Abstract
Geschwind Syndrome, a characteristic behavioral syndrome frequently described in patients affected by temporal lobe epilepsy, consists of the following features: hyper-religiosity, hypergraphia, hyposexuality, and irritability. Here we report the 9-year-clinical course of a case of Geschwind Syndrome that developed as a first and salient clinical expression of right temporal lobe variant of frontotemporal lobar degeneration. Only one patient affected by frontotemporal dementia has previously been shown to present with Geschwind Syndrome.
MS presented at age 73 with 3 years of personality and behavioral symptoms. Her early symptoms primarily included hyper-religiosity, hypergraphia, and poor emotional regulation (irritability, impulsivity, disinhibition, egocentric behavior). Over nine years, other cognitive functions (word retrieval, memory coding and recall, set-shifting, famous face and building recognition) became affected; however, hyper-religiosity, hypergraphia, and scarce emotional control remained her most prominent deficits. Longitudinal cortical thickness and volumetric analyses revealed early atrophy in the right temporal pole, right amygdala, and right hippocampus, which progressively affected homologous regions in the left hemisphere. The present case describes an unusual clinical picture associated with frontotemporal dementia, in which the most salient symptoms originated and remained consistent with Geschwind Syndrome.
Keywords: Right temporal lobe, Frontotemporal lobar degeneration, Geschwind Syndrome, Hypergraphia, Hyper-religiosity
1. Introduction
Starting with their seminal works published in 1974 and 1975, Waxman and Geschwind recognized that patients affected by temporal lobe epilepsy (TLE) could develop a specific constellation of symptoms that influenced their affect, personality, and cognition (Bear & Fedio, 1977; Waxman & Geschwind, 1975; Waxman & Geschwind, 1974, see also Devinsky & Schachter, 2009). In most cases, the characteristic behavioral syndrome described is interictal (i.e., present constantly, without a specific relationship to individual seizures) and consists of the following features: hyper-religiosity (increased interest in philosophical, moral, and religious issues), hypergraphia (excessive compulsive writing often of a religious or philosophical nature), hyposexuality, and irritability of varying degrees. Geschwind attributed the syndrome to limbic system damage that accrued during seizures (Waxman & Geschwind, 2005). In the past few decades, several group studies and case reports have described one or more features of what is now referred to as Geschwind Syndrome (Garcia-Santibanez & Sarva, 2015; Hermann, Whitman, & Arntsont, 1983; Okamura et al., 1993; Roberts, Robertson, & Trimble, 1982; Sachdev & Waxman, 1981; Tebartz van Elst et al., 2003; Woollacott et al., 2015; Wuerfel et al., 2004). Some authors have suggested a major involvement of the right hemisphere, with hyper-religiosity and hypergraphia occurring more frequently in patients with non-dominant hemisphere TLE (Roberts et al., 1982; Wuerfel et al., 2004, but see Okamura et al., 1993). Other studies have more specifically implicated bilateral (Tebartz van Elst et al., 2003) or right hemisphere (Wuerfel et al., 2004) hippocampal damage in the manifestation of Geschwind Syndrome in TLE.
Although the behavioral triad of hyper-religiosity, hypergraphia, and hyposexuality is considered unique to TLE, some patients with frontotemporal lobar degeneration (FTLD) demonstrate symptoms that partially overlap with Geschwind Syndrome. The right-temporal lobe variant (RTLV) of FTLD, which is characterized by progressive atrophy that is most prominent in the right anterior temporal lobe often associated with impaired emotional regulation and social cognition and behavior, has been associated with hyper-religiosity in 15% of cases studied (Chan et al., 2009). In this case series by Chan and colleagues, one patient exhibited both hyper-religiosity and hyposexuality (but not hypergraphia). A separate recent case report described three patients with the semantic variant of primary progressive aphasia and new-onset creative writing behavior that was similar to hypergraphia (Wu et al., 2013). To date, only one patient who met the diagnostic criteria for Frontotemporal Dementia (FTD) without epilepsy and who presented with Geschwind Syndrome symptoms has been reported in the literature (Postiglione et al., 2008).
Here, we describe the 9-year-clinical course of a patient with Geschwind Syndrome that developed as the first, most salient clinical expression of RTLV of FTLD. We report the disease progression through repeated neuropsychological assessments and longitudinal neuroimaging analysis.
2. Case description
MS, a 73-year-old, left-handed, English speaking woman presented with a 3-year history of progressive behavioral changes. MS had 12 years of education and worked as a cashier and secretary until she had children. When her children were older, she returned to work in the school system as a secretary and nurse. She first came to medical attention due to concerns that she might have Alzheimer’s Disease, although at that time no evidence of any cognitive abnormality was found. She was subsequently evaluated in the Frontotemporal Disorders Unit at Massachusetts General Hospital in Boston. During the visit, MS denied any significant problems with her memory, stating only that she occasionally went into a room in her home and forgot what she was intending to do. She denied difficulty recalling conversations, names, or words in discourse. She also denied difficulty with all activities of daily living, including dressing, hygiene, money management, and meal preparation. She continued to drive without impairment, and stated that she had never become lost or disoriented in a familiar area. The patient’s daughter concurred with the observation of no change in cognitive abilities, but reported a significant change in her mother’s emotional functioning throughout the preceding years. Specifically, she reported that her mother became angry much more frequently than she used to, without provocation, and that the level of her expressed anger was more intense. This did not fit her prior emotional state, which was described by her family as gentle and kind. MS also appeared to have become more self-centered, and would often bring conversations back to herself. We formally quantified MS’s personality change by administering the NEO-Five Factor Inventory (NEO-FFI; Costa & McCrae, 1992); see Table 1. The patient and her daughter each completed NEO-FFI ratings for MS’s current and premorbid personality. The daughter’s assessment suggested changes from the patients’ premorbid personality in the form of decreased extraversion, openness to new experience, agreeableness, conscientiousness, and increased neuroticism. In contrast, the patient rated herself inversely, revealing a lack of insight into her changes in personality.
Table 1.
Current and premorbid personality characteristics as assessed by MS and her daughter on the NEO-Five Factor Inventory
| Rater | Condition | Neuroticism | Extraversion | Openness | Agreeableness | Conscientiousness |
|---|---|---|---|---|---|---|
| Daughter | Premorbid | 35 | 55* | 45* | 67* | 66 |
| Current | 39 | 34*α | 25*α | <1*α | 46α | |
| MS | Premorbid | 37* | 53* | 42* | 51* | 59 |
| Current | 34* | 63*α | 53*α | 63*α | 64α |
Scores are t values for women;
indicates that premorbid and current scores differ categorically in terms of NEO norms;
indicates that the patients’ scores and caregiver’s scores differ categorically in terms of NEO norms.
The patient’s daughter also reported that MS had become more religious: although she had been a lifelong churchgoing Catholic, she had recently begun watching religious shows, reading religious books, and talked about God much more frequently than previously. When asked about her current mood, MS reported that she was tired, busy, and had a lot to do, although she would still describe her mood as “happy”. Scores obtained on the Geriatric Depression Scale (Yesavage et al., 1983) and at the Beck Depression Inventory-II (Beck, Steer, & Brown, 1996) were not suggestive of of depression. In addition, MS did not endorse any item on the Beck Anxiety Inventory (Beck, Epstein, Brown, Steer, & others, 1988). Her neurological exam and her serum laboratory screening tests for dementia were normal. Sleep, appetite, weight, and interest in activities had reportedly been normal. Medical history was not contributory. She and her family denied birth stress, developmental abnormalities, nor other premorbid cognitive or psychiatric symptoms. Family medical history was significant for two sisters with dementia in their 80′s, and possible dementia in her father (onset in his late 60s, died at 72 year old) and mother (onset in her 80s, died at 95 year old), although the cause of the dementia was unclear and the characteristics of symptoms were not like the patient’s. The neuropsychological evaluation estimated her premorbid intellectual abilities to fall in the average range, based on a screening measure of verbal ability. MS was well-oriented and scored 29/30 on the Mini Mental State Examination (MMSE, Folstein, Folstein, & McHugh, 1975). Performance on most tests of attention and executive functioning fell within normal range. Memory performance was notable for a low average score. All aspects of language were normal, including confrontation naming, phonemic and semantic fluency, comprehension, prosody, and written expression. Visuospatial ability was normal. Her daughter’s ratings of behavioral change, as assessed by both the Frontal System Behavioral Scale (FrSBe) (Grace, 2011) and Behavior Rating Inventory of Executive Functioning (BRIEF) (Gioia, Isquith, Guy, & Kenworthy, 2000) rating scales, indicated dramatic changes, including increased disinhibition, impulsivity, and poor emotional control.
At age 74, MS’s symptoms remained relatively stable, and included irritability, paranoia, occasional aggression, disinhibition, changes in interpersonal warmth and empathy, emotional distancing from her family, and lack of insight. Her religious interests had increased. She frequently attributed daily events to “God’s will” and proselytized to people, including strangers. She started exhibiting signs of hypergraphia; she would frequently was write cards and perform extensive mailings of church-related information in a manner that seemed almost obsessive. She also developed a new preoccupation with politics. Despite being impulsive and easily angered, her family noted that she didn’t react in a normal way to emotional events in her life. The most striking example was that, when her son died suddenly of a myocardial infarction, she did not demonstrate a clear emotional response. At his funeral, the patient’s daughter reported that she seemed to be “going through the motions” of grieving but in a manner that seemed like she was an actress following a script. At this time, considering the clinical and neuroimaging features, a diagnosis of probable behavioral variant FTD (Rascovsky et al., 2011), of a mild severity, was made.
Between the ages of 74 and 75, a progression of behavioral symptoms was noted while cognition remained relatively intact: MS continued to exhibit a lack of emotional response to stressful events and exhibited disinhibited social behavior, occasionally making inappropriate comments regarding clothing and weight in others. According to her daughter, the patient developed increasingly blunted emotional expression, although she could discuss her emotions intellectually and in a somewhat appropriate fashion. She had begun writing extensively in journals, particularly about topics related to religious beliefs and practices. She wrote long cards to people when they were suffering. MS reported that she carried on being “a good Christian”, going to church and trying to do good things for people. She continued to manage her own household and community affairs, including driving, without incident.
By age 77, a progressive but mild decline was noted in general independence in daily activities and also in memory, executive function, and language. From a functional point of view, MS had stopped cooking and she frequently complained about an assortment of body ailments or symptoms, which were often not linked to identifiable medical problems. She repeated herself very frequently and occasionally had trouble finding words, but her spontaneous speech was fluent, articulate, and grammatical. During detailed language assessment (see Table 2), MS displayed mild word retrieval difficulty on the Boston Naming Test (BNT, Goodglass, Kaplan, & Barresi, 2001; Kaplan, Goodglass, & Weintraub, 1983), with some benefit from phonemic cues. However, her semantic knowledge was generally spared, as reflected by her ability to select the appropriate target on the BNT multiple choice exam, and by her performance on a word-picture matching test (Cambridge Semantic Memory Battery, CSMB, Adlam, Patterson, Bozeat, & Hodges, 2010; Hodges & Patterson, 1995).
Table 2.
Assessment of MS’s language abilities at the age of 77. MS exhibited mild deficits in word retrieval, while semantic knowledge was generally intact. No difficulties were found in auditory and written comprehension and repetition abilities.
| Linguistic domain | Test | Raw score |
|---|---|---|
| Naming | BNT | |
| Spontaneous correct | 16/30 | |
| Correct w/semantic cue | 1/14 | |
| Correct w/phonemic cue | 8/13 | |
| Correct given multiple choice | 5/5 | |
| Object knowledge | Pyramids Palm Trees: 3-picture version | 42/52 |
| Auditory comprehension | WAB: Sequential Commands | 80/80 |
| Auditory single word comprehension | CSMB: Category Comprehension | 63/64 |
| Living | 31/32 | |
| Manmade | 32/32 | |
| Reading | WAB: Comprehension of Sentences | 32/40 |
| WAB: Reading Commands | 20/20 | |
| Repetition | WAB: Repetition | 100/100 |
BNT: Boston Naming Test (Goodglass et al., 2001; Kaplan et al., 1983); WAB: Western Aphasia Battery (Risser & Spreen, 1985); CSMB: Cambridge Semantic Memory Battery (Adlam et al., 2010; Hodges & Patterson, 1995).
Other language skills, including auditory comprehension, repetition, reading, and writing were intact. She exhibited some difficulty in recalling verbal information from memory and in task shifting, as measured through the Trail Making Test (Reitan & Wolfson, 1993). Neuropsychological data longitudinally collected are reported in Table 3. Her hyper-religiosity and hypergraphia had progressed substantially, and her daughter reported that she was writing pages and pages of material often related to religious themes. The writing was dense, and used all the available space on both sides of a sheet of paper, including the margins (Figure 1). Although there was no report of symptoms of visual agnosia in daily life and MS could recognize family members, she exhibited difficulty performing a test of famous face recognition. On this task MS was shown 15 images of highly famous faces (ex: Elvis, Ronald Regan, Marilyn Monroe) and 15 unfamiliar faces and was asked to indicate whether the individual was familiar to her, and if so, if she could recall that person’s name. Although MS endorsed all of the famous faces as being familiar, she also endorsed 12/15 of the non-famous faces as being familiar indicating an extremely liberal response bias and low discriminability on this task (d’prime = 1.09). In addition, MS was unable to recall the name for any of the famous faces presented to her, suggesting that she might have a selective semantic memory deficit for familiar people. At this time her MMSE (Folstein et al., 1975) score was 24/30.
Table 3.
Longitudinal neuropsychological data collected from MS. Raw scores for each of the 5 time points are reported.
| 73 yo | 77 yo | 78 yo | 79 yo | 80 yo | |
|---|---|---|---|---|---|
| Premorbid intellectual abilities | |||||
| American National Adult Reading Test (Venegas & Clark, 2011) | 116 | 111 | 109 | – | – |
| Dementia severity | |||||
| MMSE (Folstein et al., 1975) | 29 | 24*** | 24*** | 22*** | 22*** |
| Memory | |||||
| Logical Memory IA (Wechsler, 1987a) | 7° | 7° | 3* | 3* | 0*** |
| Logical Memory IIA | 4° | 1* | 0** | 0** | 0** |
| Attention and working memory | |||||
| Digit Span Forward (Wechsler, 1987a) | 6 | 6 | 7 | 6 | 6 |
| Digit Span Backward | 4 | 3° | 6 | 5 | 5 |
| Processing speed | |||||
| WAIS-R Digit-Symbol (Wechsler, 1987b) | 50 | 50 | 36 | 39 | 28 |
| Trails A (time - seconds) (Reitan & Wolfson, 1993) | 40 | 30 | 47 | 38 | 75*** |
| Trails A (errors) | – | 0 | 0 | 0 | 0 |
| Executive functions | |||||
| Trails B (time - seconds) | 50 | 219*** | 161° | n.e. | 178* |
| Trails B (errors) | – | 3 | 2 | – | 11* |
| Language | |||||
| Category Fluency: Animals (Morris et al., 1989) | 16 | 10* | 16 | 9* | 9* |
| Category Fluency: Vegetables | 16 | 11 | 13 | 10 | 9 |
| BNT | 26 | 16*** | 19*** | 16*** | 11*** |
| Geriatric Depression Scale (Yesavage et al., 1983) | 9 | 1 | 0 | – | 1 |
| Functional Assessment Questionnaire (FAQ, %) (Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982) | – | 31.94 | 40.38 | 41.9 | – |
mildly impaired compared to normative data;
moderated impaired;
severely impaired;
low average. Normative data reported in Weintraub et al. (2009) and Shirk et al. (2011). n.e.: not evaluable. yo: years old
Figure 1.

Examples of MS’s hypergraphia. MS wrote many pages of material characterized by specific religious content and church-related information. The writing was dense, using all the available space on a sheet of paper. The patient obsessively kept and archived her letters, filling entire drawers.
Between ages the ages of 79 and 80, MS’s cognitive functions progressively deteriorated along with her behavior. She repeated herself very frequently and was increasingly fixated on a few ideas and talked about them in a perseverative fashion. Between the ages of 80 and 82, MS progressively declined in her daily function such that she was no longer able to perform instrumental and then basic activities of daily living and required assistance. An evaluation at the age of 82 was notable for her performance on the Montreal Cognitive Assessment (Nasreddine et al., 2005) in which she scored 4/30 (she was able to name a lion, was oriented to city and state, was able to do forward digit span). Her daughter reported that she is severely apathetic, but that every afternoon she becomes more active and will often tell stories that didn’t make sense. She frequently mistakes her daughter for her sister or her granddaughter, has visual hallucinations of people (a man, a baby), and speaks to people in photographs as though they are present and has conversations with them, with responses suggestive of auditory hallucinations. She is unable to prepare food, usually eats ‘what is put in front of her’ and has gained weight. She is able to read children’s books to her grandchildren daily and frequently does art projects.
3. Quantification of structural neuroimaging changes over seven years
High-resolution T1-weighted 3D structural MR images were obtained at four time points (age 73, 74, 75 and 77) on a 3T Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany), using a 12-channel phased-array head coil. Cortical reconstructions and volumetric segmentation of the T1-weighted images were performed using the FreeSurfer analysis suite version 5.3.0 according to a procedure that has been previously described in detail (Dale, Fischl, & Sereno, 1999; Fischl & Dale, 2000; Fischl, Sereno, & Dale, 1999; Rosas et al., 2002; Salat et al., 2004). Cortical reconstructions at each time point were manually inspected to check for accuracy and any errors in the automatic segmentation of the grey/white matter boundary were manually corrected (Fischl, Liu, & Dale, 2001).
Cortical atrophy was assessed at baseline (age 73) using a 2 class general linear model (GLM) as implemented in FreeSurfer, comparing cortical thickness in MS to a group of 75 age-matched healthy controls (age: M = 72.65 yrs, SD = 4.80). The results of this analysis revealed cortical thickness that was reduced in MS compared to controls, most prominently in the right temporal pole, extending caudally into the right entorhinal, perirhinal, and parahippocampal cortex, as well as anterior portions of the middle and inferior temporal gyri. Focal atrophy was also observed, to a lesser extent, in the left temporal pole (Figure 2). The magnitude of atrophy in each subcortical structure in the FreeSurfer Desikan-Killiany atlas was quantified as the percent difference in volume for each structure between MS and the healthy control group, controlling for individual variability in estimated intracranial volume. Substantial subcortical volume loss was observed in the right amygdala (z = −1.51, atrophy = 28.81%). Although the right hippocampus was only slightly smaller in normalized volume to a group of healthy age-matched controls (z = −0.48, 8% smaller), there was a marked asymmetry in the volumes of her left and right hippocampi (right = 15% smaller than left).
Figure 2. FDG-PET and Cortical atrophy at baseline (age 73) in MS.
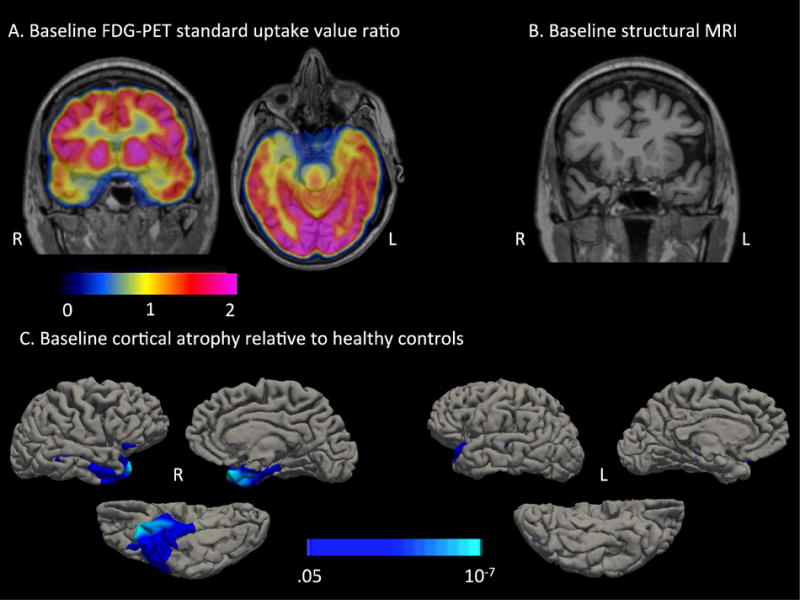
A) MS displayed hypometabolism that was most prominent in the right anterior temporal lobe. The color bar represents standard uptake value ratios (SUVR) with the pons as a reference region. B) A coronal T1-MPRAGE reveals subtle atrophy in the right temporal pole. C) A 2-class general linear model (GLM) comparing cortical thickness in MS to a group of 75 age-matched healthy controls revealed cortical thinning in the right anterior temporal lobe. The color bar represents p-values from the GLM.
To assess longitudinal changes in cortical thickness and subcortical volume, images were processed with the longitudinal stream (Reuter, Schmansky, Rosas, & Fischl, 2012) in FreeSurfer. First, a within-subject template was created using robust, inverse consistent registration (Reuter, Rosas, & Fischl, 2010). This within-subject template was then used to initialize the Talairach transforms, atlas registration, and spherical surface maps and parcellations (Reuter et al., 2012). The rate of cortical atrophy was calculated as percent change in cortical thickness per year with reference to cortical thickness in the baseline MRI taken at age 73. The results of this analysis revealed progressive bilateral atrophy in the anterior temporal lobes, medial orbitofrontal cortex, and insula, and lingual gyrus. Subcortical atrophy was quantified as % volume loss with reference to the volume of each structure at baseline at age 73 (Figure 3). By age 77, substantial subcortical atrophy was observed in the bilateral hippocampi (right: 12.97% volume loss, left: 22.50% volume loss), left amygdala (10.20% volume loss), and the left nucleus accumbens (28.16% volume loss).
Figure 3. Longitudinal cortical thinning and subcortical atrophy in MS.
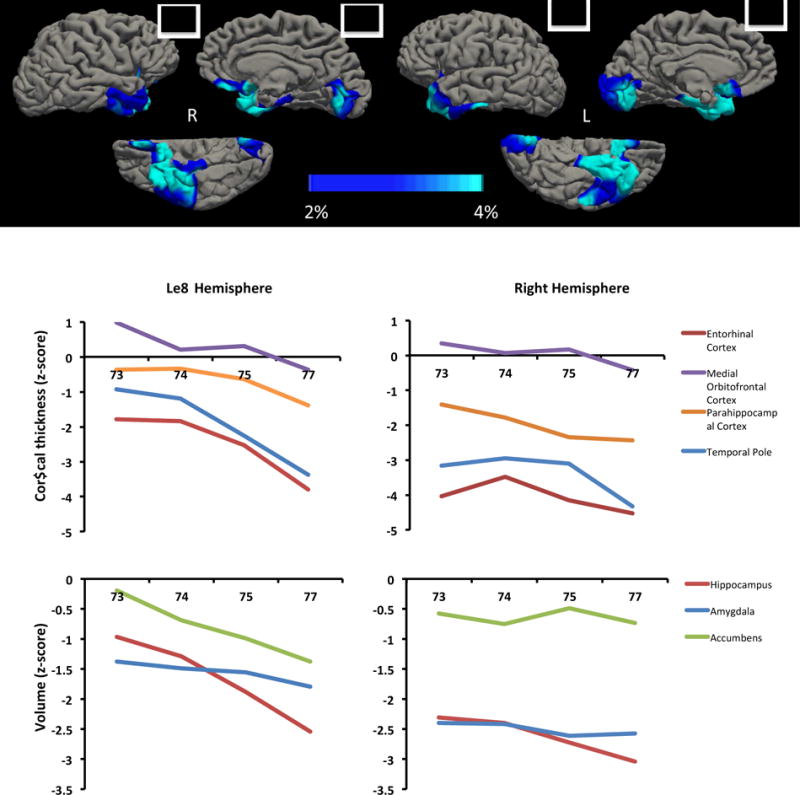
From age 73 to 77 MS displayed progressive cortical atrophy in the bilateral anterior temporal lobes, medial orbitofrontal cortex, and lingual gyri. Time points included in the longitudinal analysis were age 73, age 74, age 75 and age 77. Color bar represents the rate of cortical thinning per year as a percentage of baseline (age 73) cortical thickness.
4. Discussion
We have presented the cognitive, behavioral and anatomical features of MS, a case of RTLV of FTLD over a period of nine years. This case provides valuable insights into both the variety of clinical manifestations of this variant of FTLD and the functions of cortical and subcortical structures of the temporal lobes. MS presented at age 73 with a 3 year history of personality and behavioral symptoms. MS’s early clinical presentation was mainly characterized by: (i) increasing interest in religious issues (hyper-religiosity); (ii) excessive compulsive writing on religious themes (hypergraphia); (iii) poor emotional regulation (irritability, impulsivity, disinhibition, egocentric behavior). Over time, other cognitive functions were affected and a progressive decline was noted in general independence in daily activities. Seven years after her symptoms began, MS demonstrated deficits in word retrieval, verbal free recall, and set-shifting. She manifested additional difficulties in recognizing images of famous faces, although her semantic memory for common, non-unique items was grossly spared. Notably, hyper-religiosity, hypergraphia, and blunted socioemotional function remained the most prominent deficits throughout her disease progression.
Hyper-religiosity and hypergraphia, together with irritability of varying degree, are peculiar symptoms of Geschwind Syndrome, frequently reported in TLE patients (Bear & Fedio, 1977; Waxman & Geschwind, 1974; Waxman & Geschwind, 1975; Waxman & Geschwind, 2005, see also Devinsky & Schachter, 2009). To our knowledge, only one other case report describes the combination of Geschwind Syndrome symptoms in a patient affected by a neurodegenerative disease without TLE (Postiglione et al., 2008). In this case, the CT scan showed mild atrophy in both the frontal and temporal lobes, while a 18FDG-PET scan revealed hypometabolism bilaterally in the middle and superior prefrontal cortex, more marked on the left, and in the left mesial and lateral temporal cortex and in the head of caudate nuclei.
MS’s initial MRI scan was read clinically as unremarkable, but quantitative cortical thickness analysis showed mild cortical thinning in the right temporal pole and volume loss in the right amygdala. Longitudinal cortical thickness analysis depicted a pattern of progressive atrophy including bilateral anterior temporal lobes, medial orbitofrontal cortex, insula, and lingual gyri. Bilateral hippocampi (with a predominance to the left), left amygdala, and the left nucleus accumbens were also progressively involved. Notably, while neurodegeneration in the right hemisphere was much more prominent early in the course of MS’s disease, rapid degeneration in homologous regions of the left hemisphere was observed as the disease progressed, possibly reflecting the spread of pathogenic proteins along pathways that connect the bilateral temporal poles (Hock & Polymenidou, 2016).
Heightened interest in religion has been reported as a specific feature of TLE (Bear & Fedio, 1977; Garcia-Santibanez & Sarva, 2015; Tebartz van Elst et al., 2003; Waxman & Geschwind, 1974; Waxman & Geschwind, 1975; Waxman & Geschwind, 2005; Wuerfel et al., 2004), RTLV (Chan et al., 2009; Everhart, Watson, Bickel, & Stephenson, 2015) and right temporal stroke (Hoffmann, 2008). Chan et al. (2009) reported clinical features of hyper-religiosity in 15% of the RTLV cases studied (with no patients exhibiting signs of hypergraphia). Everhart et al. (2015) described the case of a Protestant minister who exhibited religiosity and pietism as early personality alterations of FTLD, in the absence of hypergraphia. To date, very little is known about the anatomical correlates of hyper-religiosity. In patients with TLE, heightened interest in religion has been associated with atrophy in the right hippocampus (Tebartz van Elst et al., 2003; Wuerfel et al., 2004). Although the right hippocampus in MS was similar in normalized volume to a group of healthy age-matched controls (8% smaller), there was a marked asymmetry in the volumes of her left and right hippocampi (right = 15% smaller than left), suggesting that her heightened interest in religion may be driven in part by early atrophy in this region.
The term hypergraphia, as conceptualized by Waxman and Geschwind (1974; 2005), refers to a tendency, typically found in TLE patients with right-sided non-dominant temporal lobe lesions (Roberts et al., 1982; Sachdev & Waxman, 1981), towards extensive, ritualistic, and compulsive writing. It is characterized by meticulous attention to detail and the desire to preserve what is written. The involvement of the limbic system (Waxman & Geschwind, 1974; Waxman & Geschwind, 2005), and in particular of the hippocampus (Tebartz van Elst et al., 2003) has been hypothesized in the pathogenesis of hypergraphia, possibly reflecting changes in emotional responsiveness (Okamura et al., 1993). Another type of abnormal writing behavior has been described in patients with damage to the non-dominant hemisphere due to stroke (Yamadori, Mori, Tabuchi, Kudo, & Mitani, 1986), tumors (Imamura, Yamadori, & Tsuburaya, 1992), and dementia (Frisoni, Scuratti, Bianchetti, & Trabucchi, 1993). This type of writing behavior is inappropriate, semi-automatic and inattentive and is characterized by spatial disarrangements and poor communicative value. This tendency has been referred to as automatic writing behavior, and is a form of utilization behavior in the sense that it is driven by a suppression of inhibitory function in the frontal lobes, leaving the patient susceptible to stimulus-driven behavior (van Vugt, Paquier, Kees, & Cras, 1996). In contrast, MS’s writing was cramped, dense, and characterized by specific religious content and church-related information. The patient possessively kept and archived her letters: a behavior very similar to hypergraphia as described in TLE patients (Roberts et al., 1982; Sachdev & Waxman, 1981; Waxman & Geschwind, 1974; Waxman & Geschwind, 2005).
Geschwind Syndrome itself has been matter of controversy for years, with regard to the existence and specificity of the behavioral changes in patients with TLE, as well as the hypothesized underling mechanisms (Blumer, 1999; Devinsky & Schachter, 2009; Devinsky & Najjar, 1999). Similar to the personality changes observed in MS, Geshwind Syndrome associated with TLE manifests as chronic, “not an acute change in personality, but one that develops, continues, and typically gets more striking with the passage of time; a personality that the patient manifests all the time, not an acute and self-limited change related to a seizure” (Devinsky & Schachter, 2009). Hippocampal sclerosis is considered the most common histopathologic abnormality in TLE patients (Blumcke et al., 2013; Blumcke, Coras, Miyata, & Özkara, 2012; Blumcke, Thom, & Wiestler, 2002; Cavanagh & Meyer, 1956; De Lanerolle et al., 2003), even if some studies have suggested a more widespread temporal lobe disturbance, including extra-hippocampal structures (Bernasconi, Natsume, & Bernasconi, 2005; Chabardes et al., 2005; Concha, Beaulieu, Collins, & Gross, 2009; Kahane et al., 2002; Meiners et al., 1994, 1999; Mitchell et al., 1999; Sankar, Bernasconi, Kim, & Bernasconi, 2008; Weder et al., 2006). Geschwind believed that hyper-excitibility/spiking in the limbic temporal lobe was critical to the behavioral changes in Geschwind syndrome (Devinski et al., 2009). There has been little neuroimaging work investigating the neuroanatomical substrates giving rise to the specific features of Geschwind Syndrome in TLE, however two studies have supported an association between hippocampal atrophy and the emergence of hyper-religiosity and hypergraphia (Tebartz van Elst et al., 2003; Wuerfel et al., 2004). In addition, the persistence of hyper-religious behaviors has been observed in 10 patients who underwent temporal lobectomies, suggesting that damage to temporal limbic structures, and not hyper-excitability per se, might underlie the behavioral manifestation of Geschwind Syndrome in patients with TLE. In the present case of RTLV of FTLD, the emergence of hyper-religiosity and hypergraphia closely mimicked what is typically observed in TLE patients, in the context of atrophy that progressively affected the anterior temporal and frontal lobes and the hippocampus bilaterally with more prominent neurodegeneration in the right hemisphere.
MS also showed early signs of emotional dysfunction that included disinhibition, impulsivity, and loss of empathy. She became self-centered with poor insight. MS’s early clinical profile is consistent with other reports of patients with circumscribed atrophy in the right anterior temporal lobe and amygdala. TLE has also been associated with behavioral and emotional changes, with some patients becoming more aggressive and impulsive, and less aware of social cues (Devinski & Schachter, 2009). A growing body of literature has associated the right temporal pole (Gainotti, 2015, Zahn et al., 2009) and amygdala (Armony, 2013) with social cognition and emotional processing. Prominent right hemisphere atrophy in semantic dementia (SD) has been associated with changes in behavior and personality, as well as person-recognition difficulties (Chan et al., 2009; Edwards-lee et al., 1997; Gainotti, Barbier, & Marra, 2003; Irish, Kumfor, Hodges, & Piguet, 2013; Joubert et al., 2006; Kamminga et al., 2015; Kumfor et al., 2016; Miller, Chang, Mena, Boone, & Lesser, 1993; Seeley et al., 2005; Thompson, Patterson, & Hodges, 2003; Tyrrell, Warrington, Frackowiak, & Rossor, 1990). More recently, it has been shown that right anterior temporal lobe atrophy predicts theory of mind deficits in SD (Irish, Hodges, & Piguet, 2014). The continued decline of social emotional behavior in MS is consistent with her progressive atrophy in the right temporal pole and the spread of atrophy to the medial orbitofrontal cortices (OFC) which have frequently been associated with the disruption of social-emotional behavior in FTD (Hutchings, Hodges, Piguet, & Kumfor, 2015; Kumfor, Irish, Hodges, & Piguet, 2013; Viskontas, Possin, & Miller, 2007). The OFCs are densely interconnected with the temporal poles via the uncinate fasciculus (Von Der Heide, Skipper, Klobusicky, & Olson, 2013), and are thought to be important for assigning emotional relevance to stimuli (Kumfor et al., 2013).
Starting at age 77, MS’s disease disrupted additional cognitive functions, including episodic memory, executive function, word finding, and semantic memory (famous face recognition). The longitudinal spread of atrophy in MS is consistent with previous reports of progressive atrophy in SD, and included lateral and medial temporal regions important for semantic and episodic memory (Brambati et al., 2007; Henry et al., 2014; Kumfor et al., 2016). Surprisingly, progressive cortical thinning was also observed in the lingual gyrus. This finding is consistent with a previous report of physiologic dysfunction in early visual cortex in SD patients that was predicted by atrophy in the anterior temporal lobes (Guo et al., 2013), and suggests that MS’s atrophy in the lingual gyrus may be driven in part by a loss of feed-back projections from the anterior temporal lobes to early visual cortex. This account is speculative, and more research is needed to understand the cause and functional consequences of atrophy in early visual cortex in SD.
5. Conclusion
Geschwind Syndrome has been frequently described in patients affected by TLE (Bear & Fedio, 1977; Waxman & Geschwind, 1975; Waxman & Geschwind, 1974, see also Devinsky & Schachter, 2009), with hippocampal damage being a specific anatomical contributor to its manifestation (Tebartz van Elst et al., 2003; Wuerfel et al., 2004). In the present report, a similar symptom constellation in a pathogenically different condition than TLE, as a clinical expression of RTLV of FTLD. Prior descriptions of the early symptoms of RTLV-FTLD call attention to a variety of cognitive (prosopagnosia, semantic memory impairment) and/or behavioral (disinhibition and somatization disorders, loss of empathy, compulsive behavior) features (Chan et al., 2009; Evans, Heggs, Antoun, & Hodges, 1995; Gainotti et al., 2003; Gentileschi, Sperber, & Spinnler, 2001; Gorno-Tempini et al., 2004; Joubert et al., 2006; Seeley et al., 2005; Sperber & Spinnler, 2003; Thompson et al., 2003; Tyrrell et al., 1990). However, to date only one patient with FTD without epilepsy has been reported as presenting with Geschwind Syndrome (Postiglione et al., 2008). Thus, the present case describes a different and unusual clinical picture of FTD, and sheds light on potential functions of the anterior temporal lobes.
Acknowledgments
We greatly appreciate the patient and family’s efforts in their participation in this study.
This study was supported by the National Institute of Deafness and Other Communication Disorders (R01 DC014296), National Institute on Aging (P50 AG00513421), and National Institute of Neurological Disorders and Stroke (R21 NS077059). This research was carried out in whole or in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant number(s) S10RR021110, S10RR023043, S10RR023401.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlam AL, Patterson K, Bozeat S, Hodges JR. The Cambridge Semantic Memory Test Battery: detection of semantic deficits in semantic dementia and Alzheimer’s disease. NeuroCase. 2010;16(3):193–207. doi: 10.1080/13554790903405693. https://doi.org/10.1080/13554790903405693. [DOI] [PubMed] [Google Scholar]
- Armony JL. Current Emotion Research in Behavioral Neuroscience: The Role(s) of the Amygdala. Emotion Review. 2013;5(1):104–115. https://doi.org/10.1177/1754073912457208. [Google Scholar]
- Bear DM, Fedio P. Quantitative analysis of interictal behavior in temporal lobe epilepsy. Archives of Neurology. 1977;34(8):454–467. doi: 10.1001/archneur.1977.00500200014003. https://doi.org/10.1001/archneur.1977.00500200014003. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Beck Depression Inventory-II. San Antonio. 1996:12–15. https://doi.org/10.1037/t00742-000.
- Beck AT, Epstein N, Brown G, Steer RA, et al. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. https://doi.org/10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology. 2005;65(Engel II):223–228. doi: 10.1212/01.wnl.0000169066.46912.fa. https://doi.org/10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- Blümcke I, Coras R, Miyata H, Özkara C. Defining clinico-neuropathological subtypes of mesial temporal lobe epilepsy with hippocampal sclerosis. Brain Pathology. 2012;22:402–411. doi: 10.1111/j.1750-3639.2012.00583.x. https://doi.org/10.1111/j.1750-3639.2012.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, Spreafico R. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: A Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia. 2013;54(7):1315–1329. doi: 10.1111/epi.12220. https://doi.org/10.1111/epi.12220. [DOI] [PubMed] [Google Scholar]
- Blümcke I, Thom M, Wiestler OD. Ammon’s horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathology (Zurich, Switzerland) 2002;12(2):199–211. doi: 10.1111/j.1750-3639.2002.tb00436.x. https://doi.org/10.1111/j.1750-3639.2002.tb00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer D. Evidence supporting the temporal lobe epilepsy personality syndrome. Neurology. 1999;53(5 Suppl 2):S9–12. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10496229. [PubMed] [Google Scholar]
- Brambati SM, Renda NC, Rankin KP, Rosen HJ, Seeley WW, Ashburner J, Gorno-Tempini ML. A tensor based morphometry study of longitudinal gray matter contraction in FTD. NeuroImage. 2007;35(3):998–1003. doi: 10.1016/j.neuroimage.2007.01.028. https://doi.org/10.1016/j.neuroimage.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JB, Meyer A. Aetiological aspects of Ammon’s horn sclerosis associated with temporal lobe epilepsy. British Medical Journal. 1956;2(5006):1403–1407. doi: 10.1136/bmj.2.5006.1403. https://doi.org/10.1136/bmj.2.5006.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabardes S, Kahane P, Minotti L, Tassi L, Grand S, Hoffmann D, Benabid AL. The temporopolar cortex plays a pivotal role in temporal lobe seizures. Brain. 2005;128(8):1818–1831. doi: 10.1093/brain/awh512. https://doi.org/10.1093/brain/awh512. [DOI] [PubMed] [Google Scholar]
- Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, Fox NC. The clinical profile of right temporal lobe atrophy. Brain. 2009;132(5):1287–1298. doi: 10.1093/brain/awp037. https://doi.org/10.1093/brain/awp037. [DOI] [PubMed] [Google Scholar]
- Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(3):312–319. doi: 10.1136/jnnp.2007.139287. https://doi.org/10.1136/jnnp.2007.139287. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Professional manual: revised NEO personality inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI) Odessa FL Psychological Assessment Resources. 1992;3:101. https://doi.org/10.1037//1040-3590.4.1.5. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. https://doi.org/10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- De Lanerolle NC, Kim JH, Williamson A, Spencer SS, Zaveri HP, Eid T, Spencer DD. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: Evidence for distinctive patient subcategories. Epilepsia. 2003;44(5):677–687. doi: 10.1046/j.1528-1157.2003.32701.x. https://doi.org/10.1046/j.1528-1157.2003.32701.x. [DOI] [PubMed] [Google Scholar]
- Devinsky J, Schachter S. Norman Geschwind’s contribution to the understanding of behavioral changes in temporal lobe epilepsy: The February 1974 lecture. Epilepsy and Behavior. 2009;15(4):417–424. doi: 10.1016/j.yebeh.2009.06.006. https://doi.org/10.1016/j.yebeh.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Najjar S. Evidence against the existence of a temporal lobe epilepsy personality syndrome. Neurology. 1999;53(5 Suppl 2):S13–25. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10496230. [PubMed] [Google Scholar]
- Edwards-lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, Mena I. The temporal variant of frontotemporal dementia. Brain : A Journal of Neurology. 1997;120(Pt 6):1027–40. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Evans JJ, Heggs AJ, Antoun N, Hodges JR. Progressive prosopagnosia associated with selective right temporal lobe atrophy: A new syndrome? Brain. 1995;118(1):1–13. doi: 10.1093/brain/118.1.1. https://doi.org/10.1093/brain/118.1.1. [DOI] [PubMed] [Google Scholar]
- Everhart DE, Watson EM, Bickel KL, Stephenson AJ. Right Temporal Lobe Atrophy: A Case That Initially Presented as Excessive Piety. The Clinical Neuropsychologist. 2015 Feb;4046:1–15. doi: 10.1080/13854046.2015.1104387. https://doi.org/10.1080/13854046.2015.1104387. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(Track II):11050–5. doi: 10.1073/pnas.200033797. https://doi.org/10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. https://doi.org/10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frisoni G, Scuratti A, Bianchetti A, Trabucchi M. Hypergraphia and brain damage. J Neurol Neurosurg Psychiatry. 1993;56(5):576–577. doi: 10.1136/jnnp.56.5.576-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. Is the difference between right and left ATLs due to the distinction between general and social cognition or between verbal and non-verbal representations? Neuroscience and Biobehavioral Reviews. 2015;51:296–312. doi: 10.1016/j.neubiorev.2015.02.004. https://doi.org/10.1016/j.neubiorev.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Barbier A, Marra C. Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain. 2003;126(4):792–803. doi: 10.1093/brain/awg092. https://doi.org/10.1093/brain/awg092. [DOI] [PubMed] [Google Scholar]
- Garcia-Santibanez R, Sarva H. Isolated Hyperreligiosity in a Patient with Temporal Lobe Epilepsy. Case Reports in Neurological Medicine. 2015;2015:1–3. doi: 10.1155/2015/235856. https://doi.org/10.1155/2015/235856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentileschi V, Sperber S, Spinnler H. CROSSMODAL AGNOSIA FOR FAMILIAR PEOPLE AS A CONSEQUENCE OF RIGHT INFERO POLAR TEMPORAL ATROPHY. Cognitive Neuropsychology. 2001;18(5):439–463. doi: 10.1080/02643290125835. https://doi.org/10.1080/02643290125835. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. TEST REVIEW Behavior Rating Inventory of Executive Function. Child Neuropsychology. 2000;6(3):235–238. doi: 10.1076/chin.6.3.235.3152. https://doi.org/10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination Third Edition (BDAE-3) Austin, TX: Pro-Ed; 2001. [Google Scholar]
- Gorno-Tempini ML, Rankin KP, Woolley JD, Rosen HJ, Phengrasamy L, Miller BL. Cognitive and Behavioral Profile in a Case of Right Anterior Temporal Lobe Neurodegeneration. Cortex. 2004;40(4):631–644. doi: 10.1016/s0010-9452(08)70159-x. https://doi.org/10.1016/S0010-9452(08)70159-X. [DOI] [PubMed] [Google Scholar]
- Grace J. Frontal Systems Behavior Scale. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York; 2011. pp. 1090–1093. https://doi.org/10.1007/978-0-387-79948-3_1895. [Google Scholar]
- Guo CC, Gorno-Tempini ML, Gesierich B, Henry M, Trujillo A, Shany-Ur T, Seeley WW. Anterior temporal lobe degeneration produces widespread network-driven dysfunction. Brain. 2013;136(10):2979–2991. doi: 10.1093/brain/awt222. https://doi.org/10.1093/brain/awt222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Wilson SM, Ogar JM, Sidhu MS, Rankin KP, Cattaruzza T, Seeley WW. Neuropsychological, behavioral, and anatomical evolution in right temporal variant frontotemporal dementia: A longitudinal and post-mortem single case analysis. Neurocase. 2014 Feb;20(2015):100–9. doi: 10.1080/13554794.2012.732089. https://doi.org/10.1080/13554794.2012.732089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Whitman S, Arntsont P. Hypergraphia in epilepsy : is there temporal lobe epilepsy ? a specificity to. 1983:848–853. doi: 10.1136/jnnp.46.9.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock EM, Polymenidou M. Prion-like propagation as a pathogenic principle in frontotemporal dementia. Journal of Neurochemistry. 2016 doi: 10.1111/jnc.13668. https://doi.org/10.1111/jnc.13668. [DOI] [PMC free article] [PubMed]
- Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer’s disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33(4):441–459. doi: 10.1016/0028-3932(94)00127-b. https://doi.org/10.1016/0028-3932(94)00127-B. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. Isolated right temporal lobe stroke patients present with geschwind gastaut syndrome, frontal network syndrome and delusional misidentification syndromes. Behavioural Neurology. 2008;20(3–4):83–89. doi: 10.3233/BEN-2008-0218. https://doi.org/10.3233/BEN-2008-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings R, Hodges JR, Piguet O, Kumfor F. Why should i care? Dimensions of socio-emotional cognition in younger-onset dementia. Journal of Alzheimer’s Disease. 2015;48(1):135–147. doi: 10.3233/JAD-150245. https://doi.org/10.3233/JAD-150245. [DOI] [PubMed] [Google Scholar]
- Imamura T, Yamadori A, Tsuburaya K. Hypergraphia associated with a brain tumour of the right cerebral hemisphere. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55(1):25–7. doi: 10.1136/jnnp.55.1.25. https://doi.org/10.1136/jnnp.55.9.861-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M, Hodges JR, Piguet O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain. 2014;137(4):1241–1253. doi: 10.1093/brain/awu003. https://doi.org/10.1093/brain/awu003. [DOI] [PubMed] [Google Scholar]
- Irish M, Kumfor F, Hodges JR, Piguet O. Contrasting socioemotional dysfunction in right- versus left-lateralised semantic dementia. Dement Neuropsychol. 2013;7(1):88–95. doi: 10.1590/S1980-57642013DN70100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert S, Felician O, Barbeau E, Ranjeva JP, Christophe M, Didic M, Ceccaldi M. The right temporal lobe variant of frontotemporal dementia: cognitive and neuroanatomical profile of three patients. Journal of Neurology. 2006;253(11):1447–58. doi: 10.1007/s00415-006-0232-x. https://doi.org/10.1007/s00415-006-0232-x. [DOI] [PubMed] [Google Scholar]
- Kahane P, Chabardès S, Minotti L, Hoffmann D, Benabid AL, Munari C. The role of the temporal pole in the genesis of temporal lobe seizures. Epileptic Disorders. 2002;4(SUPPL. 1) [PubMed] [Google Scholar]
- Kamminga J, Kumfor F, Burrell JR, Piguet O, Hodges JR, Irish M. Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: an examination of clinical characteristics and emotion processing. Journal of Neurology, Neurosurgery, and Psychiatry. 2015;86(10):1082–8. doi: 10.1136/jnnp-2014-309120. https://doi.org/10.1136/jnnp-2014-309120. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kumfor F, Irish M, Hodges JR, Piguet O. Discrete Neural Correlates for the Recognition of Negative Emotions: Insights from Frontotemporal Dementia. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0067457. https://doi.org/10.1371/journal.pone.0067457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F, Landin-Romero R, Devenney E, Hutchings R, Grasso R, Hodges JR, Piguet O. On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain : A Journal of Neurology. 2016;139(Pt 3):986–98. doi: 10.1093/brain/awv387. https://doi.org/10.1093/brain/awv387. [DOI] [PubMed] [Google Scholar]
- Meiners LC, Van Gils A, Jansen GH, De Kort G, Witkamp TD, Ramos LMP, Mali WPTM. Temporal lobe epilepsy: The various MR appearances of histologically proven mesial temporal sclerosis. American Journal of Neuroradiology. 1994;15(8):1547–1555. [PMC free article] [PubMed] [Google Scholar]
- Meiners LC, Witkamp TD, de Kort GA, van Huffelen AC, van der Graaf Y, Jansen GH, van Veelen CW. Relevance of temporal lobe white matter changes in hippocampal sclerosis. Magnetic resonance imaging and histology. Investigative Radiology. 1999;34(1):38–45. doi: 10.1097/00004424-199901000-00006. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9888052%5Cnhttp://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9888052. [DOI] [PubMed] [Google Scholar]
- Miller BL, Chang L, Mena I, Boone K, Lesser IM. Progressive right frontotemporal degeneration: clinical, neuropsychological and SPECT characteristics. Dementia. 1993;4(3–4):204–213. doi: 10.1159/000107324. https://doi.org/Thesis_references-Converted#130. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Jackson GD, Kalnins RM, Saling MM, Fitt GJ, Ashpole RD, Berkovic SF. Anterior temporal abnormality in temporal lobe epilepsy: a quantitative MRI and histopathologic study. Neurology. 1999;52(2):327–336. doi: 10.1212/wnl.52.2.327. https://doi.org/10.1212/WNL.52.2.327. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–65. doi: 10.1212/wnl.39.9.1159. https://doi.org/10.1212/WNL.41.4.479. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. https://doi.org/10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Okamura T, Fukai M, Yamadori A, Hidari M, Asaba H, Sakai T. A clinical study of hypergraphia in epilepsy. Journal of Neurology, Neurosurgery, and Psychiatry. 1993;56(5):556–9. doi: 10.1136/jnnp.56.5.556. https://doi.org/10.1136/jnnp.56.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. https://doi.org/10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Postiglione A, Milan G, Pappatà S, De Falco C, Lamenza F, Schiattarella V, Striano S. Fronto-temporal dementia presenting as Geschwind’s syndrome. Neurocase. 2008;14(3):264–70. doi: 10.1080/13554790802269976. https://doi.org/10.1080/13554790802269976. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(9):2456–2477. doi: 10.1093/brain/awr179. https://doi.org/10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. 2nd. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: A robust approach. NeuroImage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. https://doi.org/10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. https://doi.org/10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser AH, Spreen O. The western aphasia battery. Journal of Clinical and Experimental Neuropsychology. 1985;7(4):463–470. https://doi.org/http://dx.doi.org.ezaccess.libraries.psu.edu/10.1080/01688638508401277. [Google Scholar]
- Roberts J, Robertson M, Trimble M. The lateralising significance of hypergraphia in temporal lobe epilepsy. Journal of Neurology, Neurosurgery, and Psychiatry. 1982;45(2):131–138. doi: 10.1136/jnnp.45.2.131. https://doi.org/10.1136/jnnp.45.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58(5):695–701. doi: 10.1212/wnl.58.5.695. https://doi.org/10.1212/WNL.58.5.695. [DOI] [PubMed] [Google Scholar]
- Sachdev HS, Waxman SG. Frequency of hypergraphia in temporal lobe epilepsy: an index of interictal behaviour syndrome. Journal of Neurology, Neurosurgery, and Psychiatry. 1981;44(4):358–60. doi: 10.1136/jnnp.44.4.358. https://doi.org/10.1136/jnnp.44.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. https://doi.org/10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sankar T, Bernasconi N, Kim H, Bernasconi A. Temporal lobe epilepsy: Differential pattern of damage in temporopolar cortex and white matter. Human Brain Mapping. 2008;29(8):931–944. doi: 10.1002/hbm.20437. https://doi.org/10.1002/hbm.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, Rosen HJ. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64(8):1384–90. doi: 10.1212/01.WNL.0000158425.46019.5C. https://doi.org/10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirk SD, Mitchell MB, Shaughnessy LW, Sherman JC, Locascio JJ, Weintraub S, Atri A. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimer’s Research & Therapy. 2011;3(6):32. doi: 10.1186/alzrt94. https://doi.org/10.1186/alzrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber S, Spinnler H. Covert Person Recognition: its Fadeout in a Case of Temporal Lobe Degeneration. Cortex. 2003;39(1):57–67. doi: 10.1016/s0010-9452(08)70074-1. https://doi.org/10.1016/S0010-9452(08)70074-1. [DOI] [PubMed] [Google Scholar]
- T W, K R, B K, R K, Z M, B M. Hypergraphia in semantic variant PPA. Neurology. 2013 Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed11&NEWS=N&AN=71130727.
- Tebartz van Elst L, Krishnamoorthy ES, Bäumer D, Selai C, von Gunten A, Gene-Cos N, Trimble MR. Psychopathological profile in patients with severe bilateral hippocampal atrophy and temporal lobe epilepsy: Evidence in support of the Geschwind syndrome? Epilepsy and Behavior. 2003;4(3):291–297. doi: 10.1016/s1525-5050(03)00084-2. https://doi.org/10.1016/S1525-5050(03)00084-2. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61(9):1196–203. doi: 10.1212/01.wnl.0000091868.28557.b8. https://doi.org/10.1212/01.WNL.0000091868.28557.B8. [DOI] [PubMed] [Google Scholar]
- Tyrrell PJ, Warrington EK, Frackowiak RS, Rossor MN. Progressive degeneration of the right temporal lobe studied with positron emission tomography. Journal of Neurology, Neurosurgery, and Psychiatry. 1990;53(12):1046–50. doi: 10.1136/jnnp.53.12.1046. https://doi.org/10.1136/jnnp.53.12.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt P, Paquier P, Kees L, Cras P. Increased writing activity in neurological conditions: a review and clinical study. Journal of Neurology, Neurosurgery, and Psychiatry. 1996;61(5):510–4. doi: 10.1136/jnnp.61.5.510. https://doi.org/10.1136/jnnp.61.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas J, Clark E. National Adult Reading Test. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York; 2011. p. 1705. https://doi.org/10.1007/978-0-387-79948-3_1467. [Google Scholar]
- Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann NY Acad Sci. 2007 doi: 10.1196/annals.1401.025. annals.1401.025. https://doi.org/10.1196/annals.1401.025. [DOI] [PubMed]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain. 2013 doi: 10.1093/brain/awt094. https://doi.org/10.1093/brain/awt094. [DOI] [PMC free article] [PubMed]
- Waxman SG, Geschwind N. The interictal behavior syndrome of temporal lobe epilepsy. Archives of General Psychiatry. 1975;32(12):1580–1586. doi: 10.1001/archpsyc.1975.01760300118011. https://doi.org/10.1001/archpsyc.1975.01760300118011. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Geschwind N. Hypergraphia in temporal lobe epilepsy. Epilepsy & Behavior : E&B. 2005;6(2):282–91. doi: 10.1016/j.yebeh.2004.11.022. 1974. https://doi.org/10.1016/j.yebeh.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Waxman S, Geschwind N. Hypergraphia in temporal lobe epilepsy. Neurology. 1974;6(2):282–291. doi: 10.1016/j.yebeh.2004.11.022. https://doi.org/10.1016/j.yebeh.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for Wechsler Memory Scale - Revised. The Psychological Corporation. 1987a https://doi.org/PCA-Converted#56.
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: Psychological Corporation; 1987b. [Google Scholar]
- Weder BJ, Schindler K, Loher TJ, Wiest R, Wissmeyer M, Ritter P, Missimer J. Brain areas involved in medial temporal lobe seizures: A principal component analysis of ictal SPECT data. Human Brain Mapping. 2006;27(6):520–534. doi: 10.1002/hbm.20196. https://doi.org/10.1002/hbm.20196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Disease and Associated Disorders. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. https://doi.org/10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollacott IOC, Fletcher PD, Massey LA, Pasupathy A, Rossor MN, Caine D, Warren JD. Compulsive versifying after treatment of transient epileptic amnesia. Neurocase. 2015;21(5):548–53. doi: 10.1080/13554794.2014.953178. https://doi.org/10.1080/13554794.2014.953178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuerfel J, Krishnamoorthy ES, Brown RJ, Lemieux L, Koepp M, Tebartz van Elst L, Trimble MR. Religiosity is associated with hippocampal but not amygdala volumes in patients with refractory epilepsy. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75(4):640–642. doi: 10.1136/jnnp.2003.06973. https://doi.org/10.1136/jnnp.2003.06973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamadori Atsushi, Mori Etsuro, Tabuchi Masayasu, Kudo Yutaka, MItani Y. Hypergraphia: a right hemisphere syndrome. Journal of Neurology, Neurosurgery & Psychiatry. 1986;49(10):1160–1164. doi: 10.1136/jnnp.49.10.1160. https://doi.org/10.1136/jnnp.49.10.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. https://doi.org/10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Iyengar V, Huey ED, Tierney M, Krueger F, Grafman J. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009;132(3):604–616. doi: 10.1093/brain/awn343. https://doi.org/10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]


