Abstract
WHO guidelines recommend immediate initiation of antiretroviral therapy (ART) for all individuals at HIV diagnosis regardless of CD4 count, but concerns remain about potential low uptake or poor adherence among healthy patients with high CD4 counts, especially in resource-limited settings. This study assessed the acceptability of earlier treatment among HIV-positive South African women, median age at enrollment 25 (IQR 22–30), in a 10-year prospective cohort study by (i) describing temporal CD4 count trends at initiation in relation to WHO guidance, (ii) virological suppression rates post-ART initiation at different CD4 count thresholds, and (iii) administration of a standardized questionnaire. 158/232 (68.1%) participants initiated ART between 2006 and 2015. Mean CD4 count at initiation was 217 cells/μl (range 135–372) before 2010, and increased to 531 cells/μl (range 272–1095) by 2015 (p<0.001). Median viral load at ART initiation decreased over this period from 5.2 (IQR 4.6–5.6) to 4.1 (IQR 3.4–4.6) log copies/ml (p=0.004). Virological suppression rates at 3, 6, 12 and 18 months were consistently above 85% with no statistically significant differences for participants starting ART at different CD4 count thresholds. A questionnaire assessing uptake of early ART amongst ART-naïve women, median age 28 (IQR 24–33), revealed that 40/51 (78.4%) were willing to start ART at CD4 ≥500. Of those unwilling, 6/11 (54.5%) started ART within six months of questionnaire administration. Temporal increases in CD4 counts, comparable virological suppression rates, and positive patient perceptions confirm high acceptability of earlier ART initiation for the majority of patients.
Keywords: HIV, Antiretroviral therapy, CD4 count, South African women
Introduction
In 2013, the World Health Organization (WHO) issued revised HIV treatment guidelines, recommending to initiate antiretroviral therapy (ART) at a CD4 count threshold of 500 cells/μl for HIV-positive individuals [1]. Modeling exercises have estimated that implementation of these guidelines would save 3 million lives and prevent over 3.5 million new HIV infections between 2013 and 2025 [2]. In 2015, these guidelines were updated recommending ART initiation at any CD4 count, also known as the ‘Test and Treat’ strategy, where ART is immediately initiated at the time of HIV diagnosis [3].
Benefits of early ART initiation include better immune recovery, reduced risk of HIV transmission, and a lower chance of developing HIV-related diseases such as cardiovascular, kidney and liver diseases, neurological complications and cancers [48]. Recent randomized multicenter studies have demonstrated reductions in both morbidity and mortality among HIV-positive individuals when starting ART at a CD4 count threshold of 500 cells/μl as compared to deferring therapy [9, 10]. Furthermore, the latest ART treatment regimens are more convenient, better tolerated, and more effective than older regimens, thus reducing the burden on the patient of starting treatment at an earlier stage during the disease [11, 12]. Despite the recent evidence and WHO guidance, questions remain at the individual patient level about balancing the benefits and potential drawbacks of earlier ART, such as side effects, long-term adherence, development of drug resistance, and increased cost of life-long therapy.
As the evidence for early ART increases, it is important to understand how patients perceive starting treatment earlier. Guidelines have less impact if patients or clinicians do not find them acceptable. It is therefore important to understand how CD4 count and viral load at ART initiation have changed over time in relation to changing WHO guidelines. In addition, few studies have examined patient acceptability of early ART, especially in resource-limited settings. Of the studies that have been conducted, most HIV-positive participants thought that initiating ART earlier would help maintain health and prevent HIV transmission, however, they were also concerned about side effects, adherence to life-long treatment, community acceptance, and stigma [13, 14]. Patient reasons for either acceptance or reluctance to initiate ART at higher CD4 counts are integral to informing both programmatic and policy decisions within South Africa and the wider global community.
The objective of this study was to assess the acceptability of earlier ART in a 10-year prospective cohort study of HIV-positive South African women by (i) describing temporal trends of CD4 counts at ART initiation in relation to changing WHO guidance, (ii) determining virological suppression rates post-ART initiation at different CD4 count thresholds, and (iii) obtaining participant feedback through administration of a standardized questionnaire.
Methods
Study population
A total of 232 women were enrolled at a median age of 25 (IQR 22 – 30) into the CAPRISA 002 Acute Infection study, an ongoing prospective cohort study, which started in August 2004. Participants were recruited at two sites within the province of KwaZulu-Natal in South Africa, an urban site in the city of Durban and a rural site in Vulindlela. The original aim of the study was to describe clinical, virologic and immunologic characteristics of HIV-1 subtype C infection [15]. Women who seroconverted during the HIV-negative phase of CAPRISA 002 or during other CAPRISA seroincidence and prevention trials, including the CAPRISA 004 and CAPRISA 008 microbicide trials [16], were enrolled into the Acute HIV Infection phase, and then followed up during chronic infection, ART initiation, and up to 5 years on ART. Additional information about CAPRISA 002, including the recruitment protocol and patient eligibility criteria, were reported previously [15, 17].
Study procedures
Once HIV infection was diagnosed, study participants had at least 3-monthly follow up visits, where clinical assessments including CD4 count (FACSCalibur Flow Cytometer or TruCOUNT, BD biosciences, San Jose, CA, USA) and HIV viral load measurements (Roche Cobas Amplicor v.1.5, Taqman version 1.0 or version 2.0, Roche Diagnostics, Switzerland) were performed. Participants were offered to start ART according to WHO guidance, usually slightly ahead of its adoption by the South African treatment guidelines (Figure 1). The first participants started ART at a CD4 count threshold of <200 cells/μl in 2006. In 2010, WHO guidance changed to a treatment threshold of ≤350 cells/μl, while in South Africa only pregnant women, tuberculosis co-infected patients or those with AIDS-defining illness were eligible for ART at this threshold. This guidance was extended to the rest of the South African HIV positive population in 2013. In June 2013, the WHO recommended treatment at ≤500 cells/μl, which was then expanded to universal treatment in 2015. South Africa moved to a threshold ≤500 cells/μl in 2015, and recently introduced universal treatment in September 2016. Since January 2015, slightly ahead of the guidelines, participants of the CAPRISA 002 study have been offered to start ART at a CD4 count of 500 cells/μl or above, essentially offering immediate therapy, irrespective of CD4 count. Once on ART, participants were followed up at 1, 3, 6, 12 months after initiation, and 6-monthly thereafter. For the purpose of this study, virological suppression was defined as viral load <400 copies/ml, because this was the detectability threshold for the original Roche COBAS Amplicor version 1.5 assay allowing comparison over time. Ethical approval for the CAPRISA 002 study and the Acceptability Questionnaire were granted by the local Biomedical Research Ethics Committee (BREC) (#E013/04).
Figure 1.
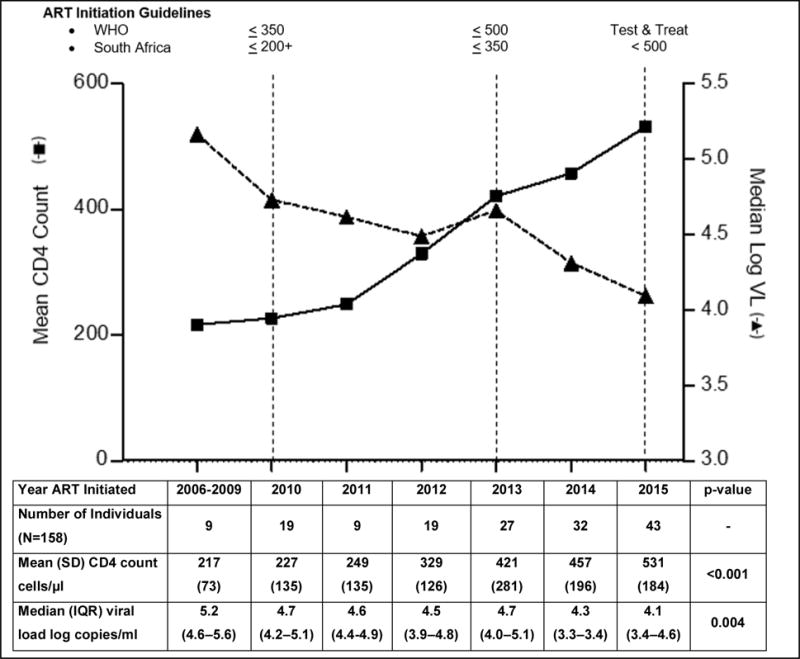
CD4 count and viral load trends at ART Initiation in CAPRISA 002
Early ART questionnaires
Between November 2014 and May 2015 a cross-sectional questionnaire was administered at both study sites to assess participant acceptability and perceptions of early ART initiation. CAPRISA 002 participants were eligible for the questionnaire, if they were ART-naïve and were willing to provide consent (n=51). Questionnaires were administered by a study nurse in either English or the local language, isiZulu, and assessed the level of agreement of participants with statements sourced from the literature. The questionnaire was created to gauge the willingness of participants to initiate ART at CD4 count 500 cells/μl or above, perceived benefits and drawbacks of early ART initiation for the individual and the public, and partner information, including the partner’s HIV and ART status and disclosure. Some questions required a yes or no answer, while others provided a number of predetermined answers and participants were instructed to choose as many as apply. There was also an option for each question that allowed participants to specify an additional answer that was not listed. For example, patients were asked if they were willing to start treatment at ≥500 cells/μl and instructed to check the box marked either ‘yes’ or ‘no’. A subsequent question stated that if a participant indicated that they were willing to start treatment at ≥500 cells/μl then they should provide all reasons for their willingness to do so. The answers that were listed were 1) ‘I feel ready to start treatment’, 2) ‘I want to improve my health’, 3) ‘I am planning to have a baby’, and 4) ‘I want to reduce my chances of getting tuberculosis’, and 5) ‘Other: Specify’. Patients were given different answers to select if they responded that they were currently unwilling to initiate ART at ≥500 cells/μl. In addition, patients were asked if they considered any of the listed factors important benefits of HIV treatment. These potential benefits were divided into two categories: potential individual benefits and potential public health benefits and participants were instructed to select all that apply as well as given an option to specify an answer that was not included in the questionnaire. Questionnaires were recorded as case report forms and faxed into the CAPRISA Data Management Centre, using the DataFax system version 2014.1.1 (Clinical DataFax Systems Inc., Ontario, Canada). Data from subsequent CAPRISA 002 visits were then used to verify, whom of the 51 participants completing the questionnaire went on to initiate ART, and at what CD4 count.
Statistical methods
Characteristics of the study participants were summarized using descriptive statistics expressed as means with standard deviation (SD) or medians with interquartile ranges (IQR) for continuous variables and proportions for categorical variables. An independent samples t-test was used to compare two means of continuous variables, while the Wilcoxon-Mann-Whitney test was used to compare two medians. Proportions were compared using Fisher’s exact test. The significance of the trend of a continuous variable was assessed with a t-test. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and graphs were prepared using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA).
Results
CD4 count and viral load trends at ART Initiation in CAPRISA 002
A total of 232 HIV-positive women were enrolled into the CAPRISA 002 acute infection study. Of these, 158 (68.1%) initiated ART between January 2006 and December 2015, and were included in the analysis. Of the remainder, 32 (13.8%) had not started therapy, 23 (9.9%) started therapy in another treatment study or at their local clinic, 11 (4.7%) were lost to follow-up, 4 (1.7%) died, and 4 (1.7%) had incomplete data available. The mean CD4 count at initiation was 217 cells/μl (range 135 – 372) before 2010, and gradually increased to 531 cells/μl (range 272 – 1095) in 2015 (p<0.001). The median viral load simultaneously decreased from 5.2 (IQR 4.6 – 5.6) to 4.1 (IQR 3.4 – 4.6) log copies/ml (p=0.004) (Figure 1).
Virological suppression rates stratified by CD4 count treatment thresholds
The proportion of participants who achieved virological suppression, defined as a viral load result of <400 copies/ml, at 3, 6, 12 or 18 months after ART initiation was analyzed. Participants were stratified according to whether they started ART below or above two CD4 count thresholds of 350 and 500 cells/μl. Overall, suppression rates were high at greater than 86% for all participants at all time-points. There was no statistically significant difference between virological suppression at 3, 6, 12 or 18 months when comparing those who started below and above 350 or 500 cells/μl (Table 1).
Table 1.
Virological Suppression stratified by CD4 count at ART Initiation
| CD4 Count at Initiation (cells/μl) | 3 Months | 6 Months | 12 Months | 18+ Months |
|---|---|---|---|---|
| <350 (n=77) | 87.9% (51/58) |
87.0% (67/77) |
87.8% (65/74) |
92.8% (65/70) |
| ≥350 (n=80) | 95.3% (61/64) |
92.7% (51/55) |
92.1% (35/38) |
100% (26/26) |
| P-value | 0.190 | 0.295 | 0.544 | 0.319 |
| <500 (n=132) | 92.2% (94/102) |
88.5% (100/113) |
88.0% (88/100) |
94.4% (85/90) |
| ≥500 (n=35) | 90.0% (18/20) |
90.0% (18/20) |
100% (12/12) |
100% (6/6) |
| P-value | 1.000 | 1.000 | 0.357 | 1.000 |
Early ART questionnaire
A total of 51 ART-naïve CAPRISA 002 participants were eligible and completed the early treatment questionnaire. All participants were female with a median age of 28 (IQR 24–33), and 28/51 (54.9%) were from the rural research site. The majority were unmarried, but with a regular partner (44/51, 86.3%) and almost one half (24/51, 47.1%) were employed. Three quarters (37/51, 72.5%) indicated that they had disclosed their HIV status to their partner. At the time of questionnaire administration, participants had a mean CD4 count of 590 cells/μl (range 260 – 1450) and a median viral load of 4.1 log copies/ml (IQR 3.7 – 4.5).
Perceived individual and public health benefit of ART
Participants were asked if they agreed with various statements about the individual and public health benefits of treatment with ART. More than 80% of participants agreed that the listed outcomes were important benefits of HIV treatment including reduced risk of contracting tuberculosis (48/51, 94.1%), reduced disease and death (47/51, 92.2%), and reduced organ disease (43/51,84.3%). Furthermore, the majority considered reduced transmission during pregnancy (46/51, 90.2%), during breastfeeding (43/51, 84.3%), and to a partner (45/51, 88.2%) important public health benefits (Figure 2A). Participant beliefs about important risks and drawbacks of treatment varied in a ‘yes’ versus ‘no’ question. Some participants were concerned about side effects to treatment (30/51, 58.8%), the need for lifelong therapy (19/51, 37.3%), having to take treatment everyday (18/51, 35.3%), having to disclose the HIV status because of treatment (29/51,56.9%), and fear of stigma (29/51, 56.9%) (Figure 2B).
Figure 2. A and B: Perceived benefits and drawbacks of earlier ART (n=51).
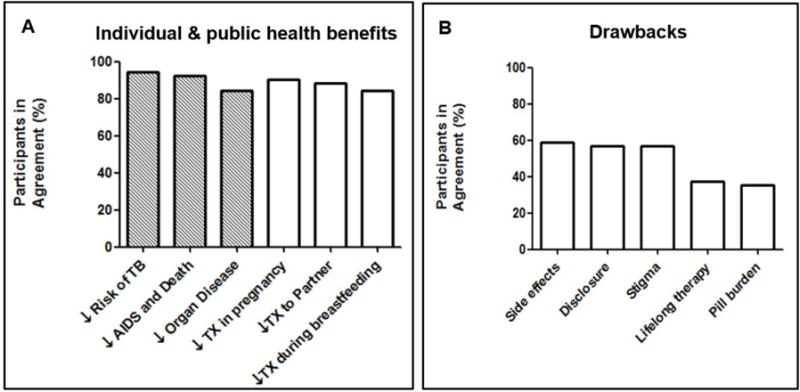
Tx = transmission
Willingness to start early treatment
Of the 51 women who completed the questionnaire, 40 (78.4%) were willing, while 11 (21.6%) were unwilling to start ART at a CD4 count ≥500 cells/μl. Of those willing, 28 (70%) stated that they felt ready to start treatment, 36 (90%) wanted to improve their health, 25 (62.5%) wanted to reduce their chances of contracting Tuberculosis, and 12 (30%) were planning a pregnancy (Figure 3A). Of the 11 women who were unwilling to start ART at CD4 ≥500 cells/μl, 10 (90.9%) stated that they felt well and therefore did not think they needed ART. Less important reasons why women did not want to initiate ART at CD4 ≥500 cells/μl were their concerns about medication side effects (5/11,45.5%), high pill burden (3/11,27.3%), and having to disclose their HIV status to the partner or to family members (3/11,27.3%) (Figure 3B).
Figure 3.
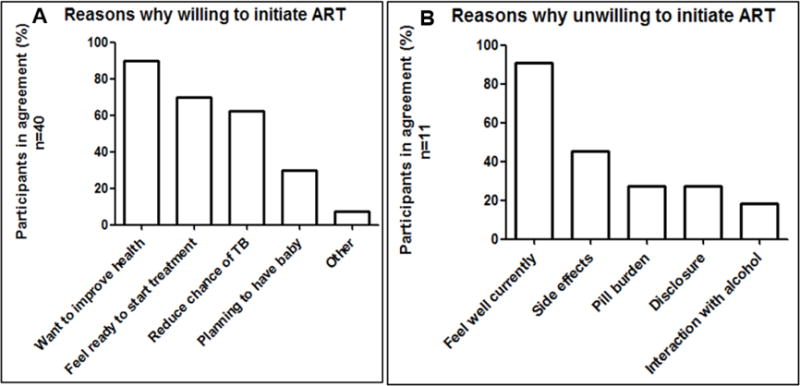
A and B: Acceptability of ART initiation at CD4 ≥500 (n=51)
High HIV prevalence and low ART coverage among male partners
Of the 51 participants who responded to the questionnaire, 48 women (94.1%) stated that they had a current regular partner, one woman (2.0%) had a casual partner and two women (3.9%) had no current partner. A total of 27/49 (55.1%) knew their partner’s status, with 22/27 (81.5%) of these partners being identified as HIVpositive. However, only 5/22 (22.7%) of them were reported to be on ART by the women.
Perception and reality of ART Initiation
Of the 51 women participating in the questionnaire, 35 (68.6%) initiated therapy within 6 months of questionnaire administration. Of the 40 participants who indicated that they were willing to initiate at CD4 ≥500, 29 (72.5%) started treatment at a mean CD4 count of 717 cells/μl (range 377 – 1191). Of the 11 participants unwilling to initiate at CD4 ≥500, 6 (54.5%) initiated ART within 6 months of questionnaire administration at a mean CD4 count of 690 cells/μl (range 322 – 1224). A total of 16/51 (31.4%) participants remained ART-naïve with a mean CD4 count of 815 cells/μl (range 436 – 1254) at the end of 2015.
Discussion
The goal of this study was to investigate participant acceptability of early ART initiation by analyzing cohort CD4 count and viral load data, and through administration of a participant questionnaire. We found that CD4 counts at ART initiation have been dramatically increasing over the last decade, and that concurrently, HIV viral loads have decreased, potentially having an important impact on HIV transmission at the individual and community level. These results suggest the successful implementation of WHO and South African Department of Health guidelines, and reveal a history of acceptance of earlier ART initiation by HIV-positive South African women who were followed up for an extended period in the CAPRISA 002 cohort. Importantly, while women started ART earlier, this did not have a negative impact on their adherence, as demonstrated by similar rates of virological suppression up to two years post-ART initiation regardless of the CD4 count at treatment start.
Behavioral questionnaires in a subset of the women revealed that a majority of nearly 80% were willing to initiate ART at a CD4 count of 500 cells/μl or higher. This is an important finding that demonstrates that most patients will likely adhere to the WHO recommendations and initiate ART soon after HIV diagnosis as the policy becomes fully implemented and accepted in South Africa. This study also found that patient perceptions on when to start can differ from their actions. Approximately one quarter of women who indicated that they were willing to start treatment early did not start within six months. This could have been explained by logistical reasons, such as the visit schedule or due to adjustment to the diagnosis and general readiness. Conversely, more than half of the women who indicated that they were unwilling to start treatment at a CD4 count of 500 cells/μl, initiated at a CD4 count >500 cells/μl within six months, demonstrating that a discussion with health care professionals about the benefits of earlier ART can promote earlier initiation among concerned patients. One study on ART initiation in the United States found that education and provider recommendation of ART were the most important factors in a patient’s decision to initiate treatment [18].
Other important factors relating to ART initiation include partner notification and disclosure. If patients do not disclose their status to partners or other family members, it can dissuade them from initiating ART, especially if they currently feel healthy. Only one half of questionnaire participants knew their partners’ status, and the majority of these partners were identified as HIV-positive, consistent with high rates of heterosexual transmission in this population. The low ART coverage among partners highlights the need for increased HIV testing strategies targeting males, but also couple counselling with regards to partner diagnosis and early ART initiation as a couple.
The main reason study participants stated for not wanting to initiate ART at a higher CD4 count was that they felt well and therefore did not see the need for therapy. There appears to be a common belief that ART does not need to be initiated until one begins to feel sick and demonstrate signs of more advanced HIV progression [19]. This shows that the results from recent studies [9, 10], which clearly demonstrate the benefits of earlier ART initiation still need to be communicated effectively to patients, so that informed decisions on treatment start can be made.
This study had some limitations. Firstly, the study population consisted of young South African women, meaning that results on early ART initiation cannot necessarily be generalized to men, children, and older populations. Secondly, these women have been participating in a prospective cohort study with regular clinic visits for some time, suggesting that this population might be more knowledgeable about ART, more health conscious, or more likely to act on health professionals’ advice than the general population. Finally, the questionnaire was administered to a relatively small number of women, and may not have covered all possible reasons for ART refusal, which may be relevant to other regions in South Africa or globally.
In this study we have shown that earlier ART initiation is acceptable to the majority of participants as evidenced by the increasing CD4 count at ART initiation over time and high levels of virological suppression among women regardless of CD4 count at treatment start. Although some concerns remain about side effects, stigma, and disclosure, appropriate education and counseling by healthcare professionals can encourage patients to start early and successful HIV therapy. At a programmatic level this may mean that ART initiation visits should be prioritized to ensure sufficient time is allocated to explain to newly diagnosed patients, the individual and prevention benefits of early ART. Our study suggests that men may not be accessing ART programs as readily as women. Future studies should investigate the acceptability of early ART among men in order to inform programming.
The decision by the South African Department of Health to implement universal treatment made more than two million additional people eligible for ART [20]. Considering this commitment, it is now important to allocate sufficient resources for HIV testing and treatment, and to monitor the quality of these programs, so that all patients and communities can reap the individual and population level benefits of early ART initiation.
Acknowledgments
We thank the CAPRISA 002 Study Team and all the CAPRISA 002 study participants who continue to make an important contribution to HIV research.
Funding: Over the past decade, the CAPRISA 002 study has received support from the National Institute of Allergy and infectious Disease (NIAID), National Institutes of Health (NIH) (grant # AI51794), from CONRAD (USAID co-operative grant #GP00-08-00005-00, subproject agreement # PPA-09-046), from the National Research Foundation (grants # 67385, # 96354), the Technology Innovation Agency, and the Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP) funded by the Fogarty International Center, NIH (grant # D43TW00231).
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
Ethical approval: All procedures performed in this study were approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal and were in accordance with the 1964 Helsinki declaration and its later amendments.
References
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013 Jun; http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf (accessed 31 July 2016) [PubMed]
- 2.Doherty M, Ford N, Vitoria M, Weiler G, Hirnschall G. The 2013 WHO guidelines for antiretroviral therapy: evidence-based recommendations to face new epidemic realities. Curr Opin HIV AIDS. 2013 Nov;8(6):528–34. doi: 10.1097/COH.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. http://apps.who.int/iris/bitstream/10665/186275/1Z9789241509565_eng.pdf (accessed 31 July 2016) [PubMed]
- 4.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001 Nov 28;286(20):2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 5.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007 Feb 1;44(3):441–6. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016 Sep 1;375(9):830–9. doi: 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010 Jun 12;375(9731):2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22(7):841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.INSIGHT START Study Group. Lundgren JD, Babiker AG, Gordin F, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015 Aug 27;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TEMPRANO ANRS 12136 Study Group. Danel C, Moh R, Gabillard D, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015 Aug 27;373(9):808–22. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 11.Nachega JB, Parienti JJ, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis. 2014 May;58(9):1297–1307. doi: 10.1093/cid/ciu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langebeek N, Sprenger HG, Gisolf EH, et al. A simplified combination antiretroviral therapy regimen enhances adherence, treatment satisfaction and quality of life: results of a randomized clinical trial. HIV Med. 2014 May;15(5):286–90. doi: 10.1111/hiv.12112. [DOI] [PubMed] [Google Scholar]
- 13.Fox MP, Mazimba A, Seidenberg P, Crooks D, Sikateyo B, Rosen S. Barriers to initiation of antiretroviral treatment in rural and urban areas of Zambia: a crosssectional study of cost, stigma, and perceptions about ART. J Int AIDS Soc. 2010 Mar 6;13:8. doi: 10.1186/1758-2652-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Tang Z, Sun H, et al. Acceptability of early anti-retroviral therapy among HIV-infected people in Anhui province in China. AIDS Care. 2015;27(5):669–74. doi: 10.1080/09540121.2014.983042. [DOI] [PubMed] [Google Scholar]
- 15.van Loggerenberg F, Mlisana K, Williamson C, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS One. 2008 Apr 16;3(4):e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mlisana K, Werner L, Garrett NJ, et al. Rapid disease progression in HIV-1 subtype C-infected South African women. Clin Infect Dis. 2014 Nov 1;59(9):1322–31. doi: 10.1093/cid/ciu573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christopoulos KA, Olender S, Lopez AM, et al. Retained in HIV Care But Not on Antiretroviral Treatment: A Qualitative Patient-Provider Dyadic Study. PLoS Med. 2015 Aug 11;12(8):e1001863. doi: 10.1371/journal.pmed.1001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz IT, Dietrich J, Tshabalala G, Essien T, Rough K, Wright AA. Understanding treatment refusal among adults presenting for HIV-testing in Soweto, South Africa: a qualitative study. AIDS Behav. 2015 Apr;19(4):704–14. doi: 10.1007/s10461-014-0920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.UNAIDS. South Africa takes bold step to provide HIV treatment for all. 2016 May 13; http://www.unaids.org/sites/default/files/20160513_PR_SouthAfrica_en.pdf (accessed 21 December 2016)


