Abstract
Objective
The enteric nervous system (ENS) undergoes neuronal loss and degenerative changes with age. The cause of this neurodegeneration is poorly understood. Muscularis macrophages (MMs) residing in close proximity to enteric ganglia maintain neuromuscular function via direct crosstalk with enteric neurons and have been implicated in the pathogenesis of gastrointestinal motility disorders like gastroparesis and post-operative ileus. The aim of this study was to assess whether aging causes alterations in macrophage phenotype that contributes to age-related degeneration of the ENS.
Design
Longitudinal muscle and myenteric plexus (LMMP) from small intestine of young, mid-aged and old mice was dissected and prepared for whole mount immunostaining, flow cytometry, Luminex immunoassays, western blot analysis, enteric neural stem cell (ENSC) isolation, or conditioned media. Bone marrow derived macrophages were prepared and polarized to classic (M1) or alternative (M2) activation states. Markers for macrophage phenotype were measured using quantitative RT-PCR.
Results
Aging causes a shift in macrophage polarization from anti-inflammatory ‘M2’ to pro-inflammatory ‘M1’ that is associated with a rise in cytokines and immune cells in the ENS. This phenotypic shift is associated with a neural response to inflammatory signals, increase in apoptosis and loss of enteric neurons and ENSCs, and delayed intestinal transit. An age-dependent decrease in expression of the transcription factor FoxO3, a known longevity gene, contributes to the loss of anti-inflammatory behavior in macrophages of old mice and FoxO3 deficient mice demonstrate signs of premature aging of the ENS.
Conclusion
A shift by macrophages towards a pro-inflammatory phenotype with aging causes inflammation-mediated degeneration of the ENS.
Introduction
Aging causes physiologic changes in gastrointestinal function that contribute to many disorders including constipation and fecal incontinence which cause significant physical, emotional, and financial burdens[1 2]. Age-dependent loss and degeneration of the enteric nervous system (ENS), the intrinsic nervous system of the gastrointestinal tract, likely plays a role in these disorders[1 3]. There is evidence that oxidative stress, alterations in neurotrophic factors, and calcium dysregulation may contribute, but the exact mechanism for these age-related changes remains poorly understood[3].
The muscular layer of the gut wall has been found to contain numerous tissue resident macrophages, termed muscularis macrophages (MMs), residing in close apposition to enteric ganglia[4–6]. Through direct crosstalk with enteric neurons, MMs are important for gastrointestinal neuromuscular function and their depletion results in prolongation of colonic transit times[6]. Macrophages have been classified as either M1 (classically activated) or M2 (alternatively activated) based on their role in inflammatory processes[7]. M1 macrophages produce pro-inflammatory mediators including cytokines and reactive oxygen and nitrogen species and function in host defense. In contrast, M2 macrophages play a role in dampening inflammation and promoting wound healing[7]. In the intestine, macrophage phenotype differs depending on their location, with macrophages in the lamina propria layer preferentially assuming a pro-inflammatory M1 phenotype while MMs displaying an anti-inflammatory M2 phenotype[4]. However, macrophages demonstrate tremendous plasticity and are capable of reversibly switching between M1 and M2 phenotypes[8]. Perturbation of the normal balance of M1/M2 macrophages appears to be an important factor in disease pathogenesis including cancer, atherosclerosis, autoimmune disease, osteoporosis and neurodegeneration[9]. Alterations in the phenotype of MMs have been implicated in gastrointestinal motility disorders including gastroparesis and post-operative ileus (POI)[10–14]. Loss of expression of mannose receptor C type 1 (CD206), an M2 marker, in MMs of Non-obese diabetic (NOD) mice has been found to correlate with development of gastric delay[11] suggesting that alterations in M1/M2 phenotype may contribute to gastrointestinal neuromuscular disorders. Whether aging causes changes in macrophage phenotype that may contribute to the degeneration of the ENS and age-related gastrointestinal disorders is unknown. Here we show that aging causes a shift in macrophage polarization from an anti-inflammatory ‘M2’ state to a pro-inflammatory ‘M1’ state. This shift is partly due to decreased expression of the transcription factor FoxO3, a gene linked to longevity, and accompanies inflammation-mediated loss of enteric neurons and enteric neural stem cells (ENSCs) and delayed intestinal transit.
Materials and Methods
Please see supplementary methods (available online only) for an expanded version of this section.
Animals
Male C57/BL6 mice of different age groups (3 months, 10–12 months, and 20–24 months) acquired from the National Institute of Aging Aged Rodent Colony, FoxO3−/− mice in FVB/N background[15], and Wnt1-cre;tdTomato mice[16] were used in experiments. All animal experiments were approved by Stanford University institutional animal care and use committees.
Tissue and cell preparation
Mice were anesthetized, euthanized, and laparotomy was performed. Small intestine was removed, lavaged, and longitudinal muscle with the adherent myenteric plexus (LMMP) was dissected as previously described[16]. Tissue was either freshly frozen in dry ice, fixed in paraformaldehyde (PFA), enzymatically digested with collagenase, or placed in media for ex vivo organotypic cultures. Tissue digestion was performed using protocol adapted from Joseph et al.[17] by sequentially incubating tissue in Digestion Buffer (consisting of M199 media (Invitrogen, Carlsbad CA), 0.1% BSA, 1mM CaCl2, 20 mM HEPES, and 150 μM P188) containing 1.1 mg/ml collagenase (Sigma-Aldrich) for 30 minutes at 37°C, followed by 10 U/ml papain (Worthington Biochemicals, Lakewood NJ) in Hank’s buffered salt solution (HBSS, Invitrogen) without calcium and magnesium, then quenched with ice-cold Digestion Buffer containing 50 U/ml DNAse I (Worthington). Tissue was mechanically dissociated by gentle trituration and filtered through a 40 μm nylon mesh cell strainer.
Flow cytometry
Dissociated cells were incubated with Zombie Aqua Viability (Biolegend, San Diego, CA) followed by blocking with 5% rat serum and mouse anti-CD16/CD32 (eBioscience, San Diego, CA) in HBSS containing 2% BCS. Samples were incubated for 30 minutes at 4°C with primary antibodies and isotype controls (supplemental table 1). After washing, cells were passed through a 40 μm nylon mesh cell strainer and sorting was performed directly into Trizol (Qiagen, Valencia CA) using a BD FACS Aria (BD Bioscience, San Jose CA). Gating strategy for sorting of macrophages is shown in supplemental figure 1. Analysis was performed using FlowJo Software (Tree Star Inc, Ashland OR).
RNA isolation and quantitative real-time PCR
RNA was extracted from cells with miRNeasy kit (Qiagen) and converted to cDNA using High-Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City CA). For sorted MMs, cDNA preamplification performed with Taqman PreAmp Master Mix kit (Applied Biosystems). Quantitative RT-PCR was performed on ABI StepOne Plus real time instrument using the Taqman expression assays listed in supplemental table 2. For analysis, each gene was normalized to the housekeeping gene GAPDH and fold change in gene expression between groups was calculated using the Pfaffl method.
Statistics
Data is expressed as mean +/− standard error of mean and was analyzed using Mann-Whitney, t-test, and one-way analysis of variance (ANOVA). Significance was deemed when p value was less than 0.05. Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software Inc, La Jolla CA).
Results
Muscularis macrophages demonstrate shift from anti-inflammatory to pro-inflammatory phenotype with aging
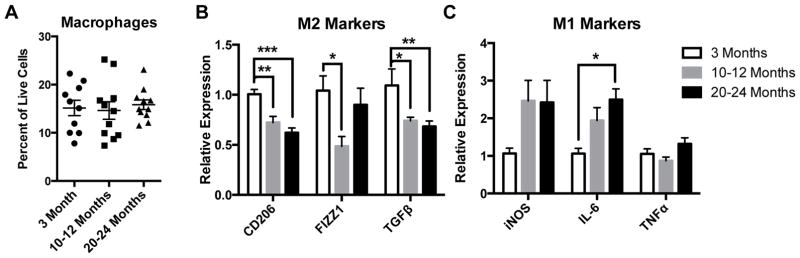
Consistent with a recent report by Gabanyi et al., which showed that macrophages in the muscularis preferentially express M2 markers compared to those in the lamina propria[4], we found the majority of MMs (in both young and old mice) to express the M2-marker CD206 by immunostaining (supplemental figure 1A). Within the muscle layers of the intestine, MMs were found to reside primarily in the myenteric plexus layer, surrounding enteric neurons (supplemental figure 1B). We performed flow cytometry on dissociated cells from LMMP of mice from 3 different age groups, young (3 month old), mid-aged (10–12 month old) and old (20–24 month old). Macrophages, defined as CD45+F4/80+ cells, were the predominant immune cell in the muscularis layer, comprising nearly 20% of total live cells and representing between 60–80% of all leukocytes or CD45+ cells (figure 1A and supplemental figure 1C). The numbers of MMs (as a percent of live cells) did not differ significantly with age (figure 1A). Next we sorted MMs (see supplemental figure 1D for gating scheme) and performed mRNA expression analysis of a variety of anti-inflammatory ‘M2’ markers including CD206, Found In Inflammatory Zone 1 (FIZZ1), and transforming growth factor β (TGFβ), and pro-inflammatory ‘M1’ markers including inducible nitric oxide synthetase (iNOS), interleukin-6 (IL-6), and tumor necrosis factor α (TNFα). MMs from mid-aged and old mice revealed reduced expression of M2 markers, CD206, FIZZ1 and TGFβ compared to young mice (figure 1B). Additionally, MMs expressed higher pro-inflammatory M1 markers with age, although only IL-6 from old mice reached statistical significance (figure 1C). Taken together, this suggests that aging is accompanied by a shift in macrophages from an anti-inflammatory to pro-inflammatory phenotype.
Figure 1. Muscularis macrophages demonstrate a shift from anti-inflammatory to proinflammatory state with aging.
On flow cytometry, MMs (CD45+F4/80+ cells) were found to be the predominant immune cell in the muscularis layer, comprising between 15–20% of all live cells, but their numbers did not differ significantly with age (A). MMs sorted from old and mid-aged mice revealed a decline in expression of M2 markers CD206, FIZZ1 and TGFβ (B), and a rise in expression of M1 markers, particularly IL-6, which reached statistical significance in old (C) compared to young mice by qRT-PCR. n≥10 for all groups, *p<.05, ***p<.001 by one-way ANOVA with Bonferroni’s multiple comparisons test.
Increased inflammation in ENS microenvironment with age
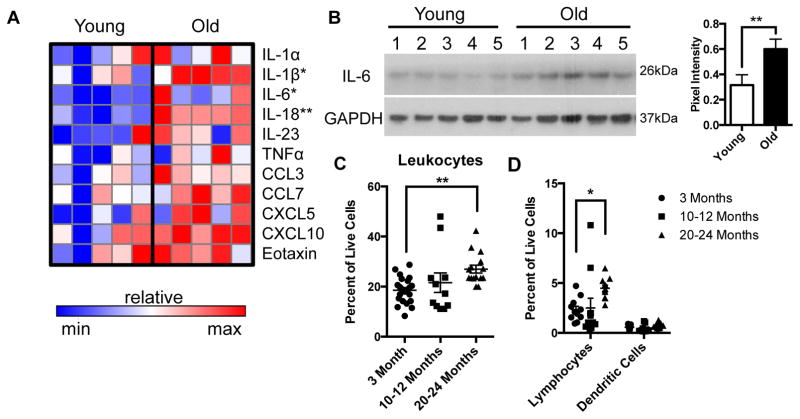
To determine whether this age-dependent shift in macrophage phenotype affects the inflammatory milieu of the ENS microenvironment, we performed Luminex immunoassays on protein extract of LMMP from young and old mice. Expression of pro-inflammatory cytokines and chemokines were increased overall in the ENS microenvironment from old mice with IL-6, IL-1β and IL-18 reaching statistical significance (figure 2A). Elevation of IL-6 was confirmed in a second cohort of young and old mice using Western Blot analysis (figure 2B). To evaluate whether alterations in immune cells accompanied these changes, we performed immunophenotyping of LMMP using flow cytometry. A statistically significant increase in leukocytes (CD45+ cells) was found in the ENS microenvironment of mid-aged and old compared to young mice (figure 2C). Although no difference was seen in the numbers of macrophages (figure 1A) and dendritic cells (CD45+CD11c+F4/80−) as a percent of live cells, a statistically significant rise in lymphocytes (CD45+CD3+CD19+) was found in old compared to young mice (figure 2D). A decline in macrophages and rise in lymphocytes as a percent of CD45+ cells was also observed in the old age group (supplemental figure 1C). In summary, we demonstrate that the age-associated shift in macrophages towards a M1 phenotype is associated with a rise in pro-inflammatory cytokines and chemokines and increased infiltration of lymphocytes in the ENS.
Figure 2. Increased inflammation in ENS microenvironment with age.
Heat map of cytokine and chemokine expression of LMMP lysate from young and old mice from Luminex analysis revealed higher pro-inflammatory cytokines and chemokines with age (A). Elevation of IL-6 was seen on a second cohort of young and old mice by Western blot, which was statistically significant based on pixel intensity normalized to GAPDH (B). Flow cytometry performed on enzymatically dissociated LMMP with gating of live, single cells revealed increased numbers of CD45+ leukocytes (as percent of live cells) in old mice compared to young (C). There was also a statistically significant rise in lymphocytes (CD3+CD19+) in old compared to young mice but no difference in dendritic cells (CD11c+ F4/80−) as a percent of live cells (D). n≥5 for each group; *p<.05, **p<.01 by Mann Whitney for (A); **p<.01 by t-test for (B); *p<.05, **p<.01 by one-way ANOVA with Bonferroni’s multiple comparisons test for (C) and (D).
Age-associated neural response to inflammatory signals is associated with increased apoptosis, loss of enteric neurons and delayed intestinal transit
In order to evaluate whether this age-dependent inflammation causes changes to the ENS, we evaluated whether Signal Transducer and Activator of Transcription 3 (STAT3), a downstream mediator of IL-6, IL-18 and other cytokines, was preferentially activated in enteric neurons of old mice. We found a 2-fold increase in percent of neurons expressing phosphorylated STAT3 (pSTAT3) in old compared to young mice, indicating increased STAT3 activity (figure 3A). An increased ratio of pSTAT3/STAT3 was also observed in old mice by western blot analysis of LMMP protein extract (supplemental figure 2A). We found a statistically significant increase in number of enteric neurons expressing cleaved caspase-3 (Asp175) (figure 3B and supplemental table 3). This perinuclear staining pattern of cleaved caspase-3 is similar to that observed in apoptotic neurons in the central nervous system[18] and was observed in enteric neurons that were also TUNEL+ (supplemental figure 2B). A reduction in the density of neurons within myenteric ganglia (figure 3C) was observed in old compared to young mice, suggesting neuronal loss with aging. This age-dependent reduction in neuronal density is similar to previous reports in human, rat and guinea pigs[3]. These neurodegenerative changes were associated with prolonged total intestinal transit time in old mice compared to young (figure 3D). These findings suggest that age-dependent changes in macrophage phenotype are associated with neural response to inflammatory signals and loss of enteric neurons.
Figure 3. Age-dependent changes to myenteric plexus are associated with delayed intestinal transit.
Immunostaining of wholemount preparations of LMMP labeled with pan-neuronal marker HuC/D (red) and pSTAT3 (green) revealed increased numbers of Hu+ neurons expressing pSTAT3 (arrows) in old compared with young mice (A). This difference expressed as a percent of neurons (pSTAT3+Hu+/total Hu+) was statistically significant. Increased STAT3 activity corresponded to an increase in number of Hu+ neurons (red) co-expressing the apoptosis marker cleaved caspase-3 (Asp175, green, arrows) in old mice compared to young (B). As a percent of neurons (Asp175+Hu+/total Hu+) this difference was statistically significant. Old mice also demonstrated a statistically significant reduction in neuronal density (Hu+ cells/ganglia area (100 μm2)) compared to young (C). Whole intestinal transit studies performed on a separate cohort of young and old mice revealed prolonged transit times in old mice (D). **p<.01, ***p<.001 by t-test. Scale bars, 50 μm.
Increased pro-inflammatory cytokines cause reduction in enteric neural stem cells with aging
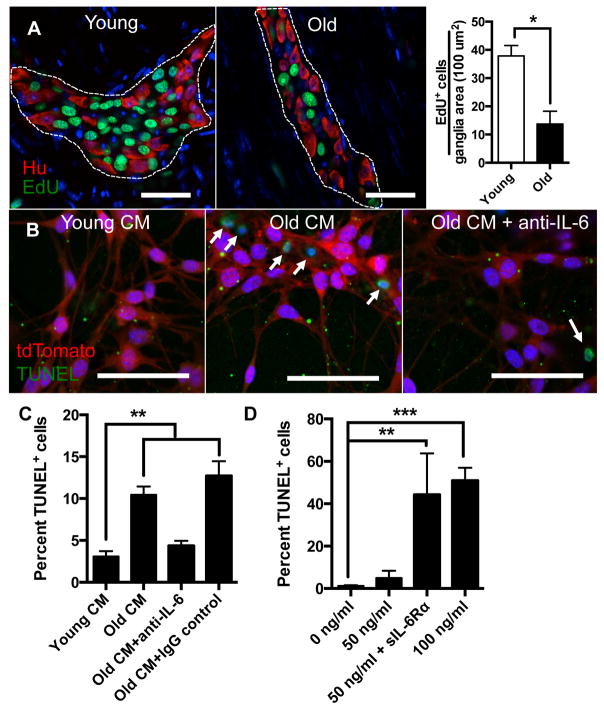
The ENS contains a resident population of multipotent stem cells termed enteric neural stem cells (ENSCs) capable of regeneration following injury[16 19 20]. In culture these stem cells, which can be identified by a variety of markers including p75, CD49b and nestin, can differentiate into neurons, glia and myofibroblasts[17]. We have previously shown that, similar to in vitro cultures, an ex vivo organotypic culturing system can be used to assess proliferation and differentiation of ENSCs. This ex vivo system, however, has the advantage of allowing their visualization within intact myenteric ganglia[16]. We cultured LMMP sections from young and old mice with the thymidine analog EdU for 40 hours to assess proliferation. We found a nearly 3-fold reduction in the number of EdU+ cells per myenteric ganglia area in old compared to young mice (figure 4A), suggesting a decline in number of ENSCs with age. These results are consistent with a prior report showing fewer cells expressing the ENSC marker p75 and decreased multipotent neurospheres isolated from guts of old mice compared to young[21]. Next we evaluated whether factors in the aged-ENS microenvironment directly impact survival of ENSCs. Using a Wnt1-cre;tdTomato mouse line in which neural crest-derived cells are fluorescently labeled[22], we enriched for ENSCs using CD49b[17] magnetic beads and cultured them in conditioned media prepared with LMMP from young (Young CM) or old mice (Old CM). Old CM caused a 2-fold increase in TUNEL+ ENSCs compared to Young CM (figure 4B and C). Luminex immunoassays revealed that Old CM contained 2-fold greater levels of IL-6 compared to Young CM (supplemental figure 3). Since IL-6 has been implicated in impaired survival of neural progenitor cells in the central nervous system[23 24], we explored whether this cytokine in particular was responsible for decreased survival of ENSCs. The addition of IL-6 neutralizing antibody (and not IgG control) reversed the pro-apoptotic effect of Old CM (figure 4B and C). Additionally, we found that recombinant murine IL-6 caused increased TUNEL-positivity when cultured with ENSCs but required over a 20-fold higher concentration (100 ng/ml) than found in Old CM (figure 4D and supplemental figure 3). Given that cells which do not express IL-6 receptor can still signal through IL-6 via soluble IL-6 receptor, termed trans-signaling[25], we wanted to evaluate whether this may explain the discrepancy. We found that adding soluble recombinant IL-6 receptor α (sIL-6Rα) caused increased TUNEL positivity at a lower concentration of IL-6 (50 ng/ml) (figure 4D) suggesting that IL-6 exerts its pro-apoptotic effect on ENSCs through trans-signaling.
Figure 4. Effect of aging and pro-inflammatory cytokines on enteric neural stem cells.
Using an ex vivo organotypic culturing system to measure proliferation, a nearly 3 fold reduction of EdU+ cells (green) were found per ganglia area in old compared to young mice (A). Using CD49b magnetic beads, ENSCs were isolated from a Wnt1-cre;tdTomato mouse (fluorescently labelled neural-crest cells) and cultured with conditioned media prepared with LMMP from young (Young CM) or old (Old CM) mice. Old CM was also cultured with either IL-6 neutralizing antibody or an IgG control. TUNEL assay revealed over 2-fold increase in numbers of TUNEL+ cells (green) when ENSCs (red) were cultured with Old CM compared to Young CM indicating increased apoptosis due to factors in the aged ENS microenvironment. This increase in TUNEL-positivity was reversed with the addition of IL-6 neutralizing antibody but not IgG control (B and C). When cultured with recombinant IL-6 at increasing concentrations, ENSCs demonstrated a statistically significant increase in TUNEL+ cells (D) at the highest concentration (100 ng/ml). The addition of soluble IL-6 Rα (200 ng/ml) augmented the number of TUNEL+ cells when added to IL-6 at a lower concentration (50 ng/ml). *p<.05 by t-test for (A); *p<.05, **p<.005 by one-way ANOVA with Bonferroni’s multiple comparisons test for (C) and (D). Scale bars, 50 μm.
Age-dependent loss of anti-inflammatory phenotype is in part intrinsic to macrophages
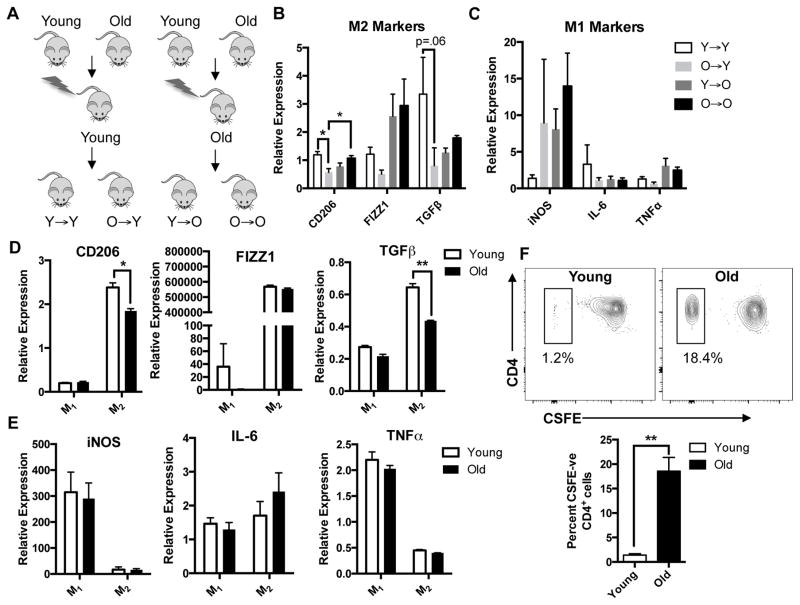
To assess whether the shift in phenotype is intrinsic to macrophages or related to extrinsic influences in aged-microenvironment, we generated congenic BM chimeras by transplanting BM from young or old mice into lethally irradiated young or old mice (figure 5A). This technique has been shown to be an effective way to deplete host MMs and replace them with macrophages derived from BM graft[13]. Similar to old mice, sorted MMs from O→Y revealed reduction in expression of M2 markers CD206 (p<0.05), FIZZ1 and TGFβ (p=0.06) compared to Y→Y mice (figure 5B), suggesting that cell intrinsic factors contribute to the age-dependent loss of suppressive phenotype. While not statistically significant we also found a trend towards increased leukocytes in O→Y compared with Y→Y (supplemental figure 4A). MMs from chimeras transplanted with old BM (O→O and O→Y) showed a trend towards increased iNOS expression suggesting a more pro-inflammatory phenotype (figure 5C). We also found a modest but statistically significant preservation of neuronal density in enteric ganglia from Y→O chimeras compared to O→O (supplemental figure 4B), supporting the possibility that young BM confers some protection against age-related neuronal loss.
Figure 5. Macrophages from old mice have intrinsic differences that make them less antiinflammatory.
Congeneic BM chimeras were generated by injecting BM harvested from young or old C57BL/6 mice into lethally irradiated young or old mice (A). MMs sorted from O→Y expressed decreased M2 markers compared to Y→Y suggesting that the age-dependent loss of anti-inflammatory phenotype is intrinsic to the macrophage (B). Although not statistically significant, mice transplanted with old BM (O→Y and O→O) had a trend towards increased expression of iNOS (C). Bone marrow derived macrophages (BMDMs) prepared from old mice and treated with IL-4 and IL-13 to polarize to M2 activation state (M2) exhibited reduced expression of M2 markers particularly CD206 and TGFβ (D). IFNγ-treated BMDMs (M1) from young and old mice did not demonstrate significant differences in expression of proinflammatory M1 markers (E). When incubated with CD4+ T cells activated with anti-CD3 and anti-CD28 and labeled with CSFE, IL-4/IL-13-treated BMDMs from old mice demonstrated reduced suppression of lymphocyte proliferation (higher percent of CSFE-negative CD4+ cells) compared to those from young (F). For (D) and (E) RNA expression was normalized to untreated (M0) macrophages from young mice. *p<.05 by one-way ANOVA with Bonferroni’s multiple comparisons test and t-test, **p<.01 by t-test. n≥5 for chimeric mouse groups.
To help confirm that aging causes intrinsic changes to macrophages, we prepared BM-derived macrophages (BMDMs) from young and old mice. BMDMs were treated with either interferon γ (IFNγ) or IL-4 and IL-13 to induce M1 or M2 polarization states respectively. When treated with IL-4 and IL-13, cells of old mice showed decreased anti-inflammatory M2 markers CD206 and TGFβ compared to young (figure 5D) by RT-PCR. There was no difference in expression of pro-inflammatory markers iNOS, IL-6 and TNFα in IFNγ-treated BMDMs from young and old mice (figure 5E). To assess whether this difference might be due to an age-related alteration in the ability to polarize, we performed flow cytometry on the BMDMs. The majority of CD45+ cells (>96%) from both young and old expressed the macrophage marker F4/80. Furthermore, we found similar expression of CD206 in BMDMs treated with either IL-4/IL-13 (supplemental figure 5A) or IFNγ (supplemental figure 5B) from young and old suggesting the differences we observed in mRNA gene expression were not due to altered polarization. To determine whether reduction in ‘M2’ markers corresponds to diminished anti-inflammatory properties, we performed a lymphocyte suppression assay. CD4+ T cells isolated from spleen of a young mouse were labeled with CSFE, activated with anti-CD3 and anti-CD28, then co-cultured with IL-4/IL-13-treated BMDMs from young or old mice for 72 hours. Co-culturing with BMDMs from young mice yielded dramatically fewer proliferated (CSFE-negative) lymphocytes (1.2% versus 18.4% of CD4+ cells) compared to old (figure 5F), indicating diminished suppressive behavior in macrophages from old mice. Taken together, these findings suggest that intrinsic changes in macrophages contribute to their loss of anti-inflammatory properties.
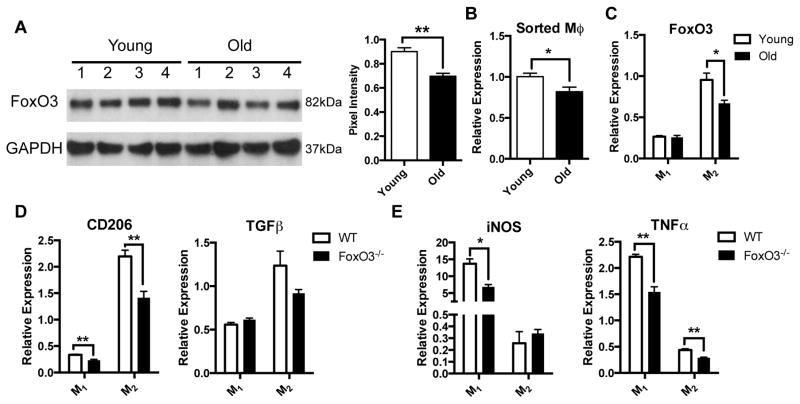
Reduction in FoxO3 expression is associated with decreased anti-inflammatory phenotype in macrophages
FoxO3, a member of the family of Forkhead transcription factors, is one of a few genes in which genetic variants have been consistently linked to longevity in humans[26]. In addition to regulating metabolism, apoptosis, autophagy, and stem cell homeostasis, FoxO3 plays a role in modulating the immune response[26]. FOXO3 genetic variants have been associated with more aggressive disease courses for patients with Crohn’s disease and rheumatoid arthritis, and FoxO3-deficient mice demonstrate an exaggerated inflammatory response to experimental colitis[27]. Since FoxO3 appears to regulate the immune response through myeloid cells[27 28], we evaluated whether alterations in its expression may account for age-related changes to MMs. We found a 20% reduction in FoxO3 expression in LMMP from old compared to young mice (figure 6A), which is similar to the reduction seen in skeletal muscle of old rats[29]. This age-dependent reduction was also seen in sorted MMs (figure 6B) and BMDMs by both qRT-PCR (figure 6C) and western blot analysis (supplemental figure 5C). To determine whether this reduction in FoxO3 expression may account for changes in macrophage phenotype, we generated BMDMs from FoxO3−/− and WT mice. As seen in old mice, BMDMs from FoxO3−/− mice treated with IL-4 and IL-13 demonstrated reduced expression of CD206 and TGFβ compared to WT (figure 6D). IFNγ-treated BMDMs from FoxO3−/− mice demonstrated decreased expression of pro-inflammatory markers (figure 6E). Despite this decline in M1 markers, we found that administration of LPS to similarly IFNγ-treated BMDMs caused increased elevation of pro-inflammatory markers relative to WT suggesting a more pro-inflammatory phenotype (supplemental figure 5D). These results suggest that an age-dependent reduction of FoxO3 accounts for the loss of suppressive phenotype of macrophages with aging.
Figure 6. Reduction of FoxO3 expression with aging contributes to decrease in anti-inflammatory phenotype.
Western blot revealed a modest but statistically significant reduction of FoxO3 expression in protein extract of LMMP from old compared to young mice based on pixel intensity normalized to GAPDH (A). A decline in FoxO3 expression was also found in sorted MMs (B) and IL-4/IL-13-treated BMDMs (C) from old mice. To evaluate whether decreased FoxO3 expression might be responsible for alterations in macrophage phenotype, BMDMs were derived from FoxO3−/− and WT mice and treated with either IFNγ (M1) or IL-4 and IL-13 (M2). M2 BMDMs from FoxO3−/− mice demonstrated reduced expression of M2 markers particularly CD206 compared to WT (D). Decreased expression of pro-inflammatory markers iNOS and TNFα was observed in M1 BMDMs from FoxO3−/− mice (E) although higher expression of M1 markers was seen following LPS stimulation (see supplemental figure 5D). For (C–E) RNA expression was normalized to untreated (M0) macrophages from WT mice. *p<.05, **p<.01 by t-test.
FoxO3 deficiency mirrors age-associated neural activation, apoptosis and enteric neuronal loss
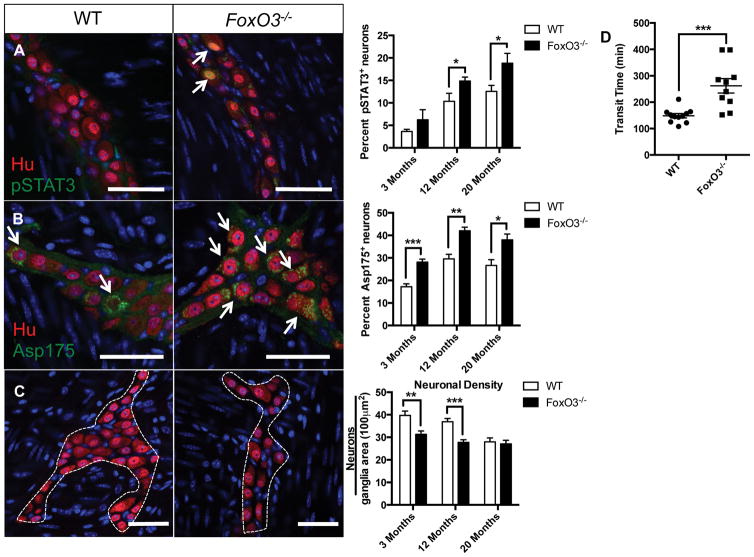
Similar to C57/BL6 mice, we found an age-dependent increase in the numbers of enteric neurons immunoreactive for pSTAT3 in the FVB mouse strain (p<.01 by two-way ANOVA). FoxO3 deficiency caused a statistically significant increase in the percent of pSTAT3+ neurons compared to WT in the mid and old age groups (figure 7A). We also found a statistically significant increase in numbers of enteric neurons expressing the apoptosis marker Asp 175 in FoxO3−/− mice compared to WT in all age groups (figure 7B and supplemental table 3). These findings corresponded to a decrease in neuronal density in FoxO3−/− mice compared to WT in young and mid-aged groups but not old (figure 7C). On a separate cohort of male mice between 14–17 months of age, FoxO3−/− mice demonstrated prolonged total intestinal transit times compared to age-matched controls (figure 7D). In summary, these findings suggest that FoxO3 deficiency mimics a premature aging phenotype in the ENS.
Figure 7. Degenerative changes to myenteric plexus and delayed intestinal transit in FoxO3−/− mice.
Immunostaining of LMMP from FoxO3−/− and WT FVB mice from 3 age groups (3 months, 12 months, and 20 months) with pan-neuronal marker HuC/D (red) and pSTAT3 (green) revealed increased numbers of Hu+ neurons expressing pSTAT3 (arrows) in FoxO3−/− compared to WT mice in the mid and old age groups (A). Expressed as percent of neurons (pSTAT3+Hu+/total Hu+), this difference was statistically significant for the mid and old age groups. An increased number of Hu+ neurons (red) co-expressing cleaved caspase-3 (Asp175, green, arrow) was also found in FoxO3−/− compared to WT mice and was statistically significant in all age groups (B). A statistically significant decline in neuronal density (Hu+ cells/ganglia area (100 μm2)) in was observed in FoxO3−/− compared to WT mice was observed in the 3 month and 12 month age groups but not the 20 month group (C). Prolonged intestinal transit times were found in FoxO3−/− mice compared to WT (performed on a separate cohort of male mice between the age of 14–17 months). Representative images are from 12-month age group. *p<.05, **p<.01, and ***p<.001 by multiple t-test. Scale bars, 50 μm.
Discussion
A growing body of evidence suggests that MMs play an important role in maintenance of gastrointestinal neuromuscular function and that perturbations in their phenotype may underlie motility disorders[4 6 10–14]. Here we show that MMs are the predominant immune cell within the muscularis layer of intestine, comprising between 60–80% of all leukocytes. Although aging does not affect their abundance, it causes a shift in macrophage polarization from anti-inflammatory M2 to pro-inflammatory M1 (figure 1). This age-dependent shift in macrophage phenotype parallels inflammatory changes in the ENS microenvironment including elevated pro-inflammatory cytokines and infiltration of immune cells particularly lymphocytes (figure 2), and functional delay in intestinal transit (figure 3D). Our findings mirror those seen following acute injury using animal models for POI where macrophages have been shown to be responsible for driving inflammation and disrupting gastrointestinal neuromuscular function[13 14]. Notably, chronic low-grade inflammation, termed ‘inflammaging’, has been observed in other tissues and organs with aging[30] and macrophages have been speculated to play a critical role[31]. In addition to macrophages, other cells may also contribute to the observed inflammation. We noted an increase in the numbers of lymphocytes with age (figure 2D). While this may be secondary to pro-inflammatory factors released by macrophages, intrinsic age-dependent alterations in lymphocyte populations, particularly regulatory and effector T cells, may contribute. Additionally, non-immune cells particularly enteric glia and senescent cells may have a role in the inflammation. Enteric glia have been implicated in production of IL-6 and MCP-1 following injury[32]. Senescent cells, which have been detected in myenteric ganglia of old mice[33], can secrete pro-inflammatory cytokines and chemokines termed senescence-associated secretory phenotype[34]. Further experiments aimed at elucidating the contributions of various cell types on age-dependent inflammation are clearly needed.
Our analysis was restricted to the muscularis layer of the small intestine, but we believe our findings are generalizable to other regions of the gut including the colon and submucosal layer. While local embryonic precursor cells of yolk sac origin maintain some macrophage populations, mucosal macrophages from intestine have been shown to derive from circulating monocytes from bone marrow[35]. Given that we observed a loss of suppressive behavior in BMDMs from old mice (figure 5E), it is likely that macrophages from other regions of the gut derived from circulating monocytes will have a similar phenotype. Nonetheless, future experiments definitively showing that MMs from adult mice are derived from circulating monocytes are clearly warranted.
While the pathogenesis of gastrointestinal neuromuscular disorders including gastroparesis, functional dyspepsia and irritable bowel syndrome remains poorly understood, inflammation appears to play a role[36–38]. In animal models of colitis, inflammation has been shown to cause a loss of myenteric neurons[39 40]. Our findings support a role for inflammation in the pathogenesis of age-related gastrointestinal disorders such as constipation. We demonstrate that inflammation in the ENS microenvironment of old mice correlates with a neural response (increased STAT3 activity in myenteric neurons), neuronal loss, and delayed intestinal transit (figure 3). These findings suggest that immunotherapies may have a role in the treatment of age-related gastrointestinal disorders. Future studies that replicate our findings in humans and manipulate the immune response to dampen age-dependent inflammation are likely to determine the potential of such therapies.
A decline in ENSCs has previously been noted in old mice[21] and our finding of reduced EdU uptake in ganglia of old mice seems to support this (figure 4A). We show that factors released from the aged ENS microenvironment, particularly IL-6, causes increased apoptosis of ENSCs (figure 4B–D). While ENSCs remain quiescent in adult mice at steady state[16 17 19], they appear capable of neurogenesis after injury[19]. Thus it suggests that elderly individuals may have more lasting damage to their ENS following acute insults such as gastroenteritis or toxin ingestion than young.
Our findings suggest that age-intrinsic differences in macrophages play a role in the observed shift in macrophage phenotype. Despite an inherent limitation in our chimera experiment, the use of congenic mice which prevented us from determining efficiency of tissue engraftment, we observed a reduction in M2 markers in O→Y compared to Y→Y (figure 5B), suggesting both successful engraftment and intrinsic differences in macrophage phenotype. Similar age-dependent reduction of M2 markers (FIZZ1, Arg1, and SOC1) has been observed in IL-4-treated macrophages derived from human peripheral blood monocytes and mouse adherent splenocytes[41 42], arguing that this difference is common to humans and other populations of macrophages. It is notable that despite the loss of anti-inflammatory phenotype, MMs from O→Y did not demonstrate a significant rise in pro-inflammatory M1 markers compared to Y→Y (figure 5C). Additionally, M1-polarized BMDMs from old mice did not demonstrate increased expression of pro-inflammatory markers compared to young either (figure 5F). This suggests that while macrophages from old mice may have less suppressive properties, extrinsic factors in the aged microenvironment are likely responsible for their assuming a pro-inflammatory phenotype. A recent study found a preference for M2 phenotype in mice deficient of IL-18 receptor[43], thus it is possible that the elevation of IL-18 we observed in the aged-microenvironment by Luminex (figure 2A) may be one such extrinsic factors that promotes a pro-inflammatory phenotype.
Our findings implicate a role for FoxO3 in the intrinsic differences observed in macrophages with age. Although one explanation for the lower M1 and M2 markers seen in IFNγ and IL-4/IL-13-treated BMDMs from FoxO3−/− mice (figure 6D and 6E) is a diminished ability to polarize, we do not believe this to be the case. We found substantial differences in particular M1 and M2 markers (over 5-fold elevation of iNOS for IFNγ-treated FoxO3−/− BMDMs and >100,000-fold elevation of FIZZ1 for IL-4/IL-13-treated FoxO3−/− BMDMs, data not shown) compared to untreated ‘M0’ BMDMs from both WT and FoxO3−/− suggesting no lack in ability to polarize. Therefore, our findings suggest that FoxO3 is necessary for maintaining suppressive and homeostatic properties of alternatively activated macrophages (figure 6D). A similar immune modulatory role for FoxO3 has been observed in human monocytes. A similar immune modulatory role for FoxO3 has been observed in human monocytes. A genetic variant of FOXO3 (rs12212067) that is associated with more aggressive Crohn’s disease and rheumatoid arthritis was found to result in decreased expression of FoxO3 and increased production of pro-inflammatory cytokines in human monocytes following stimulation with LPS[27]. While this genetic variant has not been linked to longevity, others found in close proximity have been[26]. While the mechanism by which these FOXO3 genetic variants extend life is unclear, our results suggest that these polymorphisms may maintain higher expression of FoxO3 in macrophages with age thus dampening inflammation.
FoxO3 is expressed in a variety of tissues and cells and regulates diverse biologic processes including metabolism, autophagy, apoptosis and stem cell homeostasis[26]. Conditional deletion of FoxO3 in neural stem cells causes their premature depletion within the central nervous system with aging[15 44]. The importance of FoxO3 is highlighted by the resultant delay in intestinal transit (figure 7D). We believe this is due to the role of FoxO3 in immune modulation based on the increased STAT3 activity and neuronal loss observed in the ENS of FoxO3−/− mice (figure 7A–C); however, we cannot exclude the possibility that the effect of FoxO3 deficiency on non-immune cells like neurons and glia may be involved. Thus, further studies including BM chimeras and cell-type-specific knockouts will be necessary to further elucidate the relative importance of FoxO3 deficiency in different cell types on the ENS. It is unclear why we did not observe a difference in neuronal density in FoxO3−/− and WT mice from the old age group but a possible explanation is selection bias given that only 26% of FoxO3−/− mice survived to 20 months of age (data not shown).
Based on the findings of our study, we propose a model for age-dependent motility disorders (supplemental figure 6). An age-related decline in FoxO3 expression in MMs causes loss of their suppressive properties and results in a low-grade chronic inflammatory state. With time, this pro-inflammatory state results in tissue damage, a loss of regenerative capacity, and ultimately a decline in neuromuscular function.
Supplementary Material
Immunostaining of LMMP with the macrophage markers Mac-1 (CD11b, red) and CD206 (green) revealed the majority of MMs (arrows) to co-express both markers (A). While the representative image is from a young mouse, this finding was also observed in old mice. CD206-expressing MMs (green) were observed surrounding Hu+ neurons (red) in the myenteric plexus layer but not in the circular muscle or longitudinal muscle layers (B). Expressed as percent of CD45+ cells, a statistically significant rise in lymphocytes and fall in macrophages was found in LMMP from old mice compared to young (C). There was no difference in dendritic cells between age groups. For sorting of MMs, sequential gating was performed on single cell suspensions of LMMP for live cells (based on exclusion of Zombie Aqua uptake) then immunoreactivity to CD45 before sorting F4/80+ cells (D). *p<.05, **p<.01 by one-way ANOVA with Bonferroni’s multiple comparisons test. Scale bars, 50 μm.
Elevated expression of pSTAT3 relative to STAT3 was observed in LMMP lysate from old mice compared with young by western blot (A). Based on pixel intensity, this difference in pSTAT3/STAT3 ratio was statistically significant. TUNEL assay was performed on whole mount LMMP followed by immunostaining for cleaved caspase-3 (Asp175, green) and Hu (blue) (B). TUNEL+ neurons (red) were observed to be immunoreactive for Asp 175 (arrows). *p<.05 by t-test. Scale bars, 50 μm.
Luminex immunoassays performed on conditioned media revealed levels of IL-6 to be nearly 2- fold greater in Old CM compared to Young CM.
A trend towards increased CD45+ leukocytes (as a percent of live cells) was found in the ENS of young mice transplanted with old BM (O→Y) compared to young BM (Y→Y) (A). There was a modest but statistically significant increase in neuronal density found in the myenteric ganglia of Y→O compared to O→O (B). *p<.05 by one-way ANOVA with Bonferroni’s multiple comparisons test and t-test.
Flow cytometry performed on BMDMs from young or old mice treated with either IL-4 and IL-13 (A) or IFNγ (B) revealed similar scatter plots based on F4/80 and CD206 expression. An age dependent reduction of FoxO3 expression was detected in IFNγ-treated (M1) and IL-4/IL-13- treated (M2) BMDMs by Western blot with pixel intensity normalized to GAPDH represented as bar chart on right (C). IFNγ-treated (M1) BMDMs from FoxO3−/− mice stimulated with LPS (100 ng/ml) for 4 hours demonstrate increased expression of pro-inflammatory markers relative to their unstimulated state, particularly for IL-6, when compared to WT (D). ***p<.001 by t-test.
An age-dependent decline of FoxO3 in MMs results in loss of suppressive phenotype. MMs assume a pro-inflammatory state resulting in inflammation-induced loss of enteric neurons and ENSCs, and disruption of neuromuscular function.
Summary Box.
What is already known about this subject?
Direct cross-talk between muscularis macrophages and enteric neurons controls gastrointestinal neuromuscular function.
Muscularis macrophages have been implicated in disease pathogenesis of motility disorders including gastroparesis and post-operative ileus.
Aging is associated with neurodegenerative changes to the ENS and impairment of neuromuscular function but the mechanism remains unclear.
What are the new findings?
A shift in macrophage polarization from anti-inflammatory ‘M2’ to pro-inflammatory ‘M1’ states occurs in muscularis macrophages with aging and is associated with chronic low-grade inflammation in the ENS microenvironment and delayed transit.
Intrinsic changes in macrophages, particularly age-dependent decrease in expression of the transcription factor FoxO3, account for some of the alterations in macrophage phenotype with aging.
How might it impact on clinical practices in the foreseeable future?
Our findings indicate that ‘inflammaging’, a chronic low-grade inflammatory state associated with aging, may contribute to age-dependent degeneration of the ENS and age-associated motility disorders.
This may lead to novel immunotherapies to treat common disorders like constipation and fecal incontinence.
Acknowledgments
Grant Support: Supported by NIH grants AG045098 (LB), DK103966 (LB) and AG049622 (AH).
We thank Jing Xue, Marisol Chang, Vishal Sharma, and Sidhartha Sinha for technical assistance. We thank Anne Brunet for providing resources to conduct experiments. We thank Vanda Lennon (Mayo Clinic, Rochester) for providing the HuC/D antibody.
Abbreviations
- ENS
enteric nervous system
- MM
muscularis macrophage
- LMMP
longitudinal muscle and myenteric plexus
- ENSC
enteric neural stem cell
- POI
post-operative ileus
- BMDMs
bone marrow derived macrophages
- FIZZ1
Found In Inflammatory Zone 1
- TGFβ
transforming growth factor β
- iNOS
inducible nitric oxide synthetase
- IL-6
interleukin-6
- TNFα
tumor necrosis factor α
- STAT3
Signal Transducer and Activator of Transcription 3
- Young CM
conditioned media prepared with LMMP from young mice
- Old CM
conditioned media prepared with LMMP from old mice
Footnotes
Competing Interest Statement
The authors wish to declare no competing interests.
References
- 1.Camilleri M, Cowen T, Koch TR. Enteric neurodegeneration in ageing. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2008;20(3):185–96. doi: 10.1111/j.1365-2982.2007.01072.x. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 2.Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102(3):895–901. doi: 10.1016/0016-5085(92)90175-x. S0016508592001203 [pii][published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 3.Saffrey MJ. Cellular changes in the enteric nervous system during ageing. Developmental biology. 2013;382(1):344–55. doi: 10.1016/j.ydbio.2013.03.015. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 4.Gabanyi I, Muller PA, Feighery L, et al. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell. 2016;164(3):378–91. doi: 10.1016/j.cell.2015.12.023. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikkelsen HB. Macrophages in the external muscle layers of mammalian intestines. Histology and histopathology. 1995;10(3):719–36. [PubMed] [Google Scholar]
- 6.Muller PA, Koscso B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158(2):300–13. doi: 10.1016/j.cell.2014.04.050. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3(1):23–35. doi: 10.1038/nri978. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 8.Porcheray F, Viaud S, Rimaniol AC, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clinical and experimental immunology. 2005;142(3):481–9. doi: 10.1111/j.1365-2249.2005.02934.x. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55. doi: 10.1038/nature12034. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard CE, Gibbons SJ, Mann IS, et al. Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2014;26(9):1275–84. doi: 10.1111/nmo.12389. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138(7):2399–409. 409 e1. doi: 10.1053/j.gastro.2010.02.014. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cipriani G, Gibbons SJ, Verhulst PJ, et al. Diabetic Csf1op/op mice lacking macrophages are protected against the development of delayed gastric emptying. Cellular and molecular gastroenterology and hepatology. 2016;2(1):40–47. doi: 10.1016/j.jcmgh.2015.09.001. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matteoli G, Gomez-Pinilla PJ, Nemethova A, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63(6):938–48. doi: 10.1136/gutjnl-2013-304676. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 14.Wehner S, Behrendt FF, Lyutenski BN, et al. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. 2007;56(2):176–85. doi: 10.1136/gut.2005.089615. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renault VM, Rafalski VA, Morgan AA, et al. FoxO3 regulates neural stem cell homeostasis. Cell stem cell. 2009;5(5):527–39. doi: 10.1016/j.stem.2009.09.014. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker L, Peterson J, Kulkarni S, et al. Ex vivo neurogenesis within enteric ganglia occurs in a PTEN dependent manner. PloS one. 2013;8(3):e59452. doi: 10.1371/journal.pone.0059452. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph NM, He S, Quintana E, et al. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. The Journal of clinical investigation. 2011 doi: 10.1172/JCI58186. 58186 [pii] published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang JQ, Shen W, Zhou C, et al. The human epilepsy mutation GABRG2(Q390X) causes chronic subunit accumulation and neurodegeneration. Nature neuroscience. 2015;18(7):988–96. doi: 10.1038/nn.4024. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laranjeira C, Sandgren K, Kessaris N, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. The Journal of clinical investigation. 2011 doi: 10.1172/JCI58200. 58200 [pii] published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metzger M. Neurogenesis in the enteric nervous system. Arch Ital Biol. 2010;148(2):73– 83. [PubMed] [Google Scholar]
- 21.Molofsky AV, Slutsky SG, Joseph NM, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443(7110):448–52. doi: 10.1038/nature05091. nature05091 [pii] published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker L, Kulkarni S, Tiwari G, et al. Divergent fate and origin of neurosphere-like bodies from different layers of the gut. American journal of physiology Gastrointestinal and liver physiology. 2012;302(9):G958–65. doi: 10.1152/ajpgi.00511.2011. ajpgi.00511.2011 [pii] published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–5. doi: 10.1126/science.1088417. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 24.Vallieres L, Campbell IL, Gage FH, et al. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(2):486–92. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Neuhofer P, Song L, et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. The Journal of clinical investigation. 2013;123(3):1019–31. doi: 10.1172/JCI64931. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris BJ, Willcox DC, Donlon TA, et al. FOXO3: A Major Gene for Human Longevity - A Mini-Review. Gerontology. 2015;61(6):515–25. doi: 10.1159/000375235. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JC, Espeli M, Anderson CA, et al. Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway. Cell. 2013;155(1):57–69. doi: 10.1016/j.cell.2013.08.034. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dejean AS, Beisner DR, Ch’en IL, et al. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nature immunology. 2009;10(5):504–13. doi: 10.1038/ni.1729. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furuyama T, Yamashita H, Kitayama K, et al. Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microscopy research and technique. 2002;59(4):331–4. doi: 10.1002/jemt.10213. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 30.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of ageing and development. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 31.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 32.Stoffels B, Hupa KJ, Snoek SA, et al. Postoperative Ileus Involves Interleukin-1 Receptor Signaling in Enteric Glia. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.09.030. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 33.Jurk D, Wang C, Miwa S, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging cell. 2012;11(6):996–1004. doi: 10.1111/j.1474-9726.2012.00870.x. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodier F, Campisi J. Four faces of cellular senescence. The Journal of cell biology. 2011;192(4):547–56. doi: 10.1083/jcb.201009094. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bain CC, Bravo-Blas A, Scott CL, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nature immunology. 2014;15(10):929–37. doi: 10.1038/ni.2967. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140(5):1575–85. e8. doi: 10.1053/j.gastro.2011.01.046. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nature reviews Gastroenterology & hepatology. 2010;7(3):163–73. doi: 10.1038/nrgastro.2010.4. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 38.Vanheel H, Farre R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nature reviews Gastroenterology & hepatology. 2013;10(3):142–9. doi: 10.1038/nrgastro.2012.255. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 39.Gulbransen BD, Bashashati M, Hirota SA, et al. Activation of neuronal P2X7 receptor- pannexin-1 mediates death of enteric neurons during colitis. Nature medicine. 2012;18(4):600–4. doi: 10.1038/nm.2679. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linden DR, Couvrette JM, Ciolino A, et al. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2005;17(5):751–60. doi: 10.1111/j.1365-2982.2005.00703.x. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 41.Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2012;32(1):18–26. doi: 10.1089/jir.2011.0058. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suchy D, Labuzek K, Buldak L, et al. Comparison of chosen activation markers of human monocytes/macrophages isolated from the peripheral blood of young and elderly volunteers. Pharmacological reports: PR. 2014;66(5):759–65. doi: 10.1016/j.pharep.2014.04.008. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 43.Pazos P, Lima L, Tovar S, et al. Divergent responses to thermogenic stimuli in BAT and subcutaneous adipose tissue from interleukin 18 and interleukin 18 receptor 1-deficient mice. Scientific reports. 2015;5:17977. doi: 10.1038/srep17977. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paik JH, Ding Z, Narurkar R, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell stem cell. 2009;5(5):540–53. doi: 10.1016/j.stem.2009.09.013. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunostaining of LMMP with the macrophage markers Mac-1 (CD11b, red) and CD206 (green) revealed the majority of MMs (arrows) to co-express both markers (A). While the representative image is from a young mouse, this finding was also observed in old mice. CD206-expressing MMs (green) were observed surrounding Hu+ neurons (red) in the myenteric plexus layer but not in the circular muscle or longitudinal muscle layers (B). Expressed as percent of CD45+ cells, a statistically significant rise in lymphocytes and fall in macrophages was found in LMMP from old mice compared to young (C). There was no difference in dendritic cells between age groups. For sorting of MMs, sequential gating was performed on single cell suspensions of LMMP for live cells (based on exclusion of Zombie Aqua uptake) then immunoreactivity to CD45 before sorting F4/80+ cells (D). *p<.05, **p<.01 by one-way ANOVA with Bonferroni’s multiple comparisons test. Scale bars, 50 μm.
Elevated expression of pSTAT3 relative to STAT3 was observed in LMMP lysate from old mice compared with young by western blot (A). Based on pixel intensity, this difference in pSTAT3/STAT3 ratio was statistically significant. TUNEL assay was performed on whole mount LMMP followed by immunostaining for cleaved caspase-3 (Asp175, green) and Hu (blue) (B). TUNEL+ neurons (red) were observed to be immunoreactive for Asp 175 (arrows). *p<.05 by t-test. Scale bars, 50 μm.
Luminex immunoassays performed on conditioned media revealed levels of IL-6 to be nearly 2- fold greater in Old CM compared to Young CM.
A trend towards increased CD45+ leukocytes (as a percent of live cells) was found in the ENS of young mice transplanted with old BM (O→Y) compared to young BM (Y→Y) (A). There was a modest but statistically significant increase in neuronal density found in the myenteric ganglia of Y→O compared to O→O (B). *p<.05 by one-way ANOVA with Bonferroni’s multiple comparisons test and t-test.
Flow cytometry performed on BMDMs from young or old mice treated with either IL-4 and IL-13 (A) or IFNγ (B) revealed similar scatter plots based on F4/80 and CD206 expression. An age dependent reduction of FoxO3 expression was detected in IFNγ-treated (M1) and IL-4/IL-13- treated (M2) BMDMs by Western blot with pixel intensity normalized to GAPDH represented as bar chart on right (C). IFNγ-treated (M1) BMDMs from FoxO3−/− mice stimulated with LPS (100 ng/ml) for 4 hours demonstrate increased expression of pro-inflammatory markers relative to their unstimulated state, particularly for IL-6, when compared to WT (D). ***p<.001 by t-test.
An age-dependent decline of FoxO3 in MMs results in loss of suppressive phenotype. MMs assume a pro-inflammatory state resulting in inflammation-induced loss of enteric neurons and ENSCs, and disruption of neuromuscular function.