Abstract
Melanoma has a high propensity to metastasize to the brain, and patients with melanoma brain metastases (MBM) have an extremely poor prognosis. The recent approval of several molecularly-targeted agents (e.g., BRAF, MEK inhibitors) and biologics (anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies) has brought new hope to patients suffering from this formerly untreatable and lethal disease. Importantly, there have been recent reports of success in some clinical studies examining the efficacy of both targeted agents and immunotherapies that show similar response rates in both brain metastases and extracranial disease. While these studies are encouraging, there remains significant room for improvement in the treatment of MBM, given the lack of durable response and the development of resistance to current therapies. Critical questions remain regarding mechanisms that lead to this lack of durable response and development of resistance, and how those mechanisms may differ in systemic sites versus brain metastases. One issue that may not be fully appreciated is that the delivery of several small molecule molecularly-targeted therapies to the brain is often restricted due to active efflux at the blood-brain barrier (BBB) interface. Inadequate local drug concentrations may be partially responsible for the development of unique patterns of resistance at metastatic sites in the brain. It is clear that there can be local, heterogeneous BBB breakdown in MBM, as exemplified by contrast-enhancement on T1-weighted MR imaging. However, it is possible that the successful treatment of MBM with small molecule targeted therapies will depend, in part, on the ability of these therapies to penetrate an intact BBB and reach the protected micro-metastases (so called “sub-clinical” disease) that escape early detection by contrast-enhanced MRI, as well as regions of tumor within MRI-detectable metastases that may have a less compromised BBB. The emergence of resistance in MBM may be related to several diverse, yet interrelated, factors including the distinct microenvironment of the brain and inadequate brain penetration of targeted therapies to specific regions of tumor. The tumor microenvironment has been ascribed to play a key role in steering the course of disease progression, by dictating changes in expression of tumor drivers and resistance-related signaling mechanisms. Therefore, a key issue to consider is how changes in drug delivery, and hence local drug concentrations within a metastatic microenvironment, will influence the development of resistance. Herein we discuss our perspective on several critical questions that focus on many aspects relevant to the treatment of melanoma brain metastases; the answers to which may lead to important advances in the treatment of this devastating disease.
Keywords: melanoma brain metastases, blood-brain barrier, drug delivery, active efflux, molecularly-targeted agents, drug resistance
Graphical Abstract
1) Introduction
The metastatic spread of melanoma to the brain is a major cause of morbidity and mortality in patients suffering from this disease. Metastatic melanoma, a debilitating and lethal form of skin cancer, is projected to have 87,110 new cases in 2017 in the US, with 9,730 deaths (Siegel et al. 2017). This malignancy has a high proclivity to spread to the brain, and patients with multiple brain metastases have an extremely poor prognosis, with a median overall survival (OS) of less than 6 months (Damsky et al. 2014; Sloan et al. 2009; Raizer et al. 2008; Gupta et al. 1997). The presence of brain metastases in a significant proportion (∼70%) of melanoma patients at autopsy is a clear indication of an important unmet medical need (Gorantla et al. 2013; McWilliams et al. 2003).
Despite the remarkable progress seen in the overall treatment of melanoma patients (Bates 2013), it is still difficult to treat advanced metastatic disease that has spread to the brain. Melanoma patients with multiple brain metastases can have a particularly poor survival, as short as 1-2 months. The standard of care for the management of patients with multiple brain metastases has been whole brain radiation therapy. Patients with one to three brain metastases are often candidates for surgical resection or stereotactic radiosurgery, depending on the location and size of the lesion(s) (Fife et al. 2004). Even with the advent of relatively successful targeted therapies and immunotherapies, treatments that can reliably provide major survival benefits for patients suffering from this devastating condition have still not been realized.
The small molecule targeted therapies inhibiting MAP kinase signaling (e.g., BRAF inhibitors such as vemurafenib and dabrafenib, MEK inhibitors such as trametinib and cobimetinib) and the large molecule immune checkpoint inhibitors (e.g., cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors such as ipilimumab and programmed cell death receptor (PD-1) inhibitors such as nivolumab) have shown some improvements in overall survival (OS) and progression-free survival (PFS) by a few months in patients with melanoma brain metastases (MBM) (Dummer et al. 2014; Rochet et al. 2012; Long et al. 2012; Falchook et al. 2012; Spagnolo et al. 2016; Davies et al. 2017). While encouraging, such modest improvements in OS are far from satisfactory, and do not represent a high probability of cure for patients suffering from this lethal condition. The results of some clinical studies suggest that the observed intracranial responses are poor compared to extracranial responses (Rochet et al. 2012; Larkin et al. 2014; Harding et al. 2015; McArthur et al. 2016; Spagnolo et al. 2016). However, there are important observations from other trials that indicate the intracranial responses are comparable to the extracranial responses (Gibney et al. 2015; Margolin et al. 2012; Di Giacomo, Margolin 2015; Cohen et al. 2016; Spagnolo et al. 2016; Davies et al. 2017), creating a broad spectrum of reported efficacy of these therapies in MBM. Lack of durable efficacy may be due to many factors, and in MBM it may be related to resistance mechanisms that include both inadequate delivery and specific brain microenvironment characteristics that each could lead to changes in expression of genetic drivers. While small molecule molecularly-targeted therapies target key oncogenic proteins in deregulated signaling cascades, immunotherapies act by enriching prevailing immune responses to melanoma antigens. It is well appreciated that small molecule therapies must distribute to the target site to exert their action. However, it is crucial to recognize that immune checkpoint inhibitors such as anti-CTLA-4 and anti-PD-1 therapies can cause activation and proliferation of cytotoxic T-lymphocytes in systemic sites, which are then able to gain access to the brain metastatic locations and attack the tumor cells (Engelhardt, Ransohoff 2012; Margolin 2016) thereby offering an important rationale for activity in MBM.
It is important to note that the patients with symptomatic MBM have frequently been excluded from pivotal clinical trials testing novel therapeutic agents (Davies et al. 2011; Cohen et al. 2016). This is due to the poor prognosis seen in this subset of patients and, at least in part, can be ascribed to the fact that several small molecule molecularly-targeted agents are substrates for active efflux at the blood-brain barrier (BBB), and so the delivery of these agents to brain metastases can be severely restricted (Durmus et al. 2015; Agarwal et al. 2011b; Parrish et al. 2015b; Gampa et al. 2016). Such exclusionary practices have limited progress with regard to testing the efficacy of these agents in the treatment of MBM. While few trials have been specifically tailored for testing therapies in this subset of patients, some clinical trials are currently underway and will be important in understanding the therapeutic outcomes with the use of the recently developed therapies in patients with MBM.
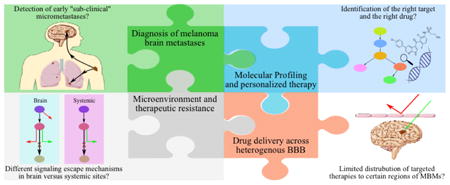
It is critical to realize that the successful treatment of MBM, or any brain metastases, with molecularly-targeted agents will depend on the development of novel therapeutic agents that are not only potent at the target site, but are also capable of penetrating an intact BBB and reaching the protected metastatic tumor cells that are not clinically detectable upon contrast-enhanced MR imaging (Murrell et al. 2016). Several critical questions surrounding the clinical diagnoses and treatment of MBM are addressed in this perspective, and adequate drug delivery is a component of each of these critical clinical questions. Figure 1 depicts the complexity involved in the discovery and development of new treatment paradigms for MBM. This “puzzle” will only be truly solved when all the key aspects influencing effective therapy are taken into consideration. For instance, it is clear that a potent drug that does not reach its intended target will be ineffective. Moreover, at least in a subset of non-responsive patients, a therapy that is initially effective in systemic (i.e, non-brain) metastases may not have efficacy in the brain due to microenvironment driven changes in tumor-growth driving targets. A broad understanding of these issues, all somewhat tied to the problem of adequate drug delivery to brain metastases, will help the scientific and medical community make appropriate decisions with respect to the discovery, development, and patient-specific selection of therapeutic agents for the treatment of MBM. Although a multi-factorial approach is needed to deal with this important problem of MBM treatment, the goal of this perspective is to focus on aspects related to drug delivery, bearing in mind other crucial factors such as microenvironment and resistance that will also need to be addressed in a comprehensive approach to this problem. In this perspective, we put forth our views on some of the critical clinical questions (Box 1) regarding the current and future treatment of MBM. However, before tackling these questions, that are largely related to drug delivery across the BBB, a brief review of the structure and function of the BBB is offered.
Figure 1.
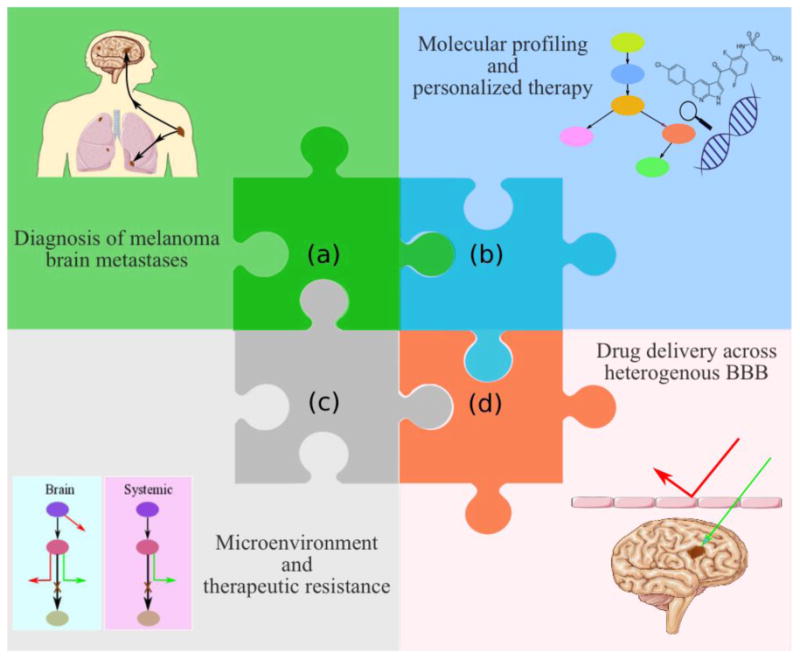
A complex, dynamic puzzle of interrelated pieces necessary to improve the treatment of melanoma brain metastases (MBM) includes: (a) improved imaging modalities for diagnosis of early “sub-clinical” micro-metastases, (b) improved identification of the brain-specific targets and selection of suitable targeted therapies, (c) improved recognition of brain-specific resistance mechanisms, and, (d) improved drug design to overcome BBB delivery issues and yield effective combination therapies. (2-column fitting image)
Box 1. Critical questions in the treatment of MBM.
Can targeted agents with limited distribution across an intact BBB be used to reliably treat MBM?
Will enhancing the brain distribution of systemically active agents improve the treatment of micro-metastatic disease in the brain?
Are therapies that can target currently “undetectable” brain micro-metastases more effective in extending progression-free or overall survival than treating larger brain metastases that are detectable by MRI?
In a related question (to #3), does the analysis of drug levels in a resected brain tumor specimen reflect drug levels in other regions of the brain that require treatment, especially in and around the micro-metastases?
Will the use of drugs that have initial efficacy in systemic (i.e., non-brain) metastases, but have poor brain penetration, lead to resistant brain metastases? If so, will this be through mechanisms different than those leading to the eventual resistance at systemic sites?
What are the mechanisms responsible for the homing of tumor cells to the brain and how does the integrity of the BBB at the metastatic site affect the homing process? Also, will the integrity of the BBB at a metastatic site influence the rate of tumor growth in individual brain metastases?
In drug discovery, design and development, how can we find drugs that are active against the relevant targets, have adequate BBB permeability, and limit the development of resistance; the critical issues currently limiting the treatment of MBM?
Are potential advances in the treatment of melanoma patients negatively impacted by the exclusion of patients with brain metastases from pivotal clinical trials testing novel targeted therapies?
2) Blood-brain barrier: An obstacle for drug delivery to brain metastases
The BBB is comprised of several cell types, leading to various mechanisms that limit the penetration of many small and large molecule compounds into the brain. The endothelial cells in the brain microvasculature are held in close proximity by tight junctions, and are enclosed by extracellular matrix elements, pericytes and astrocyte foot processes, which collectively form the neurovascular unit (NVU), i.e., the BBB (Abbott 2013). Such a complex architecture restricts the passive entry of many xenobiotics, including drug molecules, into the brain. Moreover, as shown in Figure 2, the entry of therapeutic agents into the brain is also strictly regulated by the presence of diverse transport systems belonging to the ATP-Binding Cassette (ABC) and Solute Carrier (SLC) families of proteins at the BBB (Taylor 2002; Abbott et al. 2010). Of special interest are two important efflux transporters, P-glycoprotein (P-gp, MDR1, ABCB1) and breast cancer resistance protein (BCRP, ABCG2), known to limit the CNS delivery of several small molecule therapeutics that may be used for the treatment of CNS diseases (Agarwal et al. 2011a; Wu, S 2014; Gampa et al. 2016; Durmus et al. 2015). As examples of drugs relevant to the treatment of melanoma, there is evidence from preclinical studies that indicates an interaction of several molecularly-targeted therapies, including vemurafenib, dabrafenib, trametinib, palbociclib, cobimetinib and omipalisib, with P-gp and BCRP, resulting in poor drug distribution to the brain (Choo et al. 2014; Durmus et al. 2012; Mittapalli et al. 2013; Mittapalli et al. 2012; Parrish et al. 2015a; Vaidhyanathan et al. 2014; Vaidhyanathan et al. 2016; de Gooijer et al. 2015).
Figure 2.
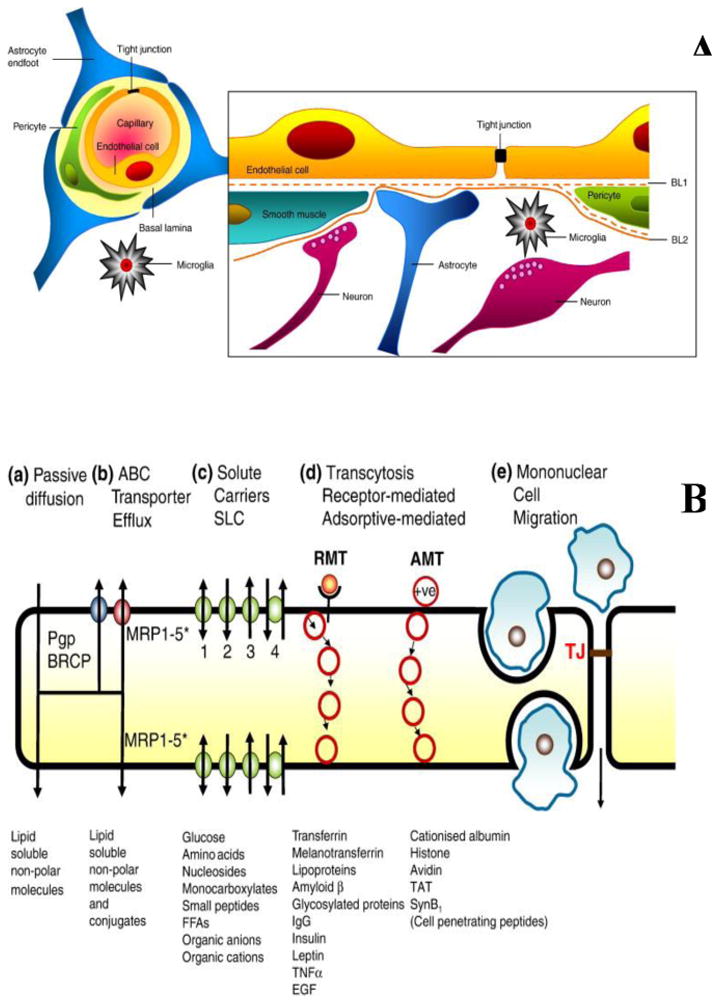
The blood-brain barrier (BBB). A) the BBB (also referred to as the neurovascular unit, NVU) architecture, and cell associations that form the BBB. B) various routes of trafficking across the BBB that includes transcellular passive diffusion, active efflux, carrier mediated influx, receptor mediated transcytosis (RMT), adsorptive-mediated transcytosis (AMT), diapedesis and modulation of tight junctions (TJ) (adapted from (Abbott 2013), with permission). (2-column fitting image)
The BBB in a brain tumor core has been widely considered to be compromised, allowing unrestricted drug delivery to tumor cells (Stewart 1994; Gerstner, Fine 2007). This belief has been supported by evidence from contrast-enhanced magnetic resonance imaging (CE-MRI) that can detect tumor locations with a permeable BBB (Fortin 2012; Essig et al. 2006). However, the BBB disruption in brain neoplasms, both primary and metastatic, have a marked heterogeneity (Essig et al. 2006) and a review of the clinical data from 24 studies shows highly variable drug levels in brain tumor specimens for different antineoplastic agents (Pitz et al. 2011). The extent of BBB compromise in brain metastases and its impact on CNS delivery of therapeutic agents is an active area of investigation. Osswald and colleagues have measured permeability of brain metastases in a preclinical melanoma mouse model by injecting sodium fluorescein dye and determining the ratio of dye in metastases versus vessels. The results indicate that ∼71% of brain metastases were non-permeable and only ∼29% were permeable (Osswald et al. 2016) Lockman et al., have reported that the analysis of brain metastases from animal models of breast cancer show only partial BBB compromise in more than 89% of lesions. The magnitude of BBB compromise was observed to vary within and between metastases, and even in the leakiest brain metastases the permeability was much lower than in systemic tumors. Moreover, the BBB permeability of the tumor correlated with degree of drug uptake and effect. As seen in Figure 3, the distribution of paclitaxel into the breast cancer brain metastases is highly variable, even within a single metastatic lesion (Lockman et al. 2010). Along these same lines, Adkins et al. observed in animal models of breast cancer brain metastases that the changes in permeability were independent of tumor size and morphology. These studies also report that less than 10% of the studied metastases exhibited a leaky enough BBB (up to ∼50-fold higher permeability) to allow therapeutic agents to reach efficacious levels in the brain (Lockman et al. 2010; Adkins et al. 2016). Similarly, another preclinical study in a breast cancer brain metastases model revealed a variability of greater than 30-fold in vinorelbine concentrations between brain metastases. Vinorelbine distribution also varied widely within a single brain metastatic site due to heterogeneous permeability of the BBB (Samala et al. 2016). These examples in breast tumor brain metastases could be applicable in the context of MBM, as exemplified by Osswald et al., (Osswald et al. 2016) and are critical in guiding future research efforts in MBM.
Figure 3.
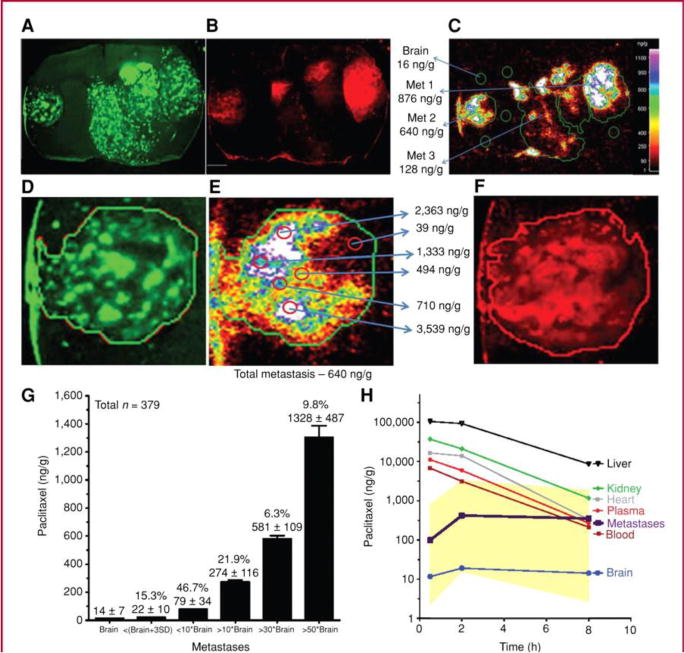
Variable paclitaxel uptake in 231-BR-HER2 brain metastases that correlates with 3-kDa Texas Red dextran accumulation. 14C-Paclitaxel distribution in 231-BR-HER2 metastases after intravenous administration of 10 mg/kg of paclitaxel (Taxol formulation). Distribution of eGFP (A), 3-kDa Texas Red dextran [10-minute circulation (B)], and 14C-paclitaxel [8 hours (C)], followed by a 30-second vascular washout. White scale bar represents 1 mm. Heterogeneous distribution is shown within one representative brain metastasis (D, eGFP; E, 14C-paclitaxel; and F, 3-kDa Texas Red dextran). G, 14C-paclitaxel concentration (ng/g) in brain and 231-BR-HER2 brain metastases. Values represent the percentage of all metastases in each group, and the mean ± SD of 14C-paclitaxel concentration in each group. H, mean brain metastasis drug concentration was measured at different times (30 minutes–8 hours) and related to that in brain distant from tumor, plasma, blood, and systemic tissues (n = 3 animals per point). Yellow areas show highest and lowest concentrations observed in brain metastases at the 3 time points. Calculated area under the curve cumulative exposure (in μg h/g) equaled 0.18 in brain, 2.9 in average brain metastasis, and 80 to 400 in systemic tissues (adapted from (Lockman et al. 2010), with permission). (2-column fitting image)
In a commentary about the Osswald publication, Steeg and colleagues discuss important ideas related to misconceptions about the permeability of brain metastases. They highlight the confusion with respect to permeability, i.e., the belief that brain metastases are permeable to drugs since many are contrast-enhancing with gadolinium. As Steeg points out, a critical question related to this is, if permeability is uniformly increased, why does chemotherapy with activity in systematic metastases not work in the brain? (Steeg et al. 2016). This commentary emphasizes the crucial observation that various independent groups have shown, using different techniques, that brain metastases exhibit a heterogeneous permeability, and a significant fraction are poorly permeable (Steeg et al. 2016). While the laboratories of Drs. Smith and Lockman used quantitative imaging following injection of dyes and radiolabeled drugs (Lockman et al. 2010), the laboratories of Drs. Foster and Chambers used gadolinium CE-MRI and high-resolution anatomic MRI to understand permeability of brain metastases in breast cancer models, and observed it to be heterogeneous (Murrell et al. 2016; Murrell et al. 2015). The laboratory of Dr. Winkler, confirms such findings of heterogeneity in permeability of brain metastases in a melanoma mouse model. They also went on to test a brain-permeable and brain-impermeable PI3K inhibitor alongside each other, observing that the brain-permeable inhibitor alone inhibited the growth of impermeable metastases. As suggested by Steeg, although a “brain-permeable” drug would often be defined differently, a useful definition in translational research is a drug that demonstrates an uptake sufficient to elicit a drug response (Steeg et al. 2016). The findings reported herein with regard to heterogeneity in brain permeability should also be tested in other brain metastasis model systems, such as spontaneous metastasis models. While responses and survival advantages have been observed with approved therapies, disease progression is frequent and development of brain-permeable drugs will possibly enhance drug efficacy. A first step in this direction will be to incorporate aspects favorable for increasing drug brain permeability (e.g., hydrogen bonding, lipophilicity, evading drug efflux) into early drug development programs (Heffron 2016).
The current gold standard technique for the clinical detection of brain tumors is CE-MRI. Brain regions with a leaky BBB allow a large hydrophilic contrast agent such as gadolinium-diethylenetriamine pentaacetic acid (GAD-DTPA, molecular weight of 938 daltons) to passively diffuse into the brain parenchyma, indicating the “tumor” regions (Essig et al. 2006). A major limitation of CE-MRI is that it can only reveal tumor locations that have a leaky BBB, while missing the detection of tumor cells in areas with an unbroken BBB, such as the micro-metastases and regions within larger tumors that have an intact BBB (Essig et al. 2006). As a consequence, this diagnostic modality can detect leaky tumors in advanced disease but not the early “sub-clinical” micro-metastases. Fidler et al., have reported that the brain metastases become leaky only after they attain a size of 0.25 mm (Fidler 2011). An outcome is that the contrast-enhancing larger metastases with a leaky BBB may have relatively unrestricted drug delivery while the “sub-clinical” micro-metastases with an intact BBB have limited drug delivery leading to sub-therapeutic drug exposure. These ideas advocate the need for the development of brain-penetrant drugs capable of reaching not only the leaky metastases but also the “sub-clinical” micro-metastases and regions within MRI-detectable larger metastases that have an intact BBB, to achieve tumor regression and prevent tumor relapse.
3) Critical questions in the treatment of melanoma brain metastases
The following discusses certain critical questions that, when successfully addressed, will almost certainly generate many hypotheses that will stimulate targeted research in MBM (see Box 1). This research, specific for the issues presented by brain metastases, will undoubtedly lead to therapeutic breakthroughs and improved clinical outcomes in the treatment of MBM.
3.1 Can targeted agents with limited distribution across an intact BBB be used to reliably treat MBM?
Limited drug delivery to tumor cells behind an intact BBB is undoubtedly an impediment to efficacy. Effective concentrations are not likely achieved for many of the small molecule molecularly-targeted agents in areas of micro-metastases and in regions of clinically detectable metastases that have an intact BBB. This is especially true where the drug BBB permeability is limited by efflux transport systems.
Given the discussion above regarding the heterogeneity of the BBB breakdown in many types of brain tumors, both metastatic and primary, instructive examples from breast cancer brain metastases illustrate how drug penetration through an intact BBB may strongly influence efficacy in brain metastases. A marked heterogeneity in the integrity of BBB in preclinical models of breast cancer corresponded with drug distribution, both within and between brain metastatic lesions (Adkins et al. 2016; Lockman et al. 2010; Samala et al. 2016). Lockman et al., and Adkins et al., report that less than 10% of brain metastatic lesions had a “leaky” enough BBB to allow the distribution of conventional chemotherapeutics, such as paclitaxel and doxorubicin, to achieve effective concentrations (Lockman et al. 2010; Adkins et al. 2016). Both of these compounds are substrates for efflux pumps at the BBB. While these drugs are not used for MBM, such studies show that there can be marked differences in drug penetration, and hence efficacy, when compounds are subject to efflux at the BBB.
Targeted therapies such as BRAF and MEK inhibitors, and immune checkpoint inhibitors such as anti-CTLA4 and anti-PD1 therapies have shown significant efficacy in patients with MBM, with some studies reporting that the intracranial responses were comparable to the extracranial responses (Gibney et al. 2015; Margolin et al. 2012; Di Giacomo, Margolin 2015; Cohen et al. 2016; Davies et al. 2017). While encouraging, the improvements in OS were limited, and certainly do not represent a possible cure for MBM patients. The absence of durable efficacy with molecularly-targeted agents, to some extent, may be related to the insufficient delivery of innovative treatments to the target sites in the brain that are behind an intact BBB. Moreover, most small molecule targeted agents have been shown to be effluxed by BBB transporters, particularly P-gp and BCRP. There is ample evidence that most of the small molecule therapies used for treatment of MBM are liable to active efflux at the BBB, which could be an important cause for the modest responses observed with these therapies. Vemurafenib has been shown to be a substrate for both P-gp and BCRP in animal models (Mittapalli et al. 2012; Durmus et al. 2012), and has very limited distribution into the CSF of melanoma patients (CSF:plasma ratio <1%) (Sakji-Dupre et al. 2015). Dabrafenib has a slightly higher permeability across an intact BBB than does vemurafenib in animal models (Mittapalli et al. 2013), and it has a slightly better efficacy in clinical trials (Long et al. 2012). Both of the approved MEK inhibitors, trametinib and cobimetinib, have also been shown to be substrates of BBB efflux transporters (Vaidhyanathan et al. 2014; Choo et al. 2014). These reports strongly suggest that future targeted agents with an enhanced BBB permeability may result in greater and more durable efficacy in MBM treatment. As such, drug design and development goals should include creation of compounds that are not substrates of efflux proteins at the BBB (Heffron 2016).
3.2 Will enhancing the brain distribution of systemically active agents improve the treatment of micro-metastatic disease in the brain?
The problem of limited drug delivery to brain tumors may be overcome by developing targeted therapies that can penetrate an intact BBB. However, important to the final result of improving delivery is the remaining question: will “therapeutic” drug levels in the brain, i.e., levels similar to those seen in systemic metastases, lead to regression of “sub-clinical” MBM? Further understanding of the brain microenvironment, and how it may be different than in the systemic metastatic sites with respect to tumor growth and development of resistance will be necessary to properly evaluate the impact of differences in systemic versus brain drug delivery.
The design and use of more BBB-penetrant drugs, in comparison to currently approved compounds, will help determine the effectiveness of such drugs and understand the emergence of different patterns of resistance. While there are a few brain-penetrant targeted drugs, a detailed evaluation of their efficacy in treating brain metastases has not yet been completed. Limited data about such brain-penetrant inhibitors exists and so it is not clear at this point if such inhibitors will be more efficacious in brain metastases. Conduct of studies testing the efficacy of such inhibitors would help us better understand enhancements in treatment outcomes, and if development of therapeutic resistance can be delayed or avoided altogether.
Rational drug design to improve brain penetration of a drug that is effective against systemic targets is exemplified by Salphati et al., who have optimized the physiochemical properties of PI3K inhibitors using in-silico tools to develop GNE-317 and GDC-0084, both capable of penetrating the BBB. In orthotopic GBM mouse models, they observed that GNE-317 (U87, GS2, and GBM10 models) and GDC-0084 (U87 and GS2 models) achieved significant tumor growth inhibition compared to pictilisib, a brain impenetrant PI3K inhibitor. Further, it was reported that GNE-317 and GDC-0084 were homogenously distributed throughout the brain using matrix-assisted-laser-desorption-ionization mass spectrometry imaging (MALDI-MSI), and also well-distributed to both contrast-enhancing and non-enhancing brain tumors in corresponding U87 and GS2 models. In the same models, pictilisib was not detected in the normal brain and non-enhancing tumors. Even in the contrast-enhancing tumors, the detected pictilisib levels were spatially heterogeneous and varied by 6-fold within the contrast-enhancing U87 tumors (Salphati et al. 2016; Salphati et al. 2012; Salphati et al. 2014).
Another enlightening example is shown by the ALK inhibitors, such as alectinib, ceritinib, AP26113 and lorlatinib (PF-06463922). While these compounds have not been employed for MBM, they have demonstrated efficacy against brain metastases from lung tumors. The intracranial efficacies in NSCLC patients of alectinib (not liable to efflux by P-gp) and ceritinib (brain-to-blood exposure ratio of ∼15 % in preclinical rat model) have been attributed to the brain penetration of these compounds (Toyokawa et al. 2015). Lorlatinib, a macrocyclic ALK inhibitor, has also been specifically developed to cross the BBB (Johnson 2014). Moreover, AZD3759, an EGFR inhibitor, also induces profound regression of brain metastases in a preclinical mouse models of NSCLC (Zeng et al. 2015; Yang et al. 2016). AZD3759 has a high brain penetration with the unbound partition coefficient, Kpuu,brain (Figure 4) of 0.86 and is not a substrate of P-gp or BCRP efflux transporters (Tan et al. 2016). These are instructive examples because it can be expected that many of the same BBB-mediated issues in drug delivery are present in lung metastases as are in intracranial melanoma metastases.
Figure 4.
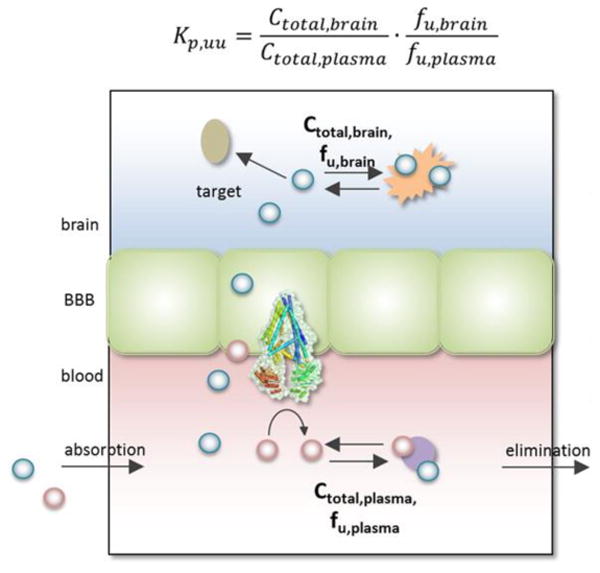
Elucidation of the unbound partition coefficient, Kpuu. The diagram represents the protein-bound and unbound drug molecules in the blood and brain compartments. The Kpuu, brain to plasma unbound drug concentration ratio, is determined using the total drug concentrations (Ctotal) and unbound fractions (fu), in both the blood and brain compartments. P-gp, an efflux transporter, is also shown on the luminal (blood) side of the BBB (adapted from, (Dolgikh et al. 2016)). (1.5-column fitting image)
3.3 Are therapies that can target currently “undetectable” brain micro-metastases more effective in extending progression-free or overall survival than treating larger brain metastases that are detectable by MRI?
A critical clinical question in treatment of MBM relates to, when do melanoma metastases first seed the brain during the progression of metastatic melanoma? Brain impenetrant drugs will not necessarily deter the initial seeding of metastatic tumor cells in the brain microenvironment and given this, would earlier initial diagnosis of brain metastases help influence therapeutic outcomes? An important corollary to this overriding question is determining if targeted therapies that can penetrate an intact BBB and achieve therapeutic levels early in disease progression will result in tumor growth inhibition or regression in brain sites.
Osswald et al. have observed in an important preclinical study, using a cranial window and multi-photon laser scanning microscopy, that targeting melanoma micro-metastases led to shrinkage of tumors in the brain. In this melanoma mouse model, the permeability of the brain metastases was determined by injecting sodium fluorescein dye and measuring the ratio of dye in metastases versus vessels. GNE-317, as indicated above, a PI3K inhibitor optimized to penetrate an intact BBB, showed responses in all brain metastases independent of the integrity of the BBB while therapy with non-brain permeable GDC-0980 (a related PI3K inhibitor with comparable effects on signaling inhibition in in-vitro experiments) led to responses only in permeable brain metastases (Figure 5). Also, GNE-317 showed significant benefits in the treatment of both non-permeable micro and macro-metastases. However, a reduction in the size of the tumor with GNE-317 therapy was observed only with micro-metastases, whereas the macro-metastases still grew (Figure 6A). Moreover, they also visualized and tracked dormant, single tumor cells in the brain. These single cells were in a BBB “impermeable” microenvironment and stable through a duration of 30 days post injection. The dormant cells, even though the surrounding BBB was intact, were observed to regress upon treatment with the BBB permeable GNE-317 (Figure 6B-E). These observations indicate that drugs engineered to have enhanced BBB permeability, thereby able to target the early micro-metastases, may prove to be more effective than less permeable drugs used to treat larger clinically detectable metastases at later stages of disease progression. While these results may seem obvious with respect to delivery across an intact BBB, it is critical that this concept be incorporated into future drug design and use for therapies in melanoma brain metastases (Osswald et al. 2016).
Figure 5.
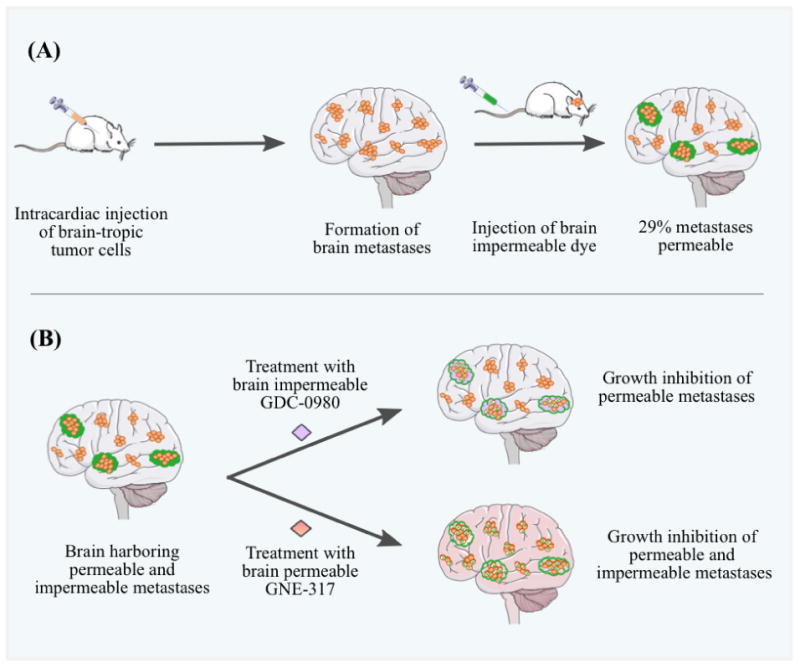
The role of BBB permeability in the efficacy of targeted therapies for the treatment of brain metastases. A, injection of brain-tropic A2058 melanoma cells (orange) into the left cardiac ventricle of mice led to the formation of MBMs. Sodium fluorescein (green), a brain-impermeable dye, was injected intravenously via tail vein, and its uptake into metastatic lesions was measured and used as a marker of brain metastasis permeability. Only ∼29% of the brain metastases were permeable to the administered dye (green clouds). B, mice harboring both permeable (green clouds) and impermeable (no clouds) brain metastases were randomized to vehicle or two PI3K inhibitors, GDC-0980, which is brain-impermeable, or GNE-317, which has demonstrated brain permeability. Both GDC-0980 and GNE-317 inhibited the growth of permeable metastases, while only the brain-permeable GNE-317 inhibited the growth and caused tumor apoptosis (white tumor cells with X) of impermeable metastases. These observations confirm a functional contribution of brain permeability to drug efficacy for brain metastases treatment (modified and redrawn from (Steeg et al. 2016)). (2-column fitting image)
Figure 6.
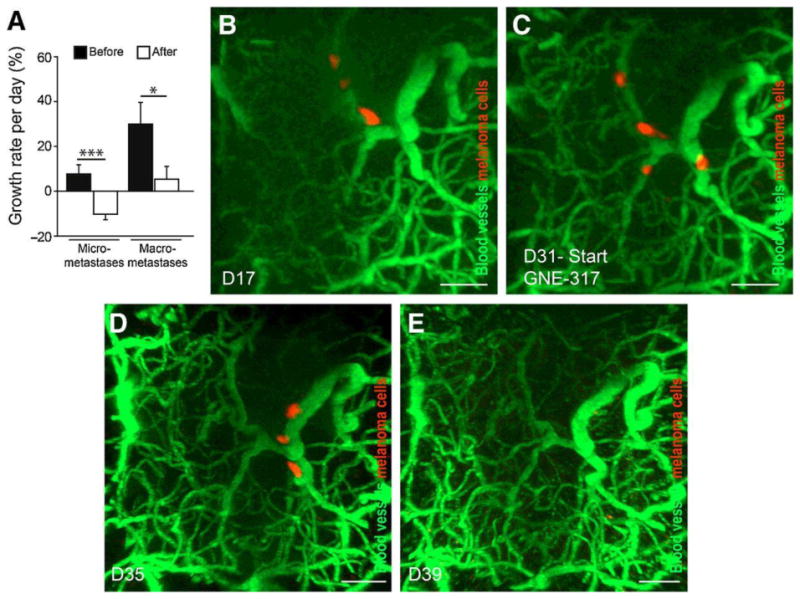
GNE-317 targets micro-metastases and dormant tumor cells. A, Subgroup analysis of GNE-317 treated metastases, separately for non-permeable micro- (n=10) and macro-metastases (n=6) (***, P < 0.001 and *, P=0.033; two- sided t tests). B–E, Treatment response of single, long-term, non-proliferating and non-regressing, thus dormant, melanoma cells. The single cells are slowly moving during dormancy. After start of treatment with GNE-317 at D31 (C), the dormant cells regressed within 8 days (E) (adapted from (Osswald et al. 2016), with permission). (2-column fitting image)
Given that the current imaging methods cannot detect micro-metastatic disease in the brain, future gains in early diagnosis will be obtained through newer cutting-edge imaging methods. Are there any such methods on the horizon? A related and equally important question is how reliably does current CE-MRI predict the condition of the BBB in larger metastatic brain tumors? Are there distinct regions within a larger metastatic site that have an intact BBB; i.e., a BBB that has heterogeneous integrity leading to regions that will have inadequate drug delivery? This is clearly an area that deserves more attention in future research.
The major diagnostic tool for primary and metastatic brain tumors is CE-MRI. While MRI has advantages over computed tomography (CT) and positron emission tomography (PET)/CT in terms of higher sensitivity, it is only moderately specific such as in differentiating between actual response and pseudoprogression. Use of newer MR and PET imaging techniques based on the detection of pathological changes like alterations in function, metabolism, etc. by assessing tissue metabolites (proton MR spectroscopy), water mobility/infiltration (diffusion weighted MRI), vascularity (DSC perfusion weighted imaging), metabolism (FDG PET) and proliferation (18F-FET PET, 18F-DOPA PET), may be valuable in providing an earlier and more clear diagnosis. Combining some of these techniques (e.g., pre Gad T1 with DSC perfusion weighted imaging) may provide additional value and minimize issues of misdiagnosis and lack of early detection (Bruzzone et al. 2012). An emerging imaging technique that has been shown to be useful in providing physiological details of a tumor such as vascularity and hemodynamic attributes is dynamic contrast-enhanced (DCE) MRI. Such information is beneficial in differentiating the aggressive hypervascular tumors from hypovascular tumors, which is not possible with conventional CE-MRI (Jung et al. 2016). Nevertheless, even with these currently existing imaging methods, future progress in early detection, and corresponding BBB integrity, will require further refinement of existing methods, or the development of new imaging methods that have greater sensitivity to detect tumor. It is important that such methods do not rely on the breakdown of the BBB to identify tumors.
3.4 In a related question (to #3), does the analysis of drug levels in a resected brain tumor specimen reflect drug levels in other regions of the brain that require treatment, especially in and around the micro-metastases?
Using total drug levels determined in a homogenized resected brain tumor as a measure of the drug delivery to that tumor is misleading. Regions of the resected tumor core may have a relatively leaky BBB allowing enhanced distribution of drug and may show higher drug levels than other regions of metastatic tumors in the brain (Gerstner, Fine 2007). However, in tumor regions that have an intact BBB, like the micro-metastases or even specific volumes within a larger tumor mass, there will be restricted delivery of those compounds that have limited BBB permeability. Therefore, drug concentrations measured by examining a homogenate of a portion, or the entire excised lesion may mask the regions of inadequate drug delivery and as such will not be a good representation of regio-specific drug delivery to areas of growing tumor behind an intact BBB.
Studies on BBB integrity conducted in animal models of metastatic breast cancer have revealed some important findings that can be instructive for brain metastases in general (Lockman et al. 2010; Mittapalli et al. 2017). More than 89% of the metastatic lesions in the brain showed only partial BBB compromise, and the magnitude of compromise was observed to vary within and between metastases. The permeability of the heterogeneous BBB in the tumor correlated with degree of drug distribution and the response (Lockman et al. 2010). Moreover, less than 10% of the brain metastases had a BBB that was adequately permeable to allow therapeutic agents to reach the desired efficacious levels (Adkins et al. 2016). Mittapalli et al. have used a novel quantitative fluorescent microscopy imaging technique to determine the permeability of brain metastases in a preclinical breast cancer metastatic model (Mittapalli et al. 2017). They observed that the average within tumor BBB permeability varied broadly among individual metastases in contrast to the relatively homogenous average tumor-to-tumor permeability in RG2 gliomas. Also, the vascular pore size in the metastatic lesions was about 10-fold smaller than that observed in the glioma model, suggesting a less leaky vasculature in metastases compared to this primary brain tumor model (Mittapalli et al. 2017). Such observations highlight the heterogeneity of drug delivery to the brain metastatic lesions. Given the intricacies of a heterogeneously leaky BBB among different metastatic lesions, methods that simultaneously measure drug concentrations and the BBB integrity in a regio-specific manner will be necessary to determine correlations between delivery and efficacy.
Any technique that can enable the measurement of drug concentrations in distinct regions of the brain, with sufficient spatial resolution to correlate local concentrations with measurements of efficacy, will be extremely valuable. The use of the MALDI-MSI technique has been an important step forward in this direction. MALDI-MSI is a continually improving methodology that can be used to visualize relative drug levels in distinct regions of a brain section, allowing one to gain a spatial and temporal understanding of brain drug distribution. Liu et al. have used MALDI-MSI to describe the regional brain distribution of three model drugs with differential BBB permeability in mice and show that the correlation of drug and heme images can be used to understand drug permeability across BBB (Liu et al. 2013). Heme was validated as a robust marker for vasculature in the brain and measured in conjunction with the model compounds. The MALDI signal for RAF265, a RAF inhibitor with poor brain penetration, was observed to co-localize with the signal for heme, suggesting the confinement of drug to the vasculature and the inability to permeate an intact BBB, however, the RAF inhibitor was able to penetrate an intracranial tumor core (Figure 7). In contrast, the signal for BKM120, a PI3K inhibitor with good brain penetration, was observed to be well dispersed throughout the brain tissue, even the “normal” brain tissue, indicating the ability of this drug to permeate an intact BBB and distribute to various regions of the brain parenchyma. Also, for the case of erlotinib, an EGFR inhibitor, studied in U87 glioma xenograft tumors, the signal intensity for heme was high around the tumor margin (indicative of disruption of the BBB) and erlotinib was able to escape the tumor vasculature to evenly distribute within the tumor tissue of the U87 model (Liu et al. 2013). However, there are significant questions about the lack of invasiveness of the U87 model, and whether it is a good model to explore spatial differences in BBB permeability to erlotinib or other targeted agents.
Figure 7.
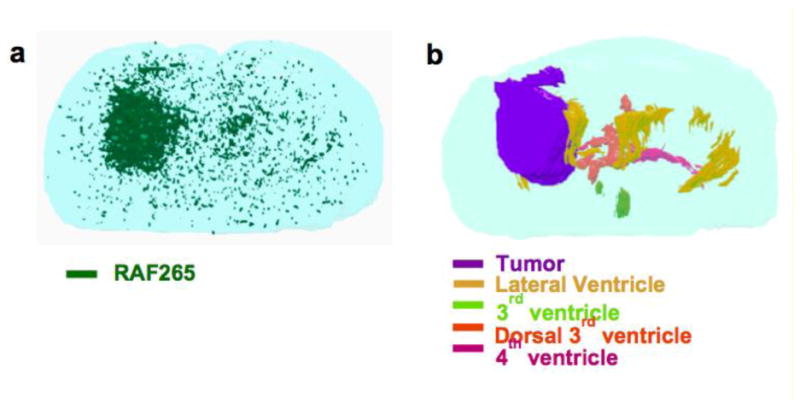
3D reconstruction of (A) RAF265 (in green), (B) tumor (in purple) and ventricles (lateral ventricles in yellow, 3rd ventricle in light green, dorsal 3rd ventricle in orange, 4th ventricle in magenta) from optical images of H&E staining sections. Serial sections were collected with 30-50 μm intervals and imaged by mass spectrometer with 100 μm spatial resolution or microscope. 3D models were reconstructed using 3D Doctor (adapted from (Liu et al. 2013), with permission). (2-column fitting image)
Recently, Aikawa et al. have studied penetration of alectinib (ALK inhibitor) to regions of non-tumor-bearing brain in preclinical mouse models using a combination of MALDI-MSI and LC-MS/MS techniques, referred to as quantitative MSI (qMSI) by the authors. The technique involved the use of serial brain sections in which the first and third sections were used for the measurement of alectinib using LC-MS/MS and the second (middle) section was used for drug analysis by MALDI-MSI (Figure 8). The integrated MSI response was then correlated to the LC-MS/MS determined drug concentrations in a homogenized slice to add a quantitative capability to the conventional MSI.
Figure 8.
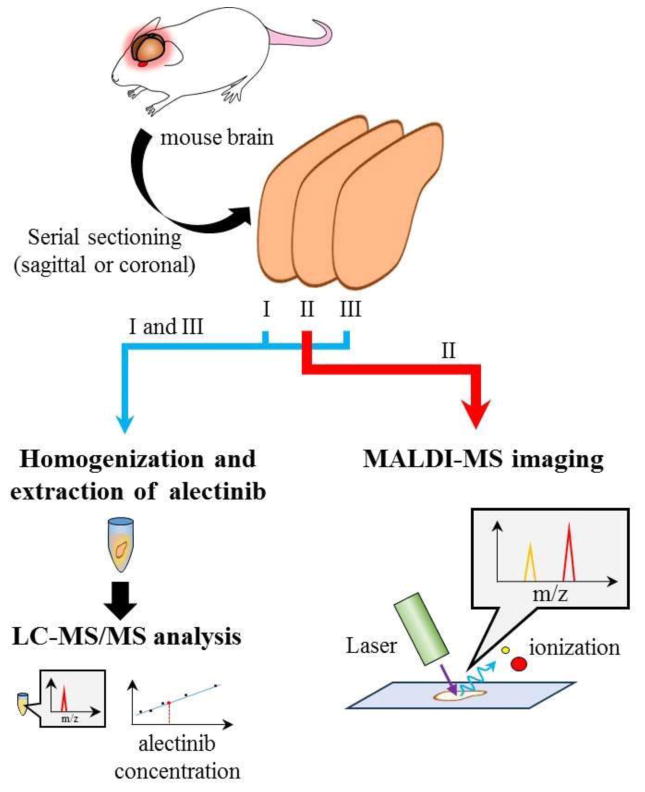
A schematic representation of the quantitative MSI (qMSI) approach. Three serial sections isolated from non-tumor bearing normal brain specimens were used to create the qMSI image. One brain section was used to detect the alectinib distribution by MALDI-MSI, whereas the other two sections were used to quantify the amount of alectinib. The signal intensity of the images detected by MALDI-MSI was converted into the absolute quantity of alectinib found in the serial section (adapted from (Aikawa et al. 2016), with permission). (1.5-column fitting image)
However, it is important to note in this situation that any spatial differences in drug concentrations within a slice will be lost by this technique, as will the power of the MALDI-MSI to determine relative concentrations with a high spatial specificity. To address that caveat, laser microdissection (LMD) of specific regions of additional serial sections followed by LC-MS/MS drug concentration measurements in the LMD specimens was performed in an attempt to confirm the quantitative distributions of alectinib obtained by qMSI (Figure 9) (Aikawa et al. 2016). The use of such advanced techniques to understand not only the drug permeability across the BBB, but also the differential distribution to distinct regions of the “normal” brain and within brain tumors is essential to better understand and correlate drug delivery to observed response. While the development of MALDI-MSI as a tool to better understand brain drug delivery is exciting, the technique has limitations in terms of quantitative measurements of drug levels. Therefore, there is still a pressing need for the development of novel highly-sensitive quantitative modalities to clearly understand how drug delivery to distinct regions of the brain and/or tumor may influence efficacy. Progress in finding effective therapies for the treatment of MBM and other brain tumors will rely on these new methods of analysis.
Figure 9.
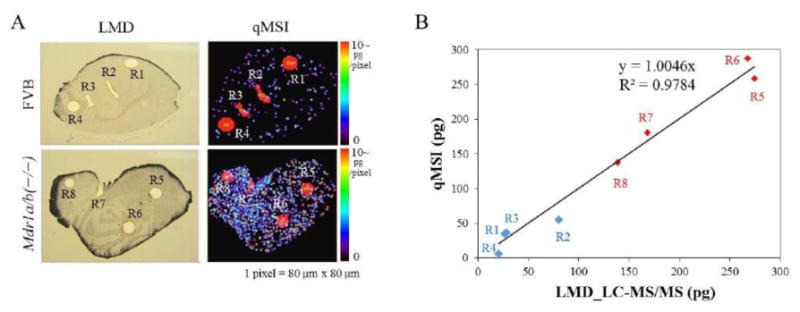
Validation of the quantitative distribution of alectinib with qMSI by using laser microdissection technique. A, mouse brain sections isolated at 1 hour after administration of 20 mg/kg alectinib were used to confirm the quantitative alectinib distribution in the qMSI image (80 μm resolution) in FVB and MDR1a/b knockout mice. Laser microdissection was used to cut out eight separate regions (R1-R8) of additional brain sections, and alectinib in those tissue regions was quantified by LC-MS/MS. B, correlation between the amounts of alectinib measured from dissected R1-R8 regions and those determined from complementary R1-R8 regions of qMSI images was examined. FVB: blue, R1-R4; MDR1a/b knockout: red, R5-R8. (adapted from (Aikawa et al. 2016), with permission). (2-column fitting image)
3.5 Will the use of drugs that have initial efficacy in systemic (i.e., non-brain) metastases, but have poor brain penetration, lead to resistant brain metastases? If so, will this be through mechanisms different than those leading to the eventual resistance at systemic sites?
It is evident that targeted therapies such as vemurafenib, dabrafenib, and their respective combinations with MEK inhibitors, cobimetinib and trametinib have improved the treatment of metastatic melanoma (Bates 2013). However, these targeted agents have poor permeability (Choo et al. 2014; Mittapalli et al. 2013; Mittapalli et al. 2012; Vaidhyanathan et al. 2014) across the BBB likely leading to subtherapeutic drug concentrations in areas of the brain where the BBB is intact. Current clinical data indicate that a significant proportion of patients stop responding to these targeted agents due to the development of resistance in systemic tumors (Welsh et al. 2016). It is certainly possible that the limited distribution of these drugs to the brain will lead to sub-therapeutic concentrations that may select for resistant tumor cells. The existence of locations within the metastatic lesions having an intact BBB can lead to sub-therapeutic drug exposures and the establishment of a sanctuary site. Such sub-therapeutic drug exposures, especially over prolonged periods, may induce the development of resistance to therapy by tumor cells. The development of resistant clones of metastatic cells at non-brain sites and dissemination of the resistant cells from such sites to further seed the brain with drug resistant tumor cells can also possibly result in the growth of resistant MBMs. Furthermore, it is possible that with the development and use of agents that are BBB-penetrant, the mechanisms that drive resistance in systemic tumors and brain may become more similar. These perspectives on site-specific resistance mechanisms need further study, and future results may more clearly inform clinicians on drug choice to combat resistance.
Also, it is important to recognize that the expression of tumor targets could be different in tumors in the brain versus systemic tumor locations, possibly driven by differences in the tumor microenvironment. The hyperactivation of PI3K-AKT pathway and loss of PTEN have been implicated in the brain but not in extracerebral melanoma metastases (Bucheit et al. 2014). It was observed in preclinical mouse xenograft studies that a number of genes were reprogrammed, i.e., multiple gene expression changes occurred when tumors cells were implanted in the brain, irrespective of the type of tumor cell (Park et al. 2011). A discordance in gene expression patterns between different metastatic sites of the same tumor type was observed, while the brain metastases from diverse tumor types were more similar to each other (Park et al. 2011) which may be reflective of the similar microenvironment (a common soil). In a retrospective study involving whole-exome sequencing of 86 matched brain metastases, primary tumors, and normal tissue samples, Brastianos et al. have observed that 53% of the patients had clinically instructive alterations in brain metastases that were not detected in primary tumors (Brastianos et al. 2015). Also interesting was that though genetically different from the primary tumors, distinct brain metastases were genetically homogenous and were associated with alterations sensitive to PI3K/AKT/mTOR, CDK, and HER2/EGFR inhibitors. Such alterations may be required for the establishment of metastatic disease in the brain, and may also influence the development of therapeutic resistance. Given these observations, characterization of the brain metastases post tissue collection from craniotomies may provide vital information not obtained from the genomic analysis of primary tumors, and would guide the selection of therapies directed towards key targets expressed in brain metastases. It is possible that diverse metastatic entities converge in common soil providing repeated, targetable features for appropriate molecularly-targeted agents (Brastianos et al. 2015; Saunus et al. 2015). The development of brain-penetrant inhibitors targeting such alterations expressed in brain metastases, and the conduct of clinical trials testing them, would delineate the clinical viability of such targets for the treatment of brain metastases, including MBM.
Given such differences in gene expression patterns between the systemic sites and the brain, it is possible that the mechanisms of resistance may be different in the brain compared to other systemic sites. However, additional research is needed in this area to clearly demarcate any such differences in resistance mechanisms. One question with respect to this: is the difference in tumor response and/or resistance between systemic sites and those in the brain related to only differences in drug delivery (i.e., achievable concentration), or are there specific influences of the different microenvironments, beyond drug delivery, that could lead to differences in efficacy and/or resistance?
We do not yet have a comprehensive understanding of other roles of the tumor microenvironment in the brain compared to the periphery (i.e., outside the brain) that may influence therapeutic outcomes. The microenvironment could be dictating the changes in gene expression profiles that, along with limited drug exposure, may lead to emergence of unique patterns of resistance and ultimately determine the choice of targets and subsequent drugs considered for the treatment of metastatic disease in the brain.
An important question related to the influence of the microenvironment is: will brain-only failure during targeted therapy portend the emergence of peripheral (i.e., non-brain) resistance? Given that the lack of adequate drug delivery and different gene expression patterns distinguishes the MBM from systemic metastases, systemic metastases could be responsive to targeted therapy and show initial tumor regression, during which time there may be “brain-only” failure to therapy. The question then becomes: will tumor cells from such resistant brain metastases reseed regions outside the brain, leading to the development of resistant systemic metastases? This is clearly an important clinical question that needs further study; in spite of its multi-factorial complexity.
3.6 What are the mechanisms responsible for the homing of tumor cells to the brain and how does the integrity of the BBB at the metastatic site affect the homing process? Also, will the integrity of the BBB at a metastatic site influence the rate of tumor growth in individual brain metastases?
Specific interactions that lead to the homing of melanoma tumor cells to the brain are not well understood. However, a few recent findings indicate some possible mechanisms implicated in the establishment of MBMs. The expression of PLEKHA5 has been found to be linked to the increased transmigration of melanoma cells across the BBB and is associated with a higher risk of MBM formation (Jilaveanu et al. 2015; Eisele et al. 2015). Also, β1 integrins have been implicated to play a vital role in furthering the metastatic process by regulating the shift of cells to active and migratory mode, likely an important step in the formation of MBM (Barkan, Chambers 2011) Another study suggests that matrix metalloproteinase proteins (MMPs) may be playing a role in the process of invasion of melanoma cells to the brain. Overexpression of MMPs such as MMP2 and MMP9 was suggested to aid the melanoma cells in permeating the BBB (Rizzo et al. 2015). Furthermore, it has been reported that claudin1, a tight junction protein, was responsible for the suppression of the malignant phenotype in melanoma (Izraely et al. 2015). It is known that claudins are important transmembrane proteins of the tight junctions between endothelial cells in the BBB. Given this, the integrity of the BBB may be playing an essential role in the process of homing of the metastatic tumor cells to the brain. A further elucidation of the key driving mechanisms responsible for homing of the metastatic melanoma cells to the brain will be useful in devising strategies for the prevention and treatment of MBM.
Osswald et.al, has examined the relationship between the integrity of BBB at the tumor site and the speed of tumor growth in preclinical animal models with MBMs. They report that the MBMs with a leaky BBB had a higher growth rate compared to that of MBMs with an intact BBB. Such an observation was attributed to a possibly more robust supply of growth factors and nutrients to the “BBB-permeable” metastases. Clearly more studies in this area are needed to further clarify the mechanisms involved (Osswald et al. 2016).
3.7 In drug discovery, design and development, how can we find drugs that are active against the relevant targets, have adequate BBB permeability, and limit the development of resistance; the critical issues currently limiting the treatment of MBM?
To achieve significant improvements in treatment outcomes in patients with MBM, it is necessary to design and develop drugs that are potent in inhibiting key oncogenic targets, limit the emergence of therapeutic resistance and are capable of penetrating an intact BBB. All of these aspects should be considered early in the drug development process to guide the design of novel drugs. When using potent combination therapies to overcome resistance, it is required that all the individual drugs in the combinations penetrate the BBB to achieve therapeutically active drug levels in the brain. Until all of these necessary elements of drug design are met, we will likely be frustrated in our quest to find effective treatments for MBM. Also important, is the use of appropriate animal models for testing new investigational drugs. Preclinical studies with high predictive and clinical relevance are invaluable in the drug development process, and can greatly expedite the search for efficacious treatments.
A few examples of such medicinal chemistry tailored approaches have been described by Heffron in a recent perspective. The physicochemical properties of kinase inhibitors recognized as CNS penetrating (n = 20; molecules with meaningful free brain concentrations or are not substrates of P-gp/BCRP) to those that have limited CNS penetration (n = 48; molecules with low brain concentrations or are substrates of P-gp/BCRP), as well as 119 marketed CNS drugs, were compared. The median and mean values of cLogP, cLogD7.4, total polar surface area (TPSA), hydrogen bond donor (HBD) count, and molecular weight between the two categories of kinase inhibitors were notably similar, and the only significant disparity was the calculated pKa (which can affect HBD count for those compounds that are sufficiently basic). The CNS penetrating inhibitors had a lower median pKa than both kinase inhibitors and CNS drugs incapable of crossing the BBB. Such examples illustrate the importance of subtle differences in the fundamental properties of the therapeutic compounds, and how they critically impact the brain delivery of these compounds. These ideas should be incorporated into future drug development efforts in the pursuit of brain-penetrant inhibitors for the treatment of CNS disorders (Heffron 2016).
In particular, Heffron's article describes the discovery of three molecules; AZD3759, lorlatinib, and TAK-960, from five compounds that were initially designed with the intent of improving brain penetration, where intramolecular hydrogen bonds were exploited to mask at least 1 HBD (this masking would not be accounted for in calculated physicochemical properties of those molecules) and would also affect the effective polar surface area. As Heffron points out, it is possible that other CNS penetrant kinase inhibitors, whether intentionally or not, also employ a similar design strategy to avoid efflux (Heffron 2016). Regardless, this is an approach that is worth consideration in the development of brain-penetrant inhibitors for treatment of CNS disorders, including brain metastases. If the lessons learned from the development of currently employed compounds are applied in a positive way, early in the development of future therapeutic compounds intended for treatments related to CNS, we will likely see more targeted therapies that are capable of penetrating the BBB and overcoming this key hurdle. Achieving this would be a major leap in the struggle to find more efficacious treatments, even cures, for CNS disorders, specifically for MBM that are protected by an uncompromised BBB.
Optimal drug design and development efforts should be complimented by appropriate subsequent preclinical testing, pertinent to the research objectives. Preclinical animal models are indispensable tools that aid in gaining mechanistic insights of the disease biology, and in screening activity of drugs in the developmental pipeline. A useful animal model should be reproducible and closely recapitulate the human disease. Such models developed to study MBM would be most beneficial if they generate spontaneous tumors and form metastatic lesions with high propensity to seed the brain, as seen clinically (Kerbel 2015). Patient-derived xenograft (PDX) models are biologically stable across passages and preserve the primary histopathological, genetic and molecular characteristics of patient tumor. Due to their value as predictive tools of clinical outcomes, PDXs are being used for various applications including preclinical drug activity testing, biomarker identification, and developing personalized medicine approaches (Tentler et al. 2012; Hidalgo et al. 2014). Establishment of PDXs is achieved by implanting tumor cells isolated from patients into immunodeficient mice (subcutaneous or orthotopic), and such an approach is suitable for melanoma (Hidalgo et al. 2014; Quintana et al. 2008). PDXs are useful as “avatars” for testing anti-cancer therapies, with a high predictive value of potential clinical outcomes (Quintana et al. 2012; Einarsdottir et al. 2014). For example, Krepler and colleagues have used mouse PDXs in combination with targeted sequencing and phosphoproteomics for the conduct of personalized “preclinical trials”, that led to successful identification of second-line targeted therapies for treating drug resistant tumors (Krepler et al. 2016). Another useful preclinical model is the genetically engineered mouse model (GEMM) that harbors mutations common to MBM, and has growth patterns resembling human disease. One advantage of GEMMs over PDX models is that the lesions develop in an immunocompetent host, allowing testing of both targeted and immunotherapies with appreciable predictive and clinical relevance (Ruggeri et al. 2014). Another valuable model is the spontaneous metastasis model that is capable of recapitulating all the events involved in the multi-step process of metastatic spread. The technique involves an in-vivo serial selection process for isolation of enriched aggressive metastatic cells, which are then orthotopically implanted in mice to allow the formation of distant visceral metastases (Kerbel 2015). The observed metastatic patterns are variable between animals, providing a realistic representation of the clinical situation (Francia et al. 2011). These models have been used in a few translational studies, and an example is a study showing the growth of relapsed spontaneous brain metastases in mice with advanced systemic metastatic melanoma but without brain disease, after systemic disease control upon drug treatment (Cruz-Munoz et al. 2008).
A major challenge with preclinical models, given the important role of tumor microenvironment in cancer biology, is the change of stromal components from predominantly human to predominantly mice. PDX and spontaneous metastases models employ immunocompromised hosts, which prevents their use for testing immunotherapies. Also, generation of models from a patient tumor isolated at a single time point cannot capture the broad diversity of the dynamic course of disease progression (Francia et al. 2011; Hidalgo et al. 2014; Tentler et al. 2012). In spontaneous metastases models, a problem is the regrowth of primary tumors at the location of surgical resection, and also resection of primary tumors itself can cause complications. Also, the selection of aggressive subpopulations of metastatic tumor cells can need multiple cycles of in-vivo selection (Francia et al. 2011; Kerbel 2015). The major challenges with the development of GEMMs are asynchronous development of tumors in the transgenic host, and lack of availability of tissue-specific promoters for developing certain cancers (Ruggeri et al. 2014). Despite the unique advantages offered, establishment of GEMMs and spontaneous metastases models is complex, expensive and time consuming, which may limit their practical utilization in testing therapeutics for MBM (Kerbel 2015; Ruggeri et al. 2014).
Given that each of the described models have certain advantages and disadvantages, the selection of an appropriate model for studying a given research question is critical, and it is beneficial to perform evaluations in more than one kind of model when feasible. A multi-model approach with spontaneous metastatic models as secondary screens in conjunction with GEMMs or PDX models can provide a broader picture of the likely outcomes of an investigational therapy in clinic (Kerbel 2015). While the development of strategies such as “humanized mice” with reconstituted human immune system (Krepler et al. 2016; Kalscheuer et al. 2012) to overcome the use of an immunocompromised host are underway, the current PDX and spontaneous metastases models are nevertheless valuable tools. Preclinical testing in the right animal model can guide the conduct of clinical studies and help make informed decisions, all of which play a pivotal role in improving the success rate of finding better therapies.
3.8 Are potential advances in the treatment of melanoma patients negatively impacted by the exclusion of patients with brain metastases from pivotal clinical trials testing novel targeted therapies?
It is important to include patients with symptomatic MBM in clinical trials to fully test the effectiveness of the novel targeted therapies. Achieving systemic disease control in a trial testing patients with detectable systemic metastases, but no brain metastases, might not be enough, as most of the patients ultimately die with brain metastases. Autopsy results show the presence of brain metastases in 70% of the studied melanoma patient population, suggesting a grave need to treat MBMs to improve therapeutic outcomes in patients with advanced melanoma or else the progress achieved with systemic disease control will remain incremental (Gorantla et al. 2013; McWilliams et al. 2003). One traditional drug development model has been to conduct clinical trials designed to test the safety and therapeutic efficacy of novel therapeutics in patients with systemic disease burden, while excluding patients with detectable brain disease. The reason for such exclusionary practices has been the dismal prognosis associated with this patient population.
The pivotal clinical trials testing novel therapeutics associated with the treatment of melanoma comprised of more than 6000 metastatic melanoma patients but excluded patients with active MBMs (Cohen et al. 2016). Smaller trials testing these agents in MBM populations were opened later and included 234 patients, representing only 4.1% of the patients enrolled; clearly insufficient (Cohen et al. 2016). Given the growing evidence that suggests differences in the microenvironment of the brain versus systemic sites, in terms of both the expression of genomic drivers and restricted drug delivery to the brain, it is critical to include patients with brain involvement early in clinical trials to gain insight into the mechanisms that may lead to therapeutic failure (Brastianos et al. 2015). For instance, the PI3K inhibitors would historically have been tested in patients with systemic disease while this target may actually be more important in brain metastases (Chen et al. 2014; Bucheit et al. 2014; Niessner et al. 2016; Peng et al. 2016). The exclusionary practices in clinical trial testing of novel therapeutic agents could result in an incomplete understanding of key concepts. Thus, inclusion of patients with active MBMs early in clinical trials testing novel therapies, along with trials specifically tailored to this population, would help accelerate the development of therapies to treat the debilitating MBMs.
4) Opportunities for the future
The eight major questions that have been posed in this perspective emphasize significant gaps in our knowledge that, if answered, will directly address several critical unmet needs in the treatment of MBM. Research that can answer these questions, both preclinical and clinical, will undoubtedly move the field toward more efficacious treatments.
One aspect of many of the questions is how we can ensure adequate drug delivery to melanoma metastases that are protected by an uncompromised BBB. The small molecule molecularly-targeted agents are restricted by their ability to permeate an intact BBB, mainly due to active efflux by BBB transporters, particularly P-gp and BCRP (Agarwal et al. 2011b; Gampa et al. 2016; Parrish et al. 2015b). Also, it has been reported that melanoma cells previously treated with targeted agents are capable of expressing the ATP-dependent efflux transporter ABCB5, while a side-population of melanoma stem cells have been identified to express ABCB5 intracellularly and ABCB1 on the plasma membrane (Chartrain et al. 2012; Luo et al. 2012). Existence of such efflux mechanisms at the level of tumor cells forms a secondary barrier, which can further limit the delivery of small molecule molecularly-targeted therapies to the tumor cells. The exposure of metastatic tumor cells to sub-therapeutic drug levels will lead to poor treatment outcomes in patients with MBM due to the establishment of the brain as a pharmacological sanctuary site, possibly leading to the development of resistance. It was observed in preclinical models of metastatic melanoma that effective control of systemic metastatic burden for a period of time can result in an increased rate of relapse of brain metastases. The efficacy benefit in controlling systemic metastatic disease is possibly allowing the seeded micro-metastases in the brain to have more time to expand into detectable macroscopic metastases (Cruz-Munoz et al. 2008). Thus, it is important to enhance the drug delivery of targeted therapies to the brain. The development of brain-penetrant inhibitors, and their evaluation in preclinical and clinical studies, will help us understand the target-engagement related improvements in efficacy by improving drug delivery to MBM, thus eliminating one variable that could affect efficacy. Also important and related to this line of questioning is the development of techniques that can facilitate reliable measurements of drug concentrations at distinct locations of the brain, with adequate spatial resolution to correlate local drug concentrations with biomarkers of target engagement and overall efficacy.
A key area that needs attention in future research is the development of imaging modalities that can detect micro-metastatic disease early in the disease progression. This would help clinicians to develop a fundamental understanding of the timing of the first appearance of the brain metastases compared to the systemic melanoma tumors, and evaluate if any improvements in treatment outcomes can be achieved by using brain-penetrant targeted therapies early in the disease progression. A deeper elucidation of the driving mechanisms responsible for homing of the metastatic melanoma cells to the brain will be extremely useful in identifying potential new targets, and devising novel strategies for both the prevention and treatment of MBM. Such advances will definitely help us better understand the disease progression, and move forward in the quest for a cure to treat MBM.
Additional research is also required to clearly demarcate any differences in resistance mechanisms in the brain versus the systemic sites. While differences in drug delivery to the target sites is an important reason for differences in tumor response and/or resistance between systemic and brain metastatic sites, it is crucial to understand if there are any other specific influences of the different microenvironments that could lead to alterations in target expression, efficacy and/or resistance. As discussed above, the microenvironment of a metastatic site could be dictating the changes in gene expression profiles that, along with limited drug exposure, may lead to emergence of unique patterns of resistance and ultimately determine the choice of targets and subsequent drugs, possibly as combination therapies, that will be considered for the treatment of metastatic disease in the brain. Given the differences in gene expression patterns between the systemic sites and the brain, it is also possible that the mechanisms of resistance may be different in the brain compared to the systemic sites, leading to a “brain-only” pattern of failure. These viewpoints on site-specific resistance mechanisms need additional evaluation, and future results may clearly inform clinicians on the apt choice of drug(s) to combat resistance.
To achieve meaningful enhancements in treatment outcomes in patients with MBM, it is vital to design and develop drugs that are potent in inhibiting key oncogenic targets, limit the emergence of therapeutic resistance and are capable of penetrating an intact BBB. All of these aspects should be considered early in the drug development process to guide the design of novel drugs (Figure 10). Drugs specifically designed with the intent of treating brain metastases, all the while recognizing the source of primary tumor (e.g., melanoma, breast, lung cancer), might be more important than screening and developing agents for the treatment of a particular systemic tumor. Testing candidates from such a screening for use in the treatment of brain metastases growing from that specific cancer type may not include important “brain-specific” development parameters, such as blood-brain barrier penetration or efficacy against unique drivers that are preferentially expressed in the brain tumor. This is essential considering the critical idea that the brain, a common soil for the systemic tumor “seeds”, may be fostering the growth of specific clones of tumor cells and also causing changes in the tumor gene expression patterns, an adaptation that may be necessary for the tumor cells to grow in the privileged microenvironment of the brain. Such observations imply that brain metastases growing from different primary tumors may be more similar in terms of gene expression patterns, compared to the primary tumors from which they originate. Thus, developing brain-penetrant inhibitors specifically designed to be effective against brain metastases emerging from various primary tumors, might be a viable strategy to test in future drug development endeavors for the treatment of brain metastases. It is important to note the fact that drug delivery to the target sites and potency of the developed therapeutic agents in inhibiting the metastatic tumor cells in the brain, both dictate the ultimate outcome seen in the treatment of the brain metastatic disease.
Figure 10.
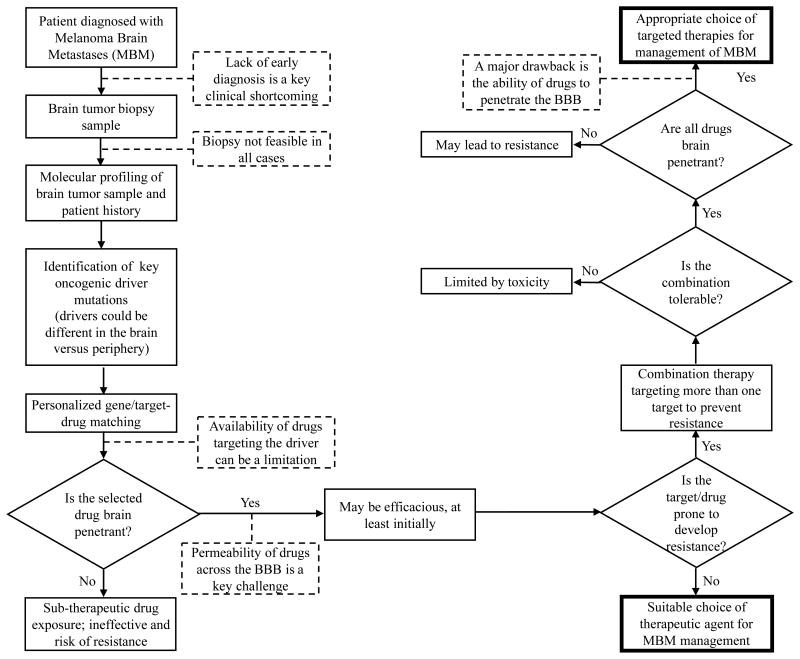
Flow chart depicting the sequence of steps that need to be considered for the treatment of a patient with MBM. Some of the key limitations in the current practice are listed in dashed boxes, the answers to which can help in the advancement of the treatment of MBMs. The ultimate goal is to find a suitable targeted therapy that is not just palliative but can provide a cure to the debilitating MBM. (2-column fitting image)
Achieving a modest improvement in the overall survival by the use of targeted therapies (e.g., vemurafenib and dabrafenib, combined with MEK inhibitors) in metastatic melanoma may be significant, but is still far from being sufficient to improve the MBM patient's dire situation. We need therapies that can treat the metastatic disease in the brain and prolong the survival to such an extent that it makes an impact on quality of life, beyond a meagre increase in survival of the patient by few months. It is therefore critical to include patients with active MBMs early in future clinical trials testing targeted agents, along with design of trials specifically tailored to test the MBM population, to help accelerate the development of therapies to treat the debilitating MBMs.
This perspective reviewed many of the critical issues that remain in developing effective treatments for MBM. The task of adequate delivery of potent agents to protected sites in the brain must be solved to avoid the possibility of brain-only failure and emergence of unique patterns of resistance. A better understanding of how the tumor microenvironment influences tumor growth is also required to help match effective drugs to appropriate patient. Great progress has been made, and future research, guided by the right questions, will undoubtedly improve the outlook for patients with MBM.
Acknowledgments
Funding: This work was partially supported by National Institutes of Health grant from: National Institute of Neurological Diseases and Stroke R01-NS077291 (WFE, JNS), R01-NS073610 (WFE), U54-CA210180 (WFE, JNS).
Abbreviations
- ABC
ATP-binding cassette
- AMT
adsorptive-mediated transcytosis
- BBB
blood-brain barrier
- BCRP
breast cancer resistance protein
- CE-MRI
contrast-enhanced magnetic resonance imaging
- CNS
central nervous system
- fu
unbound fraction
- CT
computed tomography
- CTLA-4
cytotoxic T-lymphocyte-associated antigen 4
- GAD-DTPA
gadolinium-diethylenetriamine pentaacetic acid
- GBM
Glioblastoma
- GEMM
genetically engineered mouse model
- HBD
hydrogen-bond donor
- Kpuu
unbound partition coefficient
- LC-MS/MS
liquid chromatography tandem mass spectroscopy
- LMD
laser microdissection
- MALDI
matrix assisted laser desorption ionization
- MAPK
mitogen activated protein kinase
- MBM
melanoma brain metastases
- MMP
matrix metallo-protease
- MSI
mass spectroscopy imaging
- NSCLC
non-small cell lung cancer
- NVU
neurovascular unit
- OS
overall survival
- PET
positron emission tomography
- PD-1
programmed death 1
- PD-L1
programmed death-ligand 1
- PDX
patient-derived xenograft
- PFS
progression-free survival
- P-gp
P-glycoprotein
- RMT
receptor mediated transcytosis
- SLC
solute carrier
- TJ
tight junctions
- TPSA
total polar surface area
Footnotes
Conflict of Interest Statement: The authors indicate no conflict of interest with the subject matter of this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36(3):437–49. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Adkins CE, Mohammad AS, Terrell-Hall TB, Dolan EL, Shah N, Sechrest E, et al. Characterization of passive permeability at the blood-tumor barrier in five preclinical models of brain metastases of breast cancer. Clin Exp Metastasis. 2016;33(4):373–83. doi: 10.1007/s10585-016-9784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Hartz AM, Elmquist WF, Bauer B. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des. 2011a;17(26):2793–802. doi: 10.2174/138161211797440186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med. 2011b;13:e17. doi: 10.1017/S1462399411001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa H, Hayashi M, Ryu S, Yamashita M, Ohtsuka N, Nishidate M, et al. Visualizing spatial distribution of alectinib in murine brain using quantitative mass spectrometry imaging. Sci Rep. 2016;6:23749. doi: 10.1038/srep23749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D, Chambers AF. beta1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clin Cancer Res. 2011;17(23):7219–23. doi: 10.1158/1078-0432.CCR-11-0642. [DOI] [PubMed] [Google Scholar]
- Bates SE. A sea change in melanoma. Clin Cancer Res. 2013;19(19):5282. doi: 10.1158/1078-0432.CCR-13-2219. [DOI] [PubMed] [Google Scholar]
- Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015;5(11):1164–77. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone MG, D'Incerti L, Farina LL, Cuccarini V, Finocchiaro G. CT and MRI of brain tumors. Q J Nucl Med Mol Imaging. 2012;56(2):112–37. [PubMed] [Google Scholar]
- Bucheit AD, Chen G, Siroy A, Tetzlaff M, Broaddus R, Milton D, et al. Complete loss of PTEN protein expression correlates with shorter time to brain metastasis and survival in stage IIIB/C melanoma patients with BRAFV600 mutations. Clin Cancer Res. 2014;20(21):5527–36. doi: 10.1158/1078-0432.CCR-14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrain M, Riond J, Stennevin A, Vandenberghe I, Gomes B, Lamant L, et al. Melanoma chemotherapy leads to the selection of ABCB5-expressing cells. PLoS One. 2012;7(5):e36762. doi: 10.1371/journal.pone.0036762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Chakravarti N, Aardalen K, Lazar AJ, Tetzlaff MT, Wubbenhorst B, et al. Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clin Cancer Res. 2014;20(21):5537–46. doi: 10.1158/1078-0432.CCR-13-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo EF, Ly J, Chan J, Shahidi-Latham SK, Messick K, Plise E, et al. Role of P-glycoprotein on the brain penetration and brain pharmacodynamic activity of the MEK inhibitor cobimetinib. Mol Pharm. 2014;11(11):4199–207. doi: 10.1021/mp500435s. [DOI] [PubMed] [Google Scholar]
- Cohen JV, Tawbi H, Margolin KA, Amravadi R, Bosenberg M, Brastianos PK, et al. Melanoma central nervous system metastases: current approaches, challenges, and opportunities. Pigment Cell Melanoma Res. 2016;29(6):627–42. doi: 10.1111/pcmr.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Munoz W, Man S, Xu P, Kerbel RS. Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 2008;68(12):4500–5. doi: 10.1158/0008-5472.CAN-08-0041. [DOI] [PubMed] [Google Scholar]
- Damsky WE, Theodosakis N, Bosenberg M. Melanoma metastasis: new concepts and evolving paradigms. Oncogene. 2014;33(19):2413–22. doi: 10.1038/onc.2013.194. [DOI] [PubMed] [Google Scholar]
- Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–96. doi: 10.1002/cncr.25634. [DOI] [PubMed] [Google Scholar]
- Davies MA, Saiag P, Robert C, Grob JJ, Flaherty KT, Arance A, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(17)30429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gooijer MC, Zhang P, Thota N, Mayayo-Peralta I, Buil LC, Beijnen JH, et al. P-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclib. Invest New Drugs. 2015;33(5):1012–9. doi: 10.1007/s10637-015-0266-y. [DOI] [PubMed] [Google Scholar]
- Di Giacomo AM, Margolin K. Immune checkpoint blockade in patients with melanoma metastatic to the brain. Semin Oncol. 2015;42(3):459–65. doi: 10.1053/j.seminoncol.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Dolgikh E, Watson IA, Desai PV, Sawada GA, Morton S, Jones TM, et al. QSAR Model of Unbound Brain-to-Plasma Partition Coefficient, Kp,uu,brain: Incorporating P-glycoprotein Efflux as a Variable. J Chem Inf Model. 2016;56(11):2225–33. doi: 10.1021/acs.jcim.6b00229. [DOI] [PubMed] [Google Scholar]
- Dummer R, Goldinger SM, Turtschi CP, Eggmann NB, Michielin O, Mitchell L, et al. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer. 2014;50(3):611–21. doi: 10.1016/j.ejca.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Durmus S, Hendrikx JJ, Schinkel AH. Apical ABC transporters and cancer chemotherapeutic drug disposition. Adv Cancer Res. 2015;125:1–41. doi: 10.1016/bs.acr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Durmus S, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Oral availability and brain penetration of the B-RAFV600E inhibitor vemurafenib can be enhanced by the P-GLYCOprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Mol Pharm. 2012;9(11):3236–45. doi: 10.1021/mp3003144. [DOI] [PubMed] [Google Scholar]
- Einarsdottir BO, Bagge RO, Bhadury J, Jespersen H, Mattsson J, Nilsson LM, et al. Melanoma patient-derived xenografts accurately model the disease and develop fast enough to guide treatment decisions. Oncotarget. 2014;5(20):9609–18. doi: 10.18632/oncotarget.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele SC, Gill CM, Shankar GM, Brastianos PK. PLEKHA5: A Key to Unlock the Blood-Brain Barrier? Clin Cancer Res. 2015;21(9):1978–80. doi: 10.1158/1078-0432.CCR-14-2604. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33(12):579–89. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Essig M, Weber MA, von Tengg-Kobligk H, Knopp MV, Yuh WT, Giesel FL. Contrast-enhanced magnetic resonance imaging of central nervous system tumors: agents, mechanisms, and applications. Top Magn Reson Imaging. 2006;17(2):89–106. doi: 10.1097/01.rmr.0000245464.36148.dc. [DOI] [PubMed] [Google Scholar]
- Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ. The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011;21(2):107–12. doi: 10.1016/j.semcancer.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Fife KM, Colman MH, Stevens GN, Firth IC, Moon D, Shannon KF, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293–300. doi: 10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]
- Fortin D. The blood-brain barrier: its influence in the treatment of brain tumors metastases. Curr Cancer Drug Targets. 2012;12(3):247–59. doi: 10.2174/156800912799277511. [DOI] [PubMed] [Google Scholar]
- Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11(2):135–41. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampa G, Vaidhyanathan S, Wilken-Resman B, Parrish KE, Markovic SN, Sarkaria JN, et al. Challenges in the Delivery of Therapies to Melanoma Brain Metastases. Current Pharmacology Reports. 2016;2:309–25. doi: 10.1007/s40495-016-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol. 2007;25(16):2306–12. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]
- Gibney GT, Gauthier G, Ayas C, Galebach P, Wu EQ, Abhyankar S, et al. Treatment patterns and outcomes in BRAF V600E-mutant melanoma patients with brain metastases receiving vemurafenib in the real-world setting. Cancer Med. 2015;4(8):1205–13. doi: 10.1002/cam4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla V, Kirkwood JM, Tawbi HA. Melanoma brain metastases: an unmet challenge in the era of active therapy. Curr Oncol Rep. 2013;15(5):483–91. doi: 10.1007/s11912-013-0335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G, Robertson AG, MacKie RM. Cerebral metastases of cutaneous melanoma. Br J Cancer. 1997;76(2):256–9. doi: 10.1038/bjc.1997.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JJ, Catalanotti F, Munhoz RR, Cheng DT, Yaqubie A, Kelly N, et al. A Retrospective Evaluation of Vemurafenib as Treatment for BRAF-Mutant Melanoma Brain Metastases. Oncologist. 2015;20(7):789–97. doi: 10.1634/theoncologist.2014-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron TP. Small Molecule Kinase Inhibitors for the Treatment of Brain Cancer. J Med Chem. 2016;59(22):10030–66. doi: 10.1021/acs.jmedchem.6b00618. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izraely S, Sagi-Assif O, Klein A, Meshel T, Ben-Menachem S, Zaritsky A, et al. The metastatic microenvironment: Claudin-1 suppresses the malignant phenotype of melanoma brain metastasis. Int J Cancer. 2015;136(6):1296–307. doi: 10.1002/ijc.29090. [DOI] [PubMed] [Google Scholar]
- Jilaveanu LB, Parisi F, Barr ML, Zito CR, Cruz-Munoz W, Kerbel RS, et al. PLEKHA5 as a Biomarker and Potential Mediator of Melanoma Brain Metastasis. Clin Cancer Res. 2015;21(9):2138–47. doi: 10.1158/1078-0432.CCR-14-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung BC, Arevalo-Perez J, Lyo JK, Holodny AI, Karimi S, Young RJ, et al. Comparison of Glioblastomas and Brain Metastases using Dynamic Contrast-Enhanced Perfusion MRI. J Neuroimaging. 2016;26(2):240–6. doi: 10.1111/jon.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalscheuer H, Danzl N, Onoe T, Faust T, Winchester R, Goland R, et al. A model for personalized in vivo analysis of human immune responsiveness. Sci Transl Med. 2012;4(125):125ra30. doi: 10.1126/scitranslmed.3003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS. A Decade of Experience in Developing Preclinical Models of Advanced- or Early-Stage Spontaneous Metastasis to Study Antiangiogenic Drugs, Metronomic Chemotherapy, and the Tumor Microenvironment. Cancer J. 2015;21(4):274–83. doi: 10.1097/PPO.0000000000000134. [DOI] [PubMed] [Google Scholar]
- Krepler C, Xiao M, Sproesser K, Brafford PA, Shannan B, Beqiri M, et al. Personalized Preclinical Trials in BRAF Inhibitor-Resistant Patient-Derived Xenograft Models Identify Second-Line Combination Therapies. Clin Cancer Res. 2016;22(7):1592–602. doi: 10.1158/1078-0432.CCR-15-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–76. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- Liu X, Ide JL, Norton I, Marchionni MA, Ebling MC, Wang LY, et al. Molecular imaging of drug transit through the blood-brain barrier with MALDI mass spectrometry imaging. Sci Rep. 2013;3:2859. doi: 10.1038/srep02859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–78. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–95. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- Luo Y, Ellis LZ, Dallaglio K, Takeda M, Robinson WA, Robinson SE, et al. Side population cells from human melanoma tumors reveal diverse mechanisms for chemoresistance. J Invest Dermatol. 2012;132(10):2440–50. doi: 10.1038/jid.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin K. The Promise of Molecularly Targeted and Immunotherapy for Advanced Melanoma. Curr Treat Options Oncol. 2016;17(9):48. doi: 10.1007/s11864-016-0421-5. [DOI] [PubMed] [Google Scholar]
- Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–65. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- McArthur GA, Maio M, Arance A, Nathan P, Blank C, Avril MF, et al. Vemurafenib in Metastatic Melanoma Patients with Brain Metastases: An Open-Label, Single-Arm, Phase 2 Multicentre Study. Ann Oncol. 2016 doi: 10.1093/annonc/mdw641. [DOI] [PubMed] [Google Scholar]
- McWilliams RR, Brown PD, Buckner JC, Link MJ, Markovic SN. Treatment of brain metastases from melanoma. Mayo Clin Proc. 2003;78(12):1529–36. doi: 10.4065/78.12.1529. [DOI] [PubMed] [Google Scholar]
- Mittapalli RK, Adkins CE, Bohn KA, Mohammad AS, Lockman JA, Lockman PR. Quantitative Fluorescence Microscopy Measures Vascular Pore Size in Primary and Metastatic Brain Tumors. Cancer Res. 2017;77(2):238–46. doi: 10.1158/0008-5472.CAN-16-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittapalli RK, Vaidhyanathan S, Dudek AZ, Elmquist WF. Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther. 2013;344(3):655–64. doi: 10.1124/jpet.112.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittapalli RK, Vaidhyanathan S, Sane R, Elmquist WF. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032) J Pharmacol Exp Ther. 2012;342(1):33–40. doi: 10.1124/jpet.112.192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell DH, Hamilton AM, Mallett CL, van Gorkum R, Chambers AF, Foster PJ. Understanding Heterogeneity and Permeability of Brain Metastases in Murine Models of HER2-Positive Breast Cancer Through Magnetic Resonance Imaging: Implications for Detection and Therapy. Transl Oncol. 2015;8(3):176–84. doi: 10.1016/j.tranon.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell DH, Zarghami N, Jensen MD, Chambers AF, Wong E, Foster PJ. Evaluating Changes to Blood-Brain Barrier Integrity in Brain Metastasis over Time and after Radiation Treatment. Transl Oncol. 2016;9(3):219–27. doi: 10.1016/j.tranon.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessner H, Schmitz J, Tabatabai G, Schmid AM, Calaminus C, Sinnberg T, et al. PI3K Pathway Inhibition Achieves Potent Antitumor Activity in Melanoma Brain Metastases In Vitro and In Vivo. Clin Cancer Res. 2016;22(23):5818–28. doi: 10.1158/1078-0432.CCR-16-0064. [DOI] [PubMed] [Google Scholar]
- Osswald M, Blaes J, Liao Y, Solecki G, Gommel M, Berghoff AS, et al. Impact of Blood-Brain Barrier Integrity on Tumor Growth and Therapy Response in Brain Metastases. Clin Cancer Res. 2016;22(24):6078–87. doi: 10.1158/1078-0432.CCR-16-1327. [DOI] [PubMed] [Google Scholar]
- Park ES, Kim SJ, Kim SW, Yoon SL, Leem SH, Kim SB, et al. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proc Natl Acad Sci U S A. 2011;108(42):17456–61. doi: 10.1073/pnas.1114210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish KE, Pokorny J, Mittapalli RK, Bakken K, Sarkaria JN, Elmquist WF. Efflux transporters at the blood-brain barrier limit delivery and efficacy of cyclin-dependent kinase 4/6 inhibitor palbociclib (PD-0332991) in an orthotopic brain tumor model. J Pharmacol Exp Ther. 2015a;355(2):264–71. doi: 10.1124/jpet.115.228213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish KE, Sarkaria JN, Elmquist WF. Improving drug delivery to primary and metastatic brain tumors: strategies to overcome the blood-brain barrier. Clin Pharmacol Ther. 2015b;97(4):336–46. doi: 10.1002/cpt.71. [DOI] [PubMed] [Google Scholar]
- Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016;6(2):202–16. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitz MW, Desai A, Grossman SA, Blakeley JO. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011;104(3):629–38. doi: 10.1007/s11060-011-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Piskounova E, Shackleton M, Weinberg D, Eskiocak U, Fullen DR, et al. Human melanoma metastasis in NSG mice correlates with clinical outcome in patients. Sci Transl Med. 2012;4(159):159ra49. doi: 10.1126/scitranslmed.3004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456(7222):593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizer JJ, Hwu WJ, Panageas KS, Wilton A, Baldwin DE, Bailey E, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199–207. doi: 10.1215/15228517-2007-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo A, Vasco C, Girgenti V, Fugnanesi V, Calatozzolo C, Canazza A, et al. Melanoma cells homing to the brain: an in vitro model. Biomed Res Int. 2015;2015:476069. doi: 10.1155/2015/476069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochet NM, Dronca RS, Kottschade LA, Chavan RN, Gorman B, Gilbertson JR, et al. Melanoma brain metastases and vemurafenib: need for further investigation. Mayo Clin Proc. 2012;87(10):976–81. doi: 10.1016/j.mayocp.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri BA, Camp F, Miknyoczki S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem Pharmacol. 2014;87(1):150–61. doi: 10.1016/j.bcp.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Sakji-Dupre L, Le Rhun E, Templier C, Desmedt E, Blanchet B, Mortier L. Cerebrospinal fluid concentrations of vemurafenib in patients treated for brain metastatic BRAF-V600 mutated melanoma. Melanoma Res. 2015;25(4):302–5. doi: 10.1097/CMR.0000000000000162. [DOI] [PubMed] [Google Scholar]
- Salphati L, Alicke B, Heffron TP, Shahidi-Latham S, Nishimura M, Cao T, et al. Brain Distribution and Efficacy of the Brain Penetrant PI3K Inhibitor GDC-0084 in Orthotopic Mouse Models of Human Glioblastoma. Drug Metab Dispos. 2016;44(12):1881–9. doi: 10.1124/dmd.116.071423. [DOI] [PubMed] [Google Scholar]
- Salphati L, Heffron TP, Alicke B, Nishimura M, Barck K, Carano RA, et al. Targeting the PI3K pathway in the brain--efficacy of a PI3K inhibitor optimized to cross the blood-brain barrier. Clin Cancer Res. 2012;18(22):6239–48. doi: 10.1158/1078-0432.CCR-12-0720. [DOI] [PubMed] [Google Scholar]
- Salphati L, Shahidi-Latham S, Quiason C, Barck K, Nishimura M, Alicke B, et al. Distribution of the phosphatidylinositol 3-kinase inhibitors Pictilisib (GDC-0941) and GNE-317 in U87 and GS2 intracranial 30 glioblastoma models-assessment by matrix-assisted laser desorption ionization imaging. Drug Metab Dispos. 2014;42(7):1110–6. doi: 10.1124/dmd.114.057513. [DOI] [PubMed] [Google Scholar]
- Samala R, Thorsheim HR, Goda S, Taskar K, Gril B, Steeg PS, et al. Vinorelbine Delivery and Efficacy in the MDA-MB-231BR Preclinical Model of Brain Metastases of Breast Cancer. Pharm Res. 2016;33(12):2904–19. doi: 10.1007/s11095-016-2012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunus JM, Quinn MC, Patch AM, Pearson JV, Bailey PJ, Nones K, et al. Integrated genomic and transcriptomic analysis of human brain metastases identifies alterations of potential clinical significance. J Pathol. 2015;237(3):363–78. doi: 10.1002/path.4583. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control. 2009;16(3):248–55. doi: 10.1177/107327480901600307. [DOI] [PubMed] [Google Scholar]
- Spagnolo F, Picasso V, Lambertini M, Ottaviano V, Dozin B, Queirolo P. Survival of patients with metastatic melanoma and brain metastases in the era of MAP-kinase inhibitors and immunologic checkpoint blockade antibodies: A systematic review. Cancer Treat Rev. 2016;45:38–45. doi: 10.1016/j.ctrv.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Steeg PS, Zimmer A, Gril B. Therapeutics for Brain Metastases, v3. Clin Cancer Res. 2016;22(24):5953–5. doi: 10.1158/1078-0432.CCR-16-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DJ. A critique of the role of the blood-brain barrier in the chemotherapy of human brain tumors. J Neurooncol. 1994;20(2):121–39. doi: 10.1007/BF01052723. [DOI] [PubMed] [Google Scholar]
- Tan CS, Cho BC, Soo RA. Next-generation epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor -mutant non-small cell lung cancer. Lung Cancer. 2016;93:59–68. doi: 10.1016/j.lungcan.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Taylor EM. The impact of efflux transporters in the brain on the development of drugs for CNS disorders. Clin Pharmacokinet. 2002;41(2):81–92. doi: 10.2165/00003088-200241020-00001. [DOI] [PubMed] [Google Scholar]
- Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9(6):338–50. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyokawa G, Inamasu E, Shimamatsu S, Yoshida T, Nosaki K, Hirai F, et al. Identification of a Novel ALK G1123S Mutation in a Patient with ALK-rearranged Non-small-cell Lung Cancer Exhibiting Resistance to Ceritinib. J Thorac Oncol. 2015;10(7):e55–7. doi: 10.1097/JTO.0000000000000509. [DOI] [PubMed] [Google Scholar]
- Vaidhyanathan S, Mittapalli RK, Sarkaria JN, Elmquist WF. Factors influencing the CNS distribution of a novel MEK-1/2 inhibitor: implications for combination therapy for melanoma brain metastases. Drug Metab Dispos. 2014;42(8):1292–300. doi: 10.1124/dmd.114.058339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidhyanathan S, Wilken-Resman B, Ma DJ, Parrish KE, Mittapalli RK, Carlson BL, et al. Factors Influencing the Central Nervous System Distribution of a Novel Phosphoinositide 3-Kinase/Mammalian Target of Rapamycin Inhibitor GSK2126458: Implications for Overcoming Resistance with Combination Therapy for Melanoma Brain Metastases. J Pharmacol Exp Ther. 2016;356(2):251–9. doi: 10.1124/jpet.115.229393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh SJ, Rizos H, Scolyer RA, Long GV. Resistance to combination BRAF and MEK inhibition in metastatic melanoma: Where to next? Eur J Cancer. 2016;62:76–85. doi: 10.1016/j.ejca.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Wu CP, S VA. The pharmacological impact of ATP-binding cassette drug transporters on vemurafenib-based therapy. Acta Pharm Sin B. 2014;4(2):105–11. doi: 10.1016/j.apsb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Guo Q, Wang Y, Chen K, Zhang L, Cheng Z, et al. AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med. 2016;8(368):368ra172. doi: 10.1126/scitranslmed.aag0976. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Wang J, Cheng Z, Chen K, Johnstrom P, Varnas K, et al. Discovery and Evaluation of Clinical Candidate AZD3759, a Potent, Oral Active, Central Nervous System-Penetrant, Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. J Med Chem. 2015;58(20):8200–15. doi: 10.1021/acs.jmedchem.5b01073. [DOI] [PubMed] [Google Scholar]