Abstract
Objective
Electrical neurostimulation has traditionally been limited to the use of charge-balanced waveforms. Charge-imbalanced and monophasic waveforms are not used to deliver clinical therapy, because it is believed that these stimulation paradigms may generate noxious electrochemical species that cause tissue damage.
Approach
In this study, we investigated the dissolution of platinum as one of such irreversible reactions over a range of charge densities up to 160 µC cm−2 with current-controlled first phase, capacitive discharge second phase waveforms of both cathodic-first and anodic-first polarity. We monitored the concentration of platinum in solution under different stimulation delivery conditions including charge-balanced, charge-imbalanced, and monophasic pulses.
Main results
We observed that platinum dissolution decreased during charge-imbalanced and monophasic stimulation when compared to charge-balanced waveforms.
Significance
This observation provides an opportunity to re-evaluate the charge-balanced waveform as the primary option for sustainable neural stimulation.
Keywords: neuromodulation, electrical stimulation, neurostimulation safety, charge balance, medical devices
1. Introduction
Electrical neurostimulation is a rapidly expanding line of therapy for an increasing number of neurological and physiological disorders (Pikov 2015). Future neuromodulation devices will employ a number of innovations such as microelectrodes (Rajan et al 2015) and waveforms of unconventional shape and current steering to enable higher spatial resolution (Firszt et al 2007). Progress in this field is dependent on the contemporary regulatory landscape enabling rapid patient access to the most physiologically-effective, power-efficient, and safe therapies available.
A number of limitations for safe electrical stimulation of neural tissue have been outlined in the literature (Merrill et al 2005, Cogan et al 2016). Proposed limits on waveform shape, charge per phase and charge density are based on observations from experiments in animals in which damage to neural tissue was observed at relatively high levels of electrical stimulation (Pudenz et al 1975, McCreery et al 1990). Several theories were proposed to explain the mechanism of tissue damage, including ‘overstimulation’ (excitotoxicity) due to damage from ionic imbalances, excessive neurotransmitter release, and metabolic stress; and damage from noxious electrochemical species generated during charge injection (Krauthamer et al 1991). Since the contribution of each of these factors is not very well understood, the existing stimulation limits are based on purely empirical observations (Shannon 1992). The empirical nature of this limit makes it hard to extrapolate to stimulation paradigms that are substantially different from the conditions that were initially used to establish this limit. One classical requirement is for a non-injurious stimulation waveform to be biphasic and charge-balanced, first proposed by Lilly et al (1955). The traditional hypothesis states that a charge-balanced waveform is less damaging to tissue than charge-imbalanced waveform, because electrochemically generated and potentially toxic species produced during the first phase of the waveform are consumed during the second phase of the waveform (Merrill et al 2005). However, very few studies have been performed to verify this hypothesis (Kumsa et al 2016a). Additionally, the charge-balanced concept has been challenged by in vivo studies by Mortimer and colleagues (Mortimer et al 1980, Scheiner et al 1990).
In this work, we investigated how charge-imbalance (less charge in the secondary phase) affects the generation of soluble platinum species. The dissolution of Pt electrodes occurs during charge injection on Pt and Pt-containing electrodes. Pt dissolution is an irreversible and undesirable electrochemical reaction (Agnew et al 1977, Robblee et al 1983). Our data demonstrates that charge-imbalance leads to a decrease in Pt dissolution during both cathodic-first and anodic-first pulses. This is an important observation that questions the established charge-balanced dogma and highlights the need for a more detailed evaluation of potential application of charge-imbalanced waveforms in neurostimulation.
2. Methods
2.1. Chemicals
All chemicals were purchased from Sigma-Aldrich (Sigma Aldrich, St Louis, MO) and used as received. All experiments were performed with the solution (0.2 mg ml−1) of bovine serum albumin (BSA) in phosphate-buffered saline (PBS) that was prepared using deionized water (18 MΩ cm Milli-Q). The solution was exposed to ambient atmosphere assuming equilibrium of the dissolved gases.
2.2. Electrochemical Experiments
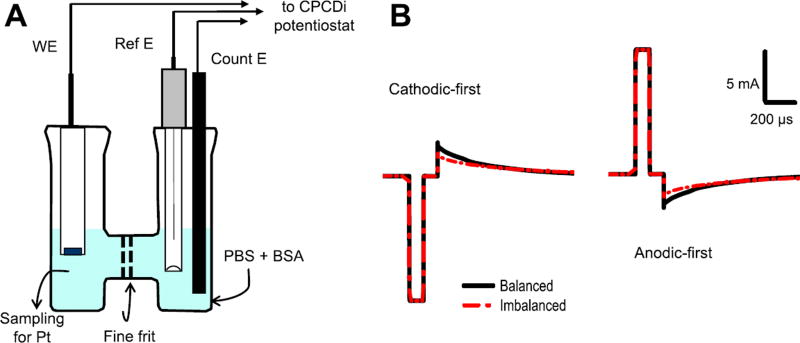
Prior to each experiment, a Pt disk electrode with a diameter of 0.1 cm and area of 0.00785 cm2 (ET075-1, eDAQ, Colorado Springs, CO) was polished on an aluminum oxide tile for 5 min and sonicated in deionized water for 3 min to ensure identical initial conditions. Measurements were performed in an open-to-atmosphere electrochemical H-cell (RRPG060, Pine Instrument, Durham, NC) at room temperature with 20 ml of PBS at pH 7.4 with BSA solution (Robblee et al 1980) in each compartment. The working electrode was placed in one compartment of the H-cell, and a Ag/AgCl reference electrode (930-00015, Gamry Instruments, Warminster, PA) and a graphite sheet counter electrode (Alfa Aesar, Ward Hill, MA) were placed in the other compartment of the H-cell (figure 1(A)).
Figure 1.
Experimental setup and sketch of the used waveforms. (A) Experiments were performed in an H-shaped bicameral cell filled with PBS solution of BSA with Ag/AgCl reference (Ref E) and carbon counter (Count E) electrodes located in the right compartment and Pt disk working electrode (WE) located in the left compartment where samples for ICP-MS analysis for Pt were taken at the end of pulsing. Current pulsing was performed with the CPCDi instrument at 50 Hz using capacitive discharge waveforms under both charge-balanced and charge-imbalanced conditions (B) in either cathodic-first or anodic-first configuration with 100 µs pulse width and 100 µs inter-phase delay.
Pulsing experiments were carried out using a Current-Pulse Capacitor Discharge instrument (CPCDi) manufactured at Case Western Reserve University (Kumsa et al 2016b). For charge-balanced waveforms, passive capacitive discharge was performed using a 1 µF capacitor. For charge-imbalanced waveforms, a 100 Ω leakage resistor was used in parallel with the capacitor. For monophasic pulsing, a diode rectifier was employed to allow current to flow only in one direction. The detailed description of the instrument is provided elsewhere (Kumsa et al 2016b). The pulsing was done at 50 Hz with a 100 µs pulse width and 100 µs inter-phase delay. Biphasic charge-balanced, charge-imbalanced and monophasic stimulations were investigated (figure 1(B)). A Tektronix oscilloscope (DPO 4034) with 10 MΩ input impedance probes was used for recording current and potential waveforms during pulsing measurements. The current amplitude varied from 1.73 mA to 12.56 mA, which corresponds to charge density of 22, 47, 77, 114 and 160 µC cm−2 per phase. This range of charge densities was chosen to cover a region of interest for stimulation parameters from k = 0.566 to k = 2.33 as defined by the Shannon equation (Shannon 1992). Each experiment was run for two hours. The level of charge imbalance was calculated by integrating recorded current versus time traces (figure 1(B)) and yielded 15% for cathodic-first and 18.2% for anodic-first biphasic pulses. For control experiments, the working electrode was left at open circuit potential. The experiments for each set of parameter were replicated three times.
2.3. Inductively coupled plasma mass spectrometry (ICP-MS)
Aliquots of the solution were taken from the H-cell compartment with the Pt disk working electrode at the end of the each run (figure 1(A)). ICP-MS analysis was performed using a 2% nitric acid matrix on a Thermo Scientific X series 2 ICP-MS instrument (Thermo Scientific, Waltham, MA). Pt concentration was converted into dissolution rate expressed in units of µg cm−2.
2.4. Data analysis
Data were processed and plots were generated using MS Excel (Microsoft, Redmond, WA) and Origin (OriginLab Corporation, Northampton, MA). All data are reported as mean and standard deviation.
3. Results
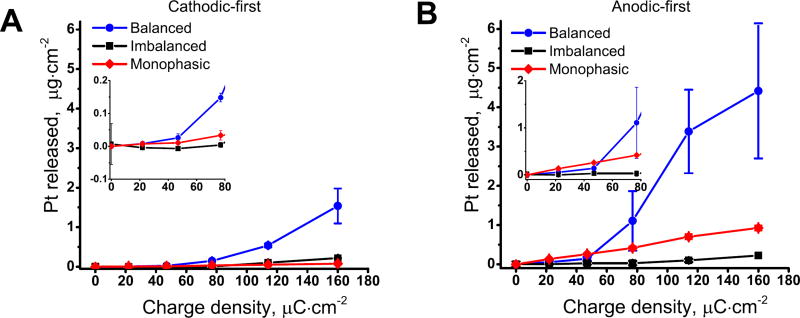
For the cathodic-first charge-balanced waveforms (figure 2(A)), the Pt dissolution increased with stimulation intensity up to 1.54 ± 0.44 µg cm−2 · after two hours for a charge injection level of 160 µC cm−2. Also, variability in measured Pt increased for higher charge injection values. When charge-imbalanced waveforms were used under the same conditions, Pt dissolution significantly decreased with a maximum dissolution of 0.218 ± 0.016 µg cm−2 after two hours of stimulation at 160 µC cm−2. Cathodic monophasic pulsing led to an even smaller amount of Pt released into the solution with a maximum dissolution of 0.078 ± 0.029 µg cm−2 after two hours of stimulation at 160 µC cm−2.
Figure 2.
Concentration of platinum in solution under different stimulation conditions. Concentration of Pt measured with ICP-MS after two hours of pulsing for cathodic-first (A) and anodic-first (B) stimulation at different charge densities at frequency of 50 Hz. Data reported for three independent replicates as mean and standard deviation. The inserts show scaled data for charge densities up to 80 µC cm−2.
For the anodic-first charge-balanced waveforms (figure 2(B)), Pt dissolution followed the same pattern of increasing dissolution rate with stimulation intensity, reaching a maximum of 4.42 ± 1.72 µg cm−2 after two hours of stimulation at 160 µC cm−2. Similar to the cathodic-first waveforms, the variability increased at higher Pt concentration, which follows the pattern that was reported earlier (Robblee et al 1980). The charge-imbalanced waveform led to a decrease in Pt dissolution with a maximum dissolution rate of 0.22 ± 0.02 µg cm−2 after two hours of stimulation at 160 µC cm−2. Conversely, the anodic-first monophasic waveform resulted in a slightly higher rate than imbalanced with Pt released to the solution with a maximum dissolution of 0.93 ± 0.06 µg cm−2 for two hours of stimulation at 160 µC cm−2.
4. Discussion
The use of charge-imbalanced waveforms to reduce electrode corrosion under cathodic-first pulses has previously been discussed in the literature (Scheiner et al 1990, Merrill et al 2005). Additionally, Pt dissolution during pulsing has been reported (Brummer et al 1977, Black and Hannaker 1979, McHardy et al 1980, Robblee et al 1980). Early work by McHardy et al (1977) demonstrated that cathodic charge imbalance reduces corrosion of stainless steel electrodes. However, direct relationship between charge-imbalanced waveform and its effect on irreversible electrochemical reactions such as Pt electrode dissolution under both cathodic-first and anodic-first pulses has not yet been established.
Our findings indicate that, for charge-balanced waveforms, Pt dissolution occurs over a wide range of charge densities including those in the region where the risk of tissue damage is considered to be minimal. For example, stimulation at charge density below 77 µC cm−2 and charge per phase of 0.6 µC for 0.1 cm diameter electrode (k = 1.66) places it in the region where no tissue damage was reported during brief stimulation in feline cortex (Pudenz et al 1975, McCreery et al 1990, Shannon 1992, Cogan et al 2016). The experiments were performed only for two hours, which makes it difficult to make predictions of the temporal profile of Pt release for a longer period of time. Furthermore, in vivo studies of Pt release during electrical stimulation have shown that the rate of Pt release does not follow monotonic behavior (Robblee et al 1983) with the glial capsule likely to provide a barrier for Pt diffusion further from the electrode into the tissue (Roitbak and Syková 1999, Prodanov and Delbeke 2016).
Interestingly, our data indicates larger release of Pt in case of anodic-first stimulation versus cathodic-first stimulation. Cathodic-first is generally considered as more efficient in evoking action potentials than anodic-first (Merrill et al 2005). Our results suggest that the sequence in stimulation polarity plays an important role not only in its electrophysiological effects, but also in the generation of potentially-noxious electrochemical species. Hence, the sequence of stimulation polarity might be an important factor in evaluation of its safety.
The charge-balanced requirement for clinical neurostimulation is based on the assumption that fewer products of irreversible electrochemical reactions are produced during charge-balanced stimulation. Our findings indicate the opposite behavior, with regards to Pt dissolution. For both charge-imbalanced and monophasic stimulation, less Pt is released in the solution for the experiments performed at the same charge density. Along with dissolution of Pt, other reactions such as reduction of oxygen with generation of reactive oxygen species (Morton et al 1994), oxidation of chloride and generation of oxychlorides (Brummer et al 1977), oxidation of endogenous compounds (Merrill et al 2005, Musa et al 2011) and electrolysis of water with shifts in pH and generation of hydrogen (H2) and oxygen (O2) are listed in the literature as potentially undesirable for neural tissue. The effect of charge-imbalance on these reactions needs to be further investigated to assess the potential of imbalanced waveform for clinical neurostimulation. Additional in vitro experiments are needed to fully characterize the effect of imbalanced waveforms on generation of other noxious species. This includes examination of electrochemical reactions occurring during pulsing. Additionally, investigation of charge-imbalance during neurostimulation in vivo in specific anatomic locations and with use of modern histological techniques is needed.
Acknowledgments
The authors would like to thank Professor J Thomas Mortimer (Case Western Reserve University) for inspiration for this work and for starting this collaboration and CWRU for loaning the CPCDi instrument. Additionally, the authors would like to thank Karl Steinke (Boston Scientific Corporation) for his comments on the manuscript and contribution to this work. Also, the authors would like to thank Dr Jose Centeno and Dr Victor Krauthamer (Office of Science and Engineering Laboratories, Center for Devices and Radiological Health, US Food and Drug Administration) for their help in managing this project. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either actual or implied endorsement of such products by the Department of Health and Human Services.
References
- 1.Agnew WF, Yuen TG, Pudenz RH, Bullara LA. Neuropathological effects of intracerebral platinum salt injections. J. Neuropathology Exp. Neurol. 1977;36:533–46. doi: 10.1097/00005072-197705000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Black RC, Hannaker P. Dissolution of smooth platinum electrodes in biological fluids. Appl. Neurophysiol. 1979;42:366–74. doi: 10.1159/000102382. [DOI] [PubMed] [Google Scholar]
- 3.Brummer SB, McHardy J, Turner MJ. Electrical stimulation with Pt electrodes: trace analysis for dissolved platinum and other dissolved electrochemical products. Brain Behav. Evol. 1977;14:10–22. doi: 10.1159/000124611. [DOI] [PubMed] [Google Scholar]
- 4.Cogan SF, Ludwig KA, Welle CG, Takmakov P. Tissue damage thresholds during therapeutic electrical stimulation. J. Neural Eng. 2016;13:21001. doi: 10.1088/1741-2560/13/2/021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Firszt JB, Koch DB, Downing M, Litvak L. Current steering creates additional pitch percepts in adult cochlear implant recipients. Otology & Neurotology. 2007;28:629–36. doi: 10.1097/01.mao.0000281803.36574.bc. [DOI] [PubMed] [Google Scholar]
- 6.Krauthamer V, Bekken M, Horowitz JL. Morphological and electrophysiological changes produced by electrical stimulation in cultured neuroblastoma cells. Bioelectromagnetics. 1991;12:299–314. doi: 10.1002/bem.2250120505. [DOI] [PubMed] [Google Scholar]
- 7.Kumsa DB, Hudak EM, Mortimer JT. Electron transfer processes occurring on platinum neural stimulating electrodes: Pulsing experiments for cathodic-first/imbalanced-charge/biphasic-pulses for 0.566 ≤ k ≤ 2.3 in rat subcutaneous tissues. J. Neural Eng. 2016a doi: 10.1088/1741-2552/aaf931. In preparation. [DOI] [PubMed] [Google Scholar]
- 8.Kumsa DW, Montague FW, Hudak EM, Mortimer JT. Electron transfer processes occurring on platinum neural stimulating electrodes: pulsing experiments for cathodic-first/charge-balanced/biphasic pulses for 0.566≤ k≤ 2.3 in oxygenated and deoxygenated sulfuric acid. J. Neural Eng. 2016b;13 doi: 10.1088/1741-2560/13/5/056001. [DOI] [PubMed] [Google Scholar]
- 9.Lilly JC, Hughes JR, Alvord EC, Galkin TW. Brief, noninjurious electric waveform for stimulation of the brain. Science. 1955;121:468–9. doi: 10.1126/science.121.3144.468. [DOI] [PubMed] [Google Scholar]
- 10.McCreery DB, Agnew WF, Yuen TG, Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans. Biomed. Eng. 1990;37:996–1001. doi: 10.1109/10.102812. [DOI] [PubMed] [Google Scholar]
- 11.McHardy J, Geller D, Brummer SB. An approach to corrosion control during electrical stimulation. Annu. Biomed. Eng. 1977;5:144–9. doi: 10.1007/BF02364014. [DOI] [PubMed] [Google Scholar]
- 12.McHardy J, Robblee LS, Marston JM, Brummer SB. Electrical stimulation with pt electrodes: IV. Factors influencing Pt dissolution in inorganic saline. Biomaterials. 1980;1:129–34. doi: 10.1016/0142-9612(80)90034-4. [DOI] [PubMed] [Google Scholar]
- 13.Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J. Neurosci. Methods. 2005;141:171–98. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Mortimer JT, Kaufman D, Roessman U. Intramuscular electrical stimulation: tissue damage. Annu. Biomed. Eng. 1980;8:235–44. doi: 10.1007/BF02364479. [DOI] [PubMed] [Google Scholar]
- 15.Morton SL, Daroux ML, Mortimer JT. The role of oxygen reduction in electrical stimulation of neural tissue. J. Electrochem. Soc. 1994;141:122–30. [Google Scholar]
- 16.Musa S, Rand DR, Bartic C, Eberle W, Nuttin B, Borghs G. Coulometric detection of irreversible electrochemical reactions occurring at Pt microelectrodes used for neural stimulation. Anal. Chem. 2011;83:4012–22. doi: 10.1021/ac103037u. [DOI] [PubMed] [Google Scholar]
- 17.Pikov V. Implantable Neuroprostheses for Restoring Function. Oxford: Elsevier; 2015. Global market for implanted neuroprostheses, in Kilgore K; pp. 383–94. [Google Scholar]
- 18.Prodanov D, Delbeke J. A model of space-fractional-order diffusion in the glial scar. J. Theor. Biol. 2016;403:97–109. doi: 10.1016/j.jtbi.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 19.Pudenz RH, Bullara LA, Jacques S, Hambrecht FT. Electrical stimulation of the brain: III. The neural damage model. Surgical Neurol. 1975;4:389–400. [PubMed] [Google Scholar]
- 20.Rajan AT, Boback JL, Dammann JF, Tenore FV, Wester BA, Otto KJ, Gaunt RA, Bensmaia SJ. The effects of chronic intracortical microstimulation on neural tissue and fine motor behavior. J. Neural Eng. 2015;12:66018. doi: 10.1088/1741-2560/12/6/066018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robblee LS, McHardy J, Agnew WF, Bullara LA. Electrical stimulation with Pt electrodes: VII. Dissolution of Pt electrodes during electrical stimulation of the cat cerebral cortex. J. Neurosci. Methods. 1983;9:301–8. doi: 10.1016/0165-0270(83)90062-6. [DOI] [PubMed] [Google Scholar]
- 22.Robblee LS, McHardy J, Marston JM, Brummer SB. Electrical stimulation with Pt electrodes: V. The effect of protein on Pt dissolution. Biomaterials. 1980;1:135–9. doi: 10.1016/0142-9612(80)90035-6. [DOI] [PubMed] [Google Scholar]
- 23.Roitbak T, Syková E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia. 1999;28:40–8. doi: 10.1002/(sici)1098-1136(199910)28:1<40::aid-glia5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Scheiner A, Mortimer DJT, Roessmann U. Imbalanced biphasic electrical stimulation: muscle tissue damage. Annu. Biomed. Eng. 1990;18:407–25. doi: 10.1007/BF02364157. [DOI] [PubMed] [Google Scholar]
- 25.Shannon RV. A model of safe levels for electrical stimulation. IEEE Trans. Biomed. Eng. 1992;39:424–6. doi: 10.1109/10.126616. [DOI] [PubMed] [Google Scholar]