Abstract.
We explore noninvasive biomarkers of microvascular invasion (mVI) in patients with hepatocellular carcinoma (HCC) using quantitative and semantic image features extracted from contrast-enhanced, triphasic computed tomography (CT). Under institutional review board approval, we selected 28 treatment-naive HCC patients who underwent surgical resection. Four radiologists independently selected and delineated tumor margins on three axial CT images and extracted computational features capturing tumor shape, image intensities, and texture. We also computed two types of “delta features,” defined as the absolute difference and the ratio computed from all pairs of imaging phases for each feature. 717 arterial, portal-venous, delayed single-phase, and delta-phase features were robust against interreader variability (). An enhanced cross-validation analysis showed that combining robust single-phase and delta features in the arterial and venous phases identified mVI (AUC ). Compared to a previously reported semantic feature signature (AUC 0.47 to 0.58), these features in our cohort showed only slight to moderate agreement (Cohen’s kappa range: 0.03 to 0.59). Though preliminary, quantitative analysis of image features in arterial and venous phases may be potential surrogate biomarkers for mVI in HCC. Further study in a larger cohort is warranted.
Keywords: radiomics, microvascular invasion, contrast-enhanced computed tomography, image features, segmentation, hepatocellular carcinoma
1. Introduction
The incidence of hepatocellular carcinoma (HCC) has quadrupled in the last three decades, surging from 2.9 new cases per 100,000 people in 1982 to 8.5 new cases per 100,000.1 HCC is the third most common cause of cancer-related death in men,1 accounting for one billion dollars in direct costs,2 2 billion in HCC related inpatient costs,3 and lost wages during peak productive years.4
45% of HCCs are localized to the liver, allowing surgical options.1 In patients with an absence of clinically relevant portal hypertension, hepatectomy remains the gold standard for very early and early stage disease, as defined by the Barcelona clinic liver cancer staging system ( or a single tumor 2 to 5 cm, respectively)5,6 while orthotopic liver transplantation (OLT) is recommended in patients with clinically evident portal hypertension and early stage HCC that meet the Milan criteria.5–7 However, 20% of patients undergoing OLT and 50% of patients undergoing hepatectomy exhibit microvascular invasion (mVI) and consequently early tumor recurrence, reducing the 5-year survival from 80% to 40% after OLT and from 50% to 70% to 20% after hepatectomy.7–10 Studies have shown that mVI is the strongest independent predictor of poor prognosis.11–16 Unfortunately, though, its presence is not reliably diagnosed on a biopsy, making preoperative diagnosis challenging. Current practice uses two surrogate biomarkers, the Milan criteria and serum alpha-fetoprotein (AFP), as predictors, with confirmation by histology after excision of the tumor. The Milan criteria, which uses tumor size and number, is imperfect though. Discordance exists between the true size and the actual number of tumors seen on explant and preoperative images.17,18 Furthermore, 30% of tumors selected by the Milan criteria for OLT have mVI and, conversely, 50% of HCCs not selected by the Milan criteria do not have mVI.7,19 Additionally, when controlled for mVI, tumor size does not appear to add any prognostic information.20 Serum AFP lacks sensitivity, and even models that combine both the Milan criteria and AFP have a modest sensitivity of 50% at a specificity of 80% and an area under curve (AUC) of 0.7,21 underscoring the need for better preoperative biomarkers for mVI. Prospective identification of mVI thus has direct implication on organ allocation, surgical technique, prognostication, and public policy.
The advent of quantitative imaging techniques provides us with the opportunity to discover a robust imaging biomarker of mVI in HCC. The use of contrast enhanced CT to predict global HCC gene expression, i.e., radiogenomics, has been reported previously. These studies used semantic observations, i.e., image characteristics annotated manually by radiologists, to predict mVI, and associated mVI with a venous invasion gene signature.22–24 Although the methods for semantic feature description are easily translatable to the clinic, they are operator dependent and, in some circumstances, might suffer from interreader variability. The emergence of quantitative imaging methods, also known as radiomics, allows the automated extraction of quantitative image features from regions of interest (ROIs) in images.25–29 Radiomics, therefore, provides an opportunity to identify features of HCC predictive of mVI that are potentially less operator dependent, requiring less operator training and being less prone to interreader variability.
In this study, we hypothesize that quantitative image features, derived from preoperative, contrast-enhanced, triphasic CT scans, are associated with mVI. Our purpose is to evaluate these quantitative image features as surrogate biomarkers for mVI and to compare them to recently proposed signatures that use semantic image features.
2. Materials and Methods
2.1. Patient Selection and Image Data Collection
This was a single institution retrospective study that was compliant with the Health Insurance Portability and Accountability Act and that was granted institutional review board approval. Between February 2010 and April 2014, we reviewed medical records and imaging for all adult patients ( years of age) who underwent surgical resection of a previously untreated (locoregional therapy naive) presumed solitary HCC. Inclusion criteria included confirmed pathological diagnosis of HCC and availability of an adequate preoperative CT defined by a in all phases and obtained within 3 months of the surgery. Fibrolamellar HCC or mixed cell tumors (HCC and cholangiocarcinoma) were excluded. Technical requirements for the preoperative CT included availability of images obtained in the arterial, portal venous, and delayed phases with maximum slice thickness of 3 mm for the arterial and portal-venous phases and optimal arterial opacification obtained using an automated bolus tracking technique.
2.2. Demographic, Clinical, and Scanning Parameters of the Cohort
Out of 46 chemotherapy naive patients, who underwent hepatic resection for HCC at our institution during the study time period, 28 patients had a preoperative CT that met our inclusion criteria and were available for analysis. Table 1 details the cohort demographics and tumor characteristics, and Table 2 provides details about the imaging acquisition and reconstruction. The median age in the cohort was 64 years (range 38 to 85 years). Mean tumor diameter in the axial plane was 7.4 cm (median 5.6 cm, range 1.5 to 20 cm, SD 4.9 cm). Median AFP was 11.6 (range 2 to 19,816). For clinical purposes, 7 patients had an , whereas 20 patients were AFP nonresponders (). Ten patients (36%) had mVI on pathology, including 1 patient with micro- and macrovascular invasion. Tumor differentiation was as follows; 8 (29%) well differentiated (WD), 17 (61%) moderately differentiated (MD), and 4 (14%) poorly differentiated (PD).
Table 1.
Demographics and clinical characteristics of the cohort. SD, standard deviation.
| Demographics | mVI present () | mVI absent () |
|---|---|---|
| Age (years): (range) | (51 to 85) | (38 to 81) |
| Sex | 8 male; 2 female | 13 male; 5 female |
| Race | ||
| White | 5 (50%) | 10 (55.6%) |
| Asian | 2 (20%) | 5 (27.8%) |
| Hispanic | 2 (20%) | 3 (16.7%) |
| African American | 1 (10%) | 0 (0%) |
| Liver disease | ||
| Hepatitis B | 3 (30%) | 7 (38.9%) |
| Hepatitis C | 1 (10%) | 7 (38.9%) |
| NASH/cryptogenic | 6 (60%) | 4 (22.2%) |
| Tumor | ||
| Max. diameter (cm): (range) | (2 to 20) | (1.5 to 17) |
| Alpha-feto protein () | ||
| (range) | (3 to 19,816) | (2 to 1145) |
| Tumor | ||
| WD | 0 (0%) | 8 (44.4%) |
| MD | 7 (70%) | 9 (50%) |
| PD | 3 (30%) | 1 (5.6%) |
Table 2.
Scanning characteristics of the cohort.
| Scanning parameters | mVI present () | mVI absent () |
|---|---|---|
| Slice thickness (mm) | ||
| Arterial: (range) | (0.625 to 3) | (0.625 to 3) |
| Portal venous: (range) | (0.625 to 3) | (0.625 to 3) |
| Delayed: (range) | (1.0 to 5) | (1.0 to 5) |
| mA | ||
| Arterial: (range) | (206 to 500) | (109 to 700) |
| Portal venous: (range) | (252 to 540) | (121 to 740) |
| Delayed: (range) | (217 to 540) | (113 to 640) |
| kVp | ||
| Arterial: (range) | (100 to 140) | (80 to 120) |
| Portal venous: (range) | (100 to 140) | (80 to 120) |
| Delayed: (range) | (100 to 140) | (80 to 120) |
2.3. Radiomics
Four radiologists (R.S., J.L., A.K., and N.K.) with 6, 10, 12, and 13 years of experience in HCC CT analysis, respectively, drew ROIs on the CT images. Operators were instructed to place three ROIs along the perceived tumor border on different cross sections of the tumor. The process was repeated for each phase of imaging available. Typically, the first ROI was selected on a centrally located slice followed by two additional slices (one more superior and one more inferior, noncontiguous). We used ePAD, a freely available quantitative imaging informatics platform to generate all ROI’s.30 We used the dice similarity coefficient to evaluate the agreement between ROIs from different radiologists for cases where the radiologists segmented the same slice. Since the readers were free to select the slices for segmentation, the slices chosen were not always the same and the number of overlapping slices decreased as a function of the number of readers: for two, three, and four readers the overlap was 148, 64, and 14 slices, respectively. We used Lin’s concordance correlation coefficient to measure the consistency of the features extracted for each ROI across radiologists and patients.31
We used a locally developed quantitative image feature pipeline to generate the radiomics features for all patients, similar to previous work.26,29,32 Using a single ROI as input, the pipeline computed 464 features, described in previous work33 for each CT phase that characterized the intensities and textures within the ROIs, the sharpness of lesion boundaries, and the boundary shapes. Table 3 lists the feature categories and the number of features in each category. The feature values were averaged over the three cross sections to capture features occurring in multiple slices. We defined delta radiomics features to be the differences and the ratios between the image features extracted from pairs of phases. This created three additional data sets representing the change between arterial and portal-venous, arterial and delayed, and delayed and portal-venous phases (Fig. 1), resulting in a total of 4176 features. We selected robust features to be those having intraclass correlation (ICC), estimated by Lin’s correlation coefficient, of across radiologists,31 resulting in 717 robust features for further analysis.
Table 3.
Quantitative feature categories and number of features in each category used in this study. For each phase and each pairwise delta feature, we computed these 464 features.
| Feature category | Number of features computed |
|---|---|
| Intensity | 22 |
| Texture | 333 |
| Shape | 7 |
| Edge | 102 |
Fig. 1.
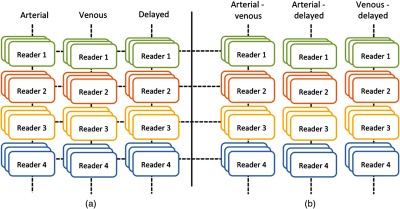
Workflow of the study with triphasic CT imaging of HCC patients. (a) Outlines are done by four readers in three positions on each of the three phases: arterial, venous and delayed. (b) Delta features (arterial-venous, arterial-delayed and venous-delayed) are created capturing the change between two phases.
2.4. Predictive Modeling Using Sparse Linear Regression
We built predictive models for mVI using image features using sparse linear regression (SLR) also known as least absolute shrinkage and selection operator (lasso).34 We chose this approach because more complex classifiers are sensitive to noise and prone to overfitting when applied to a small cohort such as ours. SLR models the dependent variable, mVI, as a linear combination of the image features (), i.e., , where the are the weights of the linear regression. SLR selects the most predictive imaging features by minimizing the least-squares error , subject to a regularization L1-norm constraint, which places an additional penalty on the weights . This penalty achieves feature selection by setting the sum of the absolute value of weights to be less than a constant value forcing nonpredictive weights to be set to zero. Selected features will have a nonzero coefficient in the SLR model.35 We evaluated the performance of the model using the area under the receiver operating characteristic (ROC) curve (AUC), which is created by plotting sensitivity against specificity at varying thresholds.36 We used a nested leave one reader out, leave one patient out cross-validation (LOR-LOP-CV) strategy to estimate the performance on a sample that was annotated by a different reader and that was not used for model building.
2.5. Comparison with Semantic Image Features and Tumor Size
We compared our model with tumor size and two recently reported signatures based on semantic features: radiogenomic venous invasion (RVI)22 and two-trait predictor of venous invasion (TTPVI).24 These signatures included the following five semantic features; the presence of internal arteries on arterial and portal-venous phases, hypodense halo and tumor-liver difference, and tumor margins on the portal-venous phase. Three of the four readers (A.K, J.L, and N.K.) provided these five semantic features, which resulted in RVI and TTPVI signatures. We evaluated the interobserver agreement using Cohen’s kappa statistic between pairwise readers. We report the minimum, average, and maximum Kappa statistic for all pairwise comparisons between readers.
3. Results
3.1. Texture Features are the Most Robust against Interreader Variation
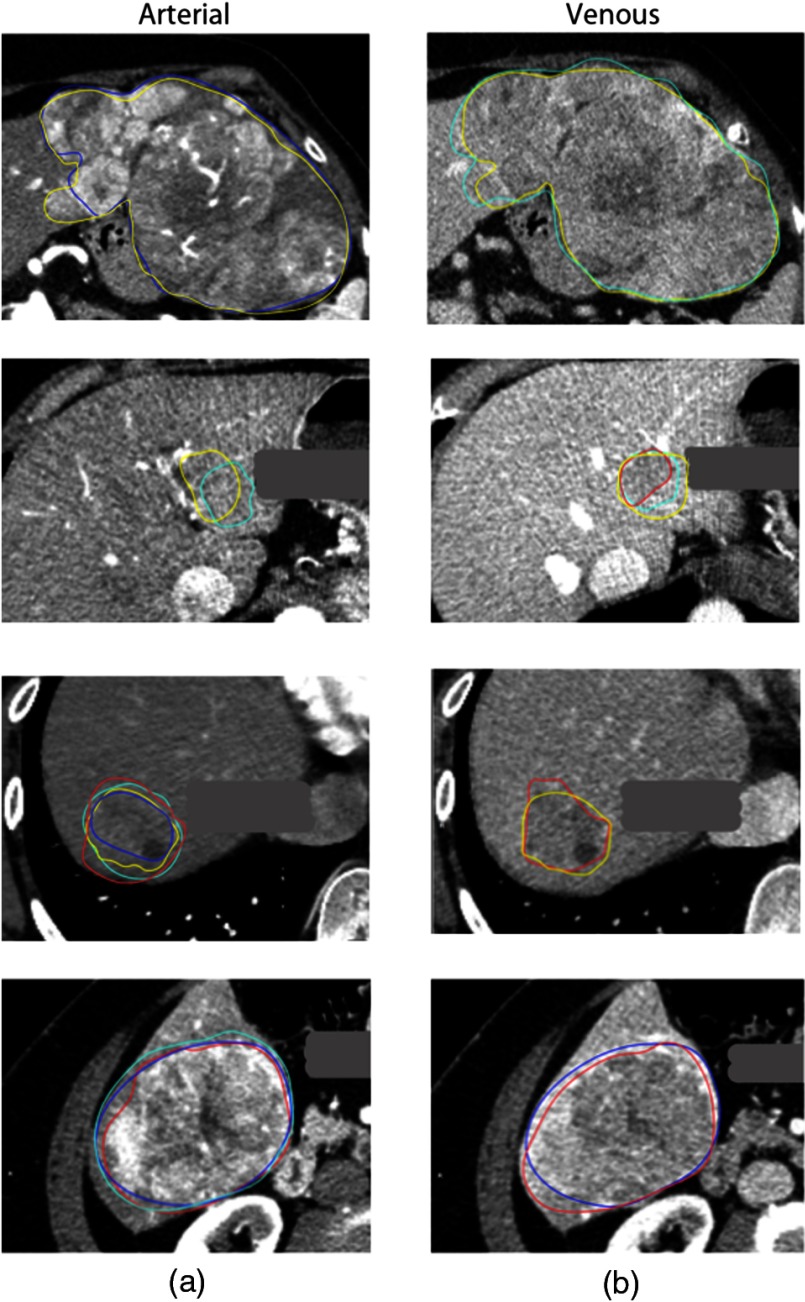
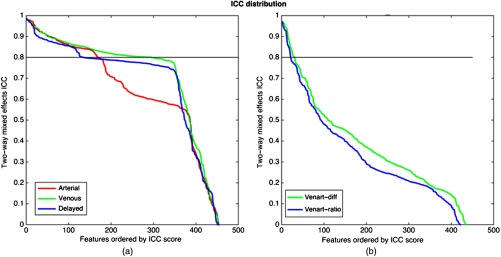
Figure 2 shows examples of high and low overlap between reader delineations on CT imaging. Overall, the dice similarity coefficient for cases where the radiologists assessed the same slice ranged from 0.19 to 0.93. Defining feature robustness as , we found 170 arterial, 295 venous, and 135 delayed features that were robust against reader delineation variability [Fig. 3(a)]. In general, the texture features were most robust across all phases, whereas the shape and sharpness features had the lowest robustness, presumably because they rely on consistent slice selection (which we did not require) and border delineation. Similarly, for the delta features [Fig. 3(b)], we found 31, 41, and 45 robust features for the difference between venous and arterial, arterial and delayed, and delayed and venous, respectively, and 20, 33, and 35 robust delta ratio features for the ratio between venous and arterial, arterial and delayed, and delayed and venous, respectively.
Fig. 2.
Example outlines for four different patients by four different readers (yellow, reader 1; teal, reader 2; red, reader 3; blue, reader 4) with different overlaps for arterial and venous images. The dice similarity coefficient, measuring agreement between readers, where they assessed the same slice ranged from 0.19 to 0.93
Fig. 3.
Feature robustness as measured by ICC for both individual phase features and delta features. (a) ICC score for each single-phase feature, ordered by ICC score. (b) ICC score for each venous-arterial delta feature ordered by ICC score.
3.2. Multivariate Analysis Using Sparse Linear Regression Finds Association of Microvascular Invasion with Quantitative Image Features across Readers
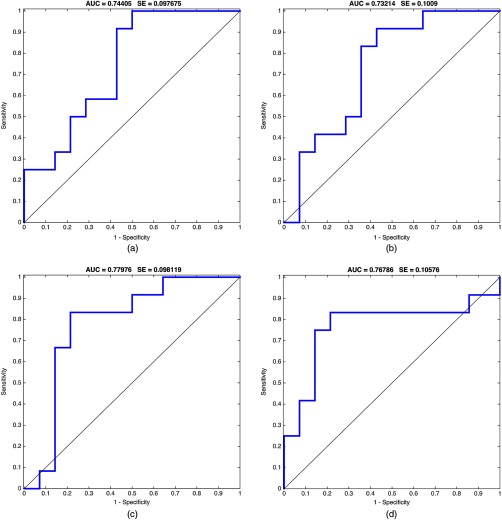
Next, within an LOR-LOP-CV framework, we used the SLR techniques described above applied to various combinations of quantitative imaging feature categories, including only features that were robust to interreader variability for mVI. Among the various resulting linear models, the highest-performing models were built using arterial–venous delta features, which were predictive of mVI. Moreover, combining these delta features with single-phase features resulted in similar performance but a narrower confidence interval (CI). The performance of these models was: and , respectively. Figure 4 shows ROC curves obtained using the delta of venous and arterial along with single phase features for each of the four readers, training on three readers and testing on the other. We compared the ROC curves of the four readers among each other according to Ref. 37. -values ranged between 0.611 and 0.913 for the pairwise comparisons, showing no statistical differences between the models.
Fig. 4.
ROC curves for the four readers using LOR-LOP-CV. Model built from single-phase features combined with venous–arterial delta phase features. (a)–(d) Curves for readers 1, 2, 3, and 4, respectively. Also shown AUC and standard error (SE) for each ROC curve ().
Table 4 show LOR-LOP-CV performance statistics of different models obtained using different combinations of input feature categories.
Table 4.
Performance measured by AUC using different combinations of the data and an LOR-LOP-CV strategy. Best performing models use single-phase features combined with arterial–venous delta features.
| Sample name | AUC mean | AUC CI ± |
|---|---|---|
| Single-phase features | ||
| Arterial | 0.45 | 0.065 |
| Venous | 0.47 | 0.081 |
| Delayed | 0.46 | 0.044 |
| Venous + arterial | 0.47 | 0.030 |
| All phases combined | 0.70 | 0.033 |
| Delta features | ||
| All delta features | 0.67 | 0.038 |
| Arterial–venous delta features | 0.75 | 0.063 |
| Combinations of single-phase and delta features | ||
| Venous + arterial + delta of venous and arterial | 0.70 | 0.034 |
| All phases + arterial–venous delta features | 0.76 | 0.018 |
| All phases combined + all delta features | 0.72 | 0.048 |
Note: Bold fonts indicate our final model, based on the best results compared to other models in this table.
Table 5 lists the top features selected for each reader within the single-phase and delta arterial–venous features. Table 5 also shows that features selected when testing on each left out reader overlapped. Texture features and delta features were selected most frequently and consistently. Specifically, Daubechies-247-venart-delta difference and Gabor-9-venart-delta difference were selected across readers indicating the importance of delta difference features and texture features. Daubechies wavelets and Gabor texture features have been previously reported in the literature as correlated with clinical variables.33,38–41
Table 5.
| Reader 1 features | Reader 2 features | Reader 3 features | Reader 4 features |
|---|---|---|---|
| Gabor-9-venart-delta difference | Daube-247-venart-delta difference | Gabor-9-venart-delta difference | Peak position-arterial |
| Peak position-arterial | Gabor-9-venart-delta difference | Daube-247-venart-delta difference | Gabor-9-venart-delta difference |
| Daube-268-venous | Daube-267-venous | Daube-285-venous | Daube-247-venart-delta difference |
| Daube-247-venart-delta difference | Peak position-arterial | Daube-267-venous | Daube-285-venous |
| Histogram-bin-7-venart-delta ratio | Histogram-bin-7-venart-delta ratio |
3.3. Comparison with Semantic Features and Tumor Size
Interobserver agreement for the semantic features was low ranging from 0.03 for the tumor-liver difference feature to 0.59 for the internal arteries feature assessed on arterial imaging. The presence of internal arteries was the most consistent as assessed on arterial imaging versus venous imaging (Cohen’s kappa 0.59 versus 0.20, Table 6). Thus, each semantic feature separately did not result in a model significantly better than a random predictor (AUC 0.47 to 0.58). Similarly, tumor size was not predictive of mVI (AUC 0.62). The RVI and TTPVI also were not better than a random predictor with AUC of 0.53 and 49, respectively.
Table 6.
Kappa statistic for interobserver agreement among three readers of five semantic features.
| Semantic image feature | Min | Average | Max |
|---|---|---|---|
| Presence of internal arteries—assessed on arterial imaging | 0.45 | 0.59 | 0.73 |
| Presence of internal arteries—assessed on venous imaging | 0.02 | 0.20 | 0.36 |
| Presence of hypodense halo | 0.16 | 0.29 | 0.44 |
| Tumor liver difference | 0.03 | 0.08 | |
| Tumor margins: smooth versus nodular | 0.05 | 0.18 | 0.37 |
4. Discussion
The presence of mVI is the leading, independent predictor of early recurrence and, consequently, survival in patients undergoing OLT or hepatectomy.11–13 Presently, mVI can only be diagnosed post hoc by a pathologist. The most widely used preoperative surrogates for mVI and tumor biology are tumor size and tumor number as established by the Milan criteria.5,7 The Milan criteria, though, are limited. Adaptation of these criteria, originally derived from pathological data, to imaging remains inaccurate.17,18 Furthermore, 30% of tumors selected by the Milan criteria for OLT have mVI while 50% of HCCs not selected by the Milan criteria do not have mVI,11,19 limiting the utility of using size as an indicator of mVI. To this point, in an analysis by Lim et al., the authors demonstrated that using multivariate analysis, mVI was predictive of survival, after which the Milan status did not add additional discriminative information.13
Noninvasive, prospective identification of mVI could potentially have a direct impact on judicious and equitable organ allocation, the extent of hepatectomy and prognostication. Early graft loss following OLT due to undiagnosed mVI could be avoided in this era of organ shortage. Further the organ shortage has been devastating for patients without HCC awaiting OLT, who in fact have a superior post OLT 5-year overall survival but a 33% lower probability of getting an organ and, subsequently, a higher waitlist mortality.43 Finally, an anatomical resection, while challenging, provides a better chance at 5-year survival in the presence of mVI than a wedge resection, making the case for preoperative determination of the vascular invasion status important to determine surgical technique.10
In this study, we evaluated radiomics image features as surrogate biomarkers of mVI in HCC and compared this approach to semantic image features. Our study shows that computational features capturing lesion texture, intensities, and shape extracted from triphasic CT images had a diagnostic accuracy (AUC) of 0.76 compared to two previously reported signatures based on semantic features, RVI22 and TTPVI,24 with AUCs of 0.53 and 0.49, respectively. In addition, in our cohort, these signatures based on semantic features suffered from high interreader variability (Table 5).
While complete and accurate tumor segmentation in all three phases followed by computation of radiomic features might provide a more complete characterization of the tumor, there are presently no reliable automatic or practical manual solutions. We based our manual approach, which requires manual outlines of three slices in each phase, on a recent study that showed that many image features of HCC are robust to slice choice and to the position of segmentation outlines.33 Our results suggest that surrogate biomarkers can be derived from a subset of the tumor acquired by slice selection and manual contouring. Until reliable automatic three-dimensional segmentation can be achieved, our approach represents a clinically translatable solution.
The rise of radiomics using semantic and quantitative image features of tumors on contrast-enhanced imaging provides us with an opportunity for the development of radiomic biomarkers that predict pathological features or clinically relevant outcomes. The success of semantic features of HCC patients has been shown previously by showing associations between semantic features and molecular characteristics of mVI.22,23 These studies demonstrate that imaging features, such as internal arteries and hypodense halo, successfully predict the presence of mVI and consequently survival.22,24 However, these results differ from ours. While these prior studies have relied on a consensus review to determine the presence or absence of a semantic feature, our study relied on each operator independently determining the same. Thus, it is likely that this methodology resulted in the interobserver variability in our cohort. On the other hand, our results show that quantitative image features exist that are robust against interobserver variability.
Our method requires a radiologist to trace the borders of these tumors, which is time-consuming. It appears, and in other similar studies,33 that quantitative features may be expressed globally and hence insensitive to the exact location of the ROIs. Thus, we believe that this task can be accomplished by radiologists, and perhaps trained technicians, as well as by computer algorithms. However, further studies are required to prove this.
Our study has the following limitations. We analyzed data in a small cohort of patients with HCC in a single institution, which likely does not fully capture the variability of the disease. In addition, all patients in our cohort underwent surgical resection. Larger cohorts, including liver transplant patients, are needed to validate our findings and the accuracy of arterial and venous radiomics features to predict mVI. Also, our model was developed on a relatively homogenous cohort with regards to CT acquisition and reconstruction while effects of these on radiomics features have been reported in the literature.44–49
Although preliminary, our work shows that our approach using radiomics analysis of CT is promising for the noninvasive preoperative assessment of mVI in HCC and suggests that further study in larger and external cohorts is warranted.
Acknowledgments
This work was supported by the National Institutes of Health (Grants No. R01 CA160251, U01 CA190214 and U01 CA187947). The authors acknowledge Dr. Daniel L. Rubin for providing access to ePAD, the software tool used to generate all semantic and ROI annotations in this study.
Biography
Biographies for the authors are not available.
References
- 1.Surveillance, Epidemiology, and End Results (SEER) Program, SEER statistics database, http://www.seer.cancer.gov (3 February 2017).
- 2.El Khoury A. C., et al. , “Economic burden of hepatitis C-associated diseases in the United States,” J. Viral Hepatitis 19(3), 153–160 (2012). 10.1111/jvh.2012.19.issue-3 [DOI] [PubMed] [Google Scholar]
- 3.Mishra A., et al. , “The inpatient economic and mortality impact of hepatocellular carcinoma from 2005 to 2009: analysis of the US nationwide inpatient sample,” Liver Int. 33(8), 1281–1286 (2013). 10.1111/liv.2013.33.issue-8 [DOI] [PubMed] [Google Scholar]
- 4.Lang K., et al. , “The burden of illness associated with hepatocellular carcinoma in the United States,” J. Hepatol. 50(1), 89–99 (2009). 10.1016/j.jhep.2008.07.029 [DOI] [PubMed] [Google Scholar]
- 5.Bruix J., Sherman M., “Management of hepatocellular carcinoma: an update,” Hepatology 53(3), 1020–1022 (2011). 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet J. M., Fuster J., Bruix J., “The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma,” Liver Transpl. 10(2 Suppl 1), S115–S120 (2004). 10.1002/(ISSN)1527-6473 [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V., et al. , “Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis,” N. Engl. J. Med. 334(11), 693–700 (1996). 10.1056/NEJM199603143341104 [DOI] [PubMed] [Google Scholar]
- 8.Portolani N., et al. , “Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications,” Ann. Surg. 243(2), 229–235 (2006). 10.1097/01.sla.0000197706.21803.a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clavien P. A., et al. , “Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report,” Lancet Oncol. 13(1), e11–e22 (2012). 10.1016/S1470-2045(11)70175-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakai T., et al. , “Anatomic resection independently improves long-term survival in patients with T1–T2 hepatocellular carcinoma,” Ann. Surg. Oncol. 14(4), 1356–1365 (2007). 10.1245/s10434-006-9318-z [DOI] [PubMed] [Google Scholar]
- 11.Mazzaferro V., et al. , “Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis,” Lancet Oncol. 10(1), 35–43 (2009). 10.1016/S1470-2045(08)70284-5 [DOI] [PubMed] [Google Scholar]
- 12.Imamura H., et al. , “Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy,” J. Hepatol. 38(2), 200–207 (2003). 10.1016/S0168-8278(02)00360-4 [DOI] [PubMed] [Google Scholar]
- 13.Lim K. C., et al. , “Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria,” Ann. Surg. 254(1), 108–113 (2011). 10.1097/SLA.0b013e31821ad884 [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Peralvarez M., et al. , “A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability,” Ann. Surg. Oncol. 20(1), 325–339 (2013). 10.1245/s10434-012-2513-1 [DOI] [PubMed] [Google Scholar]
- 15.Taketomi A., et al. , “Predictors of extrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma,” Ann. Surg. Oncol. 17(10), 2740–2746 (2010). 10.1245/s10434-010-1076-2 [DOI] [PubMed] [Google Scholar]
- 16.Chan K. M., et al. , “Characterization of hepatocellular carcinoma recurrence after liver transplantation: perioperative prognostic factors, patterns, and outcome,” Asian J. Surg. 34(3), 128–134 (2011). 10.1016/j.asjsur.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 17.Shah S. A., et al. , “Accuracy of staging as a predictor for recurrence after liver transplantation for hepatocellular carcinoma,” Transplantation 81(12), 1633–1639 (2006). 10.1097/01.tp.0000226069.66819.7e [DOI] [PubMed] [Google Scholar]
- 18.Yao F. Y., et al. , “The impact of pre-operative loco-regional therapy on outcome after liver transplantation for hepatocellular carcinoma,” Am. J. Transplant. 5(4 Pt 1), 795–804 (2005). 10.1111/j.1600-6143.2005.00750.x [DOI] [PubMed] [Google Scholar]
- 19.Grasso A., et al. , “Liver transplantation and recurrent hepatocellular carcinoma: predictive value of nodule size in a retrospective and explant study,” Transplantation 81(11), 1532–1541 (2006). 10.1097/01.tp.0000209641.88912.15 [DOI] [PubMed] [Google Scholar]
- 20.Lim K. C., et al. , “Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria,” Br. J. Surg. 99(12), 1622–1629 (2012). 10.1002/bjs.v99.12 [DOI] [PubMed] [Google Scholar]
- 21.Duvoux C., et al. , “Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria,” Gastroenterology 143(4), 986–994.e3 (2012). 10.1053/j.gastro.2012.05.052 [DOI] [PubMed] [Google Scholar]
- 22.Banerjee S., et al. , “A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma,” Hepatology 62(3), 792–800 (2015). 10.1002/hep.27877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal E., et al. , “Decoding global gene expression programs in liver cancer by noninvasive imaging,” Nat. Biotechnol. 25(6), 675–680 (2007). 10.1038/nbt1306 [DOI] [PubMed] [Google Scholar]
- 24.Renzulli M., et al. , “Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma?” Radiology 279(2), 432–442 (2016). 10.1148/radiol.2015150998 [DOI] [PubMed] [Google Scholar]
- 25.Gillies R. J., Kinahan P. E., Hricak H., “Radiomics: images are more than pictures, they are data,” Radiology 278(2), 563–577 (2016). 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aerts H. J., et al. , “Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach,” Nat. Commun. 5, 4006 (2014). 10.1038/ncomms5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Napel S., Giger M., “Special section guest editorial:radiomics and imaging genomics: quantitative imaging for precision medicine,” J. Med. Imaging 2(4), 041001 (2015). 10.1117/1.JMI.2.4.041001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar V., et al. , “Radiomics: the process and the challenges,” Magn. Reson. Imaging 30(9), 1234–1248 (2012). 10.1016/j.mri.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambin P., et al. , “Radiomics: extracting more information from medical images using advanced feature analysis,” Eur. J. Cancer 48(4), 441–446 (2012). 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin D. L., et al. , “Automated tracking of quantitative assessments of tumor burden in clinical trials,” Transl. Oncol. 7(1), 23–35 (2014). 10.1593/tlo.13796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch G. G., “Intraclass correlation coefficient,” in Encyclopedia of Statistical Sciences, Kotz S., Johnson N. L., Eds., Vol. 4, pp. 213–217, Wiley, New York: (1982). [Google Scholar]
- 32.Gevaert O., et al. , “Glioblastoma multiforme: exploratory radiogenomic analysis by using quantitative image features,” Radiology 273, 131731 (2014). 10.1148/radiol.14131731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Echegaray S., et al. , “Core samples for radiomics features that are insensitive to tumor segmentation: method and pilot study using CT images of hepatocellular carcinoma,” J. Med. Imaging 2(4), 041011 (2015). 10.1117/1.JMI.2.4.041011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tibshirani R., “Regression shrinkage and selection via the lasso,” J. R. Stat. Soc. Ser. B 73, 267–282 (1996). 10.1111/j.1467-9868.2011.00771.x [DOI] [Google Scholar]
- 35.Zou H., Hastie T., “Regularization and variable selection via the elastic net,” J. R. Stat. Soc. Ser. B 67(2), 301–320 (2005). 10.1111/j.1467-9868.2005.00503.x [DOI] [Google Scholar]
- 36.Hanley J. A., McNeil B. J., “The meaning and use of the area under a receiver operating characteristic (ROC) curve,” Radiology 143(1), 29–36 (1982). 10.1148/radiology.143.1.7063747 [DOI] [PubMed] [Google Scholar]
- 37.Hanley J. A., McNeil B. J., “A method of comparing the areas under receiver operating characteristic curves derived from the same cases,” Radiology 148(3), 839–843 (1983). 10.1148/radiology.148.3.6878708 [DOI] [PubMed] [Google Scholar]
- 38.Marcelja S., “Mathematical description of the responses of simple cortical cells,” J. Opt. Soc. Am. 70(11), 1297–300 (1980). 10.1364/JOSA.70.001297 [DOI] [PubMed] [Google Scholar]
- 39.Lambrou T., Linney A., Todd-Pokropek A., “Wavelet-based analysis and classification of liver CT,” in Medical Images and Signals IRC, p. 53 (2005). [Google Scholar]
- 40.Ilonen J., et al. , “Image feature localization by multiple hypothesis testing of Gabor features,” IEEE Trans. Image Process. 17(3), 311–325 (2008). 10.1109/TIP.2007.916052 [DOI] [PubMed] [Google Scholar]
- 41.Ayres F. J., Rangayvan R., “Characterization of architectural distortion in mammograms,” IEEE Eng. Med. Biol. Mag. 24(1), 59–67 (2005). 10.1109/MEMB.2005.1384102 [DOI] [PubMed] [Google Scholar]
- 42.Daubechies I., Ten Lectures on Wavelets, SIAM, Philadelphia: (1992). [Google Scholar]
- 43.Schlansky B., et al. , “Waiting time predicts survival after liver transplantation for hepatocellular carcinoma: a cohort study using the United Network for Organ Sharing registry,” Liver Transpl. 20(9), 1045–1056 (2014). 10.1002/lt.23917 [DOI] [PubMed] [Google Scholar]
- 44.Solomon J., et al. , “Quantitative features of liver lesions, lung nodules, and renal stones at multi-detector row CT examinations: dependency on radiation dose and reconstruction algorithm,” Radiology 279(1), 185–194 (2016). 10.1148/radiol.2015150892 [DOI] [PubMed] [Google Scholar]
- 45.Li X., et al. , “Lung nodule detection in pediatric chest CT: quantitative relationship between image quality and radiologist performance,” Med. Phys. 38(5), 2609–2618 (2011). 10.1118/1.3582975 [DOI] [PubMed] [Google Scholar]
- 46.Lo P., et al. , “Variability in CT lung-nodule quantification: effects of dose reduction and reconstruction methods on density and texture based features,” Med. Phys. 43(8), 4854–4865 (2016). 10.1118/1.4954845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu L., et al. , “Assessing agreement between radiomic features computed for multiple CT imaging settings,” PLoS One 11(12), e0166550 (2016). 10.1371/journal.pone.0166550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young S., et al. , “Variability in CT lung-nodule volumetry: effects of dose reduction and reconstruction methods,” Med. Phys. 42(5), 2679–2689 (2015). 10.1118/1.4918919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao B., et al. , “Exploring variability in CT characterization of tumors: a preliminary phantom study,” Transl. Oncol. 7(1), 88–93 (2014). 10.1593/tlo.13865 [DOI] [PMC free article] [PubMed] [Google Scholar]