Abstract
The dopamine transporter (DAT), which mediates the inactivation of released dopamine through its reuptake, is the primary molecular target for the actions of psychostimulants. An increasing number of studies support an essential role for phosphorylation of serines (Ser) in the distal amino (N) terminus of DAT in regulating its function. Still, the molecular details of the regulation of phosphorylation and its impact on function are not fully understood. To address this, we have developed and characterized two distinct phospho-antibodies that recognize human DAT when it is phosphorylated at Ser7 or Ser12. Our data show that treatment of cells with phorbol 12-myristate 13-acetate (PMA), amphetamine (AMPH) or okadaic acid (OA) leads to an increase in the phosphorylation of DAT at both residues and that these responses are dependent on the activity of protein kinase C. We also show that AMPH-induced and OA-induced phosphorylation of DAT are also dependent on Ca2+/calmodulin-dependent protein kinase α. Our data further suggest that the lipid raft localization of DAT is necessary for efficient N-terminal phosphorylation and for the associated behavioral effects of AMPH, demonstrating the potential of these novel antibodies as powerful tools to study DAT regulation and function in vivo.
Introduction
The dopamine transporter (DAT) is the plasmalemmal membrane protein that mediates the inactivation of released dopamine (DA) through its reuptake. DAT belongs to the solute carrier 6 (SLC6) family of neurotransmitter:sodium symporters, which also includes carriers for norepinephrine (NET) and serotonin (SERT) (Amara and Kuhar, 1993; Chen and Reith, 2004; Torres et al., 2003). DAT is the primary molecular target for the actions of cocaine, amphetamines (AMPH) and other psychostimulants. The mechanisms that modulate the activity of DAT in vivo are therefore critical for the spatial and temporal regulation of dopaminergic neurotransmission as well as for the behavioral consequences of drugs of abuse.
DAT is hypothesized to function via an alternating access mechanism involving a regulated transition of the transporter from an “outward-facing” to an “inward-facing” conformation (Jardetzky, 1966; Loland et al., 2003). AMPH acts as a substrate for DAT and, once inside neurons, causes release of vesicular DA into the cytoplasm and mobilization of cytoplasmic DA to the cell exterior via DAT through non-exocytic reverse transport (efflux) (Freyberg et al., 2016; Sulzer et al., 2005). It was proposed that efflux occurs via a facilitated exchange mechanism, whereby the transport of AMPH into the cell increases the number of transporters in the inward-facing conformation, leading to the transport of DA out of the cell (Fischer and Cho, 1979). However, later studies challenged this model and suggested that AMPH-induced DA efflux is at least partially uncoupled from uptake (Pifl and Singer, 1999; Scholze et al., 2002) and might involve a channel-like mode of the transporter (Kahlig et al., 2005; Sitte et al., 1998). Moreover, we and others have demonstrated that phosphorylation of serines in the distal amino (N) terminus of DAT is required for efflux (Fog et al., 2006; Goodwin et al., 2009; Khoshbouei et al., 2004; Ramamoorthy et al., 2011), but not for uptake activity, inhibitor binding, oligomerization or trafficking of the transporter in heterologous cells (Granas et al., 2003; Hastrup et al., 2001; Hastrup et al., 2003; Khoshbouei et al., 2004). Consistent with these findings, we also showed that mutation of the 5 N-terminal serines to alanine (hDAT-StoA) inhibits AMPH-induced hyperlocomotion in Drosophila melanogaster but does not interfere with the locomotor effects of methylphenidate, an uptake blocker that does not induce efflux (Pizzo et al., 2013). Further, we showed that Ca2+/calmodulin-dependent protein kinase α (CaMKIIα) plays a key role in this process (Pizzo et al., 2014). It interacts with the carboxy (C)-terminus of DAT and is required for the phosphorylation of the N-terminal serines and for AMPH-induced efflux in vitro (Fog et al., 2006), as well as for AMPH-induced behavior in Drosophila. DAT phosphorylation can also be stimulated by treatment with protein kinase C (PKC) activators such as phorbol 12-myristate 13-acetate (PMA) (Cowell et al., 2000; Foster et al., 2002; Granas et al., 2003). Furthermore, treatment with PKC inhibitors attenuates AMPH-induced DAT phosphorylation (Cervinski et al., 2005), as well as AMPH-induced DAT-mediated DA efflux (Kantor and Gnegy, 1998; Kantor et al., 2001). In contrast, the broad-spectrum protein phosphatase inhibitor Okadaic acid (OA) results in an increase in DAT phosphorylation (Huff et al., 1997; Vaughan et al., 1997), which suggests that dephosphorylation is another regulatory mechanism of DAT function and points to a significant ongoing endogenous kinase activity that is independent of exogenous kinase activators.
An increasing number of studies support an essential role for phosphorylation in regulating DAT function (Cervinski et al., 2010; Foster et al., 2008; Gnegy, 2003; Moritz et al., 2015). Still, the molecular details of the regulation of phosphorylation and its impact on function are not fully understood. Moreover, it has been difficult to ascertain the precise identities of the serines involved. The N-terminal tail of DAT contains 5 serine residues at positions 2, 4, 7, 12 and 13. Studies investigating DAT phosphorylation have relied, in large part, on metabolic phosphorylation of DAT with 32P, peptide mapping using specific proteases, and/or site-directed mutagenesis of specific serines. They showed that deletion of the first 22 amino acids of DAT (hDATdel22) or the simultaneous mutation of the five N-terminal serine residues to alanine (hDAT-StoA) eliminates the incorporation of 32P in response to PKC activation (Granas et al., 2003). Mutation of Ser7, Ser12 and Ser13 were found to reduce phosphorylation of DAT by PKC, MEK1/2, and PI3K (Lin et al., 2003). More recently, a recombinant rat DAT N-terminal peptide was shown to be phosphorylated in vitro by PKA at Ser7, CaMKII at Ser13 and at multiple sites (Ser4, Ser7, and Ser13) by PKC (Moritz et al., 2013). Mass spectrometry also revealed Ser7 as a site for phosphorylation of heterologously expressed hDAT in HEK293 cells treated with OA (Moritz et al., 2013). Furthermore, we have previously shown that expression of hDATdel22 or hDAT-StoA inhibited AMPH-induced DA efflux in heterologous cells (Fog et al., 2006; Khoshbouei et al., 2004), as well as AMPH-induced DAT-mediated hyperlocomotion in Drosophila larvae (Pizzo et al., 2014; Pizzo et al., 2013). Our studies in the StoA background also showed that single mutation to Asp of the alanines at positions 7 (Ser7) and 12 (Ser12), but not at positions 2, 4, and 13, restores AMPH-induced DA efflux (Fog et al., 2006; Khoshbouei et al., 2004). Nonetheless, the direct phosphorylation of these serines in response to AMPH has not been validated in vivo. To address this, we developed and characterized two distinct phospho-antibodies that recognize hDAT when it is phosphorylated at Ser7 or Ser12. Our data show that treatment of cells with PMA, AMPH or OA all lead to an increase in the phosphorylation of DAT at both residues and that inhibition of either PKC or CAMKII leads to a decrease in PMA-, AMPH-, and OA-induced phosphorylation of DAT. Our data also suggest that the lipid raft localization of DAT is necessary for efficient N-terminal phosphorylation and for the associated in vivo effects of AMPH, demonstrating the potential of these novel antibodies as powerful tools to study DAT regulation and function in vivo.
Results
To understand better the mechanisms and pathways that regulate DAT phosphorylation, we developed two distinct antibodies against the N-terminal domain of human DAT (hDAT) phosphorylated at either Ser7 (pSer7) or Ser12 (pSer12). We tested the antibodies in lysates of HEK293-derived EM4 cells that stably express wild-type FLAG-tagged hDAT (Hastrup et al., 2001; Hastrup et al., 2003; Khoshbouei et al., 2004). Because the antibodies also recognized off target proteins, lysates were immuno-precipitated with antibodies against the FLAG-tag and were then subjected to immunoblot analysis with pSer7, pSer12 and DAT C-terminus antibodies. Minimal DAT phosphorylation was observed in the absence of any stimulation (Figure 1A, FLAG-hDAT -PMA), suggesting that this cell line provided a suitable background for these studies. In contrast, treatment with the PKC activator PMA resulted in a dramatic increase of transporter protein phosphorylation that was readily detected by both phospho-antibodies (Figure 1A, FLAG-hDAT +PMA). To characterize the specificity of the antibodies, serines at positions 7 and 12 were individually or simultaneously replaced with alanines (S7A, S12A or S7AS12A) in the FLAG-hDAT background and the resulting mutants were stably expressed in EM4 cells. As shown in Figure 1B, the pSer7 antibody was unable to detect PMA-induced DAT phosphorylation when FLAG-S7A-hDAT or FLAG-S7A-S12A-hDAT was expressed, whereas the pSer12 antibody failed to detect DAT phosphorylation in cells expressing FLAG-S12A-hDAT or FLAG-S7A-S12A-hDAT. Similarly, both antibodies were unable to detect PMA-induced phosphorylation in cells expressing hDAT that had the first five N-terminal serines simultaneously mutated to alanines (Figure 1B, FLAG-hDAT-StoA) (Khoshbouei et al., 2004). The serines at positions 7 and 12 were restored individually or simultaneously in the FLAG-hDAT-StoA background (Figure 1C, 7S, 12S or 7S12S). A signal was detected using the pSer7 antibody when 7S or 7S12S but not 12S was expressed. pSer12 on the other hand, failed to detect phosphorylation in a 12S background. However, it did detect a signal when a 12S13S mutant was expressed (Figure 1D), suggesting that the neighboring Ser13 is part of the epitope recognized by the antibody. Taken together, these results indicate that each antibody is highly specific for its N-terminal DAT target.
Figure 1. PMA, amphetamine and okadaic acid increase phosphorylation of human DAT Ser 7 and Ser 12.
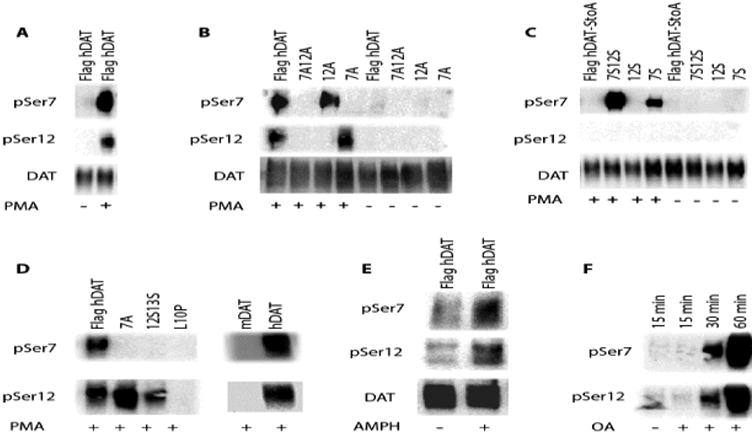
A) HEK293-derived EM4 cells stably expressing FLAG-tagged human DAT (FLAG-hDAT) were treated with 1 μM PMA (+) or vehicle (-) for 15 min. DAT was immunoprecipitated by the FLAG tag and probed for phosphorylated Ser7 (pSer7) or Ser12 (pSer12), or total DAT. Treatment with PMA led to robust phosphorylation of hDAT at Ser7 and Ser12. B) Ser7 and Ser12 in FLAG-hDAT were individually or simultaneously replaced with alanines (S7A, S12A or S7AS12A). pSer7 antibody did not detect PMA-induced DAT phosphorylation when either the S7A or S7AS12A mutant was expressed. pSer12 antibody did not detect DAT phosphorylation when either the S12A or S7AS12A mutant was expressed. C) Neither pSer7 nor pSer12 antibody detected PMA-induced DAT phosphorylation in lysates from cells expressing hDAT with all 5 N-terminal serines mutated to alanine (hDAT-StoA). Restoration of Ser7 (S7) or Ser7/Ser12 (S7S12) into hDAT-StoA led to robust signal with pSer7. Restoration of Ser12 (S12) alone did not restore the signal detected by either pSer7 or pSer12. D) Simultaneous restoration of S12 and S13 (S12S13) in hDAT-StoA background restored the signal detected by pSer12 but not pSer7. Neither pSer7 nor pSer12 detected PMA-induced DAT phosphorylation when the N-terminal Leu10 was mutated to proline in hDAT (L10P). Neither phosphoantibodies detected PMA-induced phosphorylation of mouse DAT (mDAT), as compared to a robust signal detected for human DAT (hDAT). E) Treatment with 10 μM amphetamine (AMPH) for 30 min increased phosphorylation at both Ser7 and Ser12. F) Treatment with 1 μM okadaic acid (OA) enhanced levels of Ser7 and Ser 12 phosphorylation in a time-dependent manner.
The species specificity of these antibodies was tested by expressing mouse DAT (mDAT) in EM4 cells. mDAT is 93% identical to hDAT, and sequence alignment shows that the first 27 N-terminal amino acids of hDAT are identical to those of mDAT, except for residue 10, which is a proline in mDAT as opposed to a leucine in hDAT (Bruss et al., 1999). When EM4 cells transfected with mDAT were stimulated with PMA, no DAT phosphorylation was detected using either antibody (Figure 1D). Note that position 10 was included in the peptide antigens used to create both the pSer7 and the pSer12 antibodies. We therefore hypothesized that this substitution destroys the recognition sequence of the two phospho-antibodies. To test this, we constructed a FLAG-L10P-hDAT mutant. Phosphorylation of the mutant upon PMA treatment was not detected using either antibody (Figure 1D), consistent with our hypothesis and further confirming that the antibodies unfortunately cannot be used in mouse or rat tissue due to their specificity for hDAT.
To confirm that PMA-induced DAT phosphorylation at S7 and S12 is mediated by PKC, we used two different PKC inhibitors and studied their effect in EM4 cells transfected with FLAG-hDAT and treated with PMA. G06850 is a general inhibitor of PKC (Toullec et al., 1991) whereas Ro 32-0432 preferentially targets PKCα and, to a lesser extent, PKCβ over PKCε (Wlkinson et al., 1993). Indeed, G06850 and Ro 32-0432 inhibited PMA-induced S7 phosphorylation by 87% and 84%, respectively (Figure 2A). Similarly, 12S phosphorylation was reduced by 96% and 88%, respectively (Figure 2B).
Figure 2. Inhibition of DAT phosphorylation by PKC and CaMKII inhibitors.
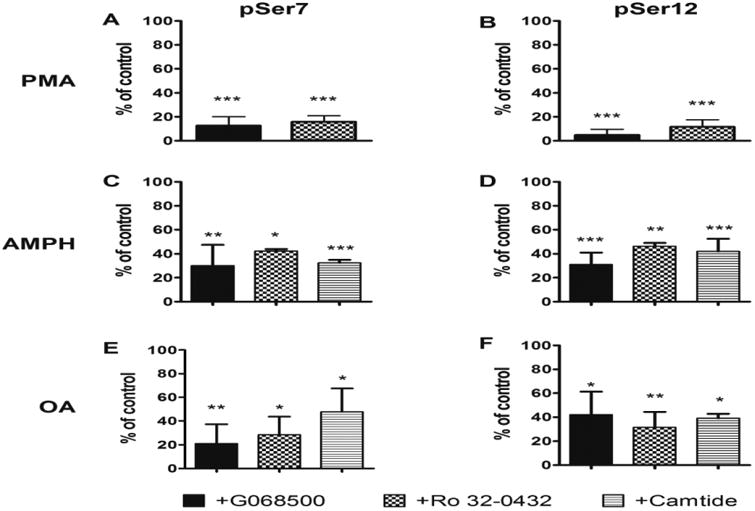
Cells stably expressing FLAG-hDAT were treated with 1 μM PMA for 15 min (A and B), 10 μM AMPH for 10 min (C and D) or 4 μM OA for 15 min (E and F) in the presence or absence of an inhibitor: cells were incubated with 1 μM G06850 (solid black bars), 0.5 μM Ro 32-0432 (checkered bars), or 5 μM Camtide (striped bars), for 30 min prior to the addition of the activator. DAT was immunoprecipitated by the FLAG tag and probed for phosphorylated Ser7 (pSer7, A, C, E) or Ser12 (pSer12, B, D, F), or total DAT. Data are presented as percent inhibition of signal in the presence of inhibitor, compared to its absence. Data = mean±S.E.M with n≥3 per experiment. Significance of inhibition was calculated using one way ANOVA, followed by a post-hoc Dunnett's multiple comparison test. * P<0.05, ** P<0.01, *** P<0.001.
Treatment with AMPH leads to DAT phosphorylation at Ser7 and Ser12 in a manner dependent on both PKC and CAMKII
Previous studies have shown that AMPH-induced DAT-mediated DA efflux and DAT phosphorylation are attenuated in the presence of PKC inhibitors (Cervinski et al., 2005; Cowell et al., 2000; Kantor and Gnegy, 1998; Kantor et al., 2001). We have previously demonstrated that binding of CaMKIIα to the DAT C-terminus facilitates phosphorylation of the DAT N-terminus and mediates AMPH-induced DA efflux in both cultured cells and striatial tissue (Fog et al., 2006). More recently we showed that inhibition of CaMKIIα in DA neurons of Drosophila larvae blocks AMPH-induced hyperlocomotion, whereas expression of a pseudophosphorylated DAT (hDAT-StoD) bypasses the need for CaMKIIα in this behavioral assay (Pizzo et al., 2014). We therefore examined whether AMPH treatment can lead to phosphorylation of DAT at Ser7 and Ser12 and whether this phosphorylation is dependent on PKC or CamKII or both. Indeed, as shown in Figure 1E, both Ser7 and Ser12 were phosphorylated following AMPH treatment, albeit less robustly than by PMA. AMPH-induced phosphorylation of Ser7 was inhibited by 70% and 58% by G06850 and Ro 32-0432, respectively (Figure 2C), whereas that of Ser12 was inhibited by 69% and 56%, respectively (Figure 2D). To determine whether CAMKIIα is also required for phosphorylation of DAT at Ser7 and Ser12 in response to stimulation by AMPH, we used CaMKIINtide, a peptide derived from an endogenous inhibitor of CaMKII (Chang et al., 1998; Lepicard et al., 2006). CaMKIINtide inhibits both Ca2+-dependent and Ca2+-independent activity and is highly specific; it inhibits both α and β isoforms of CaMKII, but not CaMKI, CaMKIV, PKC, or PKA (Chang et al., 1998). We used a form of CaMKIINtide that was made membrane-permeant by the addition of an antennapedia sequence (Ant-CaMKIINtide) (Bowton et al., 2010; Sanhueza et al., 2007). As shown in Figures 2C and 2D, the AMPH-induced increases in Ser7 and Ser12 phosphorylation were inhibited by 68% and 58%, respectively upon addition of Ant-CaMKIINtide to cells stimulated with AMPH. In contrast, addition of Ant-CaMKIINtide did not decrease PMA-induced phosphorylation of either pSer7 or pSer12 (data not shown).
Treatment with OA leads to phosphorylation at Ser7 and Ser12 in a manner dependent on both PKC and CAMKII
OA, a broad-spectrum protein phosphatase inhibitor, has been shown to stimulate the incorporation of 32P into DAT (Huff et al., 1997; Vaughan et al., 1997). This increase in phosphorylation produced in the absence of exogenous kinase activators reflects the rate of basal phosphorylation. We therefore determined whether stimulation with OA would specifically increase phosphorylation at Ser7 and Ser12 and whether this increase is dependent on PKC and/or CAMKII. Indeed, treating the cells with OA led to an increase in the phosphorylation of Ser7 (Figure 1F), which was inhibited by 79% and 71% by G06850 and Ro 32-0432, respectively (Figure 2E). OA also stimulated Ser12 phosphorylation and this was inhibited by 58% and 68% by G06850 and Ro 32-0432, respectively (Figure 2F). Treatment with Ant-CAMKIINtide prior to OA inhibited phosphorylation of Ser7 and Ser12 by 53% and 61%, respectively (Figures 2E and 2F). Thus inhibition of phosphatase activity reveals that in HEK293 cells both PKC and CaMKII are involved in ongoing phosphorylation of both Ser7 and Ser12.
Lipid raft localization is necessary for efficient N-terminal phosphorylation of DAT and the behavioral response to AMPH
We recently identified the lipid raft-associated protein Flotillin-1 (Flot1) as a novel regulator of DAT function, required to maintain the localization of DAT in cholesterol-rich regions in the plasma membrane (Cremona et al., 2011; Pizzo et al., 2013). Critically, we found that treatment of cells with nystatin or depletion of Flot1, leads to loss of DAT from rafts and inhibits AMPH-induced DA efflux without impairing DAT-mediated DA uptake (Cremona et al., 2011). We also showed that knockdown of Flot1 specifically in DA neurons of Drosophila larvae inhibits hyperlocomotion induced by AMPH but not that induced by methylphenidate, an uptake blocker that does not induce efflux (Pizzo et al., 2013). Therefore, like DAT phosphorylation, the localization of DAT to lipid rafts is required for its efflux capacity. Since many kinases, including CaMKII (Suzuki et al., 2008; Tsui et al., 2005) and PKC (Evans et al., 2006; Niggli et al., 2004), are enriched in lipid rafts, we hypothesized that the distribution of DAT within the plasma membrane may regulate its phosphorylation and thereby AMPH-induced DA efflux and behavior. To test this hypothesis, we treated cells expressing Flag-hDAT with nystatin, which binds free cholesterol and disrupts the lipid raft localization of DAT (Cremona et al., 2011), and examined its effect on PMA-induced phosphorylation of Ser7. We observed a 57% inhibition (p<0.001, n=7) of PMA-induced phosphorylation of Ser7 in response to nystatin (Figure 3A), consistent with our hypothesis that lipid raft localization of DAT is necessary for efficient N-terminal phosphorylation by raft-associated or recruited kinases.
Figure 3. Pseudophosphorylation of DAT N-terminal serines bypasses the requirement for raft localization in the actions of amphetamine.
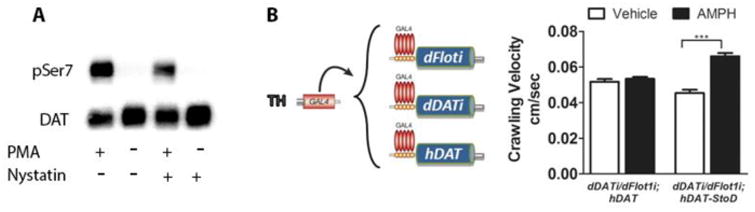
A) Cells stably expressing FLAG DAT were incubated with or without 25 μg/mL nystatin for 20 min and then treated for 15 min with 0.5 μM PMA or vehicle. DAT was immunoprecipitated by the FLAG tag and probed for phosphorylated serine 7 (pSer7) or total DAT. B) Larvae expressing UAS-driven small RNA-mediated interference against both Drosophila DAT (dDATi) and Flot1 (dFlot1i) in dopamine neurons using a Gal4 driver specific to tyrosine hydroxylase (TH)-positive neurons (TH-GAL4) exhibited a blunted AMPH response that was not rescued by expression of hDAT. In contrast, expression of hDAT/S to D did rescue the response to AMPH (***P<0.001). Data = mean±S.E.M. (n ≥ 30 larvae per group).
To test our hypothesis in vivo, we used a mutant hDAT that has the 5 N-terminal serines mutated to aspartate (hDAT-StoD), which mimics constitutive phosphorylation (pseudophosphorylation) and restores AMPH-induced DA efflux in heterologous cells (Khoshbouei et al., 2004). We have previously shown that expression of this mutant in Drosophila neurons bypasses the requirement for CaMKII in AMPH-induced hyperlocomotion (Pizzo et al., 2014). If the role of Flot1 in efflux is solely to localize DAT to lipid rafts so it can be phosphorylated, it follows that pseudophosphorylation of DAT would also be able to rescue the AMPH response that is lost due to Flot1 knockdown, despite the mislocalization of DAT away from lipid rafts. Indeed, our data show that expression of hDAT-StoD in DA neurons of Drosophila larvae rescued the blunted response to AMPH caused by Flot1 knockdown (Figure 3B). In contrast, expression of the WT hDAT control failed to rescue the response.
Discussion
While there is overwhelming evidence that AMPHs cause mobilization of intracellular DA to the cell exterior via DAT through non-exocytic efflux (Sulzer et al., 1995; Sulzer et al., 1992), the underlying mechanisms remain poorly defined. Studies from our labs and others have implicated the phosphorylation of DAT and its localization within microdomains in the plasma membrane as critical regulators of DAT-mediated efflux of DA. However, a lack of appropriate tools has made it difficult to probe directly the effect of kinases, phosphatases and other signaling processes on the phosphorylation state of DAT in vivo. Here we report the development and characterization of phospho-specific antibodies that can detect phosphorylation of hDAT at positions Ser7 and Ser12. Consistent with previous studies, our data show that Ser7 and Ser12 are major targets for PKC and CaMKII, both of which have been shown to phosphorylate the DAT N terminus (Cervinski et al., 2005; Fog et al., 2006; Foster et al., 2006; Foster et al., 2002; Gorentla et al., 2009; Granas et al., 2003; Lin et al., 2003; Moritz et al., 2013) and to play a role in DA efflux in cells and rodent brain slice (Chen et al., 2009; Gnegy, 2003; Johnson et al., 2005; Loweth et al., 2009). The precise functional relationship between the two kinases remains to be determined. They might act in parallel, although our previous data suggest a more complex interaction, as AMPH-induced hyperlocomotion in Drosophila is completely blocked by CaMKIINtide (Pizzo et al., 2014). A PKC-mediated mechanism should not be blocked unless CaMKII functions downstream of the actions of PKC. Interestingly, PKC activation leads to breakdown of PIP2, and thus can modulate transporter activity indirectly (Hamilton et al., 2014). The role of PKC is further complicated by its regulation of DAT trafficking (Cervinski et al., 2005; Foster et al., 2006; Gabriel et al., 2013; Granas et al., 2003) and the role of associated proteins such as the plasma membrane-associated GTPase Rin, which interacts with DAT in membrane microdomains (Navaroli et al., 2011). Thus, the precise role of PKC activation in DAT phosphorylation and its functional interaction with CaMKII will require further study. Furthermore, while our data support previous mutational and functional analyses that implicate Ser7 and Ser 12 as the critical serines phosphorylated in the DAT N terminus in vivo, we cannot rule out phosphorylation at other positions, as well as the potential effects of phosphorylation of one or more other sites on the phosphorylation of Ser7 and Ser12, and this will require further investigation.
Our data provide a direct link between the localization of DAT to lipid rafts, its N-terminal phosphorylation and the consequent behavioral response to AMPH. This is consistent with previous studies showing that treatment with methyl-beta-cyclodextrin (MbC), which also disrupts rafts, inhibits PMA-induced phosphorylation (Foster et al., 2008). However, it was unclear whether this effect was due to the membrane-microdomain localization of DAT or due its direct interaction with cholesterol, since MbC acts by extracting cholesterol from cell membranes (Ilangumaran and Hoessli, 1998). Nystatin, on the other hand, directly inserts into the membrane, binds to free cholesterol and physically disrupt rafts, while maintaining tightly bound cholesterol within the membrane (Coutinho and Prieto, 1995). We previously showed that treatment of cells with nystatin leads to loss of DAT from rafts, but has no effect on DA uptake (Cremona et al., 2011), consistent with the lack of effect of filipin on DA uptake reported previously (Foster et al., 2008). These data support our hypothesis that the Flot1-dependent localization of DAT to lipid rafts, and its consequent phosphorylation, play a specific role in AMPH-induced dopamine efflux through DAT without affecting transport function. Of note, treatment with MbC has been shown to inhibit transport by DAT and the serotonin transporter (SERT) (Adkins et al., 2007; Foster et al., 2008; Magnani et al., 2004). However, given our findings with nystatin and those with filipin(Foster et al., 2008), this likely relates to the removal of cholesterol tightly bound to DAT, which could impact transport through direct effects on the proteins ability to undergo the conformational changes associated with transport, rather than the localization of DAT to rafts.
Materials and Methods
Materials
All cell culture materials were obtained from Cellgro biochemicals, and gel electrophoresis materials were obtained from Biorad. The ECL kit was obtained from Amersham. PMA and OA were obtained from LC Laboratories. The PKC inhibitors, G06850 and Ro 32-0432, recombinant rabbit skeletal muscle PP1 inhibitor 2, and recombinant human kidney PP2A inhibitor 1 were obtained from Calbiochem. The CAMKII ant peptide inhibitor was a generous gift from Aurellio Galli. All other chemicals were from Sigma Chemical.
Cell culture and transfections
HEK 293-derived EM4 cells stably transfected with a macrophage scavenger receptor to promote adherence(Robbins and Horlick, 1998) using lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction and as previously described (Hastrup et al., 2001; Khoshbouei et al., 2004) were used for all experiments. Flag-hDAT, Flag-hDAT-StoA, Flag-S7A-hDAT, Flag-S12A-hDAT, Flag-S7AS12A-hDAT, Flag-7S-hDAT-StoA, Flag-12S-hDAT-StoA, Flag-7S12ShDAT-StoA, and Flag-12S13S-hDAT-StoA sequences were generated, confirmed and stably transfected. Cells were cultured in DMEM supplemented with 10% fetal bovine serum at 37°C and 5% CO2.
Custom development of phospho-specific antibodies from Phosphosolutions
The phospho-specific antibodies were generated by Phosphosolutions (Aurora, CO, USA). Immunization with a phospho-peptide can generate three different types of antibodies targeting DAT: 1) the desired phospho-specific antibody, 2) antibodies that are specific for the dephospho form of the peptide generated if endogenous phosphatases dephosphorylate the injected peptide conjugate, and 3) pan-specific antibodies against sequences/conformations of the peptide that did not involve the phosphoryl group in the epitope.
The desired phospho-specific antibodies were isolated through serial phospho- and dephospho- affinity chromatography steps. The first column used was the phosphopeptide affinity column to bind both the desired antibodies and also panspecific antibodies, which were then eluted from the phosphopeptide affinity column. The eluted phospho- and pan-specific antibodies were then applied to deplete pan specific antibodies. Thus, the flow-through contained the desired phospho-specific antibodies, which were validated as described herein.
Detection of phosphorylation of DAT at Ser7 and Ser12
HEK293 cells stably expressing Flag-hDAT were treated with 1 μM PMA, 4 μM OA or vehicle for 15 min and 10 μM AMPH for 30 min in the presence or absence of inhibitor (unless noted otherwise). The cells were incubated with the inhibitors G06850 (1 μM), Ro 32-0432 (0.5 μM) or CAMKII ant-Peptide inhibitor (5μM) for 30 min, prior to the addition of the activator. To test the effect of nystatin, cells were incubated with or without 25 μg/mL nystatin for 20 min and then treated for 15 min with 0.5 uM PMA or vehicle. The cells were washed in 5 ml ice- cold buffer (25 mM Tris-HCl, pH 7.6, with 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 50 mM sodium pyrophosphate, 50 mM NaF, 10 mM glycerophosphate, 10 mM NEM supplemented with protease inhibitors (Pefablock 2mg/ml, Aprotinin 2 mg/ml, Leupeptin 1 mg/m land pepstatinA 1mg/ml). Cells were lysed in the same buffer containing 1% triton. The lysate was immunoprecipitated by an antibody directed against the Flag (Sigma), resolved by SDS-PAGE, transferred to a PDVF membrane and blotted with the rabbit anti pSer7 and pSer12 antibody and in parallel with a Goat C-terminal DAT antibody (Santa Cruz). Bands were visualized using goat anti-rabbit and Donkey anti-goat (Santa Cruz) horseradish peroxidase-conjugated secondary antibody and detected by ECL and quantitated on a FluorChem 8900 imaging system (Alpha Innotech). Data are presented as percent inhibition of signal in the presence of inhibitor, compared to its absence. Data = mean±S.E.M with n≥3 per experiment. Significance of signal inhibition in response the relevant drug was calculated using one way ANOVA, followed by a post-hoc Dunnett's multiple comparison test. * P<0.05, ** P<0.01, *** P<0.001.
Drosophila locomotion assay
Two hundred flies (3:2, females:males) of the respective genotype were placed in bottles filled with standard medium and permitted to lay eggs with experiments commencing on the fourth day of egg-laying. twenty to 30 early third instar larvae (82- to 86-h old) per experimental group were washed with distilled water and placed onto 70% yeast paste (vehicle treatment) or 70% yeast paste in 60 mM amphetamine (Sigma, St Louis, MO) for 1 h (18 °C). Food coloring was added to the yeast paste to ascertain whether the larvae fed on the provided paste. Fed larvae were subsequently transferred onto 100-mm Petri dishes filled with 1% agar dissolved in distilled water. Each dish containing a set of 1-3 larvae was placed on a cool-operated, evenly illuminated fluorescent light box positioned underneath a video camera (Dalsa PT-41-04M60, Teledyne, Dalsa, Waterloo, Ontario, Canada), which captured a high-contrast video image of larval profiles over a featureless background. Larvae were acclimated on the agar plate for 1 min followed by 1 min of data acquisition in a designated behavior room (23–25 °C, 35–40% humidity). We used the Multi-Worm Tracker and Choreography software packages (open source availability) to track and quantify larval movement.(Pizzo et al., 2014; Pizzo et al., 2013).
Acknowledgments
This work was supported by National Institutes of Health grants R01 DA041510 (JAJ, CSK) and K05 DA022413 (JAJ).
Footnotes
Author Contribution: CSK, NS and JAJ designed the experiments. CSK and NS conducted the experimental procedures and analyzed the data. NS performed the experiments in heterologous cells, including validation of the antibodies and the investigation into the role of kinases and phosphatases in DAT phosphorylation in response to the various chemical and pharmacological reagents. CSK performed the genetic and behavioral experiments in Drosophila to investigate the relationship between DAT phosphorylation and Flotillin-1 function in AMPH-induced behavior. CSK, NS and JAJ interpreted the data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, Ramamoorthy S, Gether U. Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry. 2007;46:10484–10497. doi: 10.1021/bi700429z. [DOI] [PubMed] [Google Scholar]
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Bowton E, Saunders C, Erreger K, Sakrikar D, Matthies HJ, Sen N, Jessen T, Colbran RJ, Caron MG, Javitch JA, et al. Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. J Neurosci. 2010;30:6048–6057. doi: 10.1523/JNEUROSCI.5094-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss M, Wieland A, Bonisch H. Molecular cloning and functional expression of the mouse dopamine transporter. J Neural Transm (Vienna) 1999;106:657–662. doi: 10.1007/s007020050187. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. The Journal of biological chemistry. 2005;280:40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA. Syntaxin 1A regulates dopamine transporter activity, phosphorylation and surface expression. Neuroscience. 2010;170:408–416. doi: 10.1016/j.neuroscience.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci U S A. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Reith ME. Interaction between dopamine and its transporter: role of intracellular sodium ions and membrane potential. J Neurochem. 2004;89:750–765. doi: 10.1111/j.1471-4159.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Furman CA, Zhang M, Kim MN, Gereau RWt, Leitges M, Gnegy ME. Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J Pharmacol Exp Ther. 2009;328:912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A, Prieto M. Self-association of the polyene antibiotic nystatin in dipalmitoylphosphatidylcholine vesicles: a time-resolved fluorescence study. Biophys J. 1995;69:2541–2557. doi: 10.1016/S0006-3495(95)80125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell RM, Kantor L, Hewlett GH, Frey KA, Gnegy ME. Dopamine transporter antagonists block phorbol ester-induced dopamine release and dopamine transporter phosphorylation in striatal synaptosomes. Eur J Pharmacol. 2000;389:59–65. doi: 10.1016/s0014-2999(99)00828-6. [DOI] [PubMed] [Google Scholar]
- Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–477. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JH, Murray D, Leslie CC, Falke JJ. Specific translocation of protein kinase Calpha to the plasma membrane requires both Ca2+ and PIP2 recognition by its C2 domain. Mol Biol Cell. 2006;17:56–66. doi: 10.1091/mbc.E05-06-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther. 1979;208:203–209. [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, et al. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Cervinski MA, Gorentla BK, Vaughan RA. Regulation of the dopamine transporter by phosphorylation. Handb Exp Pharmacol. 2006:197–214. doi: 10.1007/3-540-29784-7_10. [DOI] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Vaughan RA. Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J Biol Chem. 2002;277:25178–25186. doi: 10.1074/jbc.M200294200. [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Sonders MS, Aguilar JI, Hiranita T, Karam CS, Flores J, Pizzo AB, Zhang Y, Farino ZJ, Chen A, et al. Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat Commun. 2016;7:10652. doi: 10.1038/ncomms10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel LR, Wu S, Kearney P, Bellve KD, Standley C, Fogarty KE, Melikian HE. Dopamine transporter endocytic trafficking in striatal dopaminergic neurons: differential dependence on dynamin and the actin cytoskeleton. J Neurosci. 2013;33:17836–17846. doi: 10.1523/JNEUROSCI.3284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnegy ME. The effect of phosphorylation on amphetamine-mediated outward transport. Eur J Pharmacol. 2003;479:83–91. doi: 10.1016/j.ejphar.2003.08.059. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, De Felice LJ, Khoshbouei H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284:2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorentla BK, Moritz AE, Foster JD, Vaughan RA. Proline-directed phosphorylation of the dopamine transporter N-terminal domain. Biochemistry. 2009;48:1067–1076. doi: 10.1021/bi801696n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U. N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J Biol Chem. 2003;278:4990–5000. doi: 10.1074/jbc.M205058200. [DOI] [PubMed] [Google Scholar]
- Hamilton PJ, Belovich AN, Khelashvili G, Saunders C, Erreger K, Javitch JA, Sitte HH, Weinstein H, Matthies HJ, Galli A. PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nat Chem Biol. 2014;10:582–589. doi: 10.1038/nchembio.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastrup H, Karlin A, Javitch JA. Symmetrical dimer of the human dopamine transporter revealed by cross-linking Cys-306 at the extracellular end of the sixth transmembrane segment. Proc Natl Acad Sci U S A. 2001;98:10055–10060. doi: 10.1073/pnas.181344298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastrup H, Sen N, Javitch JA. The human dopamine transporter forms a tetramer in the plasma membrane: cross-linking of a cysteine in the fourth transmembrane segment is sensitive to cocaine analogs. J Biol Chem. 2003;278:45045–45048. doi: 10.1074/jbc.C300349200. [DOI] [PubMed] [Google Scholar]
- Huff RA, Vaughan RA, Kuhar MJ, Uhl GR. Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. J Neurochem. 1997;68:225–232. doi: 10.1046/j.1471-4159.1997.68010225.x. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S, Hoessli DC. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J. 1998;335(Pt 2):433–440. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem. 2005;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci U S A. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J Pharmacol Exp Ther. 1998;284:592–598. [PubMed] [Google Scholar]
- Kantor L, Hewlett GH, Park YH, Richardson-Burns SM, Mellon MJ, Gnegy ME. Protein kinase C and intracellular calcium are required for amphetamine-mediated dopamine release via the norepinephrine transporter in undifferentiated PC12 cells. J Pharmacol Exp Ther. 2001;297:1016–1024. [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepicard EM, Mizuno K, Antunes-Martins A, von Hertzen LS, Giese KP. An endogenous inhibitor of calcium/calmodulin-dependent kinase II is up-regulated during consolidation of fear memory. Eur J Neurosci. 2006;23:3063–3070. doi: 10.1111/j.1460-9568.2006.04830.x. [DOI] [PubMed] [Google Scholar]
- Lin Z, Zhang PW, Zhu X, Melgari JM, Huff R, Spieldoch RL, Uhl GR. Phosphatidylinositol 3-kinase, protein kinase C, and MEK1/2 kinase regulation of dopamine transporters (DAT) require N-terminal DAT phosphoacceptor sites. J Biol Chem. 2003;278:20162–20170. doi: 10.1074/jbc.M209584200. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norgaard-Nielsen K, Gether U. Probing dopamine transporter structure and function by Zn2+-site engineering. Eur J Pharmacol. 2003;479:187–197. doi: 10.1016/j.ejphar.2003.08.068. [DOI] [PubMed] [Google Scholar]
- Loweth JA, Svoboda R, Austin JD, Guillory AM, Vezina P. The PKC inhibitor Ro31-8220 blocks acute amphetamine-induced dopamine overflow in the nucleus accumbens. Neurosci Lett. 2009;455:88–92. doi: 10.1016/j.neulet.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani F, Tate CG, Wynne S, Williams C, Haase J. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J Biol Chem. 2004;279:38770–38778. doi: 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- Moritz AE, Foster JD, Gorentla BK, Mazei-Robison MS, Yang JW, Sitte HH, Blakely RD, Vaughan RA. Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. J Biol Chem. 2013;288:20–32. doi: 10.1074/jbc.M112.407874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz AE, Rastedt DE, Stanislowski DJ, Shetty M, Smith MA, Vaughan RA, Foster JD. Reciprocal Phosphorylation and Palmitoylation Control Dopamine Transporter Kinetics. J Biol Chem. 2015;290:29095–29105. doi: 10.1074/jbc.M115.667055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaroli DM, Stevens ZH, Uzelac Z, Gabriel L, King MJ, Lifshitz LM, Sitte HH, Melikian HE. The plasma membrane-associated GTPase Rin interacts with the dopamine transporter and is required for protein kinase C-regulated dopamine transporter trafficking. J Neurosci. 2011;31:13758–13770. doi: 10.1523/JNEUROSCI.2649-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli V, Meszaros AV, Oppliger C, Tornay S. Impact of cholesterol depletion on shape changes, actin reorganization, and signal transduction in neutrophil-like HL-60 cells. Exp Cell Res. 2004;296:358–368. doi: 10.1016/j.yexcr.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Pifl C, Singer EA. Ion dependence of carrier-mediated release in dopamine or norepinephrine transporter-transfected cells questions the hypothesis of facilitated exchange diffusion. Mol Pharmacol. 1999;56:1047–1054. doi: 10.1124/mol.56.5.1047. [DOI] [PubMed] [Google Scholar]
- Pizzo AB, Karam CS, Zhang Y, Ma CL, McCabe BD, Javitch JA. Amphetamine-induced behavior requires CaMKII-dependent dopamine transporter phosphorylation. Mol Psychiatry. 2014;19:279–281. doi: 10.1038/mp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo AB, Karam CS, Zhang Y, Yano H, Freyberg RJ, Karam DS, Freyberg Z, Yamamoto A, McCabe BD, Javitch JA. The membrane raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Mol Psychiatry. 2013;18:824–833. doi: 10.1038/mp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins AK, Horlick RA. Macrophage scavenger receptor confers an adherent phenotype to cells in culture. Biotechniques. 1998;25:240–244. doi: 10.2144/98252st04. [DOI] [PubMed] [Google Scholar]
- Sanhueza M, McIntyre CC, Lisman JE. Reversal of synaptic memory by Ca2+/calmodulin-dependent protein kinase II inhibitor. J Neurosci. 2007;27:5190–5199. doi: 10.1523/JNEUROSCI.5049-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholze P, Norregaard L, Singer EA, Freissmuth M, Gether U, Sitte HH. The role of zinc ions in reverse transport mediated by monoamine transporters. J Biol Chem. 2002;277:21505–21513. doi: 10.1074/jbc.M112265200. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J Neurochem. 1998;71:1289–1297. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Pothos E, Sung HM, Maidment NT, Hoebel BG, Rayport S. Weak base model of amphetamine action. Ann N Y Acad Sci. 1992;654:525–528. doi: 10.1111/j.1749-6632.1992.tb26020.x. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Du F, Tian QB, Zhang J, Endo S. Ca2+/calmodulin-dependent protein kinase IIalpha clusters are associated with stable lipid rafts and their formation traps PSD-95. J Neurochem. 2008;104:596–610. doi: 10.1111/j.1471-4159.2007.05035.x. [DOI] [PubMed] [Google Scholar]
- Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, Caron MG. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J Biol Chem. 2003;278:2731–2739. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Tsui J, Inagaki M, Schulman H. Calcium/calmodulin-dependent protein kinase II (CaMKII) localization acts in concert with substrate targeting to create spatial restriction for phosphorylation. J Biol Chem. 2005;280:9210–9216. doi: 10.1074/jbc.M407653200. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem. 1997;272:15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294(Pt 2):335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]


