Abstract
Background
Extended release naltrexone (XR-NTX) injected intramuscularly monthly has been shown to reduce relapse in persons with opioid use disorder. Baseline factors, including patients’ demographics, comorbidities and lifestyle, may help identify patients who will benefit most or least from XR-NTX treatment.
Methods
Potential moderators of XR-NTX’s effect were examined in the largest North American randomized open-label effectiveness trial of XR-NTX. Relapse status (Yes/No) at 6-month follow-up was regressed on treatment group (XR-NTX, N=153; or Treatment-as-Usual [TAU], N=155), baseline covariates, and their two-way interaction to identify moderator effects. Baseline covariates included age, gender, summary scores for depression, suicidal thoughts, drug abuse risk, substance use, medical, psychiatric and employment status, socialization, legal and family/social issues, history of abuse and quality of life measures.
Results
Alcohol use to intoxication in the 30 days before randomization was a significant moderator: during the treatment phase, those who reported being recently intoxicated before randomization to XR-NTX relapsed to opioids at a rate (56%) similar to TAU (58%), while those without alcohol intoxication in the prior 30 days had a lower rate of opioid relapse (41% vs. 65%, respectively, P<0.04).
Conclusions
XR-NTX appeared to work equally well across subgroups with diverse demographic, addiction, mental health and environmental characteristics, with the possible exception of working better among those without recent alcohol intoxication. These findings should be reassuring to practitioners increasingly using XR-NTX as medical addiction therapy in diverse and often vulnerable populations.
Introduction
Opioid use disorders are a growing public health concern, with an estimated three million people in the US and 16 million people worldwide experiencing this chronic relapsing illness (Schuckit, 2016). Criminal justice system (CJS) involved populations are disproportionally affected by opioid use disorders and are especially prone to relapse and overdose immediately following incarceration (Binswanger et al., 2007). Opioid agonist therapies, including methadone and buprenorphine, have been shown to be effective in treating opioid use disorders in criminal justice settings and upon release (Chandler, Fletcher, & Volkow, 2009; Gordon et al., 2015; Kinlock et al., 2007; McKenzie et al., 2012). However, most CJS-involved persons will not receive these pharmacotherapies (Friedmann et al., 2012) and will instead receive no formal treatment or less-effective psychoeducation (Friedmann, Taxman, & Henderson, 2007).
Naltrexone for extended release injectable suspension (XR-NTX) is an FDA-approved opioid antagonist that can be administered to persons who have been tapered off of opioids to help prevent relapse. It month-long duration of action appeals to those patients and providers seeking an alternative to daily agonist treatment requiring physical dependence on opioids. This study is a secondary analysis of a previously published study which found XR-NTX reduced the rate of relapse in opioid-dependent persons released from prison in the US (Lee et al., 2016). Our aim for this secondary analysis is to determine if the effectiveness of XR-NTX was moderated by demographic, lifestyle or behavioral factors. A study published in 2015 found no evidence of moderating effects in recently detoxified opioid-dependent adults in Russia (Nunes et al., 2015). Our aim was to examine possible moderators of XR-NTX in a population of recently and active CJS-involved persons in the US.
Methods
Participants
This study, fully described elsewhere (Lee et al., 2015), was conducted at five independently funded sites throughout the United States. We recruited community-dwelling adult volunteers with a history of both criminal justice involvement and opioid dependence (DSM-IV). To participate, volunteers aged 18–60 had to have an interest in opioid-free maintenance, have a negative urine toxicology for all opioids prior to randomization, be under criminal justice supervision (parole, probation, other court ordered programs) currently or in the past 12 months, be in good health and able to provide informed consent. Exclusion criteria included alcohol dependence requiring a level of care that would interfere with trial participation.
Recruitment efforts were focused on at-risk criminal justice populations, medical clinics and addiction treatment programs (outpatient, inpatient, residential). The study was advertised in print, on the radio and on-line. Clinic directors were contacted about the study and flyers were posted in clinics. There was no direct involvement by criminal justice authorities in order to minimize potential for coercion and participants were required to score 100% on an informed consent quiz.
Study Methods
A total of 308 participants provided informed consent and were randomized in a 1:1 ratio to either XR-NTX or treatment as usual (TAU). Injection of XR-NTX was administered by study physicians or nurses at randomization and then every four weeks for six total injections. Participants randomized to TAU were encouraged by study staff to access treatment and relapse prevention resources in the community. The usual treatment in most of these communities was outpatient medication-free treatment, although approximately one-third received opioid agonist treatment.
Baseline assessments for each participant included: Risk Assessment Battery (RAB) which captured rates of drug use and unsafe sex (Watkins, Metzger, Woody, & McLellan, 1992); Addiction Severity Index Lite (ASI) which collected demographics as well as history of drug use, abuse, medical and psychiatric problems, employment status and family and social conflicts (McLellan et al., 1992); Mini-International Neuropsychiatric interview (Sheehan et al., 1998); Euroquol EQ-5D Quality of Life (QOL) scale (EuroQol, 1990); timeline follow back (TLFB) self-reporting of drug and alcohol use and days in a controlled environment; and other assessments of self-report criminal activity and at-risk behavior. Urine drug screening for all participants occurred every two weeks during the active study phase (6 months).
Study Variables and Analysis
The primary outcome was opioid relapse status, with relapse being defined as two or more consecutive positive or missing urine tests for opioids and/or 10 or more days of self-reported opioid use in any 28 day period. Potential moderators included baseline summary scores for depression, socialization, drug abuse risk, medical, psychiatric and employment status, alcohol use, legal and family/social issues. Additionally, we explored demographics, history of substance use, physical and other abuse, suicidal thoughts and quality of life measures. For Addiction Severity Index composite scores, higher scores indicate greater problem severity (McLellan et al., 1992).
A series of simple and generalized estimating equation (GEE) logistic regression models were fit with any relapse during the 6-month treatment period as the outcome and treatment group (XR-NTX or TAU), potential moderator and their interaction as independent variables. GEE models adjust for correlation among participants within study sites, but small cell sizes may lead to unstable estimates, therefore both site adjusted and unadjusted results were documented. Interaction terms were tested to identify significant moderator effects.
Results
Participants had a mean age of 44, and most were male, African American or Hispanic, had a history of heroin use, and were currently under criminal justice supervision (Table 1).
Table 1.
Baseline characteristics, XR-NTX vs. Treatment as Usual (TAU).
| XR-NTX (n=153) n (%) |
TAU (n=155) n (%) |
|
|---|---|---|
|
| ||
| Male | 129 (84.3%) | 132 (85.2%) |
|
| ||
| Age, years, mean (SD) | 44.4 (9.2) | 43.2 (9.4) |
|
| ||
| Race/Ethnicity: | ||
| Caucasian | 31 (20.4%) | 30 (19.4%) |
| African American | 81 (53.3%) | 74 (47.7%) |
| Hispanic | 37 (24.3%) | 45 (29.0%) |
|
| ||
| Years of education, mean (SD) | 11.5 (2.2) | 11.5 (1.8) |
|
| ||
| Current employment | 26 (17.0%) | 29 (18.7%) |
|
| ||
| Current CJS supervision* | 121 (79.8%) | 124 (80.0%) |
| Probation | 55 (36.0%) | 62 (40.0%) |
| Parole | 57 (37.3%) | 54 (34.8%) |
| Other | 9 (5.9%) | 8 (5.2%) |
| No CJS supervision† | 32 (20.2%) | 31 (20.0%) |
|
| ||
| Health insurance, any | 109 (71.2%) | 111 (71.6%) |
|
| ||
| Medicaid | 70 (45.8%) | 65 (41.9%) |
|
| ||
| Opioid use history, lifetime | ||
| Opioid dependence (DSM-IV) | 153 (100%) | 155 (100%) |
| Heroin use | 135 (88.8%) | 137 (88.4%) |
| Other (non-heroin) opioid use | 77 (50.7%) | 74 (47.7%) |
| Injection drug use | 64 (42.1%) | 62 (40.0%) |
|
| ||
| Opioid use, past 30 days | ||
| Heroin use | 32 (21.1%) | 43 (27.7%) |
| Other (non-heroin) opioid use | 31 (20.4%) | 26 (16.8%) |
| Any opioid use | 47 (30.9%) | 59 (38.1%) |
|
| ||
| Needed opioid detox to enter study | 13 (8.5%) | 14 (9.0%) |
|
| ||
| Cocaine use, past 30 days | 30 (19.7%) | 29 (18.7%) |
|
| ||
| Heavy alcohol use, past 30 days | 18 (11.8%) | 19 (12.3%) |
Current CJS supervision defined as parole, probation, or other (drug court, diversion, alternative to sentencing program) involvement at baseline.
No CJS supervision defined as recent criminal justice involvement (i.e., arrest, incarceration, conviction, plea bargain) 0–12 months prior to baseline, with no current CJS supervision as above.
The great majority of potential moderators did not appear to influence the effectiveness of XR-NTX (Figure 1). The only significant (P < 0.05) moderator of XR-NTX that emerged was “drank alcohol to intoxication” as defined by the Addiction Severity Index in the 30 days before randomization. Those who reported such intoxication and were assigned to XR-NTX relapsed to opioids at a rate (56%) similar to TAU (58%), while those without alcohol intoxication in the prior 30 days had a lower rate of opioid relapse (41% vs. 65%, respectively, P<0.04) (Table 2).
Figure 1. Potential Moderators of the Effectiveness of Extended-Release Naltrexone (XR-NTX).
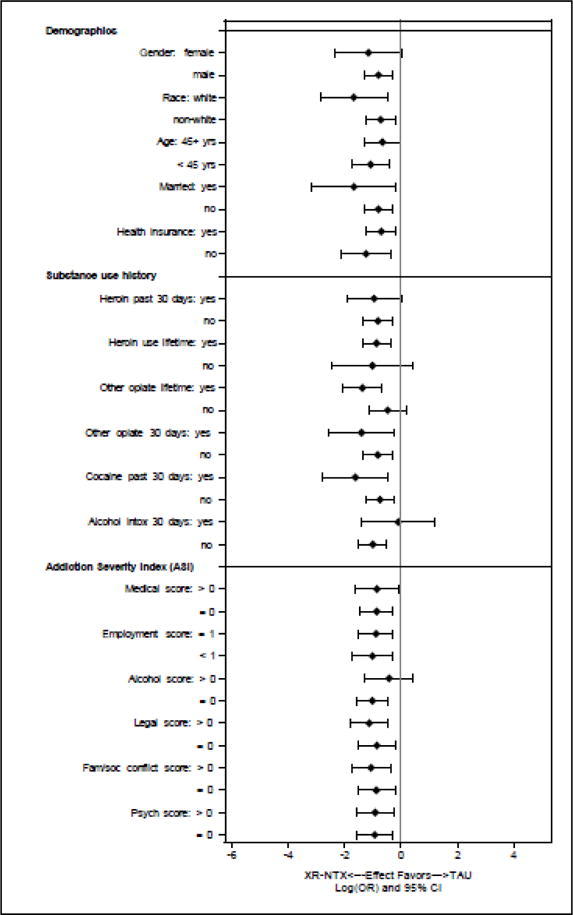
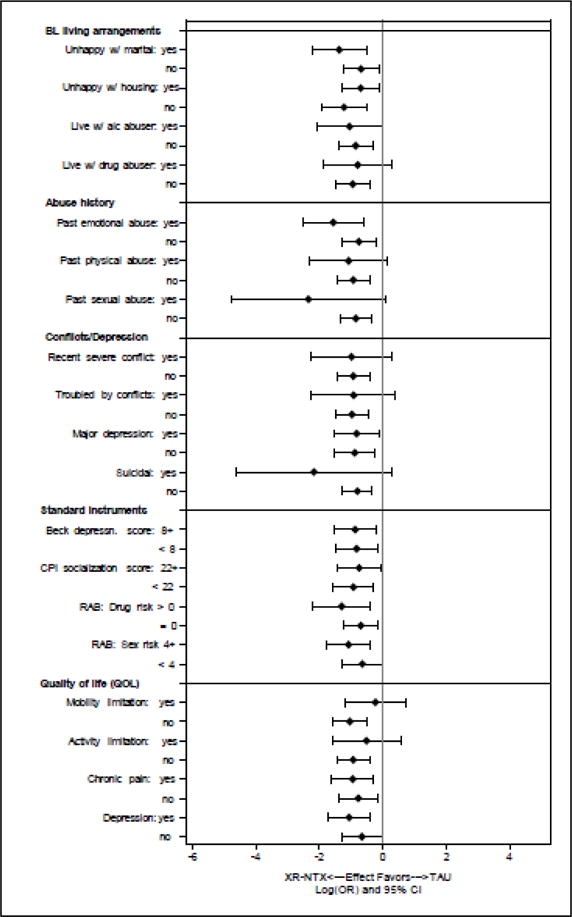
The overlap in the log (odds ratio) for relapse across levels of these attributes suggests limited effect modification.
Table 2.
Predicted relapse rates during treatment period, by treatment group and selected potential moderators
| XR-NTX | TAU | Main effect | Interaction* | ||
|---|---|---|---|---|---|
|
|
|||||
| Attribute (Potential moderator) | Proportion Relapsed (95% CI) | P value | |||
| Alcohol intoxication: 30 days† | Yes | 0.56 (0.33, 0.79) | 0.58 (0.36, 0.73) | 0.41 | 0.04 |
| No | 0.41 (0.32, 0.49) | 0.65 (0.57, 0.73) | |||
| Non-heroin opioid use: 30 days† | Yes | 0.45 (0.28, 0.63) | 0.77 (0.61, 0.93) | 0.03 | 0.14 |
| No | 0.41 (0.32, 0.50) | 0.61 (0.53, 0.70) | |||
| Risk Assessment Battery – drug risk score‡ | |||||
| 5 | 0.40 (0.27, 0.53) | 0.74 (0.61, 0.87) | 0.08 | 0.12 | |
| 0 | 0.44 (0.36, 0.53) | 0.61 (0.52, 0.69) | |||
| Addiction Severity Index – family/social score† | |||||
| 0.2 | 0.36 (0.26, 0.46) | 0.67 (0.57, 0.77) | 0.07 | 0.07 | |
| 0 | 0.46 (0.36, 0.56) | 0.63 (0.53, 0.73) | |||
| Suicidal thoughts§ | Yes | 0.36 (0.08, 0.65) | 0.83 (0.54, 1.00) | 0.09 | 0.08 |
| No | 0.43 (0.35, 0.51) | 0.62 (0.55, 0.70) | |||
| Mobility limitationsǁ | Yes | 0.47 (0.31, 0.64) | 0.53 (0.36, 0.70) | 0.20 | 0.09 |
| No | 0.42 (0.33, 0.51) | 0.67 (0.59, 0.75) | |||
Potential modifier*treatment group interaction, selected for those at P<0.15
From the Addiction Severity Index-Lite (McLellan et al., 1992).
From the Risk Assessment Battery (Watkins et al., 1992)
From the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998).
From the Euroquol EQ-5D Quality of Life scale (EuroQol, 1990)
Trends (0.05 ≤ P ≤ 0.15) were found for other potential moderators (Table 1). Subjects who reported recent non-heroin opioid use before randomization to XR-NTX tended towards greater reductions in the rate of opioid relapse (45%) compared to TAU (77%) than did those without non-heroin opioid use (41% vs. 61%, respectively, P=0.14). Those with greater drug abuse risk, more family/social conflict or suicidal thoughts also showed trends towards greater effects of XR-NTX than did those without those attributes (Table 1). Subjects with mobility limitations who were assigned to XR-NTX relapsed to opioids at a rate (47%) close to TAU (53%), while those without mobility limitations tended toward greater reductions in opioid relapse from XR-NTX compared TAU (42% vs. 67%, respectively, P<0.09).
Discussion
Because XR-NTX is FDA-approved for both opioid and alcohol use disorders, it seems logical that it would be an effective medication choice for patients with those disorders co-occurring. However, the finding that XR-NTX was less effective in preventing opioid relapse in participants with recent alcohol intoxication appears to contradict this supposition. Since one must be fully abstinent from opioids prior to XR-NTX initiation, these heavy drinking subjects might represent individuals who have substituted alcohol for opioids, and their drinking might be a marker for a more severe polysubstance addictive disorder and less stable remission that is more prone to relapse. Alternatively, XR-NTX works best for alcohol-dependent patients who achieve alcohol abstinence prior to its initiation (O’Malley, Garbutt, Gastfriend, Dong, & Kranzler, 2007), so heavy drinking might similarly have lowered the effectiveness of XR-NTX for opioid use disorder in these subjects.
Although marginal associations suggest greater effects of XR-NTX in reducing relapse among subjects with recent non-heroin opioid use, higher drug risk scores, history of family conflict, suicidal thoughts and good mobility compared to those without those attributes, in general XR-NTX appeared to work equally in all subgroups. Some studies of naltrexone for alcohol use disorder have suggested less effectiveness among women (Garbutt et al., 2005; O’Malley, Sinha, et al., 2007; Suh, Pettinati, Kampman, & O’Brien, 2008), but no decrement in response was found here for opioid use disorder. The moderator analysis of the Russian study that led to FDA approval of XR-NTX for opioid use disorder similarly found no baseline variables that indicated differential response to XR-NTX (Nunes et al., 2015).
Although this study represents the largest study of XR-NTX to date, small cell sizes for some of the variables constrain power to detect possible moderators. Because the analysis was not adjusted for multiple comparisons, the single significant relationship found might have resulted from chance, but the detection of few such relationships is somewhat reassuring. Despite these limitations, we conclude that XR-NTX appeared to work equally well across groups with diverse demographic, addiction, mental health and environmental characteristics, with the possible exception of those with co-occurring heavy alcohol consumption. These findings should be reassuring to practitioners increasingly using XR-NTX as medical addiction therapy in diverse and often vulnerable populations.
Highlights.
Potential moderators of the effect of extended release naltrexone (XR-NTX) injection XR-NTX’s effect were examined in a secondary analysis of the largest North American randomized open-label effectiveness trial.
XR-NTX appeared to work equally well across subgroups with diverse demographic, addiction, mental health and environmental characteristics, with the possible exception of working better among those without recent alcohol intoxication.
Acknowledgments
The authors thank the study participants and combined research staff across all sites. Supported by the National Institute on Drug Abuse (NIDA) through PAR-07-232 (R01DA024549, R01DA024550, R01DA024553, R01DA024554, and R01DA024555), and additional support (K24DA022412). Trial medication was provided in kind from an investigator-initiated grant from Alkermes. Dr. Lee has received investigator initiated study funding and study drug in-kind from Alkermes and Reckitt Benckiser for additional studies. Dr. Friedmann was paid an honorarium and received reimbursement for travel for attendance at an Indivior Advisory Board Meeting and served as a roundtable leader for Orexo. Dr. Gordon received investigator initiated study funding and study drug in-kind from Alkermes for an additional study. Dr. Nunes received: study drug in-kind from Alkermes/Cephalon, Inc., Reckitt-Benckiser, and Duramed Pharmaceuticals for additional studies; web-based behavioral intervention for a research study from HealthSim, LLC; devices under investigation and reimbursement for travel for investigators’ meeting from Brainsway. Dr. Nunes was paid an honorarium and received reimbursement for travel for attendance at a Lilly Advisory Board Meeting in January 2012 and received educational materials from Otsuka America Pharmaceutical, Inc. in 2013. He serves on an Advisory Board for Alkermes. Dr. O’Brien has served as a consultant to Alkermes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov number, NCT00781898.
References
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD. Release from prison–a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler RK, Fletcher BW, Volkow ND. Treating drug abuse and addiction in the criminal justice system: improving public health and safety. JAMA. 2009;301(2):183–190. doi: 10.1001/jama.2008.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Friedmann PD, Hoskinson R, Gordon M, Schwartz R, Kinlock T, Knight K, Mat Working Group Of, C. J. D. Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): availability, barriers, and intentions. Subst Abus. 2012;33(1):9–18. doi: 10.1080/08897077.2011.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Taxman FS, Henderson CE. Evidence-based treatment practices for drug-involved adults in the criminal justice system. J Subst Abuse Treat. 2007;32(3):267–277. doi: 10.1016/j.jsat.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Vivitrex Study, G Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, Schwartz RP, Couvillion KA, Sudec LJ, O’Grady KE, Shabazz H. Buprenorphine Treatment for Probationers and Parolees. Subst Abus. 2015;36(2):217–225. doi: 10.1080/08897077.2014.902787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, O’Grady K, Fitzgerald TT, Wilson M. A randomized clinical trial of methadone maintenance for prisoners: results at 1-month post-release. Drug Alcohol Depend. 2007;91(2–3):220–227. doi: 10.1016/j.drugalcdep.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Friedmann PD, Boney TY, Hoskinson RA, Jr, McDonald R, Gordon M, O’Brien CP. Extended-release naltrexone to prevent relapse among opioid dependent, criminal justice system involved adults: rationale and design of a randomized controlled effectiveness trial. Contemp Clin Trials. 2015;41:110–117. doi: 10.1016/j.cct.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA, Jr, O’Brien CP. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders. N Engl J Med. 2016;374(13):1232–1242. doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie M, Zaller N, Dickman SL, Green TC, Parihk A, Friedmann PD, Rich JD. A randomized trial of methadone initiation prior to release from incarceration. Subst Abus. 2012;33(1):19–29. doi: 10.1080/08897077.2011.609446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Krupitsky E, Ling W, Zummo J, Memisoglu A, Silverman BL, Gastfriend DR. Treating Opioid Dependence With Injectable Extended-Release Naltrexone (XR-NTX): Who Will Respond? J Addict Med. 2015;9(3):238–243. doi: 10.1097/ADM.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. J Clin Psychopharmacol. 2007;27(5):507–512. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Sinha R, Grilo CM, Capone C, Farren CK, McKee SA, Wu R. Naltrexone and cognitive behavioral coping skills therapy for the treatment of alcohol drinking and eating disorder features in alcohol-dependent women: a randomized controlled trial. Alcohol Clin Exp Res. 2007;31(4):625–634.x. doi: 10.1111/j.1530-0277.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Treatment of Opioid-Use Disorders. N Engl J Med. 2016;375(4):357–368. doi: 10.1056/NEJMra1604339. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Suh JJ, Pettinati HM, Kampman KM, O’Brien CP. Gender differences in predictors of treatment attrition with high dose naltrexone in cocaine and alcohol dependence. Am J Addict. 2008;17(6):463–468. doi: 10.1080/10550490802409074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Metzger D, Woody G, McLellan AT. High-risk sexual behaviors of intravenous drug users in- and out-of-treatment: implications for the spread of HIV infection. Am J Drug Alcohol Abuse. 1992;18(4):389–398. doi: 10.3109/00952999209051037. [DOI] [PubMed] [Google Scholar]


