Abstract
Different methamphetamine use patterns in human subjects may contribute to inconsistent findings regarding the effects of methamphetamine abuse on brain and behavior. The present study investigated whether human derived chronic and binge methamphetamine use patterns have differential effects on reward and neurochemistry in mice. Brain reward function in mice was evaluated during acute/prolonged withdrawal, and in response to methamphetamine challenge using the intracranial self-stimulation procedure. Brain dopaminergic, serotonergic and glutamatergic neurochemistry was determined with high-performance liquid chromatography. Chronic and binge regimens induced withdrawal-related decreases in reward function that were more severe during the binge regimen during cycles 1–2. Despite large differences in methamphetamine dose, both regimens induced similar reward deficits during cycles 3–4. Neither methamphetamine regimen led to persistent alterations in the sensitivity to the reward-enhancing effects of acute methamphetamine challenge. The binge regimen severely depleted striatal dopamine levels and increased brain glutamine levels. The chronic regimen had milder effects on striatal dopamine levels, and altered cortical dopamine and serotonin levels. This work highlights that the magnitude of acute/prolonged withdrawal may not reflect amount or frequency of methamphetamine intake. In contrast, the array of underlying neurochemical alterations was methamphetamine regimen-dependent. Thus, stratifying methamphetamine-dependent individuals based on use pattern may help to cater therapeutic interventions more appropriately by targeting use pattern-specific neurotransmitter systems.
Keywords: anhedonia, withdrawal, cognition, dopamine, glutamate, serotonin
INTRODUCTION
Methamphetamine dependence remains a world-wide public health problem (UNODC, 2013). Prior methamphetamine use is associated with prolonged withdrawal symptoms including anhedonia (reward deficits) and cognitive impairment (Leventhal et al., 2008; Sofuoglu et al., 2016). However, research findings are inconsistent across studies (Hart et al., 2012), which may be attributed to different methamphetamine use patterns in humans. The extent of reward and cognitive impairments associated with different methamphetamine use patterns remains largely unknown because of unreliable self-reports and study-to-study variability in which methamphetamine use factors are reported (Scott et al., 2007). Understanding how brain reward function and neurochemistry are altered by distinct use patterns will assist in identifying neurotransmitter specific targets to treat methamphetamine dependence in subgroups of individuals with different patterns of methamphetamine use. Establishing efficacious treatment strategies may help to prevent relapse and improve clinical outcomes (Sofuoglu et al., 2016).
A paucity of studies report methamphetamine use patterns in humans. Two distinct patterns of methamphetamine use have been described. Binge use features a rapid dose-escalation to high-doses over 4 days/week of use on average, followed by a period of abstinence (Cheng et al., 2010; Cho and Melega, 2002; Semple et al., 2003; Simon et al., 2002; Sommers et al., 2006). Chronic use features sustained, 6 days/week on average, use of low/moderate doses (Brecht et al., 2004; Cheng et al., 2010; Cho and Melega, 2002; Simon et al., 2002). Though binge users tend to use less frequently than chronic users, the amount of methamphetamine consumed is greater with binge users consuming approximately 1.5 times as much methamphetamine per month (~2.5 times per day of use) compared with chronic users (Cheng et al., 2010; Cho and Melega, 2002). Thus, differences in the frequency and amount of methamphetamine administered between use patterns could result in different outcomes on brain reward function, cognition and neurochemistry.
Methamphetamine-induced neurotoxicity and prolonged methamphetamine exposure have been widely studied in monkeys and rodents (Krasnova and Cadet, 2009). In rodents, methamphetamine exposures commonly feature neurotoxic regimens consisting of multiple high-dose administrations over 1–2 days or regimens consisting of low-dose escalation prior to a high-dose binge over 1–4 weeks (Krasnova and Cadet, 2009). Although these regimens do not necessarily reflect use in human subjects, they provide insight into how the pattern of methamphetamine use can differentially affect reward/cognitive outcomes. That is, memory deficits in novel object recognition (NOR) are observed after short-term, high-dose neurotoxic methamphetamine administration in rats (Belcher et al., 2008; Reichel et al., 2012) or after sustained low dose methamphetamine administration in mice (Kamei et al., 2006); but not when these regimens are preceded by dose-escalations in rats (Belcher et al., 2008; Clark et al., 2007) or mice (Kesby et al., 2015b). Chronic exposure to methamphetamine also results in depression-like behaviour. Brain reward deficits or anhedonia have been reported in rats during withdrawal from methamphetamine self-administration and non-contingent chronic methamphetamine administration (Jang et al., 2013; Miyata et al., 2011). Thus, although impairments in NOR appear to be highly exposure-dependent, little is known regarding the effects of different patterns of methamphetamine exposure on brain reward function.
Methamphetamine-induced alterations in reward and cognitive function have been associated with impairments in dopaminergic function, although other neurotransmitters such as serotonin (Muller et al., 2007) and glutamate (Ernst and Chang, 2008; Sailasuta et al., 2010) are implicated. Methamphetamine-dependent users have decreased dopamine transporter levels (a marker for dopamine terminals) in the striatum (Volkow et al., 2001). Moreover, decreases in dopamine function have been associated with deficits in memory and executive function (McCann et al., 2008), and increased risk of relapse (Wang et al., 2012). Multiple methamphetamine regimens have been shown to decrease dopamine and dopamine transporter levels in the striatum of monkeys and rodents (Lacan et al., 2013; Melega et al., 2008; Melo et al., 2012). However, few studies have directly compared the effects of human-derived patterns of methamphetamine use on brain neurochemistry and behavior.
The goal of the present study was to investigate whether two common patterns of methamphetamine use in human abusers, chronic and binge, have differential effects on brain reward function and neurochemistry in mice. Brain reward function in response to methamphetamine withdrawal and acute methamphetamine administration was assessed with the intracranial self-stimulation (ICSS) procedure. In addition, NOR was used as a readout of persistent memory deficits given previous studies suggest NOR deficits are methamphetamine regimen dependent (Belcher et al., 2008). Regional neurochemistry was assessed using high-performance liquid chromatography (HPLC). Brain regions tested included those associated with reward, motivation and cognition. The current study focussed on non-contingent administration to maintain strict control of methamphetamine dosing. In addition, the doses administered in the present study exceeded those observed in self-administration paradigms (Lacan et al., 2013; Mandyam et al., 2008). Although there is evidence of differing neurochemical responses to methamphetamine when comparing contingent versus non-contingent exposure (Lominac et al., 2012), striatal dopamine systems were similarly affected by contingent versus non-contingent methamphetamine exposure (Lacan et al., 2013). The outcomes of these studies provide insights regarding potential neurochemical mechanisms underlying the effects of two distinct human-derived methamphetamine regimens on brain reward function, neurochemistry and cognition in mice.
MATERIALS AND METHODS
Animals
A total of 60 male mice, 3–6 months old, were used (for details see Supplementary Methods). Experiments were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by the University of California San Diego Institutional Animal Care and Use Committee.
Methamphetamine regimens
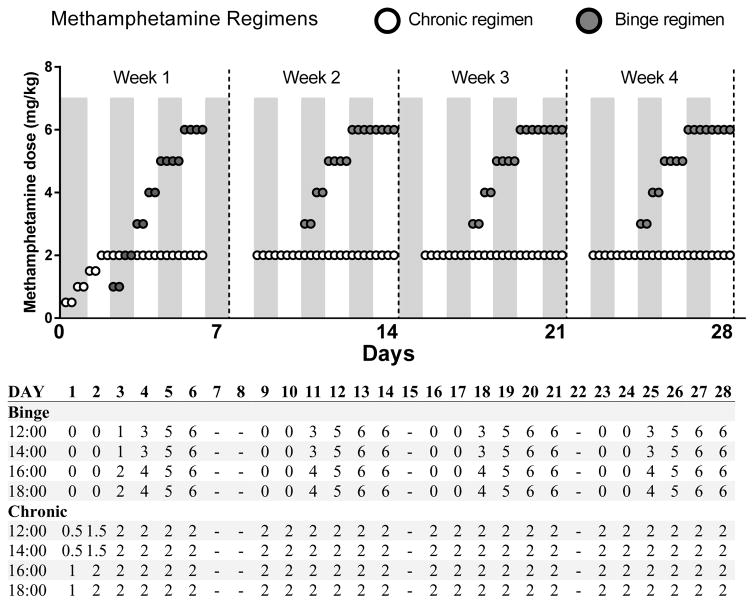
For detailed information about the development of the methamphetamine regimens see Supplementary Methods. The methamphetamine regimens (Figure 1) consisted of subcutaneous injections of saline or methamphetamine (5 ml/kg, methamphetamine hydrochloride; Sigma, St. Louis, MO, USA; reported as base concentration) for four ‘cycles’ of 6 days (four injections/day). The first cycle featured one week of dose-escalation followed by three repeated cycles of methamphetamine exposure. The chronic regimen cycles consisted of 6 days of moderate dosing (2 mg/kg). The binge regimen cycles consisted of a two-day dose-escalation (3–5 mg/kg) followed by two days of high dosing (6 mg/kg). The 6-day administration period chosen in the chronic regimen represents the average days/month of exposure observed in human chronic studies (Brecht et al., 2004; Simon et al., 2002). The 4-day administration period chosen in the binge regimen represents the average days/week of use observed in human binge studies (Cheng et al., 2010; Cho and Melega, 2002; Semple et al., 2003; Simon et al., 2002; Sommers et al., 2006) and also accounts for peak binge use during the weekend period (Friday–Monday)(Halkitis et al., 2009). The doses for both the chronic and binge regimens were selected to fit models of total use per day/month in humans. That is, binge users consume approximately 1.5 times more methamphetamine per month than chronic users (Cheng et al., 2010). Our regimens in mice reflect this aspect with the binge regimen featuring 1.7 times more methamphetamine per month than the chronic regimen. Per day of methamphetamine use, binge users commonly use 1.5–3.5 times as much methamphetamine as chronic users (Cheng et al., 2010; Cho and Melega, 2002). Thus, mice received 2.5 times the amount of methamphetamine per day of use in the binge regimen compared with the chronic regimen.
Figure 1.
Binge and chronic methamphetamine dosing regimens. The first week of treatment features a lower dose-escalation. The following three weeks of treatment are identical. The binge regimen features a two-day dose-escalation before two days of high dose administration, whereas the chronic regimen features sustained moderate dose administration throughout. Dosing for both regimens is represented graphically on top and numerically with injection times (24 h) in the table below. All doses are methamphetamine base as mg/kg. A zero value represents a saline injection administered to ensure equal injections were given for each regimen. The saline control group received saline injections at each time points.
Intracranial self-stimulation
The discrete-trial, current-intensity threshold ICSS procedure was conducted as previously described (Kesby et al., 2016a)(see Supplementary Methods). Each trial was initiated with a non-contingent stimulation and a response resulted in a second, identical contingent stimulation. By varying the current intensity level, we determine the minimal electrical current that elicits responding for the contingent stimulation, i.e., the reward threshold. Elevations in reward thresholds reflect decreased brain reward function (i.e., reward deficits or anhedonia). Conversely, lowering of thresholds reflect reward enhancement. The ICSS procedure also provides measures of response speed (latency), disinhibition/impulsivity (timeout responses), and vigor of responding (extra responses).
Novel object recognition test
NOR was assessed as previously described (Kesby et al., 2015a)(see Supplementary Methods). Mice were allowed to explore two identical wire cups for 10 min (familiarisation phase). One of the cups was then replaced with a novel cup and exploration was assessed for 10 min (test phase). The data were expressed as a discrimination ratio of the duration of object exploration: (Novel-Sample)/Total.
High-performance liquid chromatography
Neurochemistry was evaluated as previously described (Kesby et al., 2016a, b)(see Supplementary Methods). Catecholamines were analyzed using a HPLC system with electrochemical detection. Amino acids were analyzed after derivatization with fluorescence detection. Data was processed with Dionex Chromeleon software (v7.2, Thermo Scientific, CA, USA).
Experimental Design
Mice prepared with electrodes were trained in the ICSS procedure until stable reward thresholds were achieved (<10% standard deviation over three days; minimum 14 days of baseline testing). Mice were tested daily in the ICSS procedure (07:30–11:30h) prior to saline/methamphetamine administration (i.e., 12 h acute withdrawal). After termination of methamphetamine regimens, mice underwent a six-day prolonged withdrawal and continued to be tested daily on the ICSS procedure. Then, a dose-response function for acute methamphetamine (0, 0.2, 0.4 and 0.8 mg/kg, intraperitoneally, 10 ml/kg, 20 min before testing, three days apart) was assessed using a within-subject Latin square design (12 days). Three days after the final acute methamphetamine challenge, mice were tested for NOR (3 days). Finally, brain samples were collected for neurochemical analyses (23 days after completion of methamphetamine regimens; 07:30–11:30h). For details on exclusions see Supplementary Methods.
Statistical analyses
All of the analyses were performed with SPSS Statistics 20 (Chicago, IL, USA). All data were analyzed using analysis of variance (ANOVA), with Regimen as the between-subject factor, and repeated measures ANOVAs when appropriate. Data not meeting the assumption of homogeneity of variance were analyzed using Greenhouse-Geiser adjusted degrees of freedom. When appropriate, post hoc comparisons were performed using Least Significant Difference (LSD) analyses. Results are expressed as mean±SEM. Differences were considered statistically significant at p<0.05.
RESULTS
Intracranial self-stimulation
Daily withdrawal assessments
Reward thresholds data during the baseline, throughout the methamphetamine regimens and prolonged withdrawal (41 days in total) are presented in Figure 2A. Analyses revealed significant main effects of Day (F40,1160=8.9, p<0.001), Regimen (F2,29=9.7, p<0.001) and a significant interaction of Day x Regimen (F80,1160=3.6, p<0.001). To account for a different number of days of methamphetamine administration in each regimen, we compared the average reward thresholds at the 12 h withdrawal time points during each methamphetamine cycle (4-day average for binge and 6-day average for chronic regimen; Figure 2B). There were significant main effects of Week (F4,116=11.4, p<0.001), Regimen (F2,29=15.2, p<0.001) and a significant interaction of Week x Regimen (F8,116=5.2, p<0.01). Post hoc comparisons after both analyses revealed that the chronic (p<0.01) and binge (p<0.001) regimens led to significantly higher reward thresholds than saline with a greater magnitude of threshold elevations during periods of acute withdrawal (Figure 2A,B). The binge regimen led to significantly greater elevations in reward thresholds than the chronic regimen during the first two cycles of methamphetamine but not during the final two cycles or during prolonged withdrawal (Figure 2B). During prolonged 6-day withdrawal (Figure 2A), reward thresholds were significantly elevated for 2 days after both methamphetamine regimens compared with saline (p<0.05). There were no significant differences between the methamphetamine regimens for response latency, timeout responses or extra responses revealed either by total data analyses (Figure S1) or by analyses of averaged 12 h withdrawal time points during each methamphetamine cycle (Figure S2).
Figure 2.
The effects of chronic and binge methamphetamine regimens on reward thresholds for daily withdrawal (A) and during each cycle of methamphetamine exposure (average of 12 h withdrawal time points; B). Reward thresholds are presented as a percentage of baseline threshold average over the 5-days prior to beginning the methamphetamine regimens. Both regimens increased reward thresholds compared to the saline group suggesting withdrawal-associated anhedonia. However, the binge regimen elicited greater withdrawal-induced anhedonia compared to the chronic regimen during the initial two weeks of methamphetamine administration. Baseline thresholds are represented by the dotted line. Thresholds, in panel A, assessed 12 h after methamphetamine administration (which contributed to averages displayed in panel B) are represented by the shaded regions with lighter gray for the chronic regimen and darker gray for the binge regimen. Methamphetamine regimens were followed by six days of abstinence to assess prolonged withdrawal. Data are expressed as mean ± SEM.
* p<0.05 between saline and the chronic regimen (A).
#p<0.05 between saline and the binge regimen (A).
@p<0.05 between the chronic and binge regimens (A).
* p<0.05, ** p<0.01, *** p<0.001 significant difference between groups (B).
Acute methamphetamine challenge
For reward thresholds (Figure 3A), there was a significant main effect of Dose (F3,87=14.9, p<0.001) with acute methamphetamine challenge lowering reward thresholds at all doses (p<0.001). For response latency (Figure 3B), there was a significant main effect of Regimen (F2,29=5.1, p<0.05) with the binge regimen decreasing the time to respond (p<0.01) and there was a similar trend for the chronic regimen (p<0.1) when compared with saline. Both the chronic and binge regimen significantly decreased response latency at 0.4 and 0.8 mg/kg methamphetamine compared with saline (p<0.05). For extra responses (Figure 3C), there was a significant main effect of Regimen (F2,29=4.3, p<0.05) with the binge regimen increasing extra responses compared with saline (p<0.01). The binge regimen increased extra responses at 0.4 mg/kg methamphetamine when compared with saline (p<0.05) and there was a similar trend when compared with the chronic regimen (p<0.1). For timeout responses (Figure 3D), there was a significant main effect of Dose (F3,87=4.2, p<0.05) with 0.8 mg/kg methamphetamine significantly lowering timeout responses compared with all other doses (p<0.05). There were no significant interactions of Regimen x Dosing order on reward thresholds, latency, timeout responses or extra responses. There were no significant differences between regimens for latency (Figure S3A), timeout responses (Figure S3B) or extra responses (Figure S3C) on the baseline days between methamphetamine challenges.
Figure 3.
Comparisons of the long-term effects of chronic and binge methamphetamine regimens on reward thresholds (A), latency to respond (B), extra responses (C) and timeout responses (D) in response to acute methamphetamine challenge. Acute methamphetamine challenge induced reward enhancement as indicated by significantly lower reward thresholds at all doses with no significant effects of methamphetamine regimen (A). Both 0.4 and 0.8 mg/kg methamphetamine led to significantly decreased latencies after both regimens compared with saline (B). The binge regimen led to significantly elevated extra responses overall and, specifically, after 0.4 mg/kg methamphetamine compared with saline (C). A similar trend was observed for the chronic regimen when compared with saline. Acute methamphetamine challenge reduced timeout responses at the highest dose (0.8 mg/kg) with no significant effects of methamphetamine regimen (D). Data are expressed as mean ± SEM.
* p<0.05, *** p<0.001 significantly different from 0 mg/kg methamphetamine (A,D).
ap<0.05 between saline and both the binge and chronic regimens (B).
bp<0.05 between saline and the binge regimen; p<0.1 between saline and the chronic regimen (C).
# #p<0.05 significant main effect of methamphetamine regimen with the binge regimen significantly different to saline (B,C).
Novel object recognition
There were no effects of methamphetamine regimen on any measure in the NOR test. All mice significantly discriminated the NO (F1,35=110.9, p<0.001; Figure S4A). Total interaction time (Figure S4B) was lower in the test phase compared with the familiarization phase (F1,35=34.7, p<0.001). The time spent grooming (Figure S4C) was significantly different between phases (F2,70=6.5, p<0.01) with increased grooming observed in the test phase compared with the habituation (p<0.01) and familiarization (p<0.05) phases.
Neurochemistry
The results for all neurochemical measures with statistical results are reported in the Supplementary materials (Tables S1–S8). Key findings and statistical analyses are presented below.
Dopaminergic system
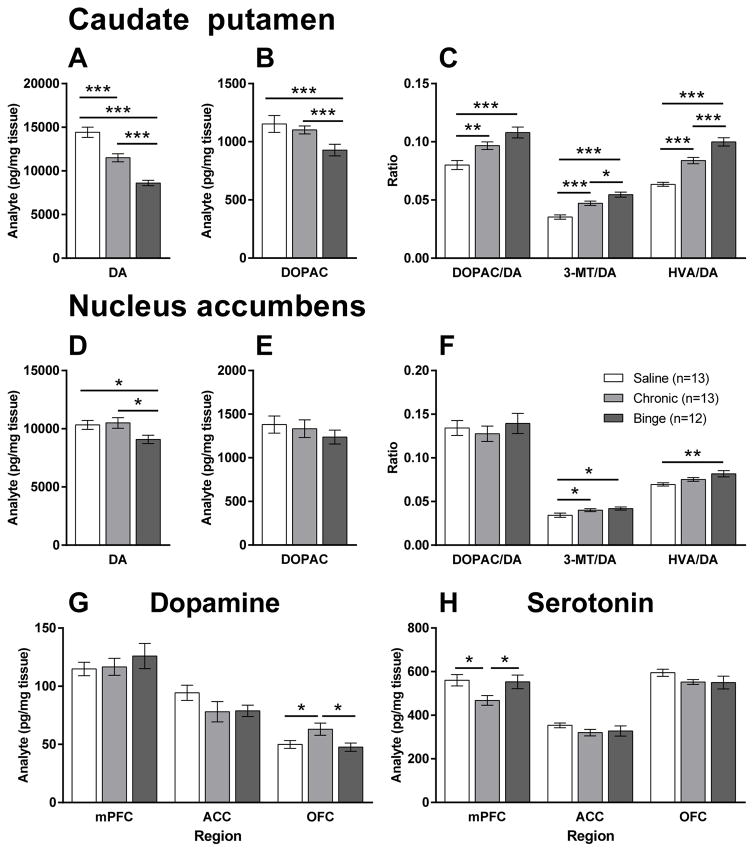
Both methamphetamine regimens had significant effects on dopamine function in the caudate putamen (CPu) and nucleus accumbens (Acb). In the CPu, there were significant group differences in the levels of dopamine (F2,35=37.1, p<0.001; Figure 4A) and DOPAC (F2,35=4.5, p<0.05; Figure 4B). A stepwise decrease in dopamine levels was observed with levels significantly lower after the chronic regimen compared with saline (20%; p<0.001), and after the binge regimen compared with both saline (40%; p<0.001) and the chronic regimen (25%; p<0.001). DOPAC levels were also significantly lower in the binge regimen compared with saline (p<0.001) and the chronic regimen (p<0.001). Correspondingly, decreased dopamine levels led to significantly increased ratios of metabolites/dopamine (Figure 4C).
Figure 4.
The effects of chronic and binge methamphetamine regimens on dopaminergic function in the caudate putamen (A–C), nucleus accumbens (D–F) and cortical subregions (G) and, serotonin levels in cortical subregions (H). In the caudate putamen, both regimens decreased dopamine (DA) levels (A) but the binge regimen resulted in greater decreases than the chronic regimen compared with saline. The binge, but not the chronic regimen also led to decreased dihydroxyphenylacetic acid (DOPAC) levels compared with saline (B). Ratios of the metabolites including DOPAC, 3-methoxytyramine (3-MT) and homovanillic acid (HVA), to DA levels were also significantly different between the regimens (C). In the nucleus accumbens, only the binge regimen resulted in decreased DA levels compared with saline (D). The effects were also less apparent than those in the caudate putamen with no differences in DOPAC levels present (E) and only minimal changes in metabolite to DA ratios (F). In the cortical subregions assessed including the medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC), the chronic regimen led to significantly elevated DA levels in the OFC (G) and reduced levels of serotonin in the mPFC (H) compared with saline and the binge regimen. Data are expressed as mean (pg/mg tissue) ± SEM.
* p<0.05, ** p<0.01, *** p<0.001.
In the Acb, dopamine levels were significantly different between groups (F2,35=3.6, p<0.05) but the effects were less dramatic than in the CPu (Figure 4D). Unlike in the CPu, dopamine was only decreased after the binge regimen with levels 10% and 13% lower than saline (p<0.05) and the chronic regimen (p<0.05), respectively. No significant differences in DOPAC levels were observed (Figure 4E). Differences in the ratios of metabolites to dopamine were minimal (Figure 4F).
Dopamine levels in the OFC (Figure 4G) were also significantly different between groups (F2,35=4.0, p<0.05). The chronic regimen led to significantly increased levels of dopamine compared with both saline (p<0.05) and the binge regimen (p<0.05).
Serotonergic system
Serotonin levels in the medial prefrontal cortex (mPFC; Figure 4H) were significantly different between groups (F2,35=3.8, p<0.05). The chronic regimen led to significantly lower levels of serotonin compared with both saline (p<0.05) and the binge regimen (p<0.05).
Amino acids
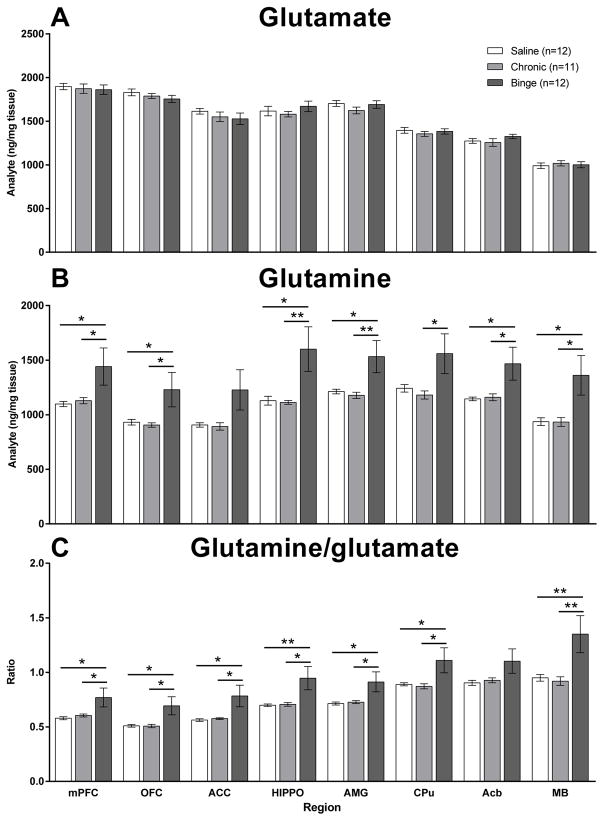
Although there was no effect of methamphetamine regimen on glutamate levels (Figure 5A), there were global alterations in glutamine levels (Figure 5B). There were significant main effects of Regimen on glutamine levels in the mPFC (F2,32=3.4, p<0.05), orbitofrontal cortex (OFC; F2,32=3.6, p<0.05), hippocampus (F2,32=5.0, p<0.05), amygdala (F2,32=4.8, p<0.05), CPu (F2,32=3.3, p<0.05), Acb (F2,32=4.0, p<0.05) and ventral midbrain (F2,32=4.9, p<0.05). In all cases glutamine levels were significantly elevated after the binge regimen. Correspondingly, there were also a significant main effects of Regimen on the glutamine/glutamate ratio (Figure 5C) in the mPFC (F2,32=3.9, p<0.05), OFC (F2,32=4.5, p<0.05), anterior cingulate cortex (F2,32=4.4, p<0.05), hippocampus (F2,32=4.9, p<0.05), amygdala (F2,32=4.1, p<0.05), CPu (F2,32=3.6, p<0.05) and ventral midbrain (F2,32=5.5, p<0.01). Again, in all cases the glutamine/glutamate ratio was significantly elevated after the binge regimen.
Figure 5.
The effects of chronic and binge methamphetamine regimens on levels of glutamate (A), glutamine (B) and the glutamine/glutamate ratio (C) in the medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), hippocampus (HIPPO), amygdala (AMG), caudate putamen (CPu), nucleus accumbens (Acb) and ventral midbrain (MB). Although glutamate levels were not altered by either regimen (A), the binge regimen led to global increases in glutamine levels (B) and an increased glutamine/glutamate ratio (C) compared with saline and the chronic regimen. Data are expressed as mean (ng/mg tissue) ± SEM.
* p<0.05, ** p<0.01.
DISCUSSION
The present studies examining two human-derived methamphetamine use patterns in mice demonstrated that key aspects of reward and neurochemistry can be either independent or dependent on methamphetamine use parameters. Despite large differences in methamphetamine administration patterns and dosage per day, binge and chronic regimens induce a similar magnitude of reward deficits during acute and prolonged withdrawal. Importantly, there were differential effects of methamphetamine regimens on brain neurochemistry. The binge regimen depleted dopamine levels in the striatum and increased glutamine levels throughout the brain. The chronic regimen had milder effects on dopamine levels in the striatum, and altered cortical dopamine and serotonin levels. Neither regimen altered NOR suggesting that these regimens were not associated with persistent memory deficits.
Reward function and methamphetamine regimens
The magnitude of threshold elevations during both the chronic and binge regimens was dynamic, with the greatest elevations observed at 12 h withdrawal and gradually returning to baseline during abstinence. The binge regimen led to more severe and prolonged withdrawal during the first two cycles of administration than the chronic regimen, consistent with a greater level of methamphetamine exposure. Interestingly, after exposure to multiple cycles, similar magnitudes of withdrawal-induced threshold elevations were observed, suggesting that reward dysfunction is not a ‘dose-dependent’ effect per se, especially given the 2.5-fold difference between the chronic and binge regimen in total methamphetamine per day. Consistent with our results, acute and prolonged withdrawal from methamphetamine resulted in elevated thresholds (Jang et al., 2013; Miyata et al., 2011), with greater threshold elevations correlating with greater levels of subsequent methamphetamine administration (Jang et al., 2013).
As expected (Bauer et al., 2013; Kesby et al., 2016a), acute methamphetamine challenge lowered ICSS reward thresholds. Surprisingly, neither regimen led to persistent alterations in acute methamphetamine-induced reward enhancement, suggesting a potential recovery of function during abstinence. Interestingly, both regimens led to decreased response latencies and increased extra responses after acute methamphetamine challenge compared with saline suggesting an impulsivity-like disinhibition of responding (Amitai et al., 2009). Although a similar pattern was observed after both regimens, the magnitude of the effect was attenuated in the chronic compared with the binge regimen, suggesting this may be a reflection of total methamphetamine exposure and the magnitude of dopamine dysfunction after each regimen. Considering that impaired response inhibition and elevated impulsivity levels are key traits of methamphetamine dependence (Everitt et al., 2007), more research is needed using behavioral tasks that specifically evaluate these constructs after exposure to chronic and binge regimens.
Neurochemical impact of methamphetamine regimens
Decreased striatal dopaminergic function in methamphetamine-dependent subjects has been associated with reward deficits that can predict relapse (Wang et al., 2012). Furthermore, rates of anhedonia are higher in subjects with a history of stimulant use compared with subjects with no history of stimulant use (Leventhal et al., 2008) suggesting sustained impairments in dopamine function. The ICSS procedure in rodents measures brain reward function that is particularly sensitive to alterations in limbic dopaminergic projections (Der-Avakian and Markou, 2012). After protracted abstinence, we observed decreased dopamine content in the CPu after both regimens and in the Acb after the binge regimen. In addition, concomitant decreases in DOPAC and increased ratios of metabolites/dopamine suggest these deficits are largely presynaptic. Although we observed regimen-dependent differences in dopamine levels, these effects were not associated with differences in reward deficits during the final cycle of methamphetamine exposure or during prolonged withdrawal. The rewarding effects of ICSS stimulation are highly dependent on dopamine tone (Hernandez et al., 2012). Thus, it would appear that even though both regimens decreased total dopamine levels, and this resulted in decreased synaptic tone (i.e., reward deficits) during the methamphetamine regimen cycles, synaptic dopamine tone was restored after cessation of the methamphetamine exposure. Given these data, we would predict that mice exposed to the methamphetamine regimens, particularly the binge regimen, would be more sensitive to methamphetamine-induced reward deficits upon re-exposure compared with those exposed to saline. This may particularly important considering relapse in humans is associated with decreases in dopamine function (Wang et al., 2012).
These data are consistent with the known impact of methamphetamine on dopaminergic transmission in animal models. For example, prolonged escalation of methamphetamine in monkeys led to decreased striatal dopamine levels after three weeks of abstinence (Melega et al., 2008). Similarly, self-administration (Brennan et al., 2010) and binges following dose-escalation (Segal and Kuczenski, 1997) have been shown to decrease striatal dopamine levels in rodents. The greater impact of the binge regimen on dopamine levels in both the CPu and Acb compared with the chronic regimen support the evidence in humans that factors such as frequency, dose and length of methamphetamine administration contribute to dopaminergic impairments (Sekine et al., 2001). However, significant recovery of dopamine transporter levels in methamphetamine-dependent individuals have been observed after as little as two weeks of abstinence (London et al., 2015). Therefore, it is possible that the observed differences in dopamine content and function between the regimens are the result of differing rates of recovery, complicating any direct associations with the previously assessed behavioral outcomes.
Neuroimaging studies have demonstrated structural and functional deficits in the PFC and OFC of methamphetamine-dependent individuals (London et al., 2015). Our studies showed that the chronic regimen, but not the binge regimen, increased dopamine levels in the OFC and decreased serotonin levels in the mPFC. These data suggest that the sustained methamphetamine exposure in the chronic regimen, rather than the restricted high-dose exposure in the binge regimen, is a critical factor in the dysregulation of cortical neurochemistry. Increased activity in the OFC of mice is associated with continued drug use despite negative consequences (Pascoli et al., 2015). This ‘compulsive drug taking’, developed over multiple sessions, is indicative of the transition to drug addiction of which the OFC plays a critical role in both rodents and humans (Everitt et al., 2007). In addition, decreased serotonin levels in the mPFC of mice is associated with increased impulsivity (Campus et al., 2016). Decreased mPFC serotonin levels may also reflect a compensatory mechanism. For example, activation of PFC serotonin receptors increases the activity of dopamine neurons in the ventral tegmental area (Bortolozzi et al., 2005). Therefore, decreased PFC serotonin after the chronic regimen may be in response to sustained methamphetamine-induced overactivity of ventral tegmental area dopamine neurons. Taken together, alterations in cortical neurochemistry may be more susceptible to the number of days used per week/month rather than the total dose of exposure.
Altered glutamatergic function has also been implicated in methamphetamine dependence and withdrawal. However, the clinical evidence using magnetic resonance spectroscopy, that cannot always distinguish glutamate from glutamine (collectively referred to as GLX), is complicated. For example, Ernst and Chang reported decreased GLX levels in the frontal cortex during the first 1–2 weeks of withdrawal (Ernst and Chang, 2008). However, levels recovered by 1–2 months and exceeded normal levels after 2 months of abstinence suggesting an excessive compensatory response. Increased glutamate levels have also been reported in the frontal cortex (Sailasuta et al., 2010) but increases in glutamate levels were more severe during early abstinence and normalised (decreased) over time. Others have reported decreased GLX levels during early abstinence that recover with time (O’Neill et al., 2015) or no differences in glutamate or GLX (Howells et al., 2014). Our results in mice showed that the binge regimen elevated glutamine levels throughout the brain after three-weeks of near abstinence, whereas the chronic regimen did not. Whether glutamine/glutamate levels are decreased during acute withdrawal or if the chronic regimen transiently alters glutamine function is not known. Collectively however, it appears that the effects of prior methamphetamine on glutamatergic function are dependent on both the use pattern and the length of abstinence.
One limitation of the present study is that it is impossible to rule out that the amount of methamphetamine, rather than the pattern, may be responsible for the neurochemical effects. Although these regimens reflect the differences observed in human subjects, future studies adjusting the doses of the chronic and/or binge regimen to generate equivalent levels of total methamphetamine exposure would be of particular interest. These studies would further clarify whether outcomes are ‘quantity of methamphetamine’ rather than ‘pattern of use’ dependent.
Cognition after methamphetamine regimens
The present study did not reveal persistent impairments in NOR after either regimen, consistent with our previous findings after an escalating methamphetamine binge regimen in mice (Kesby et al., 2015b). However, increasing memory load (i.e., time between familiarisation and test stages) may allow for the detection of long-term memory impairments, as demonstrated by others (Kamei et al., 2006; Reichel et al., 2011), after the methamphetamine regimens. Considering the observed alterations in response latency and extra responses after methamphetamine challenge in the ICSS procedure, impulsivity-like behavior may represent a useful cognitive outcome to probe in future studies. Targeting monoamine transporters with compounds such as modafinil, atomoxetine and methylphenidate have been shown to improve some measures of response inhibition in abstinent methamphetamine-dependent individuals (Sofuoglu et al., 2013). The increasing severity of alterations in both impulsivity-like behavior and dopamine depletion in the binge compared with the chronic regimen in the present study supports this behavioral and neurochemical association. Furthermore, the neurochemical changes observed in cortical regions, that are key to executive functions, suggest this cognitive domain may be particularly relevant in chronic methamphetamine users. Identifying treatments that selectively target cortical, rather than subcortical, dopaminergic and serotonergic measures may be the most relevant example of the need to stratify individuals based on use patterns before assessing the success of novel treatment strategies.
Conclusion
This work highlights that acute and prolonged signs of withdrawal do not necessarily reflect amount/frequency of methamphetamine intake. Furthermore, the array of underlying neurochemical alterations is dependent on the methamphetamine use pattern. However, more research is warranted to clarify whether the differential effects of chronic and binge regimens on brain neurochemistry are ‘methamphetamine dose’ rather than ‘pattern of use’ dependent. Our findings suggest that the use pattern of methamphetamine-dependent individuals has important implications for the neurochemical and potentially, cognitive impact. Thus, in clinical assessments of methamphetamine-dependent individuals, it is necessary to evaluate methamphetamine use patterns. The human-derived methamphetamine regimens characterized in the present study provide an avenue for future research exploring the complex relationship between methamphetamine dependence, higher cognitive function and neurochemistry.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Samuel Barnes and Dr. Karly Turner for insightful comments during the preparation of the manuscript. This work was funded by the Peter F. McManus Charitable Trust research grant for Drug Abuse awarded to Dr. James P. Kesby. The authors were also supported by the Translational Methamphetamine AIDS Research Center funded by the National Institute on Drug Abuse (P50DA26306) and the Interdisciplinary Research Fellowship in NeuroAIDS (R25MH081482) to JPK. AM has received contract research support from Forest Laboratories and Astra-Zeneca and honoraria/consulting fees from AbbVie during the past 2 years. JPK, AC, AM and SS have no competing financial interests in relation to the work described.
Footnotes
AUTHORS CONTRIBUTIONS
JPK was responsible for the study concept and design. JPK and AC contributed to the acquisition of animal data. JPK and SS completed data analysis and interpretation of findings. JPK drafted the manuscript. AC, AM and SS provided critical revision of the manuscript for important intellectual content. All authors critically reviewed the content and approved the final version for publication.
References
- Amitai N, Semenova S, Markou A. Clozapine attenuates disruptions in response inhibition and task efficiency induced by repeated phencyclidine administration in the intracranial self-stimulation procedure. Eur J Pharmacol. 2009;602:78–84. doi: 10.1016/j.ejphar.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. British Journal of Pharmacology. 2013;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O’Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: Comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Diaz-Mataix L, Scorza MC, Celada P, Artigas F. The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J Neurochem. 2005;95:1597–1607. doi: 10.1111/j.1471-4159.2005.03485.x. [DOI] [PubMed] [Google Scholar]
- Brecht ML, O’Brien A, von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addict Behav. 2004;29:89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Colussi-Mas J, Carati C, Lea RA, Fitzmaurice PS, Schenk S. Methamphetamine self-administration and the effect of contingency on monoamine and metabolite tissue levels in the rat. Brain Res. 2010;1317:137–146. doi: 10.1016/j.brainres.2009.11.069. [DOI] [PubMed] [Google Scholar]
- Campus P, Accoto A, Maiolati M, Latagliata C, Orsini C. Role of prefrontal 5-HT in the strain-dependent variation in sign-tracking behavior of C57BL/6 and DBA/2 mice. Psychopharmacology (Berl) 2016;233:1157–1169. doi: 10.1007/s00213-015-4192-7. [DOI] [PubMed] [Google Scholar]
- Cheng WS, Garfein RS, Semple SJ, Strathdee SA, Zians JK, Patterson TL. Binge Use and Sex and Drug Use Behaviors among HIV(−), Heterosexual Methamphetamine Users in San Diego. Subst Use Misuse. 2010;45:116–133. doi: 10.3109/10826080902869620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict Dis. 2002;21:21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- Clark RE, Kuczenski R, Segal DS. Escalating dose, multiple binge methamphetamine regimen does not impair recognition memory in rats. Synapse. 2007;61:515–522. doi: 10.1002/syn.20397. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J Neuroimmune Pharmacol. 2008;3:165–172. doi: 10.1007/s11481-008-9108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Annals of the New York Academy of Sciences. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Solomon TM, Moeller RW, Doig SAR, Espinosa LS, Siconolfi D, Homer BD. Methamphetamine Use among Gay, Bisexual and Non-identified Men-Who-Have- Sex-with-Men An Analysis of Daily Patterns. J Health Psychol. 2009;14:222–231. doi: 10.1177/1359105308100206. [DOI] [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? a critical review. Neuropsychopharmacology. 2012;37:586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez G, Trujillo-Pisanty I, Cossette MP, Conover K, Shizgal P. Role of dopamine tone in the pursuit of brain stimulation reward. J Neurosci. 2012;32:11032–11041. doi: 10.1523/JNEUROSCI.1051-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells FM, Uhlmann A, Temmingh H, Sinclair H, Meintjes E, Wilson D, Stein DJ. (1)H-magnetic resonance spectroscopy ((1)H-MRS) in methamphetamine dependence and methamphetamine induced psychosis. Schizophr Res. 2014;153:122–128. doi: 10.1016/j.schres.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Jang CG, Whitfield T, Schulteis G, Koob GF, Wee S. A dysphoric-like state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology. 2013;225:753–763. doi: 10.1007/s00213-012-2864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei H, Nagai T, Nakano H, Togan Y, Takayanagi M, Takahashi K, Kobayashi K, Yoshida S, Maeda K, Takuma K, Nabeshima T, Yamada K. Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol Psychiatry. 2006;59:75–84. doi: 10.1016/j.biopsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Kim JJ, Scadeng M, Woods G, Kado DM, Olefsky JM, Jeste DV, Achim CL, Semenova S. Spatial cognition in adult and aged mice exposed to high-fat diet. Plos One. 2015a;10:e0140034. doi: 10.1371/journal.pone.0140034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S. The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice. Neuropharmacology. 2016a;109:205–215. doi: 10.1016/j.neuropharm.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S. Effects of HIV/TAT protein expression and chronic selegiline treatment on spatial memory, reversal learning and neurotransmitter levels in mice. Behav Brain Res. 2016b;311:131–140. doi: 10.1016/j.bbr.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S TMARC. Cognitive deficits associated with combined HIV gp120 expression and chronic methamphetamine exposure in mice. Eur Neuropsychopharm. 2015b;25:141–150. doi: 10.1016/j.euroneuro.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacan G, Hadamitzky M, Kuczenski R, Melega WP. Alterations in the striatal dopamine system during intravenous methamphetamine exposure: Effects of contingent and noncontingent administration. Synapse. 2013;67:476–488. doi: 10.1002/syn.21654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I, Zimmerman M. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. Am J Addict. 2008;17:218–223. doi: 10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology. 2012;37:707–722. doi: 10.1038/npp.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 2015;1628:174–185. doi: 10.1016/j.brainres.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied Access to Intravenous Methamphetamine Self-Administration Differentially Alters Adult Hippocampal Neurogenesis. Biol Psychiatry. 2008;64:958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye WG, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- Melega WP, Jorgensen MJ, Lacan G, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA. Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: Brain neurotoxicity and behavioral profiles. Neuropsychopharmacology. 2008;33:1441–1452. doi: 10.1038/sj.npp.1301502. [DOI] [PubMed] [Google Scholar]
- Melo P, Magalhaes A, Alves CJ, Tavares MA, de Sousa L, Summavielle T, Moradas-Ferreira P. Methamphetamine mimics the neurochemical profile of aging in rats and impairs recognition memory. Neurotoxicology. 2012;33:491–499. doi: 10.1016/j.neuro.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Miyata H, Itasaka M, Kimura N, Nakayama K. Decreases in Brain Reward Function Reflect Nicotine- and Methamphetamine-Withdrawal Aversion in Rats. Curr Neuropharmacol. 2011;9:63–67. doi: 10.2174/157015911795017218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Progress in neurobiology. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Tobias MC, Hudkins M, London ED. Glutamatergic neurometabolites during early abstinence from chronic methamphetamine abuse. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2015:18. doi: 10.1093/ijnp/pyu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Hiver A, Luscher C. Sufficiency of Mesolimbic Dopamine Neuron Stimulation for the Progression to Addiction. Neuron. 2015;88:1054–1066. doi: 10.1016/j.neuron.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62:1119–1126. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailasuta N, Abulseoud O, Hernandez M, Haghani P, Ross BD. Metabolic Abnormalities in Abstinent Methamphetamine Dependent Subjects. Substance abuse : research and treatment. 2010;2010:9–20. doi: 10.4137/sart.s4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. An escalating dose ‘‘binge’’ model of amphetamine psychosis: behavioral and neurochemical characteristics. J Neurosci. 1997;17:2551–2566. doi: 10.1523/JNEUROSCI.17-07-02551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. The American journal of psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. Binge use of methamphetamine among HIV-positive men who have sex with men: Pilot data and HIV prevention implications. AIDS Educ Prev. 2003;15:133–147. doi: 10.1521/aeap.15.3.133.23835. [DOI] [PubMed] [Google Scholar]
- Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, Ling W. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis. 2002;21:35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive Function as a Transdiagnostic Treatment Target in Stimulant Use Disorders. Journal of dual diagnosis. 2016;12:90–106. doi: 10.1080/15504263.2016.1146383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers I, Baskin D, Baskin-Sommers A. Methamphetamine use among young adults: Health and social consequences. Addict Behav. 2006;31:1469–1476. doi: 10.1016/j.addbeh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- UNODC. World Drug Report 2013. 2013 http://www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdf.
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatr. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.