Abstract
Objectives
To describe the effect of e-liquid flavors on nicotine intake and pharmacology of e-cigarettes.
Methods
11 males and 3 females participated in a 3-day inpatient crossover study with strawberry, tobacco, and their usual flavor e-liquid. Nicotine levels were nominally 18 mg/ml in the strawberry (pH 8.29) and tobacco (pH 9.10) e-liquids and ranged between 3–18 mg/ml in the usual brands (mean pH 6.80). Each day consisted of a 15-puff session followed by 4 hours of abstinence, then 90 minutes of ad libitum use. Subjects used a KangerTech mini ProTank 3.
Results
After 15 puffs, the amount of nicotine inhaled and systemically retained were not significantly different between the strawberry and tobacco e-liquids but plasma AUC(0→180) was significantly higher with the strawberry e-liquid. While not significantly different, Cmax was 22% higher and various early time point AUCs to measure rate of rise of nicotine in blood ranged between 17–23% higher with the strawberry e-liquid compared to the tobacco e-liquid. During ad libitum use, systemic exposure to nicotine (AUC(0→90)) was the same for the tobacco and usual brand e-liquids but were both significantly lower than after using the strawberry e-liquid. The usual flavors were more liked and satisfying than the strawberry and tobacco e-liquids.
Conclusion
Flavors influence nicotine exposure through flavor liking, may affect rate of nicotine absorption possibly through pH effects, and contribute to heart rate acceleration and subjective effects of e-cigarettes. E-cigarette users titrate their nicotine exposure but the extent of titration may vary across flavors.
Keywords: E-cigarettes, flavors, nicotine delivery, nicotine pharmacokinetics, e-cigarette pharmacology
1.0. INTRODUCTION
Flavored e-liquids are commonly used in electronic cigarettes (e-cigarettes). Several flavorants are toxic and could be harmful to e-cigarette users. Diacetyl and its related compound, 2,3-pentanedione, both of which give a buttery flavor, cause bronchiolitis obliterans in humans exposed in occupational settings (Kreiss et al., 2002; van Rooy et al., 2007), and have been used as constituents of flavorants in e-liquids (Allen et al., 2016; Farsalinos et al., 2015). Some e-liquids and specific flavorants such as cinnamaldehyde, 2-methoxycinnamaldehyde, vanillin, and 2,5-dimethypyrazine (chocolate flavoring) have cytotoxic effects in in vitro studies (Bahl et al., 2012; Behar et al., 2014; Sherwood and Boitano, 2016). Flavors are also significant sources of toxic aldehydes produced during thermal decomposition of e-liquid constituents (Khlystov and Samburova, 2016).
From a regulatory perspective, use of flavors in e-liquids is controversial. Over 7000 different flavors have been identified, including tobacco, sweet flavors, menthol, and combinations (Krishnan-Sarin et al., 2015; Zhu et al., 2014). Sweet flavors, in particular, appeal to youth and may contribute to e-cigarette uptake (Kong et al., 2015; Krishnan-Sarin et al., 2015). On the other hand, flavors may be an important consideration for the acceptability of e-cigarettes to smokers who are trying to quit smoking (Farsalinos et al., 2013; Shiffman et al., 2015), a balance the U.S. Food and Drug Administration has acknowledged in its deeming rule of e-cigarettes as tobacco products (Food and Drug Administration, 2016).
Flavors might also influence nicotine pharmacokinetics from e-cigarettes, which has implications for their abuse liability. Flavors enhance the rewarding and reinforcing effect of nicotine-containing e-cigarettes (Audrain-McGovern et al., 2016), and one flavor decreased the maximum plasma nicotine concentration, Cmax, in a study of an industry e-cigarette prototype (Walele et al., 2016). To the best of our knowledge, no study has assessed differences in nicotine intake and pharmacology of flavored e-liquids when used in commercially available e-cigarettes.
The objectives of this pilot study were to describe differences in nicotine intake, pharmacokinetics, and physiologic and subjective effects during prescribed vaping, and determine the intake of nicotine and extent of titration of nicotine blood levels during ad libitum access comparing different nicotine-containing flavored e-liquids.
2.0. METHODS
We performed a 3-arm crossover study over three consecutive inpatient days in healthy e-cigarette users to examine the effects of e-liquid flavors on e-cigarette use. This paper focuses on the effects of e-liquid flavors on nicotine intake and pharmacology during a standardized session of 15 puffs and during ad libitum access. Changes in puff topography and vaping behavior with e-liquid flavors will be reported separately.
2.1. Subjects
A convenience sample of 14 subjects (3 females, 11 males) completed the study. Participants were recruited via Craigslist.com, flyers, and college campus newspapers. They were screened for eligibility at a clinical research facility. Exclusive e-cigarette users or dual users of fewer than 5 tobacco cigarettes per day, who used second and/or third generation e-cigarettes at least 25 days per month for the past 3 months or more, and had saliva cotinine levels ≥30 ng/mL were eligible. Exclusion criteria were unstable chronic medical conditions, current or past severe mental illness, pregnancy, current substance abuse other than marijuana, and sole users of first generation e-cigarettes (cig-a-likes). The study was approved by the Committee on Human Research at the University of California San Francisco. Written, informed consent was obtained from each participant and all participants were financially compensated.
2.2. Study e-liquid flavors and e-cigarette
For each of the three experimental arms, participants exclusively used one flavor of e-liquid: strawberry, tobacco or their usual brand of e-liquid. The strawberry and tobacco test e-liquids were purchased from Bulkejuice.com. We chose a strawberry flavor because it was one of the most highly rated single fruit flavors on Bulkejuice.com at the time. The tobacco flavor was chosen to represent tobacco-flavored e-liquids. Both flavors were labeled 50/50 VG/PG (vegetable glycerin/propylene glycol) and 18 mg/mL nicotine. We chose 18 mg/mL nicotine because users of smaller tanks commonly use e-liquids with high nicotine content (e.g. 18 or 24 mg/mL) (Goniewicz et al., 2013; Wagener et al., 2016).
KangerTech Mini ProTank 3 clearomizer (1.5 ohms) connected to a KangerTech 3.7 volt, 1000 mAh battery was the study e-cigarette, purchased directly from Kangertech.com. A new clearomizer was used for each assigned flavor. The nominal power of the e-cigarettes was 9.1 watts.
2.3. General procedures
The study was conducted on the Clinical Research Center (CRC) at Zuckerberg San Francisco General Hospital. Each of the three study days ran from about 4 PM to 4 PM of the next day. Subjects and study personnel could not be blinded to the e-liquid administered because each e-liquid produced a strong distinct odor. On admission to the CRC, the participants’ own e-cigarette(s) and e-liquid(s) were immediately removed. From 4–10 PM (Acclimatization Session), subjects could vape ad libitum to become acclimatized to the assigned flavor for the next day’s procedures. This time was considered sufficient for acclimatization to the e-liquid because all participants were experienced users of similar push-button tank e-cigarettes or third generation rebuildable atomizers. Participants were abstinent overnight until the morning standardized session of 15 puffs, which was followed by 4 hours of abstinence, and then a 90-minute ad libitum use session.
2.4. Standardized session experimental procedures
On each morning, participants were awakened at 7:00 AM. An intravenous (IV) line for blood sampling was placed in the forearm at 8:00 AM followed by a light breakfast. At about 8:30 AM, subjective questionnaires were administered, and three heart rate measurements were made within 10 minutes by pulse oximeter (average was used as the baseline heart rate for each day); baseline blood was sampled and urine collected at about 9:10 AM. At 9:28 AM, participants took 15 puffs, one puff every 30 seconds, from the e-cigarette. Puff duration was not controlled by the study. The amount of nicotine exhaled was collected as previously described (Havel et al., 2016; St. Helen et al., 2016a). E-cigarettes were weighed before and after vaping to determine amount of e-liquid consumed. Blood was sampled at 2, 5, 15, 30, 45, 60, 90, 120, and 180 minutes and heart rate was measured at 5, 10, 15, 20, and 30 minutes after the final puff. Subjective questionnaires were administered between the 5-minute and 15-minute blood samples.
2.5. Ad libitum session experimental procedures
After about 4 hours of abstinence, subjective questionnaires were administered and a blood sample was taken. E-cigarettes were filled with the same e-liquid used during the standardized session and weighed before and after the session. Starting at 2:00 PM, participants vaped the study e-cigarette as desired for 90 minutes. During that time, subjects watched television, browsed the Internet through their personal computers or smartphones and/or read books. Participants were not allowed to sleep or doze off. Blood samples were taken every 15 minutes and subjective questionnaires were administered again at the end of the 90-minute session.
2.6. Questionnaires
We measured nicotine withdrawal, craving, and positive and negative affective states before and after e-cigarette use with the Minnesota Nicotine Withdrawal Scale (total score) (MNWS) (Hughes and Hatsukami, 1986); the Questionnaire for Smoking Urges (QSU-Brief) modified for e-cigarettes using the total score, factor 1 subscale (a desire and intention to smoke with smoking perceived as rewarding), and factor 2 subscale (an anticipation of relief from negative affect with an urgent desire to smoke) (Cox et al., 2001); and the positive and negative affect subscales of the Positive and Negative Affect Schedule (PANAS) (Becoña et al., 1998), respectively. We used the five subscales of the modified Cigarette Evaluation Questionnaire (mCEQ) (Rose et al., 1999), further modified for e-cigarettes, to measure satisfaction, reward, aversive effects, enjoyment of sensation at the back of the throat and chest, and craving reduction. The mCEQ item “Did the e-cigarette taste good?” measured after each ad libitum session, was used as a proxy for ‘liking’ of the e-liquids.
2.7. Analytical chemistry
Nicotine was measured in the pooled 0.02 N HCl trap solution from the three gas dispersion tubes and mouthpiece and in e-liquids by LC-MS/MS using previously described methods (St. Helen et al., 2016a; Trehy et al., 2011). The LOQ was 0.5 ng/mL. Nicotine concentration in plasma was determined by GC-MS/MS (Jacob et al., 1991) modified for tandem mass spectrometry for improved sensitivity. The limit of quantitation (LOQ) was 0.2 ng/mL. The pH of all e-liquids was measured using an Accumet AB15 pH meter (Fisher Scientific, Waltham, MA). For pH measurements, 0.5 g of each e-liquid was mixed with 4.5 mL of deionized water to form a 1:10 dilution of nicotine.
2.8. Pharmacokinetic analysis
Pharmacokinetic parameters were estimated from plasma nicotine concentrations using Phoenix WinNonlin 6.3 (Pharsight Corporation, Mountain View, CA). Time to maximum plasma nicotine concentration (Tmax), Cmax, and area under the plasma nicotine concentration-time curve (AUC) from 0 to 5, 15, 30, and 180 min, and AUC from 0 to infinity (AUC0→∞) were estimated using a noncompartmental model and trapezoidal rule. Since all subjects had quantifiable plasma nicotine levels at baseline on all study days (1.3±2.0 ng/mL, mean ± SD), we corrected all measures for each morning’s baseline values as described previously (St. Helen et al., 2016a). The PK-estimated dose for the standardized session was computed as the product of average population clearance of nicotine (~1200 mL/min) and AUC(0→∞) (Hukkanen et al., 2005). We computed Tmax and baseline-corrected (afternoon baseline) Cmax and AUC from 0 to 90 minutes (AUC0→90) for the ad libitum session (St. Helen et al., 2016b).
2.9. Statistical analysis
We calculated the systemic retentions of nicotine, VG, and PG during the standardized session as: retention (%) = 100 × [(amount inhaled – amount exhaled)/amount inhaled]. The amount of nicotine, VG, and PG inhaled (mg) were estimated as the amount of e-liquid consumed (mg) × the concentration of nicotine, VG, and PG in the e-liquid, respectively. Descriptive statistics (mean and standard error of the mean) were computed for all variables by e-liquid.
Importantly, since the nicotine content of the usual brand e-liquids varied between each other (range 3–18 mg/mL) and were different from the two test flavors, our primary analyses focused on the test flavor-related differences. Analysis of data using usual flavors was a secondary analysis except for those tests where we examined titration of nicotine dose and differences in subjective effects. We utilized repeated measures analysis of variance (ANOVA) with flavor as the treatment factor (strawberry vs tobacco) and included a time factor (day of study) and flavor × time interaction term. Time (period) and flavor × time interaction terms were not significant in all models. Dependent variables of interest included amount of nicotine inhaled and systemically retained, nicotine pharmacokinetic parameters, heart rate change, and subjective effects, all in separate models. Cmax, AUCs, and Cmax and AUCs normalized for retained nicotine dose were log-transformed since they were approximately log-normally distributed. Subjective measures obtained before and after vaping were analyzed as the change in score and those obtained only after vaping were analyzed as the absolute value. Given that 11 of 14 subjects were males, we did not perform separate analyses for men and women. Including sex as a covariate did not change the effect of ‘flavors’ on the dependent variables (p-values were almost identical).
In a post hoc analysis of the effect of e-liquid pH on nicotine intake and pharmacology, we computed correlations between the pH of the usual e-liquids and nicotine intake and PK variables obtained during the standardized session. Further, nonparametric t-test was used to compare nicotine intake and PK for the acidic and basic usual e-liquids.
To assess titration of nicotine intake, differences in plasma nicotine AUC were examined across e-liquid flavors and cross-correlations between AUCs for strawberry and usual and tobacco and usual e-liquids were determined. We compared the ratio of AUC to nicotine concentration of the e-liquid across flavor conditions to estimate the degree to which subjects compensated for differences in nicotine content of the e-liquid.
Statistical tests were considered significant at α<0.05 and multiple comparisons were corrected using Tukey’s method where applicable.
3.0. RESULTS
Of 14 participants (3 females, 11 males), 9 were white, 3 were mixed-race, and 2 were Asian. The average age was 32.3 years (SD, 13.8; median, 25; range, 19–59 years). The average screening-visit expired CO and saliva cotinine were 2.7 ± 1.4 ppm (mean ± SD) and 240.3 ± 152.9 ng/mL, respectively. Twelve participants self-identified as not currently smoking tobacco cigarettes; of these, 4 self-identified as never-smokers. The participants had been using e-cigarettes for an average of 2.3 years (SD, 1.4 years; range 1–6 years). The average Penn State Electronic Cigarette Dependence Index was 9.2 (SD 3.9; range 3–15) (medium dependence) (Foulds et al., 2015).
3.1. E-liquid characteristics
Table 1 gives the names and characteristics of the e-liquids used in the study. The measured nicotine concentrations (and pH) of the strawberry and tobacco e-liquids were 19.9 mg/mL (pH 8.29) and 19.3 mg/mL (pH 9.10), respectively. The average measured nicotine concentration of the usual e-liquids flavors was 7.4 ± 3.4 mg/mL (mean ± SD) and the average pH was 6.80 ± 1.58.
Table 1.
Description of flavors, nicotine concentrations, and pH of test electronic cigarette refill liquids (e-liquids) and usual e-liquids
| Subject count | E-liquid Brand | E-liquid Flavor | Description of characterizing flavor | Nicotine on label (mg/mL) | Measured nicotine (mg/mL) | %VG | %PG | pH |
|---|---|---|---|---|---|---|---|---|
| Study test flavors | ||||||||
| Bulk e-juice | Strawberry | Strawberry | 18 | 19.9 | 60% | 40% | 8.29 | |
| Bulk e-juice | USA Tobacco | Tobacco | 18 | 19.3 | 56% | 44% | 9.10 | |
| Participant usual flavors | ||||||||
| 1 | Magic Elixir | Strapeaze | Strawberry and peach | 3 | 3.2 | 67% | 33% | 4.84 |
| 2 | Savage Juice | O’Kains Custard | Custard | 3 | 2.9 | 59% | 41% | 5.29 |
| 3 | Uncharted | 1 Night Stand | White peach and tea | 3 | 2.8 | 65% | 35% | 4.33 |
| 4 | Flavies | Yogurt | Creamy yogurt | 3 | 1.6 | 54% | 46% | 4.69 |
| 5 | Mister E-liquid | Grey Matter | Tobacco, vanilla | 3 | 3.4 | 41% | 59% | 8.54 |
| 6 | Nicoticket | The Virus | Chocolate, coffee, peanuts, honey, caramel, tobacco and maple syrup | 6 | 6.7 | 46% | 54% | 6.45 |
| 7 | Ripe Vapes | VCT | Vanilla, custard, and tobacco | 6 | 5.9 | 64% | 36% | 6.84 |
| 8 | Frisco Vapor | SOMA | Spearmint menthol, watermelon and strawberry | 6 | 6.1 | 67% | 33% | 7.53 |
| 9 | Time Zone | 1952 Washington, DC | Red sports drink | 6 | 6.2 | 89% | 11% | 5.85 |
| 10 | Lost Art Liquids | Mystery flavor (“?”) | Mixed fruit taffy candy | 6 | 4.2 | 69% | 31% | 7.29 |
| 11 | Cosmic Fog | Milk & Honey | Milk and honey | 12 | 13.5 | 57% | 43% | 7.95 |
| 12 | Velvet Cloud Vapor | Thorangine | Orange and tangerine | 18 | 14.9 | 95% | 5% | 7.96 |
| 13 | MtBakerVapor.com | Blue Ice | Blueberry and menthol | 18 | 16.7 | 81% | 19% | 8.97 |
| 14 | Vapor All | Root beer Candy | Root beer | 18 | 15.1 | 31% | 69% | 8.67 |
| Mean | 7.9 | 7.4 | 63% | 37% | 6.80 | |||
| SD | 6.0 | 5.3 | 18% | 18% | 1.58 | |||
Notes: VG = vegetable glycerin; PG = propylene glycol
3.2. Subjective ratings of taste
Average mCEQ “taste good” ratings obtained after the ad libitum session for the strawberry, tobacco, and usual e-liquids were 3.4 ± 0.4, 3.1 ± 0.5, and 5.9 ± 0.3, respectively (mean ± SEM) (maximum possible score of this item is 7). The usual flavor was rated significantly higher than the strawberry and tobacco e-liquids (p-values < 0.001) while the strawberry and tobacco e-liquids were not significantly different.
3.3. Standardized session
Data presented in this section include comparisons between strawberry and tobacco e-liquids only. The amount of nicotine inhaled from 15 puffs of the strawberry e-liquid (1.7 ± 0.2 mg) and tobacco e-liquid (1.6 ± 0.2 mg) were not significantly different (Table 2). Average systemic nicotine retention did not differ significantly between the test e-liquids.
Table 2.
Nicotine intake and pharmacokinetics following 15 puffs in a standardized session by e-liquid flavor
| Variable | Usual brand | Test Flavors | Strawberry vs Tobacco | ||
|---|---|---|---|---|---|
|
| |||||
| Strawberry | Tobacco | ‡ Mean ratio or difference (95% CI) | F (p value) | ||
| Nicotine concentration in e-liquid (mg/mL) | 7.4 (1.4) | 19.9 | 19.3 | - | - |
| Amount of e-liquid Used (mg) | 164.7 (21.6) | 99.7 (10.7) | 92.0 (10.2) | 1.08 (0.93, 1.26) | 1.30 (0.28) |
| Amount of nicotine inhaled (mg) | 0.9 (0.1) | 1.7 (0.2) | 1.6 (0.2) | 1.10 (0.94, 1.28) | 1.87 (0.21) |
| Amount of nicotine exhaled (mg) | 0.012 (0.005) | 0.021 (0.007) | 0.010 (0.003) | 1.04 (0.38, 2.83) | 0.01 (0.94) |
| Amount of nicotine retained (mg) | 0.9 (0.1) | 1.7 (0.2) | 1.5 (0.2) | 1.09 (0.94, 1.26) | 1.76 (0.22) |
| Nicotine retention (%) | 98.6 (0.5) | 98.7 (0.5) | 99.2 (0.2) | −0.5 (−1.4, 0.4) † | 1.88 (0.20) |
| Half-life (min) | 151 (13) | 124 (8) | 131 (14) | −6 (−39, 28) † | 0.14 (0.72) |
| Tmax(min) | 3.1 (0.4) | 5.4 (1.4) | 4.9 (1.2) | 0.5 (−2.8, 3.8) † | 0.12 (0.74) |
| Cmax (ng/mL) | 6.2 (1.0) | 12.1 (2.0) | 9.5 (1.2) | 1.22 (0.90, 1.66) | 2.22 (0.17) |
| AUC(0→5) (ng/mL•min) | 23 (4) | 44 (7) | 35 (5) | 1.23 (0.90, 1.68) | 2.35 (0.16) |
| AUC(0→15) (ng/mL•min) | 73 (12) | 136 (19) | 111 (14) | 1.20 (0.96, 1.52) | 3.29 (0.10) |
| AUC(0→30) (ng/mL•min) | 130 (22) | 239 (30) | 202 (25) | 1.17 (0.97, 1.41) | 3.66 (0.09) |
| AUC(0→180) (ng/mL•min) | 409 (70) | 730 (82) | 656 (78) | 1.11 (1.01, 1.22) | 6.69 (0.03) |
| AUC(0→∞) (ng/mL•min) | 663 (105) | 1078 (121) | 989 (106) | 1.08 (0.07, 1.20) | 2.58 (0.14) |
| PK-predicted nicotine dose (mg) | 0.8 (0.1) | 1.3 (0.1) | 1.2 (0.1) | 1.05 (0.97, 1.20) | 2.58 (0.14) |
| Cmax per NIC retained (ng/mL/mg) | 7.3 (0.6) | 7.2 (0.8) | 6.1 (0.5) | 1.12 (0.84, 1.51) | 0.80 (0.39) |
| AUC(0→5) per NIC retained (ng/mL•min/mg) | 27 (2) | 26 (3) | 22 (2) | 1.13 (0.85, 1.51) | 1.00 (0.34) |
| AUC(0→15) per NIC retained (ng/mL•min/mg) | 83 (6) | 82 (8) | 72 (5) | 1.11 (0.89, 1.37) | 1.14 (0.31) |
| AUC(0→30) per NIC retained (ng/mL•min/mg) | 148 (10) | 145 (12) | 132 (8) | 1.13 (0.85, 1.51) | 1.00 (0.34) |
| AUC(0→180) per NIC retained (ng/mL•min/mg) | 469 (38) | 449 (30) | 436 (31) | 1.02 (0.90, 1.15) | 0.12 (0.74) |
| AUC(0→∞) per NIC retained (ng/mL•min/mg) | 785 (71) | 669 (48) | 667 (41) | 0.99 (0.84, 1.16) | 0.03 (0.86) |
| AUC(0→180) per NIC e-liquid (ng/mL•min/mg/mL) | 59.7 (6.3) | 36.7 (4.1) | 34.0 (4.0) | 1.08 (0.98, 1.18) | 3.35 (0.10) |
| AUC(0→∞) per NIC e-liquid (ng/mL•min/mg/mL) | 101.0 (12.0) | 54.2 (6.1) | 51.3 (5.5) | 1.05 (0.94, 1.16) | 0.90 (0.37) |
| Max change in heart rate (beats per min) | 9.4 (2.4) | 17.2 (2.5) | 12.3 (2.3) | 4.6 (0.8, 8.5) † | 7.40 (0.02) |
| Heart rate AUC (beats per min•min) | 169 (53) | 245 (37) | 210 (45) | 34 (−43, 111) † | 0.98 (0.35) |
Values for the usual and test flavors are presented as mean and standard error of the mean (SEM);
Comparisons between the two test flavors are presented as mean ratios of strawberry to tobacco obtained from back-transform of least-square mean differences of log-transformed variables;
Comparisons are differences rather than ratios; PK-estimated nicotine dose is average population clearance of nicotine (~1200 mL/min) × AUC(0--∞)
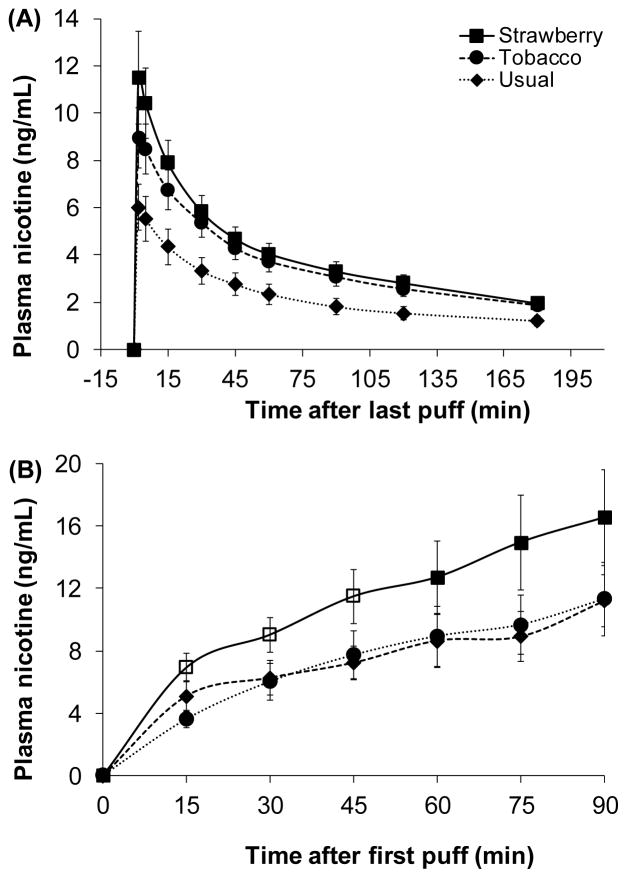
Despite not being significantly different, Cmax for the strawberry e-liquid (12.2 ng/mL) was 22% higher, on average, compared to the tobacco e-liquid (9.5 ng/mL). AUC0→5, AUC0→15, and AUC0→30 were, on average, 23%, 20%, and 17% higher, respectively, with the strawberry e-liquid compared to the tobacco e-liquid but were not significantly different. AUC0→180 was significantly higher with the strawberry e-liquid compared to the tobacco e-liquid, 11% higher on average (p = 0.03). AUC0→∞ was not significantly different between the test flavors (Table 2). PK parameters normalized by retained nicotine dose were not significantly different but were consistently higher with the strawberry compared to tobacco: Cmax per retained nicotine dose was 12% higher, on average, and AUC0→5, AUC0→15, and AUC0→30 per retained nicotine dose were between 11–13% higher, on average. Plasma nicotine concentration was not significantly different between strawberry and tobacco at any of the time points measured (Figure 1A).
Figure 1.
Average plasma nicotine concentration profiles for strawberry and tobacco test e-liquid flavors and usual brand e-liquids (mean ± SEM) after 15 puffs of the standardized session (A); average plasma nicotine concentration profiles for test e-liquid flavors and usual brand e-liquids during 90 minutes of ad libitum access to e-cigarettes (B). No differences were observed between strawberry and tobacco test flavors in plot A; open markers in plot B indicate significant differences between strawberry and tobacco test flavors.
The average maximum increase in heart rate following 15 puffs was 4.6 beats per minute (bpm) higher for the strawberry e-liquid (17.2 bpm) compared to the tobacco e-liquid (12.3 bpm) (p = 0.02). Area under the heart rate × time curve was not significantly different between the two test flavors (Table 2).
Changes in subjective measures were not significantly different between strawberry and tobacco test e-liquids (Table 4) except ratings of sensations in the throat and chest (mCEQ-sensation), which were significantly higher with the tobacco e-liquid compared to the strawberry e-liquid (p=0.05).
Table 4.
Subjective effects by e-liquid flavor in standardized and ad libitum sessions
| Variable | Usual brand | Test Flavors | Strawberry vs Tobacco | ||
|---|---|---|---|---|---|
|
| |||||
| Strawberry | Tobacco | Mean difference (95% CI) | F (p value) | ||
| A. Standardized Session | |||||
| Change in MNWS total score | −2.5 (1.5) | −3.2 (1.3) | −3.2 (1.4) | 0.2 (−2.8, 3.1) | 0.01 (0.91) |
| Change in QSU total score | −11.6 (3.7) | −20.1 (4.5) | −18.3 (4.1) | −2.2 (−6.8, 2.4) | 1.19 (0.30) |
| Change in QSU Factor 1 | −6.6 (2.4) | −13.0 (2.3) | −11.6 (2.2) | −1.8 (−5.3, 1.8) | 1.27 (0.29) |
| Change in QSU Factor 2 | −5.0 (2.0) | −7.1 (2.5) | −6.7 (2.3) | −0.4 (−2.6, 1.8) | 0.18 (0.68) |
| Change in PANAS-positive affect subscale | 2.8 (1.6) | 0.2 (1.1) | 0.4 (1.6) | −0.4 (−3.8, 3.0) | 0.08 (0.79) |
| Change in PANAS-negative affect subscale | −1.4 (0.7) | −1.9 (0.8) | −1.1 (0.7) | −0.8 (−2.7, 1.1) | 0.95 (0.36) |
| mCEQ Satisfaction subscale | 17.1 (0.9) | 12.4 (1.2) | 13.2 (1.5) | −0.6 (−2.9, 1.7) | 0.38 (0.55) |
| mCEQ Reward subscale | 21.8 (2.2) | 19.0 (2.1) | 20.2 (2.5) | −0.7 (−3.8, 2.4) | 0.30 (0.60) |
| mCEQ Aversion subscale | 3.6 (0.7) | 5.4 (0.9) | 5.5 (0.7) | −0.1 (−2.4, 2.2) | 0.01 (0.91) |
| mCEQ Sensations subscale | 5.2 (0.4) | 3.9 (0.5) | 4.9 (0.6) | −0.8 (−1.7, −0.01) | 5.40 (0.05) |
| mCEQ Craving Reduction subscale | 4.8 (0.4) | 4.6 (0.5) | 5.2 (0.4) | −0.4 (−1.3, 0.4) | 1.46 (0.26) |
| B. Ad Libitum Session | |||||
| Change in MNWS total score | −3.1 (1.6) | −4.0 (2.0) | −4.8 (1.4) | 0.9 (−2.0, 3.8) | 0.47 (0.51) |
| Change in QSU total score | −15.6 (3.2) | −16.6 (4.5) | −18.5 (3.4) | 2.0 (−5.7, 9.7) | 0.35 (0.57) |
| Change in QSU Factor 1 | −10.5 (2.1) | −10.6 (4.5) | −12.1 (2.0) | 1.6 (−4.3, 7.4) | 0.37 (0.56) |
| Change in QSU Factor 2 | −10.5 (2.1) | −5.9 (2.4) | −6.4 (1.9) | 0.4 (−3.2, 4.0) | 0.07 (0.80) |
| Change in PANAS-positive affect subscale | 1.6 (1.6) | 1.2 (1.3) | 1.4 (1.4) | −0.5 (−3.2, 2.1) | 0.20 (0.66) |
| Change in PANAS-negative affect subscale | −1.9 (0.7) | −1.4 (0.8) | −1.3 (0.7) | −0.2 (−1.5, 1.1) | 0.11 (0.75) |
| mCEQ Satisfaction subscale | 17.6 (0.7) | 12.1 (1.2) | 11.5 (1.5) | 0.5 (−1.8, 2.9) | 0.28 (0.61) |
| mCEQ Reward subscale | 21.2 (2.2) | 18.6 (2.4) | 16.7 (2.3) | 1.8 (−2.1, 5.7) | 1.07 (0.33) |
| mCEQ Aversion subscale | 2.7 (0.6) | 3.6 (0.7) | 3.2 (0.7) | 0.5 (−0.6, 1.6) | 1.05 (0.33) |
| mCEQ Sensations subscale | 5.2 (0.3) | 4.1 (0.4) | 4.1 (0.5) | 0 (−0.9, 0.9) | 0.01 (0.94) |
| mCEQ Craving Reduction subscale | 5.4 (0.4) | 4.6 (0.4) | 4.3 (0.6) | 0.3 (−0.7, 1.4) | 0.53 (0.49) |
| mCEQ Taste Good (individual item) | 5.9 (0.3) | 3.4 (0.4) | 3.1 (0.5) | 0.2 (−0.7, 1.1) | 0.30 (0.60) |
3.4. Usual e-liquids during standardized session and pH
Significant correlations with pH of the usual e-liquids are as follows: nicotine concentration of the e-liquid, 0.74 (0.002) (r, p-value); amount of nicotine inhaled, 0.84 (0.001); amount of nicotine retained, 0.84 (0.001); Tmax, 0.57 (0.03); Cmax, 0.66 (0.01); AUC0→15, 0.71 (0.04); AUC0→180, 0.78 (0.001); and, AUC0→∞, 0.77 (0.001). Cmax and all AUCs normalized for retained nicotine dose were not significantly different between the seven acidic usual e-liquids and the seven basic usual e-liquids but were consistently numerically higher with the acidic usual e-liquids (Table 5). Max heart rate change was 3 bpm higher with the acidic usual e-liquids but was not significantly different.
Table 5.
Comparison of nicotine intake and pharmacokinetics of the usual e-liquids during the standardized session when grouped into acidic (pH<7) and basic (pH>7) e-liquids. P-values in bold indicate significant differences between the usual acidic and usual basic e-liquids.
| Variable | Usual Acidic (N = 7) | Usual basic (N = 7) | p value |
|---|---|---|---|
| Amount of e-liquid Used (mg) | 149.2 (26.4) | 180.3 (35.3) | 0.44 |
| Nicotine concentration in e-liquid (mg/mL) | 4.2 (0.8) | 10.5 (2.2) | 0.04 |
| Amount of nicotine inhaled (mg) | 0.6 (0.2) | 1.3 (0.1) | 0.02 |
| Amount of nicotine exhaled (mg) | 0.011 (0.007) | 0.012 (0.008) | 0.90 |
| Amount of nicotine retained (mg) | 0.6 (0.2) | 1.3 (0.1) | 0.02 |
| Half-life (min) | 156 (17.9) | 147 (21.4) | 0.52 |
| Tmax (min) | 2.4 (0.4) | 3.7 (0.6) | 0.12 |
| Cmax (ng/mL) | 4.4 (1.2) | 8.0 (1.3) | 0.13 |
| AUC(0→5) (ng/mL•min) | 16.4 (4.7) | 30 (4.8) | 0.06 |
| AUC(0→15) (ng/mL•min) | 49 (15) | 96 (15) | 0.06 |
| AUC(0→30) (ng/mL•min) | 86 (26) | 174 (27) | 0.07 |
| AUC(0→180) (ng/mL•min) | 264 (74) | 554 (93) | 0.10 |
| AUC(0→∞) (ng/mL•min) | 460 (140) | 865 (121) | 0.10 |
| PK-predicted nicotine dose (mg) | 0.6 (0.2) | 1.0 (0.1) | 0.10 |
| Cmax per NIC retained (ng/mL/mg) | 8.2 (0.9) | 6.3 (0.8) | 0.16 |
| AUC(0→5) per NIC retained (ng/mL•min/mg) | 31 (3.2) | 24 (3.0) | 0.25 |
| AUC(0→15) per NIC retained (ng/mL•min/mg) | 90 (8.4) | 76 (8.2) | 0.16 |
| AUC(0→30) per NIC retained (ng/mL•min/mg) | 158 (16) | 137 (13) | 0.37 |
| AUC(0→180) per NIC retained (ng/mL•min/mg) | 507 (63) | 431 (42) | 0.44 |
| AUC(0→∞) per NIC retained (ng/mL•min/mg) | 891 (128) | 678 (40) | 0.16 |
| AUC(0→180) per NIC e-liquid (ng/mL•min/mg/mL) | 57 (7.6) | 62 (10.4) | 0.80 |
| AUC(0→∞) per NIC e-liquid (ng/mL•min/mg/mL) | 100 (16) | 102 (19) | 1.00 |
| Max change in heart rate (beats per min) | 11.0 (3.8) | 7.9 (3.1) | 0.65 |
| Heart rate AUC (beats per min•min) | 183 (85) | 154 (69) | 0.85 |
3.5. Ad libitum session
Participants consumed 43% more of the strawberry e-liquid and the amount of nicotine inhaled was 45% higher with the strawberry e-liquid (5.4 mg) compared to the tobacco e-liquid (4.1 mg) (Table 3). Plasma nicotine was significantly higher with the strawberry e-liquid compared to the tobacco e-liquid at 15, 30, and 45 minutes after starting the ad libitum session (Figure 1B).
Table 3.
Nicotine intake and pharmacokinetics by e-liquid flavor during a 90-minute period of ad libitum access to e-cigarettes
| Variable | Usual brand | Test Flavors | Strawberry vs Tobacco | ||
|---|---|---|---|---|---|
|
| |||||
| Strawberry | Tobacco | ‡ Mean ratio or difference (95% CI) | F (p value) | ||
| Amount of e-liquid used (mg) | 689 (103) | 316 (53) | 242 (56) | 1.43 (0.91, 2.26) | 3.14 (0.11) |
| Amount of nicotine inhaled (mg) | 3.4 (0.5) | 5.4 (0.9) | 4.1 (0.9) | 1.45 (0.92, 2.29) | 3.41 (0.10) |
| T max (min) | 79 (5) | 80 (4) | 84 (3) | −3 (−13, 7) † | 0.56 (0.47) |
| Cmax (ng/mL) | 11.5 (1.7) | 17.1 (3.1) | 11.5 (2.3) | 1.57 (0.89, 2.77) | 3.19 (0.11) |
| C90 min (ng/mL) | 11.2 (1.7) | 16.5 (3.1) | 11.3 (2.3) | 1.56 (0.91, 2.68) | 3.43 (0.10) |
| AUC(0→90) (ng/mL•min) | 628 (102) | 951 (153) | 624 (120) | 1.66 (0.94, 2.95) | 4.07 (0.07) |
| Cmax/NIC inhaled (ng/mL/mg) | 3.5 (0.2) | 3.2 (0.3) | 3.0 (0.2) | 1.08 (0.93, 1.26 | 1.33 (0.28) |
| C90 min/NIC inhaled (ng/mL/mg) | 3.4 (0.2) | 3.1 (0.3) | 2.9 (0.2) | 1.07 (0.95, 1.21) | 1.76 (0.22) |
| AUC(0→90) per NIC inhaled (ng/mL•min/mg) | 190 (12) | 183 (12) | 164 (14) | 1.15 (0.94, 1.40) | 2.38 (0.16) |
| AUC(0→90)/e-liquid NIC (ng/mL•min/mg/mL) | 105.4 (13.8) | 47.8 (7.7) | 32.3 (6.2) | 1.61 (0.91, 2.86) | 3.59 (0.09) |
Values are presented as mean and standard error of the mean (SEM);
Comparisons between the two test flavors are presented as mean ratios of strawberry to tobacco obtained from back-transform of least-square mean differences of log-transformed variables;
Comparisons are differences rather than ratios.
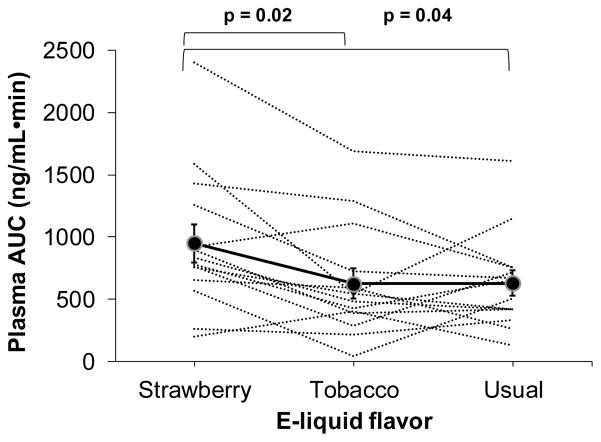
In examining titration of nicotine intake, we found significant differences in AUC0→90 across flavors (F = 3.82, p = 0.039). The ratio of plasma nicotine AUC0→90 between the 3 flavors were as follows (mean and 95% CI): strawberry vs tobacco, 1.66 (1.10–2.52), p = 0.02; strawberry vs usual, 1.55 (1.02–2.34), p = 0.04; and, tobacco vs usual, 0.93 (0.61–1.41), p = 0.72. Figure 2 shows within-subject plasma AUC0→90 and average AUC0→90 across e-liquids. Within-subject correlations between plasma AUCs were as follows: strawberry vs tobacco, 0.80 (p = 0.001); strawberry vs usual, 0.71 (p = 0.004); and, tobacco vs usual, 0.69 (p = 0.007).
Figure 2.
Within-subject area under the plasma nicotine concentration-time curve from 0 to 90 minutes (AUC0→90) and mean plasma AUC0→90 for the strawberry and tobacco test e-liquid flavors and usual brand e-liquids (mean ± SEM) during the 90-minute ad libitum session.
Changes in subjective measures were not significantly different between strawberry and tobacco test e-liquids (Table 4). When the usual e-liquid was included in the analysis, subjects reported lower average satisfaction with the strawberry (p = 0.002) and tobacco (p < 0.001) e-liquids compared to the usual brand e-liquids. Aversion was significantly higher with the strawberry e-liquid compared to the usual e-liquids (p = 0.049). Ratings of enjoyment of sensations in chest and throat were lower for both the strawberry (p = 0.022) and tobacco (p = 0.019) e-liquids compared to the usual brand e-liquids.
4.0. DISCUSSION
To the best of our knowledge, this is the first study to examine the impact of flavors on nicotine pharmacology of commercially available e-cigarettes and e-liquids. We first compared nicotine intake, pharmacokinetics, and physiologic and subjective effects using a standardized session of 15 puffs. The amount of nicotine inhaled and systemically retained were not significantly different between the strawberry and tobacco e-liquids but plasma AUC(0→180) was significantly higher with the strawberry e-liquid. While not significantly different, Cmax was 22% higher and various early time point AUCs to measure rate of rise of nicotine in blood ranged between 17–23% higher with the strawberry e-liquid compared to the tobacco e-liquid, suggestive of differences between e-liquids in the rate of nicotine absorption. The maximum change in heart rate over the first 30 minutes after vaping was, on average, 4.6 bpm higher with the strawberry compared to tobacco e-liquid, a likely effect of higher systemic nicotine exposure and/or more rapid nicotine absorption with the strawberry e-liquid. Despite higher initial nicotine exposure from use of the strawberry e-liquid, participants reported greater enjoyment of the sensations in their throat and chest from use of the tobacco flavor.
The extent of use of the two test e-liquids during ad libitum access paralleled the participants’ subjective ratings of taste; strawberry was rated slightly better than the tobacco flavor. Systemic exposure to nicotine (AUC(0→90)) was on average the same and highly correlated within subjects for the tobacco and usual brand e-liquids, suggesting that e-cigarette users titrate their nicotine intake to a desired level. We also found that AUC(0→90) was significantly higher with the strawberry compared to the tobacco or usual e-liquids, indicating that the extent of titration may differ across e-liquid flavors.
We have previously reported that flavors (the strawberry and tobacco e-liquids) do not affect e-liquid aerosolization/vaporization using vaping machines (Havel et al., 2016) and would likely not affect the amount of nicotine inhaled by users. However, vaping behavior such as puff volume, which is difficult to control in a clinical study, could have contributed to differences in nicotine intake during the 15 puffs. When normalized by the amount of nicotine systemically retained, differences in plasma nicotine AUC(0→180) were no longer significant (strawberry to tobacco ratio of 1.02), suggesting that flavors may not affect overall systemic exposure to nicotine. However, Cmax and early timepoint AUCs (AUC0→5, AUC0→15, and AUC0→30) normalized for retained nicotine dose were between 11–13% higher with the strawberry e-liquid. These differences were not statistically significant but may be indicative of modest flavor effects on the rate of nicotine absorption in the airways.
The tobacco e-liquid (pH, 9.10) was more basic than the strawberry (pH, 8.29) e-liquid, which could have contributed to slower rate of nicotine absorption compared to the strawberry e-liquid. In general, high nicotine-containing e-liquids have higher pH due to the alkalinity of nicotine but flavor additives, which may be slightly acidic, can influence the resulting e-liquid pH (Lisko et al., 2015). At higher pH, more of the nicotine in the tobacco e-liquid is expected to be in the free-base form (unionized) compared to the strawberry e-liquid (Lisko et al., 2015). Due to higher volatility of free nicotine (Pankow et al., 1997), more free nicotine from the tobacco e-liquid would leave the e-liquid solution in the vapor phase, possibly resulting in greater deposition of nicotine in the upper airways. This would lead to slower absorption and lower Cmax compared to nicotine absorbed through the lungs. The greater deposition of vapor phase nicotine in the upper airways would however, lead to increased sensory effects in the upper airways. Indeed, average rating of sensation in the throat was higher for the tobacco e-liquid than the strawberry e-liquid (Table 4). This is consistent with reports of greater upper airway sensory effects from smoking cigarettes with basic additives such as ammonia added (Henningfield et al., 2004). We also found slower rates of absorption (AUCs normalized for retained nicotine dose) and Cmax normalized for retained nicotine dose when we compared the basic usual e-liquids to the acidic usual e-liquids (Table 5). Surprisingly, even though nicotine intake from the basic usual e-liquids was double that of the acidic usual e-liquids, max increase in heart rate was, on average, 3 bpm lower with the basic usual e-liquids. This raises questions about the role e-liquid pH plays on e-cigarette physiologic effects.
Tobacco smokers switching to e-cigarettes can maintain the same nicotine exposure with e-cigarettes as they did with tobacco cigarettes (Goniewicz et al., 2016). Compensatory puffing (e.g. greater consumption) when switching from high nicotine content to low nicotine content e-liquids has been demonstrated before but self-titration was found to be incomplete, i.e. blood nicotine levels were significantly different between conditions (Dawkins et al., 2016). We found that the participants were able to replicate their overall nicotine exposure from the usual e-liquid and tobacco e-liquid with moderately high precision. Since the usual e-liquids had lower average nicotine content than the tobacco e-liquid, the participants consumed almost 3 times more usual e-liquid compared to the tobacco e-liquid to obtain similar nicotine exposure. Although average nicotine exposure between the usual and tobacco e-liquids were similar, the usual brand e-liquid was rated as more satisfying than the tobacco e-liquid, indicating that flavors play an important role in e-cigarette appeal. One study showed that while nicotine produces the throat-hit, it was the sweet flavors and not nicotine content that increased e-cigarette appeal (Goldenson et al., 2016). We did not see similar precise titration of nicotine exposure when we compared the strawberry flavor to the usual brand and tobacco e-liquids, respectively. The perceived better taste of the strawberry flavor likely made it more appealing than the tobacco flavor, and hence greater consumption and nicotine exposure (Goldenson et al., 2016).
Our research has some limitations. First, 466 brands of e-cigarettes and 7764 unique flavors have been identified (Zhu et al., 2014). In our study, we used one brand of e-cigarettes and two test e-liquids along with 14 usual brands of e-liquids. Thus, our study may not be generalizable to all e-cigarettes or flavors. We used a popular brand of e-cigarette (KangerTech) and used two flavors that represent a broad array of flavors (fruit/berry vs. tobacco) (Krishnan-Sarin et al., 2015). Second, we did not use an unflavored e-liquid control, as done previously (Walele et al., 2016). Third, participants and study personnel were not blinded to the e-liquids.
5.0. CONCLUSIONS
Flavors may influence the rate of absorption of nicotine possibly through pH effects, may affect heart rate, and contribute to satisfaction and reward of e-cigarettes. E-cigarette users titrate their overall nicotine exposure but the extent of titration varies across flavors.
Acknowledgments
We thank Ms. May Hao for clinical coordination; Dr. Natalie Nardone for project management; and Kristina Bello, Trisha Mao, and Lisa Yu for performing analytical chemistry. This study was supported by grant number 1P50CA180890-02S1 from the National Cancer Institute and Food and Drug Administration Center for Tobacco Products and P30 DA012393 and R25DA035163 from the National Institute on Drug Abuse and was carried out in part at the Clinical Research Center at Zuckerberg San Francisco General Hospital (NIH/NCRR UCSF-CTSI UL1 RR024131). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the Food and Drug Administration (FDA).
Footnotes
Contributors
Gideon St. Helen and Neal Benowitz were responsible for study concept. All authors assisted in study design. Gideon St. Helen, Christopher Havel, and Delia Dempsey assisted in clinical study procedures. Gideon St. Helen conducted the data analysis and drafted the manuscript. All authors provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
Conflict of interest
Dr. Benowitz has served on smoking cessation advisory boards for Pfizer and has been an occasional consultant to McNeil and GlaxoSmithKline, and has served as a paid expert witness in litigation against tobacco companies. All other authors declare that they have no conflicts of interest.
References
- Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, Christiani DC. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2, 3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ Health Perspect. 2016;124:733–739. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Strasser AA, Wileyto EP. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug Alcohol Depend. 2016;166:263–267. doi: 10.1016/j.drugalcdep.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl V, Lin S, Xu N, Davis B, Wang Y-h, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34:529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Becoña E, Vázquez FL, Fuentes MaJ, del Carmen Lorenzo Ma. Anxiety, affect, depression and cigarette consumption. Pers Individ Dif. 1998;26:113–119. [Google Scholar]
- Behar R, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28:198–208. doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dawkins LE, Kimber CF, Doig M, Feyerabend C, Corcoran O. Self-titration by experienced e-cigarette users: blood nicotine delivery and subjective effects. Psychopharmacology (Berl) 2016;233:2933–2941. doi: 10.1007/s00213-016-4338-2. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Kistler KA, Gillman G, Voudris V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res. 2015;17:168–174. doi: 10.1093/ntr/ntu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health. 2013;10:7272–7282. doi: 10.3390/ijerph10127272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. Final rule Fed Regist. 2016;81:28973. [PubMed] [Google Scholar]
- Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T. Development of a Questionnaire for Assessing Dependence on Electronic Cigarettes Among a Large Sample of Ex-Smoking E-cigarette Users. Nicotine Tob Res. 2015;17:186–192. doi: 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenson NI, Kirkpatrick MG, Barrington-Trimis JL, Pang RD, McBeth JF, Pentz MA, Samet JM, Leventhal AM. Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: Application of a novel methodology. Drug Alcohol Depend. 2016;168:176–180. doi: 10.1016/j.drugalcdep.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P, Benowitz NL. Exposure to Nicotine and Selected Toxicants in Cigarette Smokers Who Switched to Electronic Cigarettes: A Longitudinal Within-Subjects Observational Study. Nicotine Tob Res. 2016:ntw160. doi: 10.1093/ntr/ntw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- Havel CM, Benowitz NL, Jacob P, StHelen G. An electronic cigarette vaping machine for the characterization of aerosol delivery and composition. Nicotine Tob Res. 2016:ntw147. doi: 10.1093/ntr/ntw147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Pankow JF, Garrett BE. Ammonia and other chemical base tobacco additives and cigarette nicotine delivery: issues and research needs. Nicotine Tob Res. 2004;6:199–205. doi: 10.1080/1462220042000202472. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biol Mass Spectrom. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- Khlystov A, Samburova V. Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ Sci Technol. 2016;50:13080–13085. doi: 10.1021/acs.est.6b05145. [DOI] [PubMed] [Google Scholar]
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res. 2015;17:847–854. doi: 10.1093/ntr/ntu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347:330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Morean ME, Camenga DR, Cavallo DA, Kong G. E-cigarette use among high school and middle school adolescents in Connecticut. Nicotine Tob Res. 2015;17:810–818. doi: 10.1093/ntr/ntu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH. Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in e-cigarette cartridges and refill solutions. Nicotine Tob Res. 2015;17:1270–1278. doi: 10.1093/ntr/ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow JF, Mader BT, Isabelle LM, Luo W, Pavlick A, Liang C. Conversion of nicotine in tobacco smoke to its volatile and available free-base form through the action of gaseous ammonia. Environ Science Technol. 1997;31:2428–2433. [Google Scholar]
- Rose JE, Westman EC, Behm FM, Johnson MP, Goldberg JS. Blockade of Smoking Satisfaction Using the Peripheral Nicotinic Antagonist Trimethaphan. Pharmacol Biochem Behav. 1999;62:165–172. doi: 10.1016/s0091-3057(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Sherwood CL, Boitano S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2, 5-dimethypyrazine. Respir Res. 2016;17:57. doi: 10.1186/s12931-016-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Sembower MA, Pillitteri JL, Gerlach KK, Gitchell JG. The impact of flavor descriptors on nonsmoking teens’ and adult smokers’ interest in electronic cigarettes. Nicotine Tob Res. 2015;17:1255–1262. doi: 10.1093/ntr/ntu333. [DOI] [PubMed] [Google Scholar]
- St Helen G, Havel C, Dempsey D, Jacob P, 3rd, Benowitz NL. Nicotine delivery, retention, and pharmacokinetics from various electronic cigarettes. Addiction. 2016a;111:535–544. doi: 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen G, Ross K, Dempsey D, Havel C, Jacob P, 3rd, Benowitz N. Nicotine delivery and vaping behavior during ad libitum e-cigarette access. Tob Regul Sci. 2016b;2:363–376. doi: 10.18001/TRS.2.4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehy ML, Ye W, Hadwiger ME, Moore TW, Allgire JF, Woodruff JT, Ahadi SS, Black JC, Westenberger BJ. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J Liq Chromatogr Relat Technol. 2011;34:1442–1458. [Google Scholar]
- van Rooy FG, Rooyackers JM, Prokop M, Houba R, Smit LA, Heederik DJ. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am J Respir Crit Care Med. 2007;176:498–504. doi: 10.1164/rccm.200611-1620OC. [DOI] [PubMed] [Google Scholar]
- Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, Leavens EL, Tackett AP, Molina N, Queimado L. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control, tobaccocontrol-2016-053041. 2016 doi: 10.1136/tobaccocontrol-2016-053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walele T, Sharma G, Savioz R, Martin C, Williams J. A randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part A: Pharmacokinetics Regul Toxicol Pharmacol. 2016;74:187–192. doi: 10.1016/j.yrtph.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Zhu S-H, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3–iii9. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]