Abstract
Due to their prevalence, hallucinations are considered as one of the most frequent psychotic symptom in Alzheimer’s disease (AD). These psychotic manifestations reduce patients’ well-being, increase the burden of caregivers, contribute to early institutionalization, and are found to be related with the course of cognitive decline in AD. Considering their consequences, we provide a comprehensive account of the current state of knowledge about the prevalence and characteristics of hallucinations in AD. We provide a comprehensive and testable theoretical model about hallucinations in AD: the ALZHA (ALZheimer and HAllucinations) model. In this model, neurological, genetic, cognitive, affective, and iatrogenic factors associated with hallucinations in AD are highlighted. According to this model, hallucinations in AD first involve trait markers (i.e., cognitive deficits, neurological deficits, genetic predisposition and/or sensory deficits) to which state markers that may specifically trigger these experiences are added (e.g., psychological distress and/or iatrogenic factors). Finally, we provide recommendations for assessment and management of these psychotic manifestations in AD, with the aim to both serve patients, caregivers, and health professionals.
Keywords: Alzheimer’s disease, clinical management, hallucinations
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease. Although the hallmark of AD is progressive episodic memory impairment, neuropsychiatric symptoms, especially hallucinations, are common in the disease. Hallucinations in AD reduce patients’ well-being, increase the burden of caregivers, contribute to early institutionalization and, as we will depict later, are related with cognitive decline. Considering these consequences, we provide a comprehensive account of the current state of knowledge about hallucinations in AD. We first describe the prevalence, characteristics, and consequences of hallucinations in the disease, we then provide current neurological, genetic, and cognitive and affective explanations. We propose a theoretical framework, which captures the complexity of underlying mechanisms of hallucinations in AD. This framework, named ALZHA (ALZheimer and Hallucinations), dissociates “state” and “trait” markers of hallucinations. Following current trends in research on hallucinations [1, 2], state markers include psychological distress as well as iatrogenic factors associated with hallucinations’ occurrences, while trait markers are risk factors for hallucinations, not directly linked with the presence/absence of the experience at the time of assessment (i.e., difficulties in suppressing intrusive thoughts or memories, neurological deficits, genetic predisposition and/or sensory deficits). According to the ALZHA model, hallucinations in AD are mainly observed in patients who exhibit trait markers and who occasionally experience one or more state markers that trigger the experience. Recommendations for assessment and management of hallucinations in AD close this review to provide clinical guidelines for clinicians and caregivers.
The ALZHA model can be compared with existing models of hallucinations in other populations. In the Charles Bonnet syndrome, several theoretical models have proposed that lesions to specific neural substrates account for hallucinations [3, 4], not surprisingly as hallucinations in this syndrome are believed to be the consequence of disruption of the visual processing pathway. Prior research in the Charles Bonnet syndrome has demonstrated activation of visual cortical areas during visual hallucinations, of auditory and motor language areas during auditory-verbal hallucinations, and of somatosensory areas during somatic hallucinations. These findings suggest that, for hallucinations of a particular sensory modality in this syndrome, active involvement of the corresponding unimodal brain area is required [5]. Models on hallucinations in Parkinson’s disease have also implicated specific neurological substrates [6, 7], as well as discrepancies between conscious and non-conscious detection of stimuli [8]. As for Schizophrenia, and psychosis in general, theoretical models have been reverse engineered based on neurotransmitter properties of antipsychotic medication [9], implicated neural pathways [10], cognitive mechanisms [11–13], or combinations of neurocognitive findings [1, 14–18]. Compared with these theoretical models, the ALZHA model proposes an integrative approach that productively combines well-characterized neurological, cognitive, affective, and iatrogenic features of the disease to show how they could result in hallucinations. A similar approach was adopted by computation models in schizophrenia [19, 20]. In our view, the ALZHA model offers a comprehensive view of a variety of factors that may contribute to hallucinations, which not only offers new theoretical advances, but also informs clinical approaches to reduce the destructive effects of hallucinations on the well being of patients and caregivers.
1.1. Prevalence, characteristics, and consequences of hallucinations in AD
With a prevalence ranging from 4% to 76% (median 23%) [21], hallucinations are considered as one of the most frequent psychotic symptoms in AD [22–24]. Hallucinations in AD are mainly visual and auditory [25], but somatic, olfactory, and tactile experiences have also been reported [26]. Research suggests that visual hallucinations tend to occur at the advanced stages of AD [27]. A study has demonstrated that AD patients tend to develop visual hallucinations significantly later than patients with Dementia with Lewy bodies [28]. Thus, visual hallucinations may characterize the advanced stages of AD and the co-occurrence of vascular pathology rather than AD pathology [29, 30].
Regardless of their sensory modality, hallucinations are associated with greater cognitive impairment and a more rapid deterioration [22, 31]. Hallucinations have also been associated with an increase in mortality [22]. In a relevant study, Wilson et al. [32] assessed hallucinations and delusions in a group of 407 AD patients, monitoring the participants’ vital status for a mean duration of 3.7 years. At study onset, hallucinations were present in 41.0% of participants, and during follow-up, 146 deaths occurred. Hallucinations were associated with a 78% increase in risk of death, and risk of death was more than double in those with both auditory and visual hallucinations. Interestingly, the same study [32] did not find evidence of an association between delusions and mortality. Regarding the time of onset, hallucinations have been reported to occur at all stages of AD. A study observed hallucinations in 45 of 100 patients around the time of diagnosis [33]. Another study found that hallucinations in AD fluctuate with time but that their overall prevalence increases slowly with disease progression [34]. In the last study, the authors reported that the recurrence rate for hallucinations was 95% in AD over one year [35], suggesting that once these symptoms have occurred in the disease, they frequently recur.
On a behavioral level, hallucinations can have many consequences: for instance, they may be associated with verbal outbursts [36], aggressive behavior [37], functional decline [22], and falls [38]. A study has also reported an association between hallucinations and institutionalization in AD [22]. Hallucinations are distressing for both AD patients and caregivers, as shown by studies demonstrating that neuropsychiatric symptoms in the disease are associated with higher rates of stress, depression, and high burden of care in caregivers [39, 40]. Taken together, hallucinations have a number of adverse consequences for patients and caregivers resulting in reduced quality of life, increased caregiver distress, and higher rates of admission to hospital and residential care facilities.
After highlighting the characteristics and consequences of hallucinations in AD, we emphasize neurological, genetic, cognitive, and clinical basis of these psychotic experiences.
2. Neurological and genetic accounts for hallucinations in AD
2.1. Brain basis
Unlike the extensive body of research devoted to the exploration of the neural bases of hallucinations in schizophrenia (for review, see, [41], only limited research has specifically addressed this question in AD (for a summary, see, Table 1). A study conducted in 812 AD patients found an association between hallucinations and reduced thickness in the lateral parietal cortex [42]; however, the study was based on data from the Neuropsychiatric Inventory brief questionnaire form (NPI-Q), and, as a result, the sensory modality of hallucinations was not assessed. The same issue was addressed by another study showing smaller occipital lobes in AD patients with visual hallucinations compared to those without [43], inferring the role of pathological changes in modality-dependent cortices in these experiences. In a similar vein, a study demonstrated an association between visual hallucinations and increased occipital periventricular hyperintensities in AD [44]. Besides implicating the occipital cortex, hallucinations in AD were also found to involve frontotemporal and right parietal hypoperfusion [45]. More precisely hallucinations in AD patients have been associated with lower regional perfusion of the right and left dorsolateral frontal, left ventral striatal, and left anterior cingulate, as well as, left pulvinar and dorsolateral parietal cortex regions [46]. This prefrontal involvement may account for concomitant behaviours in AD (e.g., aggressive behavior). One study has reported an association between hallucinations in AD and the right anterior-posterior network, as well as with the anterior insula [47]. More specifically, this study assessed hallucinations with the following questions “does the patient have hallucinations, or false visions, or voices? does he/she seem to hear or see things that are not present?”. The study found associations between hallucinations and atrophy in the anterior part of the right insula, the left superior frontal gyrus, and the lingual gyri. Hallucinations were also associated with hypometabolism in the right ventral and dorsolateral prefrontal cortex, as well as with the right anterior part of the insula.
Table 1.
Summary of studies assessing neural basis of hallucinations in Alzheimer’s disease
| Study | Finding | Method |
|---|---|---|
| Blanc et al. (2014) | Association between hallucinations in AD and the right anterior-posterior network, and the anterior insula as the core region | MRI/VBM (n = 39) |
| Donovan et al (2014) | Association between hallucinations and supramarginal cortical thinning in AD | MRI/T1W (n=809) |
| Holroyd et al. (2000) | Association between visual hallucinations and visualcortex atrophy/parietal-occipitalsulcal widening in AD | 1.5T MRI/T1W (n=14) |
| Lin et al. (2006) | Association between visual hallucinations and increased occipital periventricular hyperintensities in AD | 1.5T MRI/FLAIR (n=10) |
| Lopez et al. (2001) | Association between hallucinations and frontotemporal/right parietal hypoperfusionin AD | O-water PET (n=18) |
| Mega et al. (2000) | Association between hallucinations and lower regional perfusion in the right and left dorsolateral frontal, left ventral striatal, left anterior cingulate, left pulvinar, and dorsolateral parietal cortex in AD | Tc SPECT (n=20) |
In our view, there is a relative paucity of research on the specific neuroanatomical basis of hallucinations in AD. This paucity may be attributed to a greater interest in delusions in this disease [48], i.e., those erroneous beliefs that are firmly maintained by a patient despite contradictory evidence. Critically, the research on the neuroanatomical basis of hallucinations in AD has included small sample sizes, and has also mainly assessed hallucinations regarding their occurrence and modality, rather than their phenomenological characteristics (e.g., intensity, frequency, duration).
2.2. Genetic account
A relationship has been reported between hallucinations in AD and the apolipoprotein E (ApoE) ε4 allele. The ApoE gene, located on chromosome 19, is the most important known genetic risk factor for sporadic AD [49]. A study has found significant association between ApoE ε4 and hallucinations in a large sample of 266 AD patients [50]. Although several studies confirmed this finding, some studies did not replicate this association (for a review, see [51]), a discrepancy that may be due to differences in sample sizes and diagnostic criteria.
3. Cognitive and affective accounts for hallucinations in AD
3.1. Hallucinations, unsuppressed memories?
According to Hemsley [52], inhibitory dysfunction lead to the emergence of redundant or irrelevant information from memory into awareness, generating hallucinations in Schizophrenia (for a similar view, see [53]). This model is supported by studies using the Inhibition of Currently Irrelevant Memories task. On this task, participants are typically instructed to forget previously exposed information (typically pictures) [54]. Using this task, Waters, Badcock [55] found difficulties in memory suppression in participants with schizophrenia, a difficulty that was significantly correlated with the severity of hallucinations. In the same vein, impairment in memory suppression, as observed on the Inhibition of Currently Irrelevant Memories task, was also considered responsible for intrusive thoughts in obsessive-compulsive disorder [56]. Also, difficulties on this task were observed in undergraduate-students predisposed to hallucinations [57].
Interestingly, the relationship between inhibitory mechanisms and hallucinations was also observed in AD. Hallucinations were mediated by inhibitory decline as assessed with the Stroop task in 31 patients with AD [58]. According to this study, hallucinations in AD patients may be related to difficulties in suppressing intrusive thoughts or memories.
The relationship between memory suppression and hallucinations was also assessed by studies using the directed forgetting task. In its conventional configuration, this task requires the processing of two lists of words (i.e., List 1 + List 2) [59]. Subjects are typically instructed to retain the words in List 1, after which they are asked either to continue remembering or to forget the words in it. Afterward, they are instructed to retain the words in List 2. Finally, in a recall test, they are asked to remember all of the words in both lists, regardless of the intermediary forget or remember instructions. Typically, participants with the “forget” instruction tend to show poorer memory for the items in List 1 than the “remember” participants. This effect has been attributed to a suppression effect of the forget instruction that reduces the accessibility of List 1 information [60]. The directed forgetting task has been used to demonstrate relationships between deficits in memory suppression and hallucinations in schizophrenia. A study assessed the directed forgetting list task in schizophrenia participants with and without hallucinations and found that, relatively to those without hallucinations, participants with hallucinations presented inhibitory deficits on this task (i.e., they have difficulties to suppress the List 1 items) [61]. According to Soriano, Jimenez [61], difficulties in suppressing memory representations could underlie hallucinations in schizophrenia.
An important challenge for memory retrieval is the competition between appropriate and inappropriate information. This competition is normally reduced thanks to inhibitory control processes that suppress distractive memories, intrusive or unwanted thoughts, or interfering mental images [62]. Hence, hallucinations in AD patients could be partly due to difficulties in suppressing memory representations, so that unwanted or repetitive thoughts intrude into consciousness. The relationship between inhibition and hallucinations can be further illustrated by a study in which AD patients had to remember whether they have previously told or imagined telling facts to faces of famous people [63]. Difficulties in the latter task were significantly correlated with performance on the Stroop task. Similarly, another study demonstrated significant correlations between compromise in source memory, i.e., the ability to remember the context in which information was acquired, and inhibition in AD [64]. Interestingly, a study has used the directed forgetting task to demonstrate difficulties in suppressing personal memories in AD [65].
Taken together, these lines of evidence to suggest that inhibitory dysfunction leads to the emergence of redundant or irrelevant information from long-term memory into awareness, generating hallucinations. This assumption can be supported by empirical studies showing that difficulties in intentionally inhibiting irrelevant thoughts or representations may be associated with hallucinations in Schizophrenia [66], obsessive-compulsive disorder [56], as well as in individuals prone to hallucinations [57, 61]. More specifically, the memory suppression compromise model emphasizes both inhibitory decline and memory impairment in AD and fits with the model of Hemsley [52] who have proposed an interplay between inhibitory processing and memory impairment to account for the presence of hallucinations in schizophrenia (see also [53]). This model is of particular interest as the cardinal characteristic of AD is memory compromise [67], therefore the inclusion of memory impairment in any model accounting for hallucinations aligns it with models that account for the broader cognitive and psychiatric phenomenology of the disease
3.2. Loneliness and depression
According to Fromm-Reichmann [68], loneliness makes people emotionally paralyzed and helpless, and may ultimately lead to the development of psychotic states. In a similar vein, extreme isolation in solitary confinement was reported to produce hallucinations in prisoners and hostages, perhaps, as a result of sensory deprivation [69]. In a study of 22 AD patients, we demonstrated significant correlations between hallucinations and loneliness as well as between hallucinations and social isolation [70]. This study argued that lonely AD patients generate internal stimuli to compensate for the lack of external stimulation and social contact and to fulfil their need to communicate. Hence, hallucinatory experiences may be regarded as a loophole allowing some AD patients, especially institutionalized ones, to escape the cycle of boredom, emptiness, and affective deprivation from social isolation. It is worth noting, however, that one study did not found a relationship between loneliness and hallucinations in patients with psychotic disorders [71], perhaps because the study captured only a cross-sectional snap-shot of patients with psychosis without assessing longitudinal influences of loneliness on psychosis. Regarding AD, loneliness seems to play important role in hallucinations as, contrarily to other disorders, AD has a negative impact on functional abilities (e.g., walking, communicating), which limits social interactions and deprives patients of important social resources.
Independently of their relationship with loneliness and social isolation, hallucinations in AD were also found to be associated with depression [58]. Such a relationship mirrors the frequent occurrence of depressive disorders in individuals suffering from hallucinations [72]. Individuals with severe depression commonly report auditory hallucinations, which are generally transient and limited to single words or short phrases and, usually, communicating thoughts consistent with the patient’s depressed mood [73]. Because 25% to 70% of AD patients suffer from a comorbid depressive disorder [74], whereas both depression and hallucinations are important neuropsychiatric manifestations of AD [21], it is not surprising to observe a relationship between them. However, there is a need for studies to assess potential relationships between hallucinations and anxiety and poor emotion regulationin AD.
4. Assessment and management
4.1. Assessment
Due to the subjective nature of hallucinations, several challenges limit their assessment. Some AD patients, with a preserved insight, can be reluctant to communicate about hallucinations to families in fear of negative consequences (e.g., their families may raise concerns about their mental health and/or begin to consider nursing home placement). On the other hand, anosognosia, i.e., the impaired ability to recognize the presence or appreciate the severity of cognitive deficits, limits some patients’ insight into the occurrence of hallucinations. Hence, assessment and management should engage patients as well as families and caregivers, this to determine the hallucinations’ modality, complexity, for how long the hallucinations have been present and the circumstances in which they occur. In a similar vein, it appears important to observe the patient’s behavior on several occasions, i.e., when she/he is alone or when interacting with other people.
Another issue to be considered in the assessment of hallucinations is the threshold beyond which hallucinations require psychological/medical care. Although hallucinations tend to be associated with psychotic disorders, some AD patients, especially those with mild stages of the disease, succeed to communicate these manifestations, attribute significance or personal meaning to hallucinations ((e.g. “I probably see my (deceased) wife as I am missing her”)) and finally cope with them. The latter example illustrates the importance of considering grieving when assessing hallucinations in AD. On the other hand, determining the threshold of care implies determining the degree of danger or functional risk in the short and long term for the patient and others, since hallucinations may be associated, in some circumstances, with significant confusion and/or aggressive behaviors [75].
An important tool that may be used to evaluate hallucinations in AD is the Launay–Slade Hallucination Scale [69], an auto-evaluation scale that assesses a range of psychological factors and different hallucination modalities, as well as intrusive thoughts and vivid daydreams. Another tool is the Psycho-Sensory Hallucinations Scale (PSAS), a hetero-evaluation scale that includes four hallucination modalities (auditory, visual, olfactory/gustatory, and kinesthetic modalities) as well as the vivid sensation that somebody is present nearby [76, 77]. Future research should incorporate these assessments because research on hallucinations in other clinical groups (e.g., schizophrenia) provide considerably more detail about the phenomenology of hallucinatory experience than research in AD. To overcome the effect of potential lack of insight of AD patients into the nature of hallucinatory experience [78], especially those in advanced stages of the disease, research can compare auto-evaluation scales with hetero-evaluation ones. It is worth noting, however, that one priority is to validate these scales in AD, as, to the very best of our knowledge, no published study has validated any hallucinations scales in AD.
4.2. Management
Because hallucinations in AD are mainly visual and impaired visual acuity has been associated with visual hallucinations [79], the first step in management should be visual acuity testing. In the spectrum of eye-diseases, visual hallucinations seem to be more persistent and more severe in AD patients with cataracts [79]. Therefore, visual acuity testing and cataract treatment constitutes first-line management in case of visual hallucinations. Auditory acuity testing should also be taken into account as auditory hallucinations may also occur in AD, whereas increased risk of psychosis may exist in patients with hearing impairment [80]. It is noteworthy, however, that in the advanced stages of AD, the benefit/risk balance for surgery (e.g., cataract surgery) or general anesthesia cansway the decision against such intensive measures.
General recommendations for coping with hallucinations in AD patients include environmental manipulation, such as turning off the radio or television to avoid any interference. Other environmental manipulations include removal of mirrors/objects with complex shape, avoiding any salient changes in the visual environment, focusing on the object/shape in question more precisely, approaching or trying to touch the hallucinations or looking in another direction/at another object. Leaving a light on during nighttime can also be effective to reduce shadows, although light should be adjusted to not disturb the patients’ sleep. Because hallucinations tend to worsen under conditions of increased arousal, reducing arousal may also be effective. Sleep monitoring is also a worth consideration as research indicates that sleep dysfunction may contribute to psychotic symptoms in AD [81]. Besides these general recommendations, appraisal strategies can be applied given that emotional reactions (e.g., depression and anxiety) to hallucinations are partially mediated by people’s beliefs about the voice [82]. These beliefs include the identity (who is the voice/person?), purpose (why is the person talking to me and not someone else?), and degree of omnipotence (how powerful is the voice?). Appraisal of these beliefs can provide important insight into the hallucinatory phenomena. Acceptance and Commitment Therapy can also be recommended. This therapy encourages patients to accept and experience symptoms as mental events (i.e., mindfully) rather than judging their truthfulness and reacting to them. This therapy considers hallucinations to be similar to other private experiences (such as thoughts and memories) and directs focus on hallucinations when efforts to control, eliminate or avoid them lead to poor functioning [83]. This therapy may reduce aggressive behaviors or confusion keeping in mind that severe hallucinations in dementia tend to occur in confused states with agitation [75]. Although AD patients may benefit from Mindfulness-based Cognitive Therapy to improve affective states and behavioral [84], control trials are needed to validate the effect of acceptance and commitment therapy on hallucinations.
Regarding pharmacological approaches, there are no FDA-approved antipsychotic agents for the specific treatment of hallucinations in AD [75]. The administration of second-generation atypical ant psychotics is usually favored by clinicians at the request of the family [75, 85], however, cardiac, metabolic, and cerebrovascular risks associated with these drugs raise serious concerns [85]. This view is mirrored by the French National Authority for Health according to which non-pharmacological methods should be preferred as first-line treatments for behavioral disorders [86]. The French National Authority for Health also recommends that non-pharmacological approaches should substitute the use of medication in behavioral disorders. Treatment with a psychotropic agent can be prescribed when this line of treatment is not sufficiently effective, such as in cases of refusal to cooperate, wandering, aggressive behavior, and especially, when hallucinations are severe enough to endanger the patient and the others.
Overall, management of hallucinations in AD should always take into account their modality (e.g., auditory, visual), attempt to identify potential causes and contributing factors, and consider the individual subject’s characteristics. Management should also take into account whether the targeted mechanism is a state or atrait one.
5. Summary: the ALZHA model
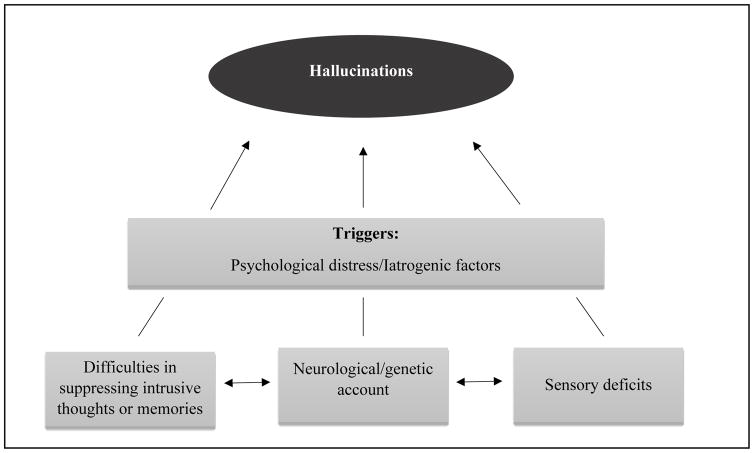
Due to their prevalence, hallucinations are considered as one of the most frequent psychotic symptoms in AD [22–24]. These manifestations are also associated with cognitive and functional decline, disruptive behaviours, falls and early institutionalization in the disease. Despite their occurrence and their negative impact on the psychological and physiological health of patients as well as their caregivers, relatively little research has been conducted on hallucinations in AD, in comparison with other neuropsychiatric and cognitive symptoms. This may be due to the fact that AD patients rarely mention their hallucinations, especially in the advanced stages of the disease due to aphasia. In this review, we attempted to highlight several cognitive and clinical accounts and integrate them into a coherent framework. Our review can be summarized in the ALZHA model (see Figure 1), according to which hallucinations in AD are mainly observed in patients who exhibit trait markers, in whom subsequent state markers will induce hallucinations’ occurrences. By proposing this model, we do not attribute hallucinations to any of the reviewed factors in isolation (e.g., psychological distress, iatrogenic factors, memory suppression, neurological deficit, sensory deficits) but rather to the complex interaction between these factors. This consideration is important as, in light of the reviewed literature, it is very difficult to identify any single factor as a main contributor to hallucinations in AD. Generally speaking, this position fits with the general view that considers AD as a clinical syndrome that encompasses a spectrum of cognitive, psychiatric and neurological manifestations with variable presence of senile plaques and neurofibrillary tangles at different brain areas partly accounting for the phenotypic variability [87].
Figure 1.
According to the ALZHA model, hallucinatory experiences in Alzheimer’s disease mainly occur in patients with trait markers (i.e., difficulties in suppressing intrusive thoughts or memories, neurological deficits, genetic predisposition and/or sensory deficits), who experience, at a given moment, one or more state markers that will trigger the experience (e.g., psychological distress and/or iatrogenic factors).
Compared with other models on hallucinations, our model has the merit to incorporate a wide variety of neurological, cognitive, affective, and iatrogenic factors that can be involved in hallucinations. Another originality of the ALZHA model is the distinction between traits (i.e., cognitive deficits, neurological deficits, genetic predisposition and/or sensory deficits) that may predispose patients to hallucinations and states that may trigger these experiences. In our view, this distinction may explain why these experiences may occur in some AD patients but not in others, as well as why such experiences may occur only at given times during AD progression
5.1. Conclusion and future directions
Research studies in AD have so far addressed isolated aspects of hallucinations (e.g., neurological, cognitive or affective dimension). As suggested by the ALZHA model, we believe that future research should consider not only the multitude of mechanisms contributing to hallucinations in AD, but also interactions between these mechanisms and carefully differentiate between state and trait mechanisms. Regarding cognitive mechanisms, we proposed the suppression hypothesis, but we also believe that more empirical research is needed regarding the potential relationship between hallucinations and source monitoring. The relationship between hallucinations and source monitoring has been extensively studied in schizophrenia, it would be of great interest to test this association in AD, especially in the light of research suggesting impaired source monitoring in AD [88, 89]. In a similar vein, there is a lack of research on the relationship between hallucinations and visual perception in AD. Future research should assess whether visual hallucinations in AD involve the ventral ‘what’ and/or dorsal ‘where’ visual processing pathways, which support object recognition and spatial location, respectively. Several hypotheses can be tested, e.g., whether visual hallucinations in AD may reflect compromise in the ability to process visual stimuli, to integrate sensory information and prior expectations, and/or to generate correct interpretations of visual input. Finally, further research is also required to compare hallucinations in AD with those in other dementias (e.g. Lewy body dementia). This comparison could pave the way for testing the specificities of hallucination in AD and identify potential links with neuropathological hallmarks (e.g., the accumulation of amyloid-beta (Aβ) plaques or tau tangles).
Hallucinations are the most frequent psychotic symptom in Alzheimer’s disease (AD)
Hallucinations in AD are associated with neurological and genetic factors
They are also associated with cognitive, affective, and iatrogenic factors
Acknowledgments
Dr. El Haj and Pr. Antoine were supported by the LABEX (excellence laboratory, program investment for the future) DISTALZ (Development of Innovative Strategies for a Transdisciplinary approach to Alzheimer disease). This research was supported in part (DK) by the Intramural Research Program of the National Institute on Aging, NIH.
Bibliography
- 1.Allen P, Laroi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32:175–91. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Whalley HC, Simonotto E, Flett S, Marshall I, Ebmeier KP, Owens DGC, et al. fMRI correlates of state and trait effects in subjects at genetically enhanced risk of schizophrenia. Brain. 2004;127:478–90. doi: 10.1093/brain/awh070. [DOI] [PubMed] [Google Scholar]
- 3.Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73:535–41. doi: 10.1136/jnnp.73.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pang L. Hallucinations Experienced by Visually Impaired: Charles Bonnet Syndrome. Optom Vis Sci. 2016;93:1466–78. doi: 10.1097/OPX.0000000000000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ffytche DH. The hodology of hallucinations. Cortex. 2008;44:1067–83. doi: 10.1016/j.cortex.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Diederich NJ, Fenelon G, Stebbins G, Goetz CG. Hallucinations in Parkinson disease. Nat Rev Neurol. 2009;5:331–42. doi: 10.1038/nrneurol.2009.62. [DOI] [PubMed] [Google Scholar]
- 7.Fenelon G, Goetz CG, Karenberg A. Hallucinations in Parkinson disease in the prelevodopa era. Neurology. 2006;66:93–8. doi: 10.1212/01.wnl.0000191325.31068.c4. [DOI] [PubMed] [Google Scholar]
- 8.Lefebvre S, Baille G, Jardri R, Plomhause L, Szaffarczyk S, Defebvre L, et al. Hallucinations and conscious access to visual inputs in Parkinson’s disease. Sci Rep. 2016;6:36284. doi: 10.1038/srep36284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel SS, Attard A, Jacobsen P, Shergill S. Acetylcholinesterase Inhibitors (AChEI’s) for the treatment of visual hallucinations in schizophrenia: a review of the literature. BMC Psychiatry. 2010;10:69. doi: 10.1186/1471-244X-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn S, Gallinat J. Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr Bull. 2012;38:779–86. doi: 10.1093/schbul/sbq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters FA, Allen P, Aleman A, Fernyhough C, Woodward TS, Badcock JC, et al. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull. 2012;38:683–93. doi: 10.1093/schbul/sbs045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho R, Wu W. Mechanisms of auditory verbal hallucination in schizophrenia. Frontiers in psychiatry. 2013;4:155. doi: 10.3389/fpsyt.2013.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson S. Accounting for the phenomenology and varieties of auditory verbal hallucination within a predictive processing framework. Conscious Cogn. 2014;30:142–55. doi: 10.1016/j.concog.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laroi F, Sommer IE, Blom JD, Fernyhough C, Ffytche DH, Hugdahl K, et al. The characteristic features of auditory verbal hallucinations in clinical and nonclinical groups: state-of-the-art overview and future directions. Schizophr Bull. 2012;38:724–33. doi: 10.1093/schbul/sbs061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aleman A, Bocker KB, Hijman R, de Haan EH, Kahn RS. Cognitive basis of hallucinations in schizophrenia: role of top-down information processing. Schizophr Res. 2003;64:175–85. doi: 10.1016/s0920-9964(03)00060-4. [DOI] [PubMed] [Google Scholar]
- 16.Fovet T, Orlov N, Dyck M, Allen P, Mathiak K, Jardri R. Translating Neurocognitive Models of Auditory-Verbal Hallucinations into Therapy: Using Real-time fMRI-Neurofeedback to Treat Voices. Frontiers in psychiatry. 2016;7:103. doi: 10.3389/fpsyt.2016.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waters FA, Collerton D, Ffytche DH, Jardri R, Pins D, Dudley R, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull. 2014;40(Suppl 4):S233–45. doi: 10.1093/schbul/sbu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen P, Modinos G, Hubl D, Shields G, Cachia A, Jardri R, et al. Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr Bull. 2012;38:695–703. doi: 10.1093/schbul/sbs066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10:48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- 20.Looijestijn J, Blom JD, Aleman A, Hoek HW, Goekoop R. An integrated network model of psychotic symptoms. Neurosci Biobehav Rev. 2015;59:238–50. doi: 10.1016/j.neubiorev.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Bassiony MM, Lyketsos CG. Delusions and hallucinations in Alzheimer’s disease: review of the brain decade. Psychosomatics. 2003;44:388–401. doi: 10.1176/appi.psy.44.5.388. [DOI] [PubMed] [Google Scholar]
- 22.Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–8. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA. Hallucinations, delusions, and cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2000;69:172–7. doi: 10.1136/jnnp.69.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162:2022–30. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- 25.Jeste DV, Finkel SI. Psychosis of Alzheimer’s disease and related dementias. Diagnostic criteria for a distinct syndrome. Am J Geriatr Psychiatry. 2000;8:29–34. doi: 10.1097/00019442-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Deutsch LH, Bylsma FW, Rovner BW, Steele C, Folstein MF. Psychosis and physical aggression in probable Alzheimer’s disease. Am J Psychiatry. 1991;148:1159–63. doi: 10.1176/ajp.148.9.1159. [DOI] [PubMed] [Google Scholar]
- 27.Hope T, Keene J, Fairburn CG, Jacoby R, McShane R. Natural history of behavioural changes and psychiatric symptoms in Alzheimer’s disease. A longitudinal study. Br J Psychiatry. 1999;174:39–44. doi: 10.1192/bjp.174.1.39. [DOI] [PubMed] [Google Scholar]
- 28.Ferman TJ, Arvanitakis Z, Fujishiro H, Duara R, Parfitt F, Purdy M, et al. Pathology and temporal onset of visual hallucinations, misperceptions and family misidentification distinguishes dementia with Lewy bodies from Alzheimer’s disease. Parkinsonism Relat Disord. 2013;19:227–31. doi: 10.1016/j.parkreldis.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ting SK, Hao Y, Chia PS, Tan EK, Hameed S. Clinicopathological correlation of psychosis and brain vascular changes in Alzheimer’s disease. Sci Rep. 2016;6:20858. doi: 10.1038/srep20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer CE, Qian W, Schweizer TA, Millikin CP, Ismail Z, Smith EE, et al. Lewy Bodies, Vascular Risk Factors, and Subcortical Arteriosclerotic Leukoencephalopathy, but not Alzheimer Pathology, are Associated with Development of Psychosis in Alzheimer’s Disease. J Alzheimers Dis. 2016;50:283–95. doi: 10.3233/JAD-150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weamer EA, Emanuel JE, Varon D, Miyahara S, Wilkosz PA, Lopez OL, et al. The relationship of excess cognitive impairment in MCI and early Alzheimer’s disease to the subsequent emergence of psychosis. Int Psychogeriatr. 2009;21:78–85. doi: 10.1017/S1041610208007734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson RS, Krueger KR, Kamenetsky JM, Tang Y, Gilley DW, Bennett DA, et al. Hallucinations and mortality in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:984–90. doi: 10.1176/appi.ajgp.13.11.984. [DOI] [PubMed] [Google Scholar]
- 33.Jost BC, Grossberg GT. The evolution of psychiatric symptoms in Alzheimer’s disease: a natural history study. J Am Geriatr Soc. 1996;44:1078–81. doi: 10.1111/j.1532-5415.1996.tb02942.x. [DOI] [PubMed] [Google Scholar]
- 34.Devanand DP, Brockington CD, Moody BJ, Brown RP, Mayeux R, Endicott J, et al. Behavioral syndromes in Alzheimer’s disease. Int Psychogeriatr. 1992;4(Suppl 2):161–84. [PubMed] [Google Scholar]
- 35.Levy ML, Cummings JL, Fairbanks LA, Bravi D, Calvani M, Carta A. Longitudinal assessment of symptoms of depression, agitation, and psychosis in 181 patients with Alzheimer’s disease. Am J Psychiatry. 1996;153:1438–43. doi: 10.1176/ajp.153.11.1438. [DOI] [PubMed] [Google Scholar]
- 36.Lerner AJ, Koss E, Patterson MB, Ownby RL, Hedera P, Friedland RP, et al. Concomitants of visual hallucinations in Alzheimer’s disease. Neurology. 1994;44:523–7. doi: 10.1212/wnl.44.3_part_1.523. [DOI] [PubMed] [Google Scholar]
- 37.Aarsland D, Cummings JL, Yenner G, Miller B. Relationship of aggressive behavior to other neuropsychiatric symptoms in patients with Alzheimer’s disease. Am J Psychiatry. 1996;153:243–7. doi: 10.1176/ajp.153.2.243. [DOI] [PubMed] [Google Scholar]
- 38.Bassiony MM, Steinberg MS, Warren A, Rosenblatt A, Baker AS, Lyketsos CG. Delusions and hallucinations in Alzheimer’s disease: prevalence and clinical correlates. Int J Geriatr Psychiatry. 2000;15:99–107. doi: 10.1002/(sici)1099-1166(200002)15:2<99::aid-gps82>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Ornstein K, Gaugler JE, Devanand DP, Scarmeas N, Zhu C, Stern Y. The differential impact of unique behavioral and psychological symptoms for the dementia caregiver: how and why do patients’ individual symptom clusters impact caregiver depressive symptoms? Am J Geriatr Psychiatry. 2013;21:1277–86. doi: 10.1016/j.jagp.2013.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocca P, Leotta D, Liffredo C, Mingrone C, Sigaudo M, Capellero B, et al. Neuropsychiatric symptoms underlying caregiver stress and insight in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2010;30:57–63. doi: 10.1159/000315513. [DOI] [PubMed] [Google Scholar]
- 41.Jardri R, Cachia A, Thomas P, Pins D. The neuroscience of hallucinations. New York: Springer Science & Business Media; 2013. [Google Scholar]
- 42.Donovan NJ, Wadsworth LP, Lorius N, Locascio JJ, Rentz DM, Johnson KA, et al. Regional cortical thinning predicts worsening apathy and hallucinations across the Alzheimer disease spectrum. Am J Geriatr Psychiatry. 2014;22:1168–79. doi: 10.1016/j.jagp.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holroyd S, Shepherd MLJ, Hunter Downs I. Occipital Atrophy Is Associated With Visual Hallucinations in Alzheimer’s Disease. The Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:25–8. doi: 10.1176/jnp.12.1.25. [DOI] [PubMed] [Google Scholar]
- 44.Lin SH, Yu CY, Pai MC. The occipital white matter lesions in Alzheimer’s disease patients with visual hallucinations. Clin Imaging. 2006;30:388–93. doi: 10.1016/j.clinimag.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 45.Lopez OL, Smith G, Becker JT, Meltzer CC, DeKosky ST. The psychotic phenomenon in probable Alzheimer’s disease: a positron emission tomography study. J Neuropsychiatry Clin Neurosci. 2001;13:50–5. doi: 10.1176/jnp.13.1.50. [DOI] [PubMed] [Google Scholar]
- 46.Mega MS, Lee L, Dinov ID, Mishkin F, Toga AW, Cummings JL. Cerebral correlates of psychotic symptoms in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2000;69:167–71. doi: 10.1136/jnnp.69.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanc F, Noblet V, Philippi N, Cretin B, Foucher J, Armspach JP, et al. Right anterior insula: core region of hallucinations in cognitive neurodegenerative diseases. PLoS One. 2014;9:e114774. doi: 10.1371/journal.pone.0114774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeves SJ, Gould RL, Powell JF, Howard RJ. Origins of delusions in Alzheimer’s disease. Neurosci Biobehav Rev. 2012;36:2274–87. doi: 10.1016/j.neubiorev.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 49.El Haj M, Antoine P, Amouyel P, Lambert JC, Pasquier F, Kapogiannis D. Apolipoprotein E (APOE) epsilon4 and episodic memory decline in Alzheimer’s disease: A review. Ageing Res Rev. 2016;27:15–22. doi: 10.1016/j.arr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zdanys KF, Kleiman TG, MacAvoy MG, Black BT, Rightmer TE, Grey M, et al. Apolipoprotein E epsilon4 allele increases risk for psychotic symptoms in Alzheimer’s disease. Neuropsychopharmacology. 2007;32:171–9. doi: 10.1038/sj.npp.1301148. [DOI] [PubMed] [Google Scholar]
- 51.DeMichele-Sweet MA, Sweet RA. Genetics of Psychosis in Alzheimer Disease: A Review. Journal of Alzheimer’s disease: JAD. 2010;19:761–80. doi: 10.3233/JAD-2010-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hemsley DR. The development of a cognitive model of schizophrenia: placing it in context. Neurosci Biobehav Rev. 2005;29:977–88. doi: 10.1016/j.neubiorev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Jardri R, Hugdahl K, Hughes M, Brunelin J, Waters F, Alderson-Day B, et al. Are Hallucinations Due to an Imbalance Between Excitatory and Inhibitory Influences on the Brain? Schizophr Bull. 2016;42:1124–34. doi: 10.1093/schbul/sbw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schnider A, Ptak R. Spontaneous confabulators fail to suppress currently irrelevant memory traces. Nat Neurosci. 1999;2:677–81. doi: 10.1038/10236. [DOI] [PubMed] [Google Scholar]
- 55.Waters FA, Badcock JC, Maybery MT, Michie PT. Inhibition in schizophrenia: association with auditory hallucinations. Schizophr Res. 2003;62:275–80. doi: 10.1016/s0920-9964(02)00358-4. [DOI] [PubMed] [Google Scholar]
- 56.Badcock JC, Waters FA, Maybery M. On keeping (intrusive) thoughts to one’s self: testing a cognitive model of auditory hallucinations. Cogn Neuropsychiatry. 2007;12:78–89. doi: 10.1080/13546800600753120. [DOI] [PubMed] [Google Scholar]
- 57.Paulik G, Badcock JC, Maybery MT. Poor intentional inhibition in individuals predisposed to hallucinations. Cogn Neuropsychiatry. 2007;12:457–70. doi: 10.1080/13546800701394329. [DOI] [PubMed] [Google Scholar]
- 58.El Haj M, Laroi F, Gely-Nargeot MC, Raffard S. Inhibitory deterioration may contribute to hallucinations in Alzheimer’s disease. Cogn Neuropsychiatry. 2015;20:281–95. doi: 10.1080/13546805.2015.1023392. [DOI] [PubMed] [Google Scholar]
- 59.Bjork EL, Bjork RA. Continuing influences of to-be-forgotten information. Conscious Cogn. 1996;5:176–96. [PubMed] [Google Scholar]
- 60.Geiselman RE, Bjork RA, Fishman DL. Disrupted retrieval in directed forgetting: a link with posthypnotic amnesia. J Exp Psychol Gen. 1983;112:58–72. doi: 10.1037//0096-3445.112.1.58. [DOI] [PubMed] [Google Scholar]
- 61.Soriano MF, Jimenez JF, Roman P, Bajo MT. Intentional inhibition in memory and hallucinations: directed forgetting and updating. Neuropsychology. 2009;23:61–70. doi: 10.1037/a0013739. [DOI] [PubMed] [Google Scholar]
- 62.El Haj M. Memory suppression in Alzheimer’s disease. Neurol Sci. 2016;37:337–43. doi: 10.1007/s10072-015-2441-5. [DOI] [PubMed] [Google Scholar]
- 63.El Haj M, Postal V, Allain P. Destination memory in Alzheimer’s Disease: when I imagine telling Ronald Reagan about Paris. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49:82–9. doi: 10.1016/j.cortex.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 64.El Haj M, Fasotti L, Allain P. Source monitoring in Alzheimer’s disease. Brain Cogn. 2012;80:185–91. doi: 10.1016/j.bandc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 65.El Haj M, Postal V, Le Gall D, Allain P. Directed forgetting of autobiographical memory in mild Alzheimer’s disease. Memory. 2011;19:993–1003. doi: 10.1080/09658211.2011.626428. [DOI] [PubMed] [Google Scholar]
- 66.Waters FA, Badcock JC, Michie PT, Maybery MT. Auditory hallucinations in schizophrenia: intrusive thoughts and forgotten memories. Cogn Neuropsychiatry. 2006;11:65–83. doi: 10.1080/13546800444000191. [DOI] [PubMed] [Google Scholar]
- 67.El Haj M, Antoine P, Nandrino JL, Kapogiannis D. Autobiographical memory decline in Alzheimer’s disease, a theoretical and clinical overview. Ageing research reviews. 2015;23:183–92. doi: 10.1016/j.arr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fromm-Reichmann F. Loneliness. Psychiatry. 1959;22:1–15. doi: 10.1080/00332747.1959.11023153. [DOI] [PubMed] [Google Scholar]
- 69.Launay G, Slade P. The measurement of hallucinatory predisposition in male and female prisoners. Pers Individ Dif. 1981;2:221–34. [Google Scholar]
- 70.El Haj M, Jardri R, Laroi F, Antoine P. Hallucinations, loneliness, and social isolation in Alzheimer’s disease. Cogn Neuropsychiatry. 2016;21:1–13. doi: 10.1080/13546805.2015.1121139. [DOI] [PubMed] [Google Scholar]
- 71.Badcock JC, Shah S, Mackinnon A, Stain HJ, Galletly C, Jablensky A, et al. Loneliness in psychotic disorders and its association with cognitive function and symptom profile. Schizophr Res. 2015 doi: 10.1016/j.schres.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 72.Birchwood M, Chadwick P. The omnipotence of voices: testing the validity of a cognitive model. Psychol Med. 1997;27:1345–53. doi: 10.1017/s0033291797005552. [DOI] [PubMed] [Google Scholar]
- 73.Chaudhury S. Hallucinations: Clinical aspects and management. Ind Psychiatry J. 2010;19:5–12. doi: 10.4103/0972-6748.77625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bierman EJ, Comijs HC, Jonker C, Scheltens P, Beekman AT. The effect of anxiety and depression on decline of memory function in Alzheimer’s disease. Int Psychogeriatr. 2009;21:1142–7. doi: 10.1017/S1041610209990512. [DOI] [PubMed] [Google Scholar]
- 75.Burghaus L, Eggers C, Timmermann L, Fink GR, Diederich NJ. Hallucinations in neurodegenerative diseases. CNS Neurosci Ther. 2012;18:149–59. doi: 10.1111/j.1755-5949.2011.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Chazeron I, Pereira B, Chereau-Boudet I, Brousse G, Misdrahi D, Fénelon G, et al. Validation of a Psycho-Sensory hAllucinations Scale (PSAS) in schizophrenia and Parkinson’s disease. Schizophr Res. 2015;161:269–76. doi: 10.1016/j.schres.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 77.Llorca PM, Pereira B, Jardri R, Chereau-Boudet I, Brousse G, Misdrahi D, et al. Hallucinations in schizophrenia and Parkinson’s disease: an analysis of sensory modalities involved and the repercussion on patients. Sci Rep. 2016;6:38152. doi: 10.1038/srep38152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El Haj M, Kapogiannis D, Antoine P. Phenomenological Reliving and Visual Imagery During Autobiographical Recall in Alzheimer’s Disease. J Alzheimers Dis. 2016;52:421–31. doi: 10.3233/JAD-151122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chapman FM, Dickinson J, McKeith I, Ballard C. Association among visual hallucinations, visual acuity, and specific eye pathologies in Alzheimer’s disease: treatment implications. Am J Psychiatry. 1999;156:1983–5. doi: 10.1176/ajp.156.12.1983. [DOI] [PubMed] [Google Scholar]
- 80.Linszen MM, Brouwer RM, Heringa SM, Sommer IE. Increased risk of psychosis in patients with hearing impairment: Review and meta-analyses. Neurosci Biobehav Rev. 2016;62:1–20. doi: 10.1016/j.neubiorev.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 81.Reeve S, Sheaves B, Freeman D. The role of sleep dysfunction in the occurrence of delusions and hallucinations: A systematic review. Clin Psychol Rev. 2015;42:96–115. doi: 10.1016/j.cpr.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chadwick P, Birchwood M. The omnipotence of voices. II: The Beliefs About Voices Questionnaire (BAVQ) Br J Psychiatry. 1995;166:773–6. doi: 10.1192/bjp.166.6.773. [DOI] [PubMed] [Google Scholar]
- 83.Gaudiano BA, Herbert JD. Acute treatment of inpatients with psychotic symptoms using Acceptance and Commitment Therapy: pilot results. Behav Res Ther. 2006;44:415–37. doi: 10.1016/j.brat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 84.Larouche E, Hudon C, Goulet S. Potential benefits of mindfulness-based interventions in mild cognitive impairment and Alzheimer’s disease: an interdisciplinary perspective. Behav Brain Res. 2015;276:199–212. doi: 10.1016/j.bbr.2014.05.058. [DOI] [PubMed] [Google Scholar]
- 85.US Food and Drug Administration FDA public advisory. Controlled trial of risperidone for the treatment of aggression, deaths with antipsychotics in elderly patients with behavioral disturbances [Google Scholar]
- 86.Santé HAd. Maladie d’Alzheimer et maladies apparentées: prise en charge des troubles du comportement perturbateurs. 2009 [Google Scholar]
- 87.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–27. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 88.El Haj M, Kessels RPC. Context Memory in Alzheimer’s Disease. Dementia and Geriatric Cognitive Disorders EXTRA. 2013;3:342–50. doi: 10.1159/000354187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.El Haj M, Kessels RP, Allain P. Source Memory Rehabilitation: A Review Toward Recommendations for Setting Up a Strategy Training Aimed at the “What, Where, and When” of Episodic Retrieval. Appl Neuropsychol Adult. 2016;23:53–60. doi: 10.1080/23279095.2014.992071. [DOI] [PubMed] [Google Scholar]