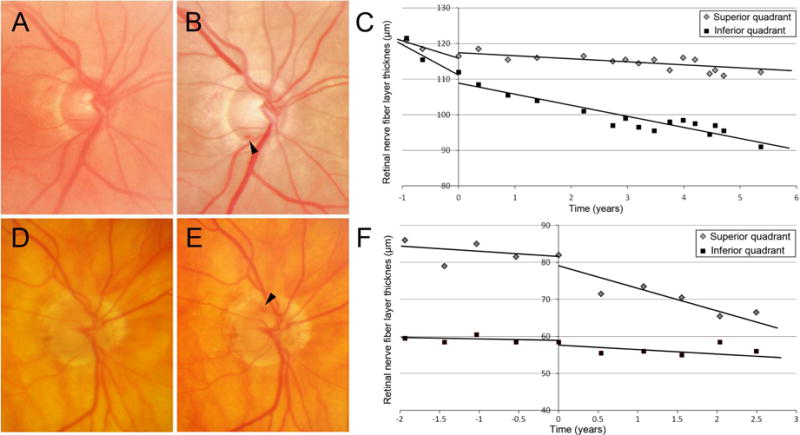
Examples of eyes with disc hemorrhage. A–C, Optic disc photograph shows no DH at the first examination (A) and inferotemporal DH at the episode of DH (B, time is set as 0). The rate of superior RNFL thinning of −3.25 μm/year before DH decreased to −0.83 μm/year after the episode of DH. The rate of inferior RNFL thinning before and after DH was −4.02 and −2.93 μm/year, respectively. The glaucoma medication was intensified from 1 to 2 eye drops after DH. D–F, Optic disc photograph shows no DH at the first examination (D) and superotemporal DH at the episode of DH (E, time is set as 0). The rate of superior RNFL thinning of −0.49 μm/year increased to −5.04 μm/year after the episode of DH. The rate of inferior RNFL thinning before and after DH was −0.21 and −1.08 μm/year, respectively. The glaucoma medication score was 2, which was not altered after DH. The RNFL slope value was calculated using mixed effects modeling.
The change in rate of RNFL thinning in eyes without treatment intensification was significantly below zero only in the DH quadrant (95% Cl: −1.70 and −0.30 μm/year), which means that the rate of RNFL thinning became faster after DH in the DH quadrant without treatment intensification (Table 4). In contrast, the change in rate of RNFL thinning in treatment intensified eyes was significantly above zero globally and in all sectors except the temporal and DH quadrants (95% Cl: global, 0.28 and 1.28 μm/year; inferior, 0.24 and 1.77 μm/year; superior, 0.90 and 3.32 μm/year; nasal, 0.05 and 0.60 μm/year; non-DH quadrant, 1.58 and 3.59 μm/year), which suggests that treatment intensification reduced the rates of RNFL thinning in those areas. Moreover, compared to eyes without a treatment change, intensification of glaucoma treatment after DH significantly reduced the global, sectoral (except temporal), non-DH quadrants and DH quadrant rates of RNFL thinning after DH compared to before DH (global, P = 0.004; inferior, P = 0.007, superior, P < 0.001, nasal, P = 0.017, non-DH quadrant, P < 0.001; DH quadrant, P = 0.005).
Figure 2 shows the differences of rate of RNFL thinning between before and after DH. The intensification of glaucoma treatment after DH significantly reduced the global and local rates of RNFL thinning after DH compared to before DH (global, −0.28±1.09 μm/year versus 0.78±1.0 μm/year, P = 0.004; DH quadrant, −0.10±1.41 μm/year versus 0.53±1.62 μm/year, P = 0.005; non-DH quadrant, −0.14±1.42 μm/year versus 2.59±2.02 μm/year, P < 0.001; nasal, −0.29±0.87 μm/year versus 0.32±0.55 μm/year, P = 0.017). It should be noted that 13 (72%) of the eyes in unchanged treatment group showed negative RNFL slope value differences at the DH quadrant, indicating that RNFL in DH quadrant predominantly decreased at a faster rate after DH without glaucoma treatment. In contrast, 5 (28%) eyes of the intensified treatment group showed faster rates of RNFL thinning in the DH quadrant after DH.
Figure 2.
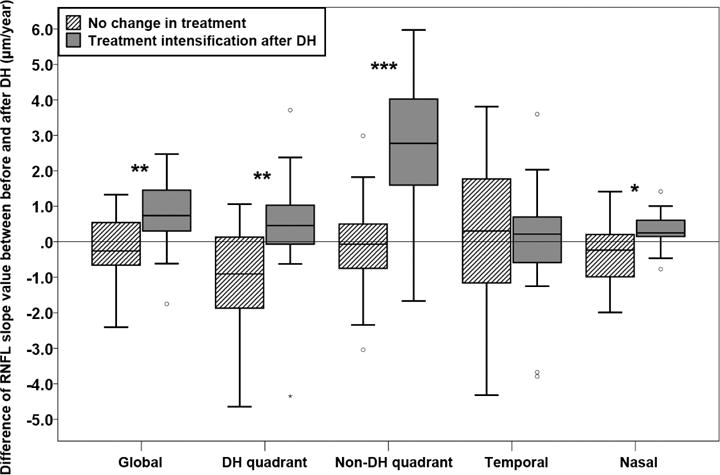
Difference of global and local retinal nerve fiber layer (RNFL) slopes before and after disc hemorrhage (DH). Positive values indicate that the rate of RNFL thinning became slower after DH compared to before DH. Negative values indicate that the rate of RNFL thinning became faster after DH compared to before DH. In global, DH, non-DH, and nasal quadrants, the rate of RNFL slope values significantly slowed more in eyes with intensified glaucoma treatment compare to eyes without treatment change. It should be noted that the slope became significantly faster (more negative) in DH quadrant in eyes without treatment change. *, **, and *** indicate P < 0.05, < 0.01, and < 0.001, respectively.
The univariate and multivariate analyses of the association between the change in global RNFL thinning before and after DH and clinical and ocular characteristics are presented in Table 5. Univariate analysis showed that change in rates of RNFL thinning was significantly associated with the change in IOP before and after DH (β, −0.176; P = 0.044) and treatment intensification (β, 1.061; P = 0.004). There was further borderline association between change in rates of RNFL thinning and marginally with visual field MD (β, 0.071; P = 0.050). In the multiple linear regression analysis with all variables in the univariate analysis, treatment intensification (β, 1.007; P = 0.005) and visual field MD (β, 0.066; P = 0.049) were significantly associated with change in global RNFL slope values. Excluding treatment intensification as a variable due to potential confounding with IOP reduction change in global RNFL slope values was significantly associated only with the change in IOP before and after DH (β, −0.176; P = 0.034).
Table 5.
Factors Influencing the Difference of Global Retinal Nerve Fiber Layer Slope Values between before and after Disc Hemorrhage.
| Univariate Model | Multivariate Model 1* | Multivariate Model 2† | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Age, per 1-yr older | 0.000 (−0.035, 0.034) | 0.98 | 0.66 | 0.82 | ||
| White race (vs. nonwhite) | −0.024 (−0.915, 0.867) | 0.96 | 0.89 | 0.95 | ||
| Female gender (vs. male) | −0.411 (−1.265, 0.444) | 0.34 | 0.30 | 0.17 | ||
| CCT, per 1-μm thicker | 0.007 (−0.001, 0.015) | 0.10 | 0.32 | 0.44 | ||
| Axial length, per 1-mm longer | −0.072 (−0.425, 0.281) | 0.68 | 0.73 | 0.85 | ||
| Glaucoma diagnosis (vs. glaucoma suspect) | −0.344 (−1.154, 0.466) | 0.39 | 0.71 | 0.67 | ||
| Baseline Visual field MD, per 1-dB better | 0.071 (0.000, 0.143) | 0.050 | 0.066 (0.000, 0.131) | 0.049 | 0.45 | |
| Baseline Global RNFL thickness, per 1-μm thicker | 0.019 (−0.007, 0.045) | 0.14 | 0.57 | 0.20 | ||
| Difference in IOP between before and after DH, per 1-mmHg higher | −0.176 (−0.346, −0.005) | 0.044 | 0.26 | −0.176 (−0.337,−0.014) | 0.034 | |
| Treatment intensification (vs. no change of treatment) | 1.061 (0.353, 1.768) | 0.004 | 1.007 (0.336, 1.678) | 0.005 | ||
CCT = central corneal thickness; DH = disc hemorrhage; IOP = intraocular pressure; MD = mean deviation; RNFL = retinal nerve fiber layer.
Values with statistical significance are shown in bold. β, regression coefficients.
Adjested for all variables in univariate regression model* or those excluding treatment intensification†.
Discussion
The current study provides evidence that intensifying treatment can slow the rate of RNFL thinning in eyes with DH. The rates of RNFL thinning were not significantly different between the DH and non-DH quadrants before DH. However, after DH, the mean rate of RNFL thinning in the non-DH quadrant was significantly reduced by treatment intensification, but unchanged if treatment was not intensified. Although the mean rate of RNFL thinning after DH in the DH quadrant was much faster than in the non-DH quadrant, the mean rate of RNFL thinning did not change after DH in the DH-quadrants of treatment intensified eyes. However in general, eyes that received intensified treatment showed significantly greater slowing of the rates of RNFL loss globally and sectorally after DH in both DH qudrants and non-DH quadrants. The discrepancy between DH and non-DH quadrants after DH might provide a clue for pathophysiology of DH on glaucoma progression.
Prior studies have already shown that DH was positively associated with glaucoma progression.9, 23 Although there have been a few reports retrospectively investigating glaucoma progression before and after DH,5, 10 or local differences of glaucoma progression after DH,7–9 there have been no reports until now investigating local changes both before and after DH, probably because of the difficulty in collecting a sufficient number of analyzable cases with DH.
Chung et al.10 reported using optic disc photography that the proportion of eyes progressing with glaucoma was not different between before and after DH. In the current study, we also found no significant differences in rates of global RNFL thinning between before and after DH, which is consistent with this earlier report that did not take into consideration intensification of treatment. This indicates that RNFL thinning before DH is likely to be disease progression and these changes in optic disc structure might be a trigger for subsequent DH.
Although treatment intensification after DH reduced the rate of RNFL loss, the efficacy of IOP-lowering treatment on the frequency of DH is controversial. Miyake et al.24 showed that IOP reduction by trabeculectomy decreased the frequency of DH in glaucoma eyes. In another study, Hendrickx et al.25 found that ocular hypotensive treatment reduced the incidence of DH in eyes with high-tension glaucoma, but not in eyes with normal-tension glaucoma (NTG). However, although these 2 studies showed that the incidence of hemorrhages was reduced with treatment, the effect of IOP reduction on glaucomatous progression was not directly evaluated in eyes with disc hemorrhages. In contrast, in the secondary analysis26 of the collaborative NTG study27, performed in only 23 eyes, it was reported that lowering of IOP did not benefit visual field progression in NTG eyes with DH. Further, the Early Manifest Glaucoma Trial (EMGT)4 reported that glaucoma treatment did not reduce the frequency of DH and suggested that DH cannot be considered an indication of insufficient IOP-lowering treatment. However, in their study, IOP-lowering treatment delayed glaucoma progression and it was not investigated whether further intensification of treatment affect subsequent glaucoma progression. Medeiros et al.6 suggested a benefit of treatment in decreasing rates of visual field progression in eyes with DH, and our results are consistent with their report. De Moraes et al.5 reported using visual field testing that the global rate of visual field deterioration was faster after DH than before DH, but they did not examine the influence of glaucoma treatment or IOP. Our findings suggest that rates of RNFL thinning associated with DH are spatially different and could be affected by the IOP-lowering treatments locally and globally.
In the current study, the rate of RNFL thinning at the DH quadrant was significantly faster than at the non-DH quadrant regardless of whether or not glaucoma treatment was intensified. It has not been clarified whether this result means that treatment independent RNFL thinning might occur in DH quadrants after DH, or more intensive IOP-lowering treatment is necessary for the DH quadrant. Either way, one possible explanation for the worsening in DH quadrant after DH may be associated with the lamina cribrosa. A recent report showed that the development DHs are related at least in part to the enlargement of focal lamina cribrosa defects.28 Suh et al.29 reported that peripapillary vessel density was significantly lower in glaucoma eyes with focal LC defects and reduction of vessel density was spatially correlated with the location of the LC defect. This suggests that loss of structural support of the laminar beams due to the focal LC defect may directly or indirectly influence local retinal microvasculature or impaired vascular supply to the laminar beams may lead to the focal disruption of the laminar structures. Although LC defects were not investigated in the current study, it is plausible that DH is closely related to LC defect and microvascular impairment in glaucoma eyes. Considering these and our results together, microvascular damage associated with DH might lead to progressive glaucoma in a location-related manner and a part of this deterioration might not be preventable under intensified glaucoma treatment.
Rates of the RNFL thinning using SD-OCT has been shown in glaucoma or suspect eyes in our previous studies. Miki et al.17 showed that the mean rate of global RNFL thinning was −2.02 μm/year in glaucoma suspect eyes that developed VFD and −0.82 μm/year in glaucoma suspect eyes that did not develop VFD. Liu et al.18 reported that mean rate of global RNFL thinning in eyes of glaucoma patients with unilateral progression was −0.89 μm/year and that in normal control eyes was −0.71 μm/year. In the current study, the mean rate of global RNFL thinning in eyes before DH was between −0.81 and −1.28 μm/year. Because the rate of global RNFL thinning in eyes before DH was not much larger than the rate of global RNFL thinning in progressing eyes in the previous studies, it would be difficult to predict DH occurrence only from the rate of RNFL thinning before DH. However, it would be helpful for a clinician to know that there is an inevitable worsening after DH in the DH quadrant and treatment intensification after DH is beneficial to inhibit further worsening.
There are several limitations of our study. The number of eyes analyzable before and after DH was small in our study, although the patients were recruited from large prospective studies. Nonetheless, spatial and temporal relationships between DH and rates of RNFL thinning were detected in this small sample size. Second, some DHs might be missed during follow-up because photographs were recorded annually. DHs have been reported to last for only 2 to 6 months.30 Third, the decision to intensity treatment was not decided randomly. Although randomized clinical trial would be more convincing to reveal an influence of treatment, it is ethically not feasible because DH is well identified as a risk factor for progression. Fourth, the rates of RNFL change using mixed-effects models before and after DH that we reported in Table 4 were not adjusted for confounders such as IOP because we wanted to show the change in actual rates with treatment intensification. However, we also modelled change in rate of RNFL loss against whether treatment was intensified and this multivariable analysis showed the efficacy of IOP-lowering treatment on DH after adjusting for several possible confounding factors (Table 5). Despite these limitations, we observed a clear relationship between local rates of RNFL thinning and DH and found a clear benefit of treatment intensification after DH.
In conclusion, although there is a worsening of the rate of RNFL thinning in a DH quadrant after DH, glaucoma treatment intensification may have a beneficial effect in reducing this rate of thinning.
Table 2.
Comparison of Rates of Retinal Nerve Fiber Layer Thinning in the Eyes with Superior or Inferior Disc Hemorrhage (36 eyes of 33 subjects)
| Before DH | After DH | P | |
|---|---|---|---|
| Follow-up period of OCT (years) | 2.49±1.45 | 2.69±1.35 | 0.75 |
| Number of OCT exam, no. | 6.3±4.2 | 6.6±4.1 | 0.62 |
| Rate of RNFL thinning (μm/year)* | |||
| Global | −1.28±0.98 | −1.03±0.69 | 0.21 |
| DH quadrant | −2.01±1.49 | −2.25±1.49 | 0.41 |
| Non-DH quadrant | −1.91±1.69 | −0.69±1.14 | 0.002 |
| Temporal | −1.09±1.07 | −0.99±1.38 | 0.75 |
| Nasal | −0.16±1.03 | −0.14±0.55 | 0.89 |
DH = disc hemorrhage; OCT = optical coherence tomography; RNFL = retinal nerve fiber layer
Values are calculated using linear mixed effects modeling.
Values are shown as mean ± standard deviation. Statistically significant values are shown in bold.
Comparison was performed using paired t-test between before and after DH
Précis.
Rates of retinal nerve fiber layer (RNFL) thinning in glaucoma patients after disc hemorrhage (DH) were faster in DH-affected quadrants than contralateral quadrants. Treatment intensification was beneficial in reducing rates of RNFL thinning.
Acknowledgments
None
Financial Disclosure(s): Tadamichi Akagi: Finalcial support – Alcon, Kowa, Pfizer, Santen, Senju; Linda M. Zangwill: Research support – Carl Zeiss Meditec, Heidelberg Engineering, National Eye Institute, Optovue, and Topcon; Luke J. Saunders: none; Adeleh Yarmohammadi: none; Patricia Isabel Manalastas: none; Min Hee Suh: none; Christopher A. Girkin: none; Liebmann: Research support – Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, National Eye Institute, Optovue, Reichert, Topcon; Consultant – Alcon, Allergan, Bausch & Lomb, Carl Zeiss Meditec, Valeant Pharmaceuticals, Reichert, Heidelberg Engineering; Robert N. Weinreb: Research support – Genentech, Heidelberg Engineering, National Eye Institute, Optos, Optovue; Consultant – Aerie Pharmaceutical, Alcon, Allergan, Bausch & Lomb, Eyenovia, Ora, Unity.
Supported in part by National Institutes of Health/National Eye Institute grants EY011008 (L.M.Z.), EY14267 (L.M.Z.), EY019869 (L.M.Z.), core grant P30EY022589; an unrestricted grant from Research to Prevent Blindness (New York, NY); grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck, and Santen; the John Mung Program from Kyoto University Global Frontier Project for Young Professionals (T.A.); Eyesight foundation of Alabama (CAG). The sponsor or funding organization had no role in the design or conduct of this research.
Abbreviations and Acronyms
- ADAGES
African Descent and Glaucoma Evaluation Study
- OCT
optical coherence tomography
- D
diopter
- DH
disc haemorrhage
- DIGS
Diagnostic Innovations in Glaucoma Study
- EMGT
Early Manifest Glaucoma Trial
- IOP
intraocular pressure
- MD
mean deviation
- NTG
normal-tension glaucoma
- RGC
retinal ganglion cell
- RNFL
retinal nerve fiber layer
- RNFLT
retinal nerve fiber layer thickness
- SAP
standard automated perimetry
- SD
spectral-domain
- SITA
Swedish interactive thresholding algorithm
- TD
time-domain
- UCSD
University of California, San Diego
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting presentation: None
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–11. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishida K, Yamamoto T, Sugiyama K, Kitazawa Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am J Ophthalmol. 2000;129:707–14. doi: 10.1016/s0002-9394(00)00441-4. [DOI] [PubMed] [Google Scholar]
- 3.Budenz DL, Anderson DR, Feuer WJ, et al. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113:2137–43. doi: 10.1016/j.ophtha.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bengtsson B, Leske MC, Yang Z, Heijl A. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology. 2008;115:2044–8. doi: 10.1016/j.ophtha.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 5.De Moraes CG, Prata TS, Liebmann CA, et al. Spatially consistent, localized visual field loss before and after disc hemorrhage. Invest Ophthalmol Vis Sci. 2009;50:4727–33. doi: 10.1167/iovs.09-3446. [DOI] [PubMed] [Google Scholar]
- 6.Medeiros FA, Alencar LM, Sample PA, et al. The relationship between intraocular pressure reduction and rates of progressive visual field loss in eyes with optic disc hemorrhage. Ophthalmology. 2010;117:2061–6. doi: 10.1016/j.ophtha.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Kernstock C, Dietzsch J, Januschowski K, et al. Optical coherence tomography shows progressive local nerve fiber loss after disc hemorrhages in glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 2012;250:583–7. doi: 10.1007/s00417-011-1825-3. [DOI] [PubMed] [Google Scholar]
- 8.Hwang YH, Kim YY, Kim HK, Sohn YH. Changes in retinal nerve fiber layer thickness after optic disc hemorrhage in glaucomatous eyes. J Glaucoma. 2014;23:547–52. doi: 10.1097/IJG.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 9.Suh MH, Park KH, Kim H, et al. Glaucoma progression after the first-detected optic disc hemorrhage by optical coherence tomography. J Glaucoma. 2012;21:358–66. doi: 10.1097/IJG.0b013e3182120700. [DOI] [PubMed] [Google Scholar]
- 10.Chung E, Demetriades AM, Christos PJ, Radcliffe NM. Structural glaucomatous progression before and after occurrence of an optic disc haemorrhage. Br J Ophthalmol. 2015;99:21–5. doi: 10.1136/bjophthalmol-2014-305349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gracitelli CP, Tatham AJ, Zangwill LM, et al. Estimated rates of retinal ganglion cell loss in glaucomatous eyes with and without optic disc hemorrhages. PLoS One. 2014;9:e105611. doi: 10.1371/journal.pone.0105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Airaksinen PJ, Mustonen E, Alanko HI. Optic disc hemorrhages. Analysis of stereophotographs and clinical data of 112 patients. Arch Ophthalmol. 1981;99:1795–801. doi: 10.1001/archopht.1981.03930020669009. [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama K, Tomita G, Kitazawa Y, et al. The associations of optic disc hemorrhage with retinal nerve fiber layer defect and peripapillary atrophy in normal-tension glaucoma. Ophthalmology. 1997;104:1926–33. doi: 10.1016/s0161-6420(97)30005-0. [DOI] [PubMed] [Google Scholar]
- 14.Diehl DL, Quigley HA, Miller NR, et al. Prevalence and significance of optic disc hemorrhage in a longitudinal study of glaucoma. Arch Ophthalmol. 1990;108:545–50. doi: 10.1001/archopht.1990.01070060093056. [DOI] [PubMed] [Google Scholar]
- 15.Rasker MT, van den Enden A, Bakker D, Hoyng PF. Deterioration of visual fields in patients with glaucoma with and without optic disc hemorrhages. Arch Ophthalmol. 1997;115:1257–62. doi: 10.1001/archopht.1997.01100160427006. [DOI] [PubMed] [Google Scholar]
- 16.Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: patterns of retinal nerve fiber layer progression. Ophthalmology. 2012;119:1858–66. doi: 10.1016/j.ophtha.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121:1350–8. doi: 10.1016/j.ophtha.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T, Tatham AJ, Gracitelli CP, et al. Rates of Retinal Nerve Fiber Layer Loss in Contralateral Eyes of Glaucoma Patients with Unilateral Progression by Conventional Methods. Ophthalmology. 2015;122:2243–51. doi: 10.1016/j.ophtha.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–45. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonas JB, Iester M. Disc hemorrhage and glaucoma. Ophthalmology. 1995;102:365–6. doi: 10.1016/s0161-6420(13)30831-8. [DOI] [PubMed] [Google Scholar]
- 21.Tatham AJ, Weinreb RN, Zangwill LM, et al. Estimated retinal ganglion cell counts in glaucomatous eyes with localized retinal nerve fiber layer defects. Am J Ophthalmol. 2013;156:578–87 e1. doi: 10.1016/j.ajo.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medeiros FA, Alencar LM, Zangwill LM, et al. Detection of progressive retinal nerve fiber layer loss in glaucoma using scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci. 2009;50:1675–81. doi: 10.1167/iovs.08-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Miyake T, Sawada A, Yamamoto T, et al. Incidence of disc hemorrhages in open-angle glaucoma before and after trabeculectomy. J Glaucoma. 2006;15:164–71. doi: 10.1097/00061198-200604000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Hendrickx KH, van den Enden A, Rasker MT, Hoyng PF. Cumulative incidence of patients with disc hemorrhages in glaucoma and the effect of therapy. Ophthalmology. 1994;101:1165–72. doi: 10.1016/s0161-6420(94)31192-4. [DOI] [PubMed] [Google Scholar]
- 26.Anderson DR, Drance SM, Schulzer M, on behalf of the Collaborative Normal-tension Glaucoma Study Group Factors that predict the benefit of lowering intraocular pressure in normal tension glaucoma. Am J Ophthalmol. 2003;136:820–9. doi: 10.1016/s0002-9394(03)00478-1. [DOI] [PubMed] [Google Scholar]
- 27.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 28.Lee EJ, Kim TW, Kim M, et al. Recent structural alteration of the peripheral lamina cribrosa near the location of disc hemorrhage in glaucoma. Invest Ophthalmol Vis Sci. 2014;55:2805–15. doi: 10.1167/iovs.13-12742. [DOI] [PubMed] [Google Scholar]
- 29.Suh MH, Zangwill LM, Manalastas PI, et al. Optical Coherence Tomography Angiography Vessel Density in Glaucomatous Eyes with Focal Lamina Cribrosa Defects. Ophthalmology. 2016;123:2309–17. doi: 10.1016/j.ophtha.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnsjo B, Dokmo Y, Krakau T. Disc haemorrhages, precursors of open angle glaucoma. Prog Retin Eye Res. 2002;21:35–56. doi: 10.1016/s1350-9462(01)00019-2. [DOI] [PubMed] [Google Scholar]


