Abstract
Aerobic exercise training is an effective therapy to improve peak aerobic power (peak VO2) in individuals with hypertension (HTN, AHA/ACC class A) and heart failure patients with preserved ejection fraction (HFpEF). High nitrate containing beetroot juice (BRJ) also improves sub-maximal endurance and decreases blood pressure in both HTN and HFpEF. We hypothesized that combining an aerobic exercise and dietary nitrate intervention would result in additive or even synergistic positive effects on exercise tolerance and blood pressure in HTN or HFpEF. We report results from two pilot studies examining the effects of supervised aerobic exercise combined with dietary nitrate in patients with controlled HTN (n=26, average age 65 ± 5 years) and in patients with HFpEF (n = 20, average age 69 ± 7 years). All patients underwent an aerobic exercise training regimen; half were randomly assigned to consume a high nitrate-containing beet juice beverage (BRJ containing 6.1 mmol nitrate for the HFpEF study consumed three times a week and 8 mmol nitrate for the HTN study consumed daily) while the other half consumed a beet juice beverage with the nitrate removed (placebo). The main result was that there was no added benefit observed for any outcomes when comparing BRJ to placebo in either HTN or HFpEF patients undergoing exercise training (p ≥ 0.14). There were within-group benefits. In the pilot study in patients with HFpEF, aerobic endurance (primary outcome), defined as the exercise time to volitional exhaustion during submaximal cycling at 75% of maximal power output, improved during exercise training within each group from baseline to end of study, 369 ± 149 sec vs 520 ± 257 sec (p = 0.04) for the placebo group and 384 ± 129 sec vs 483 ± 258 sec for the BRJ group (p = 0.15). Resting systolic blood pressure in patients with HFpEF also improved during exercise training in both groups, 136 ± 16 mm Hg vs 122 ± 3 mm Hg for the placebo group (p < 0.05) and 132 ± 12 mm Hg vs 119 ± 9 mm Hg for the BRJ group (p < 0.05). In the HTN pilot study, during a treadmill graded exercise test, peak oxygen consumption (primary outcome) did not change significantly, but time to exhaustion (also a primary outcome) improved in both groups, 504 ± 32 sec vs 601 ± 38 sec (p < 0.05) for the placebo group and 690 ± 38 sec vs 772 ± 95 sec for the BRJ group (p < 0.05) which was associated with a reduction in supine resting systolic blood pressure in BRJ group. Arterial compliance also improved during aerobic exercise training in both the HFpEF and the HTN patients for both BRJ and placebo groups. Future work is needed to determine if larger nitrate doses would provide an added benefit to supervised aerobic exercise in HTN and HFpEF patients.
Keywords: exercise, heart failure with preserved ejection fraction, hypertension, nitrate, nitric oxide, nitrite
1. INTRODUCTION
The most common form of heart failure is preserved ejection fraction (HFpEF)1; it almost exclusively affects older adults and is characterized by exercise intolerance that manifests in a poor quality of life [1–3]. Population studies show that about 90 percent of HFpEF patients have a history of chronic hypertension (HTN). HTN and HFpEF often share common cardiovascular abnormalities including increased arterial stiffness, left ventricular hypertrophy, left atrial dilation, and frequent abnormal diastolic function [4]. Symptoms of both HTN and HFpEF can be improved by aerobic exercise training [5–8]. Blood pressure in treated hypertensives is typically controlled using various drug interventions while aerobic exercise regimens can also reduce blood pressure and improve vascular function [9; 10]. Habitual exercise also improves exercise capacity [10]. To date, the only treatment confirmed in clinical trials to improve exercise capacity in patients with HFpEF is aerobic exercise training [5; 11; 12].
Low nitric oxide (NO) bioavailability results in hypertension and restoration of NO is the basis for the mechanism of action of some current medications [13–15]. Low NO bioavailability has also been suggested to contribute to poor skeletal muscle perfusion in patients with HFpEF [16], which (along with other non-cardiac factors) contributes to exercise intolerance [11; 17–19]. One attractive means to deliver NO is through the anion nitrite (NO2−) as nitrite is reduced to NO preferentially in areas of low oxygen and pH so that delivery is well-targeted to metabolically active tissue such as at the muscles involved in exercise [20]. This is often accomplished through the nitrate (NO3−)-nitrite-NO pathway [21; 22]. Plasma nitrate is derived from endogenous mechanisms (including the oxidation of NO) and from dietary consumption (especially vegetables including beets and beet root juice [23]). Bacteria in the oral cavity partially reduce salivary nitrate to nitrite [24]. Nitrate and nitrite in the gastrointestinal tract are transferred to the plasma. Nitrite is then reduced to NO, preferentially under hypoxic and acidic conditions, through mechanisms proposed to involve a variety of heme and non-heme proteins, as recently reviewed [25]. Plasma nitrate is concentrated in salivary glands and secreted back into the oral cavity so that the nitrate-nitrite-NO pathway cycles for an extended period with the half-life of plasma nitrate being about six hours [21; 26; 27].
Several studies have demonstrated the therapeutic potential of the nitrate-nitrite-NO pathway including that dietary nitrate lowers blood pressure and improves exercise performance in patients with chronic obstructive pulmonary disease [28], improves exercise performance in patients with peripheral artery disease [29], and improves exercise capacity and endurance in patients with HFpEF [30; 31]. Numerous studies have shown that dietary nitrate improves exercise efficiency or performance, or lowers blood pressure in healthy volunteers [32]. A recent double-blind, placebo controlled study demonstrated sustained blood pressure lowering due to dietary nitrate in hypertensive individuals that were both taking medication and drug-naïve [33]. In addition, it has recently been shown that infused [34] or inhaled [35] nitrite (the active metabolite of nitrate) improves hemodynamics in patients with HFpEF.
We hypothesized that the combination of oral nitrate and aerobic exercise training will improve nitric oxide bioavailability as well as blood pressure and result in improved exercise performance beyond what is observed with aerobic exercise training alone. This hypothesis is based on the notion that the effect of aerobic exercise training may be limited by poor NO bioavailability due to endothelial dysfunction. Simultaneous administration of oral nitrate could improve aerobic exercise training and subsequent outcomes. This hypothesis is supported by recent work showing that dietary nitrate results in similar physiological responses as exercise therapy in a diabetic rat model [36]. We tested our hypothesis in two separate studies: patients with HFpEF and individuals with controlled hypertension.
2. METHODS
2.1 Study Design
Both studies (HFpEF and HTN) were approved by the institutional review board, and all participants provided written, informed consent.
HFpEF Study
This pilot study was an extension of a recent study of 20 HFpEF patients (average age 69 ± 7 years) investigating dietary nitrate (but no exercise) [31]. Briefly, the recent published study examined effects of a single acute dose and one week of daily dosing of BRJ (with no exercise). This portion of the study that did not involve exercise training lasted between 12 and 22 days from the end of baseline exercise testing and the last visit of that previous study. Immediately following the final study visit of the previous report [31], all participants were entered into a 4-week exercise program and were randomized to receive either beetroot juice (BRJ, 70 ml; Beet It Sport Shot; James White Drinks, Ipswich, UK) or placebo to consume before each exercise session (Figure 1). The BRJ and placebo were identical in appearance, taste, smell, and nutrient composition except that the NO3− was removed by the manufacturer to make the placebo. As measured in our lab, the BRJ contained 0.38 g (6.1 mmol) NO3− and the placebo contained 0.0003 g (4.8 µmole) NO3−. The person responsible for dispensing the juice was not involved in study testing or data analysis.
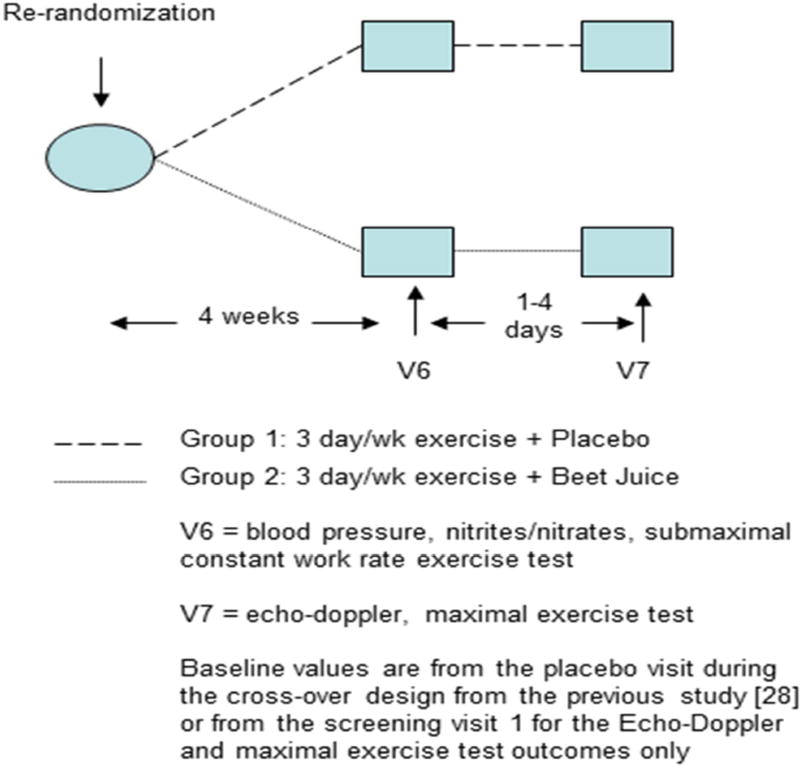
Figure 1.
For HFpEF participants, immediately following the previous study design, V5[31], all subjects were re-randomized to either 4 weeks of EX + Placebo or 4 weeks of EX + BRJ. After the 4-week intervention, subjects completed 2 follow-up visits separated by 1–4 days. Visit 6 consisted of blood pressure measurements, a blood draw, and a submaximal constant workrate exercise test. Visit 7 consisted of a resting Echo-Doppler exam and a maximal exercise test. Baseline values are from the placebo visit during the cross-over design from the previous study (either V3 or V4) or from the screening visit 1 for the Echo-Doppler and maximal exercise test outcomes only.
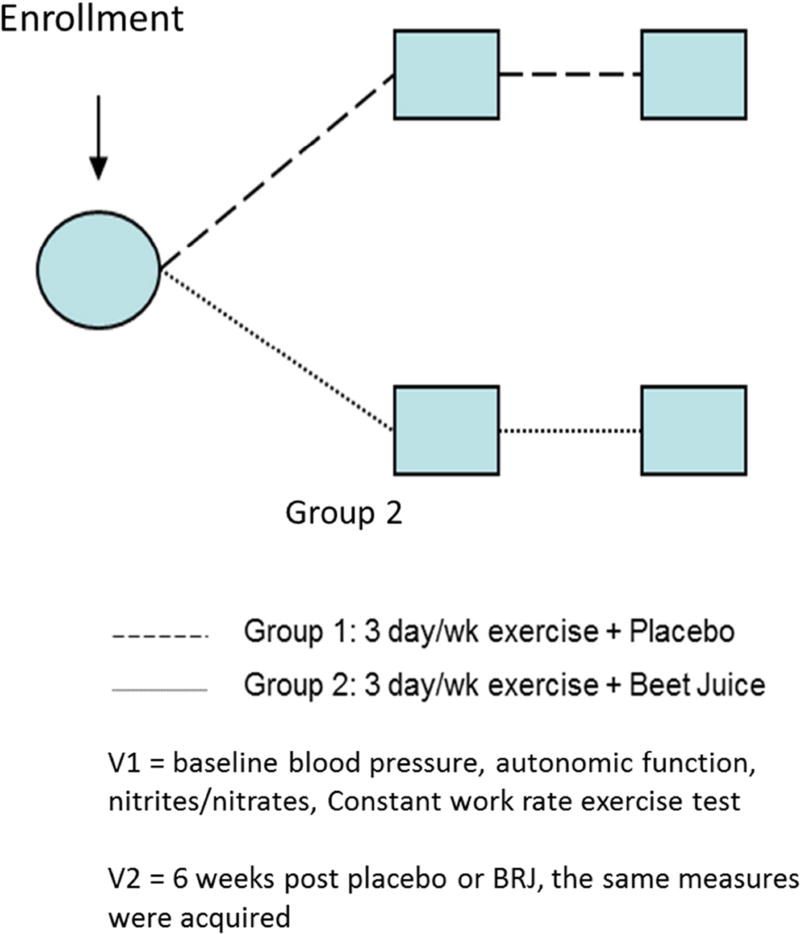
For HTN participants, immediately following the initial screening and assessment of exercise performance, cardiovascular and autonomic function at visit 1, all subjects were randomized to either 6 weeks of EX + Placebo or 6 weeks of EX + BRJ. After 6 weeks intervention, subjects returned for follow up visit 2 where the same outcomes were measured again.
Participant inclusion and exclusion criteria, recruitment, and enrollment were reported previously [31]. Participants’ characteristics are summarized in Table 1. Following the intervention, participants completed two clinic visits separated by 1–4 days. Baseline values were considered to be from the placebo crossover visit from our previous report [31]. For all visits, the participant consumed the assigned juice ~45 minutes before arriving at the clinic so that they would begin aerobic exercise at one hour after consumption.
Table 1.
Participant Characteristics - HFpEF participants
| Characteristic | EX + Placebo (N=9) |
EX + Beetroot Juice (N=10) |
P-Value |
|---|---|---|---|
| Age (years) | 70.6 ± 7.6 | 68.0 ± 6.2 | 0.43 |
| Female | 8 (89%) | 8 (80%) | 1.0 |
| White | 6 (67%) | 6 (60%) | 1.0 |
| Body Weight (kg) | 82.8 ± 15.0 | 91.9 ± 16.0 | 0.22 |
| BMI (kg/m2) | 31.5 ± 5.4 | 33.5 ± 5.8 | 0.46 |
| NYHA class | |||
| II | 8 (89%) | 5 (50%) | 0.14 |
| III | 1 (11%) | 5 (50%) | |
| Diastolic Function | |||
| Normal | 2 (22%) | 1 (10%) | 1.0 |
| Impaired Relaxation | 7 (78%) | 9 (90%) | |
| Pseudonormal | 0 (0%) | 0 (0%) | |
| Restrictive | 0 (0%) | 0 (0%) | |
| e’ (cm/s) | 5.9 ± 2.0 | 6.9 ± 1.8 | 0.26 |
| E/ e’ ratio | 14.6 ± 7.8 | 12.0 ± 5.7 | 0.42 |
| Left atrial diameter (cm) | 3.6 ± 0.4 | 3.8 ± 0.3 | 0.13 |
| Diabetes mellitus | 2 (20%) | 5 (50%) | 0.35 |
| Hx hypertension | 9 (100%) | 10 (100%) | 1.0 |
| Systolic BP (mmHg) | 147 ± 17 | 144 ± 14 | 0.74 |
| Diastolic BP (mmHg) | 69 ± 12 | 71 ± 9 | 0.66 |
| Current medication | |||
| ACE-inhibitors | 3 (33%) | 3 (30%) | 1.0 |
| Diuretics (all) | 5 (56%) | 7 (70%) | 0.65 |
| Loop diuretics | 1 (11%) | 2 (20%) | 1.0 |
| Beta-blockers | 2 (22%) | 3 (30%) | 1.0 |
| Calcium channel blockers | 2 (22%) | 5 (50%) | 0.35 |
| ARB’s | 2 (22%) | 4 (40%) | 0.63 |
| Peak VO2 (ml/kg/min) | 12.3 ± 1.7 | 11.6 ± 2.5 | 0.50 |
| Peak workload (watts) | 58 ± 17 | 59 ± 26 | 0.91 |
Data presented as mean ± SD or number (%).
Abbreviations- BMI: body mass index; NYHA: New York Heart Association; e’; mitral annulus velocity; E; early mitral velocity; Hx; history; BP: blood pressure; ACE; angiotensin converting enzyme; ARB; angiotensin receptor blocker
All participants completed a medical facility-based, supervised, 4-week exercise program. There were three exercise sessions per week held on Mondays, Wednesdays, and Fridays. The sessions consisted of a series of warm-up, flexibility, and aerobic exercises, carried out with a combination of walking (circuit and motorized treadmill with adjusted speed and grade) and cycling (Airdyne and/or recumbent bike) exercise. Participants spent approximately half of the aerobic exercise time in each mode of exercise training. The goal was to build up to at least 40 minutes of training (20 minutes walking and 20 minutes cycling) by the end of the 4-week program. The intensity started at a low to moderate intensity level (50–60% of heart rate reserve from baseline maximal exercise test) and gradually increased until the participant was able to maintain 70% of heart rate reserve for at least 20 minutes. During the cycling portion, participants exercised at 40–60% of peak workload (from the baseline maximal exercise test, measured as watts).
Subjects were asked to refrain from using mouthwash on each testing day and for 2 days prior to any scheduled consumption of the beetroot juice or placebo. Participants were instructed to consume the BRJ or placebo ~1 hour before arriving at the exercise facility along with a breakfast meal. Thus, we refer to groups as exercise + BRJ (EX + BRJ) or EX + placebo. A log of the exact time of ingestion was completed and verbal confirmation of juice consumption was obtained before each exercise session. Importantly, the BRJ or placebo was only consumed on exercise days.
HTN Study
A randomized, placebo-controlled double blind trial was performed where participants (n=26, average age 65 ± 5 years) with controlled hypertension were assigned to aerobic exercise with either nitrate rich beet root juice (BRJ, n=13, 6F) or nitrate-depleted beetroot juice placebo drink (Placebo, n=13, 7F). The BRJ (Beet It Sport Shot; James White Drinks, Ipswich, UK) for the HTN pilot study contained 0.5 g (8 mmol) NO3− and the placebo contained 0.001 g (20 µmole) NO3−. Participants were aged ≥55 years, had a systolic blood pressure between 130–160 mm Hg, were taking no more than 2 hypertensive medications, and were sedentary, defined as performing <60 minutes of moderate levels of exercise each week performed in bouts >10 minutes. Exclusion criteria included the use of tobacco products, involvement in another research study, and a Modified Mini-Mental State Exam (MMMSE) score <80. Furthermore, participants were ineligible if they were taking medications known to interfere with nitrate/nitrite metabolism, diagnosed with active neurological dysfunction, or had contraindications for participation in exercise. Patient characteristics are summarized in Table 2. The aerobic exercise intervention consisted of a center-based, individualized, moderately intense walking program of 18 sessions: three 50-minute sessions per week for 6 weeks. All participants walked on motorized Life Fitness TR-9500HR treadmills at a Borg rating of perceived exertion of 12–13. The first four sessions were used to acclimatize participants to the training protocol and progressively increase the walking time towards the goal of 50 min per session. Participants were guided through a series of stretching exercises before and after walking. The participants were instructed to consume one 70 mL (2.4 oz.) beverage each day of the week within 30 minutes of opening the bottle. On days that they were scheduled to attend the exercise intervention they were to consume the beverage 1 hour before the scheduled training session time. Daily consumption logs were completed by all participants and collected weekly. Volunteers were asked to avoid antacids, spinach, lettuce, beets, and leafy greens for the duration of the study. They were given these guidelines on a business card. We did not formally track compliance with the requested dietary restrictions. Height was measured to the nearest 0.5 cm using a stadiometer and body mass was measured to the nearest 0.1 kg using a scale. BMI (kg/m2) was calculated as body mass divided by height squared.
Table 2.
Participant Characteristics - HTN participants
| Characteristic | EX + Placebo (N=13) |
EX + Beetroot Juice (N=13) |
P-Value |
|---|---|---|---|
| Age (years) | 65.6 ± 7.6 | 65.0 ± 3.8 | 0.77 |
| Female | 7 (54%) | 6 (46%) | 0.7 |
| White | 10 (77%) | 10 (77%) | 1.0 |
| Body Weight (kg) | 100.5 ± 22.0 | 95.0 ± 11.0 | 0.47 |
| BMI (kg/m2) | 35.0 ± 6.5 | 32.3 ± 4.6 | 0.26 |
| Systolic BP (mmHg) | 129 ± 9 | 132 ± 10 | 0.5 |
| Diastolic BP (mmHg) | 74 ± 8 | 76 ± 7 | 0.6 |
| Current medication | |||
| ACE-inhibitors | 5 (38%) | 2 (15%) | 0.2 |
| Diuretics (all) | 4 (30%) | 4 (30%) | 1.0 |
| Beta-blockers | 2 (15%) | 2 (15%) | 1.0 |
| Calcium channel blockers | 0 (0%) | 0 (0%) | 1.0 |
| ARB’s | 2 (15%) | 2 (15%) | 1.0 |
| Peak VO2 (ml/kg/min) | 19.6 ± 5.0 | 17.2 ± 4.6 | 0.25 |
Data presented as mean ± SD or number (%)
Abbreviations- BMI: body mass index; BP: blood pressure; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker
2.2 Nitrite and Nitrate
Venous blood samples were drawn into BD 4 ml lithium heparin tubes and centrifuged at 4,000 rpm at room temperature for 3 minutes within 1 minute of collection. For the HTN study, blood was drawn immediately before and one hour after BRJ or placebo consumption. For the HFpEF study, blood was drawn one hour after BRJ or placebo consumption. Plasma was transferred in polypropylene microtubes containing no additives and frozen at −70°C for later analysis. Nitrite and nitrate were measured as described previously [37] using an ENO-20 nitric oxide analyzer (EICOM, San Diego, CA USA). Plasma was mixed with equal volume of 100% methanol, vortexed and centrifuged at 11,500g for 10 minutes. The supernatant was loaded into 96 well plates and nitrate and nitrate concentrations was measured using ENO-20 NOx analyzer (EICOM, San Diego, CA, USA). The nitrite and nitrate is separated via column chromatography and reacted individually with the Greiss reagent to synthesize a red diazo compound that is read at a wavelength of 540 nm by a visible light detector in the ENO-20, sample preparation described above are as per the manufacturer’s instructions. Standard nitrite and nitrate samples were made freshly each day before plasma samples were measured and used for calibration.
2.3 Exercise testing
HFpEF Study
All exercise tests were performed with the participant in an upright position on an electronically braked cycle ergometer with the pedal rate at ~ 60 rpm, as previously described [31; 38; 39]. Two exercise tests, conducted on separate visits, were completed before and after the intervention. The first exercise test was a maximal, graded (10 W per minute) exercise test performed to assess peak aerobic capacity [40]. The maximal work rate was defined as the greatest work rate that could be maintained for ≥ 30 seconds. The second exercise test was a submaximal constant work rate exercise test at ~75% of maximal work rate performed to assess submaximal aerobic endurance. The details of the submaximal constant work rate test were described previously [31]. Breath-by-breath gas exchange data (Medgraphics Ultima; Minneapolis, Minnesota) were measured continuously at rest and during exercise. All tests were performed ~ 1.5–2 hours after the participant consumed the juice. (BRJ or placebo).
HTN Study
We used a physician supervised; individualized ramp treadmill protocol, where subjects walked at a brisk pace with the speed based on their comfort and fitness. The grade was increased at a rate of 1–2 %/minute based on fitness level. Peak volume oxygen consumption (VO2 peak) was determined during the graded exercise test using a metabolic cart (Medical Graphics Ultima). Heart rate, rhythm, blood pressure, and oxygen uptake were monitored during the test. Test termination criteria include: patient request, volitional fatigue, increasing chest or leg pain, dizziness, faintness, fatigue, pallor, cyanosis, ataxic gait, cardiac arrhythmias or decompensation, hyper or hypotension, and ECG abnormalities.
2.4 Hemodynamic Measures
HFpEF Study
During all tests, heart rate and rhythm were monitored continuously using an electrocardiogram, and blood pressure was taken at rest (following 2 minutes of quiet breathing) and every two minutes during the test.
Doppler-echocardiograms were performed and analyzed according to American Society of Echocardiography recommendations and as previously described with a Phillips iE33 machine and Xcelera work station (Phillips Medical Systems, The Netherlands) [5; 12; 38; 41–44]. Doppler LV filling patterns [45; 46] and mitral annulus tissue velocities (septal) were assessed as previously described [41; 47].
HTN Study
Ambulatory 24-hour blood pressure and heart rate: Subjects were asked to wear blood pressure monitors (Spacelabs 90207, Redmond, WA) for 24 hours and keep a diary of their activities. The monitors were fitted by a trained researcher and programmed to inflate every 30 minutes from 6 am to 11 pm and every 60 minutes from 11 pm to 6 am. Subjects with at least 80% valid readings were analyzed (n=12 placebo and n=12 BRJ). Mean SBP and DBP of daytime and night-time were calculated according to self-reported sleep and awake times. The percentages of nocturnal SBP and DBP decline were determined using the following formula: [(daytime BP-night-time BP)/daytime BP] ×100. Inadequate nocturnal dipping was defined as less than 10% reduction in the mean SBP from awake to asleep [48; 49].
Measures of stroke volume (SV), cardiac output (CO), systemic vascular resistance (SVR), total arterial compliance (TAC), velocity index (VI), acceleration index (AI) and left cardiac work index (LCWI) were derived from mean arterial pressure measures obtained using impedance cardiography (ICG) machine (BioZ®, Model BZ 4110-101D) [50; 51]. This machine applies Ohm’s relationship to the thorax to allow changes in voltage and impedance due to changes in blood flow to be translated into hemodynamic parameters of cardiovascular function [52; 53].
Continuous BP and HR were acquired from noninvasive finger arterial pressure measurements for a minimum of 5 minutes in subjects in the supine position, at baseline and followup. SBP and RR intervals (RRI) files were acquired (BIOPAC acquisition software, Santa Barbara, CA) at 1000 HZ and analyzed using Nevrokard BRS software (Nevrokard BRS, Medistar, Ljubljana, Slovenia) followed by post-hoc analysis for autonomic function variables (heart rate variability (HRV), blood pressure variability (BPV) and baroreflex sensitivity (BRS)). BRS was measured using a frequency domain method as LF, HF which is the square root of the spectral density of the heart rate divided by the square root of the spectral density of systolic arterial pressure in the low frequency (LF), and high frequency (HF) range, and using a time domain method as Seq UP & DOWN (slope of the baroreflex gain curve measured by the sequence method in the UP or Down direction; Seq ALL: slope of all the baroreflex curves), while HRV was measured as power of RRI spectra in LF, HF range and their ratio (LFRRI/HFRRI) and standard deviation of normal beat-to-beat interval (SDRR) and root of mean of successive differences (rMSSD). Blood pressure variability (BPV) was measured as standard deviation of the mean arterial pressure (SDMAP) as described previously [54–56].
2.5 Study Outcomes
HFpEF Study
The predetermined primary efficacy outcome was aerobic endurance defined as the exercise time to volitional exhaustion during submaximal cycling at 75% of maximal power output. Secondary efficacy outcomes included plasma NO3− and NO2− levels, peak VO2 from the maximal exercise test, resting Echo-Doppler characteristics, VO2 and blood pressure at rest, after unloaded cycling, at 2 and 4 minutes, and at volitional exhaustion, and heart rate and other gas exchange measures at volitional exhaustion of the submaximal constant work rate exercise test. VO2 outcomes were defined as the average of the entire resting period and the average of the 30 seconds prior to each specified timepoint (i.e. 2 minutes, 4 minutes, peak)
HTN Study
The primary efficacy outcome was exercise capacity (assessed by peak oxygen consumption and time to volitional exhaustion) during a graded treadmill exercise test. Secondary outcome measures included blood pressure, autonomic and vascular function including vascular resistance, and heart rate variability (at rest or 24 hr monitoring or during the exercise test).
2.6 Ad hoc analysis
For the HTN study, the degree to which individuals converted oral nitrate to plasma nitrite was compared to improvement in exercise time. The change in nitrite was taken from levels measured before and after BRJ consumption. The groups were best-responder (BR, change greater than 150 nM), good-responder (GR, change less than 150 nM but greater than 50 nM) and non-responder (NR, change less than 50 nM). These groups had n=4, n=4, and n = 3 individuals each respectively.
2.7 Statistical Methods
Based on preliminary data from a study of chronic obstructive pulmonary disease patients, this randomized pilot study of HFpEF patients was designed to have 80% power to detect a 40% difference in the primary outcome. The HTN study did not include any a priori power calculations as it was considered a pilot study. Comparisons of participant characteristics were made by Student’s t-test for continuous variables or by Fischer’s exact test for categorical variables. For participant characteristics, NYHA class and diastolic function, in Table 2, an alternative comparison of outcome measures between the groups was made by a two way analysis of variance (ANOVA) followed up with a Tukey's characteristic-wise test. Comparison of outcome measures between intervention groups were made by analysis of covariance, with the follow-up value adjusted for the baseline value using the least square mean. A two-tailed p-value of <0.05 was required for significance. Paired samples t-tests were performed for comparison within groups (pre vs post) with a p-value <0.05 required for significance.
3. RESULTS
3.1 Retention, Adherence, Safety
HFpEF Study
All participants completed the intervention and follow-up testing. Participants attended an average of 90% of the exercise sessions. Adherence to the BRJ supplement, as measured by returned bottle count and consumption log, was 100%. There were no adverse events related to the aerobic exercise training program or BRJ supplement.
HTN Study
Adherence to the exercise intervention was 85% with 22 out of 26 volunteers having 100% adherence. Daily logs were used to assess adherence to the supplement. Only three of the 26 participants were not 100% adherent with one volunteer in the placebo group being 86% adherent in taking the supplement and two volunteers in the BRJ group being 98% adherent. There were 2 adverse events in the study, both of which were related to the exercise intervention (both participants had a fall while walking on the treadmill). One participant sustained a severe muscle strain and withdrew from the study and one participant sustained minor bruising and returned to training at the next exercise session.
3.2 Plasma NO3− and NO2−
HTN Study
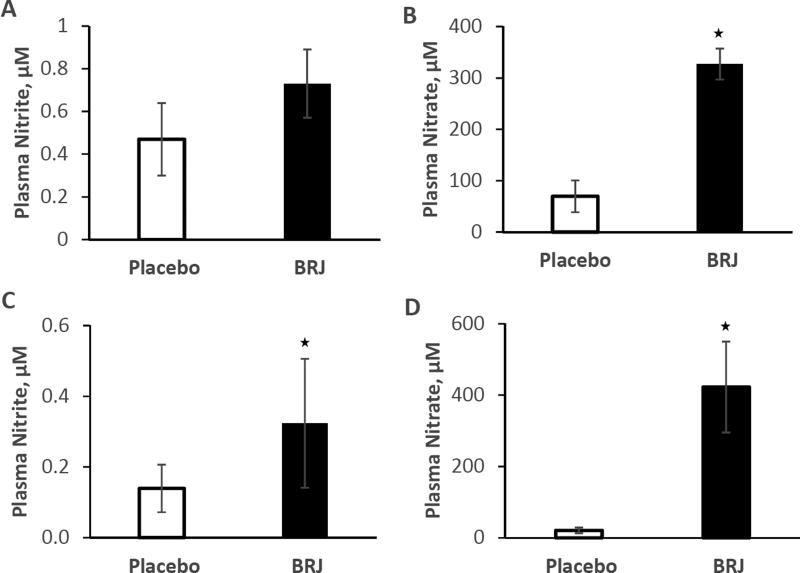
EX + BRJ resulted in both elevated nitrate and nitrite compared to EX + placebo: 422 ± 127 µM nitrate for BRJ and 21 ± 8 µM nitrate for EX + placebo, and 0.32 ± 0.18 µM nitrite for BRJ and 0.14 ± 0.07 µM nitrite for EX + placebo, p<0.001 (Figure 2). Plasma nitrite and nitrate was measured at weeks 1, 2, and 3 before and after beverage consumption. Interestingly, average plasma nitrate in volunteers taking BRJ increased from baseline at week 1 (32 µM before BRJ and 344 µM after consumption) to week 2 (114 µM before BRJ and 459 after consumption, p = 0.002) and again (compared to week 1) in week 3 (108 µM before BRJ and 467 µM after consumption, p = 0.0002). However, no changes in nitrite levels between weeks was noted nor was the change in nitrate (post-pre consumption) different at weeks 1, 2, or 3. Oddly, plasma nitrite after placebo consumption was lower in week 3 compared to week 1 (p = 0.04). No other differences in these measures were noted between weeks.
Figure 2.
Plasma NOx levels at baseline and following beverage consumption. (A) Nitrite from the HFpEF study. (B) Nitrate from the HFpEF study. (C) Nitrite from the HTN study. (D) Nitrate from the HTN study.
HFpEF Study
Plasma NO3− was significantly (p<0.001) elevated in the BRJ group (EX + BRJ: 327 ± 30 µM) compared to the EX + placebo group (EX: 70 ± 31 µM). In contrast, plasma NO2− (BRJ: 0.73 ± 0.16, Placebo: 0.47 ± 0.17 µM; p=0.27) was not different between the groups (Figure 2).
Notably, plasma NO2− and NO3− were higher in the placebo group from the HFpEF group compared to the HTN group. Part of the reason that the plasma nitrite placebo value is so high in the HFpEF study is due to a single individual whose plasma nitrite concentration was measured to be 3.15 µM. When this person's measure is excluded, the average, adjusted plasma nitrite levels for the placebo measure becomes 0.27 ± 0.17 µM (from 0.46 ± 0.17 µM when that person is included). Similarly, this same person had an extremely high level of plasma nitrate (332 µM) and excluding that value brings the average adjusted plasma nitrate values down from 70 ± 31 µM to 58 ± 34 µM. Exclusion of this single measure still did not result in a significant elevation in plasma nitrite in comparing the placebo and BRJ groups for the HFpEF study
3.3 Exercise testing
HFpEF Study
There was no significant difference in submaximal aerobic endurance (the primary outcome) between the BRJ and placebo groups (BRJ: 475 ± 60 vs. placebo: 531 ± 68 sec.; p=0.55). The expected exercise-induced improvement in submaximal aerobic endurance was observed in both populations (26% improvement in the BRJ group and 41% improvement in the placebo group, p<0.05 for placebo group, p=0.01 when groups combined) (Table 3).
Table 3.
Submaximal Constant Workload Exercise Test – HFpEF participants
| Variable | EX + Placebo | EX + Beetroot Juice | P-value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | LS Mean ± SE |
Baseline | Follow-up | LS Mean ± SE |
||
| Submax Exercise Test (n) | 8 | 8 | 8 | 10 | 10 | 10 | |
| Exercise time at constant work rate, sec | 369 ± 149 | 520 ± 257* | 531 ± 68 | 384 ± 129 | 483 ± 258 | 475 ± 60 | 0.55 |
| Resting systolic blood pressure, mmHg | 136 ± 16 | 122 ± 3* | 122 ± 2 | 132 ± 12 | 119 ± 9* | 120 ± 2 | 0.56 |
| Resting diastolic blood pressure, mmHg | 77 ± 11 | 73 ± 11 | 72 ± 3 | 72 ± 9 | 70 ± 8 | 72 ± 2 | 0.94 |
| At exhaustion: | |||||||
| VO2, ml/kg/min | 12.2 ± 1.5 | 12.7 ± 2.1 | 12.4 ± 0.4 | 11.6 ± 2.6 | 11.5 ± 2.4 | 11.7 ± 0.3 | 0.14 |
| VO2, ml/min | 1017 ± 275 | 1050 ± 281 | 1085 ± 28 | 1083 ± 355 | 1065 ± 344 | 1037 ± 25 | 0.22 |
| VCO2, ml/min | 1233 ± 406 | 1182 ± 391 | 1187 ± 48 | 1243 ± 448 | 1215 ± 453 | 1211 ± 43 | 0.72 |
| Respiratory exchange ratio | 1.20 ± 0.10 | 1.12 ± 0.08* | 1.10 ± 0.03 | 1.14 ± 0.07 | 1.12 ± 0.10 | 1.14 ± 0.03 | 0.36 |
| Heart rate, bpm | 123 ± 16 | 119 ± 16 | 116 ± 3 | 118 ± 24 | 116 ± 25 | 118 ± 3 | 0.62 |
| Systolic blood pressure, mmHg | 172 ± 12 | 173 ± 13 | 170 ± 5 | 163 ± 19 | 161 ± 19 | 163 ± 4 | 0.34 |
| Diastolic blood pressure, mmHg | 79 ± 12 | 81 ± 9 | 80 ± 2 | 76 ± 8 | 75 ± 7 | 76 ± 2 | 0.15 |
Data presented as mean ± SD or number (%) and the least-square (LS) mean ± SE of the follow-up value adjusted for the baseline value. P-value corresponds to comparison of the least-square mean (LS Mean). Mean constant work rate = 46 W for EX + Placebo and 44 W for EX + Beetroot juice. Exercise time excludes the 2-minute unloaded pedaling period.
p<0.05 for within group change by paired samples t-test (follow-up –baseline).
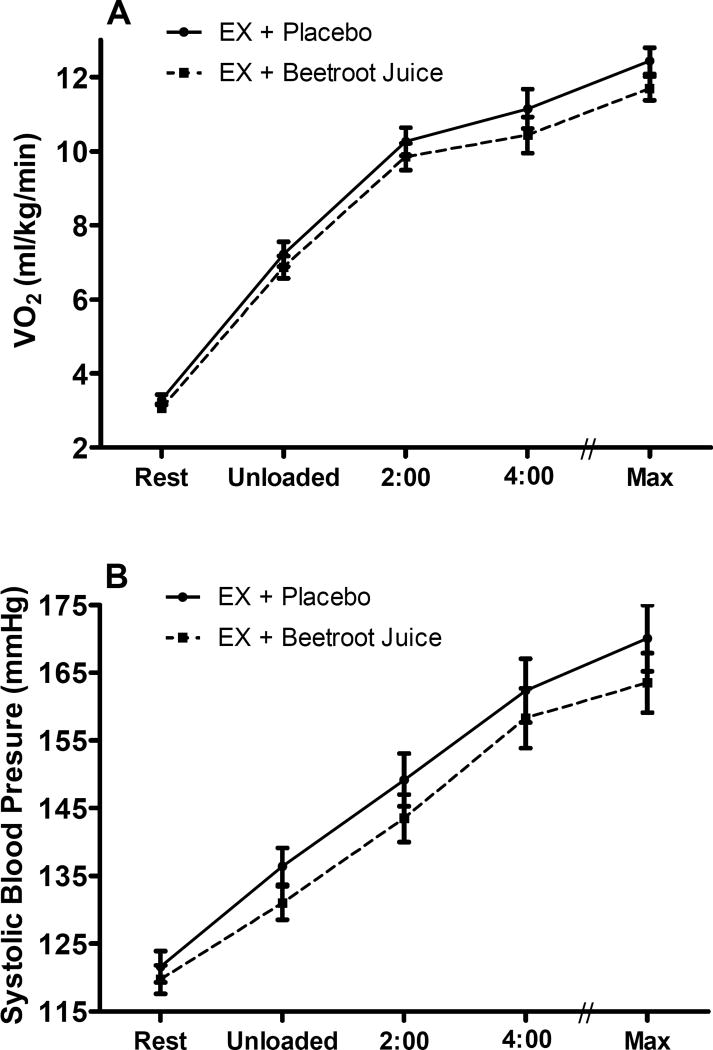
In the BRJ group there was a trend for VO2 to be lower at volitional exhaustion compared to EX + placebo (p=0.14). There were no other differences in VO2 between the BRJ and placebo groups at rest or at any time-point measured during exercise (Figure 3A). Mean respiratory exchange ratio at exhaustion was ≥ 1.12 in both groups and at both time points; indicating that although a work rate of ~75% of maximal was used, a similar, exhaustive, severe-intensity level was reached at the end of the test (as indicated by the similar respiratory exchange ratios which were not significantly different from each other). Finally, there were no differences in heart rate or any other gas exchange measure at volitional exhaustion (Table 3).
Figure 3.
VO2 (Panel A) and Systolic Blood Pressure (Panel B) at rest, after unloaded cycling, after 2 and 4 minutes, and at volitional exhaustion (max) from the submaximal constant work rate exercise test in the HFpEF study. Values are the follow-up least square means ± SE (adjusted for the baseline values). The circles and solid line represent the EX + Placebo group and the squares and dotted line represent the EX + Beetroot juice group. There were no differences between Placebo and BRJ groups,
There was no difference in peak VO2 or any other maximal exercise gas exchange measure, ventilatory anaerobic threshold, or VE/VCO2 slope comparing the BRJ and placebo groups (Table 4). Both groups improved exercise time; the change was significant in the placebo group (Table 4).
Table 4.
Maximal Exercise Test – HFpEF participants
| Variable | EX + Placebo | EX + Beetroot Juice | P-value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | LS Mean ± SE |
Baseline | Follow-up | LS Mean ± SE |
||
| Peak Exercise (n=) | 9 | 9 | 9 | 10 | 10 | 10 | |
| Exercise time (sec) | 462 ± 97 | 502 ± 99* | 507 ± 19 | 473 ± 153 | 500 ± 162 | 495 ± 18 | 0.62 |
| Workload (W) | 58 ± 17 | 64 ± 17 | 65 ± 3 | 59 ± 26 | 63 ± 27 | 62 ± 3 | 0.52 |
| VO2 (ml/kg/min) | 12.3 ± 1.7 | 12.2 ± 1.9 | 11.9 ± 0.4 | 11.6 ± 2.5 | 11.6 ± 3.0 | 12.0 ± 0.4 | 0.89 |
| VO2 (ml/min) | 1014 ± 246 | 1012 ± 244 | 1048 ± 38 | 1082 ± 347 | 1082 ± 390 | 1049 ± 36 | 0.99 |
| VCO2 (ml/min) | 1203 ± 321 | 1246 ± 352 | 1276 ± 66 | 1263 ± 480 | 1284 ± 504 | 1256 ± 63 | 0.83 |
| RER | 1.18 ± 0.13 | 1.23 ± 0.06 | 1.22 ± 0.02 | 1.15 ± 0.10 | 1.18 ± 0.07 | 1.18 ± 0.02 | 0.18 |
| HR (bpm) | 117 ± 19 | 120 ± 18 | 122 ± 5 | 123 ± 25 | 123 ± 22 | 121 ± 4 | 0.80 |
| SBP (mmHg) | 174 ± 7 | 170 ± 11 | 169 ± 4 | 165 ± 17 | 165 ± 14 | 167 ± 4 | 0.76 |
| DBP (mmHg) | 77 ± 6 | 76 ± 9 | 76 ± 3 | 77 ± 6 | 75 ± 10 | 75 ± 3 | 0.82 |
| VE/ VCO2 slope | 31.4 ± 5.2 | 33.2 ± 6.0 | 32.4 ± 1.1 | 30.0 ± 4.3 | 31.7 ± 5.6 | 32.4 ± 1.1 | 0.97 |
| VAT (ml/min) | 663 ± 195 | 669 ± 133 | 686 ± 35 | 702 ± 243 | 734 ± 270 | 718 ± 33 | 0.52 |
Data presented as mean ± SD or number (%) and the least-square mean (LS Mean) ± SE of the follow-up value adjusted for the baseline value. P-value corresponds to comparison of the least-square mean.
Abbreviations- RER: respiratory exchange ratio; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; VE: minute ventilation; VAT: ventilatory anaerobic threshold.
p<0.05 for within group change by paired samples t-test (follow-up – baseline).
HTN Study
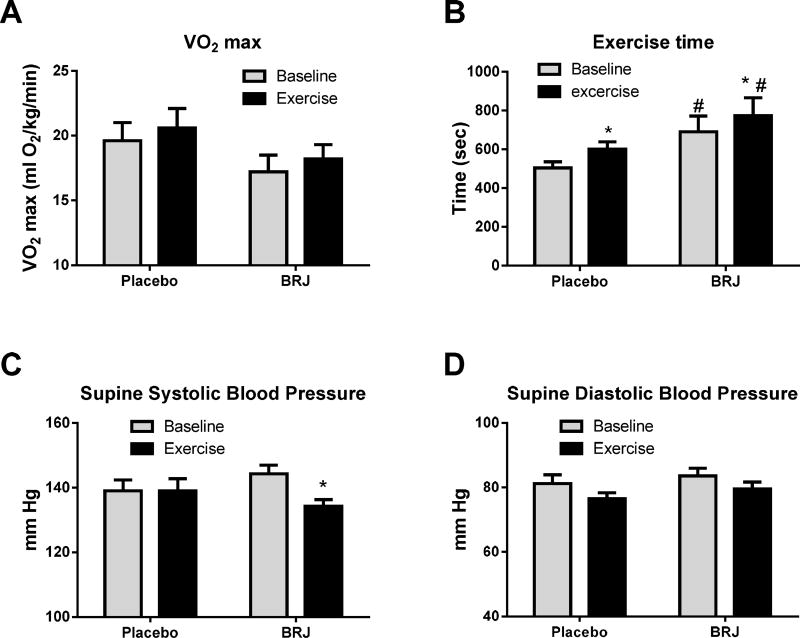
Following six weeks of self-paced treadmill walking, there was no added benefit of consuming BRJ compared to placebo when combined with exercise training for VO2peak (p>0.74). Both populations did exhibit significant increases in time to exhaustion and trends for increased peak VO2 (Fig 4A and B, Table 5).
Figure 4.
Effect of exercise (dark color panels) compared to baseline (light color panel) for the HTN study in subjects receiving either high nitrate beet root juice or placebo on VO2 (A); Exercise time (B); Supine systolic blood Pressure (C) Supine diastolic blood pressure (D). Data presented as mean ± SE. * = p<0.05 for follow up vs baseline within the same group, # = p<0.05 for BRJ vs placebo.
Table 5.
Physical performance and Hemodynamic Measures – HTN participants
| Variable | EX + Placebo | EX + Beetroot Juice | P-Value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | LS Mean ± SE |
Baseline | Follow-up | LS Mean ± SE |
||
| Graded Exercise Test (n) | 13 | 13 | 13 | 14 | 14 | 14 | |
| VO2peak | 17.0 ± 4.8 | 18.2 ± 4.1 | 19.4 ± 0.5 | 19.7 ± 5.1 | 20.6 ± 5.4 | 19.4 ± 0.5 | 0.98 |
| Exercise time (sec) | 504 ± 109 | * 601 ± 133 | 699 ± 37 | 691 ± 300 | * 773 ± 343 | 682 ± 35 | 0.74 |
| Supine resting SBP | 139 ± 12 | 139 ± 13 | 140 ± 4 | 144 ± 10 | *134 ± 14 | 133 ± 4 | 0.22 |
| Supine resting DBP | 81 ± 10 | 77 ± 6 | 77 ± 2 | 84 ± 8 | 79 ± 8 | 79 ± 1 | 0.44 |
| Peak SBP | 186 ± 22 | 186 ± 15 | 189 ± 5 | 192 ± 22 | 192 ± 28 | 190 ± 5 | 0.82 |
| Peak DBP | 88 ± 10 | 82 ± 9 | 81 ± 3 | 86 ± 13 | 83 ± 17 | 84 ± 3 | 0.51 |
| 24 hr ABPM (n) | 12 | 12 | 12 | 12 | |||
| 24 hour SBP | 128 ± 8 | 127 ± 7 | 129 ± 2 | 133 ± 11 | 131 ± 10 | 130 ± 2 | 0.88 |
| 24 hour DBP | 74 ± 9 | 72 ± 8 | 73 ± 1 | 76 ± 7 | 75 ± 7 | 74 ± 1 | 0.33 |
| 24 hour MBP | 93 ± 7 | 92 ± 6 | 93 ± 1 | 95 ± 7 | 95 ± 7 | 74 ± 1 | 0.65 |
| 24 hour HR | 73 ± 9 | 72 ± 8 | 72 ± 1 | 72 ± 11 | 69 ± 7 | 70 ± 1 | 0.28 |
| Day time SBP | 131 ± 8 | 130 ± 6 | 131 ± 2 | 134 ± 10 | 135 ± 9 | 134 ± 2 | 0.24 |
| Day time DBP | 76 ± 10 | 76 ± 8 | 76 ± 1 | 77 ± 7 | 78 ± 7 | 78 ± 1 | 0.35 |
| Day time MBP | 96 ± 9 | 95 ± 6 | 95 ± 1 | 97 ± 7 | 98 ± 7 | 98 ± 1 | 0.18 |
| Day time HR | 74 ± 9 | 75 ± 11 | 74 ± 2 | 74 ± 12 | 70 ± 7 | 70 ± 2 | 0.21 |
| Night time SBP | 125 ± 8 | 126 ± 10 | 128 ± 2 | 131 ± 13 | 127 ± 12 | 125 ± 2 | 0.36 |
| Night time DBP | 71 ± 8 | 70 ± 9 | 71 ± 2 | 74 ± 9 | 72 ± 7 | 71 ± 2 | 0.99 |
| Night time MBP | 90 ± 7 | 90 ± 9 | 91 ± 2 | 94 ± 9 | 91 ± 8 | 90 ± 2 | 0.62 |
| Night time HR | 72 ± 9 | 72 ± 9 | 71 ± 2 | 71 ± 11 | 68 ± 8 | 69 ± 2 | 0.32 |
| Hemodynamic measures (n) | 12 | 12 | 13 | 13 | |||
| SV (ml/beat) | 92 ± 26 | 101 ± 23 | 105 ± 5 | 105 ± 17 | 107 ± 15 | 104 ± 4 | 0.82 |
| CO (L/min) | 5.9 ± 1.6 | 6.0 ± 1.4 | 6.3 ± 0.2 | 6.6 ± 0.9 | 6.5 ± 0.8 | 6.2 ± 0.2 | 0.96 |
| SVR (dyne sec cm−5) | 1321 ± 399 | 1215 ± 331 | 1148 ± 48 | 1143 ± 196 | 1117 ± 209 | 1179 ± 46 | 0.65 |
| TAC (ml/mm Hg) | 15.3 ± 3.9 | * 18.4 ± 5.3 | 19.4 ± 1.1 | 18.3 ± 5.3 | * 20.5 ± 4.1 | 19.6 ± 1.0 | 0.91 |
| VI (/1000/s) | 35.5 ± 11.0 | 38.3 ± 10.4 | 39.5 ± 2.3 | 39.8 ± 9.4 | 37.5 ± 8.6 | 36.3 ± 2.2 | 0.33 |
| AI (/100/s2) | 67.1 ± 18.8 | 63.9 ± 14.6 | 63.3 ± 3.8 | 64.5 ± 21.4 | 61.2 ± 17.4 | 61.8 ± 3.6 | 0.79 |
| LCWI (kg-m /m2/min) | 3.48 ± 0.89 | 3.35 ± 0.71 | 3.49 ± 0.16 | 4.09 ± 0.57 | 3.78 ± 0.49 | 3.65 ± 0.15 | 0.48 |
Data presented as mean ± SD or number (%) and the least-square mean (LS Mean) ± SE of the follow-up value adjusted for the baseline value. P-value corresponds to comparison of the least-square mean.
p<0.05 for follow up vs baseline within the same group.
Abbreviations- ABPM: ambulatory blood pressure measurements; SBP: systolic blood pressure; DBP: diastolic blood pressure; MBP: mean blood pressure; HR: heart rate; SV: stroke volume; CO: cardiac output; SVR: systemic vascular resistance; TAC: total arterial compliance; VI; velocity index; AI; acceleration index; LCWI; left cardiac work index; Kg-m/m2: kilogram-meter per meter squared.
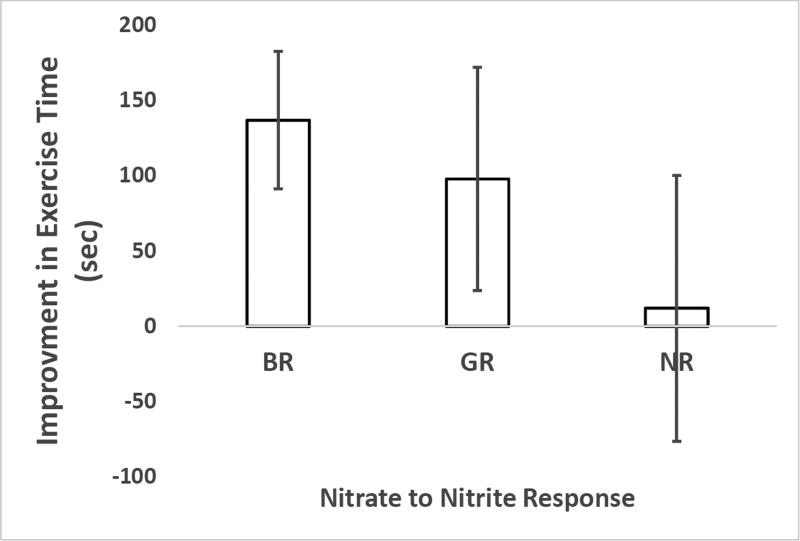
In ad hoc analyses, we compared changes in nitrite levels to the change in exercise time. As described in the methods section, participants were broken into three groups, best responders, good-responders, and non-responders based on the increases observed in plasma nitrite following BRJ consumption. The improvement in exercise time trended to be greatest for best-responders and least for non-responders (Figure 5).
Figure 5.
Relationship between nitrate to nitrite response to improvement in exercise time in the HTN study. Participants were broken into three groups: best responders (BR), good responders (GR), and non-responders (NR) based on change in plasma nitrite following BRJ consumption. Data are the average and standard error of the mean for improvement in exercise time.
3.4 Hemodynamic Measures
HFpEF Study
With BRJ, there was a trend to lower systolic blood pressure after unloaded pedaling (p=0.16) and a trend to lower diastolic blood pressure at volitional exhaustion (p=0.15) compared to placebo. There were no group differences in systolic or diastolic blood pressure at rest or at any other time point during the submaximal constant work rate exercise test (Table 3, Figure 3B). Resting systolic blood pressure was reduced following the intervention in both the BRJ (9%, p=0.007) and placebo (10%, p=0.04) groups, indicating that BRJ had no added benefit to exercise (Table 3).
There was no difference between the groups in any Echo-Doppler measures (Table 6).
Table 6.
Table 6 - Resting Echo-Doppler– HFpEF participants
| Variable | EX + Placebo | EX + Beetroot Juice | P-value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | LS Mean ± SE | Baseline | Follow-up | LS Mean ± SE | ||
| Echo-Doppler (n=) | 9 | 9 | 9 | 8 | 8 | 8 | |
| LV Mass (g) | 155.3 ± 21.8 | 159.2 ± 30.4 | 170.8 ± 5.1 | 192.0 ± 45.3 | 181.6 ± 36.8 | 168.5 ± 5.5 | 0.77 |
| E peak (cm/s) | 75 ± 18 | 79 ± 19 | 80 ± 6 | 78 ± 28 | 86 ± 24 | 85 ± 6 | 0.52 |
| A peak (cm/s) | 98 ± 26 | 99 ± 21 | 97 ± 4 | 94 ± 26 | 101 ± 26 | 103 ± 4 | 0.27 |
| E/A ratio | 0.78 ± 0.18 | 0.81 ± 0.17 | 0.84 ± 0.04 | 0.92 ± 0.50 | 0.89 ± 0.30 | 0.85 ± 0.04 | 0.99 |
| e’ (cm/s) | 5.9 ± 2.0 | 5.9 ± 1.6 | 6.2 ± 0.3 | 6.7 ± 2.0 | 6.3 ± 2.0 | 6.0 ± 0.3 | 0.66 |
| E/ e’ ratio | 11.9 ± 4.9 | 14.4 ± 5.3 | 13.7 ± 1.4 | 11.6 ± 5.0 | 15.2 ± 8.0 | 16.0 ± 1.5 | 0.63* |
| E deceleration time (ms) | 310 ± 108 | 264 ± 50 | 259 ± 15 | 245 ± 45 | 226 ± 34 | 231 ± 16 | 0.23 |
| LA diameter (cm) | 3.6 ± 0.4 | 3.6 ± 0.4 | 3.7 ± 0.03 | 3.8 ± 0.4 | 3.8 ± 0.3 | 3.7 ± 0.03 | 0.56 |
Data presented as mean ± SD or number (%) and the least-square (LS) mean ± SE of the follow-up value adjusted for the baseline value. P-value corresponds to comparison of the least-square mean.
p-value is following logarithmic transformation of this highly skewed variable.
Abbreviations- LV: left ventricular; E: early mitral velocity; A: atrial mitral velocity; e’: mitral annulus velocity (septal); LA: left atrial
HTN Study
For 24-h ambulatory blood pressure, 12 subjects in each group had >80% of the recordings valid. Blood pressure measurements at baseline and after 6 weeks of exercise training intervention in both groups are presented in Table 5. Blood pressure (systolic, diastolic and mean) and heart rate were not significantly different after exercise intervention in both groups.
Supine resting systolic blood pressure decreased significantly in the BRJ group but not in the Placebo group (Table 5, Figure 4C). The between group comparison was not significant (p=0.22), suggesting no significant added benefit of BRJ. Changes in supine resting diastolic blood pressure were not significant (Table 5, Figure 4D).
Both study groups experienced significantly improved arterial compliance compared to baseline (Table 5) and a trend towards a reduction in LCWI in BRJ group (p=0.07) (Table 5). There were no significant changes in any of the other recorded hemodynamic measures in either group (Table 5).
There was a 15–20% increase in measures of baroreflex sensitivity in both groups with no group differences (Table 1- supplement). There was also a 10–20% reduction in blood pressure variability measured as SDMAP and a 15–30% reduction in the LF/HF sympathovagal index. None of these changes reached statistical significance and there were no between group differences (p>0.21).
4. DISCUSSION
Previous work has shown that either exercise alone or dietary nitrate alone can decrease blood pressure in hypertensives and improve exercise tolerance in patients with HFpEF [5; 9–12; 30; 31; 33; 57; 58]. In addition, dietary nitrate has been shown to increase exercise efficiency, tolerance, and performance in various other populations [28; 29; 59–64]. We reasoned that an increase in perfusion due to increased NO bioavailability from dietary nitrate (as demonstrated in the case of exercising muscle in rats [65]) would allow patients to exercise at longer or higher levels of an exercise stimuli and potentially achieve a greater benefit from exercise training. We thus hypothesized that combining an exercise regimen and a dietary nitrate intervention would have an additive, if not synergistic, effect on outcomes in patients with hypertension and HFpEF. In general, the two studies presented here do not support this hypothesis; dietary nitrate appeared to have no significant added benefit compared to placebo when combined with an aerobic exercise training regimen.
In the HFpEF pilot study, the primary efficacy outcome, aerobic endurance capacity, improved due to the exercise regimen within both groups, but there was no significant added benefit of adding BRJ (p = 0.55). In fact, although there was a trend for improvement in the BRJ group, only the change observed in the placebo group reached significance. In the HTN pilot study, the primary efficacy outcome, exercise capacity, trended to improve within each group, but the trend for improvement was not greater in the BRJ group than the placebo group (p = 0.98, Figure 4A, Table 5). Exercise time increased significantly within both groups, but the improvement in exercise time for the BRJ group was not greater than that of the placebo group (p = 0.74, Figure 4B, Table 5).
Similar results were observed in secondary efficacy outcomes. In the HFpEF study, resting systolic blood pressure was significantly lowered in both groups, but there was no added benefit of BRJ (Table 3). In the HTN study there were no changes in blood pressure compared to baseline, but both total arterial compliance improved within both groups with no added benefit of BRJ (Table 5). No other significant changes were observed in any other outcome measures.
Whereas we observed no added benefit of BRJ consumption when combined with exercise, many previous studies have demonstrated benefits of BRJ consumed in the absence of a simultaneous exercise regimen. Several studies have shown that dietary nitrate reduces blood pressure [33; 57; 58; 66–71]. In particular, Kapil et al showed that dietary nitrate supplementation using a daily dose of 250 ml of beet root juice that provided about 400 mg (6.5 mmol) nitrate daily for 4 weeks, lowered pressure in both drug naïve and treated hypertensive subjects [33]. Several studies have also shown that BRJ improves exercise efficiency and/or performance [28–31; 59–61; 64; 71; 72]. Notably, in studies on patients with HFpEF, a larger acute dose of BRJ (12.9 mmol) or smaller dose (6.1 mmol) given daily for a week resulted in improved exercise outcomes [30; 31]. But not all previous studies have been positive. For example, Bondonno et al did not observe an effect of daily dietary nitrate (400 mg) on blood pressure in treated hypertensive individuals [73]. In addition, in our previously published study on patients with HFpEF, we did not observe an effect of a single, acute dose of BRJ on exercise tolerance [31]. Thus one factor that may have contributed to the lack of an effect observed in the studies reported here, as well as the previous, is the size of the nitrate dose. In the current HFpEF study, the dose (6.1 mmol) was the same as in our previous study, but the dosing schedule was lower (three times a week vs 7 times in a week). The dose in the HFpEF study presented here was lower than that in some other studies, for example 6.1 mmol (this HFpEF study) vs 12.9 mmol in the study by Zamani et al [30]. However, the nitrate dose given by Kapil et al was similar to the one in our HFpEF study and less than that in our HTN study.
Another, related factor that may have contributed to lack of additive effects of the BRJ in addition to exercise training is the variability and moderate extent to which plasma nitrite was increased in the participants in our studies (Figure 2). A recent review points out that baseline levels of fitness influence response to dietary nitrate [74] and our patients were sedentary. Moreover, for the both studies, blood was collected only one hour after BRJ consumption and thus plasma nitrite may not have reached a maximum. In previous work we have noted that plasma nitrite is close to a maximum one hour after BRJ consumption [75], but others have seen that the maximum is not reached for two to three hours [57; 67]. Thus, maximum plasma nitrite levels may have been higher than those shown in Figure 2 for the BRJ group. It is also noteworthy that the levels of nitrite measured for the placebo group in the HFpEF study were higher than normally observed (average of 0.47 µM compared to (for example) 0.14 µM for the HTN study). As discussed in the results section, part of the reason for this elevation was due to a single subject with extremely high baseline plasma nitrite. Exclusion of that person brings the average plasma nitrite for the HFpEF placebo group to 0.27 ± 0.17 µM. In addition, the lack of a restricted diet in the HFpEF study could have masked effects of BRJ. Moreover, lack of BRJ effects on plasma nitrite could be due to variability in oral bacteria that reduce nitrate to nitrite. In this regard, in ad hoc analysis of the HTN study we found evidence of a graded relationship between nitrite levels and improvement in exercise time during the exercise test (Figure 5). However, we did not observe a similar relationship in the HFpEF study. It should also be noted that our HTN study recruited patients with controlled hypertension whereas Kapil et al recruited patients with uncontrolled hypertension. It is possible that medications used by the patients in our HTN study masked any BRJ effects. Of course we recognize that a major factor in our studies is that BRJ was combined with supervised exercise which may also have masked any BRJ effects.
In cases where conversion of nitrate to nitrite is bypassed using either direct nitrite infusion [34] or inhalation [35], administration to patients with HFpEF improved hemodynamics. Success of these studies that bypass oral conversion of nitrate to nitrite may suggest that such methods of administration should be pursued in lieu of BRJ or other dietary nitrate methods. Indeed, levels of plasma nitrite achieved in these studies using inhaled or infused nitrite were an order of magnitude or higher than in our studies using BRJ [34; 35]. However, positive studies involving BRJ in a variety of conditions, including those involving patients with HFpEF in the absence of an exercise intervention [30; 31], underlines the potential of use of dietary nitrate as an inexpensive therapy or co-therapy, at least among those who respond well in terms of producing plasma nitrite.
4.1 Limitations
The studies presented have some limitations. In addition to limitations related to nitrate dose and response (in terms of increases in plasma nitrite) discussed directly above, the major limitation is the number of participants. While 20 and 26 participants may have been sufficient to detect effects of either BRJ or EX alone, the combined effect would probably have to be substantial to detect an added benefit of BRJ on top of EX. In addition a crossover design would likely have added power.
It is also possible that a longer duration of intervention is needed to observe an effect of BRJ. In the HTN study, we did not observe any between group differences in the volume of training completed during each session over the six weeks (data not shown), suggesting that a longer exercise training study would not have led to a different outcome, but we cannot be sure about this based on our data. Perhaps more importantly, more frequent BRJ dosing, particularly in the HFpEF study, may have had additional beneficial effects compared to exercise therapy alone. We have already shown that daily BRJ consumption improves exercise tolerance [31]. The rationale for BRJ dosing only 3X per week was based on the hypothesis that BRJ consumption on the day of exercise training would have an additive or synergistic effect to positive effects of exercise training alone. Whether or not daily dosing with BRJ would have had an additional beneficial effect is still an open question.
Another limitation may be due to the interval of time between when the BRJ was consumed and when testing was administered. BRJ was consumed one hour before exercise training and testing was completed within a couple of hours of consumption. As mentioned above, some studies have observed that plasma nitrite does not peak until 2–3 hours after dietary nitrate consumption. Thus, the effect of BRJ may have been bigger if exercise were started two hours later, but the optimal time is not known (perhaps beginning at one hour post consumption is best as plasma nitrite levels are elevated and would continue to rise during testing). In addition to these limitations, there are some differences in the interventions and outcomes in the two studies described.
5. CONCLUSION
In conclusion, our hypothesis that simultaneous administration of a supervised exercise and dietary nitrate interventions could improve exercise training and subsequent outcomes more than exercise training alone was not supported by the results of the two studies presented here. Further work should be conducted to see if larger nitrate doses or more frequent dosing schedules could result in additive benefits of dietary nitrate intake to those observed through supervised exercise.
Supplementary Material
Highlights.
Exercise intervention provided benefits in HTN and HFpEF pilot studies
Dietary nitrate provided no additional benefit to exercise therapy
Dose of dietary nitrate used here may have been insufficient
Acknowledgments
We thank Cassandra C. Klebous, Nicole Kus, and Eric Schmid for help in collecting data. Dr. Shaltout HA is also a faculty member in the Department of Pharmacology and Toxicology, School of Pharmacy, Alexandria University.
This work was partially supported by NIH grants R01AG18915, R01AG045551, P30AG021332, HL058091, The Kermit Glenn Phillips II Chair in Cardiovascular Medicine, Wake Forest School of Medicine, and the Moritz Chair in Geriatrics in the College of Nursing and Health Innovation at the University of Texas at Arlington. It was also partially supported by the Translational Science Center of the Reynolda Campus of Wake Forest University and the Hypertension & Vascular Research Center of Wake Forest School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: heart failure is preserved ejection fraction (HFpEF); hypertension (HTN); exercise (EX); beet root juice (BRJ); peak volume oxygen consumption (VO2 peak); stroke volume (SV); cardiac output (CO); systemic vascular resistance (SVR); total arterial compliance (TAC); velocity index (VI); acceleration index (AI); left cardiac work index (LCWI); impedance cardiography (ICG); systolic blood pressure (SBP); diastolic blood pressure (DBP); heart rate variability (HRV); blood pressure variability (BPV); and baroreflex sensitivity (BRS); low frequency (LF); and high frequency (HF); standard deviation of normal beat-to-beat interval (SDRR); root of mean of successive differences (rMSSD); Blood pressure variability (BPV); standard deviation of the mean arterial pressure (SDMAP); best-responder (BR), good-responder (GR); non-responder (NR); analysis of variance (ANOVA); body mass index (BMI); angiotensin converting enzyme (ACE); angiotensin receptor blocker (ARB); New York Heart Association (NYHA); mitral annulus velocity (e’); early mitral velocity (E); history (Hx); respiratory exchange ratio (RER); heart rate (HR); minute ventilation (VE); ventilatory anaerobic threshold (VAT); ambulatory blood pressure measurements (APBM); mean blood pressure (MBP); Power of spectrum in high frequency range (Hfa); slope of the baroreflex gain curve measured by the sequence method in the UP or Down direction (Seq UP & DOWN).
Disclosures: Dr. Kim-Shapiro is listed as a co-inventor on a patent related to use of nitrite in cardiovascular conditions, and owns stock in and serves on the scientific advisory board for Beverage Operations LLC which has licensed Wake Forest University intellectual properties and thus has a financial interest in Beverage Operations LLC. Dr. Kitzman declares the following relationships: Consultant for Abbvie, GSK, Relypsa, Regeneron, Merck, Corvia Medical, and Actavis, grant funding from Novartis, and stock ownership in Gilead Sciences and Relypsa.
No other members of the writing group have conflicts of interest to declare.
References
- 1.Kitzman DW, Gardin JM, Gottdiener JS, Arnold AM, Boineau R, Aurigemma GP, Marino E, Lyles M, Cushman M, Enright P, Group FtCHS. Importance of heart failure with preserved systolic function in patients > or = 65 Years of Age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 4.Melenovsky V, Borlaug B, Rosen B, Hay I, Ferruci L, Morrell C, Lakatta E, Najjar S, Kass D. Cardiovascular Features of Heart Failure With Preserved Ejection Fraction Versus Nonfailing Hypertensive Left Ventricular Hypertrophy in the Urban Baltimore Community: The Role of Atrial Remodeling/Dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman D, Brubaker P, Morgan T, Stewart K, Little W. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelissen VA, Smart NA. Exercise Training for Blood Pressure: A Systematic Review and Meta-analysis. Journal of the American Heart Association. 2013;2 doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagard RH, Cornelissen VA. Effect of exercise on blood pressure control in hypertensive patients. European Journal of Cardiovascular Prevention & Rehabilitation. 2007;14:12–17. doi: 10.1097/HJR.0b013e3280128bbb. [DOI] [PubMed] [Google Scholar]
- 8.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Annals of Internal Medicine. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA. Exercise and hypertension. Medicine and Science in Sports and Exercise. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 10.Seals DR, DeSouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haykowsky MJ, Kitzman DW. Exercise physiology in heart failure and preserved ejection fraction. Heart Failure Clin. 2014;10:445–452. doi: 10.1016/j.hfc.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ. Effect of endurance exercise training on endothelial function arterial stiffness in older patients with heart failure, preserved ejection fraction: A randomized controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghiadoni L, Versari D, Magagna A, Kardasz I, Plantinga Y, Giannarelli C, Taddei S, Salvetti A. Ramipril dose-dependently increases nitric oxide availability in the radial artery of essential hypertension patients. Journal of Hypertension. 2007;25:361–366. doi: 10.1097/HJH.0b013e3280115901. [DOI] [PubMed] [Google Scholar]
- 14.Cachofeiro V, Maeso R, Rodrigo E, Navarro J, Ruliope LM, Lahera V. NITRIC-OXIDE AND PROSTAGLANDINS IN THE PROLONGED EFFECTS OF LOSARTAN AND RAMIPRIL IN HYPERTENSION. Hypertension. 1995;26:236–243. doi: 10.1161/01.hyp.26.2.236. [DOI] [PubMed] [Google Scholar]
- 15.Rees DD, Palmer RMJ, Moncada S. ROLE OF ENDOTHELIUM-DERIVED NITRIC-OXIDE IN THE REGULATION OF BLOOD-PRESSURE. Proc. Natl. Acad. Sci. USA. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirai DM, Musch TI, Poole DC. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. American Journal of Physiology - Heart and Circulatory Physiology. 2015 doi: 10.1152/ajpheart.00469.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haykowsky M, Brubaker P, Morgan T, Kritchevsky S, Eggebeen J, Kitzman D. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky MJ. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal Muscle Composition and Its Relation to Exercise Intolerance in Older Patients With Heart Failure and Preserved Ejection Fraction. Am. J. Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu XL, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 21.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 22.Kapil V, Weitzberg E, Lundberg JO, Ahluwalia A. Clnical evidence demonstrating the utility of inorganic nitrate in cardiovascular health Nitric Oxide. 2014;38:45–57. doi: 10.1016/j.niox.2014.03.162. [DOI] [PubMed] [Google Scholar]
- 23.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am.J.Clin.Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Opinion - Nitrate, bacteria and human health. Nature Reviews Microbiology. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 25.Kim-Shapiro DB, Gladwin MT. Mechanisms of nitrite bioactivation. Nitric Oxide. 2014;38:58–68. doi: 10.1016/j.niox.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic. Biol. Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 27.van Velzen AG, Sips A, Schothorst RC, Lambers AC, Meulenbelt J. The oral bioavailability of nitrate from nitrate-rich vegetables in humans. Toxicology Letters. 2008;181:177–181. doi: 10.1016/j.toxlet.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Berry M, Justus NW, Hauser JI, Case AH, Basu CCHS, Rogers Z, Lewis MT, Miller GD. Dietary Nitrate Supplementation Improves Exercise Performance and Decreases Blood Pressure in COPD Patients. Nitric Oxide. 2015;48:22–30. doi: 10.1016/j.niox.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, VanBruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol. 2011;110:1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of Inorganic Nitrate on Exercise Capacity in Heart Failure With Preserved Ejection Fraction. Circulation. 2015;131:371–U1184. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One Week of Daily Dosing with Beetroot Juice Improves Submaximal Endurance and Blood Pressure in Older Patients with Heart Failure and Preserved Ejection Fraction. Journa; of the American College of Cardiology. Heart Failure. 2016;4:428–437. doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapil V, Weitzberg E, Lundberg JO, Ahluwalia A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health Nitric Oxide. 2014;38:45–57. doi: 10.1016/j.niox.2014.03.162. [DOI] [PubMed] [Google Scholar]
- 33.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary Nitrate Provides Sustained Blood Pressure Lowering in Hypertensive Patients A Randomized Phase 2, Double-Blind, Placebo-Controlled Study. Hypertension. 2015;65:320–U174. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borlaug BA, Koepp KE, Melenovsky V. Sodium Nitrite Improves Exercise Hemodynamics and Ventricular Performance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2015;66:1672–1682. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 35.Borlaug BA, Melenovsky V, Koepp KE. Inhaled Sodium Nitrite Improves Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. Circ. Res. 2016;119:880–886. doi: 10.1161/CIRCRESAHA.116.309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts LD, Ashmore T, McNally BD, Murfitt SA, Fernandez BO, Feelisch M, Lindsay R, Siervo M, Williams EA, Murray AJ, Griffin JL. Inorganic Nitrate Mimics Exercise-Stimulated Muscular Fiber-type Switching and Myokine and GABA Release. Diabetes. 2016 doi: 10.2337/db16-0843. In Press. [DOI] [PubMed] [Google Scholar]
- 37.Berry M, Justus N, Hauser J, Case A, Helms C, Basu S, Lewis RZM, Miller G. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide. 2015;48:22–30. doi: 10.1016/j.niox.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitzman DW, Hundley WG, Brubaker P, Stewart K, Little WC. A randomized controlled double-blinded trial of enalapril in older patients with heart failure preserved ejection fraction; effects on exercise tolerance, and arterial distensibility. Circ Heart Fail. 2010;3:477–485. doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott JM, Haykowsky MJ, Eggebeen J, Morgan TM, Brubaker PH, Kitzman DW. Reliability of peak exercise testing in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:1809–1813. doi: 10.1016/j.amjcard.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ACSM. ACSM's Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]
- 41.K P, GC F, DW K, IB A, TE L, RM A, M G, A A. Aldosterone antagonists and outcomes in real-world older patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2013;1:40–47. doi: 10.1016/j.jchf.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction A Randomized Clinical Trial. Jama-Journal of the American Medical Association. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottdiener JS, Bednarz J, Devereux RB, Gardin JM, Klein AL, Manning WJ, Morehead A, Kitzman DW, Oh JK, Quinones MA, Schiller NB, Stein JH, Weissman NJ. American Society of Echocardiography Recommendations for Use ofEchocardiography in Clinical Trials: A report from the American Society of Echocardiography's Guidlines and Standards Committee and the Task Force on Echocardiography in Clinical Trials. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 44.RM L, M B, RB D, FA F, E F, PA P, MH P, MJ R, J S, JS S, S S, KT S, SM SJ, WJ S. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines Standards Committee the Chamber Quantification Writing Group developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Rivas-Gotz C, Khoury DS, Manolios M, Rao L, Kopelen HA, Nagueh SF. Time interval between onset of mitral inflow and onset of early diastolic velocity by tissue Doppler: a novel index of left ventricular relaxation: Experimental studies and clinical application. J Am Coll Cardiol. 2003;42:1463–1470. doi: 10.1016/s0735-1097(03)01034-9. [DOI] [PubMed] [Google Scholar]
- 46.Gardin JM, Arnold AM, Bild DE, Smith V, Lima JAC, Klopfenstein HS, Kitzman DW. Left ventricular diastolic filling in the elderly: The Cardiovascular Health Study. Am J Cardiol. 1998;82:345–351. doi: 10.1016/s0002-9149(98)00339-7. [DOI] [PubMed] [Google Scholar]
- 47.Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y. European Society of Hypertension Working Group on Blood Pressure M, European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–68. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 49.Rizzoni D, Muiesan ML, Montani G, Zulli R, Calebich S, Agabiti-Rosei E. Relationship between initial cardiovascular structural changes and daytime and nighttime blood pressure monitoring. Am J Hypertens. 1992;5:180–6. doi: 10.1093/ajh/5.3.180. [DOI] [PubMed] [Google Scholar]
- 50.Sodolski T, Kutarski A. Impedance cardiography: A valuable method of evaluating haemodynamic parameters. Cardiol J. 2007;14:115–26. [PubMed] [Google Scholar]
- 51.Kamath SA, Drazner MH, Tasissa G, Rogers JG, Stevenson LW, Yancy CW. Correlation of impedance cardiography with invasive hemodynamic measurements in patients with advanced heart failure: the BioImpedance CardioGraphy (BIG) substudy of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) Trial. Am Heart J. 2009;158:217–23. doi: 10.1016/j.ahj.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenberg P, Yancy CW. Noninvasive assessment of hemodynamics: an emphasis on bioimpedance cardiography. Current opinion in cardiology. 2000;15:151–5. doi: 10.1097/00001573-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Osypka MJ, Bernstein DP. Electrophysiologic principles and theory of stroke volume determination by thoracic electrical bioimpedance. AACN Clin Issues. 1999;10:385–99. doi: 10.1097/00044067-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Tegeler CH, Shaltout HA, Tegeler CL, Gerdes L, Lee SW. Rightward dominance in temporal high-frequency electrical asymmetry corresponds to higher resting heart rate and lower baroreflex sensitivity in a heterogeneous population. Brain and behavior. 2015;5:e00343. doi: 10.1002/brb3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fortunato JE, Tegeler CL, Gerdes L, Lee SW, Pajewski NM, Franco ME, Cook JF, Shaltout HA, Tegeler CH. Use of an allostatic neurotechnology by adolescents with postural orthostatic tachycardia syndrome (POTS) is associated with improvements in heart rate variability and changes in temporal lobe electrical activity. Experimental brain research. 2015 doi: 10.1007/s00221-015-4499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagoner AL, Shaltout HA, Fortunato JE, Diz DI. Distinct Neurohumoral Biomarker Profiles in Children with Hemodynamically-Defined Orthostatic Intolerance May Predict Treatment Options. Am J Physiol Heart Circ Physiol. 2015 doi: 10.1152/ajpheart.00583.2015. ajpheart 00583 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. New Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 59.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiologica. 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 60.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic. Biol. Med. 2010;48:342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O(2) cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 62.Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, Gilchrist M, Benjamin N, Jones AM. Acute Dietary Nitrate Supplementation Improves Cycling Time Trial Performance. Medicine and Science in Sports and Exercise. 2011;43:1125–1131. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- 63.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O-2 cost of walking and running: a placebo-controlled study. J Appl Physiol. 2011;110:591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 64.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol. 2013;115:325–336. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 65.Ferguson SK, Hirai DM, Copp S, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol.-London. 2013;591:547–557. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hobbs DA, Kaffa N, George TW, Methven L, Lovegrove JA. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. British Journal of Nutrition. 2012;108:2066–2074. doi: 10.1017/S0007114512000190. [DOI] [PubMed] [Google Scholar]
- 67.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, MacAllister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic Nitrate Supplementation Lowers Blood Pressure in Humans. Role for Nitrite-Derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 68.Kapil V, Haydar SMA, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic. Biol. Med. 2013;55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, Gilchrist M, Winyard PG, Jones AM. Effects of short-term dietary nitrate supplementation on blood pressure O-2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol-Reg I. 2013;304:R73–R83. doi: 10.1152/ajpregu.00406.2012. [DOI] [PubMed] [Google Scholar]
- 70.Petersson J, Carlstrom M, Schreiber O, Phillipson M, Christoffersson G, Jagare A, Roos S, Jansson EA, Persson AEG, Lundberg JO, Holm L. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic. Biol. Med. 2009;46:1068–1075. doi: 10.1016/j.freeradbiomed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 71.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol-Reg I. 2010;299:R1121–R1131. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 72.Hoon MW, Johnson NA, Chapman PG, Burke LM. The Effect of Nitrate Supplementation on Exercise Performance in Healthy Individuals: A Systematic Review and Meta-Analysis. International Journal of Sport Nutrition and Exercise Metabolism. 2013;23:522–532. doi: 10.1123/ijsnem.23.5.522. [DOI] [PubMed] [Google Scholar]
- 73.Bondonno CP, Liu AH, Croft KD, Ward NC, Shinde S, Moodley Y, Lundberg JO, Puddey IB, Woodman RJ, Hodgson JM. Absence of an effect of high nitrate intake from beetroot juice on blood pressure in treated hypertensive individuals: a randomized controlled trial. The American journal of clinical nutrition. 2015;102:368–75. doi: 10.3945/ajcn.114.101188. [DOI] [PubMed] [Google Scholar]
- 74.Jonvik KL, Nyakayiru J, van Loon LJC, Verdijk LB. Can elite athletes benefit from dietary nitrate supplementation? J Appl Physiol. 2015;119:759–761. doi: 10.1152/japplphysiol.00232.2015. [DOI] [PubMed] [Google Scholar]
- 75.Presley TD, Morgan AR, Bechtold E, Clodfelter W, Dove RW, Jennings JM, Kraft RA, Bruce King S, Laurienti PJ, Rejeski WJ, Burdette JH, Kim-Shapiro DB, Miller GD. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24:34–42. doi: 10.1016/j.niox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.