Abstract
Hematopoietic cell transplantation (HCT) is an established curative treatment for a number of malignant and non-malignant diseases involving the hematopoietic system and some solid tumors. In this report, we provide information about the number of HCT procedures performed in the United States (US) in 2015 and analyze trends and outcomes of HCT as reported to the Center for International Blood and Marrow Transplant Research® (CIBMTR®). We show that the numbers of HCT performed annually continue to increase, the indications for HCT, preferred donor sources and GVHD prophylaxis continue to evolve. We report on general overall survival by indication, disease status at transplant and by transplant type. This report demonstrates a current perspective on transplant activity in the US with focus on recent trends in alternative donors and contemporary transplant practices.
Keywords: trends, HCT activity, US
Introduction
Hematopoietic Cell Transplantation (HCT) is an established curative treatment for a number of conditions including malignant hematologic diseases and non-malignant congenital and acquired diseases involving the hematopoietic system. Over a million HCTs have been reported worldwide in the last 6 decades.1 In this paper, the current trends and outcomes of HCT in the United States as reported to the Center for International Blood and Marrow Transplant Research® (CIBMTR®) are described and further discussed. The CIBMTR began as the International Bone Marrow Transplant Registry (IBMTR) in 1972. The Autologous Bone Marrow Transplant Registry (ABMTR) was started in 1989. When the IBMTR and the ABMTR merged with the National Marrow Donor Program Database (NMDP) in 2004, the CIBMTR was formed. The CIBMTR database includes data reported by more than 500 centers in 54 countries worldwide, including 205 active US centers. More than 227,906 autologous, 196,209 allogeneic related and unrelated donor and 11,225 cord blood transplant procedures have been reported through 2015. Starting in 2007, the C.W. Bill Young Stem Cell Act required that all allogeneic U.S. procedures be reported to the CIBMTR. Autologous procedures in the U.S. are still reported voluntarily and currently the CIBMTR captures 75–80% of the US autologous transplant activity. Outcomes data are prospectively collected.
Methods
Data Collection
Transplant centers collect and submit data on their transplant populations. There are two tracks, and patients are centrally assigned by CIBMTR to either a Transplant Essential Data (TED) track that collects minimal data or a Comprehensive Report Form (CRF) track that entails more detailed data collection. Data collection time points include pretransplant, 100 days, 6 months, one year and then annually until death. Repeat cellular infusions from the same donor and second transplants are also captured. All data are entered electronically into a system called FormsNet, which enforces allowable data and performs simple logical checking. Centers are audited once within a four-year audit cycle where data submitted to the research database is compared to source documents. Discrepancies are reviewed and centers may be required to submit a corrective action plan following audits. Data from allogeneic recipients are used for the Center Specific Outcomes Analysis, an annual report federally mandated for all US transplants by the C. W. Bill Young Act of 2007.
The current report use summarizes key transplant activity based on all data submitted to the CIBMTR in 2015. This summary is also available in part online.2
Statistics
Total transplant numbers are estimates based on data reported to CIBMTR on both TED forms and CRFs. Overall survival probabilities are presented according to disease, disease status, donor type, year of transplant and conditioning regimen intensity. Comparisons across survival curves are univariate and are not adjusted for potentially important contributing factors. Cause of death is as reported by centers. This analysis focuses on transplants shows trends in transplants conducted in the US from 1980 to 2015. Survival analysis using Kaplan Meier estimator was performed and reported in a three-year time point with standard error. Assessment of survival was not adjusted for other significant covariates associated with survival.
Transplant Activity in the United States
Figure 1 shows the estimated annual numbers of transplants in the US. In 2015, there were 13,658 autologous, 2351 matched related donors, 3810 unrelated adult donor, 649 cords and 653 haploidentical transplants performed. The number of autologous HCT has steadily increased since 2000, mainly for the treatment of plasma cell and lymphoproliferative disorders. There has been a significant trend in increasing autologous HCT in the last few years due to an increase in the older age and lymphoproliferative diseases populations. Allogeneic HCT from unrelated donors have steadily increased in frequency although have leveled off in the last few years, and their annual use has surpassed related donors since 2006 (Figure 2). The major contributing factors to this trend were the growth of unrelated donor registries and cord blood inventories, improvement in unrelated donor transplant outcomes and increases in the numbers of allogeneic HCT performed in patients older than 60 years with reduced intensity conditioning. Among allogeneic HCT, the trends among matched related, matched unrelated and cord transplants appear to be relatively stable since 2013; HLA mismatched related donors transplants are the only category that appear to be increasing since 2013, likely related to the increase use of haploidentical donor transplant as an option for patients without HLA-matched donors.
Figure 1.
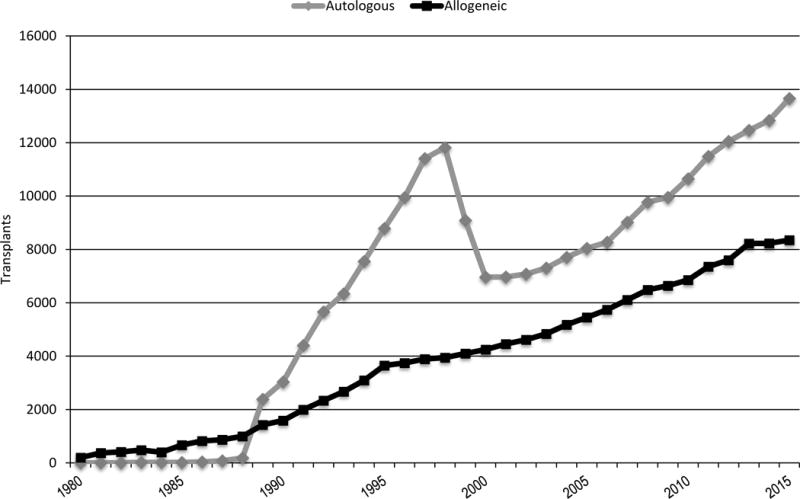
Estimated annual number of HCT in the United States
Figure 2. Allogeneic transplants in the United States by donor type.

MRD-matched related donor; MMRD-mismatched related donor; MUD-BM/PB-matched unrelated donor-bone marrow/peripheral blood
Trends in the distribution of graft sources for allogeneic HCT
Figure 2 shows the trends in distribution of graft sources for allogeneic HCT in the US over time. The annual number of unrelated donor transplants has increased in the last two decades but has remained stable since 2013. Among the pediatric population, in 2015, 33% of all unrelated donor (URD) HCT utilized umbilical cord blood grafts compared to 25% in 2000. Bone marrow has remained the preferred graft source among pediatric recipients with 81% among related and 76% among unrelated allogeneic HCT in 2015. Among adult recipients, peripheral blood remains the most common graft source in 89% related and 87% URD in 2015. The number of URD peripheral blood HCT in adults has increased consistently and comprises 50% of all adult alloHCT in 2015 compared to 9% in 2000. Mismatched related donor transplantation is an increasing modality of alloHCT in the US. In 2015, 11% of all allogeneic HCT were reported from relatives with 1 or more HLA antigen mismatch compared to 6% in 2008. Alternate graft sources are of particular importance in non-Caucasians. Among African Americans, haploidentical donor and umbilical cord HCT accounted for 18% and 19% of all HCT in 2015.
Age trends among HCT recipients
The number of both autologous and allogeneic transplants for treatment of malignant diseases in older patients continues to increase. To illustrate this trend, between 1991 and1997, 7% of allogeneic HCT occurred in patients 50 years or older; between 2000 and 2015, this increased to 38%. In 2015, 25% of all allogeneic HCT recipients were 60 years or older, increased from 5% in 2000; 4.4% were 70 years and older in 2015 compared to 0.4% in 2000.
Trends in HCT utilization by race/ethnicity
Given the increased availability of donors using alternative graft sources, we anticipate that transplant activity would increase in African Americans. The yearly transplant activity, when assessed between 2010 to 2015, show that the number of first allogeneic HCT have increased for all race/ethnic groups from 2010 to 2015, increasing from 5,553 in 2010 to 6,419 in 2015 for Caucasians (16% increase); 519 in 2010 to 744 in 2015 for African Americans (43% increase) and 332 in 2010 to 408 in 2015 for Other Races (20% increase). Alternative graft sources from relatives with either haploidentical donor or 1-antigen mismatched relative, or relative with HLA-match unknown represented 4%, 14% and 4% of donor type/graft source, respectively, for Caucasians, African Americans and Other Races in 2010 and 10%, 26% and 14% of donor type/graft source, respectively, for Caucasians, African Americans and Other Races in 2015.
Trends in the utilization of pre-transplant conditioning regimens
The CIBMTR operational definition for the intensity of conditioning regimens has been previously published.3, 4 The number of HCT using reduced-intensity conditioning has increased steadily in the last decade, particularly in patients aged 50 years and older. In 2015, 66% of all reduced-intensity conditioned HCT occurred in this age group compared to 44% in 2000. Among patients less than 50 years, the proportion of reduced-intensity conditioned HCT has also increased slightly from 2000 to 2015. The utilization of reduced-intensity conditioning varies according to the indication for transplant. Myeloablative conditioning is still used in the majority of acute leukemias and MDS; however a trend is seen toward increased use of reduced-intensity conditioning in the last 10 years reflecting an increase in HCT recipient ages.
Trends in GVHD prophylaxis
Calcineurin inhibitors and methotrexate combinations remain the most common GVHD prophylaxis since 2000, with use in 49% of all allogeneic HCT prior to 2010, and 51% in 2011–2015. The use of T-cell depletion has declined; use of in-vivo T-cell depletion with antithymocyte globulin and/or alemtuzumab was 5% in 2000–2010, and decreased to 3.9% in 2011–2015. Similarly, ex-vivo T cell depletion was 3.4% in 2000–2010, and decreased to 1% in 2011–2015. Lastly, the use of post-transplant cyclophosphamide use has risen from 0.1% in 2000–2005, to 1.7% in 2006–2010, to 7.5% in 2011–2015.
Transplant activity in 2015
Figure 3 shows the number of transplants done in the US in 2015 (N= 21,639) for various indications among adults (3A) and children (3B). In adults, more autologous HCT (N=12,709) were done than allogeneic HCT (N=7,024). There were 423 second transplants in the autologous HCT group and 234 second transplants in the allogeneic HCT group. Multiple myeloma (MM) was the most common indication for autologous HCT (N=7,760), followed by lymphoma (Hodgkin’s disease, N = 1,021; Non-Hodgkin Lymphoma, N=3,529), accounting for 58% of all autologous HCTs, in adults. Acute myeloid leukemia (AML) remained the leading indication for allogeneic transplant (N=2,935), followed by myelodysplastic syndrome/myeloproliferative neoplasms (MDS/MPN) (N=1,395) and acute lymphoblastic leukemia (ALL) (N=995), which together accounted for 70% of all allogeneic transplants in adults. In adults under age 50 years, allogeneic HCT was more commonly utilized than autologous HCT. This reversed after age 50 to more autologous than allogeneic HCT. In the pediatric population, allogeneic HCT (N= 1,304) continued to be used more commonly than autologous HCT (N=602). In children, the most common indication for allogeneic HCT is acute leukemia (AML, N=247, ALL, N=287) followed by non-malignant indications (aplastic anemia, N=128; other non-malignant diseases, N=519); lymphoma (Hodgkin’s disease, N=43; Non-Hodgkin Lymphoma, N=15) and solid tumors (N=514) are the most common indications for autologous HCT in children. The proportions of HCT for various diseases have remained fairly stable over the last 10 years in the pediatric and adult population.
Figure 3.


A. Adult HCT activity in the US (N=19,733), 2015
PCD-plasma cell disorders; NHL-non-Hodgkin Lymphoma, AML-acute myelogenous leukemia; HD-Hodgkin’s disease; ALL- acute lymphoblastic leukemia; MDS/MPN- myelodysplastic syndrome/myeloproliferative neoplasms; CLL- chronic lymphocytic leukemia; NMD- non-malignant diseases
B. Pediatric HCT activity in US (N=1,906), 2015
NHL- non-Hodgkin Lymphoma, AML- acute myelogenous leukemia; HD- Hodgkin’s disease; ALL- acute lymphoblastic leukemia; MDS/MPN- myelodysplastic syndrome/myeloproliferative neoplasms; CLL- chronic lymphocytic leukemia; NMD- non-malignant diseases
Trends in mortality
The 100 day mortality rate after autologous HCT in 2015 was 2.1 (1.9–2.4)% with disease relapse as the most common cause of death. Post-autologous HCT, the most common reported cause of death was primary disease (69%), followed by infection (8%), organ failure (4%), secondary malignancy (1%) and others (18%). We observed a trend to improved day 100 unadjusted survival since 2000, with 95 (95–95)% in 2000–2004, to 97 (97–97)% in 2005–2010, to 98 (98–98)% in 2011–2014 on univariate analysis.
The 100 day mortality among allogeneic HCT recipients in 2015 was 10.6 (10–11.2)%; 7.4 (6.5–8.4)% for MRD and 12 (11.1–12.9)% for URD HCT. The 100 day mortality was 8(2–18)% for haploidentical HCT and 9(4–14)% for umbilical cord transplants in 2015. We saw a similar trend to improved unadjusted survival rates after allogeneic HCT at 100 days with 80 (79–80)% in 2000–2004 to 86 (86–87)% in 2005–2010, to 90 (89–90)% after 2011 on univariate analysis. Among allogeneic HCT recipients, within 100 days, the most common cause of death was relapsed disease (29%) followed by infection (16%), GVHD (9%), organ failure (9%) and other (36%) in MRD HCT, and primary disease (23%), infection (18%), GVHD (10%), organ failure (11%), and other (34%) in URD HCT. After day 100, relapsed disease remained the most common cause of death (57% in MRD, 46% in URD) followed by infection (7% in MRD and 10% in URD) and GVHD (7% in MRD and 9% in URD). Table 1 shows the 3-year univariate survival outcomes for various diseases in children and adults treated with autologous and allogeneic HCT.
Table 1A.
Three year univariate OS survival for myeloid diseases and severe aplastic anemia, 2004–2014. Estimates in OS are shown as percentages with standard error.
| Matched related donor | Unrelated donor | ||||||
|---|---|---|---|---|---|---|---|
| Early | Intermediate | Advanced | Early | Intermediate | Advanced | ||
| AML | 70 ± 2 | 59 ± 4 | 30 ± 4 | 58 ± 2 | 57 ± 2 | 27 ± 2 | Pediatric |
| 57 ± 1 | 50 ± 1 | 27 ± 1 | 50 ± 1 | 46 ± 1 | 25 ± 1 | Adult | |
| ALL | 71 ± 2 | 57 ± 2 | 38 ± 5 | 67 ± 2 | 55 ± 1 | 40 ± 4 | Pediatric |
| 59 ± 1 | 38 ± 2 | 27 ± 2 | 57 ± 1 | 37 ± 1 | 24 ± 2 | Adult | |
| Matched related donor | Unrelated donor | ||||||
| MDS | Early | Advanced | Early | Advanced | |||
| 53 ± 2 | 45 ± 1 | 49 ± 1 | 40 ± 1 | ||||
| Matched | related donor | Unrelated | donor | ||||
| Myelofibrosis | 59 ± 2 | 52 ± 2 | |||||
| Other MPN | 50 ± 2 | 44 ± 2 | |||||
| Matched related donor | Unrelated donor | ||||||
| CLL | 58 ± 2 | 51 ± 1 | |||||
| Matched related donor | Unrelated donor | ||||||
| CML | Chronic | Accelerated | Blast | Chronic | Accelerated | Blast | |
| 68 ± 1 | 51 ± 3 | 26 ± 4 | 59 ± 1 | 46 ± 3 | 30 ± 4 | ||
| Matched related donor | Unrelated donor | ||||||
| SAA | 91 ± 1 | 76 ± 2 | Pediatric | ||||
| 78 ± 1 | 67 ± 2 | Adult | |||||
AML- acute myeloid leukemia, ALL- acute lymphoblastic leukemia, MDS- myelodysplastic syndrome, MPN- myeloproliferative neoplasms, CLL- chronic lymphocytic leukemia, CML- chronic myeloid leukemia, SAA- severe aplastic anemia
Conclusions
The numbers of HCT performed annually continue to increase and the indications for HCT, preferred donor sources and GVHD prophylaxis continue to evolve. As the field of HCT evolves into cellular therapies, the CIBMTR is working with academic and industry partners to build a cellular therapy database that will be able to collect data on cellular product infusions and maintain outcomes of recipients of these therapies. In addition to documenting outcomes and trends, CIBMTR data provides valuable information about the overall activity and aggregate success rates of HCT as practiced in the United States. Registry data have the advantage of capturing all allogeneic activity because of the C.W. Bill Young legislation, and are also the best source of data about autologous HCT activity even though data submission for these procedures is voluntary. Thus, our results inform the baseline success rates against which new approaches can be compared.
The data also reveal the evolving risk-balance assessments of the hematology/oncology and transplant communities between improvements in non-transplant therapies and HCT. Trends are also influenced by health care funding decisions, for example, Medicare’s agreement to fund transplants for MDS under a Coverage with Evidence Development mechanism in 2010 led to an increase in the numbers of allogeneic HCT done for MDS in older adults.5 Similar funding will be provided for multiple myeloma, myelofibrosis and sickle cell disease, which will likely impact the number of transplants performed for these indications. If benefit with HCT is proven, this could increase transplant activity for these diseases or conversely, if HCT does not deliver better outcomes, it could lead to much less HCT activity for these indications. Nonetheless, studies for coverage determination are important initiatives to understand the benefit of HCT for certain indications and in certain patient populations, and can assist in selecting patient groups likely to benefit to transplant, ultimately advancing the field and population health outcomes.
The HCT community has benefitted from the long-standing commitment of CIBMTR to collect and disseminate information that can be used to improve HCT. This report has the intent in exploring the “current state of HCT” in the U.S. so that investigators, patients and payors can have access to accurate and recent data to use.
Table 1B.
Three year univariate OS survival for common indications for common lymphomas, 2004–2014. Estimates in OS are shown as percentages with standard error.
| Autologous HCT | Allogeneic HCT | |||||
|---|---|---|---|---|---|---|
| Chemo sensitive | Chemo resistant | Chemo sensitive | Chemo resistant | |||
| MRD | URD | MRD | URD | |||
| HD | 82 ± 1 | 67 ± 2 | 63 ± 2 | 57 ± 2 | 38 ± 5 | 38 ± 3 |
| FL | 79 ± 1 | 65 ± 4 | 72 ± 2 | 66 ± 2 | 59 ± 4 | 43 ± 4 |
| DLBCL | 65 ± 1 | 45 ± 2 | 51 ± 2 | 44 ± 2 | 27 ± 3 | 23 ± 3 |
HD- Hodgkin’s Disease, FL- Follicular Lymphoma, DLBCL- Diffuse Large B Cell Lymphoma
Table 1C.
Three year univariate OS survival for common indications for multiple myeloma and AL amyloidosis, 2004–2014. Estimates in OS are shown as percentages with standard error.
| First autologous HCT | Salvage autologous HCT | |
|---|---|---|
| Multiple myeloma | 77 ± 0.2 | 67 ± 6 |
| AL amyloidosis | 79 ± 1 | Too few to analyze |
Acknowledgments
The CIBMTR is supported by the National Institutes of Health (NIH) grants # U24CA076518 and # HL069294, Health Resources and Service Administration (HRSA) contract # HHSH250201200016C. This publication is funded in part by the Research and Education Program Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and by KL2TR001438 from the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences (AD). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests
The authors have no conflicts to disclose
References
- 1.Gratwohl A, Pasquini MC, Aljurf MD, Confer DL, Baldomero H, Bouzas LF, et al. Global Hematopoietic Stem Cell Transplantation (HSCT) At One Million: An Achievement Of Pioneers and Foreseeable Challenges For The Next Decade. A Report From The Worldwide Network For Blood and Marrow Transplantation (WBMT) Blood. 2013;122(21):2133. [Google Scholar]
- 2.D’Souza A, Zhu X. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides. 2016 Available at: http://www.cibmtr.org. In, 2016.
- 3.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.57th ASH Annual Meeting and Exposition. Orlando, FL: 2015. Outcome of Patients 65 Years and Older with Myelodysplastic Syndrome (MDS) Receiving Allogeneic Hematopoietic Stem Cell Transplantation Compared to Patients 55–64 Years of Age. Blood, 2015. [Google Scholar]


