Abstract
Objective
The relationship between arthroplasty and long-term opioid use in patients with knee or hip osteoarthritis is not well studied. We examined the prevalence, patterns and predictors of persistent opioid use after hip or knee arthroplasty.
Method
Using claims data (2004–2013) from a U.S. commercial health plan, we identified adults who underwent hip or knee arthroplasty and filled ≥1 opioid prescription within 30 days after the surgery. We defined persistent opioid users as patients who filled ≥1 opioid prescription every month during the 1-year postoperative period based on group-based trajectory models. Multivariable logistic regression was used to determine preoperative predictors of persistent opioid use after surgery.
Results
We identified 57,545 patients who underwent hip or knee arthroplasty. The mean±SD age was 61.5±7.8 years and 87.1% had any opioid use preoperatively. Overall, 7.6% persistently used opioids after the surgery. Among patients who used opioids in 80% of the time for ≥4 months preoperatively (n=3,023), 72.1% became persistent users. In multivariable analysis, knee arthroplasty vs. hip, a longer hospitalization stay, discharge to a rehabilitation facility, preoperative opioid use (e.g., a longer duration and greater dosage and frequency), a higher comorbidity score, back pain, rheumatoid arthritis, fibromyalgia, migraine and smoking, and benzodiazepine use at baseline were strong predictors for persistent opioid use (C-statistic=0.917).
Conclusion
Over 7% of patients persistently used opioids in the year after hip or knee arthroplasty. Given the adverse health effects of persistent opioid use, strategies need to be developed to prevent persistent opioid use after this common surgery.
Keywords: opioid, joint arthroplasty, risk factor, osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is a highly prevalent, chronic condition, affecting nearly one-third of adults aged 65 years and older in the U.S.(1) and its prevalence continues to rise. Hip and knee OA is the most common type of OA, generally treated with a combination of non-pharmacologic interventions such as exercise, patient education and physical therapy, and medications including acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), and intra-articular glucocorticoids.(2) For patients with moderate to severe symptomatic OA resistant to the aforementioned drugs or those who cannot tolerate NSAIDs, opioid analgesics can be prescribed with caution. For patients with severe OA unrelieved by medical treatment, total joint replacement (TJR) is often considered,(2) as TJR is an effective surgical intervention leading to significant improvement in quality of life, pain and function in most patients with severe hip or knee OA.(3, 4) Between 2010 and 2011, there were an estimated 1.3–1.4 million inpatient joint replacement procedures including total knee or hip replacement or arthroplasty in the U.S.(5)
Over the past two decades, overuse of prescription opioid medications in the U.S. has become a serious public health concern.(6) Compared to the years between 1988 and 1994, when 3.4% of US adults had any use of opioid analgesics, the use of these agents has more than doubled.(6, 7) In older patients such as those with OA, due to the known cardiovascular risks of NSAIDs, the threshold for using opioids has decreased; opioids have been used more frequently in the elderly or those with cardiovascular risk factors.(8) A recent study using the Medicare Current Beneficiary Survey showed that 40% of a cohort of elderly patients with mean age of 77 received an opioid prescription in 2009.(9) However, in a Cochrane systematic review of 22 randomized controlled trials, opioids were noted to have only a small clinical benefit but a high risk of side effects, including opioid dependency and withdrawal in patients with hip and knee OA.(10) Furthermore, preoperative use of opioids may lead to persistent opioid use and poor clinical outcomes including pain, stiffness, and requirement of additional surgery following TJR.(11–14) The objective of this study was to examine the prevalence, patterns and predictors of persistent opioid use after hip or knee arthroplasty in a population-based cohort of patients who underwent hip or knee arthroplasty.
METHODS
Data source
We used claims data from a large U.S. commercial health insurance, United HealthCare/Optum Clinformatics® Data Mart Database, from January 1, 2004 to December 31, 2013. This database has been described in detail elsewhere.(15) Briefly, it includes, at any particular time point, more than 13 million fully-insured subscribers with medical and pharmacy coverage from across the United States and provides patients’ demographic information as well as longitudinal data on inpatient and outpatient medical claims, procedure claims, and pharmacy claims. The quality of these data in accurately capturing inpatient diagnoses, procedures, health care utilization and drug dispensing as well as some outpatient diagnoses is known to be high.(16) A prior validation study reported that there was 96% agreement between medical diagnoses defined using claims data and the medical records or the patient surveys.(17) The use of this de-identified database was approved by the Institutional Review Board of the Brigham and Women’s Hospital and informed consent was not required.
Study population
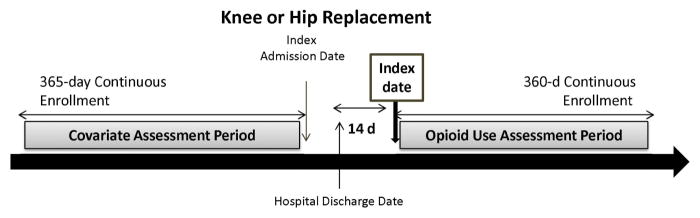
We selected adults aged ≥50 years who were hospitalized for a total hip or knee arthroplasty (i.e., index admission). The index date was defined as the 14th day following the hospital discharge date from the index admission. Patients were required to have a 1-year continuous enrollment period prior to the index admission date and a 1-year continuous enrollment period after the index date (Figure 1). Patients were excluded if they had a diagnosis of any malignancy or underwent a surgery for fracture, hip or knee arthroplasty in the year prior to the index date.
Figure 1. Overview of study design.
The index date was defined as the 14th day following the hospital discharge date from the index admission. Patients were required to have a 1-year continuous enrollment period prior to the index admission date and a 360-day continuous enrollment period after the index date.
Outcome Definition
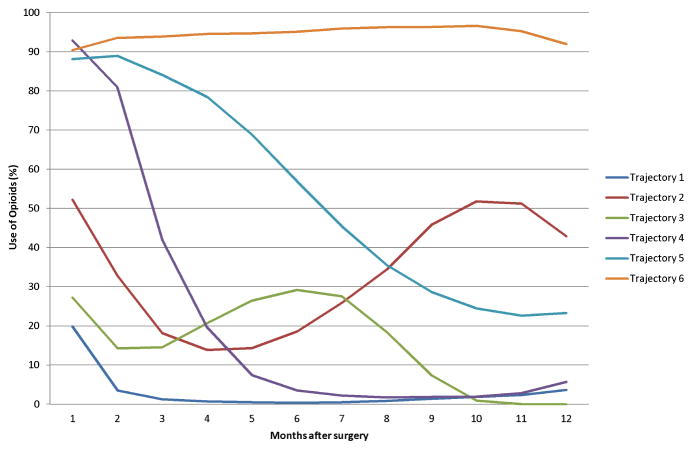
The primary outcome of interest was persistent use of opioids (i.e., hydrocodone, codeine, oxycodone, meperidine, hydromorphone, morphine, fentanyl, methadone, and oxymorphone) in the year following the index date. Persistent use was defined as having any use of opioid prescriptions in each of the 12 months continuously based on a group-based trajectory modeling (GBTM) and identified as Trajectory 6 in Figure 2. We utilized this GBTM method to identify clinically distinct, dynamic patterns of opioid use over the 12-month time period. GBTM has been used to describe longitudinal trajectories and summarize long-term medication adherence.(18–21)
Figure 2. Trajectories of opioid use after hip or knee arthroplasty.
Among 57,545 patients who underwent hip or knee arthroplasty, 7.6% (Trajectory 6) of the study cohort had persistent opioid use in the year following surgery. Trajectories 1 (51.0%), 2 (8.1%), 3 (8.8%), 4 (16.8%), and 5 (7.6%) were considered as nonpersistent users.
Covariate Assessment
During the baseline 365-day period prior to the index date, we assessed a number of pre-defined variables potentially related to persistent use of opioids. These variables were demographic factors (i.e., age, sex), type and characteristics of joint arthroplasty (i.e., surgical site, year of surgery, length of in-hospital stay, bilateral/unilateral, discharge type), preoperative opioid use characteristics [i.e., any use, duration of opioid use, single or multiple opioid agents, cumulative dose, number of months with a proportion of days covered (PDC) with opioids >80%], presence of chronic pain-related comorbidities including back pain, fibromyalgia, rheumatoid arthritis, herpes zoster with neuropathy, alcoholism, other substance abuse history, and smoking, a comorbidity score that combined 20 conditions in the Charlson and Elixhauser measures,(22) use of other prescription drugs including benzodiazepines, antidepressants, oral steroids, NSAIDs, gabapentin and pregabalin), and healthcare use pattern(23) listed in Table 1. Assuming the opioid medication was taken regularly based on the days supply and quantity dispensed, we calculated oral morphine equivalent (OME) daily dose after converting from the prescribed opioid to OME based on potency.(24)
Table 1.
Baseline characteristics of study patients in 365 days prior to joint replacement surgery
| All (N=57,545) | Persistent opioid users (N=4,394) | Non-persistent opioid users (N=53,151) | |
|---|---|---|---|
| Percentage or mean ± standard deviation | |||
|
| |||
| Demographic Factors | |||
|
| |||
| Age, years | 61.5 ± 7.8 | 59.7 ± 7.3 | 61.7 ± 7.9 |
| 50–59 | 45.5 | 56.0 | 44.6 |
| 60–69 | 39.1 | 33.5 | 39.5 |
| 70+ | 15.4 | 10.5 | 15.9 |
| Female | 57.0 | 61.8 | 56.6 |
|
| |||
| Surgical Factors | |||
|
| |||
| Bilateral surgery | 0.7 | 0.5 | 0.7 |
| Knee surgery | 68.5 | 72.4 | 68.1 |
| Year of surgery | |||
| 2004 | 0.3 | 0.2 | 0.3 |
| 2005 | 8.6 | 7.8 | 8.6 |
| 2006 | 10.4 | 9.8 | 10.5 |
| 2007 | 11.4 | 10.7 | 11.5 |
| 2008 | 14.1 | 13.7 | 14.1 |
| 2009 | 13.4 | 14.0 | 13.3 |
| 2010 | 13.9 | 14.7 | 13.8 |
| 2011 | 14.2 | 15.0 | 14.2 |
| 2012 | 13.8 | 14.2 | 13.7 |
| No. of days for in-hospital stay | |||
| 1–2 | 3.7 | 2.5 | 3.8 |
| 3–5 | 85.5 | 82.7 | 85.8 |
| 6–8 | 7.7 | 10.4 | 7.5 |
| 9–10 | 1.3 | 1.7 | 1.3 |
| 11+ | 1.8 | 2.7 | 1.7 |
| Discharge to a rehabilitation facility | 14.5 | 17.3 | 14.3 |
|
| |||
| Preoperative Opioid Use | |||
|
| |||
| Any opioid use | 87.1 | 98.9 | 86.1 |
| No. of months with opioid use | 2.3 ± 2.7 | 8.0 ± 3.8 | 1.8 ± 1.9 |
| No. of months with mean OME dose>100mg/day | 0.1 ± 1.1 | 1.4 ± 3.4 | 0.0 ± 0.4 |
| No. of months with PDC >80% | |||
| 0 | 88.7 | 31.2 | 93.4 |
| 1–3 | 6.1 | 19.2 | 5.0 |
| 4+ | 5.2 | 49.6 | 1.6 |
| Single opioid agent | 45.6 | 23.4 | 47.5 |
| Multiple opioid agents | 41.5 | 75.5 | 38.7 |
|
| |||
| Comorbidities | |||
|
| |||
| Alcoholism | 1.6 | 2.7 | 1.5 |
| Back pain | 40.8 | 63.8 | 38.9 |
| Fibromyalgia | 8.2 | 17.9 | 7.4 |
| Migraine | 7.9 | 14.8 | 7.3 |
| Osteoarthritis | 99.9 | 99.8 | 99.9 |
| Rheumatoid arthritis | 5.1 | 10.4 | 4.6 |
| SLE | 1.3 | 2.4 | 1.2 |
| Herpes zoster with neuropathy | 0.2 | 0.3 | 0.1 |
| Tobacco use | 12.3 | 19.4 | 11.7 |
| Substance abuse | 0.7 | 4.2 | 0.4 |
| Comorbidity score | 0.5 ± 1.4 | 0.9 ± 7.8 | 0.5 ± 1.4 |
|
| |||
| Medications | |||
|
| |||
| Antidepressants | 25.8 | 50.2 | 23.8 |
| Benzodiazepines | 19.0 | 44.1 | 16.9 |
| NSAIDs/Coxibs | 56.1 | 65.4 | 55.4 |
| Oral steroids | 25.9 | 38.0 | 24.9 |
| Gabapentin/pregabalin | 3.3 | 10.0 | 2.7 |
|
| |||
| Healthcare Utilization | |||
|
| |||
| No. of acute hospitalizations | |||
| 1 | 87.5 | 77.6 | 88.3 |
| 2 | 10.5 | 17 | 10.0 |
| 3+ | 2.0 | 5.4 | 1.7 |
PDC: proportion of days covered; SLE: systemic lupus erythematosus, NSAID: non-steroidal anti-inflammatory drug, Coxib: selective cyclooxygenase-2 inhibitor, OME: oral morphine equivalent,
Statistical Analysis
We assessed baseline characteristics of the entire study sample. To identify persistent users of opioids after hip or knee arthroplasty, we first created a ‘medication diary’ for each patient in our cohort indicating whether a prescription for one or more opioids was dispensed as a binary variable during each of the 12 consecutive 30-day periods after the index date. We then modeled these observed opioid dispensing patterns (i.e., 12 binary indicators) as a longitudinal response in a logistic GBTM.(25, 26) In this GBTM, the log odds of having any opioid dispensing (yes/no) during each 30-day period was estimated as a smooth function of time separately within each group. The model also estimated the probability of group membership for each patient, and patients were assigned to the group with the highest membership probability. We estimated our model using 6 adherence groups, which have been observed in prior research to provide the best overall model fit.(18) We then defined the group of patients having any opioid dispensing in each of all 12 30-day periods after the index date as “persistent users.” The model was estimated using “Proc Traj,” in SAS (SAS, Version 9.2, Cary, NC). Further details on the model and specific modeling choices have been previously described.(18) To determine baseline characteristics that predict persistent opioid use after the surgery, we constructed a multivariable logistic regression model with persistent opioid use (present versus absent) as the dependent variable and the aforementioned covariates as the independent variables. The c-statistic for predicting persistent opioid use was calculated. For internal validation of the model prediction performance, we performed 10-fold cross-validation.(27) In a sensitivity analysis, we assessed the predictors of persistent opioid use stratified by the presence of preoperative opioid use.
RESULTS
Study Population
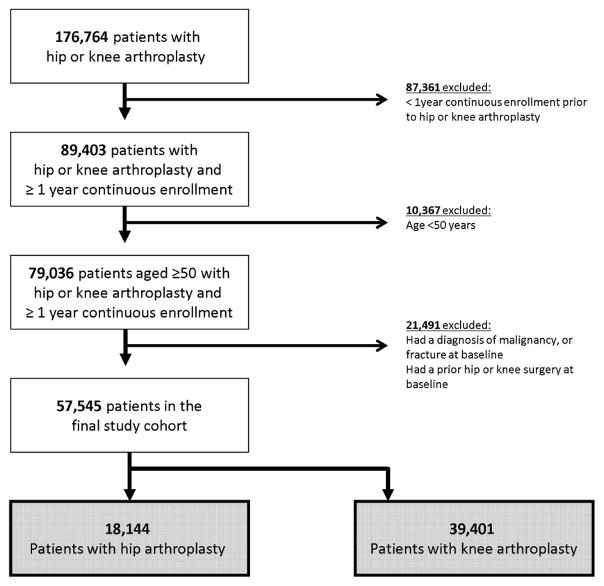
After applying inclusion and exclusion criteria, we included a total of 57,545 patients who underwent either hip or knee joint replacement between 2004 and 2013 (Figure 3). The mean (SD) age was 61.5 (7.8) years and 57.0% were female. Of these, 68.5% had a knee surgery and 31.5% had a hip surgery (Table 1). Prior to the surgery, 50,120 patients (87.1%) had at least one opioid dispensing and 10,148 (17.6%) used opioids for at least 4 months. There were 3,023 patients (5.2%) who used opioids in 80% of the time (i.e., PDC≥80%) for at least 4 months prior to the surgery. The mean (SD) duration of opioid use prior to the surgery was 2.3 (2.7) months. More than one type of opioid prescriptions were used by 41.5% of the patients. Back pain (40.8%) and fibromyalgia (8.2%) were common comorbidities. Use of NSAIDS including coxibs (56.1%) and benzodiazepines (19.0%) was also common.
Figure 3. Cohort Selection Flow.
The final study cohort included 57,545 patients who underwent either hip or knee arthroplasty.
Persistent Opioid Use
As illustrated in Figure 2, GBTM identified 6 distinct trajectories of post-TJR opioid use. There were 29,352 (51.0%) patients who had no or minimal opioid use beyond 3 months postoperatively (Trajectory 1). 4,683 (8.1%) patients had a decrease in opioid use in the first 6 months after TJR but then they had an increase in opioid use in the 6–12 month post-TJR period (Trajectory 2). Patients seen in Trajectories 3 (8.8%), 4 (16.8%) and 5 (7.6%) gradually had a decrease in the use of opioids over time. There were 4,394 (7.6%, Trajectory 6) persistent opioid users in the year following the surgery. Compared to non-persistent users (Trajectories 1 through 5), persistent opioid users (Trajectory 6) were younger, more likely to be female and have a greater preoperative use of opioids, more painful comorbidities and prescription drug use (Table 1). In both persistent and nonpersistent opioid users, hydrocodone was the most commonly used opioid agent (55.2%) preoperatively, followed by oxycodone (21.8%). After the surgery, oxycodone (50.9% in persistent opioid users and 48.2% in non-persistent users) was the most commonly used opioid at the time of hospital discharge followed by hydrocodone (42.9% in persistent opioid users and 35.1% in nonpersistent users).
Preoperative Characteristics Predicting Persistent Opioid Use
Multivariable logistic regression analysis (Table 2) showed a number of significant predictors of persistent opioid use after surgery. Patients who underwent a knee arthroplasty versus hip [adjusted odds ratio (aOR) 1.75, 95% confidence interval (CI) 1.59–1.93], who were discharged to an acute rehabilitation facility versus others (aOR 1.26, 95% CI 1.12–1.42) and who had a longer length of stay during the index admission (aOR 1.04 for an additional day of stay, 95% CI 1.02–1.06) were more likely to use opioids persistently after surgery. Preoperative opioid use patterns were strong predictors of persistent opioid use following surgery: any preoperative opioid use (aOR 1.72, 95% CI 1.27–2.33), a longer duration of opioid use preoperatively (aOR 1.47, 95% CI 1.45–1.50), ≥1 month with a OME dose ≥100mg per day (aOR 1.44, 95% CI 1.19–1.73), and a greater number of months with PDC ≥80% for opioids (aOR 4.54 for more than 7 months versus none, 95% CI 3.79–5.45). Among other medications, benzodiazepine use during the baseline period was also a predictor of persistent opioid use (aOR 1.42, 95% CI 1.29–1.56). A higher comorbidity score (aOR 1.03, 95% CI 1.00–1.06), smoking (aOR 1.20, 95% CI 1.07–1.35) and presence of chronic pain-related comorbidities such as back pain (aOR 1.28, 95% CI 1.17–1.40), fibromyalgia (aOR 1.22, 95% CI 1.07–1.39), migraine (aOR 1.19, 95% CI 1.04–1.36) and rheumatoid arthritis (aOR 1.40, 95% CI 1.20–1.65) were also significant predictors of persistent opioid use. The c-statistic of the multivariable logistic model was 0.917, indicating that the model could accurately identify persistent opioid users. The 10-fold cross-validated C statistic for the prediction model was 0.915, suggesting excellent internal validity.
Table 2.
Predictors of persistent opioid users in the entire cohort (n=57,545)
| Multivariable Odds Ratio a | 95% Confidence Interval | |
|---|---|---|
| Demographics | ||
|
| ||
| Age 50–59 | 1.02 | 0.88–1.17 |
| Age 60–69 | 0.95 | 0.83–1.10 |
| Age ≥70 | Reference | - |
| Female v. male | 1.03 | 0.94–1.13 |
|
| ||
| Surgery characteristics | ||
|
| ||
| Knee surgery v. hip | 1.75 | 1.59–1.93* |
| Discharge to a rehabilitation facility | 1.26 | 1.12–1.42* |
| No. of days for in-hospital stay | 1.04 | 1.02–1.06* |
|
| ||
| Preoperative opioid use | ||
|
| ||
| Any opioid use | 1.72 | 1.27–2.33* |
| No. of months with opioid use | 1.47 | 1.45–1.50* |
| ≥1 month with a mean OME dose>100mg/day | 1.44 | 1.19–1.73* |
| No. of months with PDC >80% | ||
| 0 | Reference | - |
| 1–3 | 2.53 | 2.25–2.84* |
| 4–6 | 3.31 | 2.79–3.94* |
| 7+ | 4.54 | 3.79–5.45* |
| Multiple opioid agents | 1.04 | 0.95–1.15 |
|
| ||
| Comorbidities | ||
|
| ||
| Alcoholism | 0.93 | 0.70–1.25 |
| Cocaine use | 3.59 | 0.95–13.5 |
| Marijuana use | 1.14 | 0.33–3.98 |
| Fibromyalgia | 1.22 | 1.07–1.39* |
| Migraine | 1.19 | 1.04–1.36* |
| Rheumatoid arthritis | 1.40 | 1.20–1.65* |
| SLE | 1.11 | 0.81–1.53 |
| Back pain | 1.28 | 1.17–1.40* |
| Tobacco use | 1.20 | 1.07–1.35* |
| Substance abuse | 1.17 | 0.84–1.64 |
| Comorbidity score | 1.03 | 1.00–1.06* |
|
| ||
| Medications | ||
|
| ||
| Antidepressants | 1.08 | 0.99–1.19 |
| Benzodiazepines | 1.42 | 1.29–1.56* |
| NSAIDs/Coxibs | 1.01 | 0.92–1.10 |
| Oral steroids | 1.02 | 0.93–1.12 |
| Gabapentin/pregabalin | 1.10 | 0.93–1.31 |
|
| ||
| Healthcare Utilization | ||
|
| ||
| No. of acute hospitalizations | 0.93 | 0.87–1.00* |
Additionally adjusted for the index year
OME: oral morphine equivalent, NSAID: non-steroidal anti-inflammatory drug, Coxib: selective cyclooxygenase-2 inhibitor, PDC: proportion of days covered, SLE: systemic lupus erythematosus
P-value <0.05
In a sensitivity analysis stratified by the preoperative use of opioids, 50,120 (87.1% of the total cohort) prior opioid users and 7,425 (12.9%) opioid-naïve patients were analyzed. After the surgery, 4,346 (8.67%) prior opioid users and 48 (0.65%) opioid-naïve patients became persistent opioid users. In patients who used opioids in 80% of the time for ≥4 months preoperatively (n=3,023), 72.1% became persistent users. Among patients with prior opioid use, multivariable logistic regression (c-statistic=0.917) showed similar significant predictors of persistent opioid use as noted in the main analysis (Table 3). Among the opioid-naïve subgroup, a greater comorbidity score (aOR 1.25, 95% CI 1.08–1.44) and discharge to an acute rehabilitation facility (aOR 2.24, 95% CI 1.21–4.13) were significant predictors of persistent opioid use. The c-statistic of the multivariable logistic model in the opioid-naïve subgroup was 0.752.
Table 3.
Predictors of persistent opioid users in the subgroup with preoperative opioid use (n=50,120)
| Multivariable Odds Ratio a | 95% Confidence Interval | |
|---|---|---|
| Demographics | ||
|
| ||
| Age 50–59 | 1.00 | 0.86–1.15 |
| Age 60–69 | 0.94 | 0.81–1.08 |
| Age ≥70 | Reference | - |
| Female v. male | 1.03 | 0.94–1.13 |
|
| ||
| Surgery characteristics | ||
|
| ||
| Knee surgery v. hip | 1.75 | 1.59–1.93* |
| Discharge to a rehabilitation facility | 1.23 | 1.09–1.39* |
| No. of days for in-hospital stay | 1.04 | 1.02–1.06* |
|
| ||
| Preoperative opioid use | ||
|
| ||
| No. of months with opioid use | 1.48 | 1.45–1.50* |
| ≥1 month with a mean OME dose>100mg/day | 1.44 | 1.20–1.74* |
| No. of months with PDC >80% | ||
| 0 | Reference | - |
| 1–3 | 2.54 | 2.26–2.85* |
| 4–6 | 3.32 | 2.79–3.95* |
| 7+ | 4.54 | 3.79–5.44* |
| Multiple opioid agents | 1.05 | 0.95–1.16 |
|
| ||
| Comorbidities | ||
|
| ||
| Alcoholism | 0.92 | 0.68–1.23 |
| Cocaine use | 3.74 | 0.99–14.1 |
| Marijuana use | 1.15 | 0.33–4.02 |
| Fibromyalgia | 1.21 | 1.06–1.38* |
| Migraine | 1.19 | 1.05–1.37* |
| Rheumatoid arthritis | 1.42 | 1.21–1.67* |
| SLE | 1.11 | 0.81–1.53 |
| Back pain | 1.27 | 1.16–1.39* |
| Tobacco use | 1.21 | 1.08–1.36* |
| Substance abuse | 1.19 | 0.85–1.66 |
| Comorbidity score | 1.02 | 0.99–1.05 |
|
| ||
| Medications | ||
|
| ||
| Antidepressants | 1.08 | 0.98–1.18 |
| Benzodiazepines | 1.43 | 1.30–1.57* |
| NSAIDs/Coxibs | 1.00 | 0.91–1.09 |
| Oral steroids | 1.02 | 0.93–1.12 |
| Gabapentin/pregabalin | 1.11 | 0.93–1.32 |
|
| ||
| Healthcare Utilization | ||
|
| ||
| No. of acute hospitalizations | 0.93 | 0.86–1.00* |
Additionally adjusted for the index year
OME: oral morphine equivalent, NSAID: non-steroidal anti-inflammatory drug, Coxib: selective cyclooxygenase-2 inhibitor, PDC: proportion of days covered, SLE: systemic lupus erythematosus
P-value <0.05
DISCUSSION
Preoperative use of opioids in patients with hip or knee OA was highly prevalent as over 87% of the study population received at least one opioid dispensing in the year prior to undergoing hip or knee arthroplasty. However, intensive preoperative opioid use was uncommon as only 5.2% of the cohort used opioids in ≥80% of the time (PDC ≥80%) for at least 4 months prior to the arthroplasty. In general, joint replacement surgery is highly effective in moderate-to-severe hip or knee OA and 51% of our study patients had no or minimal use of opioids 3 months after the surgery. However, 7% of the study population had persistent use of opioids in the year following the surgery. It is particularly important to note that among patients who had intensive opioid use preoperatively for at least 4 months, over 72% were persistent users following the surgery. Presence of chronic pain-related comorbidities and use of benzodiazepines prior to the surgery were also significant predictors of persistent opioid use following the surgery.
This study highlights several important issues in the management of moderate-to-severe hip or knee OA and pre- and post-operative pain management in patients undergoing hip or knee arthroplasty in the U.S. Similar to what has been noted in the overall U.S. population, the use of opioids even in the older age group is highly prevalent. A recent Australian-cohort study (n=9,525) showed that 44% of their patients used any opioid analgesics prior to total hip replacement compared with 87% in our study.(28) Opioids are effective in reducing acute pain, but in a Cochrane systematic review of 22 randomized controlled trials in patients with hip and knee OA they were shown to have only a small clinical benefit with a high risk of side effects as well as opioid dependency.(10) Similarly, no significant differences in pain reduction were found between opioids and NSAIDs and between less potent and potent opioids in patients with knee OA, based on a systematic review of 17 randomized controlled trials.(29) In response to the current prescription opioid crisis in the U.S., the Centers for Disease Control and Prevention (CDC) developed and published recommendations for prescribing opioids in patients with chronic non-cancer pain.(30) While it is important that patients receive appropriate pain treatment, the CDC guideline emphasizes the need to improve communication between clinicians and patients about the risks and benefits of opioid therapy for chronic pain to improve the safety and effectiveness of pain treatment, and reduce the risks associated with long-term opioid therapy, including opioid use disorder, overdose, and death. Given the limited utility of opioids for chronic management of patients with OA, use of intensive, long-term and/or high-potency opioids should be minimized in these patients. Furthermore, concurrent use of opioids and benzodiazepines which was seen in 19% of the total cohort should be avoided or at least minimized as use of both drugs at the same time put patients at greater risk for persistent opioid use as well as potentially fatal overdose.
Persistent opioid use before and after surgery may be associated with poor clinical outcomes including pain, stiffness, and revision surgery following TJR.(11–14, 31) A cohort study of 12,859 patients who had total hip replacement showed that higher OME doses in days 91–180 post-surgery were significantly associated with a greater risk of 1-year and 5-year revision.(31) Similar findings were also reported in the aforementioned Australian cohort of total hip replacement patients, (32) although whether persistent opioid use is a causative factor for revision TJR surgery or TJR failure, or a surrogate or intermediate endpoint of TJR failure needs to be further studied. Nevertheless, it would be clinically crucial to identify patients who are at risk of persistent opioid use following TJR so that clinicians can provide individualized pain management to those high-risk patients pre- and postoperatively. Particular attention needs to be paid in the preoperative evaluation and/or postoperative pain management of patients who have almost daily opioid use for more than a few months prior to the arthroplasty.
The strengths of this study include the large cohort size and use of comprehensive and longitudinal data on patients’ comorbidities, prescription drug use and health care utilization patterns. In addition, we used GBTM to determine different patterns of post-TJR opioid use and to define persistent opioid users. This modeling method estimated probabilities for multiple trajectories simultaneously and showed dynamic patterns of opioid use over time. This method shows more informative patterns of medication use over time than reporting only the proportion of patients taking opioids either as a binary or categorical variable at a given time point.(33)
This study has several limitations. First, we did not have disease or pain severity specific to knee or hip OA. Furthermore, we were unable to determine the indication for opioids. In other words, it is possible that some patients used opioids not for hip or knee OA but for a different health condition preoperatively. We included a number of common chronic-pain related conditions as baseline covariates to address this issue. Second, although the model has high predictability for persistent opioid use, our data do not include information on patients’ functional status, pain level, socioeconomic status, or other behavioral factors related to opioid use. Third, even though our study database has detailed and longitudinal data on any prescription drug dispensing before and after surgery without risk of recall bias, our study cannot assess whether or how often patients actually took the medication as prescribed. However, it is likely that patients with a monthly dispensing of a prescription drug have high adherence or persistence. Fourth, since we relied on diagnosis or procedure codes to define preoperative characteristics, there is a potential for misclassification. Lastly, our findings may not generalize to uninsured patients or those who switched to a different commercial or publicly available (e.g., Medicare) health plans because our cohort is based on a commercially insured patient population and we required patients to be continuously enrolled for at least 1 year prior to surgery.
CONCLUSION
In this population-based cohort of patients with hip or knee OA, over 7% of patients persistently used opioids in the year after hip or knee arthroplasty. The proportion of persistent opioid users after the surgery was much higher at 72.1% among patients who used opioids in ≥80% of the time (PDC ≥80%) for at least 4 months preoperatively. Patterns of preoperative opioid use and presence of other painful comorbidities such as back pain, rheumatoid arthritis, fibromyalgia and migraine and use of benzodiazepines were significant predictors of persistent opioid use following the surgery. Better preoperative pain management for both OA and other painful conditions may reduce the risk of persistent opioid use following hip or knee arthroplasty. Future study should evaluate potential strategies to minimize persistent opioid use, particularly in patients taking these drugs chronically in the preoperative period and study the impact of persistent opioid use on clinical and safety outcomes following hip or knee arthroplasty.
Acknowledgments
Other Research Funding Support: Kim is partly supported by the NIH grant R01 AR069557. Bateman is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the NIH (Bethesda, Maryland, United States) under Award Number K08HD075831.
Footnotes
Potential Conflicts of Interest: Dr. Kim has received research grants to the Brigham and Women’s Hospital from Lilly, Genentech, Pfizer, Bristol-Myers Squibb, Merck and AstraZeneca for unrelated studies. Dr. Franklin has received research grants to the Brigham and Women’s Hospital from Merck for unrelated studies. Dr. Fischer has received research grants to the Brigham and Women’s Hospital from Otsuka America and SureScripts for unrelated studies. Dr Bateman is an investigator on grants to the Brigham and Women’s Hospital from Lilly, Baxalata, GlaxoSmithKlein, and Pfizer unrelated to the topic of this manuscript. Dr Bateman consults for Optum for unrelated projects.
Authors’ contributions: All authors conceived and designed the study, interpreted the data, and critically revised the manuscript for important intellectual content. SCK drafted the paper. SCK has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest disclosures: No specific funding was given for this study.
Kim receives salary support from unrelated grants to Brigham and Women’s Hospital from Lilly, Genentech, Pfizer, Bristol-Myers Squibb, Merck and AstraZeneca for unrelated studies.
Bateman is an investigator on grants to the Brigham and Women’s Hospital from Lilly, Baxalata, GlaxoSmithKline, and Pfizer unrelated to the topic of this manuscript. Bateman consults for Optum for unrelated projects.
Franklin has received research grants to the Brigham and Women’s Hospital from Merck for unrelated studies.
Fischer has received research grants to the Brigham and Women’s Hospital from Otsuka America and SureScripts for unrelated studies.
Choudhry, Bykov, Eikermann and Lii have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.CDC. Osteoarthritis. [Google Scholar]
- 2.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 3.Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113–21. doi: 10.1001/archinternmed.2009.136. discussion 21–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daigle ME, Weinstein AM, Katz JN, Losina E. The cost-effectiveness of total joint arthroplasty: a systematic review of published literature. Best Pract Res Clin Rheumatol. 2012;26(5):649–58. doi: 10.1016/j.berh.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helmick CG. The Burden of Musculoskeletal Diseases in the United States. Table 4.9:Number of Inpatient Arthroplasty Procedures by Type, by Sex, United States, 2010/2011. [Google Scholar]
- 6.Frenk S, Porter K, Paulozzi L. NCHS data brief, no 189. Hyattsville, MD: National Center for Health Statistics; 2015. Prescription Opioid Analgesic Use Among Adults: United States, 1999–2012. [PubMed] [Google Scholar]
- 7.Paulose-Ram R, Hirsch R, Dillon C, Losonczy K, Cooper M, Ostchega Y. Prescription and non-prescription analgesic use among the US adult population: results from the third National Health and Nutrition Examination Survey (NHANES III) Pharmacoepidemiol Drug Saf. 2003;12(4):315–26. doi: 10.1002/pds.755. [DOI] [PubMed] [Google Scholar]
- 8.Parker Vail T. Preoperative pain management decisions impact outcome after total knee arthroplasty-implications for opiate use: commentary on an article by Michael G. Zywiel, MD, et al.: “Chronic opioid use prior to total knee arthroplasty”. J Bone Joint Surg Am. 2011;93(21):e1291–1. doi: 10.2106/JBJS.K.01145. [DOI] [PubMed] [Google Scholar]
- 9.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003–2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken) 2014;66(10):1489–95. doi: 10.1002/acr.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Costa BR, Nuesch E, Kasteler R, Husni E, Welch V, Rutjes AW, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2014;9:CD003115. doi: 10.1002/14651858.CD003115.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher DA, Dierckman B, Watts MR, Davis K. Looks good but feels bad: factors that contribute to poor results after total knee arthroplasty. J Arthroplasty. 2007;22(6 Suppl 2):39–42. doi: 10.1016/j.arth.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Franklin PD, Karbassi JA, Li W, Yang W, Ayers DC. Reduction in narcotic use after primary total knee arthroplasty and association with patient pain relief and satisfaction. J Arthroplasty. 2010;25(6 Suppl):12–6. doi: 10.1016/j.arth.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Pivec R, Issa K, Naziri Q, Kapadia BH, Bonutti PM, Mont MA. Opioid use prior to total hip arthroplasty leads to worse clinical outcomes. Int Orthop. 2014;38(6):1159–65. doi: 10.1007/s00264-014-2298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93(21):1988–93. doi: 10.2106/JBJS.J.01473. [DOI] [PubMed] [Google Scholar]
- 15.Kim SC, Liu J, Solomon DH. Risk of incident diabetes in patients with gout: a cohort study. Arthritis Rheumatol. 2015;67(1):273–80. doi: 10.1002/art.38918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strom BL. Overview of Automated Databases in Pharmacoepidemiology. In: Strom BL, Kimmel SE, editors. Textbook of Pharmacoepidemiology. Philadelphia: John Wiley & Sons, Ltd; 2006. pp. 167–72. [Google Scholar]
- 17.Quam L, Ellis LB, Venus P, Clouse J, Taylor CG, Leatherman S. Using claims data for epidemiologic research. The concordance of claims-based criteria with the medical record and patient survey for identifying a hypertensive population. Med Care. 1993;31(6):498–507. [PubMed] [Google Scholar]
- 18.Franklin JM, Shrank WH, Pakes J, Sanfelix-Gimeno G, Matlin OS, Brennan TA, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51(9):789–96. doi: 10.1097/MLR.0b013e3182984c1f. [DOI] [PubMed] [Google Scholar]
- 19.Twisk J, Hoekstra T. Classifying developmental trajectories over time should be done with great caution: a comparison between methods. J Clin Epidemiol. 2012;65(10):1078–87. doi: 10.1016/j.jclinepi.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Bateman BT, Franklin JM, Bykov K, Avorn J, Shrank WH, Brennan TA, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol. 2016 doi: 10.1016/j.ajog.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods. 1999;4(2):139–57. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 22.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao DD, Jiao PL, Yu JJ, Wang XJ, Zhao L, Xuan Y, et al. Higher Serum Uric Acid Is Associated with Higher Bone Mineral Density in Chinese Men with Type 2 Diabetes Mellitus. Int J Endocrinol. 2016;2016:2528956. doi: 10.1155/2016/2528956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinatra RS. Oral and Parenteral Opioid Analgesics for Acute Pain Management. In: Sinatra RS, de Leon-Cassasola OA, Viscusi ER, Ginsberg B, editors. Acute Pain Management. Cambridge ; New York: Cambridge University Press; 2009. pp. 188–203. [Google Scholar]
- 25.Nagin D. Group-based modeling of development. Harvard University Press; 2005. [Google Scholar]
- 26.Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychological methods. 1999;4(2):139. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 27.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 28.Inacio MC, Hansen C, Pratt NL, Graves SE, Roughead EE. Risk factors for persistent and new chronic opioid use in patients undergoing total hip arthroplasty: a retrospective cohort study. BMJ Open. 2016;6(4):e010664. doi: 10.1136/bmjopen-2015-010664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthritis Cartilage. 2016;24(6):962–72. doi: 10.1016/j.joca.2016.01.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 31.Namba RS, Inacio MC, Pratt NL, Graves SE, Roughead EE, Craig Cheetham T, et al. Postoperative opioid use as an early indication of total hip arthroplasty failure. Acta Orthop. 2016;87(Suppl 1):37–43. doi: 10.1080/17453674.2016.1181820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inacio MC, Pratt NL, Roughead EE, Paxton EW, Graves SE. Opioid use after total hip arthroplasty surgery is associated with revision surgery. BMC Musculoskelet Disord. 2016;17:122. doi: 10.1186/s12891-016-0970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones BL, Nagin DS. Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them. Sociol Method Res. 2007;35:542–71. [Google Scholar]